Abstract
Cryptosporidiosis is a zoonotic disease caused by a well-known parasitic protozoan called Cryptosporidium. Infection in livestock causes important economic losses among farm animals and its control has a global concern. In this study, internal white and external red layers were separated from pomegranate peels (Punica granatum) then; they were grinded to reach Nano form. Anticryptosporidial effect of their water extracts was investigated in experimentally infected mice. Also, their antioxidant activity, biochemical and histopathological changes were studied. Briefly, hot aqueous extracts of pomegranate peels were prepared regarding its good sensory attributes at concentration of 10% W/V. Analysis of total phenolics, individual phenolics by HPLC-DAD and antioxidant activities have been done. Forty-five mice were divided into five groups each one containing nine mice. The first group was healthy mice and the 2nd one was infected orally with 104Cryptosporidium parvum (C. parvum) oocysts/mice and not treated. The other 3 groups were infected and orally treated with Nitazoxanide (NTZ) for the 3rd group, pomegranate red peel extract for the 4th group and pomegranate white peel extract for the 5th group. Blood samples were collected after 1 and 2 weeks post treatment for protein profile, liver enzymes and antioxidant activity evaluation. After 3 weeks, all animals were sacrificed and ileal tissues were embedded in paraffin for histopathological examination. The results showed that pomegranate peel extracts were rich in phenolic compounds, had high antioxidant activity and therapeutic effect on C. parvum in experimentally infected mice. Red peel extract diminished C. parvum oocysts count significantly in experimentally infected mice than white peel and NTZ treatments. Also, the histopathological examination revealed that red peel treated mice ileal sections showed a great enhancement in the shape and structure of villi towards normal structure than other treated groups. Most of the measured biochemical parameters after 2 weeks’ treatment with red pomegranate peel and NTZ were enhanced in their concentrations towards the healthy normal status. In conclusion, this study showed the effectiveness of Nano-form of pomegranate white and red peel extracts against C. parvum oocysts. Pomegranate red peel extract was found to have antioxidant activity that could significantly enhance the serum biochemical parameters and oxidative stress towards the healthy normal status. Furthermore, it is suggested that pomegranate peel should be separated and used in the daily animal diet or as a functional beavarage for human as accepted from the panelists to give protective effects against this parasite as well as to improve health benefits.
Keywords: Cryptosporidium parvum, Therapeutic effect, Pomegranate peel, Nano-form, Antiparasitic beverage, Biochemical parameters, Antioxidant activity
Introduction
Cryptosporidium is a protozoan parasite that is found all over the world in humans and animals (Pumipuntu and Piratae 2018). It causes diarrhea and gradually decreases with age; the adults might harbor the parasite without any symptoms (Morsy et al. 2014). Infection of livestock with Cryptosporidium can result in mortality, retarded growth of the animals, the cost of drugs and veterinary assistance, decreased production and loss of income for the livestock sector (Shafiq et al. 2015). Control of cryptosporidiosis remained a global challenge in both veterinary and human medicine. Many agents had been tested and there was no effective or approved treatment for cryptosporidiosis. In the scope of food safety and public health, plants and their compounds offered an alternative approach to treat parasitic diseases (Hegazi et al. 2018). Some reports tested the effect of plant extracts in the treatment of cryptosporidiosis such as: garlic (Abdel Megeed et al. 2015), cinnamon and onion (Abu El Ezz et al. 2011) and pomegranate (Al-Mathal and Alsalem 2013).
Many studies were conducted to examine the effect of Punica granatum (P. granatum) in treating many diseases and microbial infections. Some of these studies indicated that P. granatum had anticestodal, antinematodal (Abdel-Ghaffar et al. 2010) and antiprotozoan activities (Dell’Agli et al. 2009). Al-Mathal and Alsalem (2012) reported that P. granatum peel is a promising treatment for Cryptosporidium parvum (C. parvum)—induced cryptosporidiosis that did not induce any side effects. There is a need for development of pomegranate both as pharmaceutical as well as dietary supplement (Wang et al. 2010). Fruit peel of P. granatum had protective effects against coccidiosis as well as it possesses an anthelmintic activity (Dkhil 2013). Punica peel extract counteracted the Eimeria papillata-induced loss of the total antioxidant capacity (Amer et al. 2015). The pomegranate peel which is normally considered to be a waste is an important antidiarrheal and antidiabetic agent (El Mageid et al. 2016).
Nano-suspensions can be prepared by top-down processes such as media milling and high-pressure homogenization. The optimum particle size of Nano suspensions for parenteral application depends on the desired biopharmaceutical properties. Rapid clearance by the phagocytic cells of liver, spleen and bone marrow is achieved in the case of particles > 150 nm (Lockman et al. 2002).
Therefore, in this study anticryptosporidial effect of P. granatum Nano-form red and white peel extracts were investigated in mice model and their effect on the histopathological and biochemical changes as well as antioxidant activity were studied.
Materials and methods
Ethical approval
Experimental animals were housed in good conditions at specific cages in the animal house of the National Research Centre (NRC), Egypt, with free access of food and water. At the end of the experiment, mice were slaughtered rapidly and painlessly. Small intestine parts were collected after mice slaughtering followed by hygienic disposal of the carcasses. The study protocol was approved by the Medical Research Ethics Committee of NRC, Egypt with the number (18–117).
Plant material
- Pomegranate Fruits: P. granatum were purchased from local market, Cairo, Egypt.
-
1.1Preparation of pomegranate peels The pomegranate was washed and then peeled off, separated into internal white and external red layers of peels. The peels were cut into small pieces, drained and dried on perforated trays by using the microwave (Samsung, Model MF245) with oven, air temperature 40 °C for 6 min (Mahmoud et al. 2018).
-
1.2Milling The pomegranate peels were carefully prepared with the separation of the internal white peel from the outer red peel. The samples were grinded so fine and a special mill (Braun KMM 30 mill), type 3045, CombiMax (Germany) then high energy planetary ball mill type MTI SFM (QM-3SP2) 250 rpm rotation was used to get ultra-fine powder (Nano form). The dried samples peels were dissolved in water with concentration of 1 g/10 ml hot water.
-
1.1
Determination of proximate composition Chemical analysis of the pomegranate peel and was evaluated, namely moisture, protein, ash, fat and crude fiber according to the methods described in the AOAC (2000).
Droplet size measurement Droplet size of Nano- and double emulsions were analyzed using a dynamic light scattering method (Zetasizer Nano Zs, Malvern Instrument, Malvern, UK). To avoid multiple scattering, all samples diluted using 0.1% water. For microstructure analysis, some images were through a Zeiss optical microscope (Germany) and analyzed by Image J software (Hosseini et al. 2015).
Determination of total phenolic compounds Peels powder (10 g) was extracted with 100 ml of hot water in an ultrasonic device (200 W, 59 kHz, Shanghai Kudos Sonication Machine Company Ltd., China) for 60 min at room temperature (Hayat et al. 2010). The Folin–Ciocalteu assay, adapted from Ramful et al. (2011) was used for the determination of total phenolics.
- Antioxidant activities
-
5.11,1-diphenyl-2-picrylhydrazyl (DPPH) radical scavenging activity Estimated according to the procedure described by Hayat et al. (2010) with some modifications. An aliquot of 0.5 mL of sample solution was mixed with 1 mL of methanolic solution of DPPH (0.2 mM). The mixture was incubated for 30 min in the darkness at room temperature. The absorbance was measured at 517 nm with spectrophotometer. Control was conducted with ethanol instead of sample. DPPH scavenging capacity was calculated by the equation:
where Ac and As are the absorbance’s at 517 nm of the control and sample, respectively. -
5.2Trolox equivalent antioxidant capacity (TEAC) assay The ABTS free radical was assayed according to Ventura et al. (2013) based on the ability of antioxidants to reduce with ABTS.+.
-
5.3Ferric reducing power (FRAP) assay The FRAP assay (Barros et al. 2012) was based on the ability of phenolics to reduce Fe3+ to Fe 2+.
