Abstract
The strategy for improving the treatment of leishmaniasis by the World Health Organization, is the development of new drugs and combination therapy. The aim of this survey was to investigate the effect of amphotericin B (AmB) in combination with selenium, in a simple or niosomal form, on Leishmania tropica (L. tropica) by in vitro advanced assays. In this study, a niosomal formulation of AmB with selenium was prepared and characterized based on size and morphology. Using MTT assay, macrophage model, flow cytometry, and qPCR, the cytotoxicity and efficiency of the niosomal formulation and simple form of combination were evaluated. No toxicity was reported for both the niosomal and simple form of the combination. The niosomal formulation significantly showed higher inhibitory effect on the promastigote and amastigote forms of L. tropica than simple combination form. Interleukin (IL)-10 significantly decreased while the level of IL-12 and metacasoase as Th-1 activator significantly increased (P < 0.001). The findings of this study indicated that niosomes are the stable carriers for this combination, easy to produce and provide promising results as an effective formulation in the inhibition of extracellular and intracellular forms of L. tropica in compared with simple combination form.
Keywords: Amphotericin B, Selenium, Niosome, Gene expression, Leishmania tropica
Introduction
Leishmaniasis is a vector-borne disease caused by the protozoan parasite, Leishmania. About 1.6 million cases of infections with visceral (VL) and cutaneous (CL) forms of leishmaniasis (Alvar et al. 2012) are reported annually. Presently, there is no vaccine available for the prevention of leishmaniasis (Kedzierski et al. 2006).
Leishmaniasis is among the neglected diseases which have limited therapeutic options. Due to toxicity, parasite resistance and high cost (Croft et al. 2006b), there have been limitations to the use of currently available drugs such as antimonial compounds as the first line of treatment and liposomal amphotericin B (AmB), miltefosine, terbinafine, pentamidine and paromomycin (Croft et al. 2006a; Moosavian Kalat et al. 2014; Farajzadeh et al. 2015a, 2016) as the second line of treatment (Freitas-Junior et al. 2012).
Consequently, the high prevalence of CL and the onset of resistance to conventional drugs requires the innovation and development of novel, nontoxic and more efficient drugs (Santos et al. 2008). Nowadays, the use of novel drug delivery systems such as niosomes is an attractive alternative treatment for leishmanisis (Frézard et al. 2009).
The strategy of niosomes is targeted delivery to tissues in order to improve the efficacy and reduce the cytotoxicity of drugs such as anticancer, antibiotics and antifungal drugs (Donowitz 1994; Owais and Gupta 2005). Niosomes are non-immunogenic, non-toxic and highly biocompatible vesicles (Wagh and Deshmukh 2015).
AmB, as the second line of leishmaniasis treatment binds to ergosterol as the main sterol in Leishmania species (Perez et al. 2016). Previous studies showed that AmB was effective against L. amazonensis, L. infantum, and L. braziliensis (Cunha et al. 2015; Duarte et al. 2016). However, liposomal formulations of AmB such as Abelcet, AmBisome (Gilead Sciences, USA), and Amphocil are more efficacious, but are more expensive and unaffordable (Agrawal et al. 2005).
Selenium, as a trace element is used in different parts of medicine such as anticancer treatment, antioxidant effects and antiviral activities (Whanger 2004; Rayman 2005, 2012). Selenium nanoparticles would easily enter into cells and inhibit bacterial growth (Yang et al. 2009; Tran and Webster 2011). In addition, selenium nanoparticles are also effective against L. major, L. tropica and L. infantum (Beheshti et al. 2013; Mahmoudvand et al. 2014; Soflaei et al. 2014).
The strategies of the World Health Organization (WHO) for improving the treatment of leishmaniasis include the development of new drugs and combination therapy (WHO 2012). The aim of drug combinations is to prevent or delay the development of resistance, shorten the duration of treatment and also increase the efficacy of drugs in the treatment of parasites, viral and bacterial infections (White 1999; Olliaro and Taylor 2003). Combination therapy are vital for the treatment of some diseases such as tuberculosis, AIDS, malaria and leishmaniasis (Kremsner and Krishna 2004; Yazdanpanah et al. 2004; Mitchison and Davies 2012).
Some studies have shown that drug combinations have been used in the treatment of leishmaniasis such as investigation of in vitro and in vivo effect and clinical trial of combining miltefosine with other standard leishmanicidal drugs (Seifert and Croft 2006; Aguiar et al. 2010; Omollo et al. 2011). The antileishmanial effect of sodium stibogluconate (SbV) combined with paromomycin, allopurinol or verapamil have been clinically and experimentally investigated (Thakur et al. 2000; Shokri et al. 2012; Riabi et al. 2013).
In order to prevent the development of resistance, shorten the duration of treatment and also reduce the side effects of conventional drugs in the treatment of leishmaniasis, this study aimed to investigate the effect of AmB combined with selenium, in the simple or niosomal form on L. tropica by an in vitro MTT assay, macrophage model, flow cytometry, and qPCR.
Materials and methods
Drug preparation
Glucantime (Sanofi-Aventis, Paris, France), AmB (India, B. No. GI50253), and selenium dioxide 99.9% (Sigma-Aldrich/Lot 079K368021) were obtained from the Provincial Health system. AmB and selenium were dissolved in sterile distilled water, according to the manufacturer’s instructions. Prior to each assay, final combination concentrations of 12.5 + 12.5, 25 + 25, 50 + 50, 100 + 100, and 200 + 200 µg/ml (Selenium + AmB) were prepared in Rosewell Park Memorial Institute (RPMI-1640) medium (Riabi et al. 2013).
Niosomal formulation
Film hydration method was used to achieve the niosomal combination of AmB plus selenium (Rogerson et al. 1987). To this end, non-ionic surfactants (Span40 and Twin40 with ratio 6/4) and cholesterol were dissolved in chloroform in a round-bottom flask and evaporated using a rotary evaporator (Buchi, Switzerland) at 180 rpm and 70 °C for 15 min. The obtained thin layer of film was hydrated with 5 ml of deionized water, which included 25 mg of AmB and 25 mg of selenium with the same potency (1%) at 70 °C for 30 min. In order to complete the hydration, niosome was kept at room temperature for 24 h. The prepared niosome was stored at 4 °C for further studies.
Characterization of niosomal formulation
The shape of noisome was determined by optical microscopy (Zeiss, Germany) and micrographs were captured. In addition, niosomal size was determined using dynamic light scattering with Master Sizer 2000 E (Malvern, UK). The tests were done in triplicate.
