Abstract
Euphorbia hirta L. from the family of Euphorbiaceae is an annual herb, which grows as a roadside weed in most tropical countries. It is prominently used by the traditional healers in rural India for the treatment of snakebites. However, the mechanisms and the major bioactive compounds behind its inhibition activity are relatively unknown. From our preliminary in silico studies, it was found that a group of pentacyclic triterpenoids from this plant are playing a major role in inhibiting the snake venom proteins. The present study was aimed at standardizing methods for obtaining callus from this medicinal plant at a much faster rate by hormone pretreatment of explants and, thus, by developing suspension cultures to obtain bioactive secondary metabolites in vitro. The results were promising that longer incubation of explants with hormone treatment showed early induction of callus. The major bioactive compounds responsible for the anti-snake venom activity were characterized from natural plant material as well as from suspension cultures, and the efficiency was found to be relatively high. The secondary metabolite analysis from suspension culture and natural plant extracts revealed that a major compound ‘Taraxerol’ and its derivatives was found abundant along with few other triterpenoids. This compound showed high inhibitory activity against pit viper snake venoms from our in silico studies with molecular docking tools. Hence, this study with identification of potential bioactive compounds against snake venom with standardization of In vitro culture methods would help in developing natural alternative medicine for snakebites in near future.
Keywords: Euphorbia hirta, Triterpenoids, Taraxerol, Callus cultures, Docking, Snake anti-venom
Introduction
Snakebite is a common medical concern and has been categorized as neglected tropical disease by World Health Organization (WHO) (2007). The traditional herbal medicine has been widely practiced in subtropical Asian countries for snakebite treatment. The scientific research to explore the mechanism of action in several medicinal plants against snake venom has gained more attention in the last 20 years. E. hirta L. commonly called as the snake weed is one of the medicinal plants (Vernacular name—Amman pacharisi in Tamil) used as a herbal medicine for snake and scorpion bite treatment in South western Ghats of India and North east coast of Tamil Nadu. In the traditional medicine, E. hirta is still used widely for the treatment of various diseases and for wound healing, removing scars, etc. (Patil et al. 2009; Shih and Cherng 2012). It is also widely known for its medicinal properties such as anti-inflammatory, anti-fungal, anti-malarial, and anti-microbial activities. It was documented that tribal people in South India to treat poisonous snakebites have used the decoction of aerial parts from E. hirta for generations (Samy et al. 2008). In general, the chemical composition of snake venom consists of 90% proteins, of which most of them have been identified as neurotoxic enzymes (Gomes et al. 2010). Local tissue necrosis and psychological sequelae have been common symptoms exhibited by victims of snakebite (Hansdak et al. 1998). Several triterpenes α-amyrin, β-amyrin, taraxerone (EH-1), taraxerol, taraxerol acetate, stigmasterol, sitosterol, β-amyrin acetate, and betulinic acid have been reported from E. hirta that are believed to neutralize snake venom (Mors et al. 2000; Wu et al. 2012; Pióro-Jabrucka et al. 2011). The similarity in the venom composition of most of the poisonous snakes found in rural places including cobra, vipers, and copperhead snakes has Phospholipase A2 (svPLA2) as its major bioactive enzyme (Kumar et al. 2016). Hence, most of the commercial anti-serum-based drugs and potential drug compounds usually target or inhibit the binding site of this enzyme to neutralize the venom in the system. The molecular mechanisms behind this inhibitory snake venom activity and characterization of corresponding bioactive metabolites from this traditionally used antidote plant are relatively unknown and a less scientifically explored topic. To explore and analyze the bioactive secondary metabolites, cell culture systems provide an ideal opportunity to study the phytochemicals with downstream applications. In addition, standardizing cell culture techniques from this plant could be useful in the aspect of conservation status, and ease of scaling up metabolites in bioreactors with commercial feasibility in future.
The current work is an attempt to explore the strategy for early callus induction in leaf explants of E. hirta and standardize the In vitro synthesis and characterization of anti-venom triterpenoids isolated from suspension cultures and natural plant extracts (root, stem, and leaves) of E. hirta.
Materials and methods
Collection of plant material and selection of explants
The plant material of E. hirta (Family: E. ceae, Order: Malpighiales, Clade: Angiosperms) were spotted and collected from surrounding barren areas in and around Karunya university campus and Siruvani foothills, Coimbatore (10.9362°N, 76.7441°E). The plant was authenticated by the research supervisor and a recognized botanist from Botanical survey of India (BSI), Coimbatore with a voucher specimen. Young leaves of naturally grown plant were selected as explants for initiation of callus under In vitro conditions.
Surface sterilization of explants
The young leaves were excised and washed thoroughly in running tap water for removal of soil, dirt, and other contaminants. Then, it was cut into smaller pieces and washed with 1% solution of Teepol (anionic detergent) for 15 min continuously until the foam is completely removed followed by rinsing in distilled water. Furthermore, the explants were taken into laminar airflow chamber for sterilization under aseptic conditions. Initially, the explants were washed with sterile distilled water for 3 min, and then treated with 5% sodium hypochlorite (NaOCl) by shaking well for 3–5 min and again washed with sterile distilled water for 3 min to remove NaOCl completely. The explants were then resterilized with 70% ethanol for 20 s and washed with sterile distilled water thrice for 3 min. The leaf explants were then dried in a sterile filter paper with the outer layers of the explant exposed in aseptic conditions. Finally, the areas adjacent to either side of the midrib region of the leaves were cut into 0.2 cm2 bits for inoculation.
