SUMMARY
Context:
Breast cancer (BC) is the most common malignancy among women in the US. Vitamin D status and intakes are thought to be inversely associated with BC occurrence.
Objectives:
In our systematic review and meta-analysis, we evaluated evidence linking serum 25(OH)D (both in serum and diet) with breast cancer (BC) occurrence.
Data sources and extraction:
Only observational studies from databases such as PubMed and Cochrane (January 1st 2000 through March 15th, 2018) were included using PRISMA guidelines. Publication bias and consistency upon replication were assessed, while harmonizing risk ratios (RR, 95% CI) of BC, per fixed increment of 5 exposures [10 ng/mL of 25(OH)D; 100 IU/d for total/dietary vitamin D intakes; vitamin D deficiency; supplement use). RRs were pooled using random effect models.
Data analysis:
Pooled findings from 22 studies suggested a net direct association between 25(OH)D deficiency and BC, with RRpooled = 1.91, 95% CI: 1.51–2.41, P < 0.001). Total vitamin D intake (RRpooled = 0.99, 95% CI: 0.97–1.00, P = 0.022, per 100 IU/d) and supplemental vitamin D (RRpooled = 0.97, 95% CI:0.95–1.00, P = 0.026) were inversely associated with BC. No evidence of publication bias was found; all 5 exposures of interest were consistent upon replication.
Conclusions:
25(OH)D deficiency was directly related to BC while total vitamin D and supplemental vitamin D intakes had an inverse relationship with this outcome. Randomized clinical trials are warranted pending further evidence from primary meta-analyses of observational studies.
Keywords: Vitamin D, Breast cancer, Meta-analysis
1. Introduction
Breast cancer (BC) is the most prevalent malignancy among US women [1]. Vitamin D is a steroid hormone known to influence multiple organ functions in our body, including the heart, the skeletal system, the lungs, the intestines and the mammary glands. Its effect on mammary gland development is mediated through the actions of the vitamin D receptor (VDR). While vitamin D is present in fatty fish, cod liver oil, egg yolk, some mushrooms and meats etc., vitamin D deficiency has become a pandemic over the last few decades [2]. Recent epidemiologic studies have reported links between vitamin D deficiency and key adverse health outcomes, namely cardiovascular and cancer-related morbidity and mortality [3]. It is known to have multiple anti-proliferative, pro-apoptotic, and pro-differentiating actions on different malignant cells, mediated by VDR [4,5] Over 90% of vitamin D is produced endogenously in the skin, subsequently undergoing two hydroxylation reactions to form the active hormonal form 1,25-dihydroxyvitamin D [1,25(OH)2D]. The half-life of 1,25(OH)2D is about 6 h, while [25(OH)D] has a half-life almost 1000 times higher than the active form [6]. It has been, therefore, more commonly used in previous studies as a vitamin D status biomarker. However, epidemiologic evidence for a relationship between plasma 25(OH)D and BC incidence and/or prevalence is limited [7]. Several longitudinal studies on serum 25(OH)D and multiple cancer risks have concluded that 25(OH)D concentrations are inversely associated with colorectal cancer incidence [8–12] but not with prostate cancer or BC incidence [10,13–15]. In relation to BC, vitamin D has mixed results when separated by menopausal status. However, a cross-sectional study of postmenopausal women in Brazil showed that low serum 25 (OH)2 vitamin D level is a risk factor for ER negative tumors [16]. Furthermore, there is a definitive lack of randomized clinical trials (RCTs) with vitamin D supplementation and risk of cancer. In short, the relationship between vitamin D status and cancer risk remains incompletely understood [17].
Following the MOOSE guidelines [18], we hypothesized that vitamin D is inversely associated with BC occurrence in pre-and post-menopausal women. Our present systematic review and meta-analysis aimed at pooling, interpreting and evaluating research evidence for the past ~18 years that links serum vitamin D and vitamin D from both food and supplements using BC as outcome. Finally, this study discusses potential biological mechanisms behind those putative associations.
2. Methods
2.1. Search strategy
A systematic review of the literature on BC was conducted using primarily PubMed and Cochrane library as a secondary search. Using PubMed search engine, focus was on serum and dietary vitamin D as specific exposures. A systematic hand search combined the keywords “vitamin D” and “BC” prior to sorting and literature search was restricted to human studies published in English between January 1st 2000 and March 15th 2018. Synonymous keywords were not included in the search to avoid heterogeneity in defining the concepts. After the initial hit (N = 5618 from PubMed and N = 85 from Cochrane database), we excluded the duplicates from the filter option. This gave us a combined N = 3826 articles, which were then sorted by the availability of free full texts online. N = 1160 studies were excluded due to their unavailability in the full form. Of the remaining 2666 articles, N = 1664 were excluded due to several reasons including but are not limited to non-human subjects, non-English language, male subjects etc. Fig. 1 shows the search result, inclusion and exclusion criteria and the number of studies included. Original research published between 2000 and 2018 was considered because the association of vitamin D with BC was studied rarely before 2000. Papers were assessed by reviewing titles and abstracts yielded by an initial search using keywords combinations within abstracts [i.e. “vitamin D” AND “breast cancer”]. A systematic literature review of BC focusing on specific exposures, namely serum and dietary vitamin D was conducted (See Appendix 1 for search details). Among studies that were included in the review, key characteristics were retrieved such as design, setting, sample size, outcome of interest and key findings. EndNote (X8.1) was used to create a reference database and we summarized extracted summary data in an Excel spreadsheet.
Fig. 1.
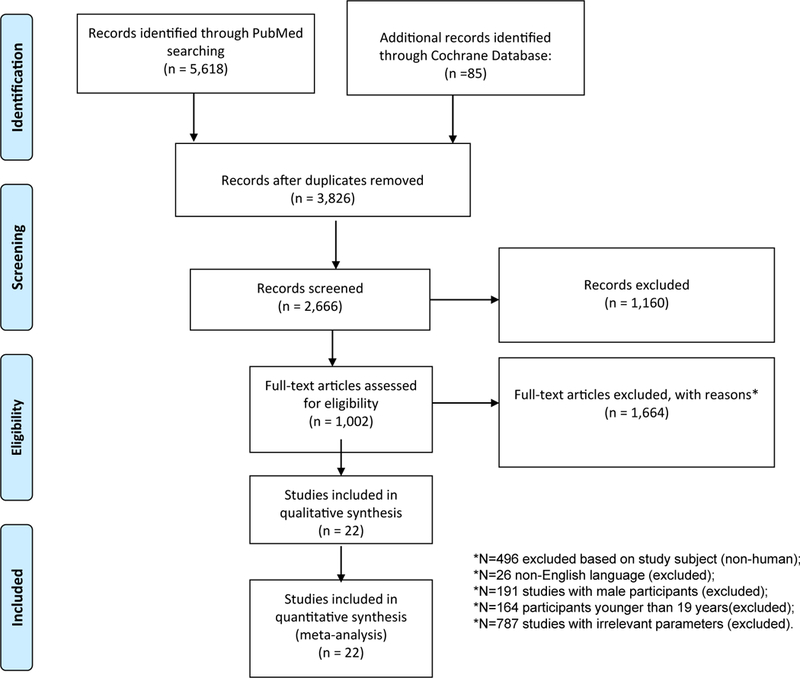
Flowchart of the study selection of articles reporting vitamin D (or related key terms) in relation to BC, PubMed and Cochrane database search 2000–2018.
2.2. Study retrieval and selection
Two reviewers (Sharmin Hossain and May Beydoun) independently worked on selecting studies for the review and meta-analysis. Study inclusion and exclusion was initiated by examining titles and abstracts. Only studies with direct relevance to our research question were retained. Table 1 list the PICO criteria study inclusion and exclusion. Full text was obtained for the selected papers, which we then screened for potential inclusion in the review and meta-analysis. The studies that were included assessed various types of associations between vitamin D status and BC incidence or prevalence. Included studies presented findings as odds ratios (OR), risk ratios (RR) or hazard ratios (HR). They used binary BC outcomes and categorical assessment of vitamin D status (usually binary). Exclusion of studies occurred for many reasons including “No relevant data available,” “Study is a randomized controlled trial,” “Study subjects are not adult women,” “Outcome is not incident or prevalent BC,” or “Exposure is not serum 25(OH)D or dietary intake of vitamin D”. Our meta-analysis summarized findings from selected observational studies, including case-control, cross-sectional, prospective and retrospective cohort studies.
Table 1.
