Abstract
Introduction
MD is considered a rare disease. An adequate model that explains MD's pathophysiology is not well established. Recently, the vascular theory of the disease has been revived.
Objectives
To characterize a MD population according to its cardiovascular risk and correlate it to the MD clinical course.
Methods
In this retrospective chart study the data of 31 MD patients observed between January 2017 and April 2018 in a tertiary university hospital were reviewed. Patients included in the study were diagnosed according to the Bárány Society criteria. Lost follow-ups, patients with autoimmune disease, atopy or allergy, major psychiatric disease and migraine were excluded. Age, gender, cardiovascular risk factors, audiometric and vestibular parameters, occurrence of MD attacks in the previous 6 months, vestibular medication in course and time course of MD were recorded and compared between groups (with and without cardiovascular risk factors).
Results
31 patients (61.3% females) mean aged 60.3 years (±9.7) were studied. 74% of the population had at least one risk factor and 51.6% of patients had attacks in the last 6 months. There was a statistically significant difference in the occurrence of MD attacks in the last 6 months (p = 0.014) between MD patients with and without risk factors. Mean PTA thresholds were higher and speech discrimination was lower in individuals with more cardiovascular risk factors.
Conclusions
Treatment of MD focusing on vascular risk factors may allow a better control of symptoms and result in a decreased need for ablative procedures in this disorder.
Keywords: Ménière disease, Cardiovascular disease, Hypertension, Prognosis
1. Introduction
Ménière's disease (MD) is a multifactorial disorder affecting the inner ear characterized by episodic vestibular symptoms associated with sensorineural hearing loss, tinnitus, and aural pressure (Semaan and Megerian, 2011). MD is a rare disease in the general population (Bruderer et al., 2017). Incidence rates are variable among studies: 8.2 to 157 per 100 000 (Espinosa-Sanchez and Lopez-Escamez, 2016). Prevalence of MD is higher in Caucasians (Simo et al., 2015). MD is often diagnosed between 20 and 50 years and its prevalence increases with age (Bruderer et al., 2017). MD is extremely rare in children and literature regarding this theme is scarce (Choung et al., 2006). There is a slight female predominance and no difference exists between the left to right affected ear ratio (Tyrrell et al., 2014). Bilateral MD has been reported in 25–40% of affected individuals (Alexander and Harris, 2010). Five distinct clinical subgroups have already been described in patients with bilateral disease (Frejo et al., 2016).
Although magnetic resonance imaging (MRI) and computed tomography (CT) scan are being increasingly used, MD is diagnosed clinically (Ito et al., 2016). The current diagnostic criteria reflect the consensus among the Bárány Society, Japan Society for Equilibrium Research, EAONO, AAO-HNS and Korean Balance Society (Espinosa-Sanchez and Lopez-Escamez, 2016).
Even though it has been known for about 150 years, MD aetiology and pathophysiology remains controversial. Currently, MD is considered a multifactorial disorder where the combined effect of genetics and environmental factors probably determine the onset of the disease and its phenotypical multiplicity. (Espinosa-Sanchez and Lopez-Escamez, 2016). Endolymphatic hydrops (EH) is a hallmark pathologic alteration of this disease. However, while EH is present in all patients with MD, not all patients with EH develop symptoms (Attyé et al., 2017; C. A. Foster and Breeze, 2013).
An adequate model that explains MD's symptoms/attacks, pathology and epidemiology is not yet established. MD attacks are thought to be a consequence of abnormal inner ear pressure combined with fluid and ion homeostasis disruption due to Reissner's membrane rupture (Gibson, 2017). On the other hand, vasospasm of inner ear small vessels has been proposed as the explanation for migraine-associated vestibular symptoms. This would in turn explain the sudden onset of auditory and vestibular symptoms in such patients (Friberg and Rask-Andersen, 2002; Liu and Xu, 2016).
Recently, the vascular theory of the disease symptoms has been proposed (C. A. Foster and Breeze, 2013; Carol A. Foster, 2015). Some authors consider MD as a cerebrovascular disease and some epidemiologic studies point to a high cardiovascular risk in MD patients, although this association has not been defined (Carol A. Foster, 2015; R. Teggi et al., 2012).
