Abstract
Acanthamoeba as free-living parasites are scattered ubiquitously, throughout the world. This study was aimed to evaluate the presence of Acanthamoeba spp. genotypes in the recreational water sources in Gorgan County, the capital of Golestan Province using both morphological and molecular approaches. Thirty water samples were collected from different recreational waters in Gorgan, the capital of Golestan Province, northern Iran during 2015–2016. Samples were filtered and followed by culture in non-nutrient agar. Acanthamoeba were identified both by morphological and molecular analysis. The pathogenical potential of positive cloned samples were also determined using tolerance test. Twenty-six percent of recreational water were identified as Acanthamoeba spp. based on the morphological analysis and from these positive samples, five samples were successfully sequenced after molecular studies. Phylogenetic analysis showed the clustering of four samples in T4 genotype group and only one sample as T15 genotype. Thermotolerance test revealed that all cloned samples were highly positive. Since the attractiveness of recreational places for people is increasing, the potential risk of this water should be monitored routinely in each region. More studies are needed to better evaluate the risk of this ubiquitous parasite for the human.
Keywords: Acanthamoeba, Genotyping techniques, Morphological, Molecular, Iran
Introduction
Acanthamoeba as free-living parasites are scattered ubiquitously, throughout the world. These protozoans are very durable and well-adapted to hard environmental conditions such as air, dusts, soil, and different water supplies and, even from the throat and nasal mucosa of healthy individuals (Marciano-Cabral and Cabral 2003; Schuster and Visvesvara 2004; Lorenzo-Morales et al. 2006; Visvesvara et al. 2007).
Recent studies on Acanthamoeba infection of recreational water sources in southern part of Iran revealed that all these sources were infected by Acanthamoeba infection (Niyyati et al. 2016a, b). Due to increasing the immunocompromised or immunosuppressed individuals in the societies, this opportunistic parasite must be more considered as a health threatening by the authorities (Forrest 2004). Contact lens users are at risk of Acanthamoeba keratitis (AK) due to wear the contaminated lens (Radford et al. 1995). Although, AK cases are uncommon, but, infectivity of corneal is sight-threatening and also can cause blindness in these patients (Lorenzo-Morales et al. 2013). Most cases of AK have a history of washing their contact lens with unclean water (Radford et al. 2002). Until now, a total of 150 cases of AK have been reported from Iran, whoever, the true number of AK maybe higher than (Niyyati and Rezaeian 2015). In addition, this parasite can act as a ‘Trojan horse’ for some amoeba-resisting bacteria (ARB) including, Legionella spp., Burkholderia cepacia, Listeria monocytogenes, Vibrio cholera, Listeria monocytogenes and Escherichia coli O157 that are existence in environmental water naturally (Pagnier et al. 2008; Thomas et al. 2010).
Detection of Acanthamoeba is based on the morphometrical and molecular methods (Lorenzo-Morales et al. 2015). Cultivation of suspected samples and contact lens in non-nutrient agar medium is a useful method for detection of Acanthamoeba parasites. Specific morphometric criteria of their trophozoites and cysts in the culture could be efficient for genus differentiating (Marciano-Cabral and Cabral 2003). However, molecular studies with precise detection were used for genotyping of parasites (Lorenzo-Morales et al. 2015). To date, 20 genotypes of Acanthamoeba are detected and the T2, T3, T4, T5, T6, T11 and T15 have been reported from Iran (Niyyati and Rezaeian 2015). Golestan Province, located in the north of Iran is well known as a tourist attractive area with many recreational water sources. This study aimed to evaluate the presence of Acanthamoeba spp. in the recreational water sources in Gorgan County using both morphological and molecular approaches.