PICOs criteria for inclusion and exclusion of studies.
| Parameters | Inclusion Criteria | Exclusion Criteria |
|---|---|---|
| Population | General population of adult females of reproductive age (pre-, postmenopausal or both) | Male |
| Intervention, Prognostic Factor, or Exposure | Total vitamin D (diet or supplements) from serum 25(OH)D | 1,25 (OH)2 D |
| Comparison | Risk ratios (RR), hazard ratios (HR) or odds ratios (OR) | Linear regression coefficients. |
| Outcome | Breast Cancer occurrence | Individual components of breast cancer were not studied (i.e. location, type, stage etc.) |
| Study Design | All types of observational studies were included in the systematic review: meta-analysis was done on case-control, cross-sectional, and prospective cohort studies. | Randomized controlled trials and retrospective cohort studies were excluded from the analyses |
2.3. Data extraction
Detailed characteristics of each study were summarized in Table 2 (e.g. gender and age composition, country), study design (e.g. case-control, cross-sectional or cohort study), sample size (e.g. total or number of cases vs. controls), exposures, covariates and quality score (QS) (described later). Selected studies in Table 2 are sorted by year of publication and first author’s last time. Type of exposure was identified as serum 25(OH)D (per 10 ng/mL), serum 25(OH)D (deficient vs. not), dietary vitamin D (per 100 IU/d), supplemental vitamin D (yes vs. no), and total vitamin D intake (per 100 IU/d). Further data extraction for use in the meta-analysis was conducted using a series Excel sheets (1 per study) in which the final effect size and its 95% CI were estimated.
Table 2.
Summary of studies selected in our meta-analysis of vitamin D and BC, PubMed 2000–2018.
| STUDY # Author/year Endnote Link |
Country | Study name; Study Design; Sample Size |
Menopausal status; Baseline age; Follow-up time |
Exposure; Outcome assessment |
25(OH)D Mean±SD % deficient |
Dietary vitamin D Mean±SD |
Supp use, % | Adjustment | Findings | Conclusion |
|---|---|---|---|---|---|---|---|---|---|---|
| QS = 0 + 1 + 1+2=4 (Range:0–8) | ||||||||||
| 1 [29] | USA | Nurses' Health Study; Nested casecontrol; Cases (N = 701) and controls (N = 724) |
Total 30–55 y Mean:~57.1 ya |
25(OH)D:IA; Self-reported diagnosis of BC during follow-up. |
Mean: 32.3 ng/mLb | n/a | n/a | BMI, menopausal status, menopausal hormone use, height, age at menarche, parity and age at first birth, weight at age 18, age at menopause, family history of BC, history of benign breast disease, alcohol intake, and smoking status. | Women in the highest quintile of 25(OH)D had RR = 0.73; (95% CI: 0.49 −1.07; P-trend = 0.06) compared with those in the lowest quintile | High levels of 25(OH)D may be modestly associated with reduced risk of BC. |
| QS = 2 + 2 + 2 + 2 = 8 (Range:0–8) | ||||||||||
| 2 [49] | USA | Cancer Prevention Study II Nutrition Cohort; Prospective cohort; N = 68,567 |
Post 50-74 y Mean: ~62 ya Up to 9 y of follow-up | Dietary/supplemental calcium and vitamin D, 68-item food frequency questionnaire (FFQ); BC incidence ascertained with NDI. | n/a | Dietary vitamin D: Mean: 167 IU/db Total vitamin D: Mean: 320 IU/d |
n/a | Age, energy, history of breast cyst, family history BC, height, weight gain since age 18, alcohol use, race, age at menopause, age at first birth and number of live births, education, mammography. History, and HRT use. | Using supplemental vitamin D intake was not associated with BC risk, overall. The association suggested a protective effect among women with estrogen receptor-positive tumors comparing highest to lowest intake of dietary vitamin D (RR = 0.74; 95% CI, 0.59 −0.93; P-trend = 0.006) | Our results support the hypothesis that dietary calcium and/or some other components in dairy products may modestly reduce risk of postmenopausal BC. The stronger inverse associations among estrogen receptorpositive tumors deserve further study. |
| QS = 0 + 2 + 2 + 2 = 6 (Range:0–8) | ||||||||||
| 3 [37] | Canada | Population-based case-control study Cases (N = 972) and Controls (N = 1135) | Total 20e69 y Mean: ~51.7 ya |
Dietary supplementation questionnaire; Ontario cancer registry: women with a pathology report indicating invasive BC, age-matched population-based controls. | n/a | n/a | Supplement use: Vitamin D/ multivitamin: Cases:15% Controls:22% |
Age, education, and ethnicity, the variables in the fully adjusted models included age at menarche (<12, 12, 13, 14+), first degree family history of BC (yes/no), ever breastfed (yes/no), and age at first birth (<20, 20–24, 25–29, 30+, nulliparous). | Reduced BC risk was associated with use of vitamin D or multivitamin supplements: OR = 0.62; 95% CI:0.49 −0.79. | Vitamin D could help prevent BC |
| QS=2 + 2 + 2 + 2 = 8(Range:0–8) | ||||||||||
| 4 [48] | USA | Iowa Women’s Health Study (IWHS) Prospective cohort N = 34,321 |
Post 55e69 y ~61.5 ya Follow-up up to 18 y |
127-item FFQ Dietary supplement questionnaire; Incident cases of BC were identified between 1986 and 2004 through linkage to the State Health Registry of Iowa, part of the National Cancer Institute's Surveillance, Epidemiology and End Results program (SEER). | n/a | Mean total vitamin D intake: ~520 IU/da | n/a | Baseline age (y), smoking status, age at menarche, age at reported menopause, first degree relative with BC, estrogen use, age at first live birth, number of live births, education category, BMI category, activity level, live on a farm, mammogram history, daily energy, fat and alcohol intakes. | The adjusted RR of BC for women consuming >800 IU/day versus <400 IU/day total vitamin D was 0.89 (95% CI: 0.77–1.03). RRs were stronger among women who were ER + or PR + status. The association of high vitamin D intake with BC was strongest in the first 5 years after baseline dietary assessment (RR = 0.66; 95% CI: 0.46e0.94 compared with lowestintake group), and diminished over time. | Vitamin D intake of >800 IU/day appears to be associated with a small decrease in risk of BC among postmenopausal women. |
| QS = 0 + 2 + 1 + 2 = 5(Range:0–8) | ||||||||||
| 5 [40] | USA | Women’s Health Initiative Nested casecontrol study within randomized trial Cases (N = 1067) and controls (N = 1067) | Post 50e79 y Mean: ~63 ya |
25(OH)D: IA; Modified block food frequency questionnaire and supplement use questionnaire; BCs were confirmed by both local and central medical record and pathology report review by trained adjudicators who were blinded to randomized allocation, with such records available in 98.2% of cases. |
–50.0 ± 20.0 nmol/Lb | Mean total vitamin D intake: 350 IU/da | Supplement use: Vitamin D/ multivitamin: Cases:47% Controls:48% | Baseline age (y), weight, and baseline percentage of energy from total fat. | Baseline 25(OH)D levels were not associated with BC risk in analyses that were adjusted for BMI and physical activity (P trend = 0.20) | 25(OH)D levels were not associated with subsequent BC risk |
| QS = 0 + 2 + 2 + 2 = 6(Range:0–8) | ||||||||||
| 6 [41] | USA | Prostate, Lung, Colorectal, and Ovarian (PLCO) Cancer Screening Trial Nested case-control study within a screening trial Cases (N = 1005) And controls (N = 1005) |
Post 55e74 y Mean: ~62 yb Mean followup time:3.9 y |
25(OH)D: IA; Incident BC cases were ascertained through self-report in an annual health survey, linkage to state cancer registries, death certificates, physician reports, and next-of-kin reports (for deceased participants). A total of 92% of the ascertained BC cases were confirmed through review of medical records. |
26.7 ng/mL | n/a | n/a | BMI at age 18 to 20, age at menarche, age at menopause, HRT use, history of benign breast disease, family history of BC, combined parity, age at first birth, smoking status, alcohol intake, and total calcium intake. | The RR of BC for the highest quintile of 25(OH)D concentration versus the lowest was 1.04 (95% CI, 0.75–1.45; P-trend = 0.81) | No inverse association between circulating 25(OH)D and BC risk |