The objectives of this original article are to characterize a MD population from a cardiovascular risk point of view and correlate these findings to the disease phenotype and evolution.
2. Methods
A cross-sectional and retrospective study was designed including patients with definite MD diagnosis according to the criteria defined by consensus among Bárány Society, Japan Society for Equilibrium Research, EAONO, AAO-HNS and Korean Balance Society (Lopez-Escamez et al., 2015). This study was approved by the Institutional Review Board for Clinical Research of our institution. Chart study data of 31 MD patients observed between January 2017 and April 2018 in a tertiary university hospital were reviewed. Data were complemented, when needed, with telephonic or clinic visits. Lost follow-ups, patients with diagnosed or suspected autoimmune disease, history of atopy or positive allergy tests, migraine history and abnormal cerebral magnetic resonances, decompensated psychiatric disease and major depression were excluded. Age, gender, cardiovascular risk factors (CVRFs), such as excessive body mass index (BMI), dyslipidaemia, hypertension, smoking status and type 2 diabetes mellitus were recorded (Table 1).
Table 1.
Cardiovascular risk factors - definition.
| Parameter | Definition |
|---|---|
| Excessive BMI | BMI ≥25 kg/m2 |
| Dyslipidaemia | Total cholesterol ≥220 mg/dL and/or Triglycerides ≥150 mg/dL or Using medication |
| Type 2 Diabetes Mellitus | HbA1c ≥ 6.5% or Fasting glucose ≥126 mg/dL or Using medication |
| Hypertension | Sustained Systolic ≥130 mmHg and/or Sustained Diastolic ≥90 mmHg or Using medication |
The population was divided into two groups: group 1 – without CVRFs (n = 8) and group 2 – with CVRFs (n = 23).
Audiometric and vestibular parameters were compared among groups. Pure Tone Audiometry (PTA) - averaged within the frequencies of 0.5, 1, 2 and 3 kHz - and Speech Discrimination Test (SDT) thresholds of the affected ear in the last audiogram, performed within the preceding year and not during a crisis, were used to characterize the audiometric patients’ profile. Percentage of vestibular hypofunction in caloric testing was used to characterize vestibular profile of patients, when available.
Caloric testing was performed with Hortmann Airmatic® system (type 51, fabrication number 851 513 220), using air cooled to 28° or heated to 44°. Data were collected and interpreted using Windows® software for PC (Ulmer VNG, Version 1.4 SYNAPSIS ®). An asymmetry of at least 20% was considered abnormal.
Regarding vestibular and audiometric parameters, in cases of bilaterality, the poorer ear was used.
Presence of MD attacks in the previous 6 months (self-reported), vestibular medication in course and MD time course (self-reported) were also documented and compared. Attacks were defined as: crises of vertigo with otological symptoms of hearing loss and/or aural fullness and/or tinnitus lasting between 20 min and 12 h.
All reported P values are two-tailed, with a P value < 0.05 indicating statistical significance. Analyses were performed with SPSS version 20.0 (SPSS Inc, IBM Corp, Armonk, NY). Normal distribution was checked using Shapiro-Wilk test or skewness and kurtosis. Categorical variables are presented as frequencies and percentages, and continuous variables as means and standard deviations, or medians and interquartile ranges for variables with skewed distributions. Comparison of the experimental groups was evaluated with the use of Student t-test or Mann-WhitneyU test, Chi-Square test, or Fisher exact test, as appropriate.
