Abstract
The current study was aimed to evaluate the frequency and epidemiological aspects of cutaneous leishmaniasis (CL) in the southwest of Iran, an endemic focus for the leishmaniasis from 2014 to 2017. In the present retrospective cross-sectional study, all suspicious CL persons who were referred to health centers affiliated to Abadan School of Medical Sciences (Abadan and Khorramshahr cities) were tested. In this regard, both clinical and parasitological (staining with Giemsa) verifications were performed to diagnose a case. Furthermore, a structured questionnaire containing some demographic details was applied for each positive patient. In total, 151 and 132 positive CL cases were identified in Abadan and Khorramshahr cities, respectively. More frequency of CL was observed during the Winter season, among male subjects, and in urban inhabitants in both cities. The incidence of CL based on age distribution was higher in the age range of 20–30 years than others. Besides, the hands and feet were the most involved organs. Although the frequency of CL has declined in the region, CL is still a public health problem. More appropriate control strategies are required to limit and eliminate the infection in the future.
Keywords: Cutaneous leishmaniasis, Leishmania, Epidemiology, Southwest of Iran
Introduction
Obligate intracellular parasites of Leishmania genus belong to family Trypanosomatidae (Order Kinetoplastida), and more than twenty species cause this vector-borne tropical/subtropical infection throughout the globe (Akhoundi et al. 2016; Foroutan et al. 2017a). Based on clinical signs and causative agent, the disease can manifest clinically at least into four forms, including cutaneous leishmaniasis (CL), diffused cutaneous leishmaniasis (DCL), mucocutaneous leishmaniasis (MCL), and visceral leishmaniasis (VL) (Akhoundi et al. 2016; Alvar et al. 2012). According to formal reports by the World Health Organization (WHO), leishmaniasis as a main global health problem with 2.4 million disability adjusted life years (DALYs) are reported in 100 countries and territories from five continents. Approximately, 12 million individuals are infected with parasites and 350 million persons are also at risk of acquiring the infection worldwide. The annual incidence of VL and CL is nearly 0.2–0.4 million and 0.7–1.2 million throughout the world, respectively. Over 70% of the total CL cases occur in the following ten countries: Peru, Costa Rica, Colombia, Brazil, Ethiopia, North Sudan, Algeria, Syria, Afghanistan, and Iran (Alvar et al. 2012). In the Eastern Mediterranean Region (EMR), CL and VL were observed from 14 out of 22 countries in this area, including: Egypt, Sudan, Jordan, Morocco, Tunisia, Palestine, Libya, Yemen, Iraq, Syria, Saudi Arabia, Pakistan, Afghanistan, and Iran (Postigo 2010). Pentavalent antimonial compounds are being prescribed as first-line drugs for the treatment of CL from the 1940s until now (Albakhit et al. 2016; Croft et al. 2006; Foroutan-Rad et al. 2016).
Anthroponotic cutaneous leishmaniasis (ACL) and zoonotic cutaneous leishmaniasis (ZCL) are two main forms of CL in Iran country, which are caused by Leishmania tropica (L. tropica) and L. major, respectively (Holakouie-Naieni et al. 2017; Namazi et al. 2018; Saki et al. 2010). Some sand fly species such as Phlebotomus sergenti (P. sergenti) and P. papatasi are the major vectors for ACL and ZCL in Iran, respectively (Karimi et al. 2014; Kavarizadeh et al. 2017). Since the ZCL is a rodent-borne infection, its expansion is dependent upon to the distribution of several reservoir hosts, including Rhombomys opimus (R. opimus), Gerbillus nanus (G. nanus), Tatera indica (T. indica), Meriones libycus (M. libycus), Meriones hurrianae (M. hurrianae), Meriones persicus (M. persicus), and Nesokia indica (N. indica) (Foroutan et al. 2017b). The first control program for leishmaniasis was launched in 1345 in Iran. Despite rigorous implemented programs and national and international investments, the infection is reported over seventeen different provinces of Iran, especially in central and southwestern regions (Holakouie-Naieni et al. 2017; Khademvatan et al. 2017). Moreover, several new endemic foci for CL have been reported during recent years (Khosravani et al. 2016; Nateghi Rostami et al. 2013; Razmjou et al. 2009).
