Abstract
Tick borne zoonotic diseases are one of the major emerging threats to live stock and public health in India, especially in Western Ghats of south India. Since livestock and wild animals share habitats and grasslands, it is important to know the species composition of major tick parasitism on live stock as well as their geographical distribution for effective control of tick and tick borne diseases. This study provides basic knowledge that is necessary to initiate Kyasanur Forest Disease (KFD) prevention programs in these areas. Ticks were sampled from Wayanad districts of Kerala from domestic animals and identified morphologically. A total of 195 cattle searched, in which 168 (86.15%) cattle were infested with ticks and a total of 3633 ticks comprising three genera and seven species were collected, Rhipicephalus microplus (52.71%) was prevalent species followed by Haemaphysalis bispinosa (16.9%), Rhipicephalus decoloratus (15.77%), Haemaphysalis turturis (11.42%), Rhipicephalus sanguineus (1.32%), Amblyomma integrum (1.15%) and Haemaphysalis spinigera (0.71%) were identified based on their morphological characters. As R. microplus was the prevalent species, the risk of transmission of babesiosis and anaplasmosis to cattle increases and the presence of Haemaphysalis sp. point out the risk of KFD in among the tribal colony people and it can be reduced by applying with acaricides on domestic animals.
Keywords: Tick, Ectoparasite, Rhipicephalus microplus, Haematophagous, Tick borne disease
Introduction
Ticks have become competent vectors, efficient distributers and amplifying agents of diverse pathogens within animal and human populations. The rate of effective contact between vector ticks with their host and the obtaining of blood meals are the driving forces of the rapid distribution of tick borne diseases within and among populations. (Peter et al. 2005; Razmi et al. 2007; Ghosh and Nagar 2014). In India, 106 tick species were recorded to be infesting domestic and wild animals (Geevarghese et al. 1997). These ticks are of very high veterinary and medical importance because of its major effect on the animal husbandry and productivity of livestock, weight gain, milk production and quality of hide (De Castro et al. 1997) and zoonotic pathogen transmission to humans. The global economic losses due to tick infestation have been estimated as US $14,000–18,000 million annually and in India it was approximately US $ 498.7 million (Singh and Rath 2013). In addition these animals are also serving as reproductive host for KFD vector ticks.
In India, the studies on ticks received importance after the discovery of KFD, transmitted by Haemaphysalis sp. in Western Ghats (Mourya et al. 2014). In human variety of tick species involved in the transmission of different pathogens which cause diseases like borreliosis or lyme diseases which is transmitted by Ixodid ticks (Maria et al. 2012), Rocky Mountain spotted fever is transmitted by Dermacentor sp. (Socolovschi et al. 2009a, b), Rickettsiosis and Boutonneuse fever transmitted by Rhipicephalus sanguineus (Socolovschi et al. 2009a, b; Levin et al. 2009). Hyalomma sp. and Haemaphysalis sp. which are responsible for viral diseases of Crimean–Congo haemorrhagic fever and Kyasanur forest disease (Mourya et al. 2014). The epidemic of Indian tick typhus was reported from Himachal Pradesh (Kumar et al. 2011) and serologically positive cases of Lyme disease and rickettsiosis were reported from Tamil Nadu and Pondicherry (Sharma et al. 1992; Stephen et al. 2018). The first instance of Crimean–Congo haemorrhagic fever was detected from Gujarat (Kumar et al. 2014). Since livestock and wild animals share habitats and grassland in these regions, studies are necessary to determine if ticks can transmit pathogens between livestock and wildlife, and even to humans in the region. This study provides very important information and basic knowledge that is necessary to initiate KFD prevention programs in these areas. In Kerala KFD is endemic in Wayanad district, however, only a few studies were done about the tick fauna in different hosts (Prakasan and Ramani 2007; Shyma et al. 2013). It is important to know the prevalence of the tick species parasitism on cattle as well as their geographical distribution for the control of tick and tick borne diseases. The aim of present study is to reveal the common tick fauna present in domestic animals of KFD endemic area in tribal settlements of Wayanad district, Kerala.
Materials and methods
Ethical clearance
All aspects involving domestic animals use were conducted in accordance with the Indian Animal Welfare Act, 2011 (section 11), and recognized standards for the care and use of animals. Written consent from cattle owners were also taken as a prior permission for tick collection from respective cattle.
