Abstract
Biochar as a carbon-rich highly porous substance has been proposed for use in agriculture and horticulture as a soil amendment. One of the main concerns of this application of biochar is its potential contamination with heavy metals (HMs) and polycyclic aromatic hydrocarbons. The aim of this research was to access the environmental risk of biochar used as a soil amendment on soil mesofauna (mites and springtails). We conducted both field and laboratory experiments with the use of wood-chip biochar from low-temperature (300 °C) flash pyrolysis. Biochar was free from polycyclic aromatic hydrocarbons (PAH), and the concentration of all tested toxic compounds was very low or even under the level of detection. Both the results of field and laboratory studies show no toxic effects on soil mesofauna. In the field studies, the biochar application of 50 t/ha in maize and oilseed rape crops significantly increased the mean number of mesofauna. This change probably resulted from improved soil chemical properties (in particular organic carbon content and cation exchange capacity) upon biochar addition. The results of the avoidance test with the use of springtail species Folsomia candida showed the possible short-term toxicity risk from a dose of 5%. The results of the reproduction test indicate the negative response of F. candida from the rate of 25% (higher than the field dose, which corresponds to 10% in laboratory tests). The reason for the short-term toxicity might be the considerable increase in soil pH after biochar addition. To our knowledge, this is the first study that has looked so widely into the effect of biochar on soil mesofauna. We encourage further studies into the risk assessment of biochar on soil organisms in both a controlled laboratory environment and in the open field.
Keywords: Biochar, Springtails, Mites, Soil quality, Avoidance, Reproduction
Introduction
Biochar is one of the products of biomass gasification in a certain range of temperatures and with limited oxygen access, also called pyrolysis. It is a solid, carbon-rich, stable, and highly recalcitrant residue (Hansen et al. 2015). Due to the specific physical and chemical nature of biochar, it has been proposed as a soil amendment to improve soil bulk density, soil pH of acid soils, to increase the stability of organic soil’s matter content, and to improve nutrient retention through cation adsorption (Lehmann et al. 2006; Liang et al. 2006; Domene et al. 2014; Marks et al. 2014; Latawiec et al. 2017b). Biochar has also been shown to improve soil aggregate stability and water availability (Ma et al. 2016; Zhang et al. 2017). Knowledge about the consequences of the use of biochar on the environment in general is, however, scarce (Kuppusamy et al. 2016; Latawiec et al. 2017a). One of the main concerns of biochar use as a soil amendment is its potential contamination with heavy metals (HMs) and polycyclicaromatic hydrocarbons (PAH) (Freddo et al. 2012). Pyrolysis technologies have been shown to increase the potential pollutant concentration of the original feedstock due to mass losses (Méndez et al. 2012; Farrell et al. 2013). Generally, the heavy metal content and alkalify of biochar can increase with the pyrolysis temperature (Chen et al. 2014). However, heavy metals are also characteristic for biochars from low-temperature pyrolysis (Domene et al. 2015a; b). Additionally, temperatures of pyrolysis up to 500 °C can result in maximum concentrations of PAH in biochar (Keiluweit et al. 2012). In the study on the effects of pyrolysis temperature on the potential toxicity of biochar, PAH were mostly produced at temperatures of 300–400 °C (Lyu et al. 2016). Also, different kinds of dioxins were found in biochars produced at temperatures from 250 to 900 °C (Hale et al. 2012).
An important aspect related to the use of biochar in agricultural crops is its effect on soil biota and ecosystem services (McCormack et al. 2013). Positive effects of biochar on microbial abundance and activity have been reported in many studies (Pietikäinen et al. 2000; Steiner et al. 2004; Birk et al. 2009; Zhang et al. 2017). Conversely, the changes in microbial community composition may affect nutrient cycles, plant growth, and the cycling of organic soil matter (Kuzyakov et al. 2009; Liang et al. 2010). The interaction between biochar and earthworm abundance and activity is also relatively well studied in field trials (Topoliantz and Ponge 2005; Tammeorg et al. 2014) and in laboratory tests (Liesch et al. 2010; Li et al. 2011; Malińska et al. 2016; Malińska et al. 2017). The response of other groups of soil biota to biochar is far less studied. Soil mesofauna are mostly represented by springtails (Hexapoda: Collembola) and soil mites (Arachnida: Acari), accounting for about 95% of the total number of arthropods inhabiting soil (Neher and Barbercheck 1998). Most mesofauna representatives live mostly in the surface soil layer, down to 10 cm (Rusek 1998; Waikhom et al. 2006; Gulvik 2007). Both groups respond relatively quickly to any changes in the soil environment and are commonly used as bioindicators of biological soil quality (Santofuro et al. 2012). Lehmann et al. (2010) claim that soil arthropods might be affected by biochar in relation to change in microorganisms’ biomass. Bunting and Lundberg (1987) found that springtails could feed on charcoal, but it is unclear whether it is beneficial to those organisms. Physical soil properties modified by biochar may also impact soil mesofauna. Large internal soil surface areas and pores are important for biological processes (Tisdall and Oades 1982). Improved bulk density and soil porosity may create better habitats for soil mesofauna (Twardowski et al. 2016). Also, the increase of pH as well as the improvement of water regime may directly affect soil microarthropod activity and abundance (Van Straalen and Verhoef 1997; Bardgett 2005).
The most widely used tests for biochar risk assessment are ecotoxicological bioassays, in particular of the well-studied springtail species Folsomia candida (Collembola: Isotomidae). Such tests have been successfully used with different biochars and doses on invertebrates (Domene et al. 2007; Domene et al. 2015a, b; Marks et al. 2014, 2016). The impact of biochar on springtails and mites, as the most abundant mesofauna representatives, within open-field conditions, fundamental for a better understanding of biochar application in practice is, however, relatively little studied. To diminish this knowledge gap, we conducted the study to determine the impact of biochar on soil mesofauna (primarily mites and springtails) in both laboratory and field conditions. The aim of our research was to evaluate the environmental risk of biochar used as a soil amendment to soil mesofauna (mites and springtails) in poor agricultural soil.
Materials and methods
Experimental design
The field experiment was set up in mid-April 2014 in the suburbs of Opole, Poland (50.5740 N, 17.8908 E) on poor (sandy and weakly acidic) agricultural soils and continued until October 2016. The climate of the area is moderately warm, with an average annual temperature of 8.4 °C and an average annual rainfall of 611 mm. The selected plants were maize and oilseed rape on account of these being common agricultural crops in Europe. Within each crop two treatments were compared: biochar and no biochar as a control, three replicates each. Each experimental plot was 3 × 3 m.
Before the start of the experiment the area was conventionally used as an agricultural field. The forecrop was maize. Pine chip biochar was applied up to a depth of 30 cm at a rate of 50 t/ha and was mixed by plowing, followed by sowing maize and oilseed rape seeds. In the field trials, no pesticides were used. Weeds were harvested manually a few times during the vegetation season. The maize variety was P8745 (FAO 250, Pioneer Company) and oilseed variety Monolit. The fertilizer in oilseed rape was ammonium sulfate 34% in a dose of 300 kg/ha, and in maize ammonium phosphate (Polydap) in a dose of 25 kg/ha. The same amount of fertilizers was applied in biochar and control treatments.