Materials and methods
Geographical information of Ardebil Province
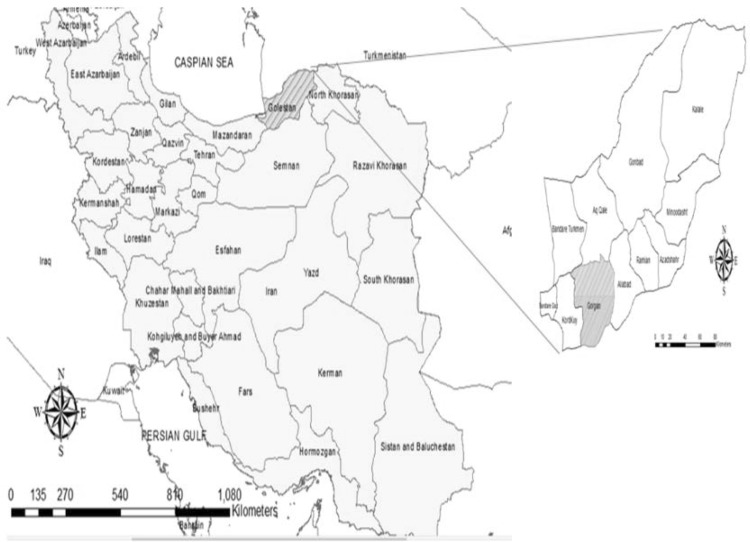
Golestan Province located in north-east of Iran, with moderate Mediterranean climate, lying within the 36°30′–38°8′N and 53°57′–56°22′E (Fig. 1). It attracts many tourists due to the presence of many recreational park and waterfall (Mollalo et al. 2015).
Fig. 1.
Map showing the region of sampling, Gorgan County, the capital of Golestan Province in north of Iran
Sampling
Thirty sample were collected from different recreational water of park ponds (22) and public squares (8) in Gorgan, the capital of Golestan Province, northern Iran during 2015–2016. Water samples belonged to three park ponds and eight public squares. The samples were placed into sterile bottles and transported to the Department of Parasitology and Mycology, School of Medicine, Shiraz University of Medical Sciences, Iran and filtered using sterile membrane filters (pore size 0.45 μm).
Morphological surveys
Filtered membranes were transferred onto 1% non-nutrient agar plates overlaid containing autoclaved Escherichia coli at 30 °C or room temperature for 1 month.
Incubated media were surveyed for Acanthamoeba parasite that could be detected easily by distinct morphology of both cyst and trophic stages (Page 1988). To access the pure media containing trophozoites of Acanthamoeba, several sub-cultures were done. For this, a few amoebae of culture were transferred to a new fresh medium. Pure media were scraped and washed with phosphate-buffered saline (PBS pH 7) to use in DNA extraction.
Tolerance assays
The axenic culture from positive samples was achieved by sub-culture method and then cloned isolates were incubated at two temperatures (37 °C and 42 °C) for different time (24, 48, and 72 h).
Molecular study
The DNA was extracted by Instagene matrix (Chelex; Biorad) protocol. Briefly, a total of 50 μl Chelex were added to approximately 1000 washed trophozoite cells and incubated at 56 °C for 20 min, and followed by 10 min incubation in Bain-marie (100 °C). the samples were centrifuged at 10,000×g for 5 min and supernatant solution was used as the DNA template for PCR reaction. PCR reactions were applied in a final volume of 30 μl including 30 ng DNA, 1.25 U Taq DNA polymerase, 1.5 mM MgCl2, 0.2 µM dNTP and 0.2 µM each primer. The diagnostic fragment 3 (DF3) region of 18S rRNA was amplified using JDP1-2 primer pairs (Schroeder et al. 2001; Booton et al. 2002). Thermal cycler program for DNA amplification was as below: Primary denaturation, 94 °C for 60 s, 35 cycles of 94 °C for 35 s, 56 °C for 45 s, 72 °C for 60 s and finally a terminal extension at 72 °C for 10 min. PCR product were electrophoresed using agarose gel 1.5% and stained with ethidium bromide (25 mg/ml). Visualisation of bands was done under UV illumination.
