Abstract
Strongyloides stercoralis hyperinfection syndrome (SHS) is a life-threatening condition that warrants early detection and management. We describe the pathogenesis, organ-specific clinical manifestations, and risk factors associated to this condition. A comprehensive review of the literature was conducted in PubMed, LILACS, EBSCO and SciELO by using the keywords: “hyperinfection syndrome”; “Strongyloides stercoralis”; “disseminated strongyloidiasis”; “systemic strongyloidiasis”, “pathogenesis” and “pathophysiology”. Relevant articles on this topic were evaluated and included by consensus. Also, a secondary search of the literature was performed. Articles in English and Spanish language were included. SHS has been described in tropical and sub-tropical regions. However, there is growing evidence of cases detected in developed countries favored by increasing migration and the advance in immunosuppressive therapies for oncologic and inflammatory diseases. SHS is characterized by massive multiplication of larvae, typically in immunocompromised hosts. Clinical manifestations vary according to the organ involved and include diarrhea, intestinal bleeding, alveolar hemorrhages, heart failure, jaundice, bacteremia among others. Despite advances in the understanding of this condition, fatality rates are near 90%. Clinicians should consider SHS in the differential diagnosis of acutely ill patients with multiple organ damage and epidemiological risk factors. Adverse outcomes are common, especially with delayed anti-parasitic treatment.
Keywords: Strongyloidiasis, Autoinfection, Massive infection, Soil-transmitted helminths, Clinical manifestations
Introduction
Strongyloides stercoralis is an intestinal nematode with a worldwide distribution. To a great extent, it affects poverty-stricken populations predominantly from rural areas; however, it may be present in regions such as the USA and Europe (Schär et al. 2013; Barros and Montes 2014; Marcos et al. 2011). Approximations of 30–100 million infected people worldwide date back to reports published in 1989 (Genta 1989) and 1996 (Jorgensen et al. 1996). Estimates on the global prevalence are challenging due to numerous limitations including inadequate diagnostic methods, small study sample size, and limited statistics (Schär et al. 2013). Most recent data reveal that this organism affects between 10 and 40% of the population in many tropical and subtropical countries and its prevalence can be as high as 60% in resource-limited settings (Schär et al. 2013; Buonfrate et al. 2013; Ketzis and Conan 2017; Puthiyakunnon et al. 2014).
Specific groups including travelers, military personnel and immigrants from endemic areas are prone to harbor and spread this infection in developed nations, where its frequency is significantly lower (Schär et al. 2013; Puthiyakunnon et al. 2014). Furthermore, with the advent of transplant and immunosuppressive therapies, S. stercoralis hyperinfection syndrome (SHS) is becoming an emergent healthcare problem (Alsharif et al. 2015; Upadhyay et al. 2001). Hence, chronically infected patients, particularly those who are asymptomatic or oligo-symptomatic, are at risk of developing SHS due to lack of healthcare policies that promote parasitological screening among patients from endemic areas receiving immunosuppressive medications (Schär et al. 2013; Barros and Montes 2014; Marcos et al. 2011; Buonfrate et al. 2013). Thus, fatal outcomes may occur, with mortality rates ranging from 60 to 87% (Vadlamudi et al. 2006; Geri et al. 2015).
The purpose of this review article is to analyze and summarize the current evidence on SHS. A comprehensive review of the literature was conducted in PubMed, LILACS, EBSCO and SciELO by using the keywords: “hyperinfection syndrome”; “Strongyloides stercoralis”; “disseminated strongyloidiasis”; “systemic strongyloidiasis”, “pathogenesis” and “pathophysiology”. A set of articles was obtained and reviewed by the authors in detail (GVR, RPR, JPP, RM, EFR) and further discussed with a senior expert (AT). Articles in English and Spanish language were included and the information was collected in summary forms. Any discrepancy was resolved by consensus. Additionally, references of these selected articles were also reviewed.
Strongyloides stercoralis: reproduction, autoinfection, hyperinfection
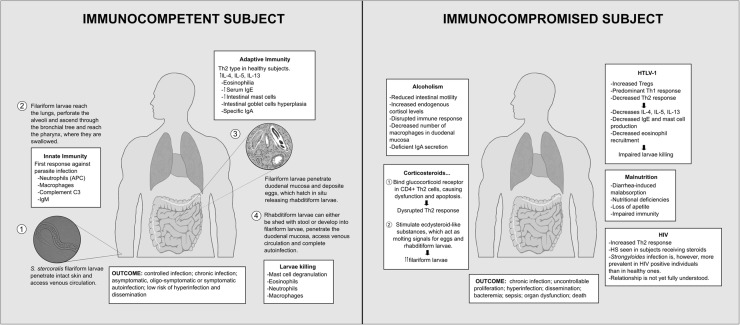
S. stercoralis reproduces through two separate life cycles: a free-living and a parasitic cycle (Fig. 1) (Centers for Disease Control and Prevention 2015). The parasitic cycle begins with the penetration of human intact skin by the filariform larvae. The larvae access venous circulation and migrate to the lungs where they penetrate the alveolar spaces and ascend across the bronchial tree, trachea, and larynx, until they reach the pharynx, where they are swallowed. Subsequently, the larvae penetrate the mucosa within the proximal small bowel, where they mature into adult females and deposit the eggs produced by parthenogenesis. These eggs hatch in situ releasing rhabditiform larvae that are eventually excreted with the stool. Once outside the human host, these larvae can mature into either free-living male and female adult worms (which can reproduce sexually, completing a free-living cycle) or into filariform larvae, ready to infect another host (Schär et al. 2013; Barros and Montes 2014; Puthiyakunnon et al. 2014). A distinctive feature of S. stercoralis is that some rhabditiform larvae may develop into filariform larvae in the intestinal lumen and penetrate the colonic mucosa or perianal skin. These filariform larvae, in turn, are capable of completing a new cycle in-host. This process is known as autoinfection and is unique to this parasite (Barros and Montes 2014; Marcos et al. 2011; Puthiyakunnon et al. 2014; Marcos et al. 2008). This phenomenon is seen in both asymptomatic and symptomatic hosts, where continuous cycles may perpetuate the infection for decades (Schär et al. 2013; Mejia and Nutman 2012). Of note, selected individuals with alterations in their immune system are at risk for developing more severe forms of disease, known as Strongyloides hyperinfection syndrome (Marcos et al. 2011; Mejia and Nutman 2012).
Fig. 1.