Analysis of biochar and soil properties
The biochar used in the experiment was the product of low-temperature flash pyrolysis (300 °C) of pine and spruce chips. It is an industrial biochar produced by Fluid Company. The heating value of the tested biochar was 25 MJ/kg. The production had the capacity of 24 m3 of the plant chips/h, which is equivalent to the product in up to 2 MG of biochar/h. Before application, selected properties of biochar (pH, organic carbon content, cation exchange capacity, and heavy metal content) were analyzed according to International Biochar Initiative (IBI) Standard Product Definition and Product Testing Guidelines (IBI 2014; Table 1). The particle size fraction of biochar applied on the field was more than 2 mm (sieve method).
Table 1.
The chemical characteristic of biochar used in the experiment
| Measured values | Limit valuesa | |
|---|---|---|
| pH H2O | 8.2 | – |
| CEC (cmol/kg) | 30.53 | – |
| Carbon content (% of DM) | 52.3 | > 50 |
| H/Corg ratio | 0.026 | < 0.7 |
| NH4+ (mg/l) | 0.04 | – |
| NO3− (mg/l) | 0.08 | – |
| Na+ (cmol/kg) | 2.09 | – |
| K+ (cmol/kg) | 5.10 | – |
| Mg2+ (cmol/kg) | 1.80 | – |
| Ca2+ (cmol/kg) | 30.5 | – |
| Pb (g/t DM) | 1.57 | < 150 |
| Mn (g/t DM) | 29.7 | |
| Cu (g/t DM) | 0.50 | < 100 |
| Hg (g/t DM) | 0.32 | < 1 |
| Zn (g/t DM) | 13.04 | < 400 |
| Cd (g/t DM) | 2.0 | < 40 |
| Total PAH (sum of 16 EPAs PAH) (g/t DM) | < 0.001 | < 6 |
| (g/t DM) | < 0.001 | – |
| (g/t DM) | 7.63 | – |
| (g/t DM) | 0.05 | – |
| (g/t DM) | – | – |
| (g/t DM) | 3.5 | – |
aAccording to IBI (2014)
Three times a year, the main soil physicochemical properties were measured (pH, organic carbon content, cation exchange capacity). Five samples were taken from each plot from a depth of 10 cm. Acidity (pH) in deionized H2O (w/v) was measured with a pH meter in soil samples in a standard dilution of 1:5 and in biochar in a modified dilution of 1:20 after 24 h equilibration on a shaker, according to Rajkovich et al. (2011). Cation exchange capacity (CEC) of control and biochar-amended soil was estimated from the equation between 1 M KCl exchangeable acidity and exchangeable Ca2+, Mg2+, Na+, and K+ measured at pH 7 with 1 M ammonium acetate (Reeuwijk 2002) on an MP 4200 Agilent spectrometer. Soil sorption capacity was estimated from the equation between 1 M KCl exchangeable acidity and CEC measured at pH 7 with 1 M ammonium acetate (Reeuwijk 2002) and compared biochar-amended soil with results from control soil.
The cation exchange capacity of biochar was calculated as a sum of Ca2+, Mg2+, Na+, K+, and NH4+ (Singh et al. 2017). Total organic carbon content was measured by dry combustion on a CHNS analyzer, CE Instruments. Total contents and bioavailable forms of Pb, Zn, Cu, Cd, Ni, and Mn in soil and biochar were measured on an MP 4200 Agilent spectrometer after sample digestion in 70% HNO3 (1:10 ratio) in a microwave system, Start D Milestone Instruments (EPA 2012) for total amounts or after extraction with distilled water in a 1:40 ratio (shaken for 2 h). Mercury content was measured in dry samples on an Hg analyzer MA-2, Nippon Instruments Corporation (NIC). Total PAH content in biochar was measured on HPLC Varian Co.
Soil mesofauna field and laboratory studies
Field studies
Soil samples for mesofauna analysis were taken from all experimental parcels during plant growth. Each year soil was taken at a frequency of 1 month on three dates from May to July. During the soil sampling in years 2015–2016, maize was in growth stages according to the BBCH scale: 10–15, 32–37, and 61–67 and oilseed rape in stages 60–69, 72–79, and 83–89. The samples were taken with the use of a soil sampler (diameter 5 cm and depth 10 cm). The volume of one sample was 196 cm2. Soil invertebrates were extracted over 24 h from the soil samples with the use of Tullgren funnels modified by Murphy (1956). After the extraction, the springtails and mites were kept in 75% ethyl alcohol. Apart from springtails and mites, other mesofauna representatives occurred in the samples: mainly nematodes, proturans, spiders, and insect larvae. The mean abundance of these groups in samples was less than 5%.
Laboratory studies
For the avoidance and reproduction test we used the Collembola species Folsomia candida Willem 1902, which is one of the recommended springtail species for ecotoxicology tests. F. candida is a parthenogenetic, predominantly fungivorus species, living in the porous soil space International Standard Organization (ISO 1999a, b; Fountain and Hopkin 2005). The genus Folsomia was one of the species found in soil samples from the field experiment. The test species comes from a certified laboratory culture, obtained from the Institute of Industrial Organic Chemistry in Pszczyna, Poland. Before the test, the springtail was reared for 6 months on a plaster of Paris/charcoal substrate and fed with dry baker’s yeast. The culture was kept in a climatic chamber at a temperature of 21 °C, with the photoperiod 12 h light and 12 h dark. The culture was synchronized before the test.
The soil used in the tests was sampled from the control plots of oilseed rape and maize in early spring 2016. Before the test soil was sieved to 5 mm. Biochar was sieved to 2 mm and mixed with soil. The homogeneous volume, soil from oilseed rape or maize crop, was prepared in the following concentrations: 1, 1.5, 2.5, 5, 10, 25, and 50%. The analyzed concentrations correspond to doses of biochar per ha from 4.8 to 404 t (calculated on the basis of weight on biochar applicate on the field, and the specific gravity of the soil from experimental plots). The dose 50 t/ha in the experiment corresponds to the volume—percentage dose 10.0% (1.88% determined by weight). For each concentration, as well as for the control soil, the water holding capacity (WHC) as well as the pH were determined. Two days before the analysis, the moisture content of each sample was optimized to 50% of maximum WHC. Moistening was carried out with deionized water.