-
5.4Determination of reducing power (RP) The reducing power of the extracts was measured according to the method of (Oyaizu 1986).
-
6.Determination of polyphenols by HPLC-DAD The HPLC system was an Agilent 1260 equipped with a quaternary pump, online degasser, autosampler and. A waters HPLC system consisting of a 600 controller pump, a temperature control Module, diode-array detector (DAD) water. Chromatographic separation was achieved on Zorbax (Luna 5 µ C18 (2) column (250 × 4.6 mm). The column temperature was maintained at 35 °C and the flow rate was 1 ml min−1, using water (A) and 0.02% trifloroacetic acid in acetonitrile (B) as the mobile phases at a flow rate of 1 ml/min. The elution programmer used was as follows: 0 min (80% A); 0–5 min (80% A); 5–8 min (40% A); 8–12 min (50% A); 12–14 min (80% A) and 14–16 min (80% A). DAD analysis was carried out at three different wave length (280, 270 and 254 nm) and calculation of peak area was at 270 nm wave length chromatogram.
-
7.Pomegranate beverage
-
7.1Preparation Pomegranate beverage was prepared by dissolving the powder in boiling water with percentages of 0.5, 1, 1.5 and 2% W/V.
-
7.2Sensory evaluation The sensory quality of the pomegranate peels beverage was evaluated according to Mahmoud et al. 2017 in terms of appearance, aroma, taste, odor and overall acceptability by an evaluation group composed of 10 trained members. The samples approximately 100 mL each were served in clear glass cups. All evaluations were performed in a well-controlled lab. at the Dept. of Food Tech, NRC. The results were presented as a score of 10 degrees for each parameter, and the full score was 50.
-
7.1
-
5.1
Experimental infection
Animals A total number of 45 male albino mice obtained from a colony at NRC, Egypt, 2–3 weeks of age, weighing 20–25 g were used. They were housed in good ventilated cages with perforated covers, supplied with standard pellet food and water.
Preparation of C. parvum oocysts C. parvum oocysts were collected from naturally-infected calf feces. Oocysts were concentrated by floatation in Sheather’s sugar solution (Current and Reese 1986) and identified by modified Ziehl–Neelsen staining technique (Henriksen and Pohlenz 1981). Sedimented oocysts were collected and stored in a 2.5% potassium dichromate solution at 4 °C. Before experiment, oocysts were concentrated and counted in a PBS solution using a hemocytometer.
Infection Mice were divided into five groups each one containing nine mice. The first group was healthy mice, non-infected, non-treated, the 2nd was experimentally infected (104C. parvum oocysts), non-treated, the 3rd was infected and treated with Cryptonaz® (100 mg Nitazoxanide) drug, the 4th was infected and treated with pomegranate red peel extract and the 5th group was infected and treated with pomegranate white peel water extract. Therapeutic dose of pomegranate peel extracts was 3 g/kg body weight were prepared freshly as 3 gm/ml peel powder in distelled water according to Al-Mathal and Alsalem (2013) and administered daily by gastric tubes 1 h before meals and for 5 consecutive days. Therapeutic doses were administered to the treated animals at the day 3 post inoculation (PI). All healthy and infected mice were sacrificed at the day 24 PI.
Fecal analysis and oocyst shedding Fecal samples were collected from the third day PI till the end of the experiment for the determination of the number of Cryptosporidium oocysts output counted for each group in 50 fields (oil immersion).
Histopathological studies
At the end of experiment, Ilea of all mice were fixed directly in 10% formalin for 24 h, dehydrated, cleared, embedded in paraffin, sectioned at 4μm and stained with H&E staining according to Bancroft and Stevens (1990).
Biochemical examination
Blood samples were collected 1- and 2-weeks post treatment, placed in plain centrifuge tubes for serum separation and used for biochemical analysis. Serum concentrations of total protein (Biuret method) and albumin were determined by coloremetric method (Cannon et al. 1974; Doumas et al. 1971) using SPECTRUM kits (BioMerieux, SA). Globulin and albumin globulin (A/G) ratio were calculated. Lipogram biochemical value was determined by measuring serum cholesterol by enzymatic colorimetric method (Zohreh et al. 2012) using a Linear Chemicals Kits. Various liver enzymes like alanine aminotransferase (ALT), aspartate aminotransferase (AST) were determined by UV enzymatic colorimetric method according to the standardized method described by Winn-Deen et al. (1988) using Linear Chemicals.S.L. Kits. The colorimetric reaction was measured using spectrophotometer.
Serum antioxidant activity
Serum activities of glutathione peroxidase (GSH-Px), catalase (CAT) as well as total antioxidant capacity (TAC) were measured using SPECTRUM kits (BioMerieux, SA). Absorbances were measured at 340 nm, 520 nm and 560 nm, respectively, by spectrophotometer.
Statistical analysis
Data of pomegranate analysis were statistically subjected to analysis of variance (ANOVA); t-test and means separation were by Snedecor and Cochran (1980). The least significant difference (L.S.D.) value was used to determine significant difference between means and to separate means at p < 0.05. Statistical significance of the biochemical examination results and serum antioxidant activity was determined using t-tests according to Petrie and Watson (1999). Data were presented as mean ± standard error (SE). All results were analyzed using Statistical SPSS for Windows, issue 15.8.
Results
Proximate composition of pomegranate peels
Table 1 showed that there was a significant difference in the composition between the internal white peel and red peel, as the red peel contained a higher proportion of fat, ash and fiber, while white peel contained higher content of moisture and protein.
Table 1.
Proximate composition of pomegranate peels processing by-products (% dw)
| Components | Moisture | Ash | Protein | Fat | Crude fiber | Total carbohydrates* |
|---|---|---|---|---|---|---|
| Red peel | 4.66 ± 1.01b | 4.2 ± 0.05a | 2.82 ± 0.04b | 4.47 ± 0.03a | 18.98 ± 1.8a | 64.87 |
| White peel | 6.50 ± 0.5a | 3.70 ± 0.03b | 4.41 ± 0.02a | 2.51 ± 0.02b | 17.26 ± 2.42a | 65.62 |
| F value | 7.72 | 193.6 | 3787.01 | 8885.77 | 0.975 | – |
| LSD | 0.0186 | 0.1004 | 0.072 | 0.058 | – | – |
All values are means of triplicate determinations ± standard deviation (SD)
Means within the same column with different letters are significantly different (P <0.05)
*Total carbohydrates: calculated by differences (100—Sum of all components)
Droplet size measurement
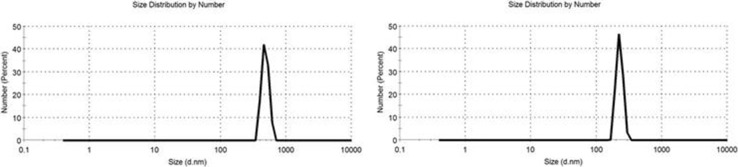
The droplet size of the test samples Fig. 1 showed that both red and white pomegranate peel powder had narrow size distribution and small diameters, while red peel had particle size of 226 nm and white peel had 571 nm.
Fig. 1.
Particle size distribution of pomegranate peel powder
Total phenolic compounds
There was a significant (p ≤ 0.05) difference between red and white peel in total phenolic content which was ranged from 180.5 mg gallic acid/gm for red peel to 209.5 mg gallic acid/gm for white (Table 2).
Table 2.
Total phenolic content and antioxidant activities of pomegranate peels
| PH | Total phenols mg gallic acid/g | % scavenging activities DPPH | TPTZ mg Trolox eq/gm sample | Reducing power (peroxide radical) | |
|---|---|---|---|---|---|
| Red Peel | 3.91 | 180.5 ± 5.9b | 54.4 ± 0.86b | 1.268 ± 0.166a | 17.87 ± 0.25b |
| White Peel | 3.53 | 209.5 ± 2.1a | 75 ± 0.77a | 1.096 ± 0.73b | 20.9 ± 0.802a |
| F value | – | 62.97 | 83.355 | 116.62 | 39.237 |
| LSD | – | 10.159 | 5.729 | 0.048 | 1.344 |
All values are means of triplicate determinations ± standard deviation (SD)
Means within the same column with different letters are significantly different (P <0.05)
Antioxidant activities
Antioxidant activity of red and white peels screened by DPPH, TBTZ and reducing power was shown in Table 2. There were significant differences (p ≤ 0.05) between the antioxidant activities of red and white layers of peels, where, white peel had more antioxidant activities than red peel.