Reproductive cycle in the immunocompetent and immunocompromised host, and summary of the immunopathogenesis
During primary infection, the host’s innate immune response is mediated primarily by granulocytes and macrophages; both able to exert effector and immunomodulatory functions (Barros and Montes 2014; Bonne-Année et al. 2013). Experiments in mice infected with S. stercoralis and S. ratti have studied the role of neutrophils and eosinophils in the early innate immune response, demonstrating their capacity to kill larvae and affect larvae migration and fecundity (Galioto et al. 2006; Watanabe et al. 2000). Moreover, robust T- helper type-2 (Th2) and regulatory T-cells (Tregs) responses are pivotal pieces of the adaptive immunity, preventing the progression of the parasitic infection to SHS (Anuradha et al. 2015a). Th2 cells secrete interleukin (IL)-4, IL-5, and IL-13, increase eosinophil recruitment, and can activate M2 macrophages and IgE antibody production. Furthermore, Th2 response can exert effects on the gastrointestinal (GI) and respiratory tract by increasing the peristalsis, and by augmenting mucus production in respiratory epithelial cells and the mast cell population of the mucosa (Marcos et al. 2011; Anuradha et al. 2015a; Bonne-Année et al. 2011).
The expression pattern of Th2 subset has been analyzed and compared to that of Th1 and Th17 subsets among uninfected and asymptomatic infected individuals, demonstrating the downregulation of Th1 and Th17 subsets and an increased frequency of CD4 + Th2 cells in those infected. Interestingly, this alternation in the phenotypic expansion of Th cells has been noticed to revert following antiparasitic treatment (Anuradha et al. 2015b). Additionally, in a parallel study, S. stercoralis-infected subjects exhibited markedly decreased levels of systemic proinflammatory cytokines, such as gamma interferon (IFN-γ), tumor necrosis factor alpha (TNF-α), and IL-1β. This suggests that Strongyloides infection could immunomodulate the host response and blunt the specific response in harmful inflammatory conditions such as autoimmune disorders (Anuradha et al. 2015a). It is unknown if these findings could correlate to a lesser extent with other metabolic abnormalities seen in infected individuals. For example, some studies have revealed that subjects who were infected by S. stercoralis and other soil-transmitted helminths may develop insulin resistance and altered glucose metabolism following antiparasitic treatment (Hays et al. 2017; Tahapary et al. 2017), suggesting a potential interplay between metabolic regulation and inflammation. On the other hand, the expansion of T-regulatory subpopulation can diminish the Th2 response, highlighting the importance of a fine balance between Th cells and T-regs to prevent the downregulation of an otherwise protective response against S. stercoralis (Montes et al. 2009).
External factors such as corticosteroid therapy or human T cell lymphotropic virus type-1 (HTLV-1) infection may alter the regular mechanisms of immunity, allowing the parasite to massively reproduce and the infection to aggravate. Hence, hyperinfection is the process by which the parasite undergoes uncontrolled proliferation, and dissemination, when it spreads to organs other than skin, lungs or GI tract (Schär et al. 2013; Barros and Montes 2014; Marcos et al. 2011; Puthiyakunnon et al. 2014). The parasite can virtually invade any organ including the liver, heart, kidneys, lymphatic ducts and nodes, and the central nervous system (Keiser and Nutman 2004; Altintop et al. 2010).
Risk factors for hyperinfection syndrome
Certain conditions have been recognized to affect the immune system, putting the host at risk for the development of SHS and dissemination. In the following lines, we describe the most common risk factors for SHS and their influence in its pathophysiology.
HTLV-1
Subjects infected with HTLV-1 show an elevated number of circulating Tregs, which downregulates the Th2 response, altering the immune balance necessary for efficient parasite eradication (Barros and Montes 2014; Barros et al. 2012). Thus, HTLV-1 is associated with an increased Th1 cytokine response, favoring the parasite burden by reduced IL-5 levels and eosinophil counts (Montes et al. 2009). Importantly, HTLV-1 infection has been associated with strongyloidiasis treatment failure among otherwise immunocompetent individuals (Terashima et al. 2002).
Corticosteroids
Corticosteroids interfere with the type-2 response through binding glucocorticoid receptors in the CD4 + Th2 cells, causing cell dysfunction and apoptosis (Marcos et al. 2011). They may also lead to an increased number of filariform larvae and dissemination through production of ecdysteroid-like substances, which act as molting signals for eggs and rhabditiform larvae (Vadlamudi et al. 2006).
Transplant
Strongyloidiasis and SHS in both solid organ transplant and hematopoietic stem cell transplantation (HSCT) have been described, often due to reactivation of chronic asymptomatic or oligo-symptomatic infections in recipients following the initiation of immunosuppressive therapy (Schär et al. 2013; Khushman et al. 2017; Mazhar et al. 2017; Izquierdo et al. 2013). Cases associated to HSCT were associated with more severe forms of disease and a higher incidence of SHS, with mortality rates at 83% (Marcos et al. 2011; Khushman et al. 2017; Al Malki and Song 2016; Wirk and Wingard 2009). There is growing evidence on donor-derived S. stercoralis infection and SHS, a unique mechanism of spread compared to other parasitic infections (Abdalhamid et al. 2015; Kim et al. 2016). Kim and colleagues (Kim et al. 2016) identified 27 cases of S. stercoralis-infected donor allograft with a 35% case fatality rate, being sepsis and bacteremia strong predictors of mortality. Both conditions were seen in 100% of subjects with fatal outcomes and are considered clinical markers of dissemination.
Alcoholism
Immunity is impaired in alcoholics due to increasing endogenous cortisol levels and reduced intestinal motility (Teixeira et al. 2016). These factors may allow the rhabditiform larvae to stay in the GI tract for a prolonged time and enabling them to develop into filariform larvae. The latter, along with a lower density of macrophages in the duodenal mucosa and deficient IgA secretion may increase the risk of SHS (Teixeira et al. 2016; Silva et al. 2016). Furthermore, recent studies have shown increased expression of Tregs in alcoholic individuals, which may attenuate the immune response and play an important role in larvae dissemination (Ribeiro et al. 2017).
Human immunodeficiency virus (HIV)
Immunosuppression secondary to HIV infection is characterized by an impaired cell-mediated response, with a progressive decay in CD4 + T cells and altered Th1 function rather than Th2 subset compromise (Siegel and Simon 2012). During the early HIV/Acquired Immune Deficiency Syndrome (AIDS) era, disseminated strongyloidiasis was considered an AIDS-defining condition. However, due to the relatively low number of cases of SHS in HIV/AIDS-affected individuals, it was removed from the list in 1987 (Schär et al. 2013; Keiser and Nutman 2004; Siegel and Simon 2012). Nowadays, HIV infection is not frequently associated to SHS (Schär et al. 2013; Salvador et al. 2013).