Study area
Wayanad district is a hill district in North-East of Kerala with an area of 2131 km2, in the part of Western Ghats lie between Latitude N 11°37′ and Longitude E 76°58′ and situated at an altitude ranging from 2500 to 3000 ft above Sea Level. Wayanad district has the highest forest cover of 83.3%, agriculture and animal husbandry is the principal occupation of the people. The district has highest tribal population of about 1.25 lakh consisting 17% of the total population. This region experiences a moderate, semitropical climate with an average temperature of 18–30 °C in summer and winter with mean rainfall of 2786 mm and the annual average relative humidity is above 60%. The area receives most of its rainfall under the influence of southwest monsoons and less rainfall under the influence of the northeast monsoons.
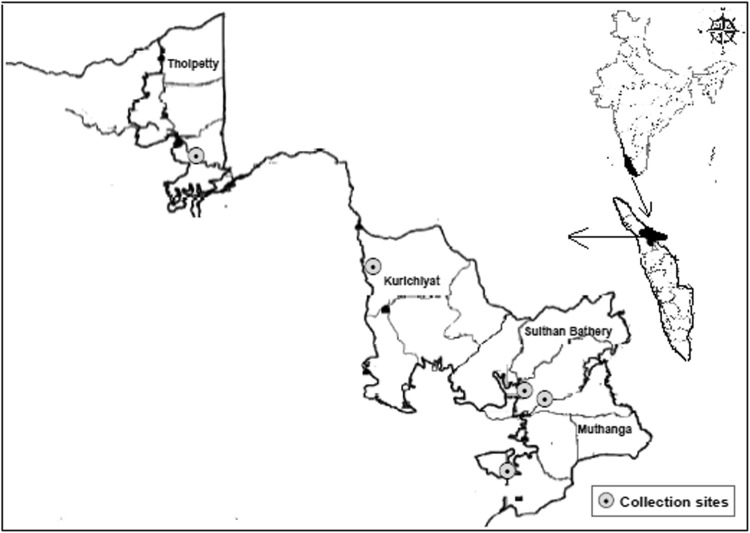
The abundance of vegetations and forest region provides favourable conditions for the survival of several tick species. The present study was performed in five different tribal villages (Tholpetty, Thottamoola, Cheeyambam, Kallumukku and Alathoor) in the northwest and southwest part of the district (Fig. 1) from January to May-2017. Cattle and goat are the main domestic livestock in the study area. Wild elephant, deer, giant squirrel, monkey, bison, boars and wolf are very abundant throughout the district, mostly in transition areas between forests and farmlands. In addition, small rodents and reptiles, which are major hosts for ticks, are also abundant in the district.
Fig. 1.
Spatial distribution map of study area inWayanad district
Tick collection and identification
Whole body of each domestic animal was searched thoroughly for the presence of ticks during day time. Precautions were taken before collecting ticks from cattle by wearing gloves, mask and applying the repellent Benzyl benzoate (I.P.25%) lotion to avoid the tick bites. The ticks were gently plucked up from the body of the host by hand with fingers were shield with rubber gloves or with the aid of blunt pointed tweezers. Ticks were grasped as close to the skin surface as possible and pulled upward with steady even pressure. Both nymph and adult ticks were collected and all were in fully blood fed condition. The specimens were kept in separate containers and preserved in 70% ethanol. Then the samples were labeled with the date, host, and locality on each container. These samples were transported to the laboratory of Medical entomology and Zoology, National Institute of Virology-Kerala unit, Alappuzha. The preserved specimens were washed, dehydrated and subjected for KOH treatment and mounted on a slide by using DPX (Distyrene, a plasticizer and Xylene) or Canada balsam for further reference. Adult ticks were identified up to species level following standard keys (Walker et al. 2013; Geevarghese and Mishra 2011) under stereomicroscope.
Site mapping and statistical analysis
Geographic coordinates of collection sites were marked using a handheld Global Positioning System (GPS). These coordinates were integrated to a GIS database using the software Arc GIS to map the distribution of ticks in domestic animals of the associated area. Spearman’s Correlation analysis was used to assess the statistical significance among tick species using IBM-SPSS statistics viewer (version 20).