Media preparation and culture conditions
The MS medium (Murashige and Skoog 1962) containing 3% sucrose and 0.8% agar (Himedia) was supplemented with NAA (naphthalene acetic acid, Himedia) and BAP (6-benzyl amino purine, Himedia) at a concentration of 1 mg L−1 after analyzing the best proportion of auxins to cytokinins and their respective concentrations for callus induction. The pH of the medium was calibrated to 5.6–5.8 and sterilized at 121 °C at 15 psi for 15 min. The inoculated explants were incubated and cultured at 25 ± 2 °C and light intensity (2000 lx) under fluorescent tube lights with a photoperiod of 16 h of light and 8 h of darkness.
Callus induction
The surface-sterilized leaf bits were inoculated horizontally on MS medium supplemented with NAA and BAP at a concentration of 1 mg L−1 in screw capped tissue culture bottles. Once the callus was initiated, it was subcultured regularly on to fresh medium supplemented with the same concentration of NAA and BAP, the calli were subcultured at a 14 day interval thrice. (Baburaj et al. 1987). The calli was maintained through subsequent subcultures at regular intervals (3–4 weeks) until the secretion and accumulation of phenolic compounds from the callus into the medium.
Establishment of cell suspension culture (growth curve)
Cell suspension culture of E. hirta was initiated by inoculating 2 g of fresh calli mass excised from the tissue culture bottles and kept in Whatman filter paper (No. 1) for few seconds to remove excess water content. It was then aseptically transferred in 100 mL of MS liquid media supplemented with similar combination of 1 mg L−1 of NAA and BAP. The cell suspensions were maintained under constant agitation through an orbital shaker set at 150 rpm and temperature of 25 ± 2 °C with 16/8 h light–dark cycle (Schripsema et al. 1990).
Hormone pretreatment of explants for early callus induction
To minimize the time taken for callus induction efficiently, a simple modified method of soaking the explants with hormones (exogenous uptake) before inoculation was performed. The surface-sterilized leaf explants were dried and pretreated with hormone combinations of NAA/BAP (1:1) incubated at different time intervals ranging from 5, 10, 20, and 30 min prior to inoculation in full-strength MS medium with the same NAA/BAP hormones at a concentration of 1 mg L−1. Untreated explants served as control for this study.
Quantification of auxin and cytokinin uptake by leaf explants
The levels of auxin and cytokinin absorbed exogenously by the leaf explants during pretreatment studies were estimated by extracting the hormones before and after treatment. It was quantified using an UV–visible spectrophotometer (Hitachi Inc.). 500 mg of fresh explants before and after pretreatment was homogenized with 10 mL of 5 mM phosphate buffer (pH 6.5) containing an internal standard (NAA) and butylated hydroxyl toluene (BHT) as an anti-oxidant. The extract was then incubated in dark for 1 h and filtered using Whatman No. 1 filter paper. The level of auxin in the filtrate was finally measured at an absorbance of 220 nm using a spectrophotometer (Heloir et al. 1996).
Similarly, the level of cytokinins in leaf explants before and after pretreatment was analyzed using extraction with modified Bielski’s solution (methanol:water:formic acid—15:4:1 v/v/v) (Tarkowski et al. 2009) with an internal standard (BAP) and the level of cytokinin in the filtrate was measured as absorbance at 270 nm using a spectrophotometer (Nilson et al. 2009).
In silico analysis
The crystal structure of PLA2 protein (phospholipase A2 enzyme) determined from pit viper snake venoms Bothrops jararacussu (PDB ID-1MG6) and Agkistrodon acutus (PDB ID-3JR8) were obtained from the RCSB Protein Data Bank (PDB—https://www.rcsb.org) and used in this computational study. The compound taraxerol, a pentacyclic triterpenoid compound from E. hirta and a potential inhibitor of PLA2 protein (Kumar et al. 2016), was retrieved from zinc database (ID: 04082498 http://www.zinc.docking.org). The molecular docking studies of taraxerol compound against PLA2 snake venom protein were performed using molecular docking tool ‘SwissDock’ (http://www.swissdock.ch) (Grosdidier et al. 2011).
Extraction of phytochemicals and analysis of secondary metabolites
The shade-dried plant parts of E. hirta (stem, leaves, and roots as separate samples and whole plant as another sample) was powdered using a laboratory mini blender for extraction steps. Hot solvent extraction (vapor by heating and condensation method) of powdered plant samples was performed in the ratio of 1:10 (w/v) using Soxhlet apparatus with the solvents such as acetone and petroleum ether. The extract was condensed to a final volume of 15 mL using rotary vacuum evaporator and stored in an airtight container for further analysis. The phytochemical analysis of the crude extract was performed for confirmation of various secondary metabolites such as Alkaloids (Meyer’s test), Flavonoids, Saponins, Phenols (Ferric chloride test), Steroids (Salkowski reaction), Glycosides, Thiols, Tannins, Anthraquinones (Borntrager’s test), and Triterpenoids (Liebermann–Burchard test) (Sivagnanam et al. 2012). The extraction of secondary metabolites from cell suspension culture was performed with acetone by maceration technique in a mortar and pestle. The extract was then centrifuged at 4000 rpm for 10 min and the supernatant was collected and stored at room temperature for further analysis.
Thin-layer chromatography (TLC)
Thin-layer chromatography of the plant crude extracts was performed in a precoated alumina plates (Merck Inc.) using the solvents Hexane and Ethyl acetate in different ratios in a linear order (10:0, 9:1, 8:2, 7:3, 6:4, 5:5, 4:6, 3:7, 2:8, 1:9, 10:0). After separation of the compounds, the TLC plates were sprayed using Liebermann–Burchard reagent (5 mL of acetic anhydride and 5 mL of concentrated sulphuric acid with 90 mL of absolute ethanol) and kept for drying at 110 °C for 10 min (Oleszek et al. 2008). Identification of triterpenoids were done on the basis of color comparison and localization of spot in the form of Rf values and compared with the standard (Oleszek et al. 2008).