| QS = 0 + 1 + 1 + 2 = 4(Range:0–8) | ||||||||||
| 7 [38] | Germany | Population-based case-control studyfrom southernGermany (Freiburgand Rhein-Neckar-Odenwald)Cases (N = 289) andcontrols (N = 595) | Pre 30–50 y Mean: ~42.6 ya | 25(OH)D: IA; Cases were identifiedthrough frequentmonitoring of hospitaladmissions, surgeryschedules andpathology reports in 38hospitals. |
51.3 nmol/L | n/a | n/a | Stratified by age and adjusted for time of blood collection, number of births, firstdegree family history, age at menarche, duration of breastfeeding, BMI, alcohol consumption. | Compared with the lowest category (<30 nmol/L), the ORs (95% CI) for the upper categories (30–45, 45 –60, ≥60 nmol/L) were 0.68 (0.43–1.07), 0.59 (0.37–0.94) and 0.45 (0.29–0.70), respectively (Ptrend = 0.0006) | There is a protective effect of vitamin D for premenopausal BC |
| QS = 0 + 1 + 1 + 2 = 4(Range:0–8) | ||||||||||
| 8 [42] | USA | Cancer Prevention Study-II (CPS-II) Nutrition Cohort Nested case-control within Prospective cohort study Cases (N = 516) and controls (N = 516) | Post 47–85 y Mean: ~69.5 yb | 25(OH)D: IA; Eligible cases included women who reported a new diagnosis of BC on a biennial CPS-II Nutrition Cohort Survey between the date of their blood draw and 30 June, 2005 (n = 514) or who did not report an incident BC but for whom fatal BC was identified through linkage with the National Death Index (n = 2). |
Mean: 49.1 −59.5 nmol/L depending on season. | n/a | n/a | Reproductive risk factors, history of benign breast disease, family history, education, alcohol use, postmenopausal hormone use, diet, recreational physical activity and zip code (for latitude). | No association between 25(OH)D and BC (OR = 1.09, 95% CI 0.70 −1.68, P = 0.60) for the top vs. bottom quintile | Results do not support an association between adulthood serum 25(OH)D and postmenopausal BC. |
| QS = 0 + 0 + 2 + 1 = 3(Range:0–8) | ||||||||||
| 9 [30] | Denmark | Case-control study Cases (N = 142) and controls (N = 420) | Total 29–87 y Mean: ~58 yb | 25(OH)D: LC; Mammography, followed by pathologic examination. Information on studied subjects from The Danish National Hospital Discharge Register and the Danish Cancer Register were also retrieved. |
Cases: 69 ± 23 nmol/L Controls: 76 ± 28 nmol/L |
n/a | n/a | Controls matched with cases on menopausal state, and time of year of blood sampling (±2 mo). | Compared with the lowest tertile of 25(OH) D levels, risk of BC was significantly reduced among women in the highest tertile (RR = 0.52; 95% CI: 0.32–0.85) | Risk of BC was inversely associated with 25(OH) D levels |
| QS = 0 + 1 + 0 + 1 = 2(Range:0–8) | ||||||||||
| 10 [43] | Finland | Finnish Maternity Cohort Nested case-control study within cohort Cases (N = 311) and controls (N = 311) |
Pre 30–34 y Mean: ~33 yb | 25(OH)D: IA; Missing information on diagnosis of BC. |
Mean: 43 nmol/La | n/a | n/a | Controls matched to cases by parity, age, year, and season | Serum 25(OH)D level was not associated with an increased risk neither at the 1st nor at the 2nd pregnancy samples (OR = 1.4, 95% CI 0.6–3.4; OR 1.4, 95% CI 0.7–2.8, respectively), but was associated with an increased risk of PABC (OR = 2.7; 95%CI 1.04–6.7) | Vitamin D may not be related to BC risk |
| QS = 0 + 1 + 1 + 2 = 4(Range:0–8) | ||||||||||
| 11[31] | France | French E3N Cohort Nested case-control within cohort Cases (N = 636) Controls (N = 1272) | Total Cases (56.9 ± 6.4) and controls (56.9 ± 6.4) | 25(OH)D: IA; Every 2–3 y, questionnaire was sent out. In each questionnaire, participants were asked whether a cancer had been diagnosed, and if so, pathology reports were requested from the attending physicians. |
Cases: 24.4 ± 10.9 ng/mL Controls: 25.1 ± 11.0 ng/mL | n/a | n/a | BMI , HRT use, history of mammography history and of breast benign disease, family history of BC, parity, smoking status, use of oral contraceptives, age at menarche, and physical activity, alcohol consumption, total energy, calcium/ vitamin D dietary intakes, vitamin D/ calcium supplement use, serum calcium, PTH, estradiol, progesterone, estradiol (pmol/L, continuous) and progesterone (nmol/L, continuous). | Found a decreased risk of BC with increasing 25(OH)D3 serum concentrations (odds ratio, 0.73; 95% confidence interval, 0.55–0.96; Ptrend = 0.02) among women in the highest tertile) | There is a decreased risk of BC associated with high 25(OH) vitamin D3 serum concentrations, especially in youngerwomen |
| QS= 0 + 1 + 1 + 2 = 4(Range:0–8) | ||||||||||
| 12 [44] | USA | Nurses' Health Study II Nested case-control study within cohort. Cases (N = 613) Controls (N = 1218) | Both All women (50.9 ± 12.6), premenopausal (39.73 ± 7.83), postmenopausal (58.68 ± 7.46) | 25(OH)D: IA; BC cases were identified on biennial questionnaires; the National Death Index was searched for nonresponders. All BC cases occurred after blood collection but before 1 June 2007. |
Cases:25.4 ± 9.5 ng/mL Controls: 25.0 ± 9.6 ng/mL | n/a | n/a | BMI at age 18 y and at the time of blood collection, ages at menarche and first birth, parity, family history of BC, and history of benign breast disease. | No significant association was observed between plasma 25(OH)D levels and BC risk (top vs. bottom quartile multivariate RR = 1.20, 95% CI (0.88–1.63), Pvalue, test for trend = 0.32) | Circulating 25(OH)D levels were not significantly associated with BC risk in this mostly pre-menopausal population |
| QS = 0 + 0 + 1 + 2 = 3(Range:0–8) | ||||||||||
| 13 [32] | USA | Case-control study Cases (N = 194) Controls (N = 194) | Total 40–70 y Mean: ~58.3 ya | 25(OH)D: IA; Histologically confirmed primary, incident, BC, with no prior cancer history except nonmelanoma skin cancer. | Cases: 32.7 ± 14.4 ng/mL Controls 37.4 ± 15.9 ng/mL | n/a | n/a | Age, race, date of blood collection, and laboratory used for vitamin D testing. | BC cases had significantly lower 25(OH)D levels than CF controls (BC: 32.7 ng/ mL vs. CF: 37.4 ng/mL; P = 0.02) | BC patients with a more aggressive molecular phenotype (basal-like) and worse prognostic indicators had lower mean 25(OH)D levels |
| QS = 0 + 2 + 2 + 2 = 6(Range:0–8) | ||||||||||
| 14 [45] | USA, | Nested Case-control From two cohorts: New York University Women’s Health Study and the Northern Sweden Mammary Screening Cohort Cases (N = 1585) Controls (N = 2940) | Total 34–65 y Mean: ~52.6 ya | 25(OH)D: IA; For the NYUWHS, incident cases of invasive BC were identified by mailed questionnaires or follow-up telephone interviews every 2–4 years after 1991, supplemented by linkages to state cancer registries in New York, New Jersey, and Florida and the US National Death Index. Medical records were reviewed to confirm self-reported cases. Using a capturerecapture analysis, we estimated that combining active and cancer registrybased follow-up resulted in a BC ascertainment rate of 95%. For the NSMSC, annual linkages to the Swedish National Cancer Registry were used to identify incident cases of BC in the cohort. |
Cases: 53.0 ± 14.9 nmol/L Controls: 54.2 ± 18.6 nmol/ mL | n/a | n/a | Age at menarche, age at first birth/parity, family history of BC, BMI, past HRT use, and alcohol consumption. | No association was observed between circulating levels of 25(OH)D and overall BC risk (multivariateadjusted model OR = 0.94; 95% CI: 0.76–1.16 for the highest vs. lowest quintile, Ptrend = 0.30) | Circulating 25(OH)D levels were not associated with BC risk overall |
| QS = 0 + 0 + 1 + 2 = 3(Range:0–8) | ||||||||||