Result
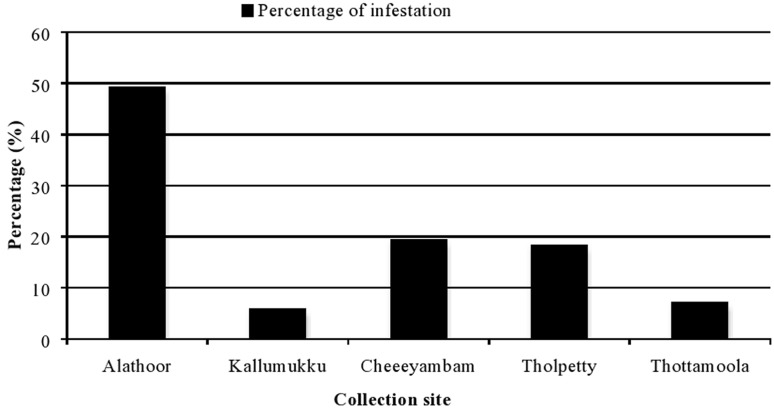
The study was conducted from January to May from five sites such as Alathoor, Cheeyambam, Kallumukku, Tholpetty and Thottamoola in Western Ghats of Wayanad district, Kerala state. A total of 195 domestic animals were searched in which 168 (86.15%) animals were found positive for ectoparasitism. The details of cattle tick infestation are presented in Table 1. Among the total animals surveyed 68.20% of cattle and 17.94% goats were infested with one or more species of ticks. Among the five sites visited, maximum number (50.91%) of ticks was collected from Alathoor followed by Kallumukku 40.13% and in other three sites such as Tholpetty 4.03%, Thottamoola 3.89% and Cheeyambam 0.94% of ticks were collected (Table 2 and Fig. 2).
Table 1.
Rate of tick infestation on cows and goats of five sites
| Sl. no. | Place | Cows | Goat | Total no. of cows and goat | % of infestation | |||
|---|---|---|---|---|---|---|---|---|
| Searched | infested | Searched | infested | Searched | Infested | |||
| 1. | Alathoor | 88 | 76 | 7 | 7 | 95 | 83 | 87.4 |
| 2. | Kallumukku | 12 | 10 | 0 | 0 | 12 | 10 | 83.3 |
| 3. | Cheyambam | 32 | 28 | 5 | 4 | 37 | 32 | 86.5 |
| 4. | Tholpetty | 12 | 12 | 23 | 19 | 35 | 31 | 88.5 |
| 5. | Thottamoola | 10 | 7 | 6 | 5 | 16 | 12 | 81.3 |
| Sub total | 154 | 133 | 41 | 35 | 195 | 168 | ||
Table 2.
Different species identified in cattle from five different sites with its sex differentiation
| Sl. no. | Site | Species name | Number | Total | % | |
|---|---|---|---|---|---|---|
| Male | Female | |||||
| 1 |
Alathoor N 11°41′04.1″ E076°21′35.3″ 2860 ft |
Rhipicephalus microplus | 120 | 886 | 1006 | 27.69 |
| R. decoloratus | 55 | 295 | 350 | 9.63 | ||
| R. sanguineus | 6 | 13 | 19 | 0.52 | ||
| Haemaphysalis bispinosa | 59 | 211 | 270 | 7.43 | ||
| H. turturis | 18 | 177 | 195 | 5.37 | ||
| Amblyomma integrum | 1 | 9 | 10 | 0.27 | ||
| Sub total | 259 | 1591 | 1850 | 50.91 | ||
| 2 |
Kallumukku N 11°41′28.0″ E076°20′38.2″ 2967 ft |
R. microplus | 80 | 743 | 823 | 22.65 |
| R. decoloratus | 46 | 149 | 195 | 5.36 | ||
| R. sanguineus | 1 | 6 | 7 | 0.19 | ||
| H. bispinosa | 64 | 185 | 249 | 6.85 | ||
| H. turturis | 14 | 165 | 179 | 4.92 | ||
| A. integrum | 0 | 6 | 6 | 0.16 | ||
| Sub total | 755 | 704 | 1459 | 40.13 | ||
| 3 |
Cheeyambam N 11°47′27.8″ E0 76°13′21.0″ 2456 ft |
R. microplus | 0 | 5 | 5 | 0.13 |
| R. sanguineus | 0 | 5 | 5 | 0.13 | ||
| H. bispinosa | 0 | 7 | 7 | 0.19 | ||
| A. integrum | 6 | 12 | 18 | 0.49 | ||
| Sub total | 6 | 29 | 35 | 0.94 | ||
| 4 |
Tholpetty N 11°52′44.6″ E0 76°04′47.7″ 2451 ft |
R. microplus | 11 | 40 | 51 | 1.40 |
| R. decoloratus | 0 | 7 | 7 | 0.19 | ||
| R. sanguineus | 0 | 13 | 13 | 0.36 | ||
| H. bispinosa | 0 | 62 | 62 | 1.70 | ||
| H. turturis | 0 | 6 | 6 | 0.16 | ||
| A. integrum | 7 | 1 | 8 | 0.22 | ||
| Sub total | 18 | 129 | 147 | 4.03 | ||
| 5 |
Thottamoola N 11°37′37.2″ E 76°19′47.9″ 2944 ft |
R. microplus | 0 | 30 | 30 | 0.83 |