Column chromatography
The plant extracts (stem, root, leaf, whole plant, and suspension cultures) of E. hirta were filtered of which 5 mL was chromatographed on Silica gel G (mesh size 100–200 μm, Himedia) in a glass column. Elution of various fractions was collected using hexane/ethyl acetate (8:2) as mobile phase. For each sample, approximately 45–50 fractions of 7 mL each were collected and the absorbance at 240–255 nm was scanned in an UV spectrophotometer to confirm the presence of triterpenoids (Consolacion and Kimberly 2014).
HPLC and LC–MS
High-performance liquid chromatography (HPLC) (Agilent 6125B SQ LC/MSD, CA, USA) provides a sensitive analytical method for resolving and quantifying triterpenoids. The different fractions which have shown positive peaks from UV spectrophotometry in the wavelength ranges from 240 to 255 nm were taken and analyzed under HPLC. The samples are dissolved in Dimethyl Sulfoxide (DMSO, Himedia) and 5 μL of the standard followed by sample solution was introduced with a Rheodyne valve equipped with a 50-μL external loop. The mobile phase flow rate was 1 mL min−1 and consisting of Hexane and Ethyl acetate double solvent system in the ratio 8:2 (Merck, HPLC Grade). The retention time and analyte peak shape were observed for the identification of the Triterpenoids. The purified samples were also sent for LC–MS analysis (liquid chromatography coupled with mass spectrometry) (Agilent 6125B SQ LC/MSD, CA, USA) to JSS College of Pharmacy, Ooty, Tamilnadu, India, for further confirmation of the presence of bioactive pentacyclic triterpenoids.
Results
Callus induction and maintenance
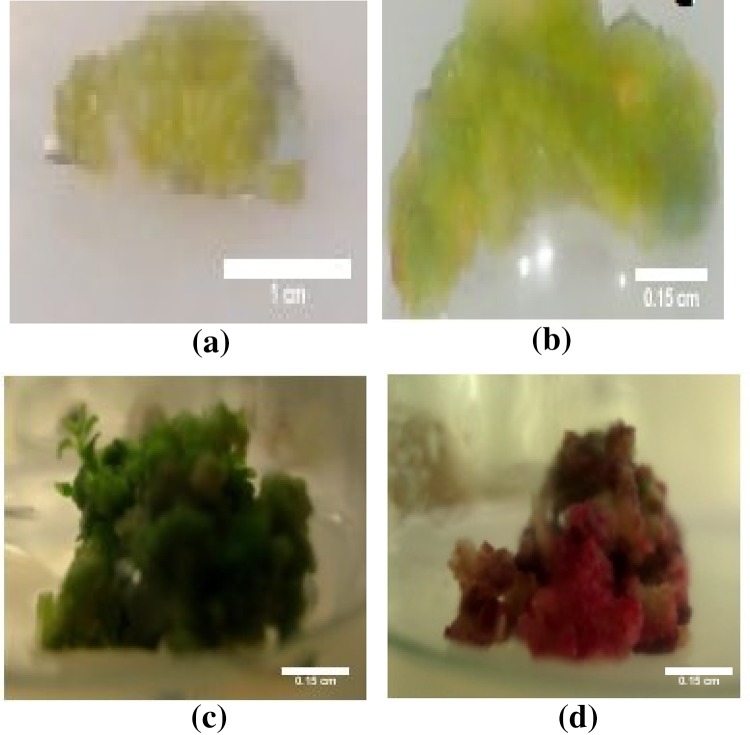
Calli was initiated from all the leaf explants inoculated on MS medium supplemented with 1 mg L−1 of NAA and BAP hormone concentrations (Fig. 1). On an average, the callus initiation started from 20 days and a well-proliferated callus was obtained from 25 to 28 days. The callus was regularly subcultured every 2–3 week interval. Different morphologies of the callus were observed such as green friable callus, pale green compact callus, pinkish red callus (phenolic compounds secretion), brittle and nodular callus, and root and shoot forming callus (early organogenesis due to fluctuation in hormone combinations) (Fig. 1).
Fig. 1.
Different callus morphologies: a callus growth after first subculture (bar 1 cm); b callus growth after second subculture (bar 1.5 cm); c mature, green, and friable callus (bar 2.5 cm); d pinkish red callus supplemented with NAA and BAP hormone (bar 3.5 cm) observed at different stages upon frequent subculturing
Early induction of callus in pretreated explants
The pretreatment of explants with hormones before inoculation in MS medium resulted in early callus induction on 11th day after inoculation when compared to 28th day in untreated explants. An average clear difference of at least 11–13 days was observed in callus induction between hormones pretreated and untreated explants. Among the different incubations, 20 and 30 min hormone pretreatment were found to be more efficient and optimal for early callus induction when compared to control.
Quantification of hormones in explants
The levels of auxin and cytokinin in pretreated explants were determined using spectrophotometer at 220 and 270 nm, respectively, and quantified through a standard graph. The levels of auxins varied in untreated and pretreated explants with time period 5, 10, 20, and 30 min (0.032, 0.039, 0.042, 0.047, and 0.058 mg mL−1) and similarly for cytokinins (0.038, 0.043, 0.054, 0.062, and 0.067 mg mL−1). A gradual increase in concentration of hormones was determined in pretreated explants when compared to control.