Direct microscopy examination, culture, immunological assays, and DNA-based techniques are the main diagnostic methods for detection of Leishmania. Among them, microscopic observation needs expertise and is not species specific, while molecular approaches have been recently developed for this purpose (Akhoundi et al. 2017; Khademvatan et al. 2011; Saki and Khademvatan 2011).
Abadan and Khorramshahr cities are considered as two main free trade zones and agricultural centers in the southwest of Iran (Khuzestan province). These cities, due to their special geographical locations, annually admitted several million workers, pilgrims, and tourists (Salmanzadeh et al. 2015). Besides, Abadan and Khorramshahr cities are the gateway for traffic of foreign nationals from Iraq (an endemic country for CL). Also, attendance of vectors (P. papatasi and P. sergenti) and some reservoir hosts (particularly, a rodent of the genus T. indica) faced these regions at risk. Thus, the above-mentioned evidences encouraged us to assess the epidemiological aspects of CL in Abadan and Khorramshahr cities during four consecutive years (2014–2017).
Materials and methods
Study area
Abadan city (30.3473°N latitudes and 48.2934°E longitudes) is located in the southwest of Iran (Khuzestan province). Abadan is confined to the East by the Bahmanshir outlet of the Karun River (the Arvand Rood) and in the West by the Arvand waterway, near the Iraq–Iran border. Its population today has reached about 350,000 people. Weather temperature is highly varied throughout the year, ranged from 53 °C in summer to − 4 °C in winter. This city has a mean temperature of 25.5 °C, average relative humidity of 45%, and mean annual precipitation of 153.3 mm (Soltani et al. 2018) (https://en.wikipedia.org/wiki/Abadan,_Iran) (Fig. 1). Khorramshahr county (30.4256° N latitudes and 48.1891° E longitudes) is an inland port city located approximately 10 km north of Abadan. The city extends to the right bank of the Arvand Rud waterway near its confluence with the Haffar arm of the Karun river. According to the 2006 census, its population was estimated 155,224, in 32,563 families (https://en.wikipedia.org/wiki/Khorramshahr_County) (Fig. 1).
Fig. 1.
Location of Abadan and Khorramshahr cities in Iran. The study regions are shown with red asterisks
Sampling and patients
The current investigation was a retrospective cross-sectional study over 4 years between 2014 and 2017 in two endemic cities (Abadan and Khorramshahr) in the southwest of Iran. During the study period, all suspicious CL patients who were referred to health centers affiliated to Abadan School of Medical Sciences were examined. Both clinical (by a trained physician) and parasitological (by expert personnel) verifications were applied to diagnose a case. In order to identify amastigote forms of the parasite, a simple direct smear was taken from the ulcer. Then, the samples were placed on a clean slide and stained with Giemsa and finally tested using a light microscope. All subjects voluntarily agreed to be tested for the cause of ulcers. Before the admission, an informed consent was obtained from adult persons and parent or guardian of children less than 15 years old. This study received the approval from the Abadan School of Medical Sciences Ethical Committee (1396.234).
Questionnaire
A structured questionnaire was designed in Excel sheet, which included the following demographic details of participants: gender (male or female), age (0–10, 10–20, 20–30, 30–40, 40–50, 50–60, 60–70, and 70–80), residence (urban or rural), season (Spring, Summer, Autumn, and Winter), location of lesions (on hands, feet, face or other places), types of lesions (dry or wet), and occupation (unemployed, housewife, etc.). For each positive subject, a questionnaire was applied.
Statistical analysis
All data were collected from Abadan and Khorramshahr health centers and were analyzed using SPSS software (version 17) (SPSS Inc., Chicago, IL, USA).