Malnutrition
S. stercoralis infections have been associated with a high risk of growth retardation and stunting in children from Cambodia (Forrer et al. 2017). Although the cause of these findings could be multifactorial, it is known that both innate and adaptive immunity can be impaired in subjects with protein-energy malnutrition, inducing thymus atrophy, and blunted Th2 immune response (Schaible and Kaufmann 2007; Takele et al. 2016). Conversely, helminth infections may disrupt the gut microbiota and have a negative impact on nutrient absorption, creating a vicious cycle that results in a higher parasitic burden (Glendinning et al. 2014). Interestingly, SHS has been also described in individuals with marginal or acceptable anthropometric parameters but severe vitamin deficiencies (Marathe and Date 2008).
Clinical manifestations and organ damage
A case series of 133 patients with SHS revealed that fever (80.6%) and respiratory symptoms (88.6%) were the most common clinical manifestations (Geri et al. 2015). This differs from data on chronic Strongyloidiasis in which urticaria and diarrhea were the most frequent complaints (Forrer et al. 2017). SHS with dissemination can exhibit varied clinical syndromes depending on the organ involved:
Gastrointestinal disease
GI manifestations described in SHS range from mild dyspepsia or abdominal pain to more ominous others such as hematemesis, abdominal distension and shock (Altintop et al. 2010; Bollela et al. 2013). Diarrhea may be present in most individuals with S. stercoralis infection; mainly as loose or watery stools (Herrera et al. 2006; Infante et al. 1998). Strongyloides infection affects the GI tract in wide extension. Initial stages of enteric disease show mild mucosal congestion and larvae restricted to the mucous membrane. This can progress to edematous enteritis with edematous wall thickening and villous atrophy (de Paola et al. 1962). Ulcerative enteritis is an ultimate severe form of disease, which presents ulcers with fibrosis and larvae throughout the entire wall, allowing bacterial translocation and subsequent bacteremia and sepsis (Puthiyakunnon et al. 2014; Kishimoto et al. 2008).
Liver and biliary tract disease
Although hepatobiliary manifestations are uncommon in SHS, severe duodenal involvement and inflammation could cause papillary stenosis and jaundice, as reported in previous studies (Keiser and Nutman 2004; Ortega et al. 2010). Autopsy findings of larvae in the gallbladder have also been reported (Basile et al. 2010). Importantly, elevation of liver enzymes could be seen in patients undergoing allogeneic transplantation and mimic the presentation of graft-versus-host disease. Thus, in a case of cholestasis or evidence of hepatic cytolysis, it may be important to screen post-transplant individuals who present with jaundice, abdominal pain, and have a compelling epidemiological background (Izquierdo et al. 2013). Also, acute pancreatitis has also been reported in the context of strongyloidiasis. In these cases, the diagnosis was achieved by assessing the biliary fluid obtained during endoscopic retrograde cholangio-pancreatography (Makker et al. 2015).
Lung disease
Pulmonary involvement is one of the most common sites affected by S. stercoralis and thus, it demands a high level of attention (Geri et al. 2015). A spectrum of clinical manifestations have been described, ranging from asthma-like symptoms such as cough and dyspnea to more severe presentations including pneumonia, diffuse bilateral infiltrates, and consolidations (Alsharif et al. 2015; Upadhyay et al. 2001; Nabeya et al. 2017). Complications such as diffuse alveolar hemorrhage, necrotizing pneumonia, and eosinophilic pleural effusions have also been described, although the data is scarce (Vadlamudi et al. 2006; Keiser and Nutman 2004; Mokhlesi et al. 2004). Asthma-like presentations are among the most challenging due to the resemblance with conventional allergic asthma. This has led to the inappropriate use of steroids as a part of the treatment, which has ultimately led to SHS and adverse outcomes (Alsharif et al. 2015; Upadhyay et al. 2001). The delay of anthelminthic therapy results in worsening respiratory function and massive parasitic reproduction, creating an unbreakable vicious cycle that culminates in death.
Pneumonia and sepsis may develop owing to repeated damage to alveolar membranes by the passage of large amounts of larvae, and due to direct transport of enteric bacteria along with larvae from the gut to the bloodstream (Nabeya et al. 2017). Thus, the host becomes susceptible for bacterial infections and further inflammation. In fact, it is quite frequent to isolate enteric bacteria in sputum samples and blood cultures of patients with SHS with pulmonary manifestations (Basile et al. 2010; Nabeya et al. 2017; Man et al. 2017). Likewise, continuous disruptions of alveolar-capillary membranes may cause inflammatory pneumonitis and alveolar hemorrhage (Mokhlesi et al. 2004). Alterations of the intestinal microbiota due to parasitic infections may hamper the ability of the host to prevent further bacterial translocation (Kinjo et al. 2015; Zaiss and Harris 2016) which again, perpetuates the burden of pneumonia, sepsis and bacteremia.
In terms of radiographic findings in SHS and lung involvement, multiple patterns have been documented to date including migratory pulmonary infiltrates and peripheral consolidations. However, none of them is specific for strongyloidiasis. Chest x-rays showing diffuse ground glass opacities, diffuse patchy infiltrates and consolidations have been associated with advanced disease (Nabeya et al. 2017; Mokhlesi et al. 2004). CT scan imaging has revealed diverse patterns of lung affection including interlobular septal thickening with ground glass opacities, cavitary lesions and abscesses (Nabeya et al. 2017; Mokhlesi et al. 2004; Hübner et al. 2013).
Cardiac disease
Two reports on disseminated strongyloidiasis involving the pericardium have been described in the literature, presenting with right-sided heart failure symptoms and exudative pericardial effusion. In both cases, S. stercoralis larvae were obtained from the pericardial fluid, and they were also detected on histopathology (Fakhar et al. 2010; Lai et al. 2002). These findings could be interpreted as an inflammatory process resulting from the passage of larvae into the pericardial space. Two risk factors associated with this presentation included corticosteroid therapy and poorly controlled diabetes.