| R. decoloratus | 0 | 21 | 21 | 0.57 | ||
| R. sanguineus | 0 | 4 | 4 | 0.11 | ||
| H. bispinosa | 0 | 26 | 26 | 0.71 | ||
| H. turturis | 0 | 35 | 35 | 0.96 | ||
| H. spinigera | 5 | 21 | 26 | 0.71 | ||
| Sub total | 11 | 131 | 142 | 3.89 | ||
| Gross total | 1649 | 1984 | 3633 | |||
Fig. 2.
Percentage of infestation in five surveyed sites
A total of 3633 ticks comprising adult 2607 (71.76%) and immature 1026 (28.24%) ticks belonging to three genera and 7 species were collected, namely Rhipicephalus, Haemaphysalis and Amblyomma are commonly prevalent in all sites studied. The highest prevalence rate was identified as Rhipicephalus microplus 1915 (52.71%) and it was the most predominant tick species, followed by R. decoloratus 573 (15.77%), Haemaphysalis bispinosa 614 (16.9%), H. turturis 415 (11.42%), R. sanguineus 48 (1.32%), Amblyomma integrum 42 (1.15%) and H. spinigera 26 (0.71%) (Table 2). Spearman’s correlation analysis between the H. bispinosa and H. turturis were found as significant (P = 0.05). Among the Rhipicephalus sp., Rhipicephalus microplus was the most prevalent species followed by R. decoloratus and R. sanguineus. Three species of Haemaphysalis such as H. bispinosa, H. turturis and H. spinigera were collected (Table 3). These results suggest that the areas are under the risk of KFD virus transmission. Average tick load per cattle of R. microplus was 11.39, R. decoloratus 3.41, R. sanguineus 0.28, Haemaphysalis bispinosa 3.65, H. turturis 2.47, H. spinigera 0.15 and Amblyomma integrum 0.25.
Table 3.
Species of ticks recovered from cattle with respect to their genera and life stage
| Sl. no. | Species name | Nymph | Adult | Total | % | Average tick load/cattle |
|---|---|---|---|---|---|---|
| 1 | Rhipicephalus microplus | 664 | 1251 | 1915 | 52.71 | 11.39 |
| 2 | R. decoloratus | 125 | 448 | 573 | 15.77 | 3.41 |
| 3 | R. sanguineus | 48 | 0 | 48 | 1.32 | 0.28 |
| 4 | Haemaphysalis bispinosa | 58 | 556 | 614 | 16.90 | 3.65 |
| 5 | H. turturis | 105 | 310 | 415 | 11.42 | 2.47 |
| 6 | H. spinigera | 26 | 0 | 26 | 0.71 | 0.15 |
| 7 | Amblyomma integrum | 0 | 42 | 42 | 1.15 | 0.25 |
| Total | 1026 | 2607 | 3633 | |||
Discussion
The Wayanad district provides favorable ecological conditions for the propagation of haematophagous arthropods including ticks due to its richness in vegetation, domestic and wild animal fauna. The present study shows that cattle are highly infested with ticks, which are responsible for transmitting harmful pathogens to both animals and human. More than 85% of domestic animals were found as infested with ticks, suggested that the area is highly under the risk of tick borne diseases. The uncontrolled movements of domestic animals in forest remain the primary reason behind the large numbers of ticks that are introduced from their territories into far regions. Earlier study from Prakasan and Ramani (2007) reported that Ixodid tick fauna of 5 genera and 18 species from nine districts of Kerala.
