Abstract
Background
The purpose of this systematic review was to evaluate the accuracy and reliability of wearable devices for objective gait measurement of Lumbar Spinal Stenosis (LSS) patients, with a focus on relevant gait metrics.
Methods
Systematic searches were conducted of five electronic databases to identify studies that assessed gait metrics by wearable or portable technology. Data was collected according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) statement guidelines.
Results
Four articles were identified for inclusion in this review. The objectives, methodology and quality of the studies varied. No single gait metric was investigated in all four studies, making comparison difficult. The most relevant metrics reported included gait cycle, gait velocity, step length and cadence, which were reported in two studies. Two studies explored gait symmetry. Differences between LSS patients and normal healthy subjects are demonstrable using wearable technology.
Conclusions
The measurements of gait cycle, cadence, step length, gait velocity, and number of steps with wearable devices can be used in the gait measurement of LSS patients for initial assessment, and objective outcomes following interventions. However, data and analysis are limited, and further studies are necessary to comment on reliability.
Keywords: Lumbar spinal stenosis, Wearables, Gait assessment, Gait metrics
Background
Lumbar Spinal Stenosis (LSS) is one of the most common indications for spine surgery, and a significant cause of pain and disability in the community. LSS patients classically present with a forward stoop (i.e. trunk flexion) with ambulation, claudicant pain, and paraesthesiae in the buttock, thigh or radiating down into the foot [1]. Decreased walking tolerance and intermittent claudication tends to be relieved by leaning forward [1]. Walking posture often correlates with patients’ symptoms, with back extension exacerbating pain and forward flexion providing variable relief. However, definitive diagnosis is based on the combination of the patient’s history, clinical examination and imaging findings on CT and MRI (CT if not MRI compatible), which need to demonstrate stenosis of the central canal surrounding the cauda equina [2, 3].
Lumbar decompression surgery using standard or minimally invasive techniques [4, 5], with or without fusion, is currently regarded as an appropriate management strategy for LSS when conservative therapies fail [2]. However, it is important to note that although the addition of fusion to lumbar decompression surgery is not associated with a significantly greater improvement than decompression alone, the overall patient quality of life is improved as seen in several studies including the Spine Patient Outcomes Research Trial (SPORT) study [6–8]. While important outcomes such as walking and pain are generally considered favourable with decompression surgery, there are limited tools for assessing these. Current assessments are primarily subjective, such as the Oswestry Disability Index (ODI) and Visual Analogue Scale (VAS), which have limitations. These limitations include: bias of reporting by patient and researcher; no firm consensus on the timing of subjective assessment; absence of continuous assessment to reveal recovery dynamics following an intervention, and the highly subjective nature of patient psychology with individual self-assessment.
The association between gait deterioration and LSS has been long established and explored in the literature. Khodedadah and Eisenstein (1993) established a correlation between gait improvement, particularly gait velocity, in patients with low back pain after lumbar fusion surgery [9]. In recent years, gait analysis has been increasingly utilised for assessing patient outcomes for various other spinal pathologies [10]. Patients with LSS have larger gait variability pre-operatively compared to post-operatively, and when compared to healthy subjects [11, 12]. A range of gait parameters including cadence, gait cycle, step length and step counts have all been proposed to correlate with spinal pathologies [13, 14]. However, traditional gait analysis in a formal laboratory setting is time consuming, equipment and labour-intensive, utilising video analysis, optical motion tracking and analysis, multiple sensors or gyroscopes, and electromyography [3, 15, 16].
Recently, the development and increased availability of wearable technologies for gait analysis has provided a faster and simplified alternative to laboratory-based gait analysis [17–19]. Wearable technologies incorporate aspects of traditional gait analysis techniques such as motion sensors, gyroscopes and accelerometers into everyday wearables such as watches and single piece devices easily attached to the clothing or body of a patient [19]. The incorporation and increased adaptation of these sensors have also decreased the cost for these wearable devices, thereby increasing affordability and facilitating their use in research.