Characterization of phenolic compounds by HPLC
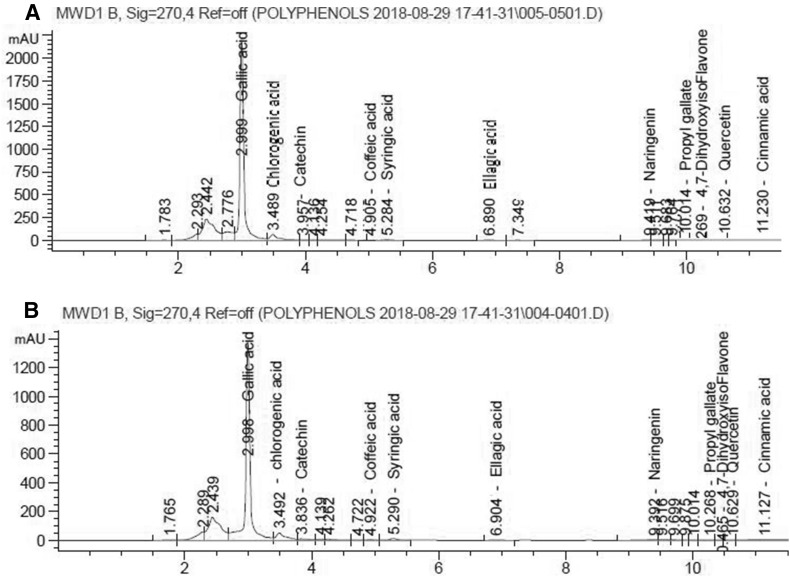
Figure 2 showed that there were 11 phenolic compounds identified by HPLC-DAD. Gallic acid was found to be the main component of phenolic represents 3.645 mg/gm sample in red peel and 4.9687 mg/gm sample in white peel followed by chlorogenic acid (0.3425 mg/gm sample in red peel and 0.3572 mg/gm sample in white peel). Red peel was rich in catechin, quercetin, caffein, coffiec acid, syringic acid and naringenin, while white peel was rich in gallic acid, chlorogenic acid and cinnamic acid.
Fig. 2.
HPLC chromatogram of red and white peels, Peaks appeared with its retention time sequentially of each phenolic acid
Sensory evaluation of pomegranate peel beverage
There was a significant difference (p ≤ 0.05) between red and white peels in sensory characteristics. There was a convergence of color and taste characteristics in the small concentrations of 0.5 and 1%, but in the higher concentrations, there was a significant difference between the sensory characteristics of the white and red peels. In general, concentration 1% was more accepted in all attributes and red pomegranate peel was more accepted by having scores more than white peel (Table 3).
Table 3.
Sensory evaluation of pomegranate peel beverage (Percentages of 0.5, 1, 1.5 and 2% W/V)
| Samples | Color (10) | Taste (10) | Flavor (10) | Overall acceptability (10) | ||||
|---|---|---|---|---|---|---|---|---|
| Red | White | Red | White | Red | White | Red | White | |
| 0.5% | 8.9*a ± 0.32 | 8.4a ± 0.52 | 8.6a ± 0.52 | 8.4a ± 0.52 | 8.9*a ± 0.32 | 8.6a ± 0.52 | 8.7a ± 0.5 | 8.5a ± 0.5 |
| 1% | 8.9*a ± 0.32 | 8.3a ± 0.48 | 8.1b ± 0.35 | 8.0a ± 0.47 | 8.2b ± 0.632 | 8.0b ± 0.47 | 8.5*a ± 0.5 | 8.2a ± 0.4 |
| 1.5% | 8.1*b ± 0.35 | 7.7b ± 0.48 | 6.7c ± 0.5 | 6.7b ± 0.48 | 7.4c ± 0.52 | 7.2c ± 0.42 | 7.3*b ± 0.48 | 7.2b ± 0.4 |
| 2% | 5.6*c ± 0.32 | 6.0c ± 0.471 | 6.2*d ± 0.42 | 5.8c ± 0.42 | 6.3d ± 0.48 | 6.3d ± 0.48 | 5.8*c ± 0.4 | 5.1b ± 0.316 |
| F value | 88.612 | 51.627 | 66.17 | 63.518 | 49.866 | 44.259 | 77.312 | 188.912 |
| LSD | 0.347 | 0.446 | 0.403 | 0.433 | 0.457 | 0.433 | 0.439 | 0.391 |
All values are mean ± standard deviation (SD)
“*”Data within the same row for each parameter are significantly different (P < 0.05), shown by t-test
Data within the same column with different small letters are significantly different (P <0.05)
Therapeutic effect of red, white peel water extracts and NTZ on C. parvum oocyst shedding in experimentally infected mice
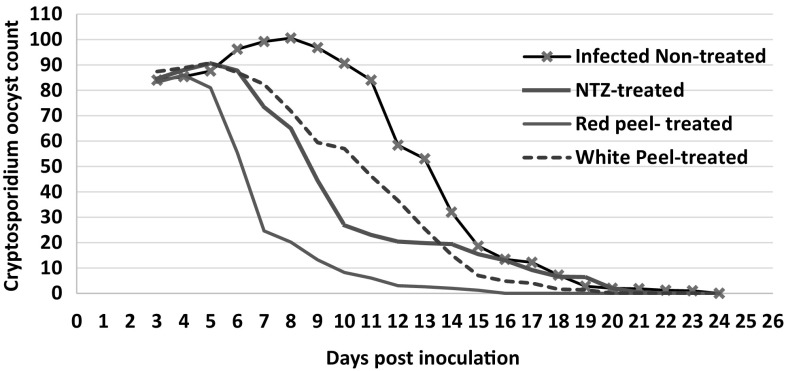
Examination of fecal smears for detection of C. parvum oocysts in 50 microscopic fields revealed that there was a gradual reduction in oocyst shedding in the infected non-treated group from the 9th day PI and continued till almost no oocysts found at day 24 PI. Also, all infected treated groups shed oocysts from the 3rd day PI and counts started to decrease from the 6th day PI (the 3rd day after treatments). There was a statistically significant reduction (P <0.05) in oocyst shedding in red peel, white peel and NTZ treated groups till reaching negligible numbers of oocysts or no oocysts found at days 15, 19 and 21 PI, respectively. Red peel extract diminished C. parvum oocysts count significantly (P <0.05) in experimentally infected mice more than white peel and NTZ treatments in almost all days PI (Fig. 3).
Fig. 3.