Sequencing
Positive samples were sequenced by Bioneer Company (Daejeon, South Korea). Sequenced samples were blasted using GenBank from the National Center for Biotechnology Information (NCBI) site (Altschul et al. 1997).
Phylogenetic analysis
Phylogenetic analysis was inferred using the 18s rRNA sequence originated from the current study with those of reference strains deposited in GenBank by the neighbour-joining method (Saitou and Nei 1987) with the MEGA version 6 computer program (Tamura et al. 2013). The evolutionary distances were constructed using the Kimura 2-parameter model (Kimura 1980) with the pairwise deletion of gap option. The statistical reliability of nodes was evaluated by bootstrap analysis with 1000 replicates. Entamoeba coli were used as out-group for construction of phylogenetic tree.
Results
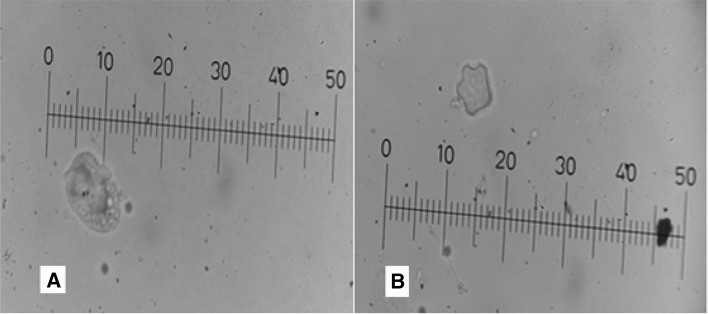
Out of 30 recreational water samples, 8 (26.6%) samples were identified as Acanthamoeba genus based on the morphological analysis of characteristic trophozoites and the double-walled cysts. From 22 samples isolated from squares, 6 samples (27.2%), were contaminated with this amoeba while, 2 of 8 (25%) samples from park ponds were positive with Acanthamoeba parasite. Acanthamoeba trophozoites were flat shape, spine-like structures and containing a prominent nucleus (Fig. 2a) while, the cysts were double walled, had a 3–6 cyst arms, with a size between 20 and 22 µm (Fig. 2b).
Fig. 2.
Light microscopy image of Acanthamoeba trophozoite (a) and cyst (b) in non-nutrient agar. Note: Each micrometre division cover 2 µm in final magnification × 400
Sub-culture of Acanthamoeba spp. to achieve the axenic media including pure parasite, were successfully performed only for 5 samples. Thermotolerance test showed that all cloned Acanthamoeba isolates were capable of growth at high temperatures including 37 °C and 42 °C. The amoeba-to-flagellate transformation test was negative for all isolates.
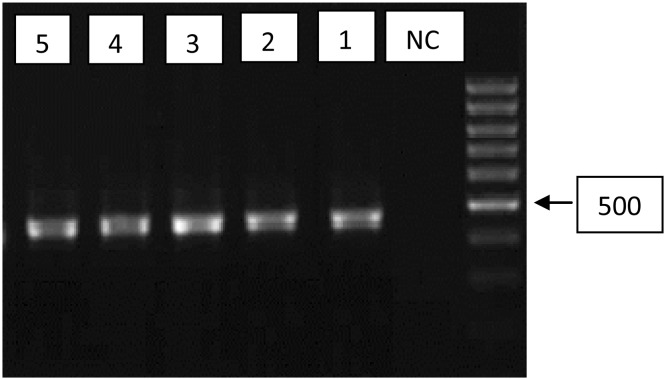
The molecular study, with genus-specific JDP primers, was carried out on these five samples. Electrophoresis of PCR product showed the 500 bp fragments on agarose gel (Fig. 3). Sequences showed the samples belonged to T4 (4/5, 80%) and T15 (1/5, 20%) genotypes (Table 1).
Fig. 3.