Cell suspension culture
Suspension cultures were initiated from callus derived from leaf explants by inoculating 0.5 to 1 g of friable callus into 250 mL Erlenmeyer flask containing 100 mL of MS broth supplemented with 1 mg L−1 of NAA, BAP, and 2 mg L−1 of NAA, BAP for growth curve study, and taraxerol extraction. The growth of cells in MS broth supplemented with 1 mg L−1 and 2 mg L−1 of NAA and BAP showed an initial lag phase for 4 days. Then, the cells grew rapidly and entered the growth phase (log) phase up to 16–20 days. After 20 days, the rate of cell growth decreased and cells entered the stationary phase. Based on the growth curve, it was found that 2 mg L−1 of NAA and BAP hormone combination exhibited better growth rate of cells.
Molecular docking studies
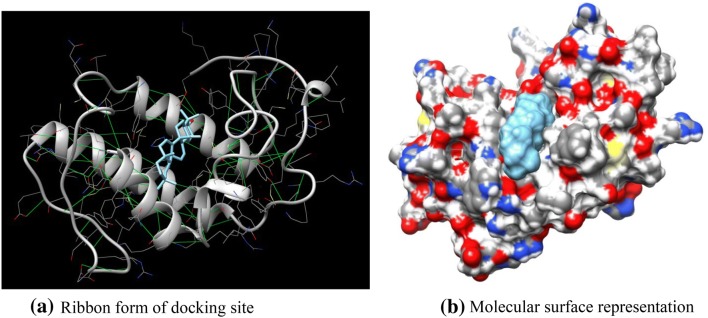
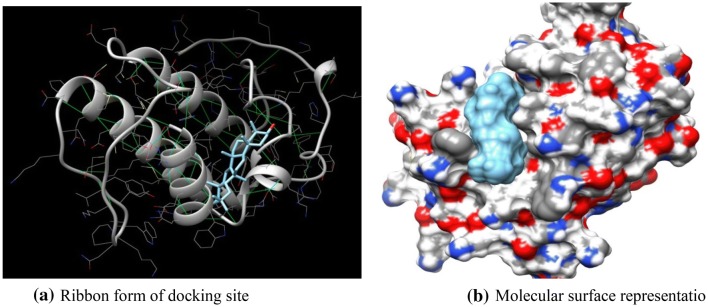
Taraxerol, a potential pentacyclic terpenoid compound, has been retrieved, modified using chemsketch as ligand docked to the active side (ASP 49) against snake venom PLA2 proteins from Protein Data Bank (PDB). The docking studies were performed using online Swissdock tool. The receptor selected for docking with compound resulted in ΔG = − 8.16 kcal/mol against PLA2 of B. jararacussu and ΔG = − 7.20 kcal/mol (Fig. 2a) against PLA2 of A. acutus (Fig. 3a). The hydrogen bonds and binding sites were also predicted with full fitness value of 928.56 kcal/mol and 876.23 kcal/mol, respectively.
Fig. 2.
a Binding site and hydrogen bond (indicated by green lines) sharing of the ligand—taraxerol with that of Bothrops jararacussu snake venom Asp 49-PLA2. b Molecular surface representation of docking site
Fig. 3.
a Binding sites and hydrogen bonds were predicted against Agkistrodon acutus PLA2 and ligand taraxerol. b Molecular surface representation of docking site
Preliminary screening for secondary metabolites
The results for phytochemical screening of acetone and petroleum ether extracts from stem, leaves, root, and whole plant of E. hirta showed the presence of flavonoids, saponins, phenols, glycosides, tannins, anthraquinones, and triterpenoids, and the absence of alkaloids, steroids, and thiols.
Analysis of triterpenoids
The presence of terpenoids was analyzed from the crude extracts of stem, leaves, roots, whole plant, and cell suspension culture of E. hirta. Only the extracts of whole plant, stem, and suspension culture have confirmed the presence of triterpenoids that was further considered for analysis by chromatography techniques. The presence of triterpenoids was confirmed through Liebermann–Burchard test (Selvakumar et al. 2012).
TLC of plant extracts
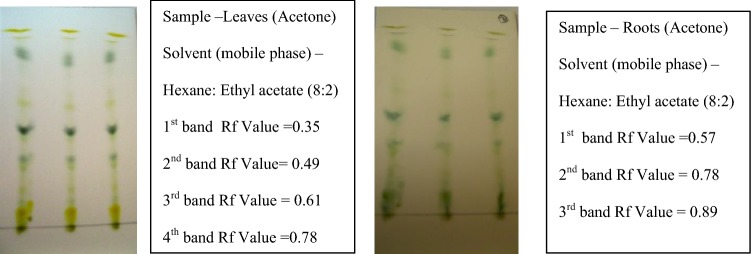
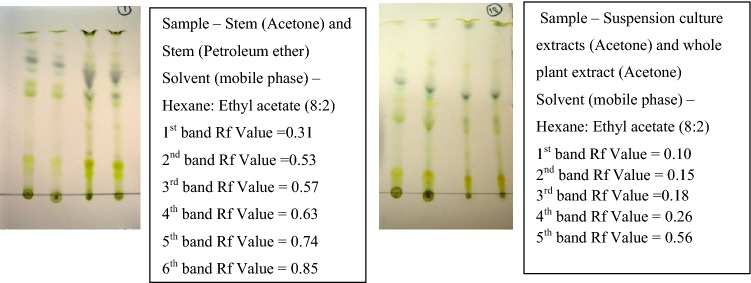
Thin-layer chromatography (TLC) were performed using different solvent ratios, Hexane and ethyl acetate in the ratios of increasing order 10:0–5:5, and the best solvent system was found for maximum separation of the bands. The leaves (acetone) were chromatographed and the Rf value was found to be 0.49, 0.35, 0.61, and 0.78. Roots (acetone) showed the Rf values 0.57, 0.78, and 0.89. The stems (acetone and petroleum ether) gave the Rf value ranges between 0.31, 0.53, 0.57, 0.63, 0.74, and 0.85. Whole plant (acetone) and suspension culture extracts (acetone) resulted in Rf values in the range 0.10, 0.15, 0.18, 0.26, and 0.56. The triterpenoids Rf values range from 0.53 to 0.57 and the samples that show the near range values were selected for eluting in column chromatography (Figs. 4, 5).