Cryptosporidium oocyst shedding in treated and non-treated experimentally infected mice
Histopathological examination findings
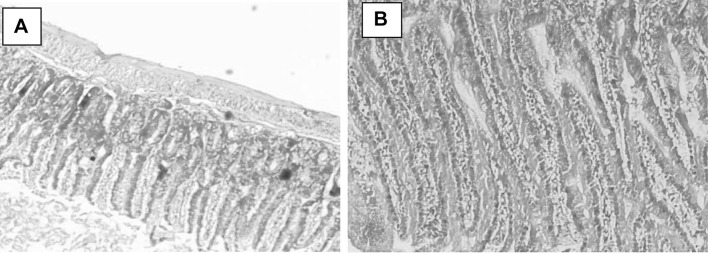
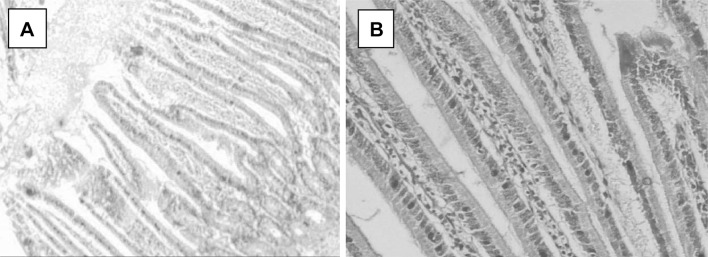
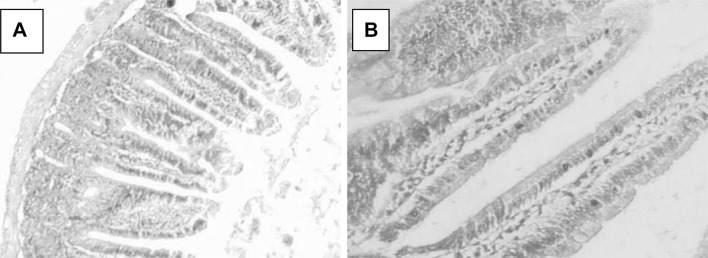
All observations were compared with healthy non-treated animal intestinal tissues (Fig. 4a, b). Light microscopic examination of C. parvum-infected ileal sections revealed the presence of altered mucosal architecture, with infiltration, sloughing and complete erosion of epithelial cells and shortening, blunting, stunting and atrophy of the intestinal villi (Fig. 5a, b). The villi of the infected treated groups; NTZ, (Fig. 6a, b), red peel (Fig. 7a, b), and white peel (8a, b) treated groups; showed slight or no pathological changes and regained their normal appearance like healthy non-infected mice. In red peel treated group, lower histopathological changes than other treated groups were found and a great enhancement was found in the shape and structure of villi with the presence of goblet cells.
Fig. 4.
Ileal tissue sections in a healthy mouse showing normal structures, H&E, a: X40, b: X100
Fig. 5.
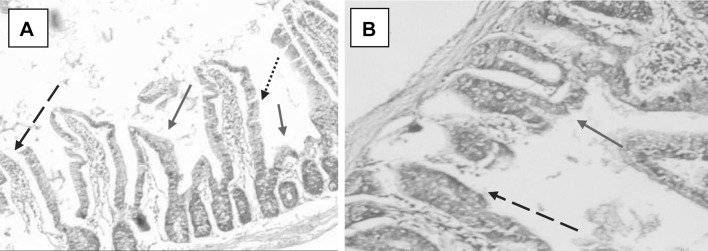
Ileal tissue sections in Cryptosporidium experimentally infected mouse showing shortening of villi (red arrows), sloughing and complete erosion of epithelial cells (dashed arrow) and goblet cells (dotted arrow) H&E, a: X40, b: X100
Fig. 6.
Ileal tissue sections of NTZ-treated Cryptosporidium experimentally infected mouse showing slight enhancement of the shape and structure of villi (solid arrows) and goblet cells (dotted arrows), H&E, a: X40, b: X100
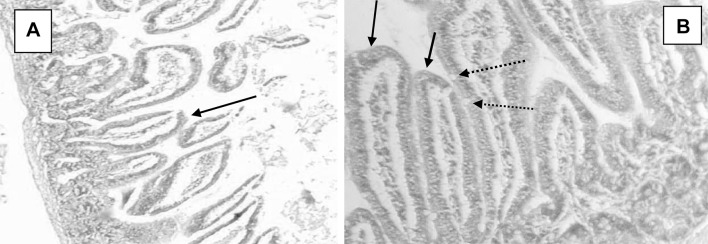
Fig. 7.
Ileal tissue sections of red peel-treated Cryptosporidium experimentally infected mouse showing great enhancement of the shape of villi tending to normal structure, H&E, a: X40, b: X100
Fig. 8.
Ileal tissue sections of white peel-treated Cryptosporidium experimentally infected mouse showing little enhancement of the shape and structure of villi, H&E, a: X40, b: X100
Biochemical examination results
Protein profile expressed in Table 4 showed a significant (P < 0.01) decrease in serum albumin and a significant (P < 0.01) increase in serum globulin concentrations in C. parvum experimentally infected mice than the healthy mice. After treatment for 1 week, it was clear that albumin concentrations significantly (P < 0.05) increased in treated either with NTZ or red pomegranate peel comparing to non-treated experimentally infected mice. However, globulin concentrations significantly (P < 0.05) decreased in red pomegranate peel treated mice. Concerning the liver enzymes, there was a significant (P < 0.01) elevation in ALT and AST levels in C. parvum experimentally infected mice compared to healthy mice. After one-week of treatments, AST levels significantly (P < 0.05) decreased in red pomegranate peel treated mice comparing to infected non-treated mice.
Table 4.
Effect of one-week post treatment by NTZ, red and white pomegranate peel on serum protein profile and liver enzymes in healthy and Cryptosporidium experimentally infected mice
| Parameters | Animals | ||||
|---|---|---|---|---|---|
| Healthy mice | Cryptosporidium experimentally infected mice | ||||
| Infected non-treated | One-weeks post treatment | ||||
| NTZ | Red pomegranate peel | White pomegranate peel | |||
| Total Protein (gm %) | 7.49 ± 0.74 | 6.15 ± 0.42 | 6.21 ± 0.62 | 7.04 ± 0.61 | 6.28 ± 0.34 |
| Albumin (gm %) | 3.72 ± 0.1** | 2.42 ± 0.04 | 3.04 ± 0.12* | 3.51 ± 0.03* | 2.77 ± 0.33 |
| Globulin (gm %) | 4.0 ± 0.05** | 4.9 ± 0.01 | 4.39 ± 0.24 | 3.97 ± 0.02* | 4.62 ± 0.12 |
| A\G ratio | 0.83 ± 0.03 | 0.91 ± 0.05 | 0.84 ± 0.07 | 0.91 ± 0.02 | 0.83 ± 0.03 |
| Cholesterol (mg %) | 176.5 ± 6.9 | 198.54 ± 11.67 | 187.31 ± 8.3 | 184.61 ± 4.76 | 199.76 ± 5.97 |
| ALT/GPT (U/L) | 25.2 ± 3.1** | 74.82 ± 4.68 | 69.31 ± 7.15 | 63.41 ± 5.27 | 77.54 ± 3.66 |
| AST/GOT (U/L) | 128.8 ± 8.2** | 259.52 ± 9.65 | 225.1 ± 11.5 | 187.29 ± 4.39* | 238.12 ± 9.24 |
* and ** Significantly different than infected non-treated group at P < 0.05 and P < 0.01, respectively
As shown in Table 5 there was a significant (P < 0.01) decrease in serum albumin and a significant (P < 0.01) elevation in ALT and AST concentrations in C. parvum experimentally infected mice after 2 weeks post treatment than the healthy mice. The results after treatment for 2 weeks showed that globulin concentrations significantly (P < 0.05) increased in red pomegranate peel treated mice comparing to infected mice. Concerning the liver enzymes level after 2 weeks’ treatment, there was a significant decrease in ALT level in NTZ, red and white pomegranate peel treated mice comparing to infected mice. However, AST level was significantly (P < 0.05) decreased only in red pomegranate peel treated mice comparing to infected mice.
Table 5.