Cytotoxicity assay
For the evaluation of drug cytotoxicity, murine macrophages (J774-A1 cells, ECACC no.91051511) were obtained from the Pasteur Institute of Iran (Tehran, Iran) and cultured in complete RPMI-1640 medium, treated with different concentrations of drugs and incubated at 37 °C in 5% CO2 for 72 h. After the incubation period, 10% MTT powder (product No. M 5655, purchased from Sigma-Aldrich®, USA) following the manufacture’s instruction was added and incubated for 3 h. Optical density was determined at 490 nm by the ELISA-reader (Bio Tek-ELX800) after the addition of isopropanol alcohol (Ganguly et al. 2006). The CC50 (cytotoxicity concentration for 50% of cells) was calculated by the probit test. Furthermore, the selectivity index (SI) = CC50/IC50 ≥ 10 (Escudero-Martínez et al. 2017) was used to check drug safety; non-toxic was assessed.
Anti-promastigote assay
Parasite growth was assessed by counting live promastigotes in a Neubauer chamber. Then 2 × 106 logarithmic-phase of L. tropica promastigotes (MHOM/IR/75/Mash2) cultured in RPMI-1640 with 100 µg streptomycin/mL and 10% (v/v) heat-inactivated fetal bovine serum (FBS) (Sigma, Aldrich, France) were treated with different concentrations of combination and niosomal form. After 72 h incubation at 24 ± 1 °C, MTT assay was performed three times and OD (optical density) was determined by spectrophotometer at 490 nm as previously mentioned. The probit test was used to calculate the IC50 value (50% inhibitory concentration).
Anti- amastigote assay
The stationary phase promastigotes of L. tropica were added to the murine macrophage cell-line. The combination adhered to the slides after 2 h incubation at 37 °C and 5% CO2, at a ratio of 10:1 promastigote: macrophage, respectively. After incubation for 24 h at 37 °C and 5% CO2, intramacrophage amastigotes were treated with different concentrations of drugs. Then, the treated cells were fixed and stained while amastigotes in 100 macrophages were counted. The infected macrophages without drugs and macrophages with no parasite and no drugs were considered as untreated and negative control, respectively. All experiments were repeated in triplicates and the IC50values were determined using the probit test.
Flow cytometry assay
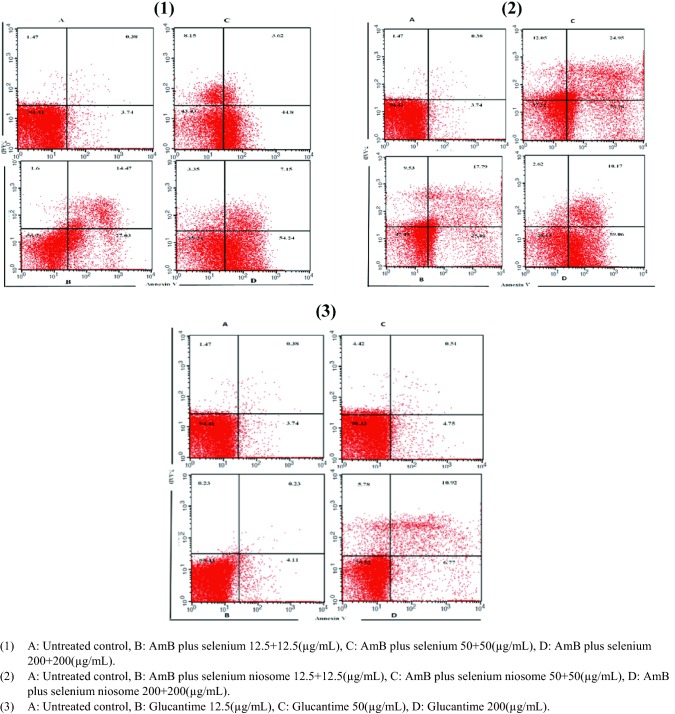
An Apoptosis Detection Kit with FITC annexin V and 7AAD (BD Pharnigen™) was used for the evaluation of apoptotic and necrotic values of treated promasigotes to indicate the externalization of phospholipid classes as an index of anti-leishmanial potential for drugs. The treated promastigotes cells with AmB plus selenium, AmB plus selenium niosome (with different concentrations of 12.5 + 12.5, 50 + 50, and 200 + 200 µg/ml), and glucantime (with concentrations of 12.5, 50, and 200 µg/ml) were incubated at 24 ± 1 °C for 72 h then the assessment of apoptotic values was carried out according to the FITC kit manufacture’s instruction. The sample containing the medium and parasite with no drugs were considered as untreated control.
Quantitative real-time PCR (qPCR) assay
RNA isolation and analysis
According to the manufacturer’s instructions, EZ-10 Spin Column Total RNA Miniprep Kit (Bio Basic Inc., Canada) was used for RNA extraction of extracellular promastigotes and intra murine macrophages amastigotes.Nanodrop ND-1000 spectrophotometer (Thermo Scientific, Wilmington, DE, USA) was used to determine the quantity and purity of RNA.
qPCR
For the detection of relative expression (IL-12, IL-10 in mouse macrophages and metacaspase in L. tropica promastigotes), quantitative real-time PCR (qPCR) assay was carried out. According to the RT reagent kit (Takara, Clontech) manufacture’s instruction, cDNA was synthesized with a total of 500 ng RNA at 37 °C for 15 min using the Revert Aid M-MuLV reverse transcriptase with a random hexamer primer (Fermentas, Vilnius, Lithuania).
Glyceraldehyde 3-phosphate dehydrogenase (GAPDH)(Chandra et al. 2008; Koutsoni et al. 2014) was used as reference gene for gene expression of IL-12 and IL-10 in murine macrophage cells (J-774 A) and RPS18 Ribosomal protein (S18) (Zhong et al. 2013; Kumar and Engwerda 2014) for gene expression of metacaspase in Leishmania (Table 1). qPCR was performed using 10 µl SYBR premix Taq™ (Takara, Clontech), 250 n/mol of the forward and reverse primers for both, and 1 μL of cDNA diluted in RNase-free water on a real-time PCR cycler (Qiagen, Chatsworth, CA) at 95 °C for 1 min; 40 cycles at 94 °C for 15 s, 60 °C for 20 s, and 72 °C for 20 s. The qPCR reactions for each sample were performed two times. Each run was performed using a positive and negative control.
