Abstract
Objective(s):
The preclinical reports have shown that specific probiotics like Bifidobacterium bifidum (B. bifidum) and Lactobacillus acidophilus (L. acidophilus) can be applied as the biotherapeutic agents in the inhibition or therapy of colorectal cancer via the modification of gut bacteria. In the previous studies, we have assessed the impact of L. acidophilus and B. bifidum probiotics on gut bacteria concentration and also their chemo-protective impact on mice colon cancer. In the following, we assessed the effects of these probiotics on the gene expression of vitamin D receptor (VDR) and the leptin receptor (LPR) and the serum biochemical parameters on mice colon cancer.
Materials and Methods:
Thirty-six male BALB/c mice were equally shared into 4 groups; (i) health with routine dietary foods without any treatment, (ii) azoxymethane (AOM)-induced mice colon cancer with common dietary foods, (iii) and (iv) AOM-induced mice colon cancer with oral consumption of L. acidophilus and B. bifidum (1×109 cfu/g) for 5 months, respectively. Then, the serum total cholesterol, triglycerides, low-density lipoprotein cholesterol (LDL), high-density lipoprotein cholesterol (HDL), alanine transaminase, alkaline phosphatase, and albumin and also VDR and LPR genes expression were evaluated.
Results:
Oral consumption of L. acidophilus and B. bifidum probiotics significantly decreased the triglycerides, alkaline phosphatase, LDL, and also the VDR and LPR gene expression in mice colon cancer (P<0.005).
Conclusion:
L. acidophilus and B. bifidum probiotics with the modification of the biochemical parameters and the expression of the VDR and LPR genes can play a key role in the protection of mouse colon cancer.
Key Words: Colon cancer, Leptin receptor, Mice, Probiotic, Vitamin D receptor
Introduction
A number of the reports have shown that the special probiotics such as Bifidobacterium and Lactobacillus can modify the gut bacteria that may be used as the biotherapeutic agents in the inhibition or therapy of colorectal cancer (CRC) (1-3). In the prior experiment, we assessed the impact of Lactobacillus acidophilus (L. acidophilus) and Bifidobacterium bifidum (B. bifidum) probiotics on the concentration of intestinal bacteria. In this context, the concentration of L. acidophilus remained almost unchanged during 5 months and showed a more stability compared to B. bifidum probiotic (4). We have also compared the role of L. acidophilus with B. bifidum probiotic on the azoxymethane (AOM)-induced mice colon cancer. In this regard, L. acidophilus probiotic consumption with a significant increase of the serum levels of interferon-γ (IFγ) and interleukin 10 (IL-10), and also the CD8+ and CD4+ cells could have a more antitumor activity than B. bifidum probiotic. Therefore, the replacement of the pathogenic bacteria by the probiotics can be a potential mechanism of the immune system responses that may modulate colon cancer severity (5).
During immune responses, the studies have demonstrated that the vitamin D receptor (VDR) activation up-regulate the expression of cathelicidin and defensin that can control the concentration of the bacterial flora, though the function of the vitamin D/VDR in regulating bacterial flora remains unidentified (1). Another study demonstrated that vitamin D3 can activate the VDR to inhibit the tumor cell development by a differentiation induction in the various tumor cells such as the intestinal cancer cells (6-8). Moreover, the recent studies have shown that the VDR−/− mice can increase the bacterial concentration in the gut and also the induction of both commensal and pathogenic bacteria (9, 10). In this context, the probiotics can modulate the anti-inflammatory signaling pathway of the VDR in colitis with an unknown mechanism. Furthermore, there is the growing evidence that the VDR can control the function of the leptin and other class I cytokines (11, 12). Leptin has been reported that can decline the renal 25-hydroxyvitamin D (3)-1α-hydroxylase expression in mice by binding to the leptin receptor (LPR) in colon cancerous cells (19). LPR is a kind of the pro-inflammatory cytokine receptor and its high circulating level has been observed in the advanced stage of CRC patients (13, 14). Although some clinical studies reported the low level of the serum leptin in CRC patients, the high serum leptin level was associated with the high incidence of colon cancer in other studies (15-18). Thus, it seems that the probiotics with an improvement of the microflora bacteria concentration can have beneficial effects on the LPR and VDR gene expression. In this respect, the present study was aimed to assess L. acidophilus and B. bifidum impacts on the VDR and LPR gene and the serum biochemical parameters on mouse colon cancer.
Materials and Methods
Materials
B. bifidum and L. acidophilus were gifted from Zist Takhmir Supplements Company (Tehran, Iran). Ketamine, xylazine, and AOM were provided from Sigma Aldrich Co. (St Louis, MO). Geneall R Hybrid-RTM-miRNA Kit (Cat.No:325-150) and M-MuLV Reverse Transcriptase (Cat. No: PR911658) (10000 units) from CinaGen Company (Tehran, Iran) were used for extracting RNA, and DNA synthesis, respectively. 5x HOT FIREPol EvaGreen qPCR Mix Plus (no ROX) were purchased from Solis BioDyne (Tehran, Iran). Biosino Biotech and Sci, Inc, Beijing, China Elisa kits were used for assessment of the biochemical parameters.