Effect of 2 weeks post treatment by NTZ, red and white pomegranate peel on serum protein profile and liver enzymes of Cryptosporidium experimentally infected mice
| Parameters | Animals | ||||
|---|---|---|---|---|---|
| Healthy mice | Cryptosporidium experimentally infected mice | ||||
| Infected Non-treated | Two weeks post treatment | ||||
| NTZ | Red pomegranate Peel | White pomegranate Peel | |||
| Total protein (gm%) | 7.34 ± 0.52 | 6.77 ± 0.16 | 6.38 ± 0.66 | 7.32 ± 0.26 | 6.08 ± 0.12 |
| Albumin (gm%) | 3.84 ± 0.02** | 3.35 ± 0.02 | 3.18 ± 0.05 | 3.73 ± 0.31 | 3.61 ± 0.05 |
| Globulin (gm %) | 4.38 ± 0.07 | 4.5 ± 0.06 | 4.3 ± 0.11 | 4.68 ± 0.01* | 4.24 ± 0.06 |
| A\G ratio | 0.89 ± 0.05 | 0.96 ± 0.08 | 0.8 ± 0.1 | 0.8 ± 0.03 | 0.82 ± 0.04 |
| Cholesterol (mg%) | 174.39 ± 5.4 | 201.65 ± 10 | 175.65 ± 9 | 177.5 ± 4.8 | 187.98 ± 8.47 |
| ALT/GPT (U/L) | 28.3 ± 2.49** | 101.8 ± 4.7 | 87.5 ± 8.8* | 37.8 ± 6.55* | 44.46 ± 8.18* |
| AST/GOT (U/L) | 132.6 ± 5.4** | 263.6 ± 6.3 | 231.2 ± 10 | 149.1 ± 5.82* | 226.3 ± 7.51 |
* and ** Significantly different than infected non-treated group at P < 0.05 and P < 0.01, respectively
Table 6 showed the changes of antioxidant activity after 1 week of treatment. GSH–Px and TAC serum activities in infected mice was significantly (P < 0.01) higher than healthy mice. However, they significantly (P < 0.01) decreased in NTZ treated mice compared with infected non-treated group.
Table 6.
Effect of one-week post treatment by NTZ, red and white pomegranate peel on GSH-Px, CAT and TAC of Cryptosporidium experimentally infected mice (Mean ± SE)
| Parameters | Animals | ||||
|---|---|---|---|---|---|
| Healthy mice | Cryptosporidium experimentally infected mice | ||||
| Infected non-treated | Two weeks post treatment | ||||
| NTZ | Red pomegranate peel | White pomegranate peel | |||
| GSH-Px (U/ml) | 8.32 ± 0.32* | 10.12 ± 0.26 | 8.44 ± 0.25* | 11.12 ± 0.26 | 10.46 ± 0.23 |
| CAT (U/ml) | 281.14 ± 9.31 | 248.56 ± 11.44 | 265.87 ± 9.35 | 276.56 ± 11.44 | 279.18 ± 10.34 |
| TAC (mM/L) | 1.98 ± 0.15* | 2.74 ± 0.06 | 1.99 ± 0.17* | 2.96 ± 0.26 | 2.50 ± 0.23 |
*Significantly different than infected non-treated group at P < 0.01
As seen in Table 7, both GSH–Px and TAC serum activities in infected mice was significantly (P < 0.01) higher than healthy mice. However, GSH–Px activity significantly (P < 0.05) increased in NTZ, red and white pomegranate peel treated mice comparing to infected non-treated group. Moreover, TAC activity significantly (P < 0.01) increased in red pomegranate peel treated mice comparing to infected non-treated group.
Table 7.
Effect of 2 weeks post treatment by NTZ, red and white pomegranate peel on serum GSH-Px, CAT and TAC of Cryptosporidium experimentally infected mice (Mean ± SE)
| Parameters | Animals | ||||
|---|---|---|---|---|---|
| Healthy mice | Cryptosporidium experimentally infected mice | ||||
| Infected Non-treated | Two weeks post treatment | ||||
| NTZ | Red pomegranate peel | White pomegranate peel | |||
| GSH-Px (U/ml) | 8.12 ± 0.26** | 6.2 ± 0.32 | 8.16 ± 0.12** | 10.62 ± 0.78* | 9.54 ± 0.78* |
| CAT (U/ml) | 278.9 ± 7.22 | 254.23 ± 8.12 | 233.2 ± 7.36 | 236.38 ± 6.52 | 227.4 ± 9.15 |
| TAC (mM/L) | 1.93 ± 0.08* | 1.32 ± ± 0.2 | 1.96 ± 0.06* | 2.51 ± 0.03** | 2.01 ± 0.11 |
* and ** Significantly different than infected non-treated group at P < 0.05 and P < 0.01, respectively
Discussion
Pomegranate is rich of bioactive compounds with pharmacological nature (Seeram et al. 2006). Singh et al. (2018) mentioned the phenolic compounds as beneficial phytochemicals in pomegranate peels. As far as we know, there is no published research on the antiparasitic effects of internal white and external red layers of P. granatum separately and based on the hypothesis that Nano-form improves the absorption as well as bioavailability of supplements in the body, this work was conducted to study the anticryptosporidial effect of this Nano-form of peels in mice model.
Pomegranate peels were by-products of the food industry. Using microwave for drying led to increase the total phenolic compounds in addition to maximizing its bioactivities (Abou-Arab et al. 2016). Results of the present study indicated that both prepared red and white pomegranate peel powders had narrow size distribution and small diameters. The particle size and its distribution might be of a great importance in determining the fate of micelles after oral administration. Morsy et al. (2018) prepared pomegranate peel Nanoparticles by lyophilization of its solubilized part. In this study, it was found that both tested pomegranate peel extracts were rich in phenolic compounds. There was a significant (p ≤0.05) difference between red and white peel in total phenolic content, ranged from 180.5 mg gallic acid/gm for red peel to 209.5 mg gallic acid/gm for white, which was higher than that reported before for peels of Egyptian pomegranate (124.34 mg GAE/g) (Moneim 2012) and Tunisian pomegranate (144.96–292.23 mg GAE/g)(Abid et al. 2017). In this study, there were 11 phenolic compounds identified by HPLC-DAD. Gallic acid was found to be the main component of phenolic represent 3.645 mg/gm sample in red peel and 4.9687 mg/gm sample in white peel followed by chlorogenic acid (0.3425 mg/gm sample in red peel and 0.3572 mg/gm sample in white peel). The results reflected that both red and white peels had high antioxidant activities and could be considered as a good cheap source of natural antioxidants. Kharchoufi et al. (2018) proved the greatest antioxidant activity of pomegranate peel water extract. The activity of these wastes might not depend on the content of phenols but depends on the quality and chemical structure of these phenols. In this study, sensory evaluation of pomegranate beverage indicated that the concentration of 1% of red peel beaverage was the most accepted in all attributes having scores than white peel. It was cleared that the extent to which the panelists accepted the pomegranate peel beverage, this might be a good indicator of the possibility of applying the production of pomegranate red peel beverage.
C. parvum is a widespread parasite and had a global significance. Development of effective therapeutics for cryptosporidiosis is undoubtedly a medical imperative. In this study, examination of fecal smears revealed that there was a gradual reduction in oocyst shedding in the infected non-treated group from the 9th day PI and continued till almost no oocysts found at day 24 PI. This agreed with previous findings that the interval which covered the natural Cryptosporidium oocyst shedding period in mice was about 24 days (Matsui et al. 2001) or about 3–4 weeks (Lacroix et al. 2001). Also, in this study, C. parvum oocysts detected in mice fecal smears were morphologically similar to those of C. parvum described in previous studies (Hassanain et al. 2011; Abdelrahman et al. 2015). In this study, a gradual reduction in oocyst shedding was noticed in all infected animal groups. Red and white pomegranate peel extracts successfully eradicated the C. parvum oocysts from feces and almost no oocysts were detected in feces by the end of the experiment. This impressive effect of pomegranate was similarly obtained using P. granatum whole peels extract on cryptosporidiosis in mice (Al-Mathal and Alsalem 2012), however in our study, the effect of Nano-form of the outer red and inner white peel extracts each separately were evaluated. Several compounds had been isolated from P. granatum and proved to have anti-proliferative and antioxidant activities (Qnais et al. 2007). NTZ treatment also caused a marked decrease in the mean number of oocysts after the initiation of therapy. Similar findings had been recorded in several studies including in vitro and in vivo studies using several animal models and in clinical trials, which had demonstrated the effectiveness of NTZ in treating diarrhea and enteritis caused by Cryptosporidium species (Abdou et al. 2013). In this study, red peel extract significantly (P < 0.05) diminished C. parvum oocysts count in experimentally infected mice than white peel and NTZ treatments in almost all days post treatment. These obvious anticryptosporidial effects might be due to the phenolic compounds found in red and white pomegranate peels. Phenolic compounds could interfere the energy generation mechanism by uncoupling the oxidative phosphorylation and also could interfere with the glycoprotein of the cell surface of the parasites and cause death (John et al. 2009). The antioxidants content of vegetables and fruits played a major role in diseases prevention (Han et al. 2008). Peel extract's phenolic compounds displayed a potent antioxidant activity. Also, crude extracts and purified fractions from pomegranate peels could offer health improvement and might be involved in food preservation and pharmaceutical purposes (Shiban et al. 2012). Also, this anticryptosporidial effect, detected in our study, might be maximized due to the Nano-form of the used peels. Nano-form might play a great role in the activity of the red peel particles which was smaller two or three times than white peel and that need further more investigations. This hypothesis was supported by Chaudhry and Castle (2011) who found that Nano-form could improve the uptake, absorption, and bioavailability of nutrients and supplements in the body compared to bulk equivalents.