Results
Abadan city
From a total of 151 smear positive cases for CL during 2014–2017, 93 (61.59%) and 58 (38.41%) were male and female, respectively. Of these, 109 (72.18%) patients lived in urban and 42 (27.82%) lived in rural areas (Table 1). Trend of CL during the study period has declined from 59 cases in 2014 to 17 patients at the end of 2017 (Fig. 2). Proportion of infection in different age-groups of 0–10, 10–20, 20–30, 30–40, 40–50, 50–60, 60–70, and 70–80 was 35 (23.17%), 29 (19.2%), 41 (27.16%), 14 (9.28%), 15 (9.94%), 12 (7.94%), 3 (1.98%), and 2 (1.33%), respectively. Based on the season, the highest and lowest rates of infection were observed in Winter 59 (39.08%) and Summer 25 (16.55%), respectively. Moreover, anatomic location of ulcers is as follows: hands 43.15%, feet 35.02%, face 16.75%, and other places 5.08%. A total of 197 lesions in 151 patients, with average number of 1.3 scar per person, was observed. Also, the majority of lesions were dry (53.8%). More details are listed in Table 1.
Table 1.
Frequency distribution of CL patients based on demographic information from 2014 to 2017 in Abadan city
| Characteristic | 2014 | 2015 | 2016 | 2017 | Total |
|---|---|---|---|---|---|
| Gender | |||||
| Male | 35 | 25 | 25 | 8 | 93 (61.59%) |
| Female | 24 | 15 | 10 | 9 | 58 (38.41%) |
| Age | |||||
| 0–10 | 10 | 9 | 12 | 4 | 35 (23.17%) |
| 10–20 | 18 | 4 | 5 | 2 | 29 (19.2%) |
| 20–30 | 14 | 13 | 9 | 5 | 41 (27.16%) |
| 30–40 | 6 | 2 | 3 | 3 | 14 (9.28%) |
| 40–50 | 5 | 5 | 4 | 1 | 15 (9.94%) |
| 50–60 | 6 | 5 | 0 | 1 | 12 (7.94%) |
| 60–70 | 0 | 0 | 2 | 1 | 3 (1.98%) |
| 70–80 | 0 | 2 | 0 | 0 | 2 (1.33%) |
| Residence | |||||
| Urban | 36 | 31 | 28 | 14 | 109 (72.18%) |
| Rural | 23 | 9 | 7 | 3 | 42 (27.82%) |
| Season | |||||
| Spring | 0 | 9 | 10 | 7 | 26 (17.21%) |
| Summer | 4 | 7 | 6 | 8 | 25 (16.55%) |
| Autumn | 13 | 12 | 14 | 2 | 41 (27.16%) |
| Winter | 42 | 12 | 5 | 0 | 59 (39.08%) |
| Location of lesions | |||||
| Hands | 42 | 17 | 20 | 6 | 85 (43.15%) |
| Feet | 26 | 17 | 15 | 11 | 69 (35.02%) |
| Face | 9 | 9 | 11 | 4 | 33 (16.75%) |
| Body | 4 | 1 | 3 | 2 | 10 (5.08%) |
| Total | 81 | 44 | 49 | 23 | 197 (100%) |
| Types of lesions | |||||
| Wet | 36 | 26 | 19 | 10 | 91 (46.2%) |
| Dry | 45 | 18 | 30 | 13 | 106 (53.8%) |
| Job | |||||
| Unemployed | 6 | 1 | 1 | 0 | 8 (5.3%) |
| Soldier | 9 | 3 | 5 | 2 | 19 (12.58%) |
| Laborer | 5 | 3 | 2 | 1 | 11 (7.3%) |
| Housewife | 14 | 12 | 2 | 7 | 35 (23.17%) |
| Other businesses | 25 | 21 | 25 | 7 | 78 (51.65%) |
Fig. 2.