Animals
Thirty-six male BALB/c mice (6-8 weeks old) were bought from Pasteur Institute of Iran and housed in the high groups under 12-hr day/night cycles. The procedures were in the agreement with Tehran University of Medical Sciences instructions for the maintenance and use of the laboratory animals and the ethical standards of the institutional and/or national research committee and with the 1964 Declaration of Helsinki and its last amendments ethical standards.
Study design
According to the study protocol, the animals were equally divided into 4 groups; (i) the health with ordinary dietary foods without any treatment, (ii) the AOM-induced colon cancer, (iii) L. acidophilus, and (iv) B. bifidum. The study design and protocol were the same as our previous work (5). In the II-IV groups, AOM (15 mg/kg, subcutaneous (SC)) was weekly injected in three continues weeks to create mouse colon cancer. Oral consumption with L. acidophilus (1×109 cfu/g Lac/002P/M) in the iii group and the nutrition with B. bifidum (1×109 cfu/g Bla/016P/M) in the iv group were started ten days before the AOM injection and daily continued sustained for 5 months (5, 19)
Blood and tissue sampling
At the end of the 20th week, the mice were finally euthanized using a cervical dislocation under the general anesthesia with the combination of the ketamine and xylazine (10:1) at a 110 mg/kg body weight dose by a SC injection. Almost 1.5 ml of the blood samples were collected and subsequently centrifuged for ten min at 3,000 rpm. Prepared serum was kept at −80 °C for future examinations. Distal colon specimens were also excised and gently removed under a routine surgery for the RNA extraction.
Hematology and blood chemistry tests
At the end of the treatment, we euthanized the mice under the general anesthesia. Blood samples were isolated and stored into the coated tubes by ethylene-diamine-tetra-acetic-acid (EDTA) for haematology, and the coated heparin for the clinical chemistry. The serum total cholesterol (TC), triglycerides (TG), low-density lipoprotein cholesterol (LDL-C) and high-density lipoprotein cholesterol (HDL-C), alanine transaminase (ALT), alkaline phosphatase (ALP), and albumin (ALB) were enzymatically measured with an Autoanalyzer System (Autoanalyser Model Biotecnica, BT 3500, Rome, Italy) and a commercial kit (Biosino Biotech & Sci, Inc, Beijing, China) (20, 21).
RNA-extraction and real-time PCR
Total RNA was extracted from the tissue of colon tumors with Gene all Hybrid-R miRNA kit (Cat No: 325-150, Lot. No: M 3250 19L) based on the manufacturer’s guidelines. The quantity and quality of the extracted RNAs were measured by Thermo Scientific Nanodrop 2000 C Spectrophotometer (USA) and an agarose gel electrophoresis (1% agarose; Gibco/BRL), respectively. The amount of the 260/280 OD ratio of all samples was confirmed between 1.8 and 2.2, showing their high purity. cDNAs were synthesized by a M-MuLV Reverse Transcriptase cDNA Synthesis Kit (10000 units) from CinaGen company (Tehran, Iran) according to the manufacturer’s instruction, and kept at −20 °C. Primers were designed in two adjacent exons by Shangaye Generay Biotech (Table 1). All of the selected primer sequences were checked with the Oligo software and the primer blast in the NCBI database. The level of the mRNA expression was normalized by using beta-actin as an internal housekeeping control gene. Real-time PCR was carried out by a light cycler tool (Applied Biosystems 7500, USA) and 5x HOT FIREPol® EvaGreen®qPCR Mix Plus (no ROX) (Solis BioDyne Inc). In a total volume of 20 μl, 14 μl of nuclease-free water, 4 μl Eva Green master mix, 1 μl of cDNA samples as well as 0.5 μl of forward and 0.5 μl reverse primers (Qiagen, Hilden, Germany) were transferred into each capillary tube. The PCR condition was involved an initial denaturation of 15 min at 95 °C, 40 cycles at 95 °C for 15 sec, 62 °C for 20 sec and 72 °C for 20 sec, respectively. The specificity of the PCR products was evaluated by confirming a single peak in the melting curve analysis. The stained 1.5% agarose gel with ethidium bromide were used for a complementary length verification (22).
Table 1.