3. Results
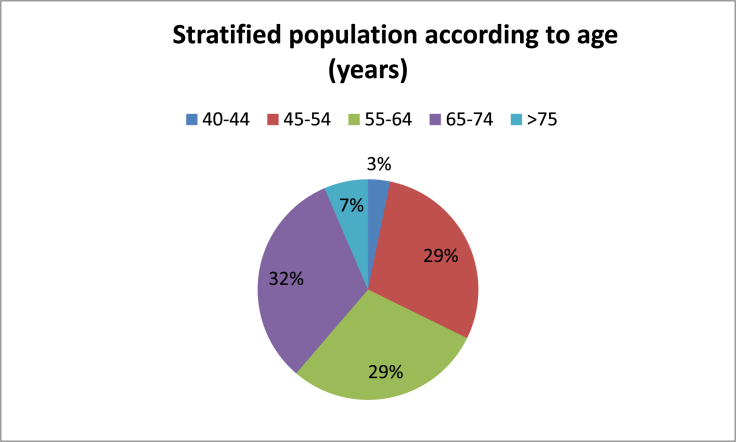
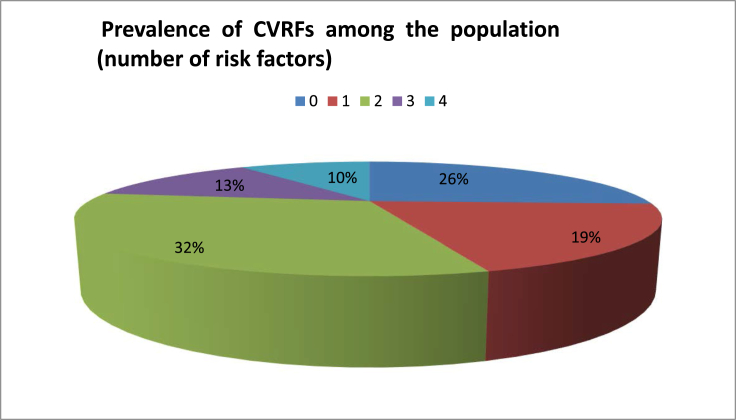
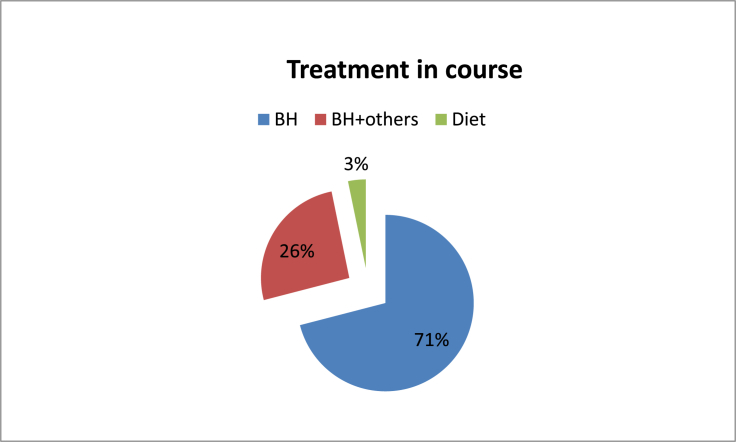
31 subjects (12 men and 19 women) mean aged 60.3 years (±9.7 years), ranging from 41 to 80 years, were studied. Stratified by age, the majority of the population was between 45 and 64 years (58%). In 16 cases the right ear was the affected one (51.6%), while only one patient had bilateral disease (3.2%). There was no statistically significant difference in right to left affected ear ratio. 74% of patients had, at least, one CVRF, while 55% had ≥2 risk factors (Graphic1, Graphic 2). The main CVRF was hypertension (42%) followed by excessive BMI (20%) and dyslipidaemia (20%). Mean PTA threshold was 60.3 dB (±23.3 dB) and median value of SDT was 79.6% (±25.6%) (Table 2). Caloric stimulation tests were performed in 48.4% of patients and were pathological in 100% of these. The mean value of vestibular function percentage loss was 54% (±22.5%) (Table 2). According to self-reported data, mean MD course disease was 7.7 years (±5.6 years) (Table 2). In general, 16 (51.6%) of the patients reported, at least, one MD attack during the previous 6 months (Table 2). The majority of the patients (71%) were using daily beta-histine at variable dosages. All patients under double medical therapy were taking a hydrochlorothiazide (50 mg) plus amiloride hydrochloride (5 mg) association, besides beta-histine in optimal dosage (Graphic 3).
Graphic1.
Stratified population according to age (years).
Graphic 2.
Prevalence of CVRFs among the population (number of risk factors).
Table 2.