Light microscopic examination of C. parvum-infected ileal sections revealed the presence of altered mucosal architecture, with sloughing and complete erosion of epithelial cells, shortening, blunting, stunting and atrophy of the intestinal villi. These findings agreed with Abu El Ezz et al. (2011) and Abdelrahman et al. (2015). Sasahara et al. (2003) found that at the time when the number of C. parvum oocysts in the ileum was maximal, absorptive and columnar goblet cells decreased in the ileal epithelium with a significant reduction in the height of the villi. In this study, concerning the villi of the infected NTZ, red and white peels treated groups, they regained their normal appearance like healthy non-infected mice. However, red peel treated group showed the greatest enhancement in the shape and structure of villi with the presence of goblet cells than other treated groups. These results might be due to the anticryptosporidial effect of red peel besides the antioxidant effect that helped the enhancement of intestinal tissue.
Cryptosporidiosis in mice might induce a chronic disease state extending throughout gastrointestinal tract or/and extra-intestinal sites leading to hepatic dysfunction (Mead et al. 1994). In the current study, the infection dynamics in mice model were characterized with screening the changes in the liver function. Mean values of serum albumin levels of Cryptosporidium experimentally infected non-treated mice were decreased all over the experiment than the healthy mice, however, serum globulin levels were increased. These results were supported by similar findings recorded by Mousa et al. (2014) and Abdel Megeed et al. (2015). This reduction in serum albumin in infected non-treated mice could be due to poor absorption of nutrients and excessive protein breakdown (Malina et al. 1994). The hyperglobulinemia could be attributed to hepatic damage associated with hypoxia (Enwezor and Sackey 2005). The decline in albumin beside the hyperglobulinemia could be a result of stresses (Hilali et al. 2006) and also might be explained as a response to the parasitic antigen (Azza 2008).
Concerning the liver enzymes measured in this study, there was an elevation in ALT and AST in Cryptosporidium experimentally infected non-treated mice. These results confirmed that cryptosporidiosis might have extra-intestinal effects (Chalmers and Davies 2010). It was found that 32% of hepatocellular carcinoma and diarrhea harbored Cryptosporidium that indicated the involvement of cryptosporidiosis as a major factor for hepatic encephalopathy (Mousa et al. 2014).
As the treatment modalities for Cryptosporidium were still limited, prevention and risk reduction became the most essential interferences (Chalmers and Davies 2010). Moreover, there was expanding epidemiological and pharmacological proof that plants contain free radical scavengers that provide good health and protection against degenerative diseases. It was known that various in vivo pathological reactions had been attributed to the role of oxygen radicals and lipid peroxides (Li et al. 2006). In this study, after 1 week, albumin concentrations increased in both NTZ and red pomegranate peel treated mice comparing to non-treated mice. However, globulin concentrations decreased in red pomegranate peel treated mice while increased at 2 weeks’ post treatment. Concerning, the liver enzymes levels after 2 weeks’ treatment, there was a significant decrease in ALT level in NTZ, red and white pomegranate peel treated mice. However, AST level decreased only in red pomegranate peel treated mice comparing to infected mice. Determination of the enzymatic antioxidant activities, such as GSH–Px and CAT as well as estimation of serum TAC was a means of assessing oxidative stress (Saleh et al. 2011). In current study, it was found that GSH–Px and TAC serum activities in infected mice increased, while, they were decreased after one-week treatment with NTZ. However, after 2 weeks of treatment, GSH–Px activity increased in NTZ, red and white pomegranate peel treated mice. Moreover, TAC activity increased in red pomegranate peel treated mice. This data reflected the oxidative stress in Cryptosporidium infected mice as the infection was linked with oxidative status, hence suggesting overload while dealing with an infection could exaggerate oxidative stress (Abo-Aziza et al. 2017). These results parallel to a previous study recorded that pomegranate seed-juice could be used as treatment for chronic diseases relative to over-production of free radicals as it was a potential source of natural antioxidant (Anahita et al. 2015). Chidambara Murthy et al. (2002) found that pretreatment with an extract of pomegranate peel enhanced the free-radical scavenging activity of the hepatic enzymes as catalase, peroxidase and super oxide dismutase. Similar results were previously reported for pomegranate bark (Tanaka et al. 1986) and leaves (Nawwar et al. 1994). Most of the measured biochemical parameters after 2 weeks’ treatment with red pomegranate peel or NTZ were enhanced in their concentrations towards the healthy normal status. This means that red pomegranate peel and NTZ treatments improved the animals’ health benefits.
Conclusion
It was concluded that the Nano-form of P. granatum peel extracts were rich in phenolic compounds, however, red peel extract had high antioxidant activity and a therapeutic effect on C. parvum in experimentally infected mice. Also, the biochemical parameters and oxidative stress in Cryptosporidium experimentally infected mice was significantly enhanced towards the healthy normal status by red pomegranate peel extract administration. Finally, pomegranate peels should be separated and used in the Nano-form in the daily human and animal diet to give protective effects against this parasite as well as to improve health benefits.
Author contributions statement
All authors prepared the research plan and the design of experiments. Aboelsoued D. separated C. parvum oocysts from calf fecal samples, carried out the experimental infection in mice, administrated the therapeutic doses, collected mice fecal pellets everyday and counted oocysts in feces throughout the experiment. Abo-Aziza F. collected the blood samples from mice, separated sera and carried out the biochemical analysis including protein profile, liver enzymes and serum antioxidant activity. Mahmoud M. prepared pomegranate peel powder by milling to achieve Nano-form, determination of proximate composition, droplet size measurement, total phenolics and polyphenols and antioxidant activities. Also, beverage preparation and sensory evaluation. Abdel Megeed K. and Abu El Ezz N. participated in dose preparation and sacrificing of mice. Abu El Ezz N, Abdel Megeed K. and Aboelsoued D. examined stained ileal sections and interpreted them histologically and pathologically. Aboelsoued D, Abo-Aziza F and Mahmoud M analyzed, interpreted the data and prepared the manuscript. All authors had critically read and approved the final manuscript.