Table 1.
Primers used for the real-time PCR
| Primers | Gene | Forward sequence (5′–3′) | Reverse sequence (5′–3′) | Product size (bp) |
|---|---|---|---|---|
| Macrophages murine cells | IL-12 P40 | CTGGAGCACTCCCCATTCCTA | GCAGACATTCCCGCCTTTG | 160 |
| IL-10 | CTTACTGACTGGCATGAGGATCA | GCAGCTCTAGGAGCATGTGC | 101 | |
| GAPDH | AGCTTCGGCACATATTTCATCTG | CGTTCACTCCCATGACAAACA | 89 | |
| Promastigotes of L. i tropica | Metacaspase | CAGCAACAATTCCTGGCGATA | AAGTTTGAAGTAAAAGGAGACAATTTGG | 140 |
| RPS18 Ribosomal protein (S18) | GTTGAGGTGCGTGGTCTGTC | TGCAGGTTGCTCAGGAGCTT | 166 |
ΔCT was calculated using the formula:
However, gene expression levels of IL-10, IL-12 and metacaspase were determined by 2−ΔCt method. In addition, Fold increase (FI) was determined by the comparative threshold assay (2−ΔΔCT).
Statistical analysis
SPSS version 22 (SPSS Inc., Chicago, IL, USA) was used for data analysis, while probit test was used to determine IC50 and CC50 values. Analysis of variance (ANOVA) and independent t test were used to evaluate the differences between the two formulations in comparison with glucantime and the untreated control. Mean 2−ΔCt for treatment and control for each cytokine were compared using GRAPHPAD PRISM 6 (GraphPad Software Inc, San Diego, CA, USA).Level of statistical significance was determined at P ≤ 0.05.
Results
Characterization of niosome
The size and morphology of selenium and AmB noisomal formulation in combination was assessed. Niosomal formulation procedure has spherical multi-layer vesicles of 50.2 ± 0.48 µm size (Fig. 1). The microscopic picture was determined in triplicates (Fig. 2).
Fig. 1.
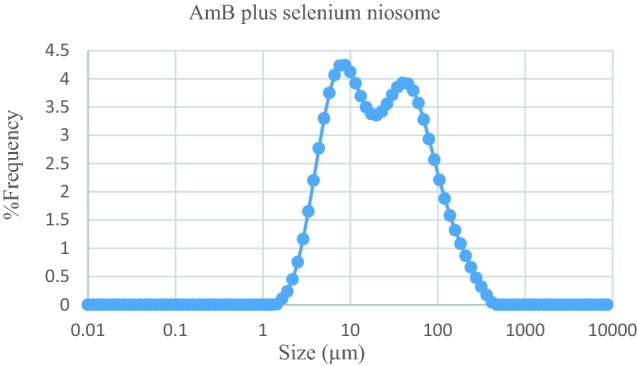
The size distribution graph in terms of the frequency of Span 40/Tween 40 (molar ratio = 6/4) of AmB plus selenium niosome
Fig. 2.
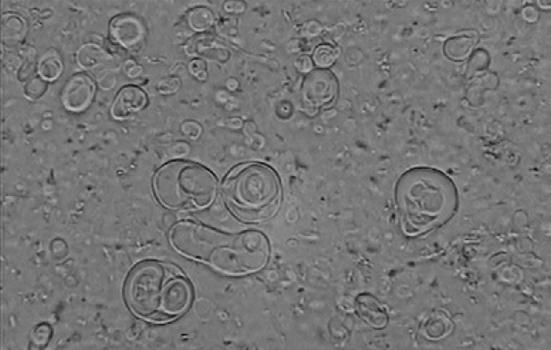
Microscopy pictures of the AmB plus selenium niosome (Span 40/Tween 40 with molar ratio: 6/4)
Cytotoxicity effects on macrophage cells
After cytotoxic analysis of the macrophage cell-line, no toxic effect was observed for the combination and its niosomal formulation at various concentrations (Fig. 3). The ratio between CC50 on macrophages murine cells and IC50 against L. tropica amastigotes (SI) was calculated. The niosomal formulation had the highest SI (Table 2).
Fig. 3.
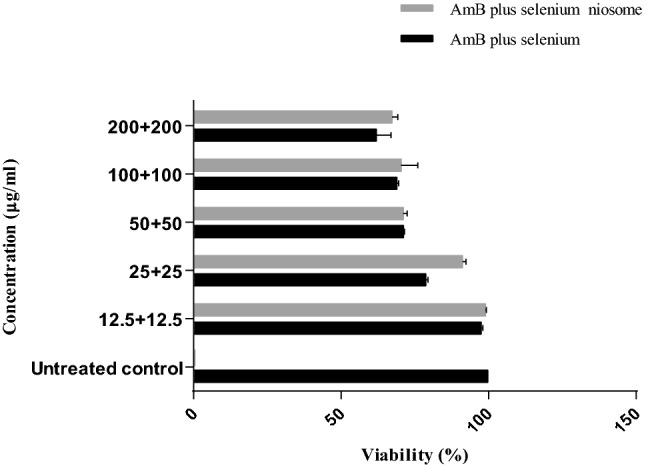
The cytotoxicity analysis of the different concentrations AmB plus selenium, AmB plus selenium niosome, and untreated control on the J774 macrophage cells
Table 2.
Comparison of the IC50 values of glucantime, AmB plus selenium and AmB plus selenium niosome on L. tropica promastigote and amastigote and CC50 values of drugs on macrophage and selectivity index (SI)
| Drug | Amastigote | Promastigote | Macrophage | *SI (selectivity index) | ||
|---|---|---|---|---|---|---|
| IC50 ± SD (µg/ml) | P value | IC50 ± SD (µg/ml) | P value | CC50 ± SD (µg/ml) | ||
| Glucantime | 222.31 ± 28.04 | P < 0.001 | 1445 ± 97.3 | P < 0.001 | 1634 ± 29.61 | 7.35 |
| AmB plus selenuim | 8.2 ± 2.2 | P < 0.001 | 18.22 ± 0.98 | P < 0.001 | 354.5 ± 48.7 | 42.13 |
| AmB plus selenuim niosome | 1.73 ± 0.4 | P < 0.001 | 6.7 ± 1.9 | P < 0.001 | 499 ± 90 | 288.43 |
IC50 of promastigotes: concentration of drug that caused 50% of growth inhibition of promastigotes
IC50 of amastigotes: concentration of drug that caused 50% of growth inhibition of amastigotes
CC50: concentration of drug that caused 50% of growth inhibition in macrophages
SI: selectivity index (CC50/IC50)
Anti-leishmnial effects on promastigote form of L. tropica
Although, both combinations showed inhibitory effects against L. tropica promastigotes, the niosomal formulation was significantly more effective (P ≤ 0.05). The IC50 values of AmB plus selenium, niosomal formulation and glucantime were 6.7 ± 1.9 µg/ml, 18.2 ± 0.9 µg/ml and 1445 ± 97.3 µg/ml, respectively (Table 2). In addition, the effect of the niosomal combination or simple form exhibited a dose- response manner (Fig. 4).