Demographic and audio-vestibulometric data of MD population.
| MD population (n = 31) | |
|---|---|
| Age, mean (±SD) | 60.3 (±9.7) |
| Gender, number of women (%) | 19 (61.3%) |
| Affected ear – right, number (%) | 16 (51.6%) |
| Bilateral, number (%) | 1 (3.2%) |
| Number of patients with ≥1 CVRfs | 23 (74%) |
| PTA, mean (±SD) | 60.3 (±23.3) |
| SDT, median (±IQR) | 79.6% (25.6%) |
| Nº of patients reporting attacks in the last 6 months (%) | 16 (51.6%) |
*CVRFs – cardiovascular risk factors, IQR – interquartile range, SD – standard deviation, SDT - speech discrimination test thresholds, PTA – pure tone audiometry thresholds.
Graphic 3.
Treatment in course.
A cerebellopontine angle and internal auditory canal evaluation through MRI was requested in 48% of patients. MRI was normal in all studied cases.
Demographic and audio-vestibulometric data of MD population are summarized in Table 2.
Comparing the two groups (group 1: without CVRFs and group 2: with CVRFs), there was statistically significant difference in the number of MD attacks stated in the previous 6 months (p = 0.014) and the more CVRFs patients had, the more probable was for them to describe an attack in the previous 6 months, independently of their age (Table 3, Table 4). There were no statistically significant differences between groups when comparing the percentage of hypovalence in caloric testing. However, patients in group 2 showed poorer results in the caloric test (Table 3).
Table 3.
Comparisons between groups.
| Group 1 (n = 8) | Group 2 (n = 23) | P value | |
|---|---|---|---|
| Presence of attacks <6 months, number of patients affected (%) |
1 (12.5%) |
15 (65.2%) |
0.014 |
| Group 1 (n = 4) |
Group (n 11) |
P Value |
|
| % of hypovalence in caloric testing, mean (±SD) | 39.8 (±18.6) | 51.5 (±21.1) | 0.34 |
Table 4.
Percentage of patients reporting at least one attack in the previous 6 months, according to the number of CVRFs.
| Number of CVRFs | Number of patients affected with this number of CVRFs (n = 31) | Mean age | % of patients reporting at least one attack in the previous 6 months |
|---|---|---|---|
| Zero | 8 | 54.9 | 12.5% |
| One | 6 | 59,6 | 33% |
| Two | 10 | 65,9 | 60% |
| Three | 4 | 64,3 | 75% |
| Four | 3 | 64,9 | 100% |
Although the mean PTA thresholds were higher and the SDT results lower in patients with CVRFs, there were no statistically significant differences between groups (Table 5). Age did not influence the results, since there were no statistically significant differences between age in groups 1 and 2 when considering disease evolution (Table 5).
Table 5.
Comparisons between groups, stratified by disease evolution.
| 0–4 years of MD (n = 11) |
p-value | 5–9 years of MD (n = 12) |
p-value | ≥10 years of MD (n = 6) |
p-value | ||||
|---|---|---|---|---|---|---|---|---|---|
| Group 1 (n = 5) | Group 2 (n = 6) | Group 1 (n = 2) | Group 2 (n = 10) | Group 1(n = 1) | Group 2(n = 5) | ||||
| PTA, mean (±SD) | 58 (±27) | 70 (± 19.7) | 0.4 | 37.5 (±10.6) | 58 (±18.6) | 0.17 | 10 | 59 (±23.8) | 0.1 |
| SDT, median (±IQR) | 92 (±12) | 69 (34.6) | 0.1 | 90 (±14.1) | 85 (±19.3) | 0.7 | 100 | 76 (±29.6) | 0.4 |
| Age, mean years (±SD) | 51(±7) | 61.1 (±9) | 0.055 | 53.5 (±21.9) | 61.9 (±7.8) | 0.31 | 68 | 68.2 (±7.0) | 0.6 |
Note.: Unknown evolution of disease −2 cases (missing data).
*SDT - speech discrimination test thresholds, PTA – pure tone audiometry thresholds.
4. Discussion
Our results are in accordance to the literature regarding age of diagnosis and laterality (Watanabe et al., 1995). There was a female preponderance of approximately 2:1, which is similar to previously reported in the literature (Bruderer et al., 2017). In our sample, we have noted that MD prevalence increases with age; this is similar to what happens regarding the cardiovascular risk factors, especially in women, when the protective effects of oestrogen disappear (Alexander and Harris, 2010; Baker et al., 2003).