Compliance with ethical standards
Conflicts of interest
The authors declare that they have no conflicts or competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abdel-Ghaffar F, Semmler M, Al-Rasheid KA, Strassen B, Fischer K, Aksu G, Klimpel S, Mehlhorn H. The effects of different plant extracts on intestinal cestodes and on trematodes. Parasitol Res. 2010;108:979–984. doi: 10.1007/s00436-010-2167-5. [DOI] [PubMed] [Google Scholar]
- Abdel Megeed KN, Hammam AM, Morsy GH, Khalil FAM, Seliem MME, Aboelsoued D. Control of cryptosporidiosis in buffalo calves using garlic (Allium sativum) and Nitazoxanide with special reference to some biochemical parameters. Glob Vet. 2015;14(5):646–655. [Google Scholar]
- Abdelrahman KA, Abdel Megeed KN, Hammam AM, Morsy GH, Seliem MME, Aboelsoued D. Molecular characterization of bubaline isolate of Cryptosporidium species from Egypt. Res J Parasitol. 2015;10(4):127–141. [Google Scholar]
- Abdou AG, Harba NM, Afifi AF, Elnaidany NF. Assessment of Cryptosporidium parvum infection in immunocompetent and immunocompromised mice and its role in triggering intestinal dysplasia. Int J Infect Dis. 2013;17(8):593–600. doi: 10.1016/j.ijid.2012.11.023. [DOI] [PubMed] [Google Scholar]
- Abid M, Yaich H, Cheikhrouhou S, Khemakhem I, Bouaziz M, Attia H, Ayadi MA. Antioxidant properties and phenolic profile characterization by LC–MS/MS of selected Tunisian pomegranate peels. J Food Sci Technol. 2017;54(9):2890–2901. doi: 10.1007/s13197-017-2727-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abo-Aziza FAM, Hendawy SHM, Zaki AA, Oda SS, El Namaky AH. Clinicohistopathological and immunological alterations in Egyptian donkeys infested by Rhinoestrus spp. during the winter season. Egypt J Vet Sci. 2017;48(2):61–71. [Google Scholar]
- Abou-Arab AA, Mahmoud MH, Abu-Salem FM. Bioactive compounds content of citrus peel as affected by drying processes. Inter J Biol Biomol Agricul Food Biotech Eng. 2016;10(4):225–228. [Google Scholar]
- Abu El Ezz NMT, Khalil AM, Shaapan RM. Therapeutic effect of onion (Allium cepa) and cinnamon (Cinnamomum zeylanicum) oils on Cryptosporidiosis in experimentally infected mice. Glob Vet. 2011;7(2):179–183. [Google Scholar]
- Al-Mathal EM, Alsalem AA. Pomegranate (Punica granatum) peel is effective in a murine model of experimental Cryptosporidium parvum. Exp Parasitol. 2012;131:350–357. doi: 10.1016/j.exppara.2012.04.021. [DOI] [PubMed] [Google Scholar]
- Al-Mathal EM, Alsalem AA. Pomegranate (Punica granatum) peel is effective in a murine model of experimental Cryptosporidium parvum ultrastructural studies of the ileum. Exp Parasitol. 2013;134:482–494. doi: 10.1016/j.exppara.2013.05.004. [DOI] [PubMed] [Google Scholar]
- Amer OS, Dkhil MA, Hikal WM, Al-Quraishy S. Antioxidant and anti-inflammatory activities of pomegranate (Punica granatum) on Eimeria papillata-induced infection in mice. BioMed Res Int. 2015;2015:219670. doi: 10.1155/2015/219670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anahita A, Asmah R, Fauziah O. Evaluation of total phenolic content, total antioxidant activity, and antioxidant vitamin composition of pomegranate seed and juice. Gen Med. 2015;3(1):1–4. [Google Scholar]
- AOAC . Official methods of analysis of the association of official analytical chemists. 17. Rockville: The Association of Official Analytical Chemists; 2000. [Google Scholar]
- Azza MK. Some biochemical, hematological and clinical studies of selected ruminal and blood constituents in camels affected by various diseases. Res J Vet Sci. 2008;1(1):16–27. [Google Scholar]
- Bancroft JD, Stevens GA. Theory and practice of histological techniques. 2. London: Churchill Livingstone; 1990. [Google Scholar]
- Barros HRM, Ferreira TAPC, Genovese MI. Antioxidant capacity and mineral content of pulp and peel from commercial cultivars of citrus from Brazil. Food Chem. 2012;134:1892–1898. doi: 10.1016/j.foodchem.2012.03.090. [DOI] [PubMed] [Google Scholar]
- Cannon DC, Olitzky I, Inkpen JA. Clinical chemistry principles and techniques of determination of total protein. 2. London: Harper and Rowpubl; 1974. [Google Scholar]
- Chalmers RM, Davies AP. Minireview: clinical cryptosporidiosis. Exp Parasitol. 2010;124:138–146. doi: 10.1016/j.exppara.2009.02.003. [DOI] [PubMed] [Google Scholar]
- Chaudhry Q, Castle L. Food applications of nanotechnologies: an overview of opportunities and challenges for developing countries. Trends Food Sci Tech. 2011;22:1–9. [Google Scholar]
- Chidambara Murthy KN, Jayaprakasha GK, Singh RP. Studies on antioxidant activity of pomegranate (Punica granatum) peel extract using in vivo models. J Agric Food Chem. 2002;50:4791–4795. doi: 10.1021/jf0255735. [DOI] [PubMed] [Google Scholar]
- Current WL, Reese NC. A comparison of endogenous development of three isolates of Cryptosporidium in suckling mice. J Protozool. 1986;33:98–108. doi: 10.1111/j.1550-7408.1986.tb05567.x. [DOI] [PubMed] [Google Scholar]
- Dell’Agli M, Galli G, Corbett Y, Taramelli D, Lucantoni L, Habluetzel A, Maschi O, Caruso D, Giavarini F, Romeo S, Bhattacharya D, Bosisio E. Antiplasmodial activity of Punica granatum L. fruit rind. J Ethnopharmacol. 2009;125:279–285. doi: 10.1016/j.jep.2009.06.025. [DOI] [PubMed] [Google Scholar]
- Dkhil MA. Anticoccidial, anthelmintic and antioxidant activities of pomegranate (Punica granatum) peel extract. Parasitol Res. 2013;112:2639–2646. doi: 10.1007/s00436-013-3430-3. [DOI] [PubMed] [Google Scholar]
- Doumas B, Watson W, Biggs M. Albumin standards and the measurement of serum albumin with bromocresyl green. Clin Chim Acta. 1971;31:87–96. doi: 10.1016/0009-8981(71)90365-2. [DOI] [PubMed] [Google Scholar]
- El Mageid MMA, Salama NA, Saleh MAM, Abo-Taleb HM. Evaluation of antdiabetic, hypocholesterolemic of pomegranate (Punica granatum L.) juice powders and peel powder extracts in male albino rats. IOSR-JPBS. 2016;11(6):53–64. [Google Scholar]
- Enwezor FNC, Sackey AKB. Camel trypanosomosis—a review. Vet Arh. 2005;75:439–452. [Google Scholar]
- Han J, Weng X, Bi K. Antioxidants from a Chinese Medicinal Herb-Lithospermum erythrorhizon. Food Chem. 2008;106(1):2–10. [Google Scholar]
- Hassanain MA, Khalil FAM, Abd El-Razik KA, Shaapan RM. Prevalence and molecular discrimination of Cryptosporidium parvum in calves in Behira provinces, Egypt. Res J Parasitol. 2011;6:101–108. [Google Scholar]
- Hayat K, Zhang X, Farooq U, Abbas S, Xia S, Jia C, Zhong F, Zhang J. Effect of microwave treatment on phenolic content and antioxidant activity of citrus mandarin pomace. Food Chem. 2010;123:423–429. [Google Scholar]
- Hegazi AG, Abdel Megeed KN, Hassan SE, Abdelaziz MM, Toaleb NI, El Shanawany EE, Aboelsoued D. Comparative ovicidal activity of Moringa oleifera leaf extracts on Fasciola gigantica eggs. Vet World. 2018;11(2):215–220. doi: 10.14202/vetworld.2018.215-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriksen SA, Pohlenz JF. Staining of cryptosporidia by a modified Ziehl-Neelsen technique. Acta Vet Scand. 1981;22:594–596. doi: 10.1186/BF03548684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilali M, Abdel-Gawad A, Nassar A, Abdel-Wahab A. Hematological and biochemical changes in water buffalo calves (Bubalus bubalis) infected with Trypanosoma evansi. Vet Parasitol. 2006;139:237–243. doi: 10.1016/j.vetpar.2006.02.013. [DOI] [PubMed] [Google Scholar]
