Abstract
Objective(s):
The aim of this study was to investigate the effect of vitamin D on glucose metabolism, as well as the expression of five key genes involved in the development of diabetes complications in liver tissue of diabetic rats.
Materials and Methods:
Twenty-four male Sprague–Dawley rats were randomly divided into three groups (8 rats in each group). The first group served as control and the other two groups received an intraperitoneal injection of 45 mg/kg streptozotocin to develop diabetes. Groups were treated for four weeks either with placebo or vitamin D (two injections of 20000 IU/kg). Thereafter, serum levels of glucose, insulin and HbA1c were assessed. Liver tissue was examined for the level of advanced glycation end products (AGEs) and the gene expression of AGE cellular receptor (AGER), glyoxalase-1 (GLO-1), aldose reductase (AR), O-linked N-acetylglucosamine transferase (OGT) and glutamine/ fructose-6-phosphate aminotransferase (GFAT).
Results:
Vitamin D injection resulted in a significant increase in plasma level of 25-hydroxycholecalciferol, which could improve hyperglycemia about 11% compared to placebo-receiving diabetic rats (P=0.005). Insulin level increased as a result of vitamin D treatment compared to control (3.31±0.65 vs. 2.15±0.79; P= 0.01). Serum HbA1c and liver AGE concentrations had a slight but insignificant reduction following vitamin D intake. Moreover, a significant decline was observed in gene expression of AGER and OGT in liver tissue (P=0.04 and P<0.001 respectively).
Conclusion:
Vitamin D might contribute in ameliorating diabetes complications not only by improving blood glucose and insulin levels, but also by suppressing AGER and OGT gene expression in the liver.
Key Words: Advanced glycation end – products, Cholecalciferol, Diabetes complications, Hexosamine pathway Vitamin D
Introduction
Global prevalence of diabetes mellitus has been reported in more than 415 million adult populations in 2015, and it is estimated that this figure, by more than 50% growth, will reach 642 million people by 2040. For this reason, the International Diabetes Federation (IDF) has symbolically named Diabetes as the third most populous country in the world. Despite different methods and medications to control blood glucose, diabetes complications such as heart disease, kidney and nerve damages impose enormous medical expenses on the governments, such that most countries spend 5 to 20% of their total health expenditure on diabetes control (1, 2). To date, several mechanisms have been proposed for the etiology and metabolic pathways involved in diabetic complications. The most important pathways include: 1) the pathway for production of advanced glycation end products (AGE) in cells, 2) Polyols pathway, 3) Hexosamine biosynthesis pathway (HBP), 4) the protein kinase C pathway, and 5) Reactive oxygen species (ROS) mitochondrial pathway (3, 4). Several strategies have been explored to decrease the complications of diabetes. Although these strategies do not result in the treatment of the disease, they can considerably decrease health costs and increase life expectancy and quality of life of diabetic patients. Modification of lifestyle and especially an appropriate diet is one of the primary solutions in this regard (5). Receiving accurate and balanced macronutrients and micronutrients play a significant role in diabetes control -as a chronic illness associated with metabolic disorders (6, 7). Vitamin D is one of the micronutrients whose relationship with diabetes has been taken into consideration recently. Several studies have shown that the incidence of type I and type II diabetes is associated with vitamin D deficiency (8, 9). Some studies have shown that receiving vitamin D supplement is associated with reduced complications such as diabetic nephropathy and retinopathy (10-12).
Considering the high prevalence of both diabetes and vitamin D deficiency in the world, the scrutiny of their relationship is of paramount importance. But, the existing evidences are controversial and inconclusive. For this purpose, the present study focused on the cellular role of vitamin D and its precise mechanism of action in reducing the complications of diabetes. Therefore, some of the most important components of pathways involved in diabetic complications were selected for further study. These components included: 1) AGE receptor (AGER) that is activated through binding to glycated compounds that are produced through non-enzymatic reactions following a high concentration of sugar in the blood, and leads to increased production of nuclear factor-kappa B (NF-κB) and its subsequent inflammatory complications. 2) Glyoxalase-1 (GLO-1) is an enzyme that decreases the AGE concentration and the possibility of their attachment to AGER by metabolizing AGE and hence decreases the activation of inflammatory pathway. 3) Aldose reductase (AR) that is the main enzyme of polyols pathway and is responsible for the production of compounds like sorbitol and galactitol and complications caused by them in diabetes. 4) O-linked N-acetylglucosamine transferase (OGT) that is an enzyme that attaches glucosamine produced in the hexosamine pathway to protein compounds and by increasing the production of glycosylated proteins contributes in the development of diabetic complications. 5) Glutamine: fructose-6-phosphate aminotransferase (GFAT) that is known as the main regulatory enzyme of hexosamine pathway (4).