Avoidance test setup
The avoidance test was carried out in accordance with the (ISO 1999a. The test was carried out in six replications for each concentration of soil/biochar concentration. In total, 84 test vessels were prepared, 42 for the soil from oilseed rape and 42 for the soil from the maize field. The plastic test vessels (9 cm length × 7 cm width × 3 cm height) were filled, half with the control soil and half with the soil/biochar concentration (in a 30- to 30-g proportion, in order to maintain the same soil level on both sites of the glass) and tightly separated with a flat piece of glass. Subsequently, the glass separating the treatments from the control was removed and 20 individuals of F. candida, 10–12 days old, were carefully transferred to the center of the container. The containers were covered with transparent plastic film perforated with small holes (in order to allow air access) and left for 48 h at a temperature of 21 °C and the photoperiod 12 h light and 12 h dark. After this time, each soil portion from each site of the vessels was flooded with water with dark ink. The soil was thoroughly mixed with the water. After a few minutes, individuals were counted. Springtails on the water surface were counted as alive.
Reproduction test setup
The reproduction test was carried out following ISO 11267 (ISO 1999b). For each concentration, five replications were prepared, while for the control eight replications. In total, 50 plastic vessels were prepared for the soil from maize and 50 for the soil from the oilseed rape field. In order to control the soil moisture and pH during the test, one additional vessel from each concentration and control was prepared. The test vessels (6 cm diameter and 7.5 cm high) were filled with 30 g of control soil and the same volume of the tested concentrations. Twelve individuals of 10–12-day-old springtails were carefully transferred to each of the containers. At the beginning of the test, and at weekly intervals, springtails were fed with 2 mg of dry baker’s yeast. The containers were covered with transparent plastic film. The vessels were kept for 28 days at a temperature of 21 °C and the photoperiod 12 h light and 12 h dark. Each week, the plastic film was removed in order to aerate the test vessels. If necessary, water was supplemented in order to maintain 40–50% WHC. After 4 weeks, the test vessels were flooded with water and a small amount of ink and photographed to count the adults and juveniles on the water surface. Individuals were counted from photographs in the graphic program (ToupView software).
Statistical analyses
The soil properties in maize and oilseed rape were compared with repeated ANOVA measures in the Agricolae Package in Software R (p ≤ 0.05). For the analyses of springtail and mite abundance the General Linear Model (GLM) was used in Statistica software, version 13.0. The qualitative factor was the experimental trial (biochar vs. control), while the quantitative factors were the year, sampling period (linked to the cropping season) and replication. The results were presented as the mean from 2 years, separately for maize and the oilseed rape crop. The effect of avoidance (A) for biochar concentrations was calculated using Eq. (1).
| 1 |
where C is the number of individuals in the control soil, T is the number of individuals in the test soil, and N is the total number of individuals
The number of individuals found in each concentration was compared with control (ANOVA, at significance level p ≤ 0.05). Additionally, both for the avoidance and reproduction tests, the dose-response model were used. The concentrations causing 50% effect (EC50) were determined from the dose-response curve, with nonlinear estimation and further least squares model estimation. The estimated function was y = b0 − b0 / (1 + (x/b2)**b1), where b0 was the expected response, b2 is the concentration for a half-maximal response, and b1 determines the slope of the function. These analyses were made in Statistica software, version 13.0.
Results
Biochar and soil properties
The tested biochar was alkaline—pH 8.2 (Table 1) and had 52.3% of carbon. The surface area of the tested biochar was low, only 16.5 m2/g, and cation exchange capacity was also lower − 39.5 cmol/kg, compared with biochars produced at higher temperatures and from other feedstock, like wheat straw, miscanthus, rice husk, or sewage sludge (Liang et al. 2006, 2010). It was free from PAH, and the concentration of all tested toxic compounds was very low or even under the level of detection, passing fixed recommendations for acceptable levels (IBI 2014).
Soil was collected only from the first 10 cm of cultivated A horizon and classified as Cambisol (FAO-WRB 2014), with typical sandy loam texture with the addition of medium gravel and pHH2O from 7.1 to 7.3, which is higher than average for Polish soils, probably due to liming done in the past. Biochar significantly increased the organic soil carbon but only in the maize crop (p = 0.002) (Table 2). Conversely, biochar decreased soil pH, whose pH was significantly higher in the control (7.22) in comparison with trials, where biochar was applied (6.49), also only in maize. The application of biochar significantly increased the values of CEC in both trials (Table 2), due to the increase of exchangeable Ca2+, Mg2+, and H+ + Al3+ (exchangeable acidity) in the soil sorption complex.
Table 2.
The soil conditions in maize and oilseed rape crop in trials where biochar was applied and control field
| Biochar | Control | F | p | |
|---|---|---|---|---|
| Maize | ||||
| Corg (%) | 0.94 | 0.78 | 16.30 | 0.002a |
| pH H2O | 6.49 | 7.22 | 5.47 | 0.04 |
| Na+ (cmol/kg) | 0.12 | 0.18 | 7.80 | 0.02 |
| Mg2+ (cmol/kg) | 2.76 | 0.86 | 18.57 | 0.001 |
| K+ (cmol/kg) | 0.34 | 0.31 | 10.83 | 0.006 |
| Ca2+ (cmol/kg) | 4.05 | 2.28 | 3.92 | 0.07 |
| Exchangeable acidity (cmol/kg) | 1.71 | 1.1 | 4.21 | 0.08 |
| CEC (cmol/kg) | 8.98 | 4.73 | 6.28 | 0.03 |
| Oilseed rape | ||||
| Corg (%) | 0.94 | 0.92 | 0.112 | 0.75 |
| pH H2O | 6.88 | 7.26 | 0.000 | 0.99 |
| Na+ (cmol/kg) | 0.20 | 0.12 | 8.92 | 0.02 |
| Mg2+ (cmol/kg) | 2.14 | 1.13 | 12.96 | 0.007 |
| K+ (cmol/kg) | 0.30 | 0.19 | 22.87 | 0.001 |
| Ca2+ (cmol/kg) | 4.12 | 2.29 | 6.28 | 0.03 |
| Exchangeable acidity (cmol/kg) | 2.0 | 1.1 | 4.56 | 0.07 |
| CEC (cmol/kg) | 8.76 | 4.84 | 5.21 | 0.05 |
aEffect of treatment (results of repeated ANOVA)
The analysis of soil and biochar properties in the soil was linked with the experiments in laboratory conditions. The pH increased from 6.54 in natural soil to 8.46 in the 50% soil-biochar mixture. The water holding capacity increased by more than 3.5 times, comparing the natural soil and 50% soil-biochar mixture (Table 3).
Table 3.
Soil pH measured in KCl and maximum water holding capacity (WHC) of soil–biochar concentrations in particular concentrations
| Dose (%) | Maize | Oilseed rape | ||
|---|---|---|---|---|
| pH in KCl | WHCmax (%) | pH in KCl | WHCmax (%) | |
| 0.0 | 6.54 | 20.88 | 6.20 | 22.36 |
| 1.0 | 6.80 | 26.22 | 6.71 | 27.42 |
| 1.5 | 6.99 | 27.24 | 6.86 | 28.96 |
| 2.5 | 7.25 | 30.31 | 7.44 | 31.59 |
| 5.0 | 7.53 | 34.20 | 7.61 | 36.40 |
| 10.0 | 7.64 | 53.50 | 7.73 | 58.45 |
| 25.0 | 8.23 | 65.35 | 8.32 | 67.87 |
| 50.0 | 8.46 | 75.06 | 8.51 | 81.42 |
Field studies on mesofauna
Maize
In the maize field, the mean abundance of three Acari suborders: Prostigmata, Gamasida, and Oribatida were significantly higher in the soil with biochar in comparison with the control (p = 0.0006, 0.04, and 0.08, respectively) (Table 4). In addition, the mean number of all mites (Acari all) was significantly higher in biochar in comparison with the control (p = 0.007). The mean number of Collembola in biochar treatment was not significantly different from the untreated one. The Collembola/Acari ratio was significantly higher in the soil with biochar, indicating better biological soil quality (p = 0.01).