| 15 [34] | Saudi Arabia | Case-control study Cases (N = 120) Controls (N = 120) | Total 47.8 ± 12.4 y | 25(OH)D: LC All women presented with invasive BC at the clinic or were receiving standard medical check-ups at the same women's clinic and were shown on medical record review to be free of cancer. | 15.4 ± 12.3 ng/mL | n/a | n/a | Age, BMI, history of cancer, parity, family history of cancer, exercise, location of exercise (indoors or outdoors), multivitamin use, presence BC in daughters, benign breast disease, menopause, and breastfeeding | In comparison with those in the highest category of vitamin D status for this population (>20 ng/ mL), the adjusted ORs (95% CIs) for invasive BC were 6.1 (2.4, 15.1) for women with a serum 25(OH)D concentration, 10 ng/ mL and 4.0 (1.6, 10.4) for women with a serum concentration of 10e20 ng/mL (Ptrend = 0.0001) | An inverse association exists between serum 25(OH)D concentrations and BC risk in Saudi Arabian women |
| QS = 0 + 0 + 1 + 2 = 3(Range:0–8) | ||||||||||
| 16 [39] | Iran | Population based case- control study Cases (N = 60) and controls (N = 116) | Pre 34–36 y Mean: ~35 yb | 25(OH)D: IA Daily intake of calcium and vitamin D and all dietary resources of mentioned factors were collected. We selected cases from patients who underwent surgery from 2010 to 2012 in Emdad Shahid Beheshti University hospital. Cases were identified from both self-reports registration and confirmed by pathological reports. The pathological feature of cases was collected from pathological reports in the pathology archive of the mentioned hospital. | Cases: 15.2 ± 8.2 ng/mL Controls: 15.5 ± 7.5 ng/mL Overall: 15.4 ng/mLa |
n/a | Vitamin D supplement, % yes: Cases: 0.0% Controls: 9.7% | Daily sunlight exposure, covering body against sunlight, calcium supplements, vitamin D supplements, fish and egg intakes and weekly profile of egg consumption. | The lack of vitamin D and calcium supplementation increased slightly the risk of premenopausal BC (p = 0.009, OR = 1.115, CI 95% = 1.049–1.187) | Vitamin D may have a role in BC incidence but it needs further proof |
| QS = 0 + 1 + 2 + 2 = 5(Range:0–8) | ||||||||||
| 17 [33] | USA | Nested case-control study within the Multiethnic Cohort Study Cases: N = 707 Controls: N = 707 | Post Mean: ~67.8 yb | 25(OH)D: LC Incident invasive BC cases were identified by linkage to the Surveillance, Epidemiology, and End Results Program registries in the states of Hawaii and California through October, 2010, including 729 eligible postmenopausal womenwith a diagnosis of invasive BC. |
Overall: Mean 25(OH)D: 31.4 ng/mLb Vitamin D deficiency (<16 ng/ mL): 7.2%a | n/a | n/a | Body mass index, parity, family history of BC, use of multivitamin and calcium supplements, season, sunburn history and engagement in strenuous sports. | 20 ng/mL increases of plasma 25(OH)D3 [OR = 0.28; 95% CI: 0.14–0.56] and 25(OH)D [OR = 0.43; 95% CI: 0.23–0.80] were inversely associated with BC risk among white women, but not among women in other race/ethnic groups. | Circulating 25(OH)D3 and 25(OH)D were associated with a reduced risk of postmenopausal BC among whites, but not in other ethnic groups, who reside in low latitude regions. |
| QS = 0 + 1 + 1 + 2 = 4(Range:0–8) | ||||||||||
| 18 [46] | USA | Nurses’ Health Study II Nested case-control within a cohort. Cases (N = 584) Controls (N = 584) | Pre 45–46 y Mean: ~45.1 ± 4.4 yb | 25(OH)D: IA BC cases were identified through the biennial questionnaires: Cases had no previously reported cancer diagnosis before blood collection and were diagnosed after blood collection but before June 1, 2007. |
Median 25(OH)D Cases:62.6 nmol/L Controls: 61.4 nmol/L | n/a | n/a | Body mass index (BMI) at age 18 and at blood collection, age at menarche, parity and age at first birth, history of benign breast disease, family history of BC, and alcohol consumption. | No association between plasma calculated free 25(OH)D and risk of BC overall (highest vs. lowest quartile RR = 1.21; 95% CI: 0.83–1.77), P-trend = 0.50) | There is no association between circulating free 25(OH)D or circulating VDBP levels with BC risk among mostly pre-menopausal women. |
| QS = 0 + 1 + 2 + 2 = 5(Range:0–8) | ||||||||||
| 19 [35] | USA | SPA6 region of South Los Angeles County in California cases = 243 and controls = 417; total N = 660 | No menopause status 21–80 years Mean: 51 y | 25(OH)D: Liquid Chromatography/ Tandem Mass Spectrometry (LC/MS/ MS) method; BC incidence/risk For cases, breast cancer status was determined by biopsy/pathology confirmed neoplasm of the breast, and only subjects who had documentation of this information were included in the study | >20 ng/mL is normal | n/a | n/a | BMI, ethnicity, age, BMI and the seasons of blood draw | The 25(OH)D3 below 20 ng/mL was significantly associated with triple negative breast cancer (TNBC) in African-Americans (OR = 5.4, p = 0.02, 95% CI), but not in the Hispanic group | There might be an association between 25(OH)D3 levels and the risk of breast cancer. |
| QS = 0 + 2 + 2 + 2 = 6(Range:0–8) | ||||||||||
| 20 [47] | USA, UK, Greece | Mendelian Randomization study 70,563 cases of cancer (22,898 prostate cancer, 15,748 breast cancer, 12,537 lung cancer, 11,488 colorectal cancer, 4369 ovarian cancer, 1896 pancreatic cancer, and 1627 neuroblastoma) and 84,418 controls. | No menopause status Total 30e55 y Mean: ~57.1 ya | Four single nucleotide polymorphisms (rs2282679, rs10741657, rs12785878 and rs6013897) Risk of incident colorectal, breast, prostate, ovarian, lung, and pancreatic cancer and neuroblastoma | n/a | n/a | n/a | n/a | OR per 25 nmol/L increase in genetically determined 25(OH)D concentrations was 1.05 (0.89–1.24) for breast cancer | Population-wide screening for vitamin D deficiency and subsequent widespread vitamin D supplementation should not currently be recommended as a strategy for primary cancer prevention. |
| QS = 0 + 2 + 1 + 2 = 5(Range:0–8) | ||||||||||
| 21 [36] | USA | The Sister Study Nested case-control Cases (N = 1611) and controls (N = 1775) | Total 35–74 y Mean: 55.6 | 25(OH)D: IC, MS; Self-reported diagnosis of BC during follow-up. | Q4 25(OH)D ≥38.0 ng/Ml Dietary vitamin D supplement use (yes vs. no) Total vitamin D intake (per 100 IU) | n/a | n/a | BMI, sunlight-related variables (e.g., latitude, physical activity, and time spent outdoors) were assessed at baseline, along with other potentially relevant covariates such as exogenous hormone use, history of osteoporosis, education, and menopausal status | 25(OH)D levels were associated with a 21% lower breast cancer hazard (highest versus lowest quartile: adjusted; OR = 0.79, CI: 0.63, 0.98) | High serum 25(OH)D levels and regular vitamin D supplement use were associated with lower rates of incident, postmenopausal breast cancer over 5 y of follow-up |
| QS = 0 + 0 + 2 + 2 = 4(Range:0–8) | ||||||||||
| 22 [4] | Pakistan | Case-control Cases (N = 42) and controls (N = 52) | No menopause status 20–75 y Mean: 40.1 y |
25(OH)D: ELISA; Diagnosed with invasive breast cancer within 6 months of all grades and of stage I-III. |
Vitamin D = 20 ng/ mL (normal) | n/a | n/a | Age, parity, BMI, sun exposure, education and economic status | Breast cancer risk was 7.8 (1.99–30.58) for women with vitamin D concentrations <20 ng/ mL from the adjusted model. | Vitamin D deficiency is associated with risk of breast cancer |
Abbreviations: 95%CI = 95% confidence interval; 25(OH)D = 25-hydroxyvitamin D; BC = BC; BMI = Body Mass Index; HR ¼ Hazard Ratio; HRT = Hormone Replacement Therapy; IA = Immunoassay; LC = Liquid Chromatography; n/a = Not applicable; NDI = National Death Index; OR = Odds Ratio; P-trend = P-value for the trend test; QS = Quality Score; RR = Risk Ratio.
Estimated based on available categorical data and ranges within each category, with sample sizes for each category.
Estimated based on available data on mean and SD for cases and controls and sample sizes of cases and control.