The most prevalent species observed in the study was R. microplus and H. bispinosa which was found infesting majority of the cattle and goat. The highest prevalence of R. microplus in Nilgiri, the adjoining area of Wayanad district in Western Ghats and also the same result was observed in KFD endemic area of Karnataka (Rajagopalan and Sreenivasan 1981; Kumar et al. 2014). It is considered as the most important tick parasite of livestock in the world and carries many pathogens (Ghosh and Nagar 2014; Kumar et al. 2014; Manaswini et al. 2017). According to Rajagopalan and Sreenivasan (1981) these species did not show any definite seasonal pattern of infestation. H. bispinosa, on the other hand, is a tick breeding principally in the cattle sheds of the KFD area of Karnataka. The infestation of nymphs and adults was observed throughout the year. Thus, the infestation of H. bispinosa on cattle and buffaloes seems to reflect their restricted breeding habits in the cattle sheds and the availability of the hosts throughout the year (Rajagopalan and Sreenivasan 1981; Bhat 1971).
Three species of Haemaphysalis such as H. bispinosa, H. turturis and H. spinigera were observed on cattle and in which H. bispinosa was the predominant species. This is a significant observation in the context of recently reported Kyasanur forest disease (KFD) incidence in the district. These species are playing an important role for the transmission of KFD in Karnataka, Kerala, Maharashtra and Goa (Rajagopalan and Sreenivasan 1981 and Mourya et al. 2014). Haemaphysalis spinigera, a major vector of KFD virus formed 0.71% of ticks on cattle. The immature of H. spinigera are known to parasitize certain species of wild mammal, small mammals and ground birds in the KFD area (Rajagopalan et al. 1968a, b, 1978; Rajagopalan and Anderson 1971; Rajagopalan 1972; Rajagopalan and Sreenivasan 1981). Cattle have become the most important hosts for the adults of H. spinigera and play a prominent role in the dissemination of larval population on the forest floor. The studies on the ixodid tick population in the forests of KFD area have shown a distinct seasonal appearance in the Western Ghats of Karnataka. The Haemaphysalis larvae start appearing on drags in September soon after the monsoon. They are abundant on the forest floor between November and December. This is followed by the appearance of nymphs in the drier months between December and January with a peak in February or March (Rajagopalan and Sreenivasan 1981). However, in our present study in Kerala (NIV Unpublished data) nymph peak density was occurred in the month of January thereafter gradually decreased and these ticks show the KFD virus prevalence during their peak months. The variation in the prevalence of ticks in this area may be due to climatic factors, climate can limit the distribution of ticks directly by affecting growth or survival. The presence of these species on cattle represents a twofold risk; cattle support their higher prevalence in the area and the bigger risk is the chance of potential vector contact/bite with the human and KFD amplifying host.
The movements and abundance of wild animals that serve as reservoirs of KFDV and hosts for Haemaphysalis spp., are profoundly affected by the climate, especially temperature. During summer season domestic animal home ranges (fringe area of forest) become wild animal refuges for food, water and shelter are common. As a result, there is a significant increase in the participation of cattle and buffaloes as hosts for ixodid ticks. The fringe area of forest is not only home range for domestic animals but also a potential KFDV circulating area between ticks and host animals. This network behavior enables the transmission of the KFD vector tick from domestic animal to wild animals vice versa. Among other species of ticks, the infestation of H. turturis, R. decoloratus and A. integrum on cattle suggests that these animals are also important hosts along with other mammals in the KFD area. R. sanguineus, the tropical dog tick, distributed globally throughout the warm countries; it is implicated as vector of B. motasi and B. ovis, and may play a role in transmission of B. equi (Ghosh and Nagar 2014). The pathogenic B. bigemina is transmitted by R. decoloratus, predominant with more than 15% in the present study, also responsible for the diseases in livestock such as Babesiosis or Redwater fever (Abdela and Jilo 2016).