In our institution, MD patients are followed annually. However, when the disease decompensates or it is in its earlier stages, trimestral or semester following are also options. The treatment options used in our sample aim to control ear and vertigo symptoms. The majority of patients (71%) are well controlled with self-adjustable doses of beta-histine, lifestyle adjustments and hypossaline diet. These findings are in line with the majority of the published data (Martín González et al., 2010).
According to our practice, caloric testing is used in cases where clinical vestibular hypofunction is noticed on the physical exam, and not as a routine to each patient diagnosed with MD. Similarly, as MRI testing is of very low yield in patients with “definite” or “probable” MD, we do not use it on a routine basis (Robinette et al., 2018).
Hearing loss in Meniere's disease early course is characterized by fluctuating hearing loss limited to the low frequencies. During the disease's evolution, hearing loss involves medium and high frequencies as well (Savastano et al., 2006). In our sample, patients with CVRFs showed higher PTA thresholds and lower speech discrimination; independently of disease course. The most expressive result was the self-reported presence of attacks, defined by crises of vertigo with ipsilateral hearing loss and/or aural fullness and/or tinnitus lasting between 20 min and 12 h. Patients in group 2 reported crisis in the last 6 months more often, and the percentage of patients reporting this was higher if more CVRFs they had. In fact, all patients with 4 CVRFs reported, at least, one MD attack in the last 6 months.
These findings point to a possible influence of the CVRFs over the course and severity of the disease. Indeed, according to our results, our MD population, when excluding potential aetiological factors, such as allergy/atopy, auto-immune disease and migraine, seem to have a high overall cardiovascular risk, especially due to hypertension. Teggi et al., in 2012, studied an elderly MD population. They found that elderly subjects presented a more “aggressive” evolution of MD and hypothesized that vascular disorders could act as a predisposing factor for MD (R. Teggi et al., 2012). Frejo et al., in 2016, studying clinical phenotypes in bilateral MD also found a group defined by synchronic hearing loss without migraine or auto-immune disease with a vascular risk profile which did not overlap with the other 4 phenotypes described. These authors recommend further studies to assess the role of inner ear microvasculature circulation in MD (Frejo et al., 2016).
Curiously, mutations in a variety of genes involved in ionic composition and/or water transport or even cardiovascular development have already been linked to MD aetiology, namely: aquaporins 1, 2, 3, 4, and 5, antiquin, potassiumvoltage-gated channel subfamily E regulatory subunit 1 and 3, Adducin 1, 2 and 3 are linked to water transport and dermatopontin and semaphoring III are linked to cardiovascular development (Chiarella et al., 2015; Espinosa-Sanchez and Lopez-Escamez, 2016; Martín-Sierra et al., 2017). On the other hand, cardiovascular risk factors such as hypertension, smoking, obesity, diabetes and altered lipid profile are well known causes of microvasculature compromise, oxidative stress, brain-blood and blood-labyrinth barriers damage making the cerebrum, and potentially the inner ear, target end-organs of the cardiovascular disease spectrum (González-marrero et al., 2013; Takemori et al., 2013). Actually, Roberto Teggi et al. focused on the levels of Chromogranin A (CgA) in MD patients. CgA plays an important role in the endothelial barrier function and vascular homeostasis; they found that levels of CgA change in MD related to the frequency of vertigo spells and time from the last attack, suggesting a relationship between the inner ear and the cardiovascular system (Teggi et al., 2015).
Besides, the relationship between inner ear and kidney has also been recently studied. Nowadays, the endolymphatic sac (ES) is considered an endocrine structure similar to the kidney at the molecular level (Møller et al., 2017; Pirodda et al., 2012). Recently, the vasopressin-aquoporin-2 system in the inner ear was proposed to mediate the endolymph homeostasis (Wu et al., 2017). Vasopressin, a neurohypophyseal peptide, is also broadly in control of the cardiovascular system (Karbek et al., 2014). Hence, elevation of plasma vasopressin, strictly related to salt-sensitive hypertension, might be one of the causative factors underlying MD symptoms (Katagiri et al., 2014; Kitahara et al., 2009; Naganuma et al., 2006).