Endomyocardial fibrosis and Löffler endocarditis have also been described in the literature although very rarely (Murali et al. 2010; Alizadeh-Sani et al. 2013; Sarangi et al. 2013; Thaden et al. 2013; Nolan et al. 2013). Afflicted patients were otherwise immunocompetent individuals who presented with heart failure symptoms as the most prominent manifestations of disease. Larvae were found in the stool microscopy and cardiac MRI revealed marked signs of endomyocardial fibrosis. Although endomyocardial fibrosis and restrictive cardiomyopathy may result from sustained eosinophil degranulation rather than direct tissue damage by the parasite, this unique situation might reflect repeated cycles of autoinfection with reactive eosinophil-mediated injury.
Renal disease
Dissemination of S. stercoralis larvae to several organs including the kidneys has been documented in autopsy studies (Keiser and Nutman 2004). Most cases of SHS with renal involvement are related to kidney transplant, described in recipients who reactivated a latent infection and in those individuals receiving infected kidney allografts from both living and cadaveric donors (Abdalhamid et al. 2015; Kim et al. 2016; Weiser et al. 2011; Hoy et al. 1981). Larvae have been also isolated in the urine, indicating that S. stercoralis is stable in a variety of tissues and fluids (Pasqualotto et al. 2009). Interestingly, strongyloidiasis has been associated with nephrotic syndrome, particularly due to minimal change disease and focal segmental glomerulosclerosis, presumably due to immune factors inducing podocyte injury and increased protein permeability (Merzkani et al. 2017; Miyazaki et al. 2010). Patients have shown improvement of their clinical status following antiparasitic treatment.
Central nervous system (CNS) disease
CNS involvement also makes the diagnosis of disseminated strongyloidiasis. SHS is characterized by the transport of gram-negative bacteria from the gut into the bloodstream, leading to bacteremia, pneumonia and meningitis (Woll et al. 2013; Greaves et al. 2013; Luvira et al. 2016; Rodríguez et al. 2012; Morgello et al. 1993). Furthermore, larvae of S. stercoralis have been found in cerebrospinal fluid (CSF), meningeal vessels and meningeal spaces (Rodríguez et al. 2012). Meningeal signs and altered mental status are the most common manifestations seen in patients with CNS strongyloidiasis (Woll et al. 2013; Rodríguez et al. 2012; Morgello et al. 1993). Cerebrospinal fluid can exhibit an aseptic meningitis-type picture on biochemical analysis or features of gram-negative meningitis, with CSF cultures that are usually positive for enteric organisms such as Escherichia coli, Proteus mirabilis, Klebsiella pneumoniae, Enterococcus faecalis and Streptococcus bovis (Keiser and Nutman 2004; Woll et al. 2013; Greaves et al. 2013). Among other CNS diseases associated with Strongyloides, it is hypothesized that larvae may cause cerebral perfusion defects through capillary obstruction which is seen on MRI as diffuse infarcts (Marcos et al. 2011). Invariably, once larvae reach the CNS, the damage is irreversible and usually associated with fatal outcomes. Antiparasitic drug levels seem to be insufficient to contain the infection (Keiser and Nutman 2004; Woll et al. 2013; Rodríguez et al. 2012).
Diagnostic approach
High level of suspicion is the cornerstone for early diagnosis to prevent the progression and high mortality of SHS and dissemination. Although the absence of eosinophilia and the presence of important extraintestinal manifestations may deviate the clinical suspicion towards other etiologies, it is important to consider SHS in the differential of patients with multiorgan dysfunction from endemic areas (Vadlamudi et al. 2006; Toledo et al. 2015).
Diagnosis of S. stercoralis can be very complicated in immunocompetent hosts, as the larvae output may be intermittent and in small concentrations (Schär et al. 2013; Barros and Montes 2014). Therefore, a relatively high index of false negative results have been described with conventional stool-based methods (Marcos et al. 2011). On the other hand, individuals who develop SHS with dissemination have a markedly increased parasite burden with presence of larvae in other body fluids and tissues, facilitating the detection to some extent (Vadlamudi et al. 2006; Geri et al. 2015; Toledo et al. 2015). For instance, Geri el al. (Geri et al. 2015) reported a detection rate of 93% in both stool and sputum samples of 133 patients with SHS.
Current diagnostic strategies include: (1) direct visualization of the larvae through body fluid microscopy (stool, sputum, bronchial-alveolar lavage, CSF), (2) direct visualization of the larvae through tissue biopsy, (3) serology, and (4) molecular assays. Among parasitological techniques, the agar plate culture is the most sensitive method (up to 90%) (Puthiyakunnon et al. 2014; Nutman 2017). However, it requires special training for accurate identification of the parasite and preparation of the culture media. Also, the incubation period may be prolonged: up to 72–96 h until the parasite can be visualized under the microscope. This is a limiting factor when there is an urge to make the diagnosis in a timely fashion.
Upper GI endoscopy has shown some utility in the diagnosis of Strongyloides as duodenal biopsy samples can be positive in up to 71.4% of the cases in patients with SHS. However, this procedure may be difficult to perform in a patient who is mechanically ventilated, supported on pressor medications or experiencing active bleeding (Kishimoto et al. 2008). Enzyme-linked immunoassay (ELISA) significantly increases the sensitivity and negative predictive value of the stool microscopy (Toledo et al. 2015). Nonetheless, antibody detection in immunocompromised individuals may be altered, leading to false negative results (Puthiyakunnon et al. 2014; Nutman 2017). Molecular diagnosis through real-time polymerase chain reaction (RT-PCR) has shown promising results on recent studies, achieving a sensitivity as high as 100% upon testing of two stool samples (Buonfrate et al. 2015). Its high specificity owes to pathogen-specific DNA target detection. Conversely, patients may test negative after receive appropriate treatment (Toledo et al. 2015). It may be a suitable test in an acute scenario, however, RT-PCR is not widely available and its cost and lack of technological support may be limiting factors in endemic areas.
Mortality and outcomes
Overall, SHS is a life-threatening condition with morality rates comparable to other serious non-communicable conditions such as acute coronary syndromes. A systematic review of 244 case reports on SHS recorded a fatality rate of 60%. Patients who did not receive any therapy had morality rates as high as 100%, while patients who were treated with albendazole (73%), thiabendazole (51%) and ivermectin (47%) exhibited improved numbers, although still high (Buonfrate et al. 2013). One of the most important factors associated to this high mortality is concomitant bacterial infection that may be detected early or late during the hospital course (Vadlamudi et al. 2006; Geri et al. 2015; Toledo et al. 2015).