The aim of this study was to investigate the effect of vitamin D consumption on the serum levels of glucose, insulin, HbA1c, and AGE, as well as the expression of five previously mentioned key genes involved in the development of diabetes complications in liver tissue.
Materials and Methods
Animals
Initially, 30 Sprague-Dawley male rats aged 3-4 months with an average weight of 300±40 g were purchased and kept in standard laboratory conditions at a temperature of 20-25 °C and with 12-hr light/dark cycle for 10 days prior to the start of the study. Throughout the study, animals had free access to drinking water and standard laboratory chow containing 0.95 to 1.0% calcium and 0.65 to 0.70% phosphorus. Animals were cared in accordance with Guide on the Care and Use of Experimental Animals, and outmost attempt was made to minimize the number of laboratory animals and also to alleviate the pain and damage caused by the experiment (13). The experimental protocol was approved by the Ethical Committee of the Tehran University of Medical Sciences, Tehran, Iran.
Study design
The rats were randomly divided into three groups and two rats were placed in each cage. At the beginning of the study, the rats in groups 2 and 3 received an intraperitoneal (IP) injection of Streptozotocin (STZ) (45 mg/kg, ~ 20 μl) and the rats in the control group were injected with the same volume of citrate buffer as placebo. STZ was purchased from Sigma (Sigma–Aldrich, St. Louis, Missouri, USA) and was dissolved in sterile sodium citrate buffer with pH of 5-6 immediately before injection. One week later, fasting plasma glucose (FPG) of all animals was measured by glucometer (Accu-check, Roche Diagnostic GmbH, Mannheim, Germany) and values greater than 250 mg/dl were considered as an indicator of diabetes. Finally, 8 rats that met the inclusion criteria were assigned to each group. Animals in group 3 on the 1st and 14th days of diabetes development received intramuscular (IM) injection of 20000 IU/kg vitamin D. Given that sesame oil was used for diluting vitamin D, it was injected to other groups as placebo. Food intake was checked and weighted per cage on a daily basis. The animals’ weight was recorded weekly and on the final day. After four weeks of vitamin D injection, all animals were anesthetized and sacrificed with IP injection of ketamine (50 mg/kg) and xylazine (30 mg/kg). Blood and liver samples of animals were immediately collected and kept in proper temperature.
Biochemical tests
Fasting blood samples were collected (between 8:00 to 10:00 AM) by cardiac puncture and centrifuged immediately to separate the serum. The serums and liver samples were immediately frozen in liquid nitrogen and stored at -80 °C until biochemical assays. Serum levels of glucose, HbA1c and calcium were evaluated by auto-analyzer (Biotecnica Instruments, Rome, Italy). Vitamin D, insulin and AGEs concentrations were measured by commercial enzyme-linked immunosorbent assay (ELISA) kits (IBL international, Hamburg, Germany).
Evaluation of gene expression
Initially, liver tissues were homogenized and mRNA was extracted using Hybrid-R RNA isolation kit (GeneAll Biotech., Seoul, South Korea). The amount and purity of RNA was evaluated by Nanodrop spectrophotometer (NanoDrop Technologies, Wilmington, Del., USA) and the ratios of 260/280 and 260/230 ~ 2.0 were considered as acceptable values. Then, cDNA was made and the expression levels of AGER, GLO-1, AR, OGT and GFAT genes were assessed by quantitative Real-time PCR using SYBR Premix Ex Taq II (Takara Bio Inc., Shiga, Japan). Sequences of primers were designed by using OligoCalc, Primer blast and GeneRunner softwares, and their specifications are presented in Table 1. Expression of mRNA was calculated with ΔCt method and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was considered as housekeeping gene.