2.4. Qualitative review and meta-analysis
The final selected 22 studies were first reviewed in a systematic way, stratifying by study design and type of exposure. The original measures of association are presented and compared across studies. Using the same 22 studies, we conducted further meta-analysis to assess BC–vitamin D strength of association among pre- and postmenopausal women combined. This analysis was thus restricted to case-control, cross-sectional, and prospective cohort studies with comparable measurements for vitamin D-related exposure. This resulted in pooled estimate measures of association across the studies, i.e. risk ratio (RR) with its 95% CI. Different exposure scales [e.g. an increase per 1 SD vs. quartiles vs. tertile vs. per 1 unit (e.g. 10 ng/mL)] dictated RR modifications. A pooled measure of association was constructed from the RRs representing the effect of a fixed incremental linear increase in the BC exposure. After converting the RR to LogeRR with its SE, each point estimate and its corresponding SE were divided by a conversion factor. Generally, means of highest and lowest tertiles lie 2.18 SD apart for a normal distribution. So, the Loge RRs for such a contrast was divided by 2.18 to obtain LogeRR per SD. When RRs contrasted quintile 5 with quintile 1, its Loge transformed version along with its SE were divided by 2.8, while those effects and SE comparing extreme quartiles were divided by 2.54, an approach adopted elsewhere [19,20]. SD values were estimated using descriptive data from cases/controls, or from the total sample when the design was either cross-sectional or cohort. They were also computed by approximating between extreme quantile differences and dividing them by the conversion factors above [21]. From this, RR per 10 ng/mL for serum 25(OH)D, or per 100 IU dietary or total vitamin D was estimated by dividing the LogeRRZ with its SEz by the estimated SD value (either reported in the study or estimated from extreme quantiles) and multiplying it by 10 and 100, respectively. The Log-transformed RR is then exponentiated thus obtaining the RR point estimate per 10 ng/mL increase in 25(OH)D or 100 IU/d of dietary/total vitamin D intake, which approximated 1 SD increase in most studies. To obtain the 95% CI, the LogeRR point estimate is used along with its SE to obtain the lower and upper confidence limits on the Loge scale. These values are then exponentiated to obtain RR’s 95% CI. A similar approach was adopted when vitamin D exposure in a specific study was reported into non-quantile categories (i.e. exposure groups with varying sample sizes). However, in this case, each contrast with a referent category is transformed into per 10 ng/mL or100 IU/d and then pooled into a common measure of association using random effects models within that study. Moreover, in the latter instance, median exposure value in each category is estimated and subtracted from the referent to obtain average increment in vitamin D exposure corresponding to each contrast. This is then used to ensure the measure of association corresponded to 10 ng/mL or 100 IU/d increase, for 25(OH)D and dietary/total vitamin D, respectively. Varying units and conversion factors in serum 25(OH)D measurement were also considered in these calculations. In studies with stratified analyses (e.g. by age group), incremental RR were estimated per strata and then pooled into one estimate with associated 95% CI, using random effects models within the study.
After the heterogeneity testing, study-specific RRs linked to an exposure of interest were examined in pooled models and demonstrated by forest plots estimated with an inverse variance weighting procedure [22]. DerSimonian and Laird’s methodology was used to ascertain random effects models that further incorporated between-study variability [22].
2.5. Harvest plot and funnel plot
A modified quality score (QS) from a previous study was utilized to measure the quality of each study [23] included (Appendix 2) [19]. For this meta-analysis, the QS scale had 4 items, namely study design, sample size, type of outcome assessment, and adjustment level for putative confounders. The score on each item ranged between 0 and 2, with a higher score reflecting better quality, yielding a total QS that could range between 0 and 8. One QS was estimated per study as only one outcome (BC) was of interest in this meta-analysis. Three QS independent item-wise assessments were carried out by three co-authors (May Beydoun, Hind Beydoun and Xiaoli Chen) and mean QS was estimated. A harvest plot was utilized to graphically represent the key findings for each exposure by QS level. This plot shows the exposure-outcome associations in each study, if they were significant and with directionality (–1 = “inverse association”, 0 = “null association”, 1 = “positive association”), while simultaneously reflecting quality. In order for a harvest plot to be represented, at least 3 study data-points per hypothesized exposure-outcome association were needed.
Finally, to ascertain publication bias, Begg’s funnel plot was used, where each OR point estimate was plotted against its corresponding standard errors (SE) on a logarithmic scale [24,25] per study, and combining all exposures (e.g. serum and dietary vitamin D). Occurrence of such bias was also tested with the Begg-adjusted rank correlation tests [26] and the Egger’s regression asymmetry test [27]. All analyses were carried out using STATA 15.0 (StataCorp, College Station, TX) [28]. Type I error was set at 0.05 for all measures of association.
3. Results
3.1. Study selection, characteristics and quality score
Out of 3826 un-duplicated titles and abstracts between 2000 and 2018, 22 original publications were selected for the systematic review and meta-analyses. Those selected studies were published between 2005 and 2017 (Mean ± SD: 2010.7 ± 3.7), with 14 being US studies, 1 Canadian, 4 European and 3 from Asia (Table 2). Moreover, most studies had a case-control or nested case-control design (n = 19), with only 3 being prospective cohort studies. Twelve studies comprised adult women of varying age ranges, while 4 included only pre-menopausal women and 6 were restricted to post-menopausal women. Overall, mean age was 53.6 with a SD of 9.8 y. The cumulative sample size is of 229,597 subjects, with a Mean ± SD: 10,436 ± 25,426 subjects per study. Considering the maximum score can be 8.0, the mean QS with SD was 4.64 ± 1.56 (range:2—8), indicating a relatively above average quality set of studies.
3.2. Qualitative review of studies
Of 19 case-control studies included in this paper, 12 studies (63.1%) showed that a reduced risk of BC was associated with high levels of 25(OH)D [4,29–36] or the use of vitamin D or multivitamin supplements that contained vitamin D [36–39] The remaining 7 case-control studies (36.8%), however, showed no associations of BC risk with 25(OH)D levels or vitamin D intake [40—47].
Of two prospective cohort studies included in this current study, both of the two studies showed that dietary calcium could reduce the risk of BC among postmenopausal women [48,49]. For example, in the Cancer Prevention Study II Nutrition Cohort (QS = 8), McCullough et al. (2005) studied dairy, calcium, and vitamin D intake and BC risk among 68,567 postmenopausal women aged 50—74 years in the US (QS = 8) [49]. Their results supported the hypothesis that dietary calcium may modestly reduce the risk of postmenopausal BC. In the Iowa Women’s Health Study of 34,321 postmenopausal women aged 55—69 years with 18 years of follow-up (QS = 8) [48], reported that vitamin D intake appeared to be associated with a small decrease in BC risk among postmenopausal women in the US. When comparing the selected studies in terms of covariate-adjustment for potential confounding, many common factors were introduced into the models including age at examination, age at menarche, age at menopause (when post-menopausal women were selected), and other key covariates such as education and use of hormone replacement therapy. However, lifestyle and health-related factors including smoking, alcohol use, physical activity, body mass index and history of cancer were adjusted several selected studies, though not in a consistent manner.
3.3. Meta-analysis: findings for serum 25(OH)D
Overall, the measures of association from 14 case-control studies on serum 25(OH)D in relation to BC were pooled. A total number of 25,515 cases matched with 97,529 controls. Our pooled findings indicated that there was no detectable association between serum 25(OH)D concentration and BC occurrence (RRpooled = 0.99, 95% CI: 0.98–1.00, P = 0.17, per 10 ng/mL). While based on 5 case-control studies (1306 cases matched with 1490 controls), serum 25(OH)D deficiency was shown to have a direct relationship with the risk of BC (RRpooled = 1.91, 95% CI: 1.51–2.41, P < 0.001) (Fig. 2A–B). It is worth noting that 9 of the 13 case-control studies used in the meta-analysis with 25(OH)D as the exposure were nested within a prospective cohort study. Due to the small number of data-points per exposure, age group stratification of the meta-analysis was not carried out.
Fig. 2.
