Abstract
The flow rate of rivers are affected when modifications are made for the benefit of mankind. Some man-made alterations carried out include dam construction. The aim of this study was to investigate the health impact of the Bui dam with respect to the prevalence and awareness level of schistosomiasis in a typical damming environment. The study was conducted in 4 riparian communities within the dam catchment area. A cross-sectional study design was employed to interview 350 individuals. Urine and stool samples were also collected from 386 participants. Results of the study showed that, knowledge of schistosomiasis was significantly greater in close communities (99.47%) than their far counterparts (50.29%) (p > 0.001; OR = 172). Schistosomiasis infection rate in the close communities (32.57%) were significantly greater in far communities (7.23%; p ≤ 0.0001). The overall prevalence of 82 (21.1%) was recorded for Schistosoma haematobium and 64 (16.1%) for Schistosoma mansoni. A significantly high prevalence of S. haematobium (43.3%) was found in the age group 15–24 with no prevalence reported for age group 5–9 (Close communities) (p = 0.012). When the same age group was further examined for S. mansonii, group 5–9 recorded a prevalence of 0% with age group 10–14 showing a high prevalence of 26.1% (p = 0.047). From the study, it was concluded that, though awareness level of Schistosomiasis knowledge on the cause, mode of transmission and symptoms were high, they were ignorant on personal preventive strategies. In addition, the study also revealed that, S. haematobium was more prevalent among inhabitants living closer to the Bui dam with children less than 14 years of age being the worst affected.
Keywords: Bui dam, Schistosomiasis, Prevalence, Awareness level, Riparian community
Introduction
The flow rate of rivers are affected when modifications are made for the benefit of mankind. Some man-made alterations carried out include dam construction (Akurugu et al. 2015). Dams are barriers created across rivers to exploit water for the generation of energy, whiles the spill over can be harnessed for agriculture (ICOLD 2000; World Commission on Dams 2000). Notwithstanding these benefits, dams can cause a plethora of negative impacts especially on people in whose immediate environment the constructions are done (Skinner et al. 2009; Akurugu et al. 2015). Chief among these are the emergence and spread of water based and water borne vector diseases (Goselle et al. 2010).
The construction of dams from rivers has led to the spread of some vector borne diseases such as schistosomiasis (Webbe 1981; Ofoezie et al. 1991). This is because newly constructed dams provide breeding sites for vectors as their habitat (stagnant or slow flowing water bodies) serve as breeding sites. Schistosoma transmission is influenced by the interplay between various factors ranging from parasite to host linked factors (Salawu and Odaibo 2016). Studies have shown that the development of dams have a high correlation with the presence and prevalence of water-related parasitic infections (including Schistosomiasis) (Chivian 2008; Patz et al. 2000; Ogbeide and Uyigue 2004). Globally, 193 million people are infected with this disease and 779 million still remain at a high risk of developing the infection (Chitsulo and Engels 2000). Schistosomiasis has been persistent despite the prolonged control and prevention efforts. This is as a result of the spread of the intermediate host as well as the migration and dependency of the people on Schistosoma-infested water (Steinmann et al. 2006). People at high risk of being infected with schistosomiasis are those who farm very close to and or live near these dams in tropical regions of the world (Rollinson and Simpson 1987).
Risk factors for Schistosoma usually include working or living near dams. The Bui dam, one such example of a water resource development project, has been in existence since 2011 (Mortey et al. 2016). Since its construction, there has not been any environmental health impact study to establish the prevalence of Schistosomiasis in its riparian communities in order to assess the health impacts of the dam. Five years after the construction of the dam, there are perceptions that the dam has triggered schistoma infection among some inhabitants within the Bui dam environs (Gyasi et al. 2018). It is therefore relevant to conduct a study to investigate whether these perceptions are valid. In view of the above, a study to explore the prevalence and intensity of schistosomiasis to assess whether the dam construction has some negative environmental impact on people in riparian communities along the dam was urgently needed. The main objective of the study was to assess the level of awareness of inhabitants on Schistosomiasis and the prevalence of the disease in selected communities within the Bui dam environs.
Methodology
Study area
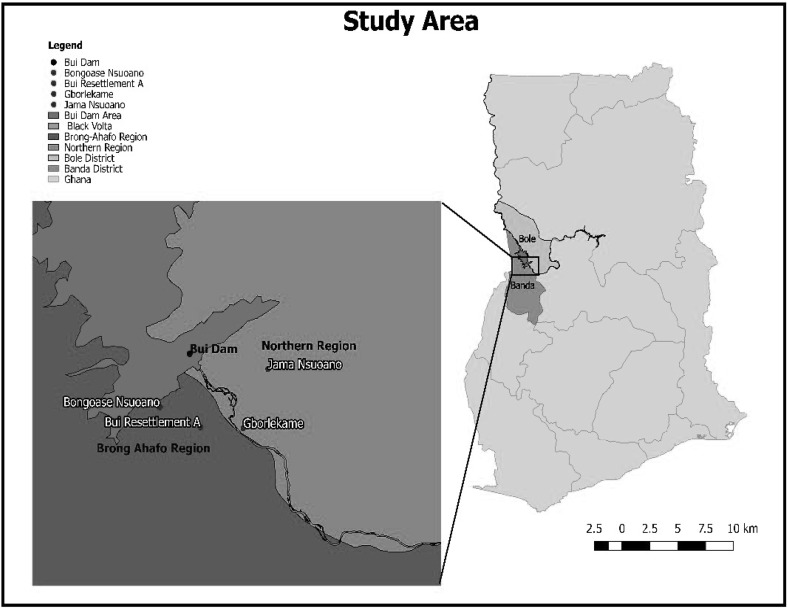
The study was conducted in 4 riparian communities within the Bui Hydroelectric Dam catchment area (Fig. 1). The dam with a total capacity of 400 MW has a net average of 980 GWH/year (Kuubeterero 2016). It is located on the border of the Brong Ahafo and the Northern regions of Ghana with coordinates 8°16′42″N 2°14′9″W/8.27833°N 2.23583°W. The study communities were Bui Resettlement “A” and Bongoase Nsuoano (located in the Banda District of the Brong Ahafo Region) and Jama Nsuoano and Gborlekame (located in the Bole District of the Northern Region). The coordinates for the study communities were Bongoase Nsuoano (8°15′0.12″N, °16′8.13″W); Jama Nsuoano (8°16′40.80″N, 2°10′50.30″W); Bui Resettlement “A” (8°14′6.43″N, 2°14′9.15″W) and Gborlekame (8°14′3.20″N, 2°12′2.85″W). Bongoase Nsuaono and Jama Nsuoano are < 2 km from the reservoir of the dam (“Near to the dam” Communities) while Bui Resettlement A and Gborlekame are 2–8 km from the reservoir of the dam (“Far from the dam” Communities).
Fig. 1.
Map of study area
Study design and population
A cross sectional study design was employed in this study. The study was conducted from December 2016 to May 2017. The target population was selected from local community members of Bongoase Nsuoano and Bui Resettlement “A” of the Banda district and Gborlekame and JamaNsuoano of the Bole district. The communities were grouped into 2 “close to the dam” communities and 2 “far from the dam” communities depending on their distance from the reservoir. The “close to the dam” communities were 0–2 km from the reservoir (Jama Nsuoano and Bongoase Nsuoano) while the “far from the dam” communities were 2–8 km from the dam (Gborlekame and Bui Resettlement “A”).