On the other hand, the location of the ES intimately nearby the sigmoid sinus/jugular bulb may explain the relationship between systemic intracranial pressure and systemic fluid volume regulation(C. A. Foster and Breeze, 2013). Moreover, coenzyme Q10 (ubiquinone), which is a potent cardiovascular modulator, is useful in arteriosclerosis, ischemic heart disease, hypertension, oxidative stress and also was found to be helpful in MD (Kimura et al., 2008).
Finally, MD treatment is based on enhancing inner ear vascularization and reducing inner ear fluid volume. Reduction of salt content in diet remains one of the most important aspects of symptomatic treatment, along with the use of diuretics. Both interact directly with cardiovascular risk by reducing arterial blood pressure (Carol A. Foster, 2015).
To date, some studies report a possible association between MD and cardiovascular comorbidities (C. A. Foster and Breeze, 2013; Carol A. Foster, 2015; Frejo et al., 2016; Pirodda et al., 2014; Teggi et al., 2012; Teggi et al., 2015; Tyrrell et al., 2014). However, only Frejo et al. established a possible vascular phenotype of bilateral MD.
Finally, Foster at al. went further, and stated that every patient with MD attacks has one or more major risk factors for cerebral ischemia, including vascular disorders and/or chronic hypoxia. They predicted that those without vascular risk factors will not have such attacks and, ultimately, they conceptualize MD as a cerebro-vascular disease(Carol A. Foster, 2015).
Limitations of this study include the small population and self-reported statement of MD onset and reference of attacks in the previous 6 months, although the definition of “attack” was well established and judicious inclusion and exclusion criteria were applied.
5. Conclusions
To our knowledge, this is the first study describing a MD population according to its cardiovascular risk factors aiming to establish a new phenotype of the disease. Cerebro-vascular dysfunction, mainly due to cardiovascular risk factors, could influence the course of MD and may be responsible for a specific disease phenotype with a worse prognosis. If this association is correct, evaluation and treatment of common vascular risk factors should be approached in every MD patient with new treatment options. MD could be, in the future, considered an early precursor of cerebrovascular disease and even vascular dementia. These findings prepare the way for future studies to identify the pathological pathways of Ménière's disease.
Disclosures
Nothing to disclosure.
Fundings
None.
Footnotes
Peer review under responsibility of PLA General Hospital Department of Otolaryngology Head and Neck Surgery.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.joto.2019.01.004.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Alexander Thomas H., Harris Jeffrey P. Current epidemiology of Meniere's syndrome. Otolaryngol. Clin. 2010;43(5):965–970. doi: 10.1016/j.otc.2010.05.001. [DOI] [PubMed] [Google Scholar]
- Attyé Arnaud. MRI of endolymphatic hydrops in patients with Meniere's disease: a case-controlled study with a simplified classification based on saccular morphology. Eur. Radiol. 2017;27(8):3138–3146. doi: 10.1007/s00330-016-4701-z. [DOI] [PubMed] [Google Scholar]
- Baker Lauren. The role of estrogen in cardiovascular disease. J. Surg. Res. 2003;115(2):325–344. doi: 10.1016/s0022-4804(03)00215-4. [DOI] [PubMed] [Google Scholar]
- Bruderer Saskia G. Population-based study on the epidemiology of Ménière’s disease. Audiol. Neurotol. 2017:74–82. doi: 10.1159/000475875. [DOI] [PubMed] [Google Scholar]