The recent surge of different medical wearables from a range of companies and research facilities, and the reliability, sensitivity and specificity of these different devices poses a potential challenge for accurate medical use and reporting. While still emerging in research, several studies have been carried out to evaluate the usefulness of these wearables for different gait related pathologies. Therefore, the aim of this study is to systematically review the available studies [20] in order to evaluate the accuracy and reliability of different gait parameters (gait cycle, cadence, step length, velocity, step counts and other metrics) for patients with LSS.
Methods
Literature analysis
The PRISMA statement guidelines were followed in identifying, screening and selecting studies for inclusion, and extracting data.
Eligibility criteria
The focus of this review was on journal articles, conference proceedings published in English since the year 2001 that described the use of wearable devices to assess gait quality for patients suffering from lumbar spinal stenosis and neurogenic claudication. The parameters that were analysed in this study include gait cycle, gait velocity, step length, cadence, step count and gait symmetry. Book chapters and review papers were excluded.
Search strategy and study selection
Relevant studies were initially identified through a systematic search for published papers in the following scientific databases: PubMed (via NLM® database), Medline (via OvidSP), CINAHL (via Ebsco), SportDiscus (via Ebsco) and Google Scholar. The search terms used were “Wearable”, “Gait”, and “Lumbar Spinal Stenosis” (see Table 1).
Table 1.
Search Strategy
| Database | Search strategy | No. of records found | No. of unique abstracts selected | No. of unique articles selected | Articles |
|---|---|---|---|---|---|
| PubMed | “gait” AND “wearable” | 680 | 17 | 0 | – |
| “gait” AND “spine” AND stenosis | 178 | 4 | 3 |
Lee 2017 [22] Loske 2018 [23] |
|
| Medline | “gait” AND “wearable” AND spine | 2 | 1 | 0 | – |
| “gait” AND “wearable” | 474 | 15 | 0 | – | |
| “gait” AND “lumbar stenosis” | 10 | 1 | 0 | – | |
| CINAHL | “gait” AND “wearable” | 209 | 0 | 0 | – |
| “gait” AND “stenosis” | 102 | 1 | 0 | – | |
| SPORTDiscus | “gait” AND “wearable” | 170 | 3 | 0 | – |
| “gait” AND “stenosis” | 17 | 2 | 0 | – | |
| Google Scholar | “gait” AND “wearable” AND “lumbar spinal stenosis” | 70 | 2 | 0 | – |
| Other (e.g. hand search of citations) | 155 | 6 | 1 | Nagai 2014 [13] | |
| Totals | 2067 | 52 | 4 |
The search of the databases was completed on 1 September 2018 by two authors (AC and RM). Titles and abstracts of all studies identified were screened for relevance. The full text of the record was reviewed if the study appeared relevance or was of uncertain relevance. Studies which were clearly not relevant based on the title and abstract screen were excluded from the review. The full text of all selected relevant records was reviewed, and eligibility was determined using the eligibility criteria defined above.
The quality of each included record was assessed by two authors (AC and RM), and relevant information was extracted. Furthermore, the references of all included studies were hand-searched for additional publications that could be included in this review. At all stages of the study selection process, decisions regarding inclusion or exclusion were made by two authors (AC and RJM). The search was revalidated by a third author (WJC).
Data extraction
Data was collected with respect to participant characteristics, study design, the sensor type uses and outcomes for each study included in our review. Participant information included number and type of participants, age, and sex. Information about the type, model, sampling frequency and location on the body of each wearable sensor was recorded. The pathology of interest, environment of the study and specific gait parameters were also recorded included walking speed, distance and time. Walking speed was labelled as “self-selected” if participants were walking at a comfortable speed for them individually, but required to maintain the same speed. If participants were allowed to walk at their own speed, it was labelled as “not controlled”.