Fig. 4.
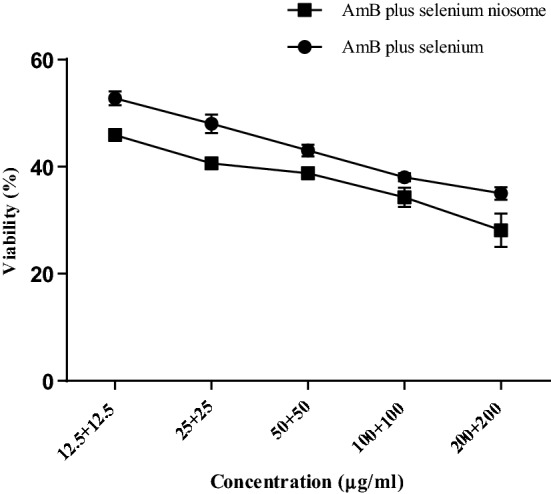
Comparison of viability effect of AmB plus selenium niosome with simple form of combination on L. tropica promastigotes by MTT assay (P < 0.05)
Anti-leishmnial effects on amastigote form of L. tropica
The mean number of macrophages with adherent amastigotes treated with both formulations was calculated. Combinations of various concentrations were able to significantly inhibit the number of amastigotes per macrophages compared with glucantime and the untreated control (P < 0.001) (Table 3). With regards to the IC50 values, the niosomal formulation was significantly more effective than the simple combination (Table 2) (IC50 = 1.73 ± 0.4 vs. 8.2 ± 2.2 µg/ml).
Table 3.
Comparison of the overall mean effect of various concentrations of AmB plus selenium, AmB plus selenium niosome and glucantime on the mean number of amastigotes in each macrophage
| Concentration | AmB plus selenui | AmB plus selenium niosome | Concentration | Glucantime | |||
|---|---|---|---|---|---|---|---|
| (µg/ml) | Mean ± SD | P value | Mean ± SD | P value | (µg/ml) | Mean ± SD | P value |
| 0 (Untreated control) | 32 ± 0.46 | NR | 32 ± 0.46 | NR | 0 (Untreated control) | 22 ± 1 | NR |
| 12.5 + 12.5 | 14.5 ± 0.35 | P ≤ 0.001 | 9.23 ± 0.28 | P ≤ 0.001 | 12.5 | 21 ± 0.26 | P ≤ 0.001 |
| 25 + 25 | 14 ± 0.53 | P ≤ 0.001 | 9.14 ± 1.16 | P ≤ 0.001 | 25 | 20 ± 0.75 | P ≤ 0.001 |
| 50 + 50 | 13.2 ± 0.26 | P ≤ 0.001 | 8.45 ± 0.08 | P ≤ 0.001 | 50 | 15 ± 0.17 | P ≤ 0.001 |
| 100 + 100 | 12.5 ± .46 | P ≤ 0.001 | 6.44 ± 0.72 | P ≤ 0.001 | 100 | 12 ± 0.26 | P ≤ 0.001 |
| 200 + 200 | 8.8 ± 0.5 | P ≤ 0.001 | 3.26 ± 0.17 | P ≤ 0.001 | 200 | 10 ± 0.62 | P ≤ 0.001 |
Flow cytometric analysis
Apoptosis and necrosis in promastigotes treated with AmB plus selenium, niosomal formulation, glucantime or untreated control were assessed using double-stained flow cytometric assay (annexin V and PI). An increase in apoptotic value from 43.25, 68.29 (12.5 + 12.5 µg/ml) to 73.67, 89.55 (200 + 200 µg/ml) was observed for AmB plus selenium and niosomal form, respectively. Programmed cell death (PCD) exhibited a dose-dependent pattern, that is, the effect was more significant at the highest niosomal formulation concentrations (200 + 200 µg/ml) compared to the simple form of combination, glucantime and untreated control (Fig. 5).
Fig. 5.
The apoptotic and necrotic profiles of Leishmania tropica promastigote with annexin V/PI at various concentrations of AmB plus selenium (1), AmB plus selenium niosome (2) and glucntime (3)
Gene expression effects
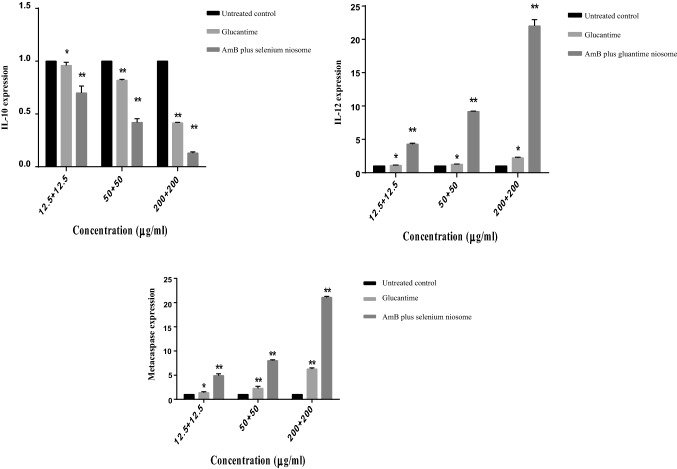
The results of gene expression showed that IL-12 and metacaspase levels significantly increased (P < 0.01). In contrast however, IL-10 level significantly decreased relative to glucantime and untreated control (P < 0.01) from the least to highest concentrations (Fig. 6).
Fig. 6.