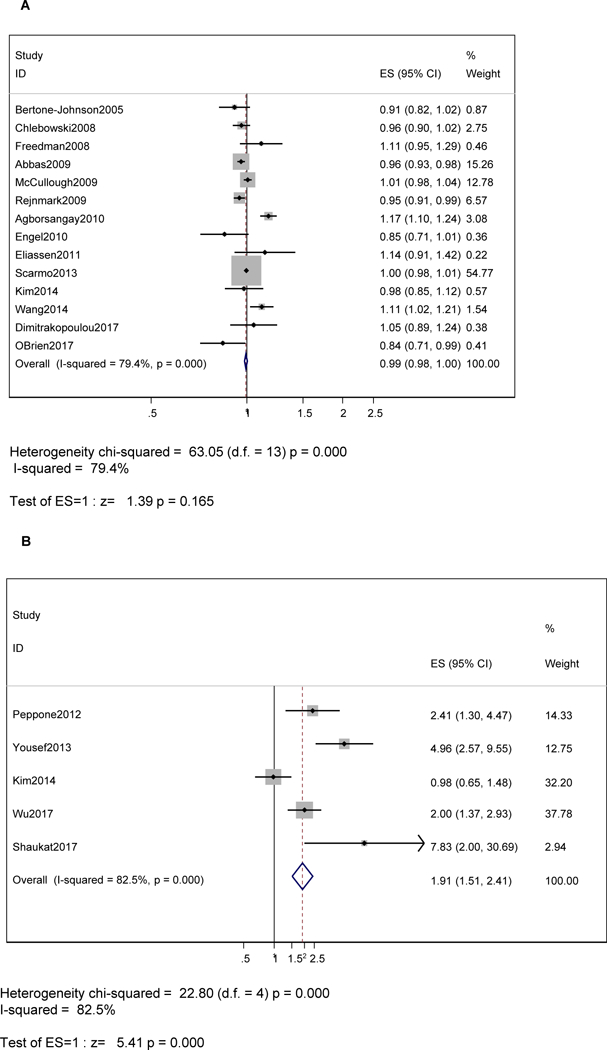
A–B Forest plot of odds ratios with 95% CI of the association between serum 25(OH)D (A) per 10 ng/mL or (B) Deficient vs. not (<10 ng/mL vs. ≥10 ng/mL (referent)) and BC, 2000–2018.
3.4. Meta-analysis: findings for dietary vitamin D (foods and supplements)
Association measured from the 5 studies on total dietary vitamin D (foods and supplements) relation to BC occurrence were pooled. Fig. 3A shows a net inverse association between total vitamin D intake and BC, with a RRpooled = 0.99, 95% CI: 0.97–1.00, P = 0.022, per 100 lU/d. This small inverse association was observed for dietary vitamin D and supplemental vitamin D usage (yes vs. no), though it attained statistical significance only for supplemental vitamin D (RRpooled = 0.97, 95% Cl: 0.95–1.00, P = 0.026).
Fig. 3.

A–C Forest plot of odds ratios with 95% CI for the associations of (A) total vitamin D (per 100 IU/d) (B) dietary vitamin D (per 100 IU/d) and (C) supplemental (yes vs. no) vitamin D with BC, 2000–2018.
3.5. Bias assessment: funnel and harvest plots
Begg’s funnel plot (Supplemental Fig. 1) indicated that most of the 28 data-points fell within the expected confidence limits when plotting Loge (RR) against its SE. There was no publication bias and asymmetry whereby Loge (RR) was not associated with its SE in terms of slope. In addition, there was no bias in terms of the directionality of Loge (RR) that were published in the literature.
The harvest plots are presented in Supplemental Fig. 2, with qualitative findings (–1 = “inverse association”, 0 = “null association” and 1 = “positive association”) against QS [50,51]. Based on the clusters, 8 of 12 studies that examined 25(OH)D in relation to BC, reported a null overall finding. Noteworthy is that those studies had a slightly higher QS compared with those that reported an inverse association, though the difference was not statistically significant (Mean QS 5.0 vs. 4.0).
4. Discussion
This study is to our knowledge, the most up to date among very few meta-analyses conducted to synthesize the literature on vitamin D exposure and BC occurrence. Pooled findings indicated that there was a net direct association between 25(OH)D deficiency and BC occurrence, with a pooled RR = 1.91, 95% Cl: 1.51–2.41, p < 0.001). A weaker inverse association was also observed for total vitamin D intake from foods and supplements (RR = 0.99, 95% Cl: 0.97–1.00, P = 0.022, per 100 IU/d) and BC. A similar association was noted for supplemental vitamin D (yes vs. no). No net association was detected between BC and serum 25(OH)D (per 10 ng/mL) or between BC and dietary vitamin D. There was no evidence of publication bias. All 5 exposures of interest came up as consistent from the harvest plots suggesting consistency. In recent years, there has been considerable interest in whether vitamin D inhibits BC development [41]. Low serum 25(OH)D levels have been reported in breast cancer patients compared to healthy controls and were also associated with poor prognosis [52,53]. The anticarcinogenic potential of vitamin D comes from the active, hormonal form of vitamin D, 1,25(OH)2D. Experimental studies indicate that 1,25(OH) 2D can also be synthesized locally from 25(OH)D in other tissues, including but not limited to breast, intestines, lung etc. [54,55] VDR is activated by 1,25(OH)D, and is found in nearly all tissues and organs in the human body. It is responsible for the transcription of numerous genes (~60) related to cell proliferation, differentiation, metastasis, and apoptosis [56]. Therefore, it is of considerable interest in relation to many cancers, including BC [57]. The circulatory levels of the biologically active metabolite 1,25(OH)2D is tightly regulated. Since circulating 25(OH) is related to 1,25(OH)2D in breast tissue and circulating 1,25(OH)2D is homeostatically controlled, circulating 25(OH)D is thought to be potentially relevant to breast carcinogenesis [41]. The final analyses were done on the selected N = 22 studies from an initial hit of over ~5500 studies. We had excluded a total of 1664 studies after the initial screening and removal of duplicates because our study focused on humans, specifically, pre- and post-menopausal women only. Therefore, we had no need for animal studies, or studies published in another language than English, had male participants, or the age group younger than 19 years of age. We also excluded studies with parameters irrelevant to our objective, e.g. efficacy of BC screening techniques, BC prognosis and survival to name a few.
To highlight the strengths of this study, first, this systematic review of the literature is, to our knowledge, one of the few to have examined a wide range of vitamin D exposures and their relationships to with BC occurrence. Second, a validated quality scoring system was used as a tool to examine heterogeneity of study results based on the quality of the data. Third, we pooled case-control studies for 25(OH)D exposures and vitamin D deficiency and prospective cohort studies for dietary and supplemental vitamin D intakes. It has been shown that since BC develops slowly, case-control studies are superior to prospective cohort studies, particularly for serum exposures. For latter case, pooling prospective cohort studies would require adjustment for length of follow-up [58–60].
Our review excluded clinical trials on vitamin D and BC primarily because of the limited number of completed trials (around the time of manuscript preparation). Those trials yielded for the most part mixed or null findings due to inadequate statistical power and/or follow-up time. One recent randomized controlled trial [61] contributed to the body of the literature, by detecting no association between supplemental vitamin D and BC risk (primary endpoint). Although this study had adequate strength and long duration, it lacked power for site-specific cancer analyses (including BC) and had a single dose of vitamin D-which could arguably contribute to the findings. However, our study results should to be interpreted with caution considering several limitations. First, our literature search was restricted to the PubMed and Cochrane databases, and did not include other electronic databases such as Embase or Web of Science. Second, we used specific key terms to perform the literature search but did not search for cross-references or unpublished studies (abstracts, conference papers, theses and dissertations). Third, while exposures included dietary and supplemental vitamin D in addition to serum 25(OH)D, some meta-analyses were low-powered given the limited number of data-points available. Fourth, evidence was mostly generated from observational studies, namely, cross-sectional, retrospective cohort and prospective cohort studies, which precludes our ability to confirm causality. Thus, a separate meta-analysis of randomized controlled trials is needed, once enough trials are made available for such analysis. Fifth, the reported associations may be confounded by other micro - and macro-nutrients, and/or lifestyle factors known to affect the risk of BC. Similarly, although dietary vitamin D is elicited from many sources, depending on the dietary assessment tool used, meat is seldom included among those sources [62]. Furthermore, compared to UVB exposure which accounts for most of the 25(OH)D variation, dietary vitamin D has little impact on vitamin D deficiency or serum level of 25(OH)D. Thus, it is less likely that an association between dietary vitamin D and BC would be detected in studies of dietary vitamin D intake. In addition to the issue of measurement error that is inherent in the dietary assessment of vitamin D with food frequency questionnaires, noteworthy is that each of the 3 studies included with supplemental vitamin D intake had different dosage of daily intake, which affects their respective findings. Similarly, despite the use of a single study design to conduct exposure-specific meta-analyses, some heterogeneity in the way exposures were measured, particularly dietary exposures, can bias the association between vitamin D and breast cancer occurrence. Nevertheless, it is likely a bias towards the null leading to attenuation of the true effect, since exposure measurement error or heterogeneity is independent of the outcome. Finally, though tested through funnel plots and other statistical methods, publication bias cannot be ruled out as an explanation for these study results, particularly when non-English literature was excluded from our review, given limited resources for translation.