Table 1.
Primer sequences for real-time PCR
| Gene | Sequence (5' → 3') | Length | Tm | GC% |
|---|---|---|---|---|
| AGER | F: ACAGAAACCGGTGATGAAGGA | 21 | 59.3 | 47 |
| R: TCTCCTCGAGTCTGGGTTG | 19 | 58.1 | 57 | |
| GLO-1 | F: TGACGAGACGCAGAGTTACC | 20 | 59.4 | 55 |
| R: GATCTTGAACGAACGCCAGAC | 21 | 59.6 | 52 | |
| AR | F: AGTAGCTGAGGAGTTTCTTCG | 21 | 57.1 | 47 |
| R: CATAGGACTGGAGTTCTAAGCA | 22 | 56.9 | 45 | |
| OGT | F: AGCTCTTCGTCTGTGTCCTA | 20 | 57.8 | 50 |
| R: CAAACTCTGGGAAGACCTCTA | 21 | 56.3 | 47 | |
| GFAT | F: TTGATTCTGATTGCTTGTGGC | 21 | 57.4 | 43 |
| R: ACAGTAGCGAAGACCCATCA | 20 | 58.4 | 50 | |
| GAPDH | F: CATTCTTCCACCTTTGATGCTG | 22 | 57.9 | 46 |
| R: TGGTCCAGGGTTTCTTACTCC | 21 | 58.9 | 52 |
AGER, advanced glycation end products receptor; GLO-1, glyoxalase-1; AR, aldose reductase; OGT, O-GlcNAc transferase; GFAT, glutamine: fructose-6-phosphate aminotransferase; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; Tm, primer melting temperature; GC%, primer guanine-cytosine content; F, forward; R, reverse
Statistical analysis
Data were expressed as mean±SD and were analyzed using Statistical Package for the Social Sciences (SPSS, version 21.0; SPSS Inc., Chicago, Illinois, USA) and the graphs were designed by GraphPad Prism (Version 7.03; GraphPad Software, La Jolla California, USA). Kolmogorov-Smirnov test was used to assess the normality of the data. In order to remove the effects of confounding factors such as body weight and food intake, analysis of covariance (ANCOVA) followed by the Bonferroni post hoc test were used to compare statistical differences between the groups. Furthermore, the non-parametric data were transformed logarithmically for analysis. P-values less than 0.05 were considered as statistically significant.
Results
At the beginning and end of this study, groups were compared in terms of body weight and food intake. No significant difference was found between groups at the beginning. However, the two diabetic groups had a lower food intake and body weight at the endpoint (P < 0.001; Table 2). Therefore, ANCOVA test was used to control the possible confounding effect of these factors.
Table 2.
Body weight and food intake of different groups
| Groups | Initial weight (g) | Final weight (g) | Food intake (g/day) |
|---|---|---|---|
| Control | 294.3±15.4 | 304.0±22.1 | 29.0±1.2 |
| Diabetic | 295.5±14.5 | 222.8±30.1* | 22.9±2.4* |
| Diabetic + vitamin D | 296.5±11.1 | 198.0±40.0* | 24.9±4.0* |
Data are presented as the Mean ± SD (n = 8 for all groups). Statistical differences were determined using paired t-test;
, P < 0.05 compared to the control group;
, P < 0.05 compared to the diabetic group.
After four weeks of treatment, the mean FPG level was increased drastically in diabetic animals in comparison with the control group (P<0.001). Two injection of vitamin D resulted in a significant increase in plasma cholecalciferol level and could improve hyperglycemia and hypoinsulinemia in comparison with diabetic rats (P=0.005 and P=0.01, respectively). Calcium level was checked and hypercalcemia was not observed in any group. Serum HbA1c and liver AGE concentrations had a slight but insignificant decrease after vitamin D injection (Table 3).
Table 3.