Quality assessment
The quality of each of the included articles was assessed using the Standard Quality Assessment Criteria for Evaluating Primary Research Papers from a Variety of Fields (QualSyst tool) [24]. Each article was evaluated by two authors (AC/ RJM) on 13 questions that considered the reporting, external validity, internal validity and power of the study. Each question had three possible answers: “Yes”, “No”, or “N/A.” A Quality Assessment score of less than 0.5 is considered poor quality, and over 0.7 good-excellent [25]. Any discrepancies in scoring between authors were discussed until an agreement was reached.
Devices
Each study used different wearable devices to measure selected gait metrics. These included:
-
i.
(Nagai et al) Triaxial accelerometers placed on cervical and lumbar spine (WAA-066, ATR Promotions Co., Japan)
-
ii.
(Lee et al) Sensorised smart-shoes (UCLA Wireless Health Institute) using pressure sensors (FSR400, Interlink Electronics, USA)
-
iii.
(Sun et al) Intelligent Device for Energy Expenditure and Activity 3 (MiniSun, LLC, Fresno, CA, USA), accelerometer and gyroscope
-
iv.
(Loske et al) RehaGait System (Hasomed GmbH, Magdeburg, Germany) with accelerometer, gyroscope and magnetometer
Results
Search results
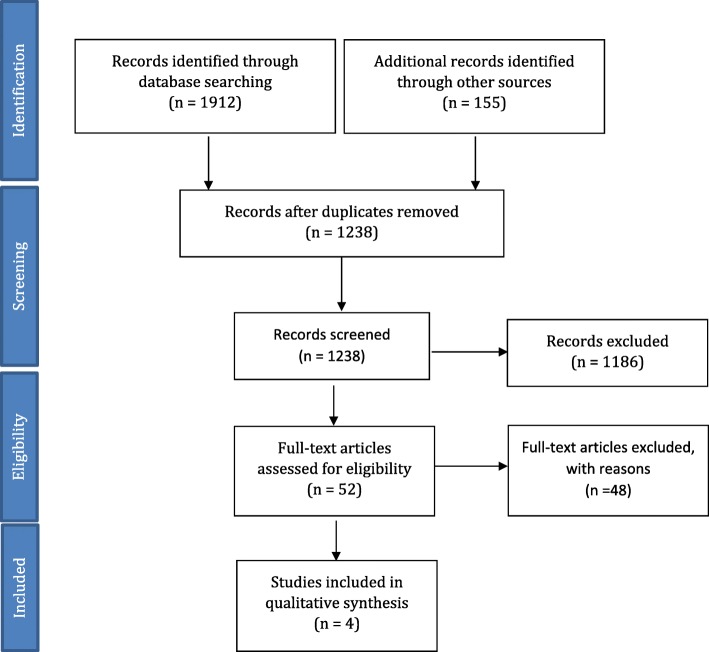
A total of 1912 articles were identified through the database search, and 155 additional articles were identified through a hand search of reference lists. Identifying duplicates, screening of the titles and abstracts led to the removal of 2015 articles, leaving 52 for full-text analysis (Fig. 1). A total of 4 articles met the inclusion criteria (see Table 2) [13, 21–23].
Fig. 1.
Flow chart outlining the process in this systematic review. Based upon the PRISMA preferred reporting for Systematic reviews [26]
Table 2.