5. Conclusions
Previous meta-analyses have demonstrated important evidence of reverse causation in studies of circulating vitamin D and BC. Only retrospective case-control studies have shown an inverse association between circulating 25(OH)D and breast cancer risk, whereas prospectively conducted studies have found no association. Our review and meta-analysis indicated that serum 25(OH)D deficiency, as well as total and supplemental vitamin D intake, were associated with BC occurrence in the general population, suggesting an inverse association between vitamin D status/intake and BC. This is an important finding in terms of future randomized controlled trials which might reveal a direct cause and effect relationship between vitamin D intake as well as serum vitamin D and the risk of BC. The clinical relevance of vitamin D deficiency makes for a strong case for BC prevention, though the impact of dietary or supplemental vitamin D on BC occurrence was weaker. Thus, increasing sunlight exposure may be a more effective way to prevent BC than diet or supplements. Nevertheless, an updated meta-analysis may be needed with more relevant studies to strengthen further those findings, particularly one using primary data from several case-control or cohort studies.
Supplementary Material
Acknowledgments
All authors read and approved the final manuscript. The authors would like to thank Gregory A. Dore and Ola S. Rostant (NIA/NIH/IRP) for their internal review of the manuscript. There is no conflict of interest. This research was supported entirely by the Intramural Research Program of the NIH, National Institute on Aging (NIA/IRP).
Footnotes
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/jxlnesp.2018.12.085.
References
- [1].Blumen H, Fitch K, Polkus V. Comparison of treatment costs for breast cancer, by tumor stage and type of service. Am Health Drug Benefits 2016;9: 23–32. [PMC free article] [PubMed] [Google Scholar]
- [2].Bodnar LM, Catov JM, Wisner KL, Klebanoff MA. Racial and seasonal differences in 25-hydroxyvitamin D detected in maternal sera frozen for over 40 years. Br J Nutr 2009;101:278–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Shi L, Nechuta S, Gao YT, Zheng Y, Dorjgochoo T, Wu J, et al. Correlates of 25-hydroxyvitamin D among Chinese breast cancer patients. PLoS One 2014;9, e86467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Shaukat N, Jaleel F, Moosa FA, Qureshi NA. Association between vitamin D deficiency and breast cancer. Pak J Med Sci 2017;33:645–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Lee MS, Huang YC, Wahlqvist ML, Wu TY, Chou YC, Wu MH, et al. Vitamin D decreases risk of breast cancer in premenopausal women of normal weight in subtropical taiwan. J Epidemiol 2011;21:87–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Bjork A, Andersson A, Johansson G, Bjorkegren K, Bardel A, Kristiansson P. Evaluation of sun holiday, diet habits, origin and other factors as determinants of vitamin D status in Swedish primary health care patients: a cross-sectional study with regression analysis of ethnic Swedish and immigrant women. BMC Fam Pract 2013;14:129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Bertrand KA, Rosner B, Eliassen AH, Hankinson SE, Rexrode KM, Willett W, et al. Premenopausal plasma 25-hydroxyvitamin D, mammographic density, and risk of breast cancer. Breast Canc Res Treat 2015;149:479–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Ma H, Lin H, Hu Y, Li X, He W, Jin X, et al. Serum 25-hydroxyvitamin D levels are associated with carotid atherosclerosis in normotensive and euglycemic Chinese postmenopausal women: the Shanghai Changfeng study. BMC Car-diovasc Disord 2014;14:197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Lee JE, Li H, Chan AT, Hollis BW, Lee I-M, Stampfer MJ, et al. Circulating levels of vitamin D and colon and rectal cancer: the physicians’ health study and a meta-analysis of prospective studies. Cancer Prev Res 2011;4:735–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Gandini S, Boniol M, Haukka J, Byrnes G, Cox B, Sneyd MJ, et al. Meta-analysis of observational studies of serum 25-hydroxyvitamin D levels and colorectal, breast and prostate cancer and colorectal adenoma. Int J Canc 2011;128: 1414–24. [DOI] [PubMed] [Google Scholar]
- [11].Touvier M, Chan DSM, Lau R, Aune D, Vieira R, Greenwood DC, et al. Meta-analyses of vitamin D intake, 25-hydroxyvitamin D status, vitamin D receptor polymorphisms, and colorectal cancer risk. Cancer Epidemiol Biomark Prev 2011;20:1003–16. [DOI] [PubMed] [Google Scholar]
- [12].Yin L, Grandi N, Raum E, Haug U, Arndt V, Brenner H. Meta-analysis: longitudinal studies of serum vitamin D and colorectal cancer risk. Aliment Pharmacol Ther 2009;30:113–25. [DOI] [PubMed] [Google Scholar]
- [13].Yin L, Grandi N, Raum E, Haug U, Arndt V, Brenner H. Meta-analysis: serum vitamin D and breast cancer risk. Eur J Cancer 2010;46:2196–205. Oxford, England : 1990. [DOI] [PubMed] [Google Scholar]
- [14].Yin L, Raum E, Haug U, Arndt V, Brenner H. Meta-analysis of longitudinal studies: serum vitamin D and prostate cancer risk. Cancer epidemio 2009;33: 435–45. [DOI] [PubMed] [Google Scholar]
- [15].Chen P, Hu P, Xie D, Qin Y, Wang F, Wang H. Meta-analysis of vitamin D, calcium and the prevention of breast cancer. Breast Canc Res Treat 2010;121: 469–77. [DOI] [PubMed] [Google Scholar]
- [16].de Sousa Almeida-Filho B, De Luca Vespoli H, Pessoa EC, Machado M, Nahas-Neto J, Nahas EAP. Vitamin D deficiency is associated with poor breast cancer prognostic features in postmenopausal women. J Steroid Biochem Mol Biol 2017;174:284–9. [DOI] [PubMed] [Google Scholar]
- [17].Ordonez-Mena JM, Schottker B, Haug U, Muller H, Kohrle J, Schomburg L, et al. Serum 25-hydroxyvitamin d and cancer risk in older adults: results from a large German prospective cohort study. Cancer Epidemiol Biomark Prev : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology 2013;22:905–9;16. [DOI] [PubMed] [Google Scholar]
- [18].Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of Observational Studies in Epidemiology (MOOSE) group. Jama 2000;283:2008–12. [DOI] [PubMed] [Google Scholar]
- [19].Leermakers ET, Darweesh SK, Baena CP, Moreira EM, Melo van Lent D, Tielemans MJ, et al. The effects of lutein on cardiometabolic health across the life course: a systematic review and meta-analysis. Am J Clin Nutr 2016;103: 481–94. [DOI] [PubMed] [Google Scholar]
- [20].Beydoun MA, Chen X, Jha K, Beydoun HA, Zonderman AB, Canas JA. Carot-enoids, vitamin A, and their association with the metabolic syndrome: a systematic review and meta-analysis. Nutr Rev 2019;77(1):32–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. Introduction to meta-analysis. 2009. [Google Scholar]
- [22].Petitti DB. Statistical methods in meat-analysis In: Petitti DB, editor. Meta-analysis. Decision Analysis, and cost-effectiveness analysis. 2nd ed New York, NY: Oxford University Press; 2000. [Google Scholar]
- [23].Carter P, Gray LJ, Troughton J, Khunti K, Davies MJ. Fruit and vegetable intake and incidence of type 2 diabetes mellitus: systematic review and meta-analysis. BMJ 2010;341:c4229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Egger M, Smith GD, Altman DG. Systematic Reviews in health care: meta-analysis in context. 2nd ed London. UK: The BMJ Publishing Group; 2001. [Google Scholar]
- [25].Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50:1088–101. [PubMed] [Google Scholar]
- [27].Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. Br Med J 1997;315:629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].STATA. Statistics/data analysis: release 15.0. Texas: Stata Corporation; 2017. [Google Scholar]
- [29].Bertone-Johnson ER, Chen WY, Holick MF, Hollis BW, Colditz GA, Willett WC, et al. Plasma 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D and risk of breast cancer. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the. American Society of Preventive Oncology 2005;14:1991–7. [DOI] [PubMed] [Google Scholar]
- [30].Rejnmark L, Tietze A, Vestergaard P, Buhl L, Lehbrink M, Heickendorff L, et al. Reduced prediagnostic 25-hydroxyvitamin D levels in women with breast cancer: a nested case-control study. Cancer epidemiology, biomarkers & prevention : A publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology 2009;18: 2655–60. [DOI] [PubMed] [Google Scholar]