Sample population
The sample population comprised inhabitants from age 7 to 70 who agreed to participate. The exclusion criteria were inhabitants below 7 years and above 70 years.
Questionnaire data collection and statistical analysis
Two research assistants who could read and understand the English Language as well as properly translate to the Twi, the local dialect of the study inhabitants were recruited and trained to assist in administration of the questionnaires. The training covered data collection tools, field procedures and interview techniques. Questionnaires were pretested before being used on the field. Both primary and secondary data collection tools were employed in this research. Primary data collection tools were limited to qualitative and quantitative methods. The quantitative method included the use of closed and open ended questions to collect data from respondents based on their general perception of the Bui dam and the prevalence and intensity of schistosomiasis. Data collected were augmented using qualitative methods that included in depth interviews, focus group discussions and personal observation. Questionnaires were used to collect participants’ demographic data and their knowledge on schistosomiasis.
Study approach
House to house visits were embarked upon and 175 respondents were randomly selected from the 2 “far from the dam” communities and interviewed. Another set of 175 respondents were randomly selected from the “close to the dam” communities. Data collected focused on the inhabitants’ general knowledge on the causes, symptoms and prevention of schistosomiasis. With the help of selected family heads and opinion leaders of the individual communities, in-depth interviews were carried out as part of the study. Focus group discussions were conducted for same sex and similar age group categories. The interviews were conducted in Twi, the local dialect commonly used in the study communities. Data collected was later transcribed into the English language. Information provided by the participants remained confidential, shared only among the research team and used only for the purpose of this study. The research team ensured that all the information provided in each community and the questionnaires were kept safely.
Sample collection and laboratory analysis
A total of 368 participants were recruited using random sampling technique in accordance with the inclusion criteria. All participants recruited for the study in each community were registered and given a unique identification number. Each participant was given two 30 ml sterile capped containers. The container was given a unique identification number for each participant. The age and sex of the participant were also recorded on the containers. The participants were asked to deposit about one-third full of stool in one sample container and 30 ml of midstream and terminal urine in the other container. Samples were stored at 4 °C and quickly transported to the laboratory for analysis.
Urine and stool samples were processed within a day of collection. Formol Ether concentration method was used to concentrate eggs from the stool samples. One gram (1 g) of each stool sample was thoroughly mixed with 7 ml of 10% formol water and strained into a centrifuge tube. Three milliliters (3 ml) of Ether was added and the mixture shaken for 1 min. They were then centrifuged by initial slow acceleration and later increased to a speed of 2000 rpm for 1 min. The debris on the surface and at the interface between the 2 liquids were loosened from the wall of the tube with an applicator stick and the supernatant discarded. The upper part of the tube was wiped clear of fatty debris. The small deposit was shaken up and poured onto a slide (Clara et al. 2012). All prepared specimens were first observed under the 10× objective for focusing and later under the 40× objective for detail examination. For the urine analysis, the colour of each sample was first recorded. The sedimentation method as described by Cheesbrough was used to concentrate ova from the urine samples (Cheesbrough 2005). Each urine sample was well mixed after which only 10 ml was transferred into a conical tube. The samples were centrifuged at 2000 rpm for 1 min and allowed to settle. The supernatant was discarded and the sediment was poured onto a microscopic slide, covered with a cover slip and examined under the microscope for detection of Schistosoma haematobium eggs (Cheesbrough 2005). All prepared specimens were first observed under the 10× objective for focusing and later under the 40× objective for detail examination.
Ethical consideration
The study was approved by the Committee on Human Research, Publication and Ethics, School of Medical Sciences, Kwame Nkrumah University of Science and Technology (CHRPE/AP/486/16) as well as the management of district hospitals in both districts. In all communities, permission was sought from traditional authorities and the consent of individuals, parents or guardians (in the case of minors) was secured before taking samples or responding to questionnaires. Participation in the study was strictly voluntary.
Data analysis
The data was statistically analysed using the Chi square test provided by statistical GraphPad Prism software version 5.0. Percentages for answers were determined using Microsoft excel.
Results
Stratification of the social demographic data based on location showed that respondents within the age category 31–43 in the close communities (28.0%) were significantly higher than those in the “far from the dam” communities (18.86%; p = 0.0435; Table 1). The age category 57–70 was more common in the far communities (13.70%) compared to the close communities (odds ratio = 0.7663). With respect to gender, the respondents were generally evenly distributed. When respondents demographic data were stratified based on educational status, the results of the study showed that those in the “close to the dam” communities who had received up to primary level education (46.86%) was significantly higher than those in the “far from the dam” communities (28.75%; p = 0.0004). The occupation of the respondents when analysed based on location showed that, there were significantly more farmers in the “far from the dam” communities (22.8%) than the close communities (2.29%). A significantly higher proportion of the respondents in the “close to the dam communities” (40.0%) were fishermen compared to their “far from the dam” communities counterparts (20%; p < 0.0001).
Table 1.
Respondents social demography stratified by location
| Variables | % close (175)a | % far (175) | % total (350) | p value | Odds ratio |
|---|---|---|---|---|---|
| Age | |||||
| 7–17 | 50 (28.57) | 59 (33.71) | 109 (31.14) | 0.2989 | 0.7864 |
| 18–30 | 36 (20.57) | 30 (17.14) | 66 (18.86) | 0.4123 | 1.252 |
| 31–43 | 49 (28.00) | 33 (18.86) | 82 (23.43) | 0.0435 | 1.673 |
| 44–56 | 21 (12.00) | 29 (16.57) | 50 (14.28) | 0.2217 | 0.6865 |
| 57–70 | 19 (10.86) | 24 (13.71) | 43 (12.29) | 0.4156 | 0.7663 |
| Sex | |||||
| Male | 83 (47.43) | 86 (49.14) | 169 (48.28) | 0.7483 | 0.9336 |
| Female | 92 (52.57) | 89 (50.86) | 181 (51.71) | 0.7483 | 1.071 |
| Marital status | |||||
| Single | 67 (38.29) | 75 (42.86) | 142 (40.57) | 0.3838 | 0.8272 |
| Married | 86 (49.14) | 88 (50.23) | 174 (49.71) | 0.8307 | 1.047 |
| Divorced | 15 (8.57) | 8 (4.57) | 23 (6.57) | 0.131 | 1.957 |
| Widowed | 7 (4.00) | 4 (2.29) | 11 (3.14) | 0.358 | 1.781 |
| Educational status | |||||
| Primary | 82 (46.86) | 50 (28.57) | 132 (37.71) | 0.0004 | 2.204 |
| JSS | 40 (22.86) | 44 (25.54) | 84 (24.00) | 0.6166 | 0.8822 |
| SHS | 23 (13.14) | 32 (18.28) | 55 (15.71) | 0.1862 | 0.6762 |
| Tertiary | 7 (4.00) | 1 (0.57) | 8 (2.28) | 0.0319 | 7.25 |
| Never | 23 (13.14) | 48 (27.43) | 71 (20.28) | 0.0009 | 0.4004 |
| Occupation | |||||
| Trading | 16 (9.14) | 20 (11.43) | 36 (10.28) | 0.4815 | 0.7799 |
| Farming | 4 (2.29) | 40 (22.86) | 44 (12.57) | < 0.0001 | 0.07895 |
| Fishing | 70 (40.00) | 35 (20.00) | 105 (30.00) | < 0.0001 | 2.667 |
| Fish monger | 24 (13.71) | 4 (2.29) | 28 (8.00) | < 0.0001 | 6.795 |
| Unemployed | 53 (30.28) | 71 (40.57) | 124 (35.43) | 0.0443 | 0.6363 |
| Other | 8 (4.57) | 5 (2.86) | 13 (3.71) | 0.3965 | 1.629 |
| Religion | |||||
| Christianity | 112 (64.00) | 92 (52.57) | 204 (58.28) | 0.0302 | 1.604 |
| Islamic | 0 (0.00) | 66 (37.71) | 66 (18.86) | < 0.0001 | 0.004691 |
| Traditional | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 | |
| None | 63 (36.00) | 17 (9.71) | 80 (22.86) | < 0.0001 | 5.228 |
JSS Junior secondary school, SHS Senior secondary school
aClose communities (close to the dam) were communities that were less than 2 km from the reservoir and those greater than 2 km but less than 8 km from the reservoir were the far communities (far from the dam)
The study also investigated respondents' awareness level of Schistosomiasis based on their proximity to the reservoir of the dam. Findings from the study revealed that proximity to the dam reservoir did not affect whether or not respondents had heard about schistosomiasis before (Table 2). Their source of the knowledge was either from school, media or both. Knowledge of schistosomiasis was significantly greater in close communities (99.47%) than the “far from the dam” communities (50.29%) with an odds ratio of 172. Notwithstanding, awareness of the forms of schistosomiasis between close (2.86%) and far communities (1.14%) yielded no significant difference (p = 0.252). A greater proportion of respondents who were aware of schistosomiasis in both close (96.00%) and far (35.43%) communities were only aware of urinary schistosomiasis (p < 0.0001). The study further investigated if any of the respondents or their relatives had had schistosomiasis infection before. Schistosomiasis infection rate in the close communities (32.57%) was significantly greater than the rate in far communities (7.23%; p ≤ 0.0001).