Despite the malignant nature of this condition, there are no guidelines for the standardized management of SHS. Oral ivermectin has been considered the standard of care for strongyloidiasis according to the World Health Organization (Barros and Montes 2014; Marcos et al. 2011; Puthiyakunnon et al. 2014; Mejia and Nutman 2012; Dacal et al. 2018); however, the appropriate duration and management of treatment failure has not been addressed in the literature. This is reflected in the variety of therapeutic regimens reported which may presumably contribute to different outcomes as well. Appropriate follow up on this group of patients and the incidence of re-infection or recrudescence of disease are yet to be elucidated in future studies. Thus far, the recommended treatment regimen for a patient with SHS is oral ivermectin 200 μg/kg/day for 2 days, and then repeated during the second and fourth week with later stool microscopy for parasite clearance surveillance (Barros and Montes 2014; Mejia and Nutman 2012). The lack of parenteral formulations may be another constraint. Additionally, clinicians may consider starting broad-spectrum antibiotics in case of further neurological decline and imminent bacteremia.
Conclusion
In conclusion, SHS remains one of the most neglected conditions in tropical and general medicine. Despite its high mortality rate, the paucity of reports, the lack of health policies to identify individuals at risk and the limited diagnostic support may be associated with its under-recognition among physicians in the intensive care unit. Due to the advance of medical therapies including transplant and immunosuppressive chemotherapy, more cases of SHS may be seen in developed countries. The spread of this parasite through donor-specific organs warrants special attention, particularly with the popularization of transplantation. SHS can virtually affect any organ and should be considered in the differential of patients with multiple organ failure refractory to conventional antibiotic therapy, especially when the pertinent epidemiological correlate is present. Survival rates are dramatically affected when appropriate therapy is delayed, and particularly when the CNS has been involved. Ivermectin remains the therapy of choice, although the appropriate dose for CNS disease, management of treatment failure and specific follow up remain obscure. Future studies should focus on affordable screening methods, and easy-to-carry diagnostic techniques that can be applied in developed and developing countries.
Acknowledgements
The authors would like to express their gratitude to the Laboratory of Parasitology at the Alexander von Humboldt Tropical Medicine Institute for their support to the elaboration of this manuscript.
Author’s contribution
GVR and RPR drafted the manuscript. GVR, RPR, JPR, RM, EFR and AT critically reviewed the literature, designed the study and made significant contributions to it. AT provided expert consultation for this topic. All the authors reviewed the manuscript and approved the final version.
Compliance with ethical standards
Conflict of interest
All authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abdalhamid BA, Al Abadi AN, Al Saghier MI, Joudeh AA, Shorman MA, Amr SS. Strongyloides stercoralis infection in kidney transplant recipients. Saudi J Kidney Dis Transplant. 2015;26(1):98–102. doi: 10.4103/1319-2442.148752. [DOI] [PubMed] [Google Scholar]
- Al Malki MM, Song JY. Strongyloides hyperinfection syndrome following haematopoietic stem cell transplantation. Br J Haematol. 2016;172(4):496. doi: 10.1111/bjh.13814. [DOI] [PubMed] [Google Scholar]
- Alizadeh-Sani Z, Vakili-Zarch A, Kiavar M, Bahadorian B, Nabavi A. Eosinophilic endomyocardial fibrosis and Strongyloides stercoralis: a case report. Res Cardiovasc Med. 2013;2(2):104–105. doi: 10.5812/cardiovascmed.9370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alsharif A, Sodhi A, Murillo LC, Headley AS, Kadaria D. Wait!!! no steroids for this asthma. Am J Case Rep. 2015;16:398–400. doi: 10.12659/AJCR.893729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altintop L, Cakar B, Hokelek M, Bektas A, Yildiz L, Karaoglanoglu M. Strongyloides stercoralis hyperinfection in a patient with rheumatoid arthritis and bronchial asthma: a case report. Ann Clin Microbiol Antimicrob. 2010;9:27. doi: 10.1186/1476-0711-9-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anuradha R, Munisankar S, Bhootra Y, Jagannathan J, Dolla C, Kumaran P, et al. Systemic cytokine profiles in Strongyloides stercoralis infection and alterations following treatment. Infect Immun. 2015;84(2):425–431. doi: 10.1128/IAI.01354-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anuradha R, Munisankar S, Dolla C, Kumaran P, Nutman TB, Babu S. Parasite antigen-specific regulation of Th1, Th2, and Th17 responses in Strongyloides stercoralis. J Immunol. 2015;195(5):2241–2250. doi: 10.4049/jimmunol.1500745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barros N, Montes M. Infection and hyperinfection with Strongyloides stercoralis: clinical presentation, etiology of disease, and treatment options. Curr Trop Med Rep. 2014;1(4):223–228. [Google Scholar]
- Barros N, Woll F, Watanabe L, Montes M (2012) Are increased Foxp3 + regulatory T cells responsible for immunosuppression during HTLV-1 infection? Case reports and review of the literature. BMJ Case Report, pp 1–5 [DOI] [PMC free article] [PubMed]
- Basile A, Simzar S, Bentow J, Antelo F, Shitabata P, Peng SK, et al. Disseminated Strongyloides stercoralis: hyperinfection during medical immunosuppression. J Am Acad Dermatol. 2010;63(5):896–902. doi: 10.1016/j.jaad.2009.09.037. [DOI] [PubMed] [Google Scholar]
- Bollela VR, Feliciano C, Teixeira AC, Junqueira AC, Rossi MA. Fulminant gastrointestinal hemorrhage due to Strongyloides stercoralis hyperinfection in an AIDS patient. Rev Soc Bras Med Trop. 2013;46(1):111–113. doi: 10.1590/0037-868215522013. [DOI] [PubMed] [Google Scholar]