Fig. 4.
Standardization of mobile phase for isolation of triterpenoids from leaf and root sample by thin-layer chromatography
Fig. 5.
Standardization of mobile phase for isolation of triterpenoids from stem and cell suspension culture sample by thin-layer chromatography
Column chromatography
About 5 mL of the whole plant, stem and cell suspension culture extracts were chromatographed on a silica gel column using hexane–ethyl acetate (8:2) as mobile phase. A total of 45–50 fractions of 7 mL/sample each were collected for optimum separation. The collected fractions were preliminary analyzed using UV spectrophotometer for absorbance at 240–255 nm to confirm the presence of triterpenoid in fractions collected from the column. The fractions 18, 39 from stem (acetone), fractions 46, 47, 49 from whole plant (acetone), and fractions 25 and 27 from suspension culture extract (acetone) exhibited absorbance between 240 and 255 nm.
HPLC analysis
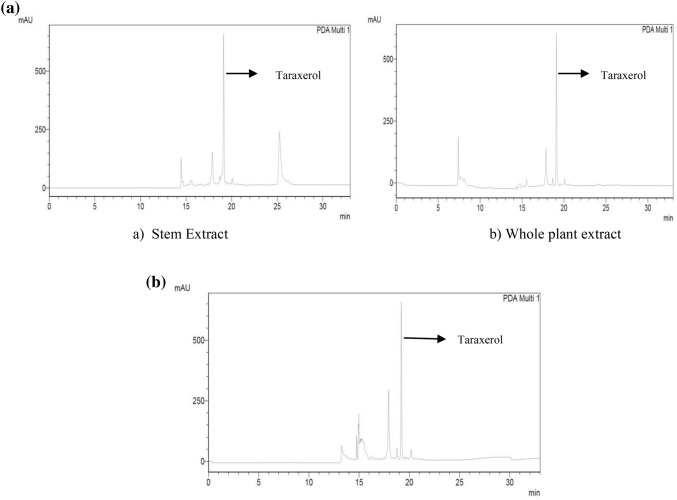
The chromatogram fractions of stem, whole plant, and cell suspension culture which has shown positive for the presence of triterpenoid were analyzed using HPLC C18 column (Shimadzu Inc.) with UV detector (240–255 nm). The volume of injected sample was 5 μL and the temperature of column was maintained. The obtained peaks were compared with the standard peak of Taraxerol chromatogram from the literature (Gantait et al. 2010). From the results obtained, the presence of taraxerol was confirmed in 39th fraction of stem sample (Fig. 6a), 47th fraction of whole plant extract, and 25th fraction of cell suspension culture (Fig. 6b).
Fig. 6.
a HPLC chromatogram of the samples from stem extract (a) and the whole plant extracts (b) confirms the presence of triterpenoid Taraxerol. b HPLC chromatogram of the samples from suspension culture extract also confirms the presence of increased levels of taraxerol with few other derivatives
LC–MS analysis
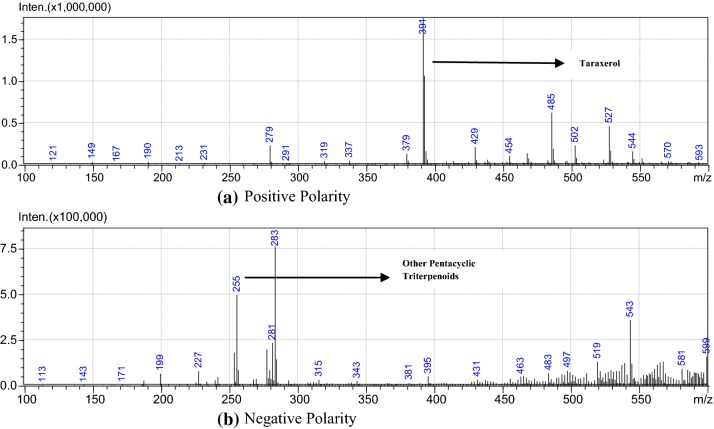
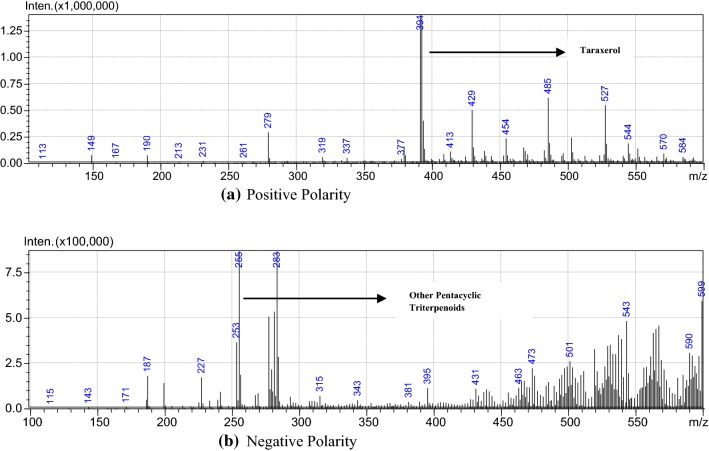
Pentacyclic triterpenoids class of compounds that are known to be abundant in most of E. ceae plant species dominates the phytochemical constituent of E. hirta also. LC–MS analysis of acetone plant extract revealed the presence of taraxerol (C30H50O). The LC–MS spectrum confirmed the presence of triterpenoids with the retention time of 121, 149, 167, 190, 213, 231, 279, 291, 319, 337, 379, 391, and 429, respectively. Interpretation of mass spectrum LC–MS was conducted using mass fragmentation tools of SIS MS tool.