- Chiarella Giuseppe, Petrolo C., Cassandro E. The genetics of Ménière’s disease. Appl. Clin. Genet. 2015;8:9–17. doi: 10.2147/TACG.S59024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choung Y.H. Rare cases of Ménière’s disease in children. J. Laryngol. Otol. 2006;120(4):343–352. doi: 10.1017/S0022215106000569. http://www.ncbi.nlm.nih.gov/pubmed/16623983 [DOI] [PubMed] [Google Scholar]
- Espinosa-Sanchez J.M., Lopez-Escamez J.A. first ed. Elsevier B.V; 2016. 137 Handbook of Clinical Neurology Menière’s Disease. [DOI] [PubMed] [Google Scholar]
- Foster C.A., Breeze R.E. The Meniere attack: an ischemia/reperfusion disorder of inner ear sensory tissues. Med. Hypotheses. 2013;81(6):1108–1115. doi: 10.1016/j.mehy.2013.10.015. [DOI] [PubMed] [Google Scholar]
- Foster Carol A. Optimal management of Ménière’s disease. Therapeut. Clin. Risk Manag. 2015;11:301–307. doi: 10.2147/TCRM.S59023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frejo Lidia. Clinical subgroups in bilateral Meniere disease. Front. Neurol. 2016;7(OCT):1–10. doi: 10.3389/fneur.2016.00182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friberg Ulla, Rask-Andersen Helge. “Vascular occlusion in the endolymphatic sac in Meniere's disease. Ann. Otol. Rhinol. Laryngol. 2002;111(3 Pt 1):237–245. doi: 10.1177/000348940211100308. [DOI] [PubMed] [Google Scholar]
- Gibson William P.R. vol. 4. 2017. pp. 4–5. (Revisiting the Cause of the Attacks of Vertigo during Meniere ’ S Disease). [Google Scholar]
- González-marrero Ibrahim. 2013. High Blood Pressure Effects on the Blood to Cerebrospinal Fluid Barrier and Cerebrospinal Fluid Protein Composition : A Two-Dimensional Electrophoresis Study in Spontaneously Hypertensive Rats. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito T. Endolymphatic space size in patients with Meniere's disease and healthy controls. Acta Otolaryngol. 2016;6489:1–4. doi: 10.3109/00016489.2016.1169556. January 2017. [DOI] [PubMed] [Google Scholar]
- Karbek Basak. Copeptin, a surrogate marker for arginine vasopressin, is associated with cardiovascular risk in patients with polycystic ovary syndrome. J. Ovarian Res. 2014;7(1):31. doi: 10.1186/1757-2215-7-31. http://ovarianresearch.biomedcentral.com/articles/10.1186/1757-2215-7-31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katagiri Yoshiaki, Takumida Masaya, Hirakawa Katsuhiro, Anniko Matti. “Long-Term administration of vasopressin can cause Ménière’s disease in mice. Acta Otolaryngol. 2014;134(10):990–1004. doi: 10.3109/00016489.2014.902989. http://www.tandfonline.com/doi/full/10.3109/00016489.2014.902989 [DOI] [PubMed] [Google Scholar]
- Kimura Ikuko. vol. 54. 2008. pp. 571–575. (Can Coenzyme Q 10 Lead to Improvement of Essential Hypertension ?: A Long-Term Case Study). 5. [Google Scholar]
- Kitahara Tadashi. vol. 30. Otology & neurotology : official publication of the American Otological Society, American Neurotology Society [and] European Academy of Otology and Neurotology; 2009. pp. 812–819.http://www.ncbi.nlm.nih.gov/pubmed/19638944 (Plasma Vasopressin and V2 Receptor in the Endolymphatic Sac in Patients with Delayed Endolymphatic Hydrops). 6. [DOI] [PubMed] [Google Scholar]
- Liu Yuan F., Xu Helen. The intimate relationship between vestibular migraine and Meniere disease: a Review of pathogenesis and presentation. Behav. Neurol. 2016;2016 doi: 10.1155/2016/3182735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Escamez, Jose A. Diagnostic criteria for Menière’s disease. J. Vestib. Res.: Equilib. Orientat. 2015;25(1):1–7. doi: 10.3233/VES-150549. [DOI] [PubMed] [Google Scholar]
- Martín-Sierra Carmen. Variable expressivity and genetic heterogeneity involving DPT and SEMA3D genes in autosomal dominant familial Meniere's disease. Eur. J. Hum. Genet. 2017;25(2):200–207. doi: 10.1038/ejhg.2016.154. http://www.nature.com/doifinder/10.1038/ejhg.2016.154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martín González C. Medical Management of Ménière’s disease: a 10-year case series and Review of literature. Eur. Arch. Oto-Rhino-Laryngol. 2010;267(9):1371–1376. doi: 10.1007/s00405-010-1265-4. [DOI] [PubMed] [Google Scholar]