Summary of articles included for review
| Authors | Participant characteristics | Sensor type | Location of sensor | Gait metrics measures | Sampling frequency | Environment | Gait speed | Gait distance/time | Quality assessment score |
|---|---|---|---|---|---|---|---|---|---|
| Nagai et al., 2014 [13] |
N = 11 (3 female, 8 male) Mean age = 72.8 yrs |
Wearable device with triaxial accelerometer (WAA-066, ATR Promotions Co., Japan) | Lumbar spine and cervical spine; 2 sensors) |
Postural sway Walking capacity Gait cycle |
1 occasion | Indoor horizontal walkway | Self-selected | 50 m repetitions until tired (maximum 548 m) | 0.54 |
| Lee et al., 2017 [22] |
N = 15 (11 female, 4 male) Mean age = 58.5 yrs |
Smart-shoes (UCLA Wireless Health Institute) with pressure sensors (FSR400, Interlink Electronics, USA) | Insole of shoe (heel, lateral plantar, toe); 5 sensors |
Plantar pressure distribution Gait symmetry |
1 occasion (preoperative) | Indoor hospital ward | Self-selected | 40 m | 0.46 |
| Sun et al., 2018 [3, 21] |
N = 20 (sex not specified) Mean age = 58 yrs |
Wearable device with accelerometers, gyroscopes (Intelligent Device for Energy Expenditure and Activity 3; MiniSun, LLC, Fresno, CA, USA) | Chest, thigh ankles and plantar surface of foot; 7 sensors |
Gait cycle Cadence Step length Gait velocity |
1 occasion | Indoor, well-lit environment | Self-selected | 16 m | 0.71 |
| Loske et al., 2018 [23] |
N = 20 (sex not specified) |
Inertial sensors with accelerometer, gyroscope and magnetometer (RehaGait System, Hasomed GmbH, Magdeburg, Germany) | Lateral shoe, lower and upper legs, pelvis; 7 sensors |
Gait cycle Gait symmetry Step length Gait velocity Cadence |
3 occasions (preoperatively, 10 weeks postop, 12 months postop) | Indoors (clinic) | Self selected | 6 min | 0.67 |
Quality assessment
The results of the quality assessment are outlined in Table 3.
Table 3.
Quality assessment scores of included studies
| Study | Question/objective | Study design | Subject and/or comparison group selection | Subject and/or comparison group characteristics | Random allocation | Blinding of investigators | Blinding of participants | Outcome and exposure measures | Sample size | Analytic measures | Estimate of variance | Confounding | Results | Conclusion | Summary score |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nagai et al. 2014 [13] | Yes | Partial | No | Yes | N/A | N/A | N/A | Partial | Partial | Partial | Yes | No | Partial | Yes | 0.54 |
| Lee et al. 2017 [22] | Partial | Partial | Partial | Yes | N/A | N/A | N/A | Partial | Partial | Partial | Yes | No | Partial | Partial | 0.46 |
| Sun et al. 2018 [3, 21] | Yes | Yes | Partial | Yes | N/A | No | N/A | Yes | Partial | Partial | Yes | No | Yes | Yes | 0.71 |
| Loske et al. 2018 [23] | Yes | Yes | Partial | Yes | N/A | No | N/A | Partial | Partial | Partial | Yes | No | Yes | Yes | 0.67 |
Gait metrics measured
Gait metrics measured across the studies included gait cycle time, gait symmetry, gait velocity, cadence, step length, number of steps, postural symmetry and plantar pressure distribution [21–23]. No single gait metric was measured in any study.
Gait cycle, gait velocity, step length, and cadence were measured in two of the four studies [21, 23]. Gait cycle was defined as the time between initial contact of one foot with the ground and contact of the same foot, denoting a single stride (synonymous with stride duration). Nagai et al. reported stride frequency in Hertz (i.e. steps per second), which is related to gait cycle [13]. Gait velocity was defined as distance travelled per unit of time in metres per second. Step length was defined as distance between the point of contact of one heel with the point of contact of the alternate heel. Cadence refers to the rate at which a person walks, and was defined as the number of steps per minute. Lee et al. and Loske et al. measured gait symmetry, which was defined as identical gait parameters in each leg, and measured according to the formula symmetry = (right leg stride time – left leg stride time)/(0.5 x [right leg stride time + left leg stride time] [22, 23]. Step count, or number of steps, was measured in one study by Sun et al., and was defined as the number of steps taken over 40 m.
The primary metric measured by Lee et al. was pressure distribution on the plantar surface of the foot during gait, describing a complex set of measurements developed by the authors based on multiple plantar pressure sensors. Lack of correlation to other more well-established gait metrics described above makes the significance of this metric uncertain.