Table 4.
The mean abundance and ecological indices of mites and springtails in trials where biochar was applied and control in maize field
| Biochar | Control | F* | p | |
|---|---|---|---|---|
| Prostigmata | 10.94 | 3.5 | 11.95 | 0.0006 |
| Astigmata | 3.78 | 4.7 | 0.27 | 0.60 |
| Gamasida | 4.02 | 2.62 | 8.38 | 0.04 |
| Oribatida | 8.05 | 1.71 | 3.12 | 0.08 |
| Acari (all) | 21.59 | 12.5 | 7.42 | 0.007 |
| Collembola (all) | 4.32 | 4.60 | 0.03 | 0.85 |
| Coll/Acari ratio | 7.0 | 3.0 | 6.32 | 0.012 |
F and p are results of general linear model (GLM)
Oilseed rape
The mean number of mites from Prostigmata and Astigmata groups was significantly higher in biochar in comparison with the control (p = 0.018 and 0.035, respectively) (Table 5). Also, mites and springtails as whole communities were significantly more abundant in biochar in comparison with the control (p = 0.013 and 0.0006, respectively). The mean number of Collembola species, as well as the Collembola/Acari ratio, did not differ significantly.
Table 5.
The mean abundance and ecological indices of mites and springtails in trials where biochar was applied and control in oilseed rape field
| Biochar | Control | F | p | |
|---|---|---|---|---|
| Prostigmata | 27.66 | 15.19 | 5.63 | 0.018 |
| Astigmata | 21.44 | 12.84 | 4.48 | 0.035 |
| Gamasida | 8.86 | 9.18 | 0.02 | 0.87 |
| Oribatida | 5.67 | 6.79 | 0.50 | 0.48 |
| Acari (all) | 65.09 | 42.57 | 6.18 | 0.013 |
| Collembola (all) | 27.59 | 8.14 | 12.01 | 0.0006 |
| Coll/Acari ratio | 1.45 | 1.09 | 0.77 | 0.38 |
F and p are results of general linear model (GLM)
Laboratory experiment on springtails
Maize
The mortality of Folsomia candida, both in the avoidance and reproduction tests, was less than 20% in all test vessels and did not differ significantly between treatments. In the avoidance test, doses from 5 to 50% significantly decreased the number of juveniles (Fig. 1).
Fig. 1.
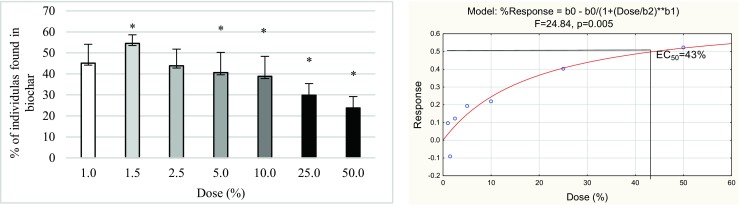
Avoidance behavior (a) and dose-response curve (b) of F. candida in all biochar concentrations mixed with soil from the maize field measured after 2 days of exposure. *p ≤ 0.05, significantly higher or lower value in comparison with control (ANOVA)
At the same time, avoidance was not observed in the first three doses of biochar (1, 1.5, and 2.5%). The half maximal effective concentration (EC50) for this test was 43%. In the reproduction test, only the four last doses (from 25 to 50%) significantly inhibited the reproduction of Collembola (Fig. 2). The lower doses did not have any effect on the number of juveniles. The EC50 for the reproduction test was 17%.
Fig. 2.
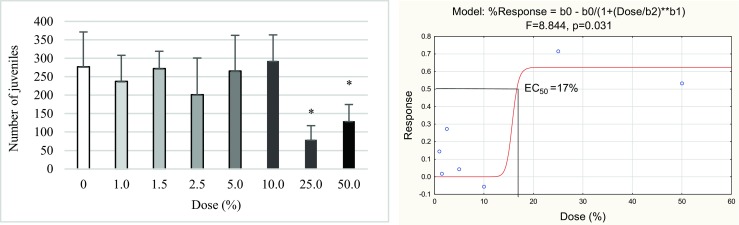
The mean number of juveniles (a) and the dose-response model of the reproduction test (b) found in seven biochar concentrations and control soil from the maize field after 28 days of incubation. *p ≤ 0.05, significantly higher or lower value in comparison with control (ANOVA)
Oilseed rape
In all test vessels with soil from the oilseed rape crop, the mortality of F. candida was lower than 20%, both in the avoidance and reproduction tests. In the avoidance test, all doses had a significant impact (negative or positive) on F. candida (Fig. 3). The first three doses (from 1 to 2.5%) significantly increased the number of individuals in soil with biochar. From the 5.0% doses, avoidance was observed in the test vessels with biochar. The EC50 for the avoidance test was 32%.
Fig. 3.
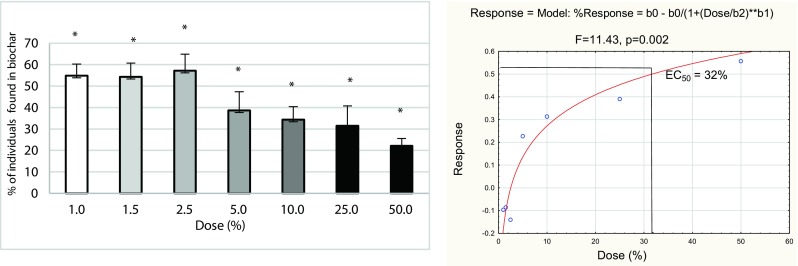
Avoidance behavior (a) and dose-response curve (b) of F. candida in all biochar concentrations mixed with soil from the oilseed rape field measured after 2 days of exposure. *p ≤ 0.05, significantly higher or lower value in comparison with control (ANOVA)
In the reproduction test, only the last two doses (25 and 50%) negatively impacted F. candida reproduction (Fig. 4). The mean number of juveniles in other doses did not differ significantly between the particular biochar concentration and the control. EC50 for this test was 27%.
Fig. 4.