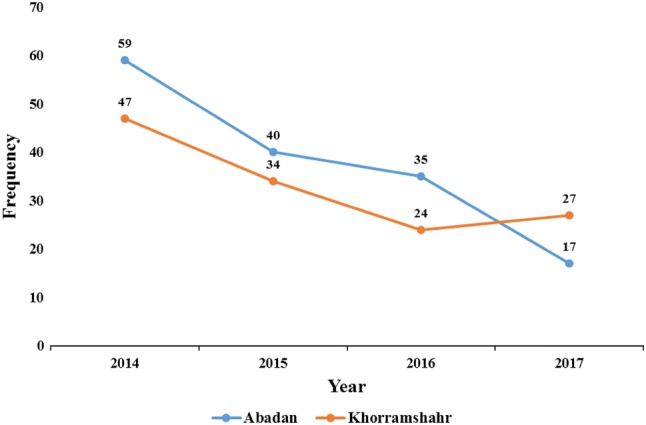
Trend of CL in Abadan and Khorramshahr cities during 2014–2017
Khorramshahr city
During 2014–2017, a total of 132 CL patients were identified, so that 83 (62.88%) of them were male and the rest were female 49 (37.12%). Out of 132 cases, 117 (88.63%) and 15 (11.37%) were from urban and rural regions (Table 2). The highest and lowest frequencies of CL were recorded as 47 in 2014 and 24 in 2016, respectively (Fig. 2). Over 63.63% (84 individuals) of CL patients were under 30 years old. The proportion of infections in different seasons are as follows: Spring 14 (10.6%), Summer 10 (7.58%), Autumn 33 (25%), and Winter 75 (56.82%). Anatomic location of the lesions were mainly on hands 46.62% and followed by feet 37.42%, face 9.82%, and other places 6.14%. A total of 163 scars in 132 patients, with average number of 1.23 lesion per person, was observed. Also, the majority of ulcers were dry (81%). More details are presented in Table 2.
Table 2.
Frequency distribution of CL patients based on demographic information from 2014 to 2017 in Khorramshahr city
| Characteristic | 2014 | 2015 | 2016 | 2017 | Total |
|---|---|---|---|---|---|
| Gender | |||||
| Male | 33 | 19 | 12 | 19 | 83 (62.88%) |
| Female | 14 | 15 | 12 | 8 | 49 (37.12%) |
| Age | |||||
| 0–10 | 11 | 8 | 3 | 7 | 29 (21.97%) |
| 10–20 | 12 | 5 | 4 | 2 | 23 (17.43%) |
| 20–30 | 13 | 8 | 7 | 4 | 32 (24.25%) |
| 30–40 | 3 | 4 | 2 | 5 | 14 (10.6%) |
| 40–50 | 6 | 5 | 7 | 7 | 25 (18.94%) |
| 50–60 | 1 | 3 | 0 | 0 | 4 (3.03%) |
| 60–70 | 0 | 1 | 1 | 2 | 4 (3.03%) |
| 70–80 | 0 | 0 | 0 | 0 | 0 (0%) |
| 80–90 | 1 | 0 | 0 | 0 | 1 (0.75%) |
| Residence | |||||
| Urban | 43 | 30 | 23 | 21 | 117 (88.63%) |
| Rural | 4 | 4 | 1 | 6 | 15 (11.37%) |
| Season | |||||
| Spring | 0 | 6 | 5 | 3 | 14 (10.6%) |
| Summer | 0 | 2 | 7 | 1 | 10 (7.58%) |
| Autumn | 12 | 12 | 1 | 8 | 33 (25%) |
| Winter | 35 | 14 | 11 | 15 | 75 (56.82%) |
| Location of lesions | |||||
| Hands | 28 | 19 | 14 | 15 | 76 (46.62%) |
| Feet | 26 | 14 | 9 | 12 | 61 (37.42%) |
| Face | 3 | 5 | 3 | 5 | 16 (9.82%) |
| Body | 5 | 2 | 3 | 0 | 10 (6.14%) |
| Types of lesions | |||||
| Wet | 12 | 5 | 5 | 9 | 31 (19%) |
| Dry | 50 | 35 | 24 | 23 | 132 (81%) |
| Job | |||||
| Unemployed | 2 | 2 | 2 | 1 | 7 (5.30%) |
| Soldier | 9 | 3 | 3 | 5 | 20 (15.15%) |
| Laborer | 2 | 3 | 2 | 2 | 9 (6.82%) |
| Housewife | 4 | 5 | 3 | 1 | 13 (9.85%) |
| Other businesses | 30 | 21 | 14 | 18 | 83 (62.88%) |
Discussion
In the current study, the frequency of CL was recorded in Abadan and Khorramshahr cities during 2014–2017. The results of this study revealed that 151 and 132 positive CL cases with an average annual frequency of 37.75 and 33 cases were reported in Abadan and Khorramshahr cities, respectively.