Table 2.
Respondents general awareness of Schistosomiasis infections based on location
| Variables | % close (175) | % far (175) | % total (350) | p value | Odds ratio |
|---|---|---|---|---|---|
| Have you heard of schistosomiasis before? | |||||
| Yes | 174 (99.43) | 88 (50.29) | 262 (74.86) | < 0.0001 | 172 |
| No | 1 (0.57) | 87 (49.71) | 88 (25.14) | < 0.0001 | 0.005813 |
| Where did you hear it? | |||||
| School | 2 (1.14) | 11 (6.28) | 13 (3.71) | 0.011 | 0.1724 |
| Home | 134 (76.57) | 38 (21.71) | 172 (49.14) | <0.0001 | 11.78 |
| School/home | 17 (9.71) | 16 (9.14) | 33 (9.43) | 0.8549 | 1.069 |
| Home/media | 3 (1.71) | 0 (0.00) | 3 (0.86) | 0.0819 | 7.122 |
| Hospital | 0 (0.00) | 11 (6.28) | 11 (3.14) | 0.0008 | 0.04075 |
| Home/school/media | 18 (10.29) | 12 (6.86) | 30 (8.57) | 0.2519 | 1.557 |
| Not applicable | 1 (0.57) | 87 (49.71) | 88 (25.14) | < 0.0001 | 0.005813 |
| What are the types of Schistosomiasis you know? | |||||
| Urinary schistosomiasis | 168 (96.00) | 62 (35.43) | 230 (65.71) | <0.0001 | 43.74 |
| Intestinal schistosomiasis | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 | 0 |
| Both | 5 (2.86) | 2 (1.14) | 7 (2.00) | 0.252 | 2.544 |
| Don’t know | 1 (0.57) | 24 (13.71) | 25 (7.14) | <0.0001 | 0.03616 |
| Have you been infected with Schistosomiasis before? | |||||
| Yes | 57 (32.57) | 13 (7.23) | 70 (20.00) | < 0.0001 | 6.02 |
| No | 118 (67.43) | 162 (92.57) | 280 (80) | < 0.0001 | 0.1661 |
| Has any of your relative been infected with this disease before? | |||||
| Yes | 49 (28.00) | 8 (4.57) | 57 (16.28) | < 0.0001 | 7.929 |
| No | 99 (56.57) | 144 (82.28) | 243 (69.43) | < 0.0001 | 0.2804 |
| Don’t know | 27 (15.43) | 23 (13.14) | 50 (14.28) | 0.5412 | 1.206 |
Inhabitants who responded affirmative to the question “Have you heard of schistosomiasis before?” were further interrogated to find out if they were aware of the cause, route of transmission, signs and symptoms of the infection (Table 3). Respondents from the close communities that attributed the cause to a worm were 55.5%. This was significantly higher than the 10.86% of respondents from the far communities (p < 0.0001). Respondents with no knowledge of the cause of Schistosomiasis were 30.85% in far communities and 10.28% in close communities (p < 0.0001). Also, 61.14% of close community respondents attributed Schistosomiasis infection to occur through contact with infested water as against 5.14% of respondents from far communities (p < 0.0001). Most of the respondents in close communities (69.71%) and few respondents from the far communities (34.28%) confirmed blood in urine as possible symptom of schistosomiasis (p < 0.0001).
Table 3.
Respondents’ knowledge on the causes, transmission, signs and symptoms of schistosomiasis stratified by location
| Variables | % close (175) | % far (175) | % total (350) | p value | Odds ratio |
|---|---|---|---|---|---|
| What causes schistosomiasis? | |||||
| Worms | 97 (55.43) | 19 (10.86) | 116 (33.14) | < 0.0001 | 10.21 |
| Stagnant water | 15 (8.57) | 1 (0.57) | 16 (4.57) | 0.0003 | 16.21 |
| River/lake | 44 (25.14) | 14 (8.00) | 58 (16.57) | < 0.0001 | 58.44 |
| Don’t know | 18 (10.28) | 54 (30.85) | 72 (20.57) | < 0.0001 | 0.2569 |
| How is schistosomiasis acquired? | |||||
| Contact with water bodies | 64 (36.57) | 63 (36.00) | 127 (36.28) | 0.9115 | 1.025 |
| Drinking dirty water | 3 (1.71) | 2 (1.14) | 5 (1.43) | 0.6524 | 1.509 |
| Contact with infested water bodies | 107 (61.14) | 9 (5.14) | 116 (33.14) | < 0.0001 | 29.02 |
| Don’t know | 0 (0.00) | 14 (8.00) | 14 (4.00) | < 0.0001 | 0.03173 |
| What are the signs and symptoms of schistosomiasis? | |||||
| Blood in urine | 122 (69.71) | 60 (34.28) | 182 (52.00) | < 0.0001 | 4.412 |
| Blood in stool | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 | 0 |
| Blood in urine and itchy skin | 15 (8.57) | 20 (11.53) | 35 (10.00) | 0.373 | 0.7266 |
| Blood in urine, painful urination and itchy skin | 21 (12.00) | 2 (1.14) | 23 (6.57) | < 0.0001 | 11.8 |
| Blood in urine and stool | 5 (2.86) | 2 (1.14) | 7 (2.00) | 0.0011 | 0.2157 |
| Blood in urine and painful urination | 11 (6.28) | 4 (2.28) | 15 (4.28) | 0.0647 | 2.867 |
Participants who responded positively to the question “Have you heard of Schistosomiasis before?” were further interrogated on how schistosomiasis could be prevented and or controlled. The notion that schistosomiasis can be prevented by not coming into contact with water bodies was evenly distributed in both close (36.57%) and far communities as shown in Table 4 below (36.00%; p = 0.9115; OR = 1.025).