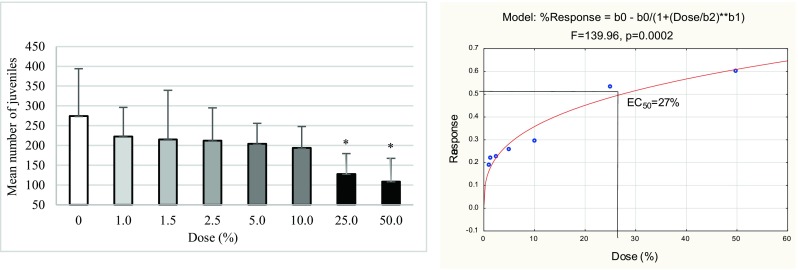
Mean number of juveniles (a) and the dose-response model of the reproduction test (b) found in seven biochar concentrations and control soil from the oilseed rape field after 28 days of incubation. *p ≤ 0.05, significantly higher or lower value in comparison with control (ANOVA)
Discussion
Our study is presumably the first attempt to study the interactions between biochar and soil-dwelling mesofauna (springtails and mites), simultaneously in field and laboratory conditions. The low-temperature pine/wood chip biochar used in the experiment had a high carbon content but low surface area and ability for nutrient storage, which was in agreement with the findings of other authors (Lee et al. 2016; Jiang et al. 2017). The analysis of metal concentration did not report any chemical interference by biochar amendment in the agro-ecosystem.
Potential impacts on soil biota and soil services related to biochar addition to soil can only be discussed at present, where specific biochar, soil type, and other experimental factors occur (Hilber et al. 2017). The application of biochar at the 50 t/ha rate in maize and oilseed rape crops significantly increased the mean number of mites and springtails, as well as the Acari/springtails ratio. The higher number of these organisms, as well as the higher value of ecological indices, shows the increase in soil biological quality in comparison with the control. Considering the results of other field experiments on biochar use in field conditions, no response of soil faunal feeding activity to biochar was found in the rate ranges from 0 to 30 t/ha (Domene et al. 2014). In rates from 12 to 48 t/ha, Zhang et al. (2013) found a significant increase of fungivorous nematode abundance and a decrease of plant parasites. Tammeorg et al. (2014) observed the positive response of earthworms to biochar (30 t/ha), but the result was not significant. Regarding epigeic arthropods, Castracani et al. (2015) did not find interaction between biochar and the abundance of epigeic macroarthropods in the rate of 14 t/ha. Concurrently with the findings of other authors (Neher and Barbercheck 1998; Gruss et al. 2018), we stipulate that the positive response of soil mesofauna to biochar addition was the result of improved physicochemical soil properties, in particular soil pH and organic matter content. In our research, the observed increase of organic carbon that followed biochar application is in agreement with a large number of previous observations (Lehmann 2007; Sohi et al. 2010; Xie et al. 2015; Vaccari et al. 2015). The liming effect of biochar was not observed under either of the tested plants. Acidity decrease after biochar addition can be explained by the release of organic acids and ammonia from material during microbial decomposition but can also be affected by crop cultivation. The addition of wood chip biochar significantly increased the sorption capacity of the tested soils, which was observed in higher cation exchange capacity and water content. These effects were also demonstrated in other field experiments (Conte et al. 2013; Baronti et al. 2014; Głąb et al. 2016; Yang et al. 2017). In our experiment, the increase in pH of an already alkaline soil did not influence the composition and abundance of target organisms, suggesting that this macroarthropod community is generally well adapted to alkaline environments.
In most of the bioassays on biochar on soil fauna, the concentrations are determined by weight. The biochar used for bioassays in our study had low particle size and great volume in small weight. Therefore, we determined volume-percentage concentrations. Our concentrations respond to the weight doses from 0.2 to 15%. The results of avoidance and reproduction tests were similar in the maize and oilseed rape crops. However, significant avoidance was noted at lower doses in comparison with the reproduction test.
In the short-term response, F. candida showed significant avoidance to biochar in doses from 5 to 50% (0.9 to 15% by weight). Furthermore, the 1.5% doses in maize and from 1 to 2.5% in oilseed rape significantly increased the number of F. candida in the test vessels with biochar. The reason might be the considerable increase in pH, from 6.54 (maize) and 6.20 oilseed rape to 8.46 and 8.51 in the dose of 50%. Van Straalen and Verhoef (1997) found that different mites and springtail species preferred soil pH varying from 2.9 to 7.6 tested in the maximum range 2 to 9. That means that these organisms preferred the more acid environment and avoided alkaline. Greenslade and Vaughan (2003) found that the highest rate of reproduction of Folsomia candida was observed in the range of pH form 3.5 to 6.6.
Domene et al. (2015b), after 2 days of incubation of Folsomia candida, found significant avoidance to maize stover biochar at a rate of 2%, which corresponds to 10% in our trials. In this case, biochar only slightly increased pH (form 7 to 7.5), but this probably caused the avoidance. Conti et al. (2017) found that the rates of different gasification biochars above 5% (in weight) caused the avoidance in F. candida. As the main reasons, authors report pH, PAH, and heavy metals. Li et al. (2011) found the avoidance of earthworm species Eisenia fetida to apple wood chip biochar in doses of 0.1 and 0.2%. While in this study traces of PAH were detected, the authors claim that avoidance might be the effect of insufficient moisture in soil with biochar (Li et al. 2011). In our tests, the soil moisture was controlled at 50% WHC. The relation between biochar contaminants and toxicity to soil faunal organisms often remains unexplained (Hilber et al. 2017).
In the reproduction test, we found a significant negative response to biochar from the rate 25% (5.0% by weight). In the study by Marks et al. (2014) biochars from pine feedstock obtained in pyrolysis and fast pyrolysis, as well as biochar obtained in poplar feedstock, positively affected the reproduction of F. candida in all doses. Domene et al. (2015b) did not find any significant effect of biochar obtained for different biomass sources (including pine wood) on F. candida reproduction in the rates from 0.2 to 14% (similar to our dose range). In our study, negative effects of biochar after 28 days of incubation were observed in relatively high concentrations, which are not applied in conventional agriculture.
Conclusions
Soil mesofauna response to biochar was studied simultaneously in field and laboratory experiments. The analyzed biochar was obtained as a product of low-temperature flash pyrolysis (300 °C) of pine and spruce chips, and free from PAH, while the concentration of all tested toxic compounds was very low or even under the level of detection. In the field studies, biochar was applied on the field in the rate 50 t/ha in two crops—maize and oilseed rape. In laboratory tests (reproduction and avoidance test on Folsomia candida), the biochar risk assessment was conducted using soil from the field experiment. The dose 10% in the test corresponds to the dose 50 t/ha of biochar in the field studies. The application of the analyzed biochar in maize and oilseed rape crops significantly increased the mean number of mites and springtails. The Collembola/Acari ratio shows higher biological soil quality in the field where biochar was applied in comparison with the control. This positive effect probably resulted from improved soil chemical properties (in particular organic carbon content and cation exchange capacity) upon biochar addition. Considering the laboratory studies, in the short-term response (avoidance test), Folsomia candida showed a possible short-term toxicity risk from a dose of 5%. The results of the long-term response (reproduction test) indicate the negative response of F. candida from the rate of 25% (higher than the field dose, which corresponds to 10% in laboratory tests). It was concluded that the main reason for avoidance was the considerable increase in pH, which was also observed by other authors. Summarizing, the biochar used in the experiment positively affected soil invertebrates in the field studies, while in the laboratory studies, only short-term risk was observed. In would be interesting to research the impacts of biochars produced in different conditions on the same soils and organisms.