Agarose gel separation of representative PCR products of the 18 s rRNA gene. Lane 1–5, positive isolates; NC negative control, DNA ladder 100 bp
Table 1.
The results of PCR, culture and sequenced samples isolated from park pounds and square water sources in Gorgan County, North Iran
| Code | Source | Culture/PCR | Genotype | Accession number |
|---|---|---|---|---|
| S1 | Park ponds | +/+ | T4 | KX870199 |
| S2 | Park ponds | +/+ | T4 | KX870200 |
| S3 | Square | +/+ | T4 | KX870201 |
| S4 | Square | +/− | – | – |
| S5 | Square | +/− | – | – |
| S6 | Square | +/− | – | – |
| S7 | Square | +/+ | T4 | KX870202 |
| S8 | Square | +/+ | T15 | KX870203 |
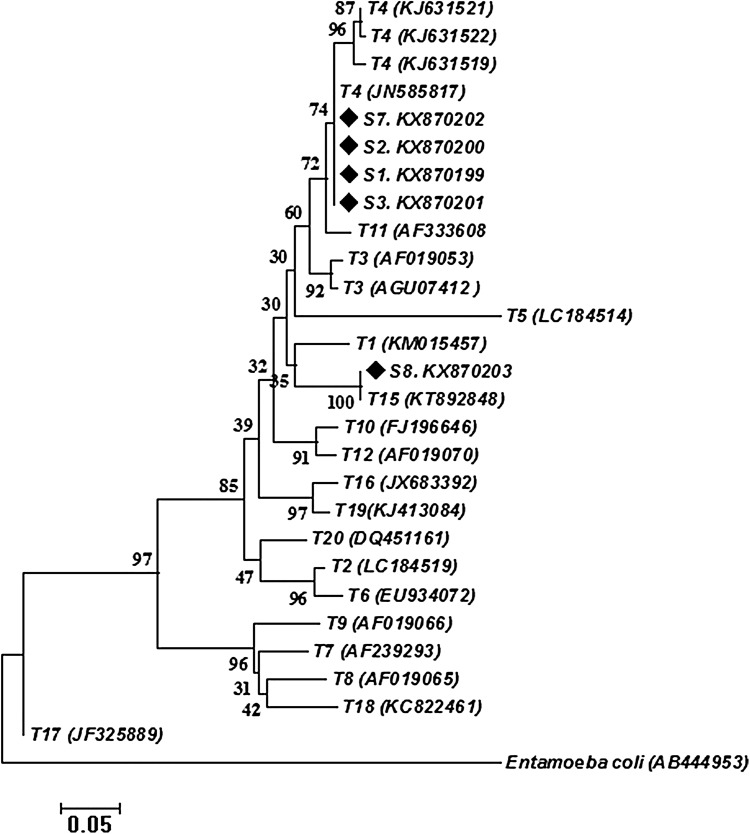
Sequenced samples and obtained sequences from GenBank database were used for phylogenetic tree construction. The 5 sequences of the current study were clustered within the genotype T4 (4/5) and T15 (1/5) with 78 and 99% bootstrap support, respectively (Fig. 4).
Fig. 4.