Biochemical factors in different experimental groups
| Groups | FPG (mg/dl) |
Insulin (mIU/l) |
HbA1c (%) |
Liver AGE (ng/ml) |
Vitamin D (ng/ml) |
Calcium (mg/dl) |
|---|---|---|---|---|---|---|
| Control | 85.87±12.63 | 3.37±0.83 | 4.65±0.50 | 103.78±13.71 | 20.93±2.49 | 9.27±0.59 |
| Diabetic | 479.37±27.90* | 2.15±0.79* | 8.75±0.48* | 123.98±10.79* | 21.33±2.44 | 9.47±0.41 |
| Diabetic + vitamin D | 428.87±37.74*# | 3.31±0.65# | 8.30±0.55* | 118.72±12.58* | 35.42±3.96*# | 9.52±0.95 |
Data are presented as the Mean ± SD (n = 8 for all groups). FPG, fasting plasma glucose; AGE, advanced glycation end products. Statistical differences were determined using ANCOVA followed by a Bonferroni post hoc test;
, P < 0.05 compared to the control group;
, P < 0.05 compared to the diabetic group.
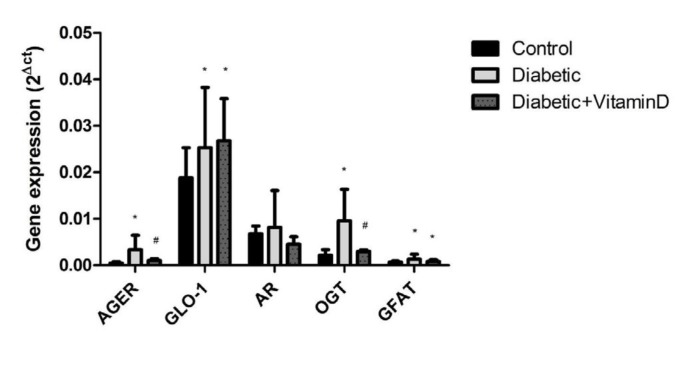
Expression of AGER mRNA was dramatically increased in diabetic group (P<0.001). However, vitamin D could down-regulate it significantly (P=0.04). The expression of GLO-1, another key enzyme in AGE pathway, was elevated in all diabetic rats (P=0.02) and vitamin D treatment did not show any additional effect (P=0.7). Aldose reductase gene expression did not change neither in diabetic nor vitamin D-receiving rats (P=0.2). In the hexosamine pathway, OGT and GFAT expression was elevated in diabetic group (P<0.001 and P=0.02, respectively), but only OGT improved as a result of vitamin D injection (P<0.001; Figure 1).
Figure 1.
Gene expression of advanced glycation end products receptor (AGER), glyoxalase-1 (GLO-1), aldose reductase (AR), O-GlcNAc transferase (OGT) and glutamine: fructose-6-phosphate aminotransferase (GFAT) in the liver tissue of different experimental groups. Data are presented as the Mean±SD (n=8 for all groups). *, P<0.05 compared to the control group; #, P<0.05 compared to the diabetic group
Discussion
In recent years, numerous epidemiological and experimental studies have indicated that vitamin D deficiency can play a role in the incidence and progression of both type I and type II diabetes (14, 15). Although low levels of vitamin D have been observed in patients with diabetes, clear and sufficient data has not been obtained from clinical trials to confirm the causative or therapeutic effect of vitamin D in diabetes. According to the results of this study, two injections of vitamin D (20000 IU/kg) and its increased serum levels could lead to a significant reduction in blood glucose. This dose was chosen based on the results of our pilot study to elevate the serum level of vitamin D up to 30 ng/ml. In addition, HbA1c showed a moderate decline, which was not statistically significant probably due to the short period of study. It seems that substantial increase in the insulin level is one of the main reasons for the improvement of blood glucose in this experiment. Previous studies also suggest that adequate serum levels of vitamin D can protect pancreatic beta cells and help insulin synthesis and secretion (16-18). Also, other mechanisms have been proposed to explain the role of vitamin D in improving blood glucose levels. For example, it has been reported that vitamin D can increase the glucose transporter 4 (GLUT4) gene expression or improve the performance of glucagon-like peptide 1 (GLP-1), and these pathways were not investigated in the present study (19, 20).