The gene expression profiles of IL-12p40, metacaspase, and IL-10 on the Leishmania.tropica treated by the AmB plus selenium niosome and glucantime in comparison with untreated control (**P < 0.001, *P < 0.01), as measured by using real-time PCR
Discussion
The first line of treatment for leishmanisis consists of the application of pentavalent antimonials including meglumine antimoniate (MA) and sodium stibogluconate (SbV).Other treatments such as ketoconazole (Momeni et al. 2003), paramomycine, gentamycine (Tolouei et al. 2011), verapamil (Shokri et al. 2012), terbinafine (Farajzadeh et al. 2015a, b) and dapsone (Osorio et al. 1998) have been used for CL treatment but have limited efficacy. Presently, there have been intense researches on developing new strategies for leishmaniasis treatment (Sundar et al. 2014).
Co-administration of antileishmanial drugs with synergistic activity increased drug efficacy, prevented drug resistance, reduced dosage and decreased drug cytotoxicity (Yazdanpanah et al. 2004; White 1999; Farajzadeh et al. 2015a).
Niosomes as a drug carrier authorize the entrapment of a large number of drugs with a wide range of solubility, stability, long shelf life and enable drug delivery to target site in a controlled manner. Therefore, they can reduce drug toxicity and also enhance efficacy (Mahale et al. 2012; Bayindir et al. 2015; Duarte et al. 2016).
The current study presents an evaluation of the in vitro leishmanicidal activities of drug combinations including non-toxic concentrations of AmB plus selenium as a noisome or simple form. The results of the study showed a positive interaction between AmB and selenium. The niosomal combination of drugs decreased the proliferations of L. tropica extra-cellular promastigotes by about 4-fold in contrast to the use of simple form of combination. Consequently, this also resulted in inhibition of L. tropica amastigotes at between 54.7 and 72.5%. In addition, the niosomal combination caused programmed cell death and increased metacaspase in L. tropica promastigotes and decreased IL-10 expression for Th-2 inhibition and increased IL-12 for Th-1 activation to destroy the parasites.
However, AmB showed effective lishmanicidal activity against Leishmania species by bonding with the membrane sterols of the parasites (Torrado et al. 2008; Prajapati et al. 2011; Chattopadhyay and Jafurulla 2011), amidst high toxicity levels(Annaloro et al. 2009).Ambisome®, Abelcet®, SinaAmphoLeish®, Amphocil®are efficient against Leishmania species and are fairly safe, but highly unstable and expensive; thereby, limiting their use. Additionally, liposomal formulations of AmB are usually administered by an intravenous route for the treatment of leishmaniasis (Frankenburg et al. 1998; Yardley and Croft 2000; Sundar and Chakravarty 2010; Varikuti et al. 2017; Wijnant et al. 2018). All these are in agreement with the results of this study.
Previous studies showed a connection between selenium as an antitumor, antioxidant and antiparasitic agents, especially against Tripanasoma. Selenium has a potent and selective effect against some species of Leishmania (Lobanov et al. 2016; Cassago et al. 2006). Other studies have reported the antileishmanial activities of selenium and nano-selenium against L. infantum, L. major, L. tropica and L. braziliensis (Beheshti et al. 2013; Mahmoudvand et al. 2014; Martín-Montes et al. 2017).
The IC50 values showed that the AmB niosomal formulation plus selenium was more effective against L. tropica at various stages with a high safety index (SI = 261.57) and also, exhibited inhibitory effect in comparison to simple form of combination.
From the results of the current study, the IC50 values for both formulations on promastigotes were significantly higher than the amastigotes forms. These findings are corroborated by previous studies that showed that extra cellular form of Leishmania were 2-60 times more resistant in comparison with the intra cellular form due to biochemical and physiological differences in terms of their modes of action in response to the drugs (Lira et al. 1999).
Programmed cell death in Leishmani spp. was due to reactive oxygen species (ROS), hydrogen peroxide (H2O2), cytochrome c release, phosphatidylethanolamine (PE), activation of peptidase (such as metacaspase) and mitochondrial depolarization when treated with drugs (Debrabant et al. 2003; Murray et al. 2005; Kaye and Scott 2011). The metacaspase, as a cysteine peptidases in L. major and L. donovani plays an important role in cell death (Lee et al. 2002; Ambit et al. 2008; Zalila et al. 2011; Raina and Kaur 2012).The results of flow cytometry of intra cellular form of L. tropica treated with combination of niosomal or simple form showed a high level of apoptosis especially in the niosomal form. In addition, metacaspase increased which is indicative of programmed cell death compared with glucantime as a standard drug.
Some cytokines, like tumor necrosis factor-α (TNF-α), IL-12, colony-stimulating factor (GM-CSF) and interferon-γ (IFN-γ) are Th1-type immunity activation markers against Leishmania infection. On the other hand, other cytokines which are activators of Th2-type cell response such as IL-4, IL-10, IL-13, and the transforming growth factor-β (TGF-β) enables the development of the disease (Prajapati et al. 2011; Gannavaram et al. 2016). The results of this study showed that IL-12 levels increased, while IL-10 levels decreased significantly from a dose response effect of 12.5–200 µg/ml, as expected. Therefore, the niosomal combination of AmB plus selenium can modulate the immune system against the disease.
Conclusion
AmB and selenium are effective against Leishmania species. Our results showed that a combination of the niosomal formulation of these drugs showed superior efficacy in the treatment of CL due to L. tropica in an in vitro model. The niosomes prepared a suitable environment for better drug efficiency by acting as perfect carriers for the drug delivery. These findings suggest that niosomes are stable carrier’s for this combination and are easy to produce and affordable. Consequently, they provide promising results for the effective formulation of CL treatment in clinical settings.
Acknowledgements
This project (Protocol no. 94/691) was reviewed and approved by the Leishmaniasis Research Center and received financial support from the Vice Chancellor of research at the Kerman University of Medical Sciences located in Kerman in Iran. This study is a part of a PhD dissertation for pursuit of a PhD by research degree in leishmaniasis and skin diseases.
Author Contributions
SF and MM conceived and designed the study. PK contributed to preparation of niosomal formulation. HS analyzed the data. IS and MM contributed to study concept, data interpretation and manuscript preparation. All authors contributed in reviewed, revised, and confirmed the manuscript.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interests.