There are currently many Haemaphyslis sp. capable of transmitting human and wildlife KFD virus in Western Ghats of India (Rajagopalan et al. 1968a, b, 1978; Rajagopalan and Anderson 1971; Rajagopalan 1972; Rajagopalan and Sreenivasan 1981). The presence of domestic animal host and their contributions to supporting different tick species can vary considerably across the forest. Previous studies in Kerala and Karnataka characterising ticks and their host have reported that domestic animal populations supporting vector ticks in Western Ghats of India (Rajagopalan and Sreenivasan 1981 and Kumar et al. 2002). The changing ecology, particularly deforestation, reforestation, plantation and associated increases in wildlife and domestic animal populations provide increased proximal host contact with the widely distributed blood-feeding ectoparasites in these areas. Further studies of tick–host relationships are necessary for better understand tick species distributions, population dynamics prevalence of tick-borne pathogens, and their potential effects on human and animal health.
Acknowledgements
Authors are grateful to Director, National Institute of virology, Pune, for his support and encouragement. Dr.MD Gokhale, National Institute of virology, Pune for critical review of the article and his valuable suggestions. The Authors acknowledge the Department of Health Research (DHR), for financial support on Project ID concept proposal: 2014-0495.
Compliance with ethical standards
Conflict of interest
The authors report no conflicts of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abdela N, Jilo K. Bovine babesiosis and its current status in Ethiopia. Adv Biol Res. 2016;10:138–146. [Google Scholar]
- Bhat HR. Localised mass breeding of Haemaphysalis bispinosa Neumann, 1897 (Acarina: Ixodidae) in Kyasanur forest disease area, Shimoga district, Mysore State, India. J Bombay Nat Hist Soc. 1971;68:485. [Google Scholar]
- De Castro JJ, James AD, Minjauw B, Di Giulio G, Permin A, Pegram RG. Long-term studies on the economic impact of ticks on Sanga cattle in Zambia. Exp Appl Acarol. 1997;21:3–19. [PubMed] [Google Scholar]
- Geevarghese G, Mishra AC. Haemaphysalis ticks of India. London: Elsevier; 2011. [Google Scholar]
- Geevarghese G, Fernande S, Kulkarni SM. A check list of Indian ticks (Acari: Ixodoidea) Indian J Anim Sci. 1997;67:566–574. [Google Scholar]
- Ghosh S, Nagar G. Problem of ticks and tick-borne diseases in India with special emphasis on progress in tick control research. J Vector Borne Dis. 2014;51:259–270. [PubMed] [Google Scholar]
- Kumar K, Balakrishnan N, Katyal R, Gill KS. Prevalence of ixodid ticks in Nilgiri district of Tamil Nadu state (India) Int J Infect Dis. 2002;34:124–127. [PubMed] [Google Scholar]
- Kumar K, Jain SK, Sharma AK. Outbreak of Indian Tick Typhus amongst residents of Deol village, District, Kangra, Himachal Pradesh (India) Int J Med Public Health. 2011;1:67–71. doi: 10.5530/ijmedph.3.2011.11. [DOI] [Google Scholar]
- Kumar K, Balakrishnan N, Abhay Kumar S. Studies on the vertical distribution of ticks of domestic animals and their public health importance in Nilgiri Hills and adjoining areas of Tamil Nadu State (India) Int J Zool. 2014;1:1–6. doi: 10.1155/2014/359812. [DOI] [Google Scholar]
- Levin ML, Killmaster L, Eremeeva ME, Dasch GA. Effects of Rickettsia conorii infection on the survival of Rhipicephalus sanguineus ticks. Clin Microbiol Infect. 2009;15:277–278. doi: 10.1111/j.1469-0691.2008.02234.x. [DOI] [PubMed] [Google Scholar]
- Manaswini D, Mitra RP, Bijayendranath M, Hembram Ananta, Trilochan M, Adhikari S. Ixodid ticks infesting cattle and associated risk factors in coastal districts of Odisha. J Entomol Zool Stud. 2017;5:129–132. [Google Scholar]