Genetic relationships of obtained genotypes in the current study (displayed with diamond symbol) and reference sequences related genotypes of Acanthamoebaas well as Entamoeba coli as the out-group. The relationships were inferred based on phylogenetic tree (18srRNAgene). The phylogenetic tree was constructed using neighbour-joining tree implemented in MEGA software version 6
Discussion
Free-living amoeba including Acanthamoeba spp. that are scattered everywhere, are more considered and investigated in recent years. This is due to the pathogenicity of these parasites in human (Todd et al. 2015). Increasing of immunocompromised individuals in societies and also more dealing with people with sources of mentioned protozoan, led to report in more cases of this opportunistic parasite in the world, including Iran (Marciano-Cabral and Cabral 2003; Nazar et al. 2011). One of the most known diseases related to Acanthamoeba in Iran is AK that is due to poor hygiene in contact lens users. It was showed that the cases of AK are increasing in the country (Niyyati and Rezaeian 2015). Recreational water, as an important habitat of Acanthamoeba spp. are very frequent in the studied region (Gorgan County) and many tourists attract to these places every year. Therefore, evaluation of potential risk of these waters for people should be more considered by health authorities. The current study evaluated the presence of Acanthamoeba spp. in park pounds and square water in Gorgan County and parasite was detected from 26.6% of analysed water samples. Of the five Acanthamoeba detected by molecular methods in this study, 4 samples belonged to T4 genotype and only one sample detected as T15 genotype. As the previous studies, T4 is showed as the most prevalent genotype in environmental in the world including Iran (Niyyati and Rezaeian 2015; Wagner et al. 2016). As with previous studies, in the current study, T4 and T15 genotypes were reported as the only genotypes isolated in water sources (Niyyati et al. 2012; Montalbano Di Filippo et al. 2015). Many studies were stressed out on free-living amoeba in Iran and presence of these amoebas were showed in hot spring (Solgi et al. 2012a, b), recreational pool (Niyyati et al. 2016a, b), and even drink water sources (Niyyati et al. 2015). Due to the pathogenicity of this genotype, infectivity risk with this genotype should be reminded for people. Thermo-tolerance test indicated the surviving of the parasite in 37 °C and 42 °C. whoever; growing of parasite in more temperatures and even in hot springs had been indicated before (Solgi et al. 2012a, b). Phylogenetic analysis showed the genomic identical in T4 and T15 genotypes of the current study with the sequences of genotypes deposited in GenBank. To the author knowledge’s, this study was the first to evaluate the recreational water for the presence of Acanthamoeba spp. in Gorgan, the capital of Golestan Province. But there are its limitations, the most noticeable limitation is restriction of sampling to only park pound and square waters. While, pool and tap water sources that people are more dealing with them, could be considered in the future studies. The findings showed the contamination of recreational water with this parasite and presence of pathogenic genotype in these water sources. The tourists are potentially at risk for parasite in these recreational areas. Due to frequent activities of human in these areas, the presence of Acanthamoeba could be hazardous for native and tourist people.
Conclusions
The presence of Acanthamoeba in recreational water in the Gorgan County is an alarm for health authorities. Since the attractiveness of recreational places for people is increasing, the potential risk of this water should be monitored routinely in each region. More studies are needed to better evaluate the risk of this ubiquitous parasite for the human.
Acknowledgements
The authors would like to express their gratitude to Mrs. Shahrbanoo Naderi for her useful collaboration.
Authors’ contributions
All authors contributed to the study design. RS was leader of the research. SM, EM, AT, MHM and MAY carried out experimental tests and prepared the Manuscript. All authors read and approved the final version of the manuscript.