Acknowledgments
We kindly thank the Fluid S.A. Company for free-of-charge delivery of biochar for the experiment. We also thank Mr. Heinrich Lempke for providing free of charge the experimental field, as well as technical and substantive support during the field experiment. We are greatly indebted to Mrs. Kamila Twardowska and Joanna Magiera-Dulewicz for support in laboratory experiments.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Bardgett R. The biology of soil: a community and ecosystem approach. Oxford: Oxford University Press; 2005. [Google Scholar]
- Baronti S, Vaccari FP, Miglietta F, Calzolari C, Lugato E, Orlandini S, Pini R, Zulian Genesio CL. Impact of biochar application on plant water relations in Vitis vinifera (L.) Eur J Agron. 2014;53:38–44. doi: 10.1016/j.eja.2013.11.003. [DOI] [Google Scholar]
- Birk JJ, Steiner C, Teixiera WC, Zech W, Glaser B (2009) Microbial response to charcoal amendments and fertilization of a highly weathered tropical soil. In: Woods WI, Teixeira WG, Lehmann J, Steiner C, Winkler Prins A, Rebellato L (eds) Amazonian dark earths, Wim Sombroek’s vision. Springer, pp 309–324
- Bunting BT, Lundberg J. The humus profile e concept, class and reality. Geoderma. 1987;40:17–36. doi: 10.1016/0016-7061(87)90011-5. [DOI] [Google Scholar]
- Castracani C, Maienza A, Grasso DA, Genesio L, Malcevschi A, Miglietta F, Vaccari FP, Mori A. Biochar–macrofauna interplay: searching for new bioindicators. Sci Total Environ. 2015;536:449–456. doi: 10.1016/j.scitotenv.2015.07.019. [DOI] [PubMed] [Google Scholar]
- Chen T, Zhang Y, Hontao W, Lu W. Influence of pyrolysis temperature on characteristics and heavy metal adsorptive performance of biochar derived from municipal sewage sludge. Bioresour Technol. 2014;164C:47–54. doi: 10.1016/j.biortech.2014.04.048. [DOI] [PubMed] [Google Scholar]
- Conte P, Marsala V, De Pasquale C, Bubici S, Valagussa M, Pozzi A, Alonzo G. Nature of water-biochar interface interactions. GCB Bioenergy. 2013;5:116–121. doi: 10.1111/gcbb.12009. [DOI] [Google Scholar]
- Conti F, Visioli G, Malcevschi A, Menta C. Safety assessment of gasification biochars using Folsomia candida (Collembola) ecotoxicological bioassays. ESPR. 2017;25(7):6668–6679. doi: 10.1007/s11356-017-0806-4. [DOI] [PubMed] [Google Scholar]
- Domene X, Alcañiz JM, Andrés P. Ecotoxicological assessment of organic wastes using the soil collembolan Folsomia candida. Appl Soil Ecol. 2007;35:461–472. doi: 10.1016/j.apsoil.2006.10.004. [DOI] [Google Scholar]
- Domene X, Mattana S, Hanley K, Enders A. Medium-term effects of corn biochar addition on soil biota activities and functions in a temperate soil cropped to corn. Soil Biol Biochem. 2014;72:152–162. doi: 10.1016/j.soilbio.2014.01.035. [DOI] [Google Scholar]
- Domene X, Enders A, Hanley K, Lehmann J. Ecotoxicological characterization of biochars: role of feedstock and pyrolysis temperature. Sci Total Environ. 2015;512–513:552–561. doi: 10.1016/j.scitotenv.2014.12.035. [DOI] [PubMed] [Google Scholar]
- Domene X, Hanley K, Enders A, Lehmann J. Short-term mesofauna responses to soil additions of corn stover biochar and the role of microbial biomass. Appl Soil Ecol. 2015;89:10–17. doi: 10.1016/j.apsoil.2014.12.005. [DOI] [Google Scholar]
- Environmental Protection Agency (EPA) (2012) Method 3050B: acid digestion of sediments, sludges, and soils. https://www.epa.gov/homeland-security-research/epa-method-3050b-acid-digestion-sediments-sludges-and-soils. Accessed 25 March 2019
- FAO-WRB (2014) World reference base for soil resources. International soil classification system for naming soils and creating legends for soil maps. Food and Agriculture Organization of the United Nations. World Soil Resources Reports 106, Rome. https://www.fao.org/3/i3794en/I3794en.pdf. Accessed 25 April 2019
- Farrell M, Rangott G, Krull E. Difficulties in using soil-based methods to assess plant availability of potentially toxic elements in biochars and their feedstocks. J Hazard Mater. 2013;250–251:29–36. doi: 10.1016/j.jhazmat.2013.01.073. [DOI] [PubMed] [Google Scholar]
- Fountain MT, Hopkin SP (2005) Folsomia candida (Collembola): A “Standard” Soil Arthropod. Annu Rev Entomol. 50: 201-222. 10.1146/annurev.ento.50.071803.130331 [DOI] [PubMed]
- Freddo A, Cai C, Reid BJ. Environmental contextualization of potential toxic elements and polycyclic aromatic hydrocarbons in biochar. Environ Pollut. 2012;171:18–24. doi: 10.1016/j.envpol.2012.07.009. [DOI] [PubMed] [Google Scholar]
- Głąb T, Palmowska J, Zaleski T, Gondek K. Effect of biochar application on soil hydrological properties and physical quality of sandy soil. Geoderma. 2016;28:11–20. [Google Scholar]
- Greenslade P, Vaughan GT. A comparison of Collembola species for toxicity testing of Australian soils. Pedobiologia. 2003;47:171–179. doi: 10.1078/0031-4056-00180. [DOI] [Google Scholar]
- Gruss I, Twardowski JP, Hurej M. Influence of 90-year potato and winter rye monocultures under different fertilization on soil mites. PPS. 2018;54:31–38. [Google Scholar]
- Gulvik M. Mites (Acari) as indicators of soil biodiversity and land use monitoring: a review. Pol J Ecol. 2007;55:415–440. [Google Scholar]
- Hale SE, Lehmann J, Rutherford D, Zimmerman AR, Bachmann RT, Shitumbanuma V, O'Toole A, Sundqvist KL, Arp HPH, Cornelissen G. Quantifying the total and bioavailable polycyclic aromatic hydrocarbons and dioxins in biochars. Environ Sci Technol. 2012;46:2830–2838. doi: 10.1021/es203984k. [DOI] [PubMed] [Google Scholar]
- Hansen V, Müller-Stöver D, Ahrenfeldt J, Holm JK, Henriksen UB, Hauggaard-Nielsen H. Gasification biochar as a valuable by-product for carbon sequestration and soil amendment. Biomass Bioenergy. 2015;72:300–308. doi: 10.1016/j.biombioe.2014.10.013. [DOI] [Google Scholar]