- Bonne-Année S, Hess JA, Abraham D. Innate and adaptive immunity to the nematode Strongyloides stercoralis in a mouse model. Immunol Res. 2011;51(2–3):205–214. doi: 10.1007/s12026-011-8258-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonne-Année S, Kerepesi LA, Hess JA, O’Connell AE, Lok JB, Nolan TJ, et al. Human and mouse macrophages collaborate with neutrophils to kill larval Strongyloides stercoralis. Infect Immun. 2013;81(9):3346–3355. doi: 10.1128/IAI.00625-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buonfrate D, Requena-Mendez A, Angheben A, Muñoz J, Gobbi F, Van Den Ende J, et al. Severe strongyloidiasis: a systematic review of case reports. BMC Infect Dis. 2013;13:78. doi: 10.1186/1471-2334-13-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buonfrate D, Formenti F, Perandin F, Bisoffi Z. Novel approaches to the diagnosis of Strongyloides stercoralis infection. Clin Microbiol Infect. 2015;21(6):543–552. doi: 10.1016/j.cmi.2015.04.001. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (2015) Global health, division of parasitic diseases. Parasites-strongyloides, biology. [cited 2018 Aug 28]. https://www.cdc.gov/parasites/strongyloides/biology.html
- Dacal E, Saugar JM, Soler T, Azcárate JM, Jiménez MS, Merino FJ, et al. Parasitological versus molecular diagnosis of strongyloidiasis in serial stool samples: how many? J Helminthol. 2018;92(1):12–16. doi: 10.1017/S0022149X17000050. [DOI] [PubMed] [Google Scholar]
- de Paola, Dias L, Da Silva J (1962) Enteritis due to Strongyloides stercoralis. A report of 5 fatal cases. Am J Dig Dis 7:1086–98 [DOI] [PubMed]
- Fakhar M, Gholami Z, Banimostafavi ES, Madjidi H. Respiratory hyperinfection caused by Strongyloides stercoralis in a patient with pemphigus vulgaris and minireview on diagnosis and treatment of strongyloidiasis. Comp Clin Pathol. 2010;19(6):621–625. [Google Scholar]
- Forrer A, Khieu V, Schär F, Hattendorf J, Marti H, Neumayr A, et al. Strongyloides stercoralis is associated with significant morbidity in rural Cambodia, including stunting in children. PLoS Negl Trop Dis. 2017;11(10):e0005685. doi: 10.1371/journal.pntd.0005685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galioto AM, Hess JA, Nolan TJ, Schad GA, Lee JJ, Abraham D. Role of eosinophils and neutrophils in innate and adaptive protective immunity to larval Strongyloides stercoralis in mice. Infect Immun. 2006;74(10):5730–5738. doi: 10.1128/IAI.01958-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genta RM. Global prevalence of strongyloidiasis: critical review with epidemiologic insights into the prevention of disseminated disease. Rev Infect Dis. 1989;11(5):755–767. doi: 10.1093/clinids/11.5.755. [DOI] [PubMed] [Google Scholar]
- Geri G, Rabbat A, Mayaux J, Zafrani L, Chalumeau-Lemoine L, Guidet B, et al. Strongyloides stercoralis hyperinfection syndrome: a case series and a review of the literature. Infection. 2015;43(6):691–698. doi: 10.1007/s15010-015-0799-1. [DOI] [PubMed] [Google Scholar]
- Glendinning L, Nausch N, Free A, Taylor DW, Mutapi F. The microbiota and helminths: sharing the same niche in the human host. Parasitology. 2014;141(10):1255–1271. doi: 10.1017/S0031182014000699. [DOI] [PubMed] [Google Scholar]
- Greaves D, Coggle S, Pollard C, Aliyu SH, Moore EM. Strongyloides stercoralis infection. BMJ. 2013;347(7919):1–6. doi: 10.1136/bmj.f4610. [DOI] [PubMed] [Google Scholar]
- Hays R, Giacomin P, Olma L, Esterman A, McDermott R. The relationship between treatment for Strongyloides stercoralis infection and type 2 diabetes mellitus in an Australian aboriginal population: a three-year cohort study. Diabetes Res Clin Pract. 2017;134:8–16. doi: 10.1016/j.diabres.2017.09.012. [DOI] [PubMed] [Google Scholar]
- Herrera J, Marcos L, Terashima A, Alvarez H, Samalvides F, Gotuzzo E. Factores asociados a la Infección por Strongyloides stercoralis en individuos de una zona endémica en el Perú. Rev Gastroenterol Peru. 2006;26(4):357–362. [PubMed] [Google Scholar]
- Hoy WE, Roberts NJ, Jr, Bryson MF, Bowles C, Lee JC, Rivero AJ, et al. Transmission of strongyloidiasis by kidney transplant? disseminated strongyloidiasis in both recipients of kidney allografts from a single cadaver donor. JAMA. 1981;246(17):1937–1939. [PubMed] [Google Scholar]
- Hübner MP, Layland LE, Hoerauf A. Helminths and their implication in sepsis—a new branch of their immunomodulatory behaviour? Pathog Dis. 2013;69(2):127–141. doi: 10.1111/2049-632X.12080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Infante R, Terashima A, Maguina C, Tello C, Alvarez H, Gotuzzo E. Estudio clínico parasitológico de pacientes con autoinfección por Strongyloides stercoralis en el Hospital Cayetano Heredia 1973–1991. Rev Gastroenterol Perú. 1998;18:37–41. [Google Scholar]
- Izquierdo I, Briones J, Lluch R, Arqueros C, Martino R. Fatal Strongyloides hyperinfection complicating a gram-negative sepsis after allogeneic stem cell transplantation: a case report and review of the literature. Case Rep Hematol. 2013;2013:860976. doi: 10.1155/2013/860976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen T, Montresor A, Savioli L. Effectively controlling strongyloidiasis. Parasitol Today. 1996;12(4):164. doi: 10.1016/0169-4758(96)80806-4. [DOI] [PubMed] [Google Scholar]
- Keiser PB, Nutman TB. Strongyloides stercoralis in the immunocompromised population. Clin Microbiol Rev. 2004;17(1):208–217. doi: 10.1128/CMR.17.1.208-217.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ketzis JK, Conan A. Estimating occurrence of Strongyloides stercoralis in the Caribbean island countries: implications for monitoring and control. Acta Trop. 2017;171:90–95. doi: 10.1016/j.actatropica.2017.03.037. [DOI] [PubMed] [Google Scholar]