- Hosseini A, Jafari SM, Mirzaei H, Asghari A, Akhavan S. Application of image processing to assess emulsion stability and emulsification properties of Arabic gum. Carbohydr Polym. 2015;126:1–8. doi: 10.1016/j.carbpol.2015.03.020. [DOI] [PubMed] [Google Scholar]
- John J, Mehta A, Shukla S, Mehta P. A report on anthelmintic activity of Cassia tora leaves. J Sci Technol. 2009;31(3):269–271. [Google Scholar]
- Kharchoufi S, Licciardello F, Siracusa L, Muratore G, Hamdi M, Restuccia C. Antimicrobial and antioxidant features of ‘Gabsiʼ pomegranate peel extracts. Ind Crops Prod. 2018;11:345–352. [Google Scholar]
- Lacroix S, Mancassola R, Naciri M, Laurent F. Cryptosporidium parvum-specific mucosal immune response in C57BL/6 neonatal and gamma interferon-deficient mice: role of tumor necrosis factor alpha in protection. Infect Immun. 2001;69:42–1635. doi: 10.1128/IAI.69.3.1635-1642.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Guo C, Yang J, Wei J, Xu J, Cheng S. Evaluation of antioxidant properties of pomegranate peel extract in comparison with pomegranate pulp extract. Food Chem. 2006;96(2):254–260. [Google Scholar]
- Lockman PR, Mumper RJ, Khan MA, Allen DD. Nanoparticle technology for drug delivery across the blood brain barrier. Drug Dev Ind Pharm. 2002;28:1–13. doi: 10.1081/ddc-120001481. [DOI] [PubMed] [Google Scholar]
- Mahmoud MH, Seleet FL, Foda MI. Effect of different concentration techniques in some properties of fresh and storage Pomegranate juice. Asian J Sci Res. 2017;10(4):290–298. [Google Scholar]
- Mahmoud MH, Wahba HM, Mahmoud MH, Abu-Salem FM. Antagonizing the hazardous impact of increased oxidative stress in Wistar rats by biscuits with dried orange peel. J Biol Sci. 2018;18(1):21–31. [Google Scholar]
- Malina JM, Rodriguez-ponce E, Ferrer O, Cutierrez AC, Herndez S. Biopathological data of goat kids with cryptosporidiosis. Vet Rec. 1994;135:67–68. doi: 10.1136/vr.135.3.67. [DOI] [PubMed] [Google Scholar]
- Matsui T, Fujino T, Kajima J, Tsuji M. Infectivity and oocyst excretion patterns of Cryptosporidium muris in slightly infected mice. J Vet Med Sci. 2001;63:20–319. doi: 10.1292/jvms.63.319. [DOI] [PubMed] [Google Scholar]
- Mead JR, Nurcan I, Xiangdong Y, Yelena B, Michael J, Michael TF, Raymond FS. Infection dynamics and clinical features of cryptosporidiosis in SCID mice. Infect Immun. 1994;62(5):1691–1695. doi: 10.1128/iai.62.5.1691-1695.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moneim AEA. Antioxidant activities of Punica granatum (pomegranate) peel extract on brain of rats. J Med Plants Res. 2012;6(2):195–199. [Google Scholar]
- Morsy GH, Megeed KNA, Hammam AM, Seliem MME, Khalil FAM, Aboelsoued D. Prevalence of Cryptosporidium infection in buffalo calves with special reference to urea and creatinine levels. Glob Vet. 2014;13(5):662–667. [Google Scholar]
- Morsy M, Mekawi E, Elsabagh R. Impact of pomegranate peel nanoparticles on quality attributes of meatballs during refrigerated storage. LWT. 2018;89:489–495. [Google Scholar]
- Mousa N, Ahmed A, El-Nahas H, Atef E, Mohammad A, Nabih M, Magdy H, Mohammad E, Narmin E, Walled E. Cryptosporidiosis in patients with diarrhea and chronic liver diseases. J Infect Dev Ctries. 2014;8(12):1584–1590. doi: 10.3855/jidc.5166. [DOI] [PubMed] [Google Scholar]
- Nawwar MAM, Hussein SAM, Merfort I. Leaf phenolics of Punica granatum. Phytochemistry. 1994;37:1175–1177. [Google Scholar]
- Oyaizu M. Studies on antioxidative activities of browning reaction products prepared from glucosamine. Jpn J Nutr. 1986;44:307–315. [Google Scholar]
- Petrie A, Watson P. Statistics for veterinary and animal science. 1. Oxford: The Blackwell Sci. Ltd; 1999. pp. 90–99. [Google Scholar]
- Pumipuntu N, Piratae S. Cryptosporidiosis: a zoonotic disease concern. Vet World. 2018;11(5):681–686. doi: 10.14202/vetworld.2018.681-686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qnais EY, Elokda AS, Abu Ghalyun YY, Abdulla FA. Antidiarrheal activity of the aqueous extract of Punica granatum (pomegranate) peels. Pharm Biol. 2007;45(9):715–720. [Google Scholar]
- Ramful D, Tarnus E, Aruoma OI, Bourdon E, Bahorun T. Polyphenol composition, vitamin C content and antioxidant capacity of Mauritian citrus fruit pulps. Food Res Inter. 2011;44:2088–2099. [Google Scholar]
- Saleh MA, Mahran OM, Al-Salahy BM. Circulating oxidative stress status in dromedary camels infested with sarcoptic mange. Vet Res Commun. 2011;35(1):35–45. doi: 10.1007/s11259-010-9450-x. [DOI] [PubMed] [Google Scholar]
- Sasahara T, Maruyama H, Aoki M, Kikuno R, Sekiguchi T, Takahashi A, Satoh Y, Kitasato H, Takayama Y, Inoue M. Apoptosis of intestinal crypt epithelium after Cryptosporidium parvum infection. J Infect Chemother. 2003;9(3):278–281. doi: 10.1007/s10156-003-0259-1. [DOI] [PubMed] [Google Scholar]
- Seeram NP, Schulman RN, Heber D. Pomegranates: ancient roots to modern medicine. Boca Raton: Taylor and Francis Group; 2006. pp. 5–8. [Google Scholar]
- Shafiq MAB, Maqbool A, Khan UJ, Lateef M, Ijaz M. Prevalence, water borne transmission and chemotherapy of cryptosporidiosis in small ruminants. Pak J Zool. 2015;47(6):1715–1721. [Google Scholar]
- Shiban MS, Al-Otaibi MM, Al-Zoreky NS. Antioxidant activity of pomegranate (Punica granatum L.) fruit peels. Food Nutr Sci. 2012;3:991–996. [Google Scholar]
- Singh B, Singh JP, Kaur A, Singh N. Phenolic compounds as beneficial phytochemicals in pomegranate (Punica granatum L.) peel: a review. Food Chem. 2018;261:75–86. doi: 10.1016/j.foodchem.2018.04.039. [DOI] [PubMed] [Google Scholar]
- Snedecor GW, Cochran WG. Statistical methods. 7. Ames: Iowa State University Press; 1980. [Google Scholar]
- Tanaka T, Nonaka GI, Nishioka I. Tannins and related compounds. XLI. Isolation and characterization of novel ellagitannins, punicacorteins A, B, C and D and punigluconin from the bark of Punica granatum L. Chem Pharm Bull. 1986;34:656–663. [Google Scholar]
- Ventura J, Alarcón-Aguilar F, Roman-Ramos R, Campos-Sepulveda E, Reyes-Vega ML, Boone-Villa VD, Jasso-Villagómez EI, Aguilar CN. Quality and antioxidant properties of a reduced-sugar pomegranate juice jelly with an aqueous extract of pomegranate peels. Food Chem. 2013;136:109–115. doi: 10.1016/j.foodchem.2012.07.039. [DOI] [PubMed] [Google Scholar]
- Wang R, Ding Y, Liu R, Xiang L, Du L. Pomegranate: constituents, bioactivities and pharmacokinetics. Fruit Veg Cereal Sci Biotechnol. 2010;4:77–87. [Google Scholar]
- Winn-Deen ES, David H, Sigler G, Chavez R. Determination of total and pancreatic α-amylase in human serum with 2-chloro-4-nitrophenyl-α-d-maltotrioside as substrate. Clin Chem. 1988;34:2005. [Google Scholar]
- Zohreh K, Parvaneh K, Solmaz C, Soheila KN. Comparative study of serum lipid profile in chicken, ostrich, cattle and sheep. Comp Clin Pathol. 2012;21:259–263. [Google Scholar]