Table 4.
Respondents’ knowledge on the prevention and control schistosomiasis based on location
| Variables | % close (175) | % far (175) | % total (350) | p value | Odds ratio |
|---|---|---|---|---|---|
| Schistosomiasis prevention | |||||
| Not coming into contact with water bodies | 64 (36.57) | 63 (36.00) | 127 (36.28) | 0.9115 | 1.025 |
| Not drinking dirty water | 3 (1.71) | 2 (1.14) | 5 (1.43) | 0.6524 | 1.509 |
| Not coming into contact with infested water bodies | 107 (61.14) | 9 (5.14) | 116 (33.14) | < 0.0001 | 29.02 |
| Don’t know | 0 (0.00) | 14 (8.00) | 14 (4.00) | < 0.0001 | 0.03173 |
The study also investigated the overall prevalence of parasitic infections based on both sex and age (Tables 5, 6). A prevalence of 24% and 19.2% of Schistosoma haematobium infection were reported in males and females respectively but were not statistically significant (p = 0.256). Prevalence of Schistosoma mansonii was 16.8% in males and 16.4% in females (p = 0.932). Schistosoma haematobium infection for age group 10–14 had the highest prevalence (36.1%) followed by age group 15–24 (30.4%) and 50–70 recording the least (10%). The differences in the prevalence among age categories were statistically significant (p = 0.001). Age 50–70 surprisingly recorded the highest prevalence (23.3%) with respect to Schistosoma mansoni infection while age group 25–49 had the least (12.5%).
Table 5.
Overall prevalence of S. haematobium and S. mansonii Infection
| Schistosoma haematobium | Schistosoma mansonii | |
|---|---|---|
| No. examined | Positive (% prevalence) | Positive (% prevalence) |
| 386 | 82 (21.2) | 64 (16.6) |
Table 6.
Prevalence of S. haematobium and S. mansonii infection stratified by gender and age
| Variable | No. examined | S. haematobium | p value | S. mansoni | p value |
|---|---|---|---|---|---|
| No. positive (% prevalence) | No. positive (% prevalence) | ||||
| Gender | 0.256 | 0.932 | |||
| Female | 219 | 42 (24.0) | 36 (16.4) | ||
| Male | 167 | 40 (19.2) | 139 (16.8) | ||
| Age | 0.001 | 0.382 | |||
| 5–9 | 60 | 9 (15.0) | 9 (15.0) | ||
| 10–14 | 61 | 22 (36.1) | 12 (19.7) | ||
| 15–24 | 69 | 21 (30.4) | 12 (17.4) | ||
| 25–49 | 136 | 24 (17.6) | 17 (12.5) | ||
| 50–70 | 60 | 6 (10.0) | 14 (23.3) |
Schistosoma haematobium infection had the highest prevalence and was reported in Gborlekame (30.8%) whiles the lowest was recorded in Bui Resettlement “A” community (p = 0.032) as shown in Table 7. Gborlekame and Bui Resettlement “A” communities had the highest (26.9%) and the least (7.2%) prevalence of Schistosoma mansonii infection respectively. The difference between the study communities was significant (p = 0.005).
Table 7.
Prevalence of S. haematobium and S. mansonii infection in individual communities
| No. examined | S. haematobium | p value | S. mansonii | p value | |
|---|---|---|---|---|---|
| No. positive (% prevalence) | No. positive (% prevalence) | ||||
| Jama Nsuoano | 133 | 32 (24.1) | 0.032 | 18 (13.5) | 0.005 |
| BongoaseNsuoano | 118 | 25 (21.2) | 26 (22.0) | ||
| Bui Resettlement A | 83 | 9 (10.8) | 6 (7.2) | ||
| Gborlekame | 52 | 16 (30.8) | 14 (26.9) |
Analysis of the results based on gender and age in the individual communities showed that, males in Jama Nsuoano had higher prevalence for both S. haematobium infection and S. mansonii infections compared to females (Table 8). The prevalence of all the parasitic infection in Bongoase Nsuoano was higher in females than males. Males in Bui Resettlement “A” had a higher prevalence for all the parasitic infection than females. Within the Gborlekame community, a higher prevalence of S. mansonii infection (29.6%) was recorded in males. This was also seen with S. haematobium infection (32.0%) for females.
Table 8.
Prevalence of S. haematobium and S. mansonii infection among the individual communities stratified by gender
| Community | Sex (No. examined) | S. haematobium | p value | S. mansonii | p value |
|---|---|---|---|---|---|
| No. positive (% prevalence) | No. positive (% prevalence) | ||||
| Jama-Nsuoano | Males (64) | 17 (26.6) | 0.516 | 9 (14.1) | 0.864 |
| Females (69) | 15 (21.7) | 9 (13.0) | |||
| Bongoase-Nsuoano | Males (49) | 10 (20.4) | 0.862 | 9 (18.4) | 0.418 |
| Females (69) | 15 (21.7) | 17 (24.6) | |||
| Bui Resettlement “A” | Males (27) | 5 (18.5) | 0.118 | 2 (7.4) | 0.965 |
| Females (56) | 4 (7.1) | 4 (7.1) | |||
| Gborlekame | Males (27) | 8 (29.6) | 0.853 | 8 (29.6) | 0.647 |
| Females (25) | 8 (32.0) | 6 (24.0) |
Within the Jama Nsuoano community, a significantly higher prevalence of S. haematobium (43.3%) was found in the age group 15–24 with no prevalence being reported in age group 5–9 (Table 9; p = 0.012). When the same age group was further examined for S. mansonii, group 5–9 recorded a prevalence of 0% with age group 10–14 showing a high prevalence of 26.1% (p = 0.047). In the Bongoase Nsuoano community, S. haematobium was recorded in all the age categories except age group 50–70. Group 10–14 reported a significantly higher prevalence of 43.8% (p = 0.015). S. mansoni in age group 15–24 had the highest prevalence of 29.4% with 10–14 group recording a prevalence of 12.5% (p = 0.769). In Bui Resettlement “A”, group 25–49 had the highest prevalence of 26.1% with group 10–14 having a prevalence of 0% (p = 0.093). Age group 10–14 and 15–24 recorded the least prevalence (0%) for S. mansoni while group 50–70 recorded the highest non significance prevalence of 21.4%. Within Gborlekame community, the least prevalence of S. haematobium (22.2%) was found in age 25–49 and 50–70 with the highest prevalence of 53.8% seen in group 10–14. Age group 50–70 recorded the highest prevalence of 55.6% with age 15–24 recording the least non-significant prevalence of 0% in S. mansoni (p = 0.161).
Table 9.