The sequences of the specific primers for the β-actin, LPR, and VDR
| Gene name | Reverse primer | Forward primer |
|---|---|---|
| VDR | 5’-TGATCGTGTTCTCTCCCAGTTC-3’ | 5’-AGAAGTGCCCAAAGGGAAGTC-3’ |
| LPR | 5’-GCTCCATTCTTGATCCCAGAG-3’ | 5’-TAGACCAGCAGTGGCTTGTTG-3’ |
| β-actin | 5’-CCAGTTGGTAACAATGCCATGT-3’ | 5’-GGCTGGTATTCCCCTCCATCG-3’ |
VDR: Vitamin D receptor; LPR: Leptin receptor
Statistical analysis
Comparison among groups was determined by the analysis of variance (ANOVA) and Tukey tests. Chi-square test was also used for a ratio comparison. Statistical significance was defined by mean±SD. Statistical analysis was performed applying the SPSS statistical software version 20.
Results
Biochemical assay and the serum level of the lipid profile
We have measured the serum level of the lipid profile including TC, HDL-C, LDL-C, and TG with the ELISA method. We have found that the LDL-C and TG were significantly increased in the AOM group (34.0±8 and 158±49 mg/dl, respectively) in comparison with the health group (9.7±5.8 mg/dl for LDL and 86±12 mg/dl for TG) (P˂0.05). Our results have also demonstrated a considerable reduction of the LDL-C and TG in L. acidophilus (7.0±3.6 and 91±23 mg/dl, respectively) and B. bifidum (12±13 and 90±13 mg/dl, respectively) groups in comparison with the AOM group (34.0±8 and 158±49 mg/dl, respectively) (P˂0.05). Compared to the health group (70±6 mg/dl), the HDL was non-significantly decreased in the AOM group (53±23 mg/dl). However, in comparison with the AOM group (53±23 mg/dl), a significant and non-significant increase of the HDL serum level was seen in L. acidophilus (72±15 mg/dl) and B. bifidum (63±11 mg/dl) groups, respectively. Furthermore, B. bifidum non-significantly declined the TC (90±43 mg/dl) in comparison with the AOM group (103±39 mg/dl), but L. acidophilus did not demonstrate a considerable change in the TC (Table 2).
Table 2.
Effects of Lactobacillus acidophilus and Bifidobacterium bifidum probiotics on the biochemical parameters in comparison with the AOM and health groups in a mouse model of colon cancer
| Groups | Health | AOM | L. acidophilus | B. bifidum |
|---|---|---|---|---|
| Parameters | ||||
| Cholesterol (mg/dl) | 107 ± 32 | 103 ± 39 | 102 ± 43 | 90 ± 43 |
| TG (mg/dl) | 86 ± 12 | 158 ± 49 * | 91 ± 23 # | 90 ± 13 # |
| HDL (mg/dl) | 70 ± 6 | 53 ± 23 * | 72 ± 15 # | 63 ± 11 |
| LDL (mg/dl) | 9.7 ± 5.8 | 34 ± 8 * | 7 ± 3.6 # | 12 ± 13 # |
| ALP (U/L) | 128 ± 20 | 200 ± 33 * | 143 ± 50 # $ | 181 ± 11 |
| ALT (U/L) | 64 ± 7.8 | 102 ± 26 * | 90 ± 14 | 89 ± 17 |
| ALB (g/dl) | 2.8 ± 0.14 | 2.9 ± 0.14 | 3.1 ± 0.1 | 2.9 ± 0.2 |
Data reported are mean ± SD;
HDL: High-density lipoprotein; LDL: low-density lipoprotein; TG: triglycerides; ALT: alanine transaminase; ALP: alkaline phosphatase; ALB: serum albumin; AOM: azoxymethane; Lactobacillus acidophilus: L. acidophilus; Bifidobacterium bifidum: B. bifidum.
P<0.05 compared to the health group;
P<0.05 compared to the AOM group;
P<0.05 compared to the B. bifidum.
Measurements of the other biochemical markers including the ALP, ALT, and ALB were performed in our study. In this regard, oral consumption of L. acidophilus significantly and non-significantly decreased the ALP (143±50 U/L) and ALT (90±14 U/L) in contrast to the AOM group (200±33 U/L for the ALP and 102±26 U/L for the ALT), respectively (P˂0.05). Moreover, we found that B. bifidum non-significantly decreased the ALP (181±11 U/L) and ALT (89±17 U/L) versus the AOM group. In spite of the ALP and ALT, a considerable change of the ALB was not seen in L. acidophilus and B. bifidum groups compared to the AOM group (Table 2).