The results of this study also showed that vitamin D independent of its effects on the control of glucose metabolism might reduce the complications of diabetes by influencing cellular pathways like AGE. Previous studies have shown that there is a direct relationship between serum levels of AGE and the incidence of diabetic microvascular complications such as nephropathy, neuropathy and retinopathy (21-23). According to the results of this one-month examination, injections of vitamin D had no significant effect on AGE level in liver tissue. However, vitamin D decreased the expression of AGE cellular receptor gene. Therefore, it is expected that vitamin D may delay the complications of diabetes (24). The possible changes in chemokines, cytokines, adhesion molecules, and other compounds that increase following the activation of AGE pathway can be the subject of future studies. On the other hand, the hypothesis that vitamin D can impact on diabetes complications via the changes in the activity of glyoxalase enzyme (the enzyme that catalyzes and eliminates glycosylated compounds from the body) was rejected due to lack of change in GLO-1 gene expression. The level of this enzyme was elevated in all diabetic rats, which might be a protective response to hyperglycemia and activation of AGE pathway (25). Nevertheless, vitamin D treatment did not cause any additional effect.
Moreover, vitamin D had no effect on aldose reductase gene expression in liver tissue. Previous studies have shown that increased expression and function of this gene in diabetic people can increase the speed of incidence of diabetes complications, while inhibiting this enzyme can improve the patient’s condition (26, 27). Considering the fact that no significant change was observed in aldose reductase gene expression in diabetic rats, it seems that the duration of this study has not been enough to measure detectable changes in this gene. Besides, the possible protective effect of vitamin D was not also detectable.
Based on our findings, OGT enzyme levels had a significant increase in the liver of diabetic rats. This enzyme catalyzes the attachment of N-acetylglucosamine unit to the serine and threonine residues of proteins. Glycosylated proteins play a role in the development of diabetic complications (28). Moreover, it is likely that this enzyme by affecting the structure and function of proteins such as insulin receptor substrate 1 (IRS1) and serine/threonine-protein kinase 2 (AKT2) as well as by reducing insulin signaling cause insulin resistance (29). In addition, the results of Inbathamizh et al. analysis suggest that probably calcitriol in terms of three-dimensional structure and active sites might have an inhibitory effect on glucosamine transferase enzyme (30). In this study, vitamin D significantly reduced OGT gene expression in the liver. The expression of GFAT gene, as an important enzyme in hexosamine pathway, increased in diabetic rats and this change was not improved by vitamin D consumption within one-month study period.
Examination of the weight and food intake of rats showed a mild decrease in food intake accompanied by a dramatic weight loss in diabetic groups. Hence, the role of these confounding variables was eliminated in all statistical comparisons.
The main strength of this study was to investigate the role of vitamin D in regulating gene expression of key receptors and enzymes involved in developing the long term complications of diabetes for the first time. However, measuring the levels of proteins and enzymes activity could certainly improve the reliability of present data and it may be the subject of future investigations.
Conclusion
In summary, this study showed that injection of vitamin D and its increased serum levels lead to a significant improvement in blood glucose and insulin levels as well as a decrease in AGER and OGT gene expression in the liver of diabetic rats. Thus, it can be concluded that vitamin D may contribute in reducing diabetes complications mainly through AGE and hexosamine pathways.
Acknowledgment
This study was funded by the Tehran University of Medical Sciences, Tehran, Iran. Assistance of the staffs is gratefully appreciated. The results described in this paper were part of student thesis.
Conflicts of Interest
All contributing authors declare that they have no conflicts of interest.