References
- Agrawal S, Rai M, Sundar S. Management of visceral leishmaniasis: Indian perspective. J Postgrad Med. 2005;51(Suppl 1):S53–S57. [PubMed] [Google Scholar]
- Aguiar MG, Pereira AMM, et al. Reductions in skin and systemic parasite burdens as a combined effect of topical paromomycin and oral miltefosine treatment of mice experimentally infected with Leishmania (Leishmania) amazonensis. Antimicrob Agents Chemother. 2010;54:4699–4704. doi: 10.1128/AAC.00809-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvar J, Vélez ID, Bern C, et al. Leishmaniasis worldwide and global estimates of its incidence. PLoS ONE. 2012;7:e35671. doi: 10.1371/journal.pone.0035671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambit A, Fasel N, Coombs GH, Mottram JC. An essential role for the Leishmania major metacaspase in cell cycle progression. Cell Death Differ. 2008;15:113–122. doi: 10.1038/sj.cdd.4402232. [DOI] [PubMed] [Google Scholar]
- Annaloro C, Olivares C, Usardi P, et al. Retrospective evaluation of amphotericin B deoxycholate toxicity in a single centre series of haematopoietic stem cell transplantation recipients. J Antimicrob Chemother. 2009;63:625–626. doi: 10.1093/jac/dkn549. [DOI] [PubMed] [Google Scholar]
- Bayindir ZS, Be AB, Yüksel N. Paclitaxel-loaded niosomes for intravenous administration: pharmacokinetics and tissue distribution in rats. Turk J Med Sci. 2015;45:1403–1412. doi: 10.3906/sag-1408-129. [DOI] [PubMed] [Google Scholar]
- Beheshti N, Soflaei S, Shakibaie M, et al. Efficacy of biogenic selenium nanoparticles against Leishmania major: in vitro and in vivo studies. J Trace Elem Med Biol. 2013;27:203–207. doi: 10.1016/j.jtemb.2012.11.002. [DOI] [PubMed] [Google Scholar]
- Cassago A, Rodrigues EM, Prieto EL, et al. Identification of Leishmania selenoproteins and SECIS element. Mol Biochem Parasitol. 2006;149:128–134. doi: 10.1016/j.molbiopara.2006.05.002. [DOI] [PubMed] [Google Scholar]
- Chandra D, Naik S, Naik S. Leishmania donovani infection down-regulates TLR2-stimulated IL-12p40 and activates IL-10 in cells of macrophage/monocytic lineage by modulating MAPK pathways through a contact-dependent mechanism. Clin Exp Immunol. 2008 doi: 10.1111/j.1365-2249.2008.03741.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattopadhyay A, Jafurulla M. A novel mechanism for an old drug: amphotericin B in the treatment of visceral leishmaniasis. Biochem Biophys Res Commun. 2011;416:7–12. doi: 10.1016/j.bbrc.2011.11.023. [DOI] [PubMed] [Google Scholar]
- Croft SL, Seifert K, Yardley V. Current scenario of drug development for leishmaniasis. Indian J Med Res. 2006;123:399–410. [PubMed] [Google Scholar]
- Croft SL, Sundar S, Fairlamb AH. Drug resistance in Leishmaniasis. Clin Microbiol Rev. 2006;19:111–126. doi: 10.1128/CMR.19.1.111-126.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha MA, de Cassia Soler R, Leão ACQ, Lindoso JAL. Efficacy and safety of liposomal amphotericin B for the treatment of mucosal leishmaniasis from the new world: a retrospective study. Am J Trop Med Hyg. 2015;93:1214–1218. doi: 10.4269/ajtmh.15-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debrabant A, Lee N, Bertholet S, et al. Programmed cell death in trypanosomatids and other unicellular organisms. Int J Parasitol. 2003;33:257–267. doi: 10.1016/S0020-7519(03)00008-0. [DOI] [PubMed] [Google Scholar]
- Donowitz GR. Tissue-directed antibiotics and intracellular parasites: complex interaction of phagocytes, pathogens, and drugs. Clin Infect Dis. 1994;19:926–930. doi: 10.1093/clinids/19.5.926. [DOI] [PubMed] [Google Scholar]
- Duarte MC, Tavares GSV, Valadares DG, et al. Antileishmanial activity and mechanism of action from a purified fraction of Zingiber officinalis Roscoe against Leishmania amazonensis. Exp Parasitol. 2016;166:21–28. doi: 10.1016/j.exppara.2016.03.026. [DOI] [PubMed] [Google Scholar]
- Escudero-Martínez JM, Pérez-Pertejo Y, Reguera RM, et al. Antileishmanial activity and tubulin polymerization inhibition of podophyllotoxin derivatives on Leishmania infantum. Int J Parasitol Drugs Drug Resist. 2017;7:272–285. doi: 10.1016/j.ijpddr.2017.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farajzadeh S, Esfandiarpour I, Haghdoost AA, et al. Comparison between combination therapy of oral terbinafine and cryotherapy versus systemic meglumine antimoniate and cryotherapy in cutaneous leishmaniasis: a randomized clinical trial. Iran J Parasitol. 2015;10:1–8. [PMC free article] [PubMed] [Google Scholar]
- Farajzadeh S, Heshmatkhah A, Vares B, et al. Topical terbinafine in the treatment of cutaneous leishmaniasis: triple blind randomized clinical trial. J Parasit Dis. 2015;1:1. doi: 10.1007/s12639-014-0641-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farajzadeh S, Heshmatkhah A, Vares B, et al. Topical terbinafine in the treatment of cutaneous leishmaniasis: triple blind randomized clinical trial. J Parasit Dis. 2016;40:1159–1164. doi: 10.1007/s12639-014-0641-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankenburg S, Glick D, Klaus S, Barenholz Y. Efficacious topical treatment for murine cutaneous leishmaniasis with ethanolic formulations of amphotericin B. Antimicrob Agents Chemother. 1998;42:3092–3096. doi: 10.1128/AAC.42.12.3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freitas-Junior LH, Chatelain E, Kim HA, Siqueira-Neto JL. Visceral leishmaniasis treatment: What do we have, what do we need and how to deliver it? Int J Parasitol Drugs Drug Resist. 2012;2:11–19. doi: 10.1016/j.ijpddr.2012.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frézard F, Demicheli C, Ribeiro RR. Pentavalent antimonials: new perspectives for old drugs. Molecules. 2009;14:2317–2336. doi: 10.3390/molecules14072317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganguly S, Bandyopadhyay S, Sarkar A, Chatterjee M. Development of a semi-automated colorimetric assay for screening anti-leishmanial agents. J Microbiol Methods. 2006;66:79–86. doi: 10.1016/j.mimet.2005.10.011. [DOI] [PubMed] [Google Scholar]