The highest incidence rate of infection was observed in 2014 in both cities and has decreased from 59 to 17 and 47 to 24 cases per year, in 2017 and 2016 in Abadan and Khorramshahr cities, respectively. Based on our results, the proportion of CL in urban regions (72.18% and 88.63%) was significantly higher than rural zones for Abadan and Khorramshahr cities, respectively. This is similar to India (80%) (Aara et al. 2013), Northern Khorasan province (60.8%) (Alavinia et al. 2009), and Kermanshah province (67%) (Nazari et al. 2012), while in some studies, higher frequency of the infection was reported in rural regions such as Mazandaran province (64.52%) (Youssefi et al. 2011) and Poledokhtar town (86.77%) (Amraee et al. 2012). The distribution and expansion of CL from endemic to non-endemic regions could be explained by several environmental variables, including progress of agriculture projects in the area, urbanization, demographic changes, irregular immigration, water supply projects, etc. These factors lead to altering the distribution pattern of CL which imposes economic wastage of societies (Holakouie-Naieni et al. 2017; Khademvatan et al. 2017).
In the present study, the frequency of CL was higher in males (61.59% and 62.88% in Abadan and Khorramshahr cities, respectively) than females, which is corresponding to previous investigations such as in Pakistan (70.58%) (Jamal et al. 2013), Iraq (57%) (AlSamarai and AlObaidi 2009), Isfahan province (61.8%) (Karami et al. 2013), Mazandaran province (59.67%) (Youssefi et al. 2011), and Qom province (59.2%) (Nateghi Rostami et al. 2013). This difference may be due to the following reasons: outdoors social activities, less coverage of body with cloth that increases the contact with sand flies, traffic in desert regions, etc. (Khademvatan et al. 2017). However, in recently published article by Amraee et al. (2012) in Poledokhtar town, the higher incidence of infection in women was documented. Its reason could be justified by the cultural customs in a certain zone, such as women’s work in livestock breeding or on farming lands.
In the current investigation, the CL was seen in all age-groups. However, the incidence of CL based on age distribution was higher in the age range of 20–30 years in both cities. This is in agreement with the results of Kermanshah province (Nazari et al. 2012) and Poledokhtar town (Amraee et al. 2012). This may be because individuals of this age-group are more likely to be exposed to the source of infection than others, while in some studies persons under 10 years were the most affected group such as Yaghoobi-Ershadi et al. (2006) in Isfahan at age-group 0–4 (40%) and Sofizadeh et al. (2012) in Gonbad Kavoos town at age-group 0–9 (41.2%).
The results of this study, indicated that the majority of the lesions were found on the hands and feet, so that similar findings have been reported from Hormozgan province (63.35% on the hands and feet) (Ahmadizadeh et al. 2013), Fasa town (43.24% and 20.44% on hands and feet, respectively) (Khosravani et al. 2016), and Poledokhtar town (44.84% and 21.29% on hand and feet, respectively) (Amraee et al. 2012). Perhaps high temperature and less or non-covered parts of the body in these places are the feasible reasons. Besides, the lesion sites are dependent on the types of vectors, climates, social and cultural behaviors of people (Khademvatan et al. 2017). However, in some studies in Pakistan (Jamal et al. 2013), Isfahan city (Ebadi and Hejazi 2003), and Bam county (Sharifi et al. 2011), the majority of the lesions were identified on the face. Based on the season, the highest rate of infection was observed in Winter and Autumn, which is in agreement with some studies (Faulde et al. 2008; Khajedaluee et al. 2014; Khosravani et al. 2016).
L. tropica and L. major are the two most common species causing CL (Holakouie-Naieni et al. 2017; Namazi et al. 2018; Saki et al. 2010). Based on clinical presentations, the results showed that most patients have dry scars (53.8% and 81% in Abadan and Khorramshahr, respectively).