Prevalence of S. haematobium and S. mansonii infection in the individual communities stratified by age
| Variable/parasite | Jama Nsuoano n (% prevalence) |
p value | Bongoase Nsuoano n (% prevalence) |
p value | Bui Resettlement “A” n (%prevalence) |
p value | Gborlekame n (% prevalence) |
p value |
|---|---|---|---|---|---|---|---|---|
| S. haematobium | 0.012 | 0.015 | 0.093 | 0.361 | ||||
| 5–9 | 0 (0.0) | 4 (22.2) | 1 (5.3) | 4 (23.5) | ||||
| 10–14 | 8 (34.8) | 7 (43.8) | 0 (0.0) | 7 (53.8) | ||||
| 15–24 | 13 (43.3) | 6 (35.3) | 1 (5.6) | 1 (25.0) | ||||
| 25–49 | 8 (14.8) | 8 (16.0) | 6 (26.1) | 2 (22.2) | ||||
| 50–70 | 3 (15.0) | 0 (0.0) | 1 (7.1) | 2 (22.2) | ||||
| S. mansoni | 0.047 | 0.769 | 0.140 | 0.161 | ||||
| 5–9 | 0 (0.0) | 3 (16.7) | 2 (10.5) | 4 (23.5) | ||||
| 10–14 | 6 (26.1) | 2 (12.5) | 0 (0.0) | 4 (30.8) | ||||
| 15–24 | 7 (23.3) | 5 (29.4) | 0 (0.0) | 0 (0.0) | ||||
| 25–49 | 3 (5.6) | 12 (24.0) | 1 (4.3) | 1 (11.1) | ||||
| 50–70 | 2 (10.0) | 4 (23.5) | 3 (21.4) | 5 (55.6) |
The various p values in the table refer to the p value for all the age category for each of the communities based on whether the infection is by S. haematobium or S. mansoni
Analysis of result with respect to sex showed that out of the 386 participants examined, 23.83% had more than one infection (Table 10). Of the 216 females examined, 5.5% had both S. haematobium and S. mansonii infections. Out of the 167 males examined, 6.6% had both S. haematobium and S. mansonii infections. However, there was no significant difference between males and females with respect to any of the double infections. Double infections with respect to age group were also investigated. Analysis of the results showed that, the highest prevalence of both S. haematobium and S. mansonii infections was recorded in the age group 10–14 (13.1%) with the least prevalence observed in age group 25–49 (2.9%). The difference in prevalence was statistically not significant (p = 0.125) as shown in Table 10 below.
Table 10.
Prevalence of Multiple Infection in stratified by gender and age
| Variable | S. haematobium + S. mansonii | p value |
|---|---|---|
| No. positive (%) | ||
| Gender | 0.697 | |
| Female | 12 (5.5) | |
| Male | 11 (6.6) | |
| Age | 0.125 | |
| 5–9 | 2 (3.3) | |
| 10–14 | 8 (13.1) | |
| 15–24 | 6 (8.7) | |
| 25–49 | 4 (2.9) | |
| 50–70 | 3 (5.0) |
S. haematobium + S. mansoni means a single individual has both S. haematobium and S. mansonii infections
S. mansonii infections had the least mean intensities (Table 11). Males had higher mean intensity of S. haematobium than females in all the study communities. S. haematobium had the highest mean intensity of 45.20 observed in males from Bongoase Nsuoano with females from Bui Resettlement “A” community recording the least (5.25). Females from Jama Nsuoano also recorded the highest mean intensity (5.78) of S. mansonii infection with males from Bui Resettlement “A” recording the least (2.50) for the same disease.
Table 11.
Mean intensity of the individual infections in the study communities based on gender
| Community | Sex | S. haematobium | S. mansonii |
|---|---|---|---|
| Mean intensity | Mean intensity | ||
| Jama Nsuoano | Male | 41.18 | 5.11 |
| Female | 29.60 | 5.78 | |
| Bongoase Nsuoano | Male | 45.20 | 5.44 |
| Female | 24.00 | 5.00 | |
| Bui Resettlement A | Male | 9.40 | 2.50 |
| Female | 5.25 | 4.25 | |
| Gborlekame | Male | 20.38 | 3.13 |
| Female | 12.88 | 4.50 |
These mean intensities were compared between the study communities to establish whether there was any difference between the communities. There was a significant difference among the individual communities when the 4 study areas were compared (p = 0.001) with respect to S. haematobium. Mean intensity of S. mansonii in the study communities was not significantly different among study communities (Table 12).
Table 12.
Comparing the intensity of S. haematobium and S. mansonii infection among the individual communities within the study area
| Infection | Jama Nsuoano | Bongoase Nsuoano | Bui Resettlement “A” | Gborlekame | p value | Significant pairs |
|---|---|---|---|---|---|---|
| S. haematobium | ||||||
| Median (Q1−Q3) | 25.50 (12.25–49.50) | 14.00 (10.00–40.00) | 5.00 (3.00–6.50) | 11.00 (5.25–21.25) | 0.001 | 1v3, 2v3 |
| Mean intensity | 35.75 | 32.48 | 7.556 | 16.63 | ||
| S. mansonii | ||||||
| Median (Q1−Q3) | 5.00 (3.00–8.00) | 5.00 (3.00–7.25) | 3.50 (2.00–5.25) | 3.00 (2.00–6.00) | 0.128 | |
| Mean intensity | 5.444 | 5.154 | 3.667 | 3.714 | ||
(Q1−Q3) means 1st quartile minus 3rd quartile; figures in brackets in the table refers to 1st quartile minus 3rd quartile (intensity) of that particular community
The mean intensity of the individual parasitic infections in the study communities based on age group were also assessed in this study. Analysis of the results showed that, within the Jama Nsuoano community, the highest mean intensity of S. haematobium (44.33 eggs/10 ml of urine) and S. mansonii (7.00 eggs/1 g of stool) were reported in age group 50–70 as shown in Table 13. Other age groups that recorded intensities of S. haematobium included group 10–14 (41.88 eggs/10 ml of urine), 15–24 (35.62 eggs/10 ml of urine) and 25–49 (26.62 eggs/10 ml of urine). The age groups 10–14, 15–24 and 25–49 recorded 5.50 eggs/1 g, 5.14 eggs/1 g and 5.00 eggs/1 g of stool of S. mansonii respectively. However, age group 5–9 recorded no intensity for S. haematobium and S. mansonii. Within the Bongoase Nsuoano community, age group 15–24 recorded the highest mean intensity of S. haematobium of 52.82 eggs/10 ml of urine with age groups 5–9, 10–14 and 25–49 recording mean intensity of 12.00 eggs/10 ml, 17.86 eggs/10 ml, and 40.25 eggs/10 ml respectively. Age group 50–70 recorded no mean intensity of S. haematobium. The highest mean intensity of S. mansonii was recorded in age group 15–29 (6.60 eggs/1 g), followed by age group 5–9 (5.33 eggs/1 g) with age group 10–14 and 50–70 having equal mean intensity of 4.50 eggs/1 g of stool. When the mean intensity of S. haematobium was examined among the age groups in Bui Resettlement “A” community, it was observed that the highest mean intensity of 9.00 eggs/10 ml of urine was recorded in age group 24–49 while the least mean intensity of 3.00 eggs/10 ml of urine was recorded in age group 5–9. Age group 10–14 recorded a zero mean intensity. For S. mansonii infection, age group 25–49 had the highest mean intensity of 5.00 eggs/1 g of stool with age group 5–9 and 50–70 recording mean intensity of 4.50 eggs/1 g of stool and 2.67 eggs/1 g of stool respectively. However, age group 10–14 and 15–24 did not show any mean intensity.