Nagai et al. measured postural sway during walking using cervical and lumbar accelerometer sensors [13]. The focus of their study was walking capacity, recording walking difficulty at various distances and whether this was correlated with increased postural sway. However, they did not correlate this with gait symmetry or gait variability or compare these results to age-matched healthy participants. Therefore, whether postural sway per se is an important gait metric cannot be determined.
Principal gait metrics
Gait cycle, gait velocity, step length and cadence are the most important metrics for patients with lumbar spinal stenosis based on current research. Despite the limited evidence available, these metrics appear to be amenable to wearable/portable gait metric analysis. However, variation in study objectives and methodology make conclusions regarding statistical significance difficult to draw.
-
i.
Gait Cycle
Gait cycle was measured in two studies, by Sun et al. and Loske et al.. Sun et al. found a gait cycle of 1.082 ± 0.637 s in healthy volunteers, which was shorter compared to 1.111 ± 0.109 s in patients with LSS. However, a small sample size of only 12 healthy subjects and 20 LSS patients was used, and the authors did not provide statistical comparison between these groups. Loske et al. compared gait cycle preoperatively in 29 LSS patients to 27 healthy subjects, reporting 1.15 ± 0.17 s in LSS patients compared to 1.06 ± 0.08 s in healthy subjects. Loske et al. also compared preoperative gait cycle time to 10 weeks post lumbar decompression, noting a decrease in mean gait cycle to 1.09 ± 0.16 s. Statistical significance was not provided, however both studies indicate that increased gait cycle time in LSS patients can be demonstrated using wearable technology. Nagai et al. reported stride frequency in Hertz (Hz), comparing LSS patients at the beginning of the walking exercise to the end of the walking exercise (1 Hz vs 0.9 Hz). They did not find a statistical difference, and did not compare their values to healthy controls.
-
ii.
Gait Velocity
Both Sun et al. and Loske et al. noted decreased gait velocity in LSS patients pre-operatively using their respective wearable devices. Sun et al. report gait velocity of 1.023 ± 0.215 m/second in the LSS cohort preoperatively, compared to 1.158 ± 0.109 m/second in the healthy group, however do not provide further comparison postoperatively. Loske et al. show a similarly decreased gait velocity in LSS patients preoperatively of 1.09 ± 0.34 m/second, compared to 1.29 ± 0.27 m/second in healthy controls. They also reported an increase in gait velocity at 10 weeks postoperatively to 1.16 ± 0.28 m/second.
-
iii.
Step Length
Sun et al. reported a decreased step length in LSS patients compared to health controls (0.557 ± 0.085 m versus 0.625 ± 0.513 m). Loske et al. report decreased described stride length rather than step length (which consists of two step lengths, right and left combined), and reported decreased stride length LSS patients preoperatively (1.18 ± 0.26 m) compared to healthy controls (1.34 ± 0.26 m). They reported some improvement at 10 weeks postoperatively (1.21 ± 0.20 m), however this was not marked.
-
iv.
Cadence
The two studies that measured cadence found conflicting results with respected to cadence in LSS patients compared to healthy controls. Sun et al. report cadence of 107.626 ± 7.102 steps/min in healthy subjects, compared to 109.219 ± 10.499 steps/min in LSS patients. On the contrast, Loske et al. report cadence of 106.6 ± 14.7 steps/min in preoperative LSS patients compared to 113 ± 12.7 steps/min in healthy subjects, increasing to 111.6 ± 13.5 steps/min at 10 weeks postoperatively.
-
v.
Step count
Of the three studies, only Sun et al. measured step count. They reported mean step count of 94.000 ± 36.245 in LSS patients over a distance of 16 m, however did not provide data for healthy subjects. In terms of total daily step counts however, this data is still lacking.
-
vi.
Gait symmetry
Lee et al. and Loske et al. examined gait symmetry in LSS patients. Lee et al. stated that gait symmetry was recorded in all patients, however did not directly report the results of gait symmetry in their cohort. They stated that there was no correlation between gait symmetry and their outcome of interest, the ODI, but found statistically significant correlation when gait symmetry was combined with other measurements of pressure distribution. This data is difficult to interpret in isolation. Loske et al. found greater gait asymmetry in LSS patients in all phases of the gate cycle, which persisted at 10 weeks follow up but had normalised by 12 months.