Funding
Funding was provided by Golestan University of Medical Sciences.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25(17):3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booton GC, Kelly DJ, Chu YW, Seal DV, Houang E, Lam DS, Byers TJ, Fuerst PA. 18S ribosomal DNA typing and tracking of Acanthamoeba species isolates from corneal scrape specimens, contact lenses, lens cases, and home water supplies of Acanthamoeba keratitis patients in Hong Kong. J Clin Microbiol. 2002;40(5):1621–1625. doi: 10.1128/JCM.40.5.1621-1625.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrest G. Gastrointestinal infections in immunocompromised hosts. Curr Opin Gastroenterol. 2004;20(1):16–21. doi: 10.1097/00001574-200401000-00005. [DOI] [PubMed] [Google Scholar]
- Kimura M. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J Mol Evol. 1980;16(2):111–120. doi: 10.1007/BF01731581. [DOI] [PubMed] [Google Scholar]
- Lorenzo-Morales J, Ortega-Rivas A, Martínez E, Khoubbane M, Artigas P, Periago MV, Foronda P, Abreu-Acosta N, Valladares B, Mas-Coma S. Acanthamoeba isolates belonging to T1, T2, T3, T4 and T7 genotypes from environmental freshwater samples in the Nile Delta region, Egypt. Acta Trop. 2006;100(1):63–69. doi: 10.1016/j.actatropica.2006.09.008. [DOI] [PubMed] [Google Scholar]
- Lorenzo-Morales J, Martin-Navarro CM, Lopez-Arencibia A, Arnalich-Montiel F, Pinero JE, Valladares B. Acanthamoeba keratitis: an emerging disease gathering importance worldwide? Trends Parasitol. 2013;29(4):181–187. doi: 10.1016/j.pt.2013.01.006. [DOI] [PubMed] [Google Scholar]
- Lorenzo-Morales J, Khan NA, Walochnik J. An update on Acanthamoeba keratitis: diagnosis, pathogenesis and treatment. Parasite. 2015;22:10. doi: 10.1051/parasite/2015010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marciano-Cabral F, Cabral G. Acanthamoeba spp. as agents of disease in humans. Clin Microbiol Rev. 2003;16(2):273–307. doi: 10.1128/CMR.16.2.273-307.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mollalo A, Alimohammadi A, Shirzadi M, Malek M. Geographic information system-based analysis of the spatial and spatio-temporal distribution of zoonotic cutaneous leishmaniasis in Golestan Province, north-east of Iran. Zoonoses Public Health. 2015;62(1):18–28. doi: 10.1111/zph.12109. [DOI] [PubMed] [Google Scholar]
- Montalbano Di Filippo M, Santoro M, Lovreglio P, Monno R, Capolongo C, Calia C, Fumarola L, D’Alfonso R, Berrilli F, Di Cave D. Isolation and molecular characterization of free-living amoebae from different water sources in Italy. Int J Environ Res Public Health. 2015;12(4):3417–3427. doi: 10.3390/ijerph120403417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazar M, Haghighi A, Niyyati M, Eftekhar M, Tahvildar-Biderouni F, Taghipour N, Abadi A, Nazemalhosseini Mojarad E, Athari A. Genotyping of Acanthamoeba isolated from water in recreational areas of Tehran, Iran. J Water Health. 2011;9(3):603–608. doi: 10.2166/wh.2011.152. [DOI] [PubMed] [Google Scholar]
- Niyyati M, Rezaeian M. Current status of Acanthamoeba in Iran: a narrative review article. Iran J Parasitol. 2015;10(2):157. [PMC free article] [PubMed] [Google Scholar]
- Niyyati M, Lasjerdi Z, Nazar M, Haghighi A, Nazemalhosseini Mojarad E. Screening of recreational areas of rivers for potentially pathogenic free-living amoebae in the suburbs of Tehran, Iran. J Water Health. 2012;10(1):140–146. doi: 10.2166/wh.2011.068. [DOI] [PubMed] [Google Scholar]
- Niyyati M, Lasgerdi Z, Lorenzo-Morales J. Detection and molecular characterization of potentially pathogenic free-living amoebae from water sources in Kish Island, Southern Iran. Microbiol Insights. 2015;8(Suppl 1):1–6. doi: 10.4137/MBI.S24099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niyyati M, Saberi R, Latifi A, Lasjerdi Z. Distribution of Acanthamoeba genotypes isolated from recreational and therapeutic geothermal water sources in Southwestern Iran. Environ Health Insights. 2016;10:69–74. doi: 10.4137/EHI.S38349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niyyati M, Saberi R, Lorenzo-Morales J, Salehi R. High occurrence of potentially-pathogenic free-living amoebae in tap water and recreational water sources in South-West Iran. Trop Biomed. 2016;33(1):95–101. [PubMed] [Google Scholar]