The spectra of the unknown compounds were compared with the spectral peaks of known taraxerol and other triterpenoid compound structure from the literature. The name, molecular weight, molecular formula, and structure of the compound of the test material were also determined from databases. The spectrum retention time fragmentation reveals 391–429, completely related to the steroidal moiety, and the hydrogen present in the structures which it clearly elucidates to confirm the structure of taraxerol, its derivatives, and other related terpenoids (Figs. 7, 8). Due to lack of appropriate standard, LC–MS data were used to confirm as shown in Figs. 7 and 8. Based on these mass spectra, the same was confirmed. It was evident that suspension culture has substantially higher accumulation of bioactive compounds to that of natural plant extracts (Fig. 8).
Fig. 7.
Full-scan product ion spectra from LC–MS of the stem extract samples: positive polarity (a) and negative polarity (b)
Fig. 8.
Full-scan product ion spectra from LC–MS of the suspension culture extract samples: positive polarity (a) and negative polarity (b)
Discussion
Apart from various medicinal properties reported from this plant such as anti-malarial, anti-fungal, anti-bacterial anti-oxidant, anti-fertility anti-inflammatory, and galactogenic activities (Kumar et al. 2010), very few studies have shown that it has the potential for snake bite treatment from its phytochemicals (Mors et al. 2000; Grenand et al. 1987). Extracting and scaling up the targeted metabolites from the naturally growing non-cultivated plant such as E. hirta can be tricky, and hence, cell suspension culture provides an ideal opportunity. Proliferation of calli from the cut edges of leafy explants from E. hirta was also previously reported when cultured in MS medium supplemented with similar hormone combinations of NAA and BAP observed after many weeks period of time (Baburaj et al. 1987). In contrast, our study revealed the callus initiation from inoculated leaf explant of E. hirta on MS medium supplemented with 1 mg L−1 of NAA and 1 mg L−1 of BAP was observed in 3–4 weeks. Investigation using various combinations and concentration gradients in plant growth regulators was performed and found that 1 mg L−1 concentration of NAA and BAP was the optimum concentration for callus that initiated on 21st day. In addition, during the maintenance of cultures, green, compact, friable, and pinkish callus was developed through subsequent subcultures after every 4 weeks. Monitoring of biomass of the callus cultures at regular interval revealed that the growth of the suspension cultures showed some physical changes that include color and texture. During the first week to second week, the light greenish colored callus cells start changing their color to green; later by 4th week, it was friable and dark green with 200.38 ± 1.56 g/L of biomass; after 8 weeks, they become brown with 191.41 ± 1.28 g/L of biomass (Matam et al. 2017).
This is the first attempt made for minimizing the number of days for callus induction from leaf explants of E. hirta. It is widely believed that the proliferation rate of callus will be high if there is more exogenous uptake of phytohormones by the explants. A previous study reported that a yellowish callus induced from leaf explant of Euphorbia helioscopia revealed high proliferation rate in MS medium supplemented with optimum concentration of 2,4-D (2,4,dichlorophenoxyacetic acid) at 3 mg L−1 externally (Yang et al. 2009). Likewise, the proliferation rate and callus initiation from pretreated explants showed higher rate than the untreated leaf explants after 3 week interval. This strategy might be useful in downstream applications and in large-scale bioreactors for producing bioactive compounds at a much faster rate (Fernandes-Ferreira et al. 1989). There are also some studies, which have shown that exogenous uptake of phytohormones has a positive effect on the development of callus (Štefančič et al. 2007). Media combinations with cytokinins and auxins were selected with the intention to augment the production in cell suspension cultures (Devi et al. 2018). In this study, the growth curve of E. hirta cell suspension cultures in MS medium supplemented with 1 mg L−1 and 2 mg L−1 of NAA and BAP has showed that the initial lag phase (4 days) period and entered growth phase (16th day) which continued by exhibiting the optimum concentration at 2 mg L−1 of NAA and BAP. Similarly, a typical growth curve of suspension cultures of E. characias was maintained on MS medium supplemented with 2, 4-D and BAP which revealed the initial lag phase for 7 days and exponential phase for 14 days (Fernandes-Ferreira et al. 1989). Among the various combinations of growth regulators tested (2,4-D and Kin; NAA and Kin; BAP and NAA), MS medium supplemented with BAP (8.88 μM) and NAA (5.37 μM) induced maximum callus formation (Saad et al. 2018).
The phytochemical analysis of different plant extracts and suspension cultures revealed the presence of secondary metabolites including triterpenoids in high abundance. In a similar study, ethanolic extracts of stem and leaves of E. hirta showed the presence of various secondary metabolites such as tannins, saponins, glycosides, and steroids (Selvakumar et al. 2012). Among different active enzymes in the venom, Snake venom phospholipase (svPLA2) is one of the major toxic venom proteins having multi functionality and causes severe tissue damage and inflammation. The In silico analysis of taraxerol compound interacted with PLA2 protein from venom of B. jararacussu revealed ΔG = − 8.16 kcal/mol when compared to the compound reserpine from Rauvolfia serpentina interacted with PLA2 that resulted in ΔG = − 9.49 kcal/mol (Sreekumar et al. 2014; Consolacion and Kimberly 2014). Based on TLC analysis, the extracts of E. hirta resulted in identification of triterpenoids with similar Rf values to that of potential bioactive compounds. Likewise, in another study, the identification of triterpenoids (Taraxerol) on a TLC plate from petroleum ether extracts of roots of H. pilosella resulted with similar Rf values (Gawronska-Grzywacz and Krzaczek 2007). The separation of compounds was studied based on fractions collected from silica based column chromatography. According to one of the studies, the petroleum extract of E. myrsinites, which, upon fractionation, indicated the presence of taraxerol (Aynehchi et al. 1972; Gawrońska-Grzywacz and Krzaczek 2007). In our study, the presence of taraxerol was found in abundance from various fractions of different extracts eluted through column.