Gene expression patterns
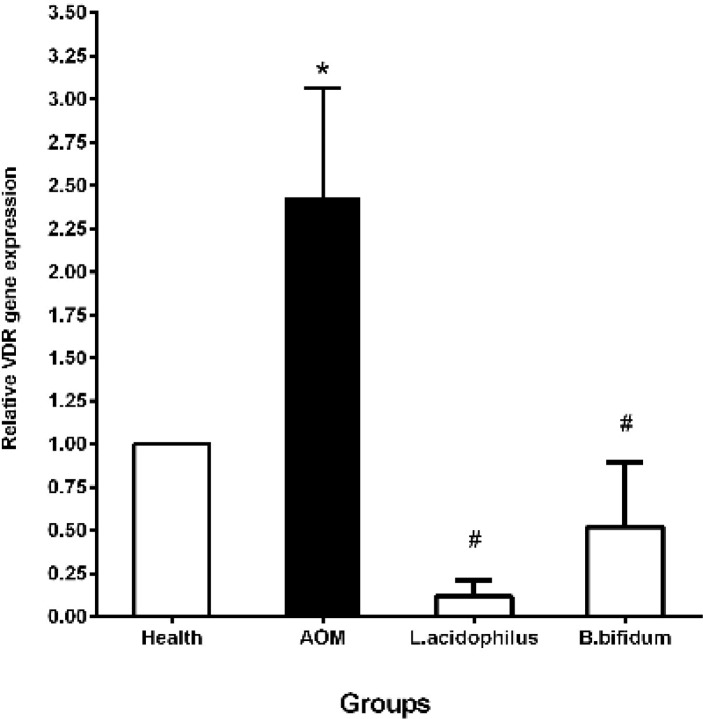
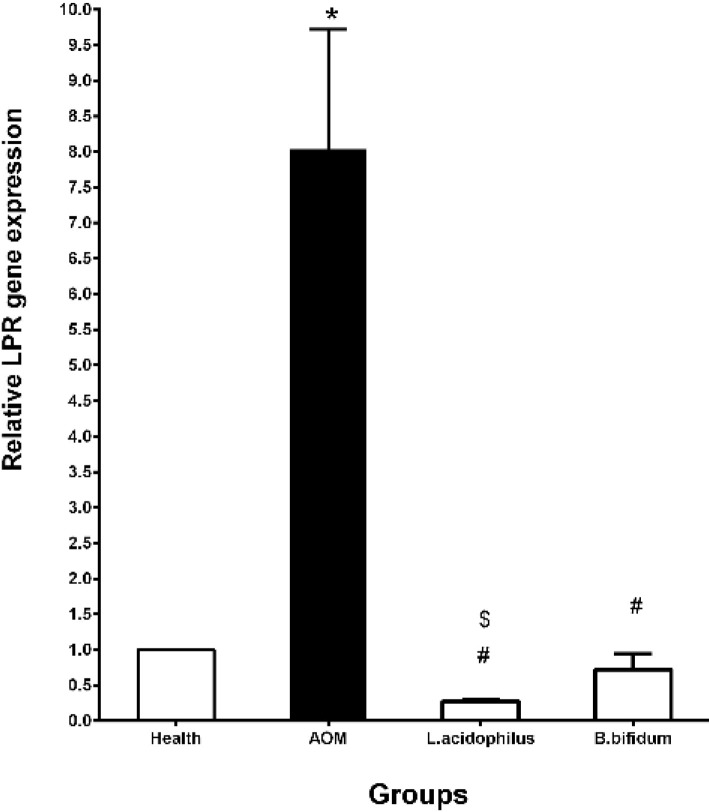
The expression of the VDR and LPR genes was evaluated in each of 4 groups with the real-time PCR method. Results in the present study indicated that the probiotics decreased the VDR and LPR gene expressions in comparison with the AOM and health groups. In this context, the VDR gene expression was significantly decreased in B. bifidum (0.52±0.37) and L. acidophilus (0.12±0.09) groups in comparison with the AOM group (2.43±0.63) (P˂0.05). Compared to the health group, the expression of the VDR gene was considerably increased in the AOM group (2.43±0.63) (P˂0.05) (Figure 1). Moreover, we found that L. acidophilus (0.27±0.03) and B. bifidum (0.72±0.22) probiotics significantly decreased the expression of the LPR gene against the AOM group (8.04±1.68) and the healthy group after 20 weeks. Similar to the VDR expression pattern, the LPR gene expression was significantly gone up in the AOM group (8.04±1.68) compared to the health group (Figure 2). Likewise, L. acidophilus (0.27±0.03) considerably declined the LPR gene expression versus B. bifidum (0.72±0.22) probiotic (Figure 2).
Figure 1.
Effects of L. acidophilus and B. bifidum probiotics on the VDR gene expression in a mouse model of colon cancer
Data reported are mean±SD; *P<0.05 compared to the health group; #P<0.05 compared to the AOM group; $P<0.05 compared to the B. bifidum; VDR=vitamin D receptor; AOM: azoxymethane, B. bifidum: Bifidobacterium bifidum, L. acidophilus: Lactobacillus acidophilus:
Figure 2.