- Hilber I, Bastos AC, Loureiro S, Soja G, Marsz A, Cornelissen G, Bucheli TD. The different faces of biochar: contamination risk versus remediation tool. J Environ Eng Landsc Manag. 2017;25:86–104. doi: 10.3846/16486897.2016.1254089. [DOI] [Google Scholar]
- International Biochar Initiative (IBI) (2014) Standardized product definition and product testing guidelines for biochar that is used in soil (Aka IBI biochar standards). IBI-STD-1.1.https://www.biochar-international.org/wp-content/uploads/2018/04/IBI_Biochar_Standards_V2.1_Final.pdf. Accessed 25 March 2019
- International Organization for Standardization (ISO)(1999a) Soil quality—avoidance test for determining the quality of soils and effects of chemicals on behavior—part 2: test with collembolans (Folsomia candida). Guideline 17512-2. International Standardization Organization, Geneva
- International Organization for Standardization (ISO) (1999b) Soil quality—inhibition of reproduction of Collembola (Folsomia candida) by soil pollutants. Guideline 11267. International Standardization organization, Geneva
- Jiang S, Tuan N, Victor R, Yang H, Zhang D, Sik OY, Huang L. Characterization of hard- and softwood biochars pyrolyzed at high temperature. Environ Geochem Health. 2017;39:403–415. doi: 10.1007/s10653-016-9873-6. [DOI] [PubMed] [Google Scholar]
- Keiluweit M, Kleber M, Sparrow MA, Simoneit BR, Prahl FG. Solvent-extractable polycyclic aromatic hydrocarbons in biochar: influence of temperature of pyrolysis and feedstock. Environ Sci Technol. 2012;46:9333–9341. doi: 10.1021/es302125k. [DOI] [PubMed] [Google Scholar]
- Kuppusamy S, Thavamani P, Megharaj M, Venkateswarlu K, Naidu R. Agronomic and remedial benefits and risks of applying biochar to soil: current knowledge and future research directions. Environ Int. 2016;87:1–12. doi: 10.1016/j.envint.2015.10.018. [DOI] [PubMed] [Google Scholar]
- Kuzyakov Y, Subbotina I, Chen H, Bogomolova I, Xu X. Black carbon decomposition and incorporation into microbial biomass estimated by 14C labeling. Soil Biol Biochem. 2009;41:210–219. doi: 10.1016/j.soilbio.2008.10.016. [DOI] [Google Scholar]
- Latawiec AE, Królczyk JB, Kubon M, Szwedziak K, Drosik A, Polanczyk E, Grotkiewicz K, Strassburg BN. Willingness to adopt biochar in agriculture: the producer’s perspective. Sustainability. 2017;9:655. doi: 10.3390/su9040655. [DOI] [Google Scholar]
- Latawiec AE, Peake L, Baxter H, et al. A reconnaissance-scale GIS-based multicriteria decision analysis to support sustainable biochar use: Poland as a case study. J Environ Eng Landsc. 2017;25:208–222. doi: 10.3846/16486897.2017.1326924. [DOI] [Google Scholar]
- Lee J, Hawkins B, Kidder M, Evans B, Buchanan AC, Day D. Characterization of biochars produced from peanut hulls and pine wood with different pyrolysis conditions. BIOB. 2016;3:15. [Google Scholar]
- Lehmann J. Bio-energy in the black. Front Ecol Environ. 2007;5(7):381–387. doi: 10.1890/1540-9295(2007)5[381:BITB]2.0.CO;2. [DOI] [Google Scholar]
- Lehmann J, Gaunt J, Rondon M. Biochar sequestration in terrestrial ecosystems—a review. Mitig Adapt Strat Gl. 2006;11:403–427. doi: 10.1007/s11027-005-9006-5. [DOI] [Google Scholar]
- Lehmann J, Rillig MC, Thies J, Masiello CA, Hockaday WC, Crowley D. Biochar effects on soil biota—a review. Soil Biol Biochem. 2010;43:1812–1836. doi: 10.1016/j.soilbio.2011.04.022. [DOI] [Google Scholar]
- Li D, Hockaday WC, Masiello CA, Alvarez PJJ. Earthworm avoidance of biochar can be mitigated by wetting. Soil Biol Biochem. 2011;43:1732–1737. doi: 10.1016/j.soilbio.2011.04.019. [DOI] [Google Scholar]
- Liang B, Lehmann J, Solomon D, Kinyangi J, Grossman J, O’Neill B, Skjemstad JO, Thies J, Luizao FJ, Petersen J, Neves EG. Black carbon increases cation exchange capacity in soils. Soil Sci Soc Am J. 2006;70:1719–1730. doi: 10.2136/sssaj2005.0383. [DOI] [Google Scholar]
- Liang B, Lehmann J, Sohi SP, Thies J, O’Neill B, Truillo L, Gaunt J, Solomon D, Grossman J, Neves EG, Luizao FJ. Black carbon affects the cycling of non-black carbon in soil. Org Geochem. 2010;41:206–213. doi: 10.1016/j.orggeochem.2009.09.007. [DOI] [Google Scholar]
- Liesch AM, Weyers SL, Gaskin JW, Das KC. Impact of two different biochars on earthworm growth and survival. AES. 2010;4:1–9. doi: 10.1080/09735070.2010.11886356. [DOI] [Google Scholar]
- Lyu H, He Y, Tang J, Hecker M, Liu Q, Jones PD, Codling G, Giesy JP. Effect of pyrolysis temperature on potential toxicity of biochar if applied to the environment. Environ Pollut. 2016;218:1–7. doi: 10.1016/j.envpol.2016.08.014. [DOI] [PubMed] [Google Scholar]
- Ma N, Zhang L, Zhang Y, Yang L, Yu C, Yin G, Doane TA, Wu Z, Zhu P, Ma X. Biochar improves soil aggregate stability and water availability in a mollisol after three years of field application. PLoS One. 2016;11:1–10. doi: 10.1371/journal.pone.0154091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malińska K, Zabochnicka-Świątek M, Cáceres R, Marfà O. The effect of precomposted sewage sludge mixture amended with biochar on the growth and reproduction of Eisenia fetida during laboratory vermicomposting. Ecol Eng. 2016;90:35–41. doi: 10.1016/j.ecoleng.2016.01.042. [DOI] [Google Scholar]
- Malińska K, Golańska M, Cáceres R, Rorat A, Weisser P, Ślęzak E. Biochar for integrated composting and vermicomposting—the effect of biochar on the activity of Eisenia fetida and the obtained vermicompost. Bioresour Technol. 2017;225:206–214. doi: 10.1016/j.biortech.2016.11.049. [DOI] [PubMed] [Google Scholar]