Purification of compounds based on targets using HPLC was used for separating the target compounds from others. Peaks of potential compounds from extracts were visualized using chromatogram for triterpenoids and from fractions compared with that of standard chromatogram. Therefore, LC/MS method was performed for the eluted compounds in the extracts of E. hirta. In our study, compounds that belong to pentacyclic triterpenoids were identified which revealed the presence of taraxerol and compared with the literatures and database for their structural data.
Conclusion
The only available medicine for snakebite treatment in the current scenario is the polyvalent serum-based anti-venoms, which are still not affordable to many in rural places and the availability is very limited. It has post-treatment complications that could lead to diverse negative reactions in the system. The production of serum is also a century old technique where they inject the venom in horses to get antibodies. Unlike the anti-venom sera, plant-based compounds provide an alternate, cost-effective treatment for snakebites with negligible or no adverse effect on the system. The current study is one among the very few studies that attempted to unravel the mechanisms and characterized some of the few bioactive compounds for snakebite in this less investigated E. hirta plant system. The recent advancements such as molecular farming, metabolic engineering, and by combining ethnobotanical approach with the traditional knowledge, these potential triterpenoid-based drug compounds from E. hirta could be a very effective alternate plant medicine for snakebite treatment in near future.
Acknowledgements
The authors would like to thank the Department of Biosciences and technology, Karunya Institute of Technology and Sciences, Coimbatore for their support, laboratory facilities, and for providing fund from Karunya student projects scheme. No ethical considerations were involved in this project. The authors would like to thank Dr. Jebasingh, Department of Chemistry, KITS for HPLC analysis, Dr. Premnath, Department of Bioinformatics, KITS for compound separation analysis and docking studies, and JSS College of pharmacy, Ooty, for LC–MS analysis.
Author contributions
The authors RAS and RSDP conceived the idea and under the guidance of RSDPR the experiments were performed by RAS. RSDP edited and proof read the manuscript.
Compliance with ethical standards
Conflict of interest
The authors declare that no potential conflict of interest was intended and declared none.
References
- Aynehchi Y, Mojtabaii M, Yazdizadeh K. Chemical examination of E. myrsinitis linn. J Pharm Sci. 1972;2:292–293. doi: 10.1002/jps.2600610237. [DOI] [PubMed] [Google Scholar]
- Baburaj S, Dhamotharan R, Santhaguru K (1987) Regeneration in leaf callus cultures of E. hirta Linn. Curr Sci 56(4):194–194. http://www.jstor.org/stable/24090380
- Consolacion YR, Kimberly BC. Triterpenes from E. hirta and their cytotoxicity. Chin J Nat Med. 2014;11(5):528–533. doi: 10.3724/sp.j.1009.2013.00528. [DOI] [PubMed] [Google Scholar]
- Devi MKA, Kumar SS, Giridhar P. High yield production of folates from soybean callus cultures in response to elicitors. 3 Biotech. 2018;8:80. doi: 10.1007/s13205-018-1101-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes-Ferreira M, Novais JM, Salomé M, Pais S. Calli and suspension cultures for biomass production of E. characias L. subsp. characias. Biotechnol Lett. 1989;11(4):259–264. doi: 10.1007/BF01031574. [DOI] [Google Scholar]
- Gantait A, Sahu A, Venkatesh P, Dutta P, Mukherjee P. Isolation of taraxerol from Coccinia grandis and its standardization. J Planar Chromatogr Mod TLC. 2010;23(5):323–325. doi: 10.1556/JPC.23.2010.5.3. [DOI] [Google Scholar]
- Gawrońska-Grzywacz M, Krzaczek T. Identification and determination of triterpenoids in Hieracium pilosella L. J Sep Sci. 2007;30(5):746–750. doi: 10.1002/jssc.200600253. [DOI] [PubMed] [Google Scholar]
- Gomes A, Das R, Sarkhel S, Mishra R, Mukherjee S, Bhattacharya S, Gomes A. Herbs and herbal constituents active against snakebite. Indian J Exp Biol. 2010;48(9):865–878. [PubMed] [Google Scholar]
- Grenand P, Moretti C, Jacquemin H (1987) PharmacopeÂes traditionelles en Guyane. Creoles, Palikur WayaÄpi Editions l’Orstom, Paris
- Grosdidier A, Zoete V, Michielin O. SwissDock, a protein-small molecule docking web service based on EADock DSS. Nucleic Acids Res. 2011;39(Web Server issue):W270–W277. doi: 10.1093/nar/gkr366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansdak SG, Lallar KS, Pokharel P, Shyangwa P, Karki P, Koirala S. A clinico-epidemiological study of snakebite in Nepal. Trop Doct. 1998;28:223–226. doi: 10.1177/004947559802800412. [DOI] [PubMed] [Google Scholar]