Effects of L. acidophilus and B. bifidum probiotics on the LPR gene expression in a mouse model of colon cancer
Data reported are mean±SD; *P<0.05 compared to the health group; #P<0.05 compared to the AOM group; $P<0.05 compared to the B. bifidum group; LPR= Leptin receptor; AOM: azoxymethane, B. bifidum: Bifidobacterium bifidum, Lactobacillus acidophilus: L. acidophilus
Discussion
The major aim of the current study was to assess L. acidophilus and B. bifidum effects on the serum biochemical parameters, and the VDR and LPR genes in mice colon cancer. Our present study results have demonstrated that L. acidophilus and B. bifidum significantly decreased the serum level of the LDL and TG, and the gene expression of the LPR and VDR in comparison with the AOM group. Unlike B. bifidum, L. acidophilus considerably increased the serum level of the HDL in comparison with the AOM group. Furthermore, the oral consumption of L. acidophilus and B. bifidum significantly and non-significantly decreased the ALP, respectively. Thus, an oral consumption of L. acidophilus and B. bifidum probiotics could be effective on the serum biochemical parameters, and the VDR and LPR genes in mice colon cancer.
The data collected from in vitro studies were verified the anti-cancer effects of the Lactobacillus probiotic in CRC studies through various pathways. For instance, Lactobacillus strains have the ability to inhibit the growth of the HT-29 cells via the Bax/Bcl-2 pathway or NO production (23). Moreover, the exopolysaccharides from nine Lactobacillus strains have shown the inhibitory effects on the HT-29 cells via an induction of the apoptosis and up-regulation of the caspase-3 activity (24). Furthermore, L. acidophilus exopolysaccharides represented the anti-cancer potentialities against the CaCo-2 cells through the apoptotic and NF-κB inflammatory pathways (25). Moreover, our previous study indicated that unlike B. bifidum, the amount of L. acidophilus did not have any change in the mice colon cancer for 5 months (4). We have also concluded that the stability of L. acidophilus in the microflora concentration has the beneficial effects in the decrease of the CRC development with a significant decrease of the incidence and size of the colon tumor, and CA19-9 and CEA markers (5). The collected data from the previous studies have also demonstrated that there is a positive correlation between the serum lipids including the TC, LDL-C, and TG with a risk of the CRC (26). The current results have indicated that 20 weeks oral consumption of L. acidophilus and B. bifidum could meaningfully decrease the LDL-C and TG compared to the AOM group. Moreover, L. acidophilus with a significant increase of the HDL serum level has a more beneficial effect than B. bifidum. In this regard, the supplementation of L. acidophilus in combination with other probiotics and phytosterols could decrease the TC and LDL-C in hypercholesterolemic rats (27). In addition, yogurt containing L. acidophilus and B. lactis probiotics has dropped the TC and LDL-C concentrations in the type 2 diabetes patients (28). We have also assessed the other biochemical parameters such as the ALP and ALT that have been shown to increase about 30% of the patients with the liver metastases of the CRC (10). In this context, our study results have also indicated that L. acidophilus has significantly declined the ALP as compared to the B. bifidum and the AOM groups. An in vitro study demonstrated that the incubation of Lactobacillus rhamnosus GG in the Caco-2 cells caused the non-significant change in the ALP activity (29). Moreover, the consumption of Bifidobacterium longum and L. acidophilus indicated no effect on the ALP level of the fecal sample in the injected rats with 1, 2-dimethylhydrazine dihydrochloride (30). In contrast, the administration of Bifidobacterium spp decreased the TC, HDL-C, LDL-C, TG, leptin, and ALT in the high-fat diet-induced obese rats (31). Therefore, it seems that a reduction of the TC, LDL-C, TG, and ALP with L. acidophilus and B. bifidum probiotics might be a method for the protection of the CRC (26, 32).
Our results also demonstrated that the VDR gene expression was considerably climbed in the AOM group in comparison with the health group. In the preclinical studies, the contradictory outcomes have observed the VDR gene expression in colon cancer models. In this context, a high-level expression of the VDR has been observed in an early colorectal tumor progression, but a loss of the VDR expression was reported during the tumor dedifferentiation. Moreover, a high VDR expression was observed in the proximal colon compared to the distal colon, which can be correlated with the gut bacterial concentration (1). In our study, L. acidophilus and B. bifidum probiotics significantly declined the VDR gene expression in comparison with the AOM group. Treatment with the VSL (a probiotic including eight different strains of bacteria) has meaningfully raised the VDR expression in the proximal and distal colons in a rat model of colitis-associated cancer (33). This contradictory in results may be due to the difference in the type of animal models and/or the probiotic strains. On the other hand, there are associations between the VDR expression and the gut bacterial concentration. Thus, the probiotics with the modulation of the gut bacterial concentration can have useful effects on the VDR gene expression. However, the biological mechanisms between the VDR functions and gut bacteria are unclear and remain a main challenge in the future.