- Maria AD, Hoen AG, Cislo P, Brinkerhoff R, Sarah A, Hamer Rowland M, Cortinas R, Vourc’h G, Melton F, Graham JH, Tsao JI, Bunikis J, Barbour AG, Kitron U, Piesman J, Fish D. Human Risk of Infection with Borrelia burgdorferi, the Lyme Disease Agent, in Eastern United States. Am J Trop Med Hyg. 2012;86:320–327. doi: 10.4269/ajtmh.2012.11-0395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mourya DT, Yadav PD, Patil DY. Highly infectious tick borne viral diseases: Kyasanur forest disease and Crimean–Congo hemorrhagic fever in India. J Public Health. 2014;3:8–21. doi: 10.4103/2224-3151.206890. [DOI] [PubMed] [Google Scholar]
- Peter RJ, Van den Bossche P, Penzhorn BL, Sharp B. Tick, fly, and mosquito control-lessons from the past, solutions for the future. Vet Parasitol. 2005;132:205–215. doi: 10.1016/j.vetpar.2005.07.004. [DOI] [PubMed] [Google Scholar]
- Prakasan K, Ramani N. Human infesting ixodid ticks of Kerala. Vet Parasitol. 2007;27:108–112. [Google Scholar]
- Rajagopalan PK. Ixodid ticks (Acarina: Ixodidae) parasitizing wild birds in the Kyasanur forest disease area of Shimoga district, Mysore State, India. J Bombay Nat Hist Soc. 1972;69:55. [Google Scholar]
- Rajagopalan PK, Anderson CR. Further studies on ticks of wild monkeys of Kyasanur forest disease area, Shimoga district. Indian J Med Res. 1971;59:847. [PubMed] [Google Scholar]
- Rajagopalan PK, Sreenivasan MA. Ixodid ticks on cattle and buffaloes in the Kyasanur forest disease area of Karnataka State. Indian J Med Res. 1981;73:880–889. [Google Scholar]
- Rajagopalan PK, Patil AP, Jorge Boshell M. Studies on ixodid tick populations on the forest floor in the Kyasanur forest disease area. Indian J Med Res. 1968;56:497. [PubMed] [Google Scholar]
- Rajagopalan PK, Patil AP, Jorge Boshell M. Ixodid ticks on their mammalian hosts in the Kyasanur forest disease area of Mysore State, India. Indian J Med Res. 1968;56:510. [PubMed] [Google Scholar]
- Rajagopalan PK, Sreenivasan MA, Anderson CR. Ixodid ticks of red spurfowl (Galloperdix spadicea spadicea) in the KFD area, Karnataka State. Indian J Med Res. 1978;68:949. [PubMed] [Google Scholar]
- Razmi GR, Glinsharifodini M, Sarvi S. Prevalence of ixodid ticks on cattle in Mazandaran province, Iran. Korean J Parasitol. 2007;45:307–310. doi: 10.3347/kjp.2007.45.4.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma RS, Verghese T, Gupta RS, Chattopadhya D (1992) Epidemiology of Lyme disease in the Nilgiri hills—1992—a preliminary report. In: Proceedings of the 3rd symposium of vectors and vector borne diseases India
- Shyma KP, Stanley B, Ray DD, Ghosh S. Prevalence of cattle and buffalo ticks in northern Kerala. Vet Parasitol. 2013;27:55–56. [Google Scholar]
- Singh NK, Rath SS. Epidemiology of ixodid ticks in cattle population of various agro-climatic zones of Punjab India. Asian Pac J Trop Med. 2013;6:947–951. doi: 10.1016/S1995-7645(13)60169-8. [DOI] [PubMed] [Google Scholar]
- Socolovschi C, Mediannikov O, Raoult D, Parola P. The relationship between spotted fever group Rickettsiae and Ixodid ticks. Vet Res. 2009;40:34. doi: 10.1051/vetres/2009017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Socolovschi C, Matsumoto K, Brouqui P, Raoult D, Parola P. Experimental infection on Rhipicephalus sanguineus with Rickettsia conorii conorii. Clin Microbiol Infect. 2009;15:324–325. doi: 10.1111/j.1469-0691.2008.02259.x. [DOI] [PubMed] [Google Scholar]
- Stephen S, Ambroise S, Gunasekaran D, Hanifah M, Sangeetha B, Pradeep J, Sarangapani K. Serological evidence of spotted fever group rickettsiosis in and around Puducherry, south India A three years study. J Vector Borne Dis. 2018;55:144–150. doi: 10.4103/0972-9062.242562. [DOI] [PubMed] [Google Scholar]
- Walker AR, Bouattour A, Camicas JL, Estrada Pena A, Horak IG, Latif, Pegram RG, Preston PM. Ticks of domestic animals in Africa, a guide to identification of species. 2. Edinburgh: Bioscience Reports; 2013. [Google Scholar]