Conclusions
Although the incidence of CL has declined during recent years, the infection is still a public health concern. Hence, health education, improvement in public awareness about the infection consequences, regular monitoring of travelers and tourists from Iraq country, skin protection from different sand fly bites by using spraying insecticides, usage of bed nets, destruction the habitats of some reservoir rodents (such as T. indica in Khuzestan province) to limit their traffic near human societies, and other appropriate control strategies are recommended in the future. At next studies, it is advised to consider the following items for a more comprehensive assessment on demographic information: the history of traveling to endemic countries during recent months, the nationality of CL subjects to evaluate the imported cases, perform PCR-based techniques to detect the species of collected Leishmania spp. samples, and using from ArcGIS software to determine the spatial distribution of CL in the target area.
Acknowledgements
The authors would like to thank all staff at the healthcare centers in Abadan and Khorramshahr cities. We are very grateful to Dr Faezeh Hedayati-Rad (Department of Environmental Sciences, Gorgan University of Agricultural Sciences and Natural Resources, Gorgan, Iran) for her helpful consultation and comments on the manuscript.
Author contributions
SS and MF designed the study protocol; SS, MH, HA, and MSK collected the data and involved in statistical analysis; MF and SS drafted the manuscript. All authors read and approved the final version of the manuscript.
Funding
This study was financially supported by Grant No (96ST-0141) from Student Research Committee, Abadan School of Medical Sciences, Abadan, Iran.
Compliance with ethical standards
Conflict interest
No potential conflict of interest relevant to this article was reported.
Ethical statement
All subjects voluntarily agreed to be tested. An informed consent was obtained from adult persons and parent or guardian of children less than 15 years old. This study received the approval from the Abadan School of Medical Sciences Ethical Committee (1396.234). Ethical issues (including plagiarism, misconduct, data fabrication and/or falsification, double publication and/or submission, redundancy, etc.) have been completely observed by the authors.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Shahrzad Soltani, Email: shahrzadsoltani225@yahoo.com.
Masoud Foroutan, Email: masoud_foroutan_rad@yahoo.com.
Maryam Hezarian, Email: maryamhezarian1996@gmail.com.
Hamed Afshari, Email: afshari.h@ajums.ac.ir.
Mehdi Sagha Kahvaz, Email: mehdikahvazi@gmail.com.
References
- Aara N, et al. Clinco-epidemiologic study of cutaneous leishmaniasis in Bikaner, Rajasthan, India. Am J Trop Med Hyg. 2013;89(1):111–115. doi: 10.4269/ajtmh.12-0558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmadizadeh E, Soleimani K, Hosseinpour M, Zakeri A, Ahmadizadeh A. Epidemiology of cutaneous leishmaniasis in Hormozgan province (2007–2011) Life Sci. 2013;10(3):1473–1475. [Google Scholar]
- Akhoundi M, et al. A historical overview of the classification, evolution, and dispersion of Leishmania parasites and sandflies. PLoS Negl Trop Dis. 2016;10(3):e0004349. doi: 10.1371/journal.pntd.0004349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akhoundi M, et al. Leishmania infections: molecular targets and diagnosis. Mol Asp Med. 2017;57:1–29. doi: 10.1016/j.mam.2016.11.012. [DOI] [PubMed] [Google Scholar]
- Alavinia S, Arzamani K, Reihani M, Jafari J. Some epidemiological aspects of cutaneous leishmaniasis in northern Khorasan Province, Iran. Iran J Arthropod Borne Dis. 2009;3(2):50–54. [PMC free article] [PubMed] [Google Scholar]