Similar to the VDR expression pattern, we have found that the LPR gene expression has significantly decreased in the AOM group versus the health group. A high level of the LPR expression was observed in the AOM group (34). Moreover, the results of other studies have indicated that the expression of the leptin and its receptor in the CRC patients could correlate with the tumor differentiation grade and depth of bowel wall invasion (13, 35). Furthermore, the results of a clinical study in the United States demonstrated that there is a relationship between the concentration of the microflora bacteria and the LP/LPR (36). Moreover, a preclinical study has shown that the VSL could decline the leptin expression in the mesenteric adipose tissue in the Crohn’s patients (37). In the present study, we have also found that 20 weeks oral consumption of L. acidophilus and B. bifidum probiotics considerably decreased the LPR gene expression in the mouse model of the AOM-induced colon cancer. Thus, L. acidophilus and B. bifidum probiotics with an improvement of the microflora bacteria have a decreasing effect on the LPR gene expression.
Conclusion
The concentration of the microflora bacteria, leptin/LPR, and vitamin D/VDR has been associated with colon cancer as environmental factors. Moreover, the previous studies displayed that the high serum level of the LDL-C and TG and also the high activity of the ALP are correlated with the CRC. Our results in the present study demonstrated that L. acidophilus and B. bifidum have significantly decreased the LDL-C, TG, LPR, and VDR gene expression compared to the AOM group. It seems that L. acidophilus with a significant increase in the HDL serum level and a significant decrease in the ALP activity has a more beneficial effect than B. bifidum in mouse colon cancer. Thus, L. acidophilus and B. bifidum probiotics with the modification of the microflora bacteria concentration and their effect on the LPR, VDR, and the mentioned biochemical parameters may play an essential role on the CRC. However, the biological mechanisms between the mentioned options and gut bacteria are unclear, and future evaluations are necessary for defining an exact mechanism.
Acknowledgment
This study was supported by Tehran University of Medical Sciences (Grant Number: 17733). None of the funding sources had any role in the study design, collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the article for publication.
Compliance with ethical standards
Ethical approval
All procedures performed in studies involving animals were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Conflict of Interest
The authors of the manuscript have no conflicts of interest to declare and are responsible for the content of the paper.
References
- 1.Lu R, Wu S, Xia Y, Sun J. The vitamin D receptor, inflammatory bowel diseases, and colon cancer. Curr Colorectal Cancer Rep. 2012;8:57–65. doi: 10.1007/s11888-011-0114-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kaji R, Kiyoshima-Shibata J, Nagaoka M, Nanno M, Shida K. Bacterial teichoic acids reverse predominant IL-12 production induced by certain lactobacillus strains into predominant IL-10 production via TLR2-dependent ERK activation in macrophages. J Immunol. 2010;184:3505–3513. doi: 10.4049/jimmunol.0901569. [DOI] [PubMed] [Google Scholar]
- 3.Ranji P, Akbarzadeh A, Rahmati-Yamchi M. Associations of probiotics with vitamin D and leptin receptors and their effects on colon cancer. Asian Pac J Cancer Prev. 2015;16:3621–3627. doi: 10.7314/apjcp.2015.16.9.3621. [DOI] [PubMed] [Google Scholar]
- 4.Khavari-Daneshvar H, Mosavi M, Khodayari H, Rahimi E, Ranji P, Mohseni AH, et al. Modifications of mice gut microflora following oral consumption of Lactobacillus acidophilus and Bifidobacterium bifidum probiotics. Turk J Med Sci. 2017;47:689–694. doi: 10.3906/sag-1504-28. [DOI] [PubMed] [Google Scholar]
- 5.Agah S, Alizadeh AM, Mosavi M, Ranji P, Khavari-Daneshvar H, Ghasemian F, et al. More Protection of Lactobacillus acidophilus than Bifidobacterium bifidum probiotics on azoxymethane-induced mouse colon cancer. Probiotics Antimicrob Proteins. 2018;10:1. doi: 10.1007/s12602-018-9425-8. [DOI] [PubMed] [Google Scholar]
- 6.Samuel S, Sitrin MD. Vitamin D’s role in cell proliferation and differentiation. Nutr Rev . 2008;66:116–124. doi: 10.1111/j.1753-4887.2008.00094.x. [DOI] [PubMed] [Google Scholar]