- Marks EAN, Mattana S, Alcañiz JM, Domene X. Biochars provoke diverse soil mesofauna reproductive responses in laboratory bioassays. Eur J Soil Biol. 2014;60:104–111. doi: 10.1016/j.ejsobi.2013.12.002. [DOI] [Google Scholar]
- Marks EAN, Mattana S, Alcañiz JM, Péres-Herrero E, Domene X. Gasifier biochar effects on nutrient availability, organic matter, mineralization, and soil fauna activity in a multi-year Mediterranean trial. Agric Ecosyst Environ. 2016;215:30–39. doi: 10.1016/j.agee.2015.09.004. [DOI] [Google Scholar]
- McCormack S, Ostle N, Bardgett RD, Hopkins DW, Vanbergen AJ. Biochar in bioenergy cropping systems: impacts on soil faunal communities and linked ecosystem processes. GCB Bioenergy. 2013;5:81–95. doi: 10.1111/gcbb.12046. [DOI] [Google Scholar]
- Méndez A, Gómez A, Paz-Ferreiro J, Gascó G. Effects of sewage sludge biochar on plant metal availability after application to a Mediterranean soil. Chemosphere. 2012;89:1354–1359. doi: 10.1016/j.chemosphere.2012.05.092. [DOI] [PubMed] [Google Scholar]
- Murphy PW (1956) A modified funnel method for extracting soil mesofauna. Proceedings of the transactions on 6th International Congress of Soil Science; Paris, pp 255–262
- Neher DA, Barbercheck ME (1998) Diversity and function of soil mesofauna. In: Qualset CO, Collins WW (eds) Biodiversity & Agroecosystems. CRC Press, pp 27–47
- Pietikäinen J, Kiikkilä O, Fritze H. Charcoal as a habitat for microbes and its effects on the microbial community of the underlying humus. Oikos. 2000;89:231–242. doi: 10.1034/j.1600-0706.2000.890203.x. [DOI] [Google Scholar]
- Rajkovich S, Enders A, Hanley K, Hyland C, Zimmerman AR, Lehmann J. Corn growth and nitrogen nutrition after additions of biochars with varying properties to a temperate soil. Biol Fertil Soils. 2011;48:271–284. doi: 10.1007/s00374-011-0624-7. [DOI] [Google Scholar]
- Reeuwijk L (2002) Procedures for soil analysis. 6th edition. Technical Paper/International Soil Reference and Information Centre. https://www.isric.org/sites/default/files/ISRIC_TechPap09.pdf. Accessed 25 March 2019
- Rusek J. Biodiversity of Collembola and their functional role in the ecosystem. Biodivers Conserv. 1998;7:1207–1219. doi: 10.1023/A:1008887817883. [DOI] [Google Scholar]
- Santofuro L, Van Gestek CAM, Rocco A, Maisto G. Soil invertebrates as bioindicators of urban soil quality. Environ Pollut. 2012;161:57–63. doi: 10.1016/j.envpol.2011.09.042. [DOI] [PubMed] [Google Scholar]
- Singh B, Camps-Arbestain M, Lehmann J (2017) Biochar: a guide to analytical methods. Csiro Publishing, pp 1–320
- Sohi SP, Krull E, Lopez-Capel E, Bol R (2010) A review of biochar and its use and function in soil. Advances in agronomy. Elsevier Academic, pp 47–82
- Steiner C, Teixeira WG, Lehmann J, Zech W. Microbial response to charcoal amendments of highly weathered soil and Amazonian dark earths in Central Amazonia: preliminary results. In: Glaser B, Woods WI, editors. Amazonian dark earths: explorations in time and space. Heidelberg: Springer; 2004. pp. 95–212. [Google Scholar]
- Tammeorg P, Parviainen T, Nuutinen V, Simojoki A, Vaara E, Helenius J. Effects of biochar on earthworms in arable soil: avoidance test and field trial in boreal loamy sand. Agric Ecosyst Environ. 2014;191:150–157. doi: 10.1016/j.agee.2014.02.023. [DOI] [Google Scholar]
- Tisdall JM, Oades JM. Organic matter and water-stable aggregates in soils. J Soil Sci. 1982;33:141–163. doi: 10.1111/j.1365-2389.1982.tb01755.x. [DOI] [Google Scholar]
- Topoliantz S, Ponge JF. Charcoal consumption and casting activity by Pontoscolex corethrurus (Glossoscolecidae) Appl Soil Ecol. 2005;28:217–224. doi: 10.1016/j.apsoil.2004.08.003. [DOI] [Google Scholar]
- Twardowski JP, Hurej M, Gruss I. Diversity abundance of springtails (Hexapoda: Collembola) in soil under 90-year potato monoculture in relation to crop rotation. Arch Agron Soil Sci. 2016;62:1158–1168. [Google Scholar]
- Vaccari FP, Maienza A, Miglietta F, Baronti S, Di Lonardo S, Giagnoni L, Lagomarsino L, Pozzi A, Pusceddu E, Ranieri R, Valboa G, Genesio L. Biochar stimulates plant growth but not fruit yield of processing tomato in a fertile soil. Agric Ecosyst Environ. 2015;207:163–170. doi: 10.1016/j.agee.2015.04.015. [DOI] [Google Scholar]
- Van Straalen NM, Verhoef HA. The development of a bioindicator system for soil acidity based on arthropod pH preferences. J Appl Ecol. 1997;34:217–232. doi: 10.2307/2404860. [DOI] [Google Scholar]
- Waikhom MD, Singh TB, Devi LJ. Vertical distribution pattern of Collembola in a sub-tropical forest floor of Manipur. Zoos Print J. 2006;21:2331–2332. doi: 10.11609/JoTT.ZPJ.1459.2331-2. [DOI] [Google Scholar]
- Xie T, Reddy KR, Wang C, Yargicoglu E, Spokas K. Characteristics and applications of biochar for environmental remediation: a review. Crit Rev Environ Sci Technol. 2015;45:939–969. doi: 10.1080/10643389.2014.924180. [DOI] [Google Scholar]
- Yang D, Yunguo L, Liu S, Huang X, Li Z, Tan X, Zeng G, Zhou L. Potential benefits of biochar in agricultural soils: a review. Pedosphere. 2017;27:645–661. doi: 10.1016/S1002-0160(17)60322-9. [DOI] [Google Scholar]
- Zhang X, Li Q, Liang W, Zhang M, Bao X, Xie Z. Soil nematode response to biochar addition in a Chinese wheat field. Pedosphere. 2013;23:98–103. doi: 10.1016/S1002-0160(12)60084-8. [DOI] [Google Scholar]
- Zhang M, Cheng G, Feng H, Sun B, Zhao Y, Chen H, Chen J, Dyck M, Wang X, Zhang J, Zhang A. Effects of straw and biochar amendments on aggregate stability, soil organic carbon, and enzyme activities in the Loess Plateau, China. Environ Sci Pollut Res Int. 2017;24:10108–10120. doi: 10.1007/s11356-017-8505-8. [DOI] [PubMed] [Google Scholar]