Table 13.
Mean intensity of S. haematobium and S. mansonii infection among the individual communities within the study area stratified by age group
| Variable/parasite | Jama Nsuoano | Bongoase Nsuoano | Bui Resettlement A | Gborlekame |
|---|---|---|---|---|
| S. haematobium | ||||
| 5–9 | 0.00 | 12.00 | 3.00 | 33.25 |
| 10–14 | 41.88 | 17.86 | 0.00 | 11.14 |
| 15–24 | 35.62 | 52.83 | 5.00 | 7.00 |
| 25–49 | 26.63 | 40.25 | 9.00 | 15.00 |
| 50–70 | 44.33 | 0.00 | 6.00 | 9.00 |
| S.mansonii | ||||
| 5–9 | 0.00 | 5.33 | 4.50 | 3.50 |
| 10–14 | 5.50 | 4.50 | 0.00 | 3.75 |
| 15–24 | 5.14 | 6.60 | 0.00 | 0.00 |
| 25–49 | 5.00 | 4.83 | 5.00 | 6.00 |
| 50–70 | 7.00 | 4.50 | 2.67 | 3.40 |
Discussion
Many studies on the knowledge, attitude and practices relating to Schistosomiasis in different parts of the world have indicated that misconceptions concerning the disease still exist (Pearson 2004; Ibidapo 2005). The results of this study revealed that the study communities had some level of knowledge on Schistosomiasis. The awareness level of Schistosomiasis in this study was observed to be high but knowledge on the cause, mode of transmission, symptoms and control remained very low. Most of the participants reported to have heard of Schistosomiasis. This could be as a result of personal experience, experience from relatives and access to mass media. This finding is not surprising since most of the respondents had had some form of formal education and it is consistent with other findings (Rassi et al. 2016; Musuva et al. 2014; Sady et al. 2013). Rassi et al. (2016) in their study conducted in Mozambique found out as many as 91% of the participants responded to have heard of Schistosomiasis before. Also 92.4% of the respondents from Yemen were aware of Schistosomiasis in a study done by Sady et al. (2013). However our finding is in contrast with other studies where they found a low awareness level of Schistosomiasis (Midzi et al. 2014; Poole et al. 2014; Odhiambo et al. 2014).
It was again observed that, participants who were aware of Schistosomiasis were more in close communities than far communities with a high statistical difference. The significantly high awareness level of the disease in close communities as compared to the far communities could be due to the difference in prevalence level in these communities. Those in the close communities were more exposed to the parasite and the disease itself and therefore may have high level of awareness than those in the far communities. Greater proportion of the respondents as well as their relatives in far communities had had Schistosomiasis before as compared to those in the close communities and this was not surprising.
The awareness of intestinal Schistosomiasis in both close and far communities were very low. This could be due to the little attention people pay to their fecal matter discharge based on ones’ location as well as the unpleasant aesthetic nature of this discharge compared to urine. This finding is consistent with a similar study conducted by Rassi et al. (2016). Fewer populations of the respondents were aware of the mode of transmission of the disease. This is consistent with the study of Sady et al. (2013) who found out that only 49.8% of the respondents in his study were aware of the correct mode of transmission of schistosomiasis. Rassi et al. (2016) again revealed that only 10% of the respondents who were aware of schistosomiasis knew at least one correct route of transmission. Misconceptions such as coming into contact with water bodies and drinking non-potable water were also cited as the route of transmission of the disease. The proportion of respondent from close communities who knew the correct mode of transmission of the disease were more in the close communities than far. This could be as a result of the significant difference in the awareness level of the disease in both communities.
Blood in urine was the most common symptoms recorded in the study population as compared to other symptoms such as blood in stool and painful urination. This finding could be due to the easy identification of change in the colour of urine as compared to stool. This is in agreement with other studies conducted elsewhere (Rassi et al. 2016). Higher percentages of these symptoms were recorded in close communities as compared to far communities. This may be as a result of the close communities being closer to the vectors of the disease from the dam than the far communities. Low knowledge on the mode of transmission and prevention was recorded in the study communities. This could also be due to the inadequate education on Schistosomiasis in the study communities. Lower knowledge on the transmission and prevention of Schistosomiasis in far communities as compared to close communities could be due to the lower infection rate in far communities.
The study further showed a generally high prevalence of Schistosomiasis in all the study communities. This could be attributed to the lack of toilet facilities in the study communities. Most inhabitants in the affected communities practiced open defecation and this could be a major contribution to the prevalence of the Schistosomiasis disease in all the study communities. It is noteworthy, that the absence of functional toilet facility has frequently been associated with outbreak of infections in studies conducted elsewhere (Abou-Zeid et al. 2012; Ugbomoiko et al. 2012). The prevalence and intensity of the disease increased with proximity to the potentially infested water bodies with Bongoase Nsuoano, Jama Nsuoano and Gborlekame which were closer to the reservoir or river being more infected than Bui Resettlement “A” (far). The mean intensity of Schistosoma infections recorded in Jama Nsuoano, Bongoase Nsuoano and Gborlekame were also higher than those recorded in Bui Resettlement “A”. Similar to our findings, Mtethiwa (2016) recorded a significant higher prevalence of Schistosomiasis in the communities closer to the water reservoir in Malawi than communities far from the reservoir. Other investigators have also demonstrated higher prevalence in areas proximal to the reservoir (Mewabo et al. 2017; Isa et al. 2015; Steinmann et al. 2006). A higher mean intensity of 31.69 eggs/10 ml of urine was recorded in Dutsin-Ma Local Government Area where Zobe Dam was located compared to the mean intensity of 19.66 eggs/10 ml of urine in Safana Local Government Area (which is far from the dam itself) (Atalabi et al. 2016).
The highly significant differences in community prevalence of Schistosomiasis recorded in our study could be attributed to the increase water contact frequency by the communities close to the reservoir as opposed to far communities. The higher the frequency of water contact, the more the chances of acquiring Schistosomiasis (Mtethiwa 2016). From our results, the inhabitants of Bongoase Nsuoano, Jama Nsuoano and Gborlekame frequently had contact with the dam and the river for various activities such as swimming, washing, fishing and washing of fish with relatively fewer people in the Bui Resettlement “A” area. This could have accounted for the differences in prevalence observed for the various communities.
Access to potable drinking water (for drinking and domestic purpose) could also be the cause of this significant difference since lack of potable water has been identified as a major risk factor in the spread of Schistosomiasis (Reuben et al. 2013). This may be due to higher exposure to infected water during the fetching of water by community inhabitants. In a study conducted by Atalabi et al. (2016), it was revealed that respondents who depended on unwholesome water source had a higher prevalence of Schistosomiasis. A highly significant association between Schistosomiasis and the usage of unsafe water for both drinking and domestic use has been recorded in Yemen (Sady et al. 2013). From the current study, it was revealed that the dam reservoir and the river in the communities served as the main sources of water for drinking and domestic use for the inhabitants of Jama Nsuoano, Bongoase Nsuoano and Gborlekame while Boreholes served as the main domestic water source for the people of Bui Resettlement “A”.