- 7.Zheng W, Wong KE, Zhang Z, Dougherty U, Mustafi R, Kong J, et al. Inactivation of the vitamin D receptor in APCmin/+ mice reveals a critical role for the vitamin D receptor in intestinal tumor growth. Int J Cancer . 2012;130:10–19. doi: 10.1002/ijc.25992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bouillon R, Carmeliet G, Verlinden L, van Etten E, Verstuyf A, Luderer HF, et al. Vitamin D and human health: lessons from vitamin D receptor null mice. Endocr Rev. 2008;29:726–776. doi: 10.1210/er.2008-0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lagishetty V, Misharin AV, Liu NQ, Lisse TS, Chun RF, Ouyang Y, et al. Vitamin D deficiency in mice impairs colonic antibacterial activity and predisposes to colitis. Endocrinology. 2010;151:2423–2432. doi: 10.1210/en.2010-0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu XZ, Ma F, Wang XL. Serological diagnostic factors for liver metastasis in patients with colorectal cancer. World J Gastroenterol. 2010;16:4084–4088. doi: 10.3748/wjg.v16.i32.4084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Menendez C, Lage M, Peino R, Baldelli R, Concheiro P, Dieguez C, et al. Retinoic acid and vitamin D (3) powerfully inhibit in vitro leptin secretion by human adipose tissue. J Endocrinol. 2001;170:425–431. doi: 10.1677/joe.0.1700425. [DOI] [PubMed] [Google Scholar]
- 12.Wu S, Liao AP, Xia Y, Li YC, Li J-D, Sartor RB, et al. Vitamin D receptor negatively regulates bacterial-stimulated NF-κB activity in intestine. Am J Pathol. 2010;177:686–697. doi: 10.2353/ajpath.2010.090998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hui L, Desen W, Zhizhong P, Lijing C, Xiaojun W, Zhenhai L, et al. Expression and biological significance of leptin, leptin receptor, VEGF, and CD34 in colorectal carcinoma. Cell Biochem Biophys. 2011;60:241–244. doi: 10.1007/s12013-010-9145-5. [DOI] [PubMed] [Google Scholar]
- 14.Endo H, Hosono K, Uchiyama T, Sakai E, Sugiyama M, Takahashi H, et al. Leptin acts as a growth factor for colorectal tumours at stages subsequent to tumour initiation in murine colon carcinogenesis. Gut. 2011;60:1363–1371. doi: 10.1136/gut.2010.235754. [DOI] [PubMed] [Google Scholar]
- 15.Bolukbas FF, Kilic H, Bolukbas C, Gumus M, Horoz M, Turhal NS, et al. Serum leptin concentration and advanced gastrointestinal cancers: a case controlled study. BMC Cancer. 2004;4:29. doi: 10.1186/1471-2407-4-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kumar M, Nagpal R, Verma V, Kumar A, Kaur N, Hemalatha R, et al. Probiotic metabolites as epigenetic targets in the prevention of colon cancer. Nutr Rev. 2013;71:23–34. doi: 10.1111/j.1753-4887.2012.00542.x. [DOI] [PubMed] [Google Scholar]
- 17.Drew JE. Molecular mechanisms linking adipokines to obesity-related colon cancer: focus on leptin. Proc Nutr Soc. 2012;71:175–180. doi: 10.1017/S0029665111003259. [DOI] [PubMed] [Google Scholar]
- 18.Matsunuma A, Horiuchi N. Leptin attenuates gene expression for renal 25-hydroxyvitamin D 3-1α-hydroxylase in mice via the long form of the leptin receptor. Arch Biochem Biophys. 2007;463:118–127. doi: 10.1016/j.abb.2007.02.031. [DOI] [PubMed] [Google Scholar]
- 19.Heydari Z, Alizadeh AM, Agah SH, Khalighfard S, Bahmani S. Effects of Lactobacillus acidophilus and Bifidobacterium bifidum probiotics on the expression of microRNAs 135b, 26b, 18a and 155, and their involving genes in mice colon cancer. Probiotics Antimicrob Proteins. 2018:1–8. doi: 10.1007/s12602-018-9478-8. [DOI] [PubMed] [Google Scholar]
- 20.Kavosi A, Noei SH, Madani S, Khalighfard S, Khodayari S, Khodayari H, et al. The toxicity and therapeutic effects of single-and multi-wall carbon nanotubes on mice breast cancer. Sci Rep. 2018;8:8375. doi: 10.1038/s41598-018-26790-x. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 21.Mohsenikia M, Farhangi B, Alizadeh AM, Khodayari H, Khodayari S, Khori V, et al. Therapeutic effects of dendrosomal solanine on a metastatic breast tumor. Life Sci. 2016;148:260–267. doi: 10.1016/j.lfs.2016.02.008. [DOI] [PubMed] [Google Scholar]
- 22.Khori V, Shalamzari SA, Isanejad A, Alizadeh AM, Alizadeh S, Khodayari S, et al. Effects of exercise training together with tamoxifen in reducing mammary tumor burden in mice: Possible underlying pathway of miR-21. Eur J Pharm. 2015;765:179–187. doi: 10.1016/j.ejphar.2015.08.031. [DOI] [PubMed] [Google Scholar]