References
- 1.Ogurtsova K, da Rocha Fernandes J, Huang Y, Linnenkamp U, Guariguata L, Cho N, et al. IDF Diabetes Atlas: Global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res Clin Pract. 2017;128:40–50. doi: 10.1016/j.diabres.2017.03.024. [DOI] [PubMed] [Google Scholar]
- 2.Herman WH. The Global burden of diabetes: An overview. diabetes mellitus in developing countries and underserved communities. Springer; 2017. pp. 1–5. [Google Scholar]
- 3.Madonna R, De Caterina R. Cellular and molecular mechanisms of vascular injury in diabetes—part I: pathways of vascular disease in diabetes. Vascul Pharmacol . 2011;54:68–74. doi: 10.1016/j.vph.2011.03.005. [DOI] [PubMed] [Google Scholar]
- 4.Forbes JM, Cooper ME. Mechanisms of diabetic complications. Physiol Rev. 2013;93:137–188. doi: 10.1152/physrev.00045.2011. [DOI] [PubMed] [Google Scholar]
- 5.Group DPPR. The 10-year cost-effectiveness of lifestyle intervention or metformin for diabetes prevention. Diabetes Care. 2012;35:723–730. doi: 10.2337/dc11-1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Evert AB, Boucher JL, Cypress M, Dunbar SA, Franz MJ, Mayer-Davis EJ, et al. Nutrition therapy recommendations for the management of adults with diabetes. Diabetes Care. 2013;36:3821–3842. doi: 10.2337/dc13-2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ley SH, Hamdy O, Mohan V, Hu FB. Prevention and management of type 2 diabetes: dietary components and nutritional strategies. Lancet. 2014;383:1999–2007. doi: 10.1016/S0140-6736(14)60613-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hyppönen E, Läärä E, Reunanen A, Järvelin M-R, Virtanen SM. Intake of vitamin D and risk of type 1 diabetes: a birth-cohort study. Lancet. 2001;358:1500–1503. doi: 10.1016/S0140-6736(01)06580-1. [DOI] [PubMed] [Google Scholar]
- 9.Pittas AG, Lau J, Hu FB, Dawson-Hughes B. The role of vitamin D and calcium in type 2 diabetes A systematic review and meta-analysis. J Clin Endocrinol Metab. 2007;92:2017–2029. doi: 10.1210/jc.2007-0298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaur H, Donaghue KC, Chan AK, Benitez-Aguirre P, Hing S, Lloyd M, et al. Vitamin D deficiency is associated with retinopathy in children and adolescents with type 1 diabetes. Diabetes Care. 2011;34:1400–1402. doi: 10.2337/dc11-0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shehab D, Al-Jarallah K, Mojiminiyi O, Al Mohamedy H, Abdella N. Does Vitamin D deficiency play a role in peripheral neuropathy in Type 2 diabetes? Diabet Med. 2012;29:43–49. doi: 10.1111/j.1464-5491.2011.03510.x. [DOI] [PubMed] [Google Scholar]
- 12.Diaz VA, Mainous AG, Carek PJ, Wessell AM, Everett CJ. The association of vitamin D deficiency and insufficiency with diabetic nephropathy: implications for health disparities. J Am Board Fam Med. 2009;22:521–527. doi: 10.3122/jabfm.2009.05.080231. [DOI] [PubMed] [Google Scholar]
- 13.Olfert ED, Cross BM, McWilliam AA. Guide to the care and use of experimental animals. Canadian Council on Animal Care Ottawa; 1993. [Google Scholar]
- 14.Husemoen LLN, Thuesen BH, Fenger M, Jørgensen T, Glümer C, Svensson J, et al. Serum 25 (OH) D and type 2 diabetes association in a general population. Diabetes Care. 2012;35:1695–1700. doi: 10.2337/dc11-1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zipitis CS, Akobeng AK. Vitamin D supplementation in early childhood and risk of type 1 diabetes: a systematic review and meta-analysis. Arch Dis Child. 2008;93:512–517. doi: 10.1136/adc.2007.128579. [DOI] [PubMed] [Google Scholar]
- 16.Park S, Kim DS, Kang S. Vitamin D deficiency impairs glucose-stimulated insulin secretion and increases insulin resistance by reducing PPAR-γ expression in nonobese Type 2 diabetic rats. J Nutr Biochem. 2016;27:257–265. doi: 10.1016/j.jnutbio.2015.09.013. [DOI] [PubMed] [Google Scholar]
- 17.Jayanarayanan S, Anju TR, Smijin S, Paulose CS. Vitamin D 3 supplementation increases insulin level by regulating altered IP3 and AMPA receptor expression in the pancreatic islets of streptozotocin-induced diabetic rat. J Nutr Biochem. 2015;26:1041–1049. doi: 10.1016/j.jnutbio.2015.04.011. [DOI] [PubMed] [Google Scholar]