- Albakhit S, Khademvatan S, Doudi M, Foroutan-Rad M. Antileishmanial activity of date (Phoenix dactylifera L) fruit and pit extracts in vitro. J Evid Based Complement Altern Med. 2016;21(4):NP98–NP102. doi: 10.1177/2156587216651031. [DOI] [PubMed] [Google Scholar]
- AlSamarai AM, AlObaidi HS. Cutaneous leishmaniasis in Iraq. J Infect Dev Ctries. 2009;3(2):123–129. doi: 10.3855/jidc.59. [DOI] [PubMed] [Google Scholar]
- Alvar J, et al. Leishmaniasis worldwide and global estimates of its incidence. PLoS ONE. 2012;7(5):e35671. doi: 10.1371/journal.pone.0035671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amraee K, Rastegar HA, Beiranvand E. An epidemiological study of cutaneous leishmaniasis in Poledokhtar district, Lorestan province, southwestern of Iran, 2001–2011. Jundishapur J Health Sci. 2012;5(1):55–62. [Google Scholar]
- Croft SL, Sundar S, Fairlamb AH. Drug resistance in leishmaniasis. Clin Microbiol Rev. 2006;19(1):111–126. doi: 10.1128/CMR.19.1.111-126.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebadi M, Hejazi H. The epidemiological study of cutaneous leishmaniasis situation in the students of primary school in Isfahan Borkhar region. J Kerman Univ Med Sci. 2003;10(2):92–98. [Google Scholar]
- Faulde M, Schrader J, Heyl G, Amirih M. Differences in transmission seasons as an epidemiological tool for characterization of anthroponotic and zoonotic cutaneous leishmaniasis in northern Afghanistan. Acta Trop. 2008;105(2):131–138. doi: 10.1016/j.actatropica.2007.10.011. [DOI] [PubMed] [Google Scholar]
- Foroutan M, et al. A systematic review and meta-analysis of the prevalence of Leishmania infection in blood donors. Transfus Apher Sci. 2017;56(4):544–551. doi: 10.1016/j.transci.2017.07.001. [DOI] [PubMed] [Google Scholar]
- Foroutan M, et al. Prevalence of Leishmania species in rodents: a systematic review and meta-analysis in Iran. Acta Trop. 2017;172:164–172. doi: 10.1016/j.actatropica.2017.04.022. [DOI] [PubMed] [Google Scholar]
- Foroutan-Rad M, Khademvatan S, Saki J, Hashemitabar M. Holothuria leucospilota extract induces apoptosis in Leishmania major promastigotes. Iran J Parasitol. 2016;11(3):339–349. [PMC free article] [PubMed] [Google Scholar]
- Holakouie-Naieni K, Mostafavi E, Boloorani AD, Mohebali M, Pakzad R. Spatial modeling of cutaneous leishmaniasis in Iran from 1983 to 2013. Acta Trop. 2017;166:67–73. doi: 10.1016/j.actatropica.2016.11.004. [DOI] [PubMed] [Google Scholar]
- Jamal Q, Shah A, Ali N, Ashraf M, Awan MM, Lee CM. Prevalence and comparative analysis of cutaneous leishmaniasis in Dargai region in Pakistan. Pak J Zool. 2013;45(2):537–541. [Google Scholar]
- Karami M, Doudi M, Setorki M. Assessing epidemiology of cutaneous leishmaniasis in Isfahan, Iran. J Vector Borne Dis. 2013;50(1):30–37. [PubMed] [Google Scholar]
- Karimi A, Hanafi-Bojd AA, Yaghoobi-Ershadi MR, Akhavan AA, Ghezelbash Z. Spatial and temporal distributions of phlebotomine sand flies (Diptera: Psychodidae), vectors of leishmaniasis, in Iran. Acta Trop. 2014;132:131–139. doi: 10.1016/j.actatropica.2014.01.004. [DOI] [PubMed] [Google Scholar]
- Kavarizadeh F, Khademvatan S, Vazirianzadeh B, Feizhaddad MH, Zarean M. Molecular characterization of Leishmania parasites isolated from sandflies species of a zoonotic cutaneous leishmaniasis in Musiyan south west Iran. J Parasit Dis. 2017;41(1):274–281. doi: 10.1007/s12639-016-0792-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khademvatan S, Neisi N, Maraghi S, Saki J. Diagnosis and identification of Leishmania spp. from Giemsa-stained slides, by real-time PCR and melting curve analysis in south-west of Iran. Ann Trop Med Parasitol. 2011;105(8):559–565. doi: 10.1179/2047773211Y.0000000014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khademvatan S, et al. Spatial distribution and epidemiological features of cutaneous leishmaniasis in southwest of Iran. Alex J Med. 2017;53(1):93–98. doi: 10.1016/j.ajme.2016.03.001. [DOI] [Google Scholar]