- Heloir M, Kevers C, Hausman J, Gaspar T. Changes in the concentrations of auxins and polyamines during rooting of in vitro-propagated walnut shoots. Tree Physiol. 1996;16(5):515–519. doi: 10.1093/treephys/16.5.515. [DOI] [PubMed] [Google Scholar]
- Kumar S, Malhotra R, Kumar D. E. hirta: its chemistry, traditional and medicinal uses and pharmacological activities. Pharmacogn Rev. 2010;4(7):58. doi: 10.4103/0973-7847.65327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar GP, Samkumar RA, Premnath D, Paulraj RSD. Molecular docking and dynamic simulation studies of pentacyclic triterpenoids from E. ceae plants with snake venom phospolipase A2 and acostatin proteins. Int J Pharm Bio Sci. 2016;7(4):402–411. doi: 10.22376/ijpbs.2016.7.4.b402-411. [DOI] [Google Scholar]
- Matam P, Parvatam G, Shetty NP. Enhanced production of vanillin flavour metabolites by precursor feeding in cell suspension cultures of Decalepis hamiltonii Wight & Arn., in shake flask culture. 3 Biotech. 2017;7:376. doi: 10.1007/s13205-017-1014-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mors WB, Nascimento MC, Pereira BM, Pereira NA. Plant natural products active against snakebite—the molecular approach. Phytochemistry. 2000;55(6):627–642. doi: 10.1016/S0031-9422(00)00229-6. [DOI] [PubMed] [Google Scholar]
- Murashige T, Skoog F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant. 1962;15(3):473–497. doi: 10.1111/j.1399-3054.1962.tb08052.x. [DOI] [Google Scholar]
- Nilson A, Arruda Sandra C, Martinelli Adriana P, Carrilho E. Direct determination of plant-growth related metabolites by capillary electrophoresis with spectrophotometric UV detection. J Braz Chem Soc. 2009;20(1):183–187. doi: 10.1590/S0103-50532009000100027. [DOI] [Google Scholar]
- Oleszek W, Stochmal A, Kapusta I. TLC of triterpenes (including Saponins) Thin Layer Chromatogr Phytochem Chromatogr Sci Ser. 2008 doi: 10.1201/9781420046786.ch20. [DOI] [Google Scholar]
- Patil SB, Nilofar NS, Chandrakant CS. Review on phytochemistry and pharmacological aspects of E. hirta Linn. Asian J Pharm Res and Health Care. 2009;1(1):113–133. [Google Scholar]
- Pióro-Jabrucka EP, Pawelczak A, Prezbyl JL, Baczek K, Weglarz Z. Accumulation of phenolic and sterol compounds in E. hirta (L.) Herba Polonica. 2011;57(2):30–37. [Google Scholar]
- Saad KR, Parvatam G, Shetty NP. Medium composition potentially regulates the anthocyanin production from suspension culture. 3 Biotech. 2018;8:134. doi: 10.1007/s13205-018-1146-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samy RP, Thwin MM, Gopalakrishnakone P, Ignacimuthu S. Ethnobotanical survey of folk plants for the treatment of snakebite in southern part of Tamilnadu. Indian J Ethnopharmacol. 2008;115:302–312. doi: 10.1016/j.jep.2007.10.006. [DOI] [PubMed] [Google Scholar]
- Schripsema J, Meijer AH, van Iren F, ten Hoopen HJG, Verpoorte R. Dissimilation curves as a simple method for the characterization of growth of plant cell suspension cultures. Plant Cell Tiss Org. 1990;22:55–64. doi: 10.1007/BF00043699. [DOI] [Google Scholar]
- Selvakumar P, Kaniakumari D, Loganathan V. Preliminary phytochemical investigation of extract of leaves and stem of E. hirta. Int J Curr Sci. 2012;2017:48–51. [Google Scholar]
- Shih MF, Cherng JY. Potential applications of E. hirta in pharmacology. In: Omboon Vallisuta., editor. Drug discovery research in pharmacognosy. Croatia: InTech; 2012. pp. 165–180. [Google Scholar]
- Sivagnanam SK, Rao RK, Mudiganti Dar UM, Gh. Jeelani P. Preliminary phytochemical analysis of Artemesia amygdalina, Nerium odorum and Strychnos potatorum. J Pharm Res. 2012;5(7):3734–3739. [Google Scholar]
- Sreekumar S, Nisha NC, Biju CK, Krishnan PN. Identification of potential lead compounds against cobra venom in Rauvolfia serpentina (L.) Benth. Ex kurz through molecular docking. Int J Pharm Res Dev. 2014;6:32–43. [Google Scholar]
- Štefančič M, Štampar F, Veberič R, Osterc G. The levels of IAA, IAAsp and some phenolics in cherry rootstock ‘GiSelA 5’ leafy cuttings pretreated with IAA and IBA. Sci Hortic. 2007;112(4):399–405. doi: 10.1016/j.scienta.2007.01.004. [DOI] [Google Scholar]
- Tarkowski P, Ge L, Yong JWH, Tan SN. Analytical methods for cytokinins. Trends Anal Chem. 2009;28(3):323–335. doi: 10.1016/j.trac.2008.11.010. [DOI] [Google Scholar]
- WHO . Rabies and Envenomings: a neglected public health issue: report of a consultative meeting. Geneva: WHO; 2007. [Google Scholar]
- Wu Y, Qu W, Geng D, Liang J, Luo Y. Phenols and flavonoids from the aerial part of E. hirta. Chin J Nat Med. 2012;10(1):40–42. doi: 10.3724/SP.J.1009.2012.00040. [DOI] [PubMed] [Google Scholar]
- Yang ZS, Chen GD, Li YX, Chen J. Characterization of callus formation in leaf of E. helioscopia. Afr J Plant Sci. 2009;3(6):122–126. [Google Scholar]