- Gannavaram S, Bhattacharya P, Ismail N, et al. Modulation of innate immune mechanisms to enhance Leishmania vaccine-induced immunity: role of coinhibitory molecules. Front Immunol. 2016;7:187. doi: 10.3389/fimmu.2016.00187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaye P, Scott P. Leishmaniasis: complexity at the host–pathogen interface. Nat Rev Microbiol. 2011;9:604–615. doi: 10.1038/nrmicro2608. [DOI] [PubMed] [Google Scholar]
- Kedzierski L, Zhu Y, Handman E. Leishmania vaccines: progress and problems. Parasitology. 2006;133:S87. doi: 10.1017/S0031182006001831. [DOI] [PubMed] [Google Scholar]
- Koutsoni O, Barhoumi M, Guizani I, Dotsika E. Leishmania eukaryotic initiation factor (LeIF) inhibits parasite growth in murine macrophages. PLoS ONE. 2014;9:1–10. doi: 10.1371/journal.pone.0097319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremsner PG, Krishna S (2004) Combinations of anti malaria drugs—Malaria site. Lancet (London, England). https://www.malariasite.com/antimalarial-combinations/. Accessed 11 Jun 2018
- Kumar R, Engwerda C. Vaccines to prevent leishmaniasis. Clin Transl Immunol. 2014;3:e13. doi: 10.1038/cti.2014.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee N, Bertholet S, Debrabant A, et al. Programmed cell death in the unicellular protozoan parasite Leishmania. Cell Death Differ. 2002;9:53–64. doi: 10.1038/sj.cdd.4400952. [DOI] [PubMed] [Google Scholar]
- Lira R, Sundar S, Makharia A, et al. Evidence that the high incidence of treatment failures in Indian Kala-Azar is due to the emergence of antimony-resistant strains of Leishmania donovani. J Infect Dis. 1999;180:564–567. doi: 10.1086/314896. [DOI] [PubMed] [Google Scholar]
- Lobanov AV, Gromer S, Salinas G, Gladyshev VN. Selenium metabolism in Trypanosoma: characterization of selenoproteomes and identification of a Kinetoplastida-specific selenoprotein. Nucl Acids Res. 2016;1:1. doi: 10.1093/nar/gkl541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahale NB, Thakkar PD, Mali RG, et al. Niosomes: novel sustained release nonionic stable vesicular systems—an overview. Adv Colloid Interface Sci. 2012;183–184:46–54. doi: 10.1016/j.cis.2012.08.002. [DOI] [PubMed] [Google Scholar]
- Mahmoudvand H, Shakibaie M, Tavakoli R, et al. In vitro study of leishmanicidal activity of biogenic selenium nanoparticles against Iranian isolate of sensitive and glucantime-resistant Leishmania tropica. Iran J Parasitol. 2014;9:452–460. [PMC free article] [PubMed] [Google Scholar]
- Martín-Montes Á, Plano D, Martín-Escolano R, et al. Library of seleno-compounds as novel agents against Leishmania species. Antimicrob Agents Chemother. 2017;61:1–13. doi: 10.1128/AAC.02546-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchison D, Davies G. The chemotherapy of tuberculosis: past, present and future [State of the art] Int J Tuberc Lung Dis. 2012;16:724–732. doi: 10.5588/ijtld.12.0083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Momeni AZ, Aminjavaheri M, Omidghaemi MR. Treatment of cutaneous leishmaniasis with ketoconazole cream. J Dermatolog Treat. 2003;14:26–29. doi: 10.1080/09546630305552. [DOI] [PubMed] [Google Scholar]
- Moosavian Kalat SA, Khamesipour A, Bavarsad N, et al. Use of topical liposomes containing meglumine antimoniate (Glucantime) for the treatment of L. major lesion in BALB/c mice. Exp Parasitol. 2014;143:5–10. doi: 10.1016/j.exppara.2014.04.013. [DOI] [PubMed] [Google Scholar]
- Murray HW, Flanders KC, Debra D, et al. Antagonizing deactivating cytokines to enhance host defense and chemotherapy in experimental visceral leishmaniasis antagonizing deactivating cytokines to enhance host defense and chemotherapy in experimental visceral leishmaniasis. Infect Immun. 2005;73:3903–3911. doi: 10.1128/IAI.73.7.3903-3911.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olliaro PL, Taylor WRJ. Antimalarial compounds: from bench to bedside. J Exp Biol. 2003;206:3753–3759. doi: 10.1242/jeb.00653. [DOI] [PubMed] [Google Scholar]
- Omollo R, Alexander N, Edwards T, et al. Safety and efficacy of miltefosine alone and in combination with sodium stibogluconate and liposomal amphotericin B for the treatment of primary visceral leishmaniasis in East Africa: study protocol for a randomized controlled trial. Trials. 2011;12:166. doi: 10.1186/1745-6215-12-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osorio LE, Palacios R, Chica ME, Ochoa MT. Treatment of cutaneous leishmaniasis in Colombia with dapsone. Lancet. 1998;351:498–499. doi: 10.1016/S0140-6736(05)78687-6. [DOI] [PubMed] [Google Scholar]
- Owais M, Gupta C. Targeted drug delivery to macrophages in parasitic infections. Curr Drug Deliv. 2005;2:311–318. doi: 10.2174/156720105774370177. [DOI] [PubMed] [Google Scholar]
- Perez AP, Altube MJ, Schilrreff P, et al. Topical amphotericin B in ultradeformable liposomes: formulation, skin penetration study, antifungal and antileishmanial activity in vitro. Colloids Surf B Biointerfaces. 2016;139:190–198. doi: 10.1016/j.colsurfb.2015.12.003. [DOI] [PubMed] [Google Scholar]
- Prajapati VK, Awasthi K, Gautam S, et al. Targeted killing of Leishmania donovani in vivo and in vitro with amphotericin B attached to functionalized carbon nanotubes. J Antimicrob Chemother. 2011;66:874–879. doi: 10.1093/jac/dkr002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raina P, Kaur S. Knockdown of LdMC1 and Hsp70 by antisense oligonucleotides causes cell-cycle defects and programmed cell death in Leishmania donovani. Mol Cell Biochem. 2012;359:135–149. doi: 10.1007/s11010-011-1007-y. [DOI] [PubMed] [Google Scholar]