This present study has shown that infections with S. haematobium were higher than S. mansonii. This finding is consistent with findings by Sady et al. (2013) who recorded higher prevalence of S. haematobium infections as compared to S. mansonii infections in children in Yemen with as much as 75% of Schistosoma infections attributable to S. haematobium. Similar findings were also recorded in Ibb province (Goselle et al. 2010). Elsewhere in Malawi, Alebie et al. (2014) also recorded a high prevalence of S. heamatobium compared to S. mansoni (Alebie et al. 2014). The findings by Alebie et al. (2014) were further investigated and confirmed by Mtethiwa (2016) where a higher prevalence of S. haematobium (51.2%) compared to S. mansoni (9.5%) was observed. They opined that the difference in the prevalence rate of these two Schistosoma species may be attributed to the variations in the abundance of the respective intermediate snail hosts. The Bulinus snail which is the intermediate host for S. haematobium may be more in the study area than the Biomphalaria snail species present and thus transmitting more S. haematobium than S. mansoni infections (Gyasi et al. 2018).
The study investigated the difference in prevalence of the parasitic infections between males and females. Generally, males were more infected with all the studied parasitic infections than females though there were no statistically significant difference for any of the diseases. This study recorded a high prevalence of both S. haematobium and S. mansoni in males than females. This is probably due to increase contact with infected water bodies by males than female as a result of engagement in swimming and or fishing. This result is consistent with studies conducted elsewhere by several investigators (Kabiru et al. 2013; Sady et al. 2013). Sady et al. (2013), however, commented that though in Yemen and other Islamic countries, females are prohibited from swimming in open water bodies, they are responsible for fetching water, washing clothes and utensils at these water sources and hence might have equal exposure to the disease as their male counterparts. Interestingly, other investigators elsewhere have shown a high female prevalence of the disease (Satayathum et al. 2006; Rudge et al. 2008).
The study further demonstrated differences in prevalence among the various age groups of community dwellers. The high prevalence and mean intensity of Schistosomiasis in groups 10–14 and 15–24 in Jama Nsuoano, Bongoase Nsuoano and Gborlekame as compared to Bui Resettlement “A” may be attributed to the proximity to the potential source of infection. The high affinity of this age group to water bodies for the purposes of swimming, playing, fetching water and helping their parents in agricultural activities such as fishing could also be another reason (Sady et al. 2013). Similar finding was made by Kabiru et al.(2013) in their community-based study conducted in Wamakko town in Nigeria. They reported that the age group 10–14 were mostly found swimming in water bodies without exhibiting shyness (Kabiru et al. 2013). They were also involved in fetching water from rivers to assist in household chores.
Conclusion
Results from the study has shown that, people living close to the dam‘s impoundment had some level of knowledge on Schistosomiasis. They were also informed on how one get infected, the possible mode of transmission and even some basic clinical symptoms of disease. However, they were doubtful on basic control measures for prevention and control. The study further concluded that S. haematobium was more prevalent than S. mansoni with severity being closer to the impoundment biased. In addition, children less than 14 years were also more affected and this is even more disturbing. It is therefore being recommended that research be intensified in this area to unravel other health related impacts of the dam. In addition, it is being recommended that education ought to be intensified to educate ‘the close to the dam’ communities on basic preventive measures to control schistosomiasis among these riparian communities.
Acknowledgements
We are grateful to the chiefs, opinion leaders and people of the study communities for their permission and willing participation for this study. We are also grateful to Dr Eric Ofosu Antwi for his immense support during data collection.
Author’s contribution
SFG conceived the idea of the study, assisted in the study design, analysed and interpreted data and wrote the discussion and conclusion. AAB wrote the introduction, collected field data, wrote methods and reported the results. EA assisted in shaping of the conceived the idea of the study and assisted in the study designed the experiment. EOA assisted in data collection and the preparation of the final manuscripts.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Samuel Fosu Gyasi, Email: samuel.gyasi.fosu@gmail.com.
Abigail Antwiwaa Boateng, Email: aabigail2010@gmail.com.
Esi Awuah, Email: esiawuahrt@gmail.com.
Eric Ofosu Antwi, Email: eric.ofosu@uenr.edu.gh.
References
- Abou-Zeid AH, Abkar TA, Mohamed RO. Schistosomiasis and soil-transmitted helminths among an adult population in a war affected area, Southern Kordofan state, Sudan. Parasite Vectors. 2012;5:133. doi: 10.1186/1756-3305-5-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akurugu BA, Zango MS, Abanyie SK, Ampofo S, Region UE. Assessing the impact of a dam on the livelihood of surrounding communities: a case study of Vea dam in the upper east region of Ghana. J Environ Earth Sci. 2015;5(4):20–26. [Google Scholar]
- Alebie G, Berhanu E, Mulugeta A, Petros B. Epidemiological study on Schistosoma mansoni infection in Sanja area, Amhara region, Ethiopia. Parasites Vectors. 2014;20147:15. doi: 10.1186/1756-3305-7-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atalabi TE, Lawal U, Ipinlaye SJ. Prevalence and intensity of genito-urinary schistosomiasis and associated risk factors among junior high school students in two local government areas around Zobe Dam in Katsina State, Nigeria. Parasites Vectors. 2016;9(1):388. doi: 10.1186/s13071-016-1672-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheesbrough M. Parasitological tests. District laboratory practice in tropical countries. Cambridge: Tropical Health Technologies; 2005. pp. 178–306. [Google Scholar]
- Chitsulo L, Engels D. The global status of schistosomiasis and its control. Acta Trop. 2000;77(1):41–51. doi: 10.1016/S0001-706X(00)00122-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chivian E (ed) (2008) Biodiversity: its importance to human health. Interim executive summary. http://chge.med.harvard.edu/publications/documents/Biodiversity_v2_screen.pdf. Accessed 31 Aug 2008
- Clara J, Bourgeois D, Muller-Bolla M. DMF from WHO basic methods to ICDAS II advanced methods: a systematic review of literature. Odontostomatol Trop Trop Dental J. 2012;35(139):5–11. [PubMed] [Google Scholar]
- Goselle NO, Imandeh AD, Dakul GN, Onwuliri DA, Abba ACF, Udeh OJ, Abelau AM. Schistosoma mansoni infections amongst school children in Jos, Nigeria. Sci World J. 2010;5(1):42–45. doi: 10.4314/swj.v5i1.61485. [DOI] [Google Scholar]