- Møller, Martin Nue. The human endolymphatic sac expresses natriuretic peptides. Laryngoscope. 2017;127(6):E201–E208. doi: 10.1002/lary.26074. http://doi.wiley.com/10.1002/lary.26074 [DOI] [PubMed] [Google Scholar]
- Naganuma Hideaki, Kawahara Katsumasa, Tokumasu Koji, Okamoto Makito. Water may cure patients with Meniere disease. Laryngoscope. 2006;116(8):1455–1460. doi: 10.1097/01.mlg.0000225904.78569.0c. http://www.ncbi.nlm.nih.gov/pubmed/16885753 [DOI] [PubMed] [Google Scholar]
- Pirodda Antonio, Arrigo Francesco Giuseppe Cicero, Borghi Claudio. Kidney disease and inner ear impairment: a simpler and closer pathogenic analogy? Intern. Emerg. Med. 2012;7(Suppl. 2):93–95. doi: 10.1007/s11739-011-0703-7. [DOI] [PubMed] [Google Scholar]
- Pirodda Antonio, Arrigo Francesco Giuseppe Cicero, Brandolini Cristina, Borghi Claudio. Inner ear symptoms: can we use them to approach cardiovascular diseases? Intern. Emerg. Med. 2014;9(8):825–827. doi: 10.1007/s11739-014-1130-3. [DOI] [PubMed] [Google Scholar]
- Robinette Kyle. Diagnostic yield of MRI of the brain and IAC in patients with neurotologic complaints. Am. J. Otolaryngol. 2018;39(6):664–669. doi: 10.1016/j.amjoto.2018.06.012. [DOI] [PubMed] [Google Scholar]
- Savastano Marina, Guerrieri Vincenzo, Marioni Gino. Evolution of audiometric pattern in Meniere's disease: long-term survey of 380 cases evaluated according to the 1995 guidelines of the American academy of otolaryngology-head and neck surgery. J. Otolaryngol. 2006;35(1):26–29. doi: 10.2310/7070.2005.4092. [DOI] [PubMed] [Google Scholar]
- Semaan Maroun T., Megerian Cliff A. Ménière’s disease: a challenging and relentless disorder. Otolaryngol. Clin. 2011;44(2):383–403. doi: 10.1016/j.otc.2011.01.010. [DOI] [PubMed] [Google Scholar]
- Simo Hermann. Meniere's disease: importance of socioeconomic and environmental factors. Am. J. Otolaryngol - Head and Neck Med. Surg. 2015;36(3):393–398. doi: 10.1016/j.amjoto.2015.01.009. [DOI] [PubMed] [Google Scholar]
- Takemori Kumiko, Murakami Tetsuo, Kometani Takashi, Ito Hiroyuki. Possible involvement of oxidative stress as a causative factor in blood-brain barrier dysfunction in stroke-prone spontaneously hypertensive rats. Microvasc. Res. 2013;90:169–172. doi: 10.1016/j.mvr.2013.08.005. [DOI] [PubMed] [Google Scholar]
- Teggi R. Does Ménière’s disease in the elderly present some peculiar features? J. Aging Res. 2012;2012:10–15. doi: 10.1155/2012/421596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teggi Roberto. Altered chromogranin A circulating levels in Meniere's disease. Dis. Markers. 2015;2015 doi: 10.1155/2015/643420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyrrell Jessica S. Prevalence, associated factors, and comorbid conditions for Ménière’s disease. Ear Hear. 2014;35(4):e162–e169. doi: 10.1097/AUD.0000000000000041. http://content.wkhealth.com/linkback/openurl?sid=WKPTLP:landingpage&an=00003446-201407000-00018 [DOI] [PubMed] [Google Scholar]
- Watanabe Yukio. Epidemiological and clinical characteristics of Meniere's disease in Japan. Acta Otolaryngol. 1995;115(S519):206–210. doi: 10.3109/00016489509121906. [DOI] [PubMed] [Google Scholar]
- Wu Jing. A mysterious role of arginine vasopressin levels in Ménièreʼs disease—meta-analysis of clinical studies. Otol. Neurotol. 2017;38(2):161–167. doi: 10.1097/MAO.0000000000001310. http://content.wkhealth.com/linkback/openurl?sid=WKPTLP:landingpage&an=00129492-201702000-00001 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.