- Khajedaluee M, et al. Epidemiology of cutaneous leishmaniasis in population covered by Mashhad University of Medical Sciences in 2011. Med J Mashhad Univ Med Sci. 2014;57(4):647–654. [Google Scholar]
- Khosravani M, Moemenbellah-Fard MD, Sharafi M, Rafat-Panah A. Epidemiologic profile of oriental sore caused by Leishmania parasites in a new endemic focus of cutaneous leishmaniasis, southern Iran. J Parasit Dis. 2016;40(3):1077–1081. doi: 10.1007/s12639-014-0637-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namazi MJ, Dehkordi AB, Haghighi F, Mohammadzadeh M, Zarean M, Hasanabad MH. Molecular detection of Leishmania species in northeast of Iran. Comp Clin Pathol. 2018;27(3):729–733. doi: 10.1007/s00580-018-2658-9. [DOI] [Google Scholar]
- Nateghi Rostami M, Saghafipour A, Vesali E. A newly emerged cutaneous leishmaniasis focus in central Iran. Int J Infect Dis. 2013;17(12):e1198–e1206. doi: 10.1016/j.ijid.2013.07.003. [DOI] [PubMed] [Google Scholar]
- Nazari N, Faraji R, Vejdani M, Mekaeili A, Hamzavi Y. The prevalence of cutaneous leishmaniases in patients referred to Kermanshah hygienic centers. Zahedan J Res Med Sci. 2012;14(8):77–79. [Google Scholar]
- Postigo JA. Leishmaniasis in the world health organization Eastern Mediterranean Region. Int J Antimicrob Agents. 2010;36(Suppl 1):S62–S65. doi: 10.1016/j.ijantimicag.2010.06.023. [DOI] [PubMed] [Google Scholar]
- Razmjou S, Hejazy H, Motazedian MH, Baghaei M, Emamy M, Kalantary M. A new focus of zoonotic cutaneous leishmaniasis in Shiraz, Iran. Trans R Soc Trop Med Hyg. 2009;103(7):727–730. doi: 10.1016/j.trstmh.2008.12.013. [DOI] [PubMed] [Google Scholar]
- Saki J, Khademvatan S. A molecular study on cutaneous leishmaniasis lesions in Khuzestan province (South west of Iran) Jundishapur J Microbiol. 2011;4(4):283–288. [Google Scholar]
- Saki J, et al. Mini-exon genotyping of Leishmania species in Khuzestan province, southwest Iran. Iran J Parasitol. 2010;5(1):25–34. [PMC free article] [PubMed] [Google Scholar]
- Salmanzadeh S, Foroutan-Rad M, Khademvatan S, Moogahi S, Bigdeli S. Significant decline of malaria incidence in southwest of Iran (2001–2014) J Trop Med. 2015;2015:523767. doi: 10.1155/2015/523767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharifi I, et al. Emergence of a new focus of anthroponotic cutaneous leishmaniasis due to Leishmania tropica in rural communities of Bam district after the earthquake, Iran. Trop Med Int Health. 2011;16(4):510–513. doi: 10.1111/j.1365-3156.2011.02729.x. [DOI] [PubMed] [Google Scholar]
- Sofizadeh A, Cherabin M, Mehravaran A. Cutaneous leishmaniasis in Gonbad Kavoos, North of Iran (2009–11): an epidemiological study. J Gorgan Univ Med Sci. 2012;14(4):100–106. [Google Scholar]
- Soltani S, Foroutan M, Afshari H, Hezarian M, Kahvaz MS. Seroepidemiological evaluation of Toxoplasma gondii immunity among the general population in southwest of Iran. J Parasit Dis. 2018 doi: 10.1007/s12639-018-1047-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaghoobi-Ershadi MR, et al. Evaluation of deltamethrin-impregnated bed nets and curtains for control of zoonotic cutaneous leishmaniasis in a hyperendemic area of Iran. Bull Soc Pathol Exot. 2006;99(1):43–48. doi: 10.3185/pathexo2818. [DOI] [PubMed] [Google Scholar]
- Youssefi MR, Shojaei J, Jalahi H, Amoli SA, Ghasemi H, Javadian M. Prevalence of cutaneous leishmaniasis during 2010 in Mazandaran Province, Iran. Afr J Microbiol Res. 2011;5(31):5793–5795. [Google Scholar]