- Khushman M, Morris MI, Diaz L, Goodman M, Pereira D, Fuller K, et al. Syndrome of inappropriate anti-diuretic hormone secretion secondary to Strongyloides stercoralis infection in an allogeneic stem cell transplant patient: a case report and literature review. Transplant Proc. 2017;49(2):373–377. doi: 10.1016/j.transproceed.2016.12.012. [DOI] [PubMed] [Google Scholar]
- Kim JH, Kim DS, Yoon YK, Sohn JW, Kim MJ. Donor-derived strongyloidiasis infection in solid organ transplant recipients: a review and pooled analysis. Transplant Proc. 2016;48(7):2442–2449. doi: 10.1016/j.transproceed.2015.11.045. [DOI] [PubMed] [Google Scholar]
- Kinjo T, Nabeya D, Nakamura H, Haranaga S, Hirata T, Nakamoto T, et al. Acute respiratory distress syndrome due to Strongyloides stercoralis infection in a patient with cervical cancer. Intern Med. 2015;54(1):83–87. doi: 10.2169/internalmedicine.54.3284. [DOI] [PubMed] [Google Scholar]
- Kishimoto K, Hokama A, Hirata T, Ihama Y, Nakamoto M, Kinjo N, et al. Endoscopic and histopathological study on the duodenum of Strongyloides stercoralis hyperinfection. World J Gastroenterol. 2008;14(11):1768–1773. doi: 10.3748/wjg.14.1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai CP, Hsu YH, Wang JH, Lin CM. Strongyloides stercoralis infection with bloody pericardial effusion in a non-immunosuppressed patient. Circ J. 2002;66(6):613–614. doi: 10.1253/circj.66.613. [DOI] [PubMed] [Google Scholar]
- Luvira V, Trakulhun K, Mungthin M, Naaglor T, Chantawat N, Pakdee W, et al. Comparative diagnosis of strongyloidiasis in immunocompromised patients. Am J Trop Med Hyg. 2016;95(2):401–404. doi: 10.4269/ajtmh.16-0068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makker J, Balar B, Niazi M, Daniel M. Strongyloidiasis: a case with acute pancreatitis and a literature review. World J Gastroenterol. 2015;21(11):3367–3375. doi: 10.3748/wjg.v21.i11.3367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Man WH, de Steenhuijsen Piters WA, Bogaert D. The microbiota of the respiratory tract: gatekeeper to respiratory health. Nat Rev Microbiol. 2017;15(5):259–270. doi: 10.1038/nrmicro.2017.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marathe A, Date V. Strongyloides stercoralis hyperinfection in an immunocompetent patient with extreme eosinophilia. J Parasitol. 2008;94(3):759–760. doi: 10.1645/GE-1392.1. [DOI] [PubMed] [Google Scholar]
- Marcos LA, Terashima A, DuPont HL, Gotuzzo E. Strongyloides hyperinfection syndrome: an emerging global infectious disease. Trans R Soc Trop Med Hyg. 2008;102(4):314–318. doi: 10.1016/j.trstmh.2008.01.020. [DOI] [PubMed] [Google Scholar]
- Marcos LA, Terashima A, Canales M, Gotuzzo E. Update on strongyloidiasis in the immunocompromised host. Curr Infect Dis Rep. 2011;13(1):35–46. doi: 10.1007/s11908-010-0150-z. [DOI] [PubMed] [Google Scholar]
- Mazhar M, Ali IA, Agudelo Higuita NI. Strongyloides hyperinfection in a renal transplant patient: always be on the lookout. Case Rep Infect Dis. 2017;2017:2953805. doi: 10.1155/2017/2953805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mejia R, Nutman TB. Screening, prevention, and treatment for hyperinfection syndrome and disseminated infections caused by Strongyloides stercoralis. Curr Opin Infect Dis. 2012;25(4):458–463. doi: 10.1097/QCO.0b013e3283551dbd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merzkani M, Uppal NN, Ross DW, Gautam-Goyal P, Malhotra P, Shah HH, et al. Strongyloides stercoralis-associated tip variant focal segmental glomerulosclerosis. Kidney Int Rep. 2017;3(1):14–18. doi: 10.1016/j.ekir.2017.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki M, Tamura M, Kabashima N, Serino R, Shibata T, Miyamoto T, et al. Minimal change nephrotic syndrome in a patient with strongyloidiasis. Clin Exp Nephrol. 2010;14(4):367. doi: 10.1007/s10157-010-0273-4. [DOI] [PubMed] [Google Scholar]
- Mokhlesi B, Shulzhenko O, Garimella PS, Kuma L, Monti C. Pulmonary Strongyloidiasis: the varied clinical presentations. Clin Pulm Med. 2004;11(1):6–13. doi: 10.1097/01.cpm.0000107609.50629.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montes M, Sanchez C, Verdonck K, Lake JE, Gonzalez E, Lopez G, et al. Regulatory T cell expansion in HTLV-1 and strongyloidiasis co-infection is associated with reduced IL-5 responses to Strongyloides stercoralis antigen. PLoS Negl Trop Dis. 2009;3(6):4–11. doi: 10.1371/journal.pntd.0000456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgello S, Soifer FM, Lin CS, Wolfe DE. Central nervous system Strongyloides stercoralis in acquired immunodeficiency syndrome: a report of two cases and review of the literature. Acta Neuropathol. 1993;86(3):285–288. doi: 10.1007/BF00304143. [DOI] [PubMed] [Google Scholar]
- Murali A, Rajendiran G, Ranganathan K, Shanthakumari S. Disseminated infection with Strongyloides stercoralis in a diabetic patient. Indian J Med Microbiol. 2010;28(4):407–408. doi: 10.4103/0255-0857.71854. [DOI] [PubMed] [Google Scholar]
- Nabeya D, Haranaga S, Parrott GL, Kinjo T, Nahar S, Tanaka T, et al. Pulmonary strongyloidiasis: assessment between manifestation and radiological findings in 16 severe strongyloidiasis cases. BMC Infect Dis. 2017;17(1):320. doi: 10.1186/s12879-017-2430-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolan MT, Ali F, Sultan T, Mcgavigan AD, Selvanayagam JB, Joseph MX. Loeffler’s endocarditis caused by Strongyloides infection. Int J Cardiol. 2013;164(3):19–21. doi: 10.1016/j.ijcard.2012.09.141. [DOI] [PubMed] [Google Scholar]
- Nutman TB. Human infection with Strongyloides stercoralis and other related Strongyloides species. Parasitology. 2017;144(3):263–273. doi: 10.1017/S0031182016000834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortega CD, Ogawa NY, Rocha MS, Blasbalg R, Caiado AH, Warmbrand G, et al. Helminthic diseases in the abdomen: an epidemiologic and radiologic overview. Radiographics. 2010;30(1):253–267. doi: 10.1148/rg.301095092. [DOI] [PubMed] [Google Scholar]