- [31].Engel P, Fagherazzi G, Boutten A, Dupre T, Mesrine S, Boutron-Ruault MC, et al. Serum 25(OH) vitamin D and risk ofbreast cancer: a nested case-control study from the French E3N cohort. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology 2010;19:2341–50. [DOI] [PubMed] [Google Scholar]
- [32].Peppone LJ, Rickles AS, Janelsins MC, Insalaco MR, Skinner KA. The association between breast cancer prognostic indicators and serum 25-OH vitamin D levels. Ann Surg Oncol 2012;19:2590–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Kim Y, Franke AA, Shvetsov YB, Wilkens LR, Cooney RV, Lurie G, et al. Plasma 25-hydroxyvitamin D3 is associated with decreased risk of postmenopausal breast cancer in whites: a nested case-control study in the multiethnic cohort study. BMC Canc 2014;14:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Yousef FM, Jacobs ET, Kang PT, Hakim IA, Going S, Yousef JM, et al. Vitamin D status and breast cancer in Saudi Arabian women: case-control study. Am J Clin Nutr 2013;98:105–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Wu Y, Sarkissyan M, Clayton S, Chlebowski R, Vadgama JV. Association of vitamin D3 level with breast cancer risk and prognosis in African-American and Hispanic women. Cancers 2017;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].O’Brien KM, Sandler DP, Taylor JA, Weinberg CR. Serum vitamin D and risk of breast cancer within five years. Environ Health Perspect 2017;125:077004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Knight JA, Lesosky M, Barnett H, Raboud JM, Vieth R Vitamin D and reduced risk of breast cancer: a population-based case-control study. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research. Am Soc Prev Oncol 2007;16:422–9. cosponsored by the. [DOI] [PubMed] [Google Scholar]
- [38].Abbas S, Chang-Claude J, Linseisen J. Plasma 25-hydroxyvitamin D and premenopausal breast cancer risk in a German case-control study. Int J Canc 2009;124:250–5. [DOI] [PubMed] [Google Scholar]
- [39].Bidgoli SA, Azarshab H. Role of vitamin D deficiency and lack of sun exposure in the incidence of premenopausal breast cancer: a case control study in Sabzevar, Iran. Asian Pac J Cancer Prev APJCP : Asian Pac J Cancer Prev APJCP 2014;15:3391–6. [DOI] [PubMed] [Google Scholar]
- [40].Chlebowski RT, Johnson KC, Kooperberg C, Pettinger M, Wactawski-Wende J, Rohan T, et al. Calcium plus vitamin D supplementation and the risk of breast cancer. J Natl Cancer Inst 2008;100:1581–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Freedman DM, Chang SC, Falk RT, Purdue MP, Huang WY, McCarty CA, et al. Serum levels of vitamin D metabolites and breast cancer risk in the prostate, lung, colorectal, and ovarian cancer screening trial. Cancer epidemiology, biomarkers & prevention : A publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology 2008;17:889–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].McCullough ML, Stevens VL, Patel R, Jacobs EJ, Bain EB, Horst RL, et al. Serum 25-hydroxyvitamin D concentrations and postmenopausal breast cancer risk: a nested case control study in the Cancer Prevention Study-II Nutrition Cohort. Breast Cancer Res 2009;11:R64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Agborsangaya CB, Surcel HM, Toriola AT, Pukkala E, Parkkila S, Tuohimaa P, et al. Serum 25-hydroxyvitamin D at pregnancy and risk ofbreast cancer in a prospective study. Eur J Cancer 2010;46:467–70. Oxford, England : 1990. [DOI] [PubMed] [Google Scholar]
- [44].Eliassen AH, Spiegelman D, Hollis BW, Horst RL, Willett WC, Hankinson SE. Plasma 25-hydroxyvitamin D and risk of breast cancer in the Nurses’ Health Study II. Breast cancer research. BCR 2011;13:R50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Scarmo S, Afanasyeva Y, Lenner P, Koenig KL, Horst RL, Clendenen TV, et al. Circulating levels of 25-hydroxyvitamin D and risk of breast cancer: a nested case-control study. Breast Cancer Res 2013;15:R15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Wang J, Eliassen AH, Spiegelman D, Willett WC, Hankinson SE. Plasma free 25-hydroxyvitamin D, vitamin D binding protein, and risk of breast cancer in the Nurses’ Health Study II. Cancer causes & control. CCC (Cancer Causes Control) 2014;25:819–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Dimitrakopoulou VI, Tsilidis KK, Haycock PC, Dimou NL, Al-Dabhani K, Martin RM, et al. Circulating vitamin D concentration and risk of seven cancers: mendelian randomisation study. BMJ 2017;359:j4761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Robien K, Cutler GJ, Lazovich D. Vitamin D intake and breast cancer risk in postmenopausal women: the Iowa Women’s Health Study. Cancer causes & control. CCC (Cancer Causes Control) 2007;18:775–82. [DOI] [PubMed] [Google Scholar]
- [49].McCullough ML, Rodriguez C, Diver WR, Feigelson HS, Stevens VL, Thun MJ, et al. Dairy, calcium, and vitamin D intake and postmenopausal breast cancer risk in the Cancer Prevention Study II Nutrition Cohort. Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology 2005;14:2898–904. [DOI] [PubMed] [Google Scholar]
- [50].Crowther M, Avenell A, MacLennan G, Mowatt G. A further use for the Harvest plot: a novel method for the presentation of data synthesis. Res Synth Methods 2011;2:79–83. [DOI] [PubMed] [Google Scholar]
- [51].Ogilvie D, Fayter D, Petticrew M, Sowden A, Thomas S, Whitehead M, et al. The harvest plot: a method for synthesising evidence about the differential effects of interventions. BMC Med Res Methodol 2008;8:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Imtiaz S, Siddiqui N, Raza SA, Loya A, Muhammad A. Vitamin D deficiency in newly diagnosed breast cancer patients. IndianJEndocrinol Metabol 2012;16: 409–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Hatse S, Lambrechts D, Verstuyf A, Smeets A, Brouwers B, Vandorpe T, et al. Vitamin D status at breast cancer diagnosis: correlation with tumor characteristics, disease outcome, and genetic determinants of vitamin D insufficiency. Carcinogenesis 2012;33:1319–26. [DOI] [PubMed] [Google Scholar]
- [54].Segersten U, Holm PK, Bjorklund P, Hessman O, Nordgren H, Binderup L, et al. 25-Hydroxyvitamin D3 1alpha-hydroxylase expression in breast cancer and use of non-1alpha-hydroxylated vitamin D analogue. Breast cancer research: BCR 2005;7:R980–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Friedrich M, Rafi L, Mitschele T, Tilgen W, Schmidt W, Reichrath J. Analysis of the vitamin D system in cervical carcinomas, breast cancer and ovarian cancer. Recent results in cancer research Fortschritte der Krebsforschung Progres dans les recherches sur le cancer 2003;164:239–46. [DOI] [PubMed] [Google Scholar]
- [56].Garland CF, Garland FC, Gorham ED, Lipkin M, Newmark H, Mohr SB, et al. The role of vitamin D in cancer prevention. Am J Public Health 2006;96:252–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Neuhouser ML, Sorensen B, Hollis BW, Ambs A, Ulrich CM, McTiernan A, et al. Vitamin D insufficiency in a multiethnic cohort of breast cancer survivors. Am J Clin Nutr 2008;88:133–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Grant WB. Effect of interval between serum draw and follow-up period on relative risk of cancer incidence with respect to 25-hydroxyvitamin D level: implications for meta-analyses and setting vitamin D guidelines. Derma- toendocrinol 2011;3:199–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Grant WB. Effect of follow-up time on the relation between prediagnostic serum 25-hydroxyvitamin D and all-cause mortality rate. Dermatoendocrinol 2012;4:198–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Grant WB. 25-hydroxyvitamin D and breast cancer, colorectal cancer, and colorectal adenomas: case-control versus nested case-control studies. Anti-cancer Res 2015;35:1153–60. [PubMed] [Google Scholar]
- [61].Manson JE, Cook NR, Lee IM, Christen W, Bassuk SS, Mora S, et al. Vitamin D supplements and prevention of cancer and cardiovascular disease. N Engl J Med 2018;380(1):33–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Crowe FL, Steur M, Allen NE, Appleby PN, Travis RC, Key TJ. Plasma concentrations of 25-hydroxyvitamin D in meat eaters, fish eaters, vegetarians and vegans: results from the EPIC-Oxford study. Publ Health Nutr 2011;14:340–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