- Gyasi SF, Boamah B, Awuah E, Otabil KB. A perspective analysis of dams and water quality: the Bui power project on the Black Volta, Ghana. J Environ Public Health. 2018;2018:10. doi: 10.1155/2018/6471525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibidapo CA. Perception of causes of malaria and treatment-seeking behaviour of nursing mothers in a rural community. Aust J Rural Health. 2005;13:214–218. doi: 10.1111/j.1440-1584.2005.00704.x. [DOI] [PubMed] [Google Scholar]
- International Commission on Large Dams (ICOLD) Dams and the environment: a viewpoint from the international commission on large dams. Irrigation Company of Upper Region (ICOUR) (1995) Ghana: ICOUR Information Handbook, ICOUR Ltd; 2000. [Google Scholar]
- Isa Y, Modu AM, Naphtali RS. A study on Schistosomiasis in three communities along Lake Alau, Konduga Local Government Area, Borno State, Nigeria. Int J Sci Technol. 2015;2:23–79. [Google Scholar]
- Kabiru M, Ikeh EI, Aziah I, Julia O, Fabiyi JP, Muhamed RA. Prevalence and intensity of Schistosoma haematobium infections: a community-based survey among school children and adults in Wmakko town, Sokoto State, Nigeria. Int J Trop Med Public Health. 2013;2(1):12–21. [Google Scholar]
- Kuubeterero DP (2016) Post inundation effects of Bui hydro electric dam on the large mammals in the Bui National Park in the Brong Ahafo Region of Ghana. An M.Sc. Thesis, KNUST
- Mewabo AP, Moyou RS, Kouemeni LE, Ngogang JY, Kaptue L, Tambo E. Assessing the prevalence of urogenital Schistosomaisis and transmission risk factors amongst school-aged children around Mapé dam ecological suburbs in Malantouen district, Cameroon. Infect Dis Poverty. 2017;6(1):40. doi: 10.1186/s40249-017-0257-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Midzi N, Mduluza T, Chimbari MJ, Tshuma C, Charimari L, Mhlanga G, et al. Distribution of Schistosomiasis and soil transmitted Helminthiasis in Zimbabwe: towards a national plan of action for control and elimination. PLoS Negl Trop Dis. 2014;8(8):e3014. doi: 10.1371/journal.pntd.0003014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortey EM, Ofosu EA, Kolodko DV. Kabobah AT (2016) Sustainability assessment of the bui hydropower system. Environments. 2017;2017(4):25. doi: 10.3390/environments4020025. [DOI] [Google Scholar]
- Mtethiwa AHN. Prevalence and intensity of Schistosomiasis in communities around water reservoirs in Malawi. J Trop Dis. 2016;4(1):1–6. doi: 10.4172/2329-891X.1000183. [DOI] [Google Scholar]
- Musuva RM, Awiti A, Omedo M, Ogutu M, Secor WE, Montgomery SP, Mwinzi PNM. Community knowledge, attitudes and practices on Schistosomiasis in Western Kenya—The SCORE Project. Am J Trop Med Hyg. 2014;90(4):646–652. doi: 10.4269/ajtmh.13-0488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odhiambo GO, Musuva RM, Atuncha VO, Mutete ET, Odiere MR, Onyango RO, et al. Low levels of awareness despite high prevalence of Schistosomiasis among communities in Nyalenda informal settlement, Kisumu City, Western Kenya. PLoS Negl Trop Dis. 2014;8(4):e2784. doi: 10.1371/journal.pntd.0002784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ofoezie IE, Imevbore AMA, Balogun MO, Ogunkoya OO, Asaolu SO. A study of an outbreak of schistosomiasis in two resettlement communities near Abeokuta, Ogun State, Nigeria. J Helminthol. 1991;65:95–105. doi: 10.1017/S0022149X00010531. [DOI] [PubMed] [Google Scholar]
- Ogbeide HE, Uyigue E (2004) Access to safe drinking water and schistosomiasis in Nigeria: survey on Ipogun Community, Ondo State of Nigeria. Submitted to the Society for Water and Public Health Protection (SWAPHEP)
- Patz JA, Graczyk TK, Geller N, Vittor AY. Effects of environmental change on emerging parasitic diseases. Int J Parasitol. 2000;30:1395–1405. doi: 10.1016/S0020-7519(00)00141-7. [DOI] [PubMed] [Google Scholar]
- Pearson A. Knowledge, attitudes and practices with regard to malaria control in an endemic rural area of Myanmar. Southeast Asian J Trop Med Public Health. 2004;35:53–62. [PubMed] [Google Scholar]
- Poole H, Terlouw DJ, Naunje A, Mzembe K, Stanton M, Betson M, Lalloo DG, Stothard JR. Schistosomiasis in pre-school aged children and their mothers in Chikhwawa district, Malawi with notes on characterisation of schistosomes and snails. Parasites Vectors. 2014;7:153. doi: 10.1186/1756-3305-7-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rassi C, Kajungu D, Martin S, Arroz J, Tallant J, Zegers de Beyl C, et al. Have you heard of Schistosomiasis? Knowledge, attitudes and practices in Nampula Province, Mozambique. PLoS Negl Trop Dis. 2016;10(3):e0004504. doi: 10.1371/journal.pntd.0004504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuben RC, Tanimu H, Musa JA. Epidemiology of urinary schistosomiasis among secondary school students in Lafia, Nasarawa State, Nigeria. J Biol Agric Health. 2013;3(2):73–83. [Google Scholar]
- Rollinson D, Simpson AJG. The biology of Schistosomiasis. London: Academic Press Limited; 1987. [Google Scholar]
- Rudge JW, Stothard JR, Basáñez MG, Mgeni AF, Khamis IS, Khamis AN, Rollinson D. Micro-epidemiology of urinary schistosomiasis in Zanzibar: local risk factors associated with distribution of infections among schoolchildren and relevance for control. Actatropica. 2008;105(1):45–54. doi: 10.1016/j.actatropica.2007.09.006. [DOI] [PubMed] [Google Scholar]
- Sady H, Al-Mekhlafi HM, Mahdy MAK, Lim YAL, Mahmud R, Surin J. Prevalence and associated factors of Schistosomiasis among children in Yemen: implications for an effective control programme. PLoS Negl Trop Dis. 2013;7(8):233. doi: 10.1371/journal.pntd.0002377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salawu OT, Odaibo AB. Schistosomiasis transmission; socio-demographic, knowledge and practices as transmission risk factors in pregnant women. J Parasit Dis. 2016;40:93. doi: 10.1007/s12639-014-0454-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satayathum SA, Muchiri EM, Ouma JH, Whalen CC, King CH. Factors affecting infection or reinfection with Schistosoma haematobium in coastal Kenya: survival analysis during a nine-year, school-based treatment program. Am J Trop Med Hyg. 2006;75:83–92. doi: 10.4269/ajtmh.2006.75.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner J, Niasse M, Haas L (eds) (2009) Sharing the benefits of large dams in West Africa. Natural Resource Issues No. 19 International Institute for Environment and Development, London
- Steinmann P, Keiser J, Bos R, Tanner M, Utzinger J. Schistosomiasis and water resources development: systematic review, meta-analysis, and estimates of people at risk. Lancet Infect Dis. 2006;6(7):411–425. doi: 10.1016/S1473-3099(06)70521-7. [DOI] [PubMed] [Google Scholar]
- Ugbomoiko US, Dalumo V, Danladi YK, Heukelbach J, Ofoezie IE. Concurrent urinary and intestinal schistosomiasis and intestinal helminthic infections in schoolchildren in Ilobu, South-western Nigeria. Actatropica. 2012;123(1):16–21. doi: 10.1016/j.actatropica.2012.03.002. [DOI] [PubMed] [Google Scholar]
- Webbe G. Schistosomiasis: some advances. BMJ. 1981;283:18. doi: 10.1136/bmj.283.6299.1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Commission on Dams (2000) Dams and development: a new framework for decision-making. The report of the World Commission on Dams. www.dams.org/report