- Pasqualotto AC, Zborowski MF, dos Anjos M, Poloni JA, dos Santos AP, Torelly AP. Strongyloides in the urine. Trans R Soc Trop Med Hyg. 2009;103(1):106–107. doi: 10.1016/j.trstmh.2008.08.011. [DOI] [PubMed] [Google Scholar]
- Puthiyakunnon S, Boddu S, Li Y, Zhou X, Wang C, Li J, et al. Strongyloidiasis—an insight into its global prevalence and management. PLoS Negl Trop Dis. 2014;8(8):e3018. doi: 10.1371/journal.pntd.0003018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro SR, Covre LP, Stringari LL, da Penha Zago-Gomes M, Gomes DC, Pereira FE. Peripheral blood CD4 +/CD25 + regulatory T cells in alcoholic patients with Strongyloides stercoralis infection. Parasitol Res. 2017;116(3):1071–1074. doi: 10.1007/s00436-016-5355-0. [DOI] [PubMed] [Google Scholar]
- Rodríguez M, Flores P, Ahumada V, Vázquez-Vázquez L, Alvarado-de la Barrera C, Reyes-Terán G. Central nervous system strongyloidiasis and cryptococcosis in an HIV-infected patient starting antiretroviral therapy. Case Rep Med. 2012;2012:575470. doi: 10.1155/2012/575470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvador F, Molina I, Sulleiro E, Burgos J, Curran A, Van den Eynde E, et al. Tropical diseases screening in immigrant patients with human immunodeficiency virus infection in Spain. Am J Trop Med Hyg. 2013;88(6):1196–1202. doi: 10.4269/ajtmh.12-0714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarangi S, Phillips SD, Burkhart HM. Strongyloides-associated left ventricular thrombus. Ann Thorac Surg. 2013;96(4):1487. doi: 10.1016/j.athoracsur.2013.01.089. [DOI] [PubMed] [Google Scholar]
- Schaible UE, Kaufmann SH. Malnutrition and infection: complex mechanisms and global impacts. PLoS Med. 2007;4(5):e115. doi: 10.1371/journal.pmed.0040115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schär F, Trostdorf U, Giardina F, Khieu V, Muth S, Marti H, et al. Strongyloides stercoralis: global distribution and risk factors. PLoS Negl Trop Dis. 2013;7(7):e2288. doi: 10.1371/journal.pntd.0002288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel MO, Simon GL. Is human immunodeficiency virus infection a risk factor for Strongyloides stercoralis hyperinfection and dissemination. PLoS Negl Trop Dis. 2012;6(7):7–9. doi: 10.1371/journal.pntd.0001581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva ML, Inês Ede J, Souza AB, Dias VM, Guimarães CM, Menezes ER, et al. Strongyloides stercoralis infection and cortisol secretion in alcoholic patients. Acta Trop. 2016;154:133–138. doi: 10.1016/j.actatropica.2015.11.010. [DOI] [PubMed] [Google Scholar]
- Tahapary DL, de Ruiter K, Martin I, Brienen EAT, van Lieshout L, Djuardi Y, et al. Effect of anthelmintic treatment on leptin, adiponectin and leptin to adiponectin ratio: a randomized-controlled trial. Nutr Diabetes. 2017;7(10):e289. doi: 10.1038/nutd.2017.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takele Y, Adem E, Getahun M, Tajebe F, Kiflie A, Hailu A, et al. Malnutrition in healthy individuals results in increased mixed cytokine profiles, altered neutrophil subsets and function. PLoS ONE. 2016;11(8):1–18. doi: 10.1371/journal.pone.0157919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teixeira MC, Pacheco FT, Souza JN, Silva ML, Inês EJ, Soares NM. Strongyloides stercoralis infection in alcoholic patients. Biomed Res Int. 2016;2016:4872473. doi: 10.1155/2016/4872473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terashima A, Alvarez H, Tello R, Infante R, Freedman DO, Gotuzzo E. Treatment failure in intestinal strongyloidiasis: an indicator of HTLV-I infection. Int J Infect Dis. 2002;6(1):28–30. doi: 10.1016/s1201-9712(02)90132-3. [DOI] [PubMed] [Google Scholar]
- Thaden J, Cassar A, Vaa B, Phillips S, Burkhart H, Aubry M, et al. Eosinophilic endocarditis and Strongyloides stercoralis. Am J Cardiol. 2013;112(3):461–462. doi: 10.1016/j.amjcard.2013.03.053. [DOI] [PubMed] [Google Scholar]
- Toledo R, Muñoz-Antoli C, Esteban JG. Strogyloidiasis with emphasis on human infections and its different clinical forms. Adv Parasitol. 2015;88:165–241. doi: 10.1016/bs.apar.2015.02.005. [DOI] [PubMed] [Google Scholar]
- Upadhyay D, Corbridge T, Jain M, Shah R. Pulmonary hyperinfection syndrome with Strongyloides stercoralis. Am J Med. 2001;110(2):167–169. doi: 10.1016/s0002-9343(01)00708-2. [DOI] [PubMed] [Google Scholar]
- Vadlamudi RS, Chi DS, Krishnaswamy G. Intestinal strongyloidiasis and hyperinfection syndrome. Clin Mol Allergy. 2006;4:8. doi: 10.1186/1476-7961-4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe K, Noda K, Hamano S, Koga M, Kishihara K, Nomoto K, et al. The crucial role of granulocytes in the early host defense against Strongyloides ratti infection in mice. Parasitol Res. 2000;86(3):188–193. doi: 10.1007/s004360050030. [DOI] [PubMed] [Google Scholar]
- Weiser JA, Scully BE, Bulman WA, Husain S, Grossman ME. Periumbilical parasitic thumbprint purpura: strongyloides hyperinfection syndrome acquired from a cadaveric renal transplant. Transpl Infect Dis. 2011;13(1):58–62. doi: 10.1111/j.1399-3062.2010.00516.x. [DOI] [PubMed] [Google Scholar]
- Wirk B, Wingard JR. Strongyloides stercoralis hyperinfection in hematopoietic stem cell transplantation. Transpl Infect Dis. 2009;11(2):143–148. doi: 10.1111/j.1399-3062.2008.00360.x. [DOI] [PubMed] [Google Scholar]
- Woll F, Gotuzzo E, Montes M. Strongyloides stercoralis infection complicating the central nervous system. Handb Clin Neurol. 2013;114:229–234. doi: 10.1016/B978-0-444-53490-3.00017-0. [DOI] [PubMed] [Google Scholar]
- Zaiss MM, Harris NL. Interactions between the intestinal microbiome and helminth parasites. Parasite Immunol. 2016;38(1):5–11. doi: 10.1111/pim.12274. [DOI] [PMC free article] [PubMed] [Google Scholar]