- 23.Chen Z-Y, Hsieh Y-M, Huang C-C, Tsai C-C. Inhibitory effects of probiotic Lactobacillus on the growth of human colonic carcinoma cell line HT-29. Molecules. 2017;22:107. doi: 10.3390/molecules22010107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Di W, Zhang L, Yi H, Han X, Zhang Y, Xin L. Exopolysaccharides produced by Lactobacillus strains suppress HT-29 cell growth via induction of G0/G1 cell cycle arrest and apoptosis. Oncol Lett. 2018;16:3577–3586. doi: 10.3892/ol.2018.9129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.El-Deeb NM, Yassin AM, Al-Madboly LA, El-Hawiet A. A novel purified Lactobacillus acidophilus 20079 exopolysaccharide, LA-EPS-20079, molecularly regulates both apoptotic and NF-kappaB inflammatory pathways in human colon cancer. Microb Cell Fact. 2018;17:29. doi: 10.1186/s12934-018-0877-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sun ZJ, Huang YH, Wu JS, Yang YC, Chang YF, Lu FH, et al. The association of serum lipids with the histological pattern of rectosigmoid adenoma in Taiwanese adults. BMC Gastroenterol. 2011;11:54. doi: 10.1186/1471-230X-11-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Awaisheh S, Khalifeh M, Al-Ruwaili M, Khalil O, Al-Ameri O, Al-Groom R. Effect of supplementation of probiotics and phytosterols alone or in combination on serum and hepatic lipid profiles and thyroid hormones of hypercholesterolemic rats. J Dairy Sci. 2013;96:9–15. doi: 10.3168/jds.2012-5442. [DOI] [PubMed] [Google Scholar]
- 28.Ejtahed H, Mohtadi-Nia J, Homayouni-Rad A, Niafar M, Asghari-Jafarabadi M, Mofid V, et al. Effect of probiotic yogurt containing Lactobacillus acidophilus and Bifidobacterium lactis on lipid profile in individuals with type 2 diabetes mellitus. J Dairy Sci. 2011;94:3288–3294. doi: 10.3168/jds.2010-4128. [DOI] [PubMed] [Google Scholar]
- 29.Turner P, Wu Q, Piekkola S, Gratz S, Mykkänen H, El-Nezami H. Lactobacillus rhamnosus strain GG restores alkaline phosphatase activity in differentiating Caco-2 cells dosed with the potent mycotoxin deoxynivalenol. Food Chem Toxicol. 2008;46:2118–2123. doi: 10.1016/j.fct.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 30.Oda T, Seto Y, Hashiba H. Supplementation of Bifidobacterium longum to a high-fat, low-calcium diet lowers cytolytic activity of fecal water in rats injected with 1, 2-dimethylhydrazine dihydrochloride. J Nutr Sci Vitaminol (Tokyo) 1998;44:187–194. doi: 10.3177/jnsv.44.187. [DOI] [PubMed] [Google Scholar]
- 31.An HM, Park SY, Lee DK, Kim JR, Cha MK, Lee SW, et al. Antiobesity and lipid-lowering effects of Bifidobacterium spp in high fat diet-induced obese rats. Lipids Health Dis. 2011;10:116. doi: 10.1186/1476-511X-10-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li C, Li J, Bai P, Lv Y. Characteristic analysis of plasma lipid metabolism level in patients with colorectal adenoma. Beijing Da Xue Xue Bao. 2011;43:432–435. [PubMed] [Google Scholar]
- 33.Appleyard CB, Cruz ML, Isidro AA, Arthur JC, Jobin C, De Simone C. Pretreatment with the probiotic VSL# 3 delays transition from inflammation to dysplasia in a rat model of colitis-associated cancer. Am J Physiol Gastrointest Liver Physiol. 2011;301:1004–1013. doi: 10.1152/ajpgi.00167.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bartucci M, Svensson S, Ricci-Vitiani L, Dattilo R, Biffoni M, Signore M, et al. Obesity hormone leptin induces growth and interferes with the cytotoxic effects of 5-fluorouracil in colorectal tumor stem cells. Endocr Relat Cancer. 2010;17:823–833. doi: 10.1677/ERC-10-0083. [DOI] [PubMed] [Google Scholar]
- 35.Joshi RK, Lee S-A. Obesity related adipokines and colorectal cancer: a review and meta-analysis. Asian Pac J Cancer Prev. 2014;15:397–405. doi: 10.7314/apjcp.2014.15.1.397. [DOI] [PubMed] [Google Scholar]
- 36.Slattery M, Ballard-Barbash R, Edwards S, Caan BJ, Potter JD. Body mass index and colon cancer: an evaluation of the modifying effects of estrogen (United States) Cancer Causes Control. 2003;14:75–84. doi: 10.1023/a:1022545017867. [DOI] [PubMed] [Google Scholar]
- 37.Mencarelli A, Distrutti E, Renga B, D’Amore C, Cipriani S, Palladino G, et al. Probiotics modulate intestinal expression of nuclear receptor and provide counter-regulatory signals to inflammation-driven adipose tissue activation. PLoS One. 2011;6:e22978. doi: 10.1371/journal.pone.0022978. [DOI] [PMC free article] [PubMed] [Google Scholar]