- Page FC. A new key to freshwater and soil gymnamoebae. Ambleside: Freshwater Biological Association; 1988. [Google Scholar]
- Pagnier I, Raoult D, La Scola B. Isolation and identification of amoeba-resisting bacteria from water in human environment by using an Acanthamoeba polyphaga co-culture procedure. Environ Microbiol. 2008;10(5):1135–1144. doi: 10.1111/j.1462-2920.2007.01530.x. [DOI] [PubMed] [Google Scholar]
- Radford CF, Bacon AS, Dart JK, Minassian DC. Risk factors for Acanthamoeba keratitis in contact lens users: a case-control study. BMJ. 1995;310(6994):1567–1570. doi: 10.1136/bmj.310.6994.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radford C, Minassian D, Dart J. Acanthamoeba keratitis in England and Wales: incidence, outcome, and risk factors. Br J Ophthalmol. 2002;86(5):536–542. doi: 10.1136/bjo.86.5.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitou N, Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987;4(4):406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- Schroeder JM, Booton GC, Hay J, Niszl IA, Seal DV, Markus MB, Fuerst PA, Byers TJ. Use of subgenic 18S ribosomal DNA PCR and sequencing for genus and genotype identification of Acanthamoebae from humans with keratitis and from sewage sludge. J Clin Microbiol. 2001;39(5):1903–1911. doi: 10.1128/JCM.39.5.1903-1911.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuster FL, Visvesvara GS. Free-living amoebae as opportunistic and non-opportunistic pathogens of humans and animals. Int J Parasitol. 2004;34(9):1001–1027. doi: 10.1016/j.ijpara.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Solgi R, Niyyati M, Haghighi A, Mojarad EN. Occurrence of thermotolerant Hartmannella vermiformis and Naegleria spp. in Hot Springs of Ardebil Province, Northwest Iran. Iran J Parasitol. 2012;7(2):47–52. [PMC free article] [PubMed] [Google Scholar]
- Solgi R, Niyyati M, Haghighi A, Taghipour N, Tabaei SJ, Eftekhar M, Nazemalhosseini Mojarad E. Thermotolerant Acanthamoeba spp. isolated from therapeutic hot springs in Northwestern Iran. J Water Health. 2012;10(4):650–656. doi: 10.2166/wh.2012.032. [DOI] [PubMed] [Google Scholar]
- Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol. 2013;30(12):2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas V, McDonnell G, Denyer SP, Maillard J-Y. Free-living amoebae and their intracellular pathogenic microorganisms: risks for water quality. FEMS Microbiol Rev. 2010;34(3):231–259. doi: 10.1111/j.1574-6976.2009.00190.x. [DOI] [PubMed] [Google Scholar]
- Todd CD, Reyes-Batlle M, Pinero JE, Martinez-Carretero E, Valladares B, Streete D, Lorenzo-Morales J, Lindo JF. Isolation and molecular characterization of Acanthamoeba genotypes in recreational and domestic water sources from Jamaica, West Indies. J Water Health. 2015;13(3):909–919. doi: 10.2166/wh.2015.232. [DOI] [PubMed] [Google Scholar]
- Visvesvara GS, Moura H, Schuster FL. Pathogenic and opportunistic free-living amoebae: Acanthamoeba spp., Balamuthia mandrillaris, Naegleria fowleri, and Sappinia diploidea. FEMS Immunol Med Microbiol. 2007;50(1):1–26. doi: 10.1111/j.1574-695X.2007.00232.x. [DOI] [PubMed] [Google Scholar]
- Wagner C, Reyes-Batlle M, Hernan A, Rojas E, Perez G, Lopez-Arencibia A, Sifaoui I, Martinez-Carretero E, Pinero JE, Valladares B, Lorenzo-Morales J. High occurrence of Acanthamoeba genotype T4 in soil sources from Bolivar State, Venezuela. Acta Parasitol. 2016;61(3):466–470. doi: 10.1515/ap-2016-0063. [DOI] [PubMed] [Google Scholar]