- Rayman MP. Selenium in cancer prevention: a review of the evidence and mechanism of action. Proc Nutr Soc. 2005;64:527–542. doi: 10.1079/PNS2005467. [DOI] [PubMed] [Google Scholar]
- Rayman MP. Selenium and human health. Lancet. 2012;379:1256–1268. doi: 10.1016/S0140-6736(11)61452-9. [DOI] [PubMed] [Google Scholar]
- Riabi TR, Sharifi I, Mohammadi AM, et al. Evaluation of a possible synergistic effect of meglumine antimoniate with paromomycin, miltefosine or allopurinol on in vitro susceptibility of Leishmania tropica resistant isolate. Iran J Parasitol. 2013;8:396–401. [PMC free article] [PubMed] [Google Scholar]
- Rogerson A, Cummings J, Florence AT. Adriamycin-loaded niosomes: drug entrapment, stability and release. J Microencapsul. 1987;4:321–328. doi: 10.3109/02652048709021824. [DOI] [PubMed] [Google Scholar]
- Santos DO, Coutinho CER, Madeira MF, et al. Leishmaniasis treatment—a challenge that remains: a review. Parasitol Res. 2008;103:1–10. doi: 10.1007/s00436-008-0943-2. [DOI] [PubMed] [Google Scholar]
- Seifert K, Croft SL. In vitro and in vivo interactions between miltefosine and other antileishmanial drugs. Antimicrob Agents Chemother. 2006;50:73–79. doi: 10.1128/AAC.50.1.73-79.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shokri A, Sharifi I, Khamesipour A, et al. The effect of verapamil on in vitro susceptibility of promastigote and amastigote stages of Leishmania tropica to meglumine antimoniate. Parasitol Res. 2012;110:1113–1117. doi: 10.1007/s00436-011-2599-6. [DOI] [PubMed] [Google Scholar]
- Soflaei S, Dalimi A, Abdoli A, et al. Anti-leishmanial activities of selenium nanoparticles and selenium dioxide on Leishmania infantum. Comp Clin Path. 2014;23:15–20. doi: 10.1007/s00580-012-1561-z. [DOI] [Google Scholar]
- Sundar S, Chakravarty J. Liposomal amphotericin B and leishmaniasis: dose and response. J Glob Infect Dis. 2010;2:159–166. doi: 10.4103/0974-777X.62886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundar S, Singh A, Singh OP. Strategies to overcome antileishmanial drugs unresponsiveness. J Trop Med. 2014;2014:1–7. doi: 10.1155/2014/646932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakur CP, Kanyok TP, Pandey AK, et al. A prospective randomized, comparative, open-label trial of the safety and efficacy of paromomycin (aminosidine) plus sodium stibogluconate versus sodium stibogluconate alone for the treatment of visceral leishmaniasis. Trans R Soc Trop Med Hyg. 2000;94(4):429–431. doi: 10.1016/S0035-9203(00)90130-5. [DOI] [PubMed] [Google Scholar]
- Tolouei S, Hasheminia S, Narimani M, et al. Leishmanicidal activity of films containing paromomycin and gentamicin sulfate both in vitro and in vivo. Iran J Parasitol. 2011;6:60–65. [PMC free article] [PubMed] [Google Scholar]
- Torrado JJ, Espada R, Ballesteros MP, Torrado-Santiago S. Amphotericin B formulations and drug targeting. J Pharm Sci. 2008;97:2405–2425. doi: 10.1002/jps.21179. [DOI] [PubMed] [Google Scholar]
- Tran PA, Webster TJ. Selenium nanoparticles inhibit Staphylococcus aureus growth. Int J Nanomed. 2011;6:1553–1558. doi: 10.2147/IJN.S21729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varikuti S, Oghumu S, Saljoughian N, et al. Topical treatment with nanoliposomal amphotericin B reduces early lesion growth but fails to induce cure in an experimental model of cutaneous leishmaniasis caused by Leishmania mexicana. Acta Trop. 2017;173:102–108. doi: 10.1016/j.actatropica.2017.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagh VD, Deshmukh OJ. Niosomes as ophthalmic drug delivery systems: a review. J Pharm Res. 2015;3:1558–1563. [Google Scholar]
- Whanger PD. Selenium and its relationship to cancer: an update. Br J Nutr. 2004;91:11–28. doi: 10.1079/BJN20031015. [DOI] [PubMed] [Google Scholar]
- White NJ. Delaying antimalarial drug resistance with combination chemotherapy. Parassitologia. 1999;41:301–308. [PubMed] [Google Scholar]
- Wijnant G-J, Van Bocxlaer K, Yardley V, et al. Relation between skin pharmacokinetics and efficacy in Am Bisome treatment of murine cutaneous leishmaniasis. Antimicrob Agents Chemother. 2018;62:e02009-17. doi: 10.1128/AAC.02009-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization (2012) Research priorities for Chagas disease, human African trypanosomiasis and leishmaniasis. World Health Organ Tech Rep Ser (975):v–xii, 1–100 [PubMed]
- Yang J, Huang K, Qin S, et al. Antibacterial action of selenium-enriched probiotics against pathogenic Escherichia coli. Dig Dis Sci. 2009;54:246–254. doi: 10.1007/s10620-008-0361-4. [DOI] [PubMed] [Google Scholar]
- Yardley V, Croft SL. A comparison of the activities of three amphotericin B lipid formulations against experimental visceral and cutaneous leishmaniasis. Int J Antimicrob Agents. 2000;13:243–248. doi: 10.1016/S0924-8579(99)00133-8. [DOI] [PubMed] [Google Scholar]
- Yazdanpanah Y, Sissoko D, Egger M, et al. Clinical efficacy of antiretroviral combination therapy based on protease inhibitors or non-nucleoside analogue reverse transcriptase inhibitors: indirect comparison of controlled trials. BMJ. 2004;328:249. doi: 10.1136/bmj.37995.435787.A6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalila H, González IJ, El-Fadili AK, et al. Processing of metacaspase into a cytoplasmic catalytic domain mediating cell death in Leishmania major. Mol Microbiol. 2011;79:222–239. doi: 10.1111/j.1365-2958.2010.07443.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong M, Wang X, Wen J, et al. Selection of reference genes for quantitative gene expression studies in the house fly (Musca domestica L.) using reverse transcription quantitative real-time PCR. Acta Biochim Biophys Sin (Shanghai) 2013;45:1069–1073. doi: 10.1093/abbs/gmt111. [DOI] [PubMed] [Google Scholar]