- 18.Yang Z, Liu F, Qu H, Wang H, Xiao X, Deng H. 1, 25 (OH) 2 D 3 protects β cell against high glucose-induced apoptosis through mTOR suppressing. Mol Cell Endocrinol. 2015;414:111–119. doi: 10.1016/j.mce.2015.07.023. [DOI] [PubMed] [Google Scholar]
- 19.Manna P, Jain SK. Vitamin D up-regulates glucose transporter 4 (GLUT4) translocation and glucose utilization mediated by cystathionine-γ-lyase (CSE) activation and H2S formation in 3T3L1 adipocytes. J Biol Chem. 2012;287:42324–42332. doi: 10.1074/jbc.M112.407833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Enciso PL, Wang L, Kawahara Y, Sakamoto S, Shimada S, Takeichi Y, et al. Dietary vitamin D 3 improves postprandial hyperglycemia in aged mice. Biochem Biophys Res Commun. 2015;461:165–171. doi: 10.1016/j.bbrc.2015.04.008. [DOI] [PubMed] [Google Scholar]
- 21.Beisswenger PJ, Howell SK, Russell GB, Miller ME, Rich SS, Mauer M. Early progression of diabetic nephropathy correlates with methylglyoxal-derived advanced glycation end products. Diabetes Care. 2013;36:3234–3239. doi: 10.2337/dc12-2689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sveen KA, Karimé B, Jørum E, Mellgren SI, Fagerland MW, Monnier VM, et al. Small-and large-fiber neuropathy after 40 years of type 1 diabetes. Diabetes Care. 2013;36:3712–3717. doi: 10.2337/dc13-0788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Choudhuri S, Dutta D, Sen A, Chowdhury IH, Mitra B, Mondal LK, et al. Role of N–epsilon-carboxy methyl lysine, advanced glycation end products and reactive oxygen species for the development of nonproliferative and proliferative retinopathy in type 2 diabetes mellitus. Mol Vis . 2013;19:100–13. [PMC free article] [PubMed] [Google Scholar]
- 24.Ramasamy R, Vannucci SJ, Du Yan SS, Herold K, Yan SF, Schmidt AM. Advanced glycation end products and RAGE: a common thread in aging, diabetes, neurodegeneration, and inflammation. Glycobiology. 2005;15:16–28. doi: 10.1093/glycob/cwi053. [DOI] [PubMed] [Google Scholar]
- 25.Brouwers O, Niessen PM, Ferreira I, Miyata T, Scheffer PG, Teerlink T, et al. Overexpression of glyoxalase-I reduces hyperglycemia-induced levels of advanced glycation end products and oxidative stress in diabetic rats. J Biol Chem. 2011;286:1374–1380. doi: 10.1074/jbc.M110.144097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kawai T, Takei I, Tokui M, Funae O, Miyamoto K, Tabata M, et al. Effects of epalrestat, an aldose reductase inhibitor, on diabetic peripheral neuropathy in patients with type 2 diabetes, in relation to suppression of N ɛ-carboxymethyl lysine. J Diabetes Complications. 2010;24:424–432. doi: 10.1016/j.jdiacomp.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 27.Hashimoto Y, Yamagishi SI, Mizukami H, Yabe-Nishimura C, Lim SW, Kwon HM, et al. Polyol pathway and diabetic nephropathy revisited: early tubular cell changes and glomerulopathy in diabetic mice overexpressing human aldose reductase. J Diabetes Investig. 2011;2:111–122. doi: 10.1111/j.2040-1124.2010.00071.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Akimoto Y, Miura Y, Toda T, Wolfert MA, Wells L, Boons G-J, et al. Morphological changes in diabetic kidney are associated with increased O-GlcNAcylation of cytoskeletal proteins including α-actinin 4. Clin Proteomics. 2011;8:15. doi: 10.1186/1559-0275-8-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Park SY, Ryu J, Lee W. O-GlcNAc modification on IRS-1 and Akt2 by PUGNAc inhibits their phosphorylation and induces insulin resistance in rat primary adipocytes. Exp Mol Med. 2005;37:220–229. doi: 10.1038/emm.2005.30. [DOI] [PubMed] [Google Scholar]
- 30.Inbathamizh L, Padmini E. In silico studies on the inhibitory effects of calcitriol and 5, 5’-dithiobis-2-nitrobenzoic acid on human glucosaminyl N-acetyl transferase 1 activity. Asian J Exp Biol Sci. 2012;3:14–21. [Google Scholar]