Discussion
LSS is a disabling condition with significant economic, physical and psychological cost. It represents the most common indication for spine surgery in people older than 65 years. Most studies regarding LSS and outcomes of surgery are based on patient-reported information which may be subjective, inaccurate or incomplete [27, 28]. There is little objective data on functional changes following surgical intervention for LSS. The use of accelerometers to evaluate activity post spinal surgery is a promising avenue to provide objective measurements as compared to self-completed questionnaires or formal laboratory-based gait assessment [29]. Based on the limited data available from the 4 identified studies, we conclude that the measurements of gait velocity, cadence, step length, number of steps and gait symmetry are useful in the assessment of decline and recovery in patients with LSS. The small number of studies and variation in methodology used indicate that further studies investigating the capacity of wearable gait metric measurement to provide reliable results are necessary.
Normal gait speed of adults and LSS patients has been studied by Bohannon et al. and Conrad et al. respectively [30, 31]. Comfortable gait speed for adults aged 20–79 has been reported to be a mean of 1.33–1.46 m/s for men and 1.27–1.41 m/s for women [30]. LSS patients have been found to have generally slower gait speed, with Conrad reporting figures of 1.01 ± 0.33 m/s for men and 0.75 ± 0.24 m/s for women [31]. The two studies that measured gait velocity in the current review demonstrated decreased gait velocity in LSS patients. Step and stride length was also identified as a significant metric for the LSS patient, and have been shown to be reduced in LSS patients in the available studies with the time frame for postoperative improvement uncertain in the LSS patient. Our review also found that the two wearable systems used by Sun et al. and Loske et al. demonstrated increased gait cycle in LSS patients compared to normal controls.
In general, the mean step length and velocity in LSS patients is less than for normal adults, which is likely to reflect pain or neurological deficit in these patients. The shorter step length and slower velocity may also represent compensation to maintain stability and reduce the falls risk. Data comparing preoperative and postoperative gait metrics in LSS patients in the same study cohort would be clinically helpful for surgeons to provide objective outcome information that could be correlated against subjective symptomatic and functional outcomes. Only one study (Loske et al) compared preoperative and postoperative measurements in their gait analysis, which speaks to the need for more and better quality data to aid clinical practice.
There are limitations in this systematic review. The five primary gait parameters examined in this study are essential to clinically evaluate patients’ gait. Although more gait parameters, such as gait phase and joint angle may be analysed for improved assessment of patients’ gait, the authors believe that a simple (single point fixation) wearable provides adequate assessment of decline and recovery for the LSS patient. Further studies to assess the reliability of different wearable technologies for gait analysis are necessary. However preliminary evidence and pilot studies suggest that simple wearables are as effective and more convenient in providing standard gait metrics compared to traditional in-laboratory gait analysis.
Although the current available studies that uses gait parameters as an evaluation tool for LSS is sparse, our findings indicate the above parameters are suitable to be used to aid monitoring surgical and treatment outcomes. The recent incorporation and increased availability of wearable measurement devices into daily gadgets such as smartphones and smartwatches ease the process of data collection for these parameters even expanding to an outside-laboratory setting. With the advancement of such devices, a larger volume of data regarding patient activity can be collected and analysed, and may even be translated into future clinical assessment to assist in evaluating patients’ outcome.
Conclusion
Although the data on gait metrics from wearable devices for LSS patients is limited, the available evidence suggests that this group exhibits decreased step length and gait velocity compared to normal subjects. Helpful gait metrics are gait cycle, step length, velocity and number of steps, which have the potential to be easily measured using new wearable devices for preoperative and postoperative assessment of LSS patients.
Acknowledgements
Not applicable.
Abbreviations
- LSS
Lumbar Spinal Stenosis
- ODI
Oswestry Disability Index
- VAS
Visual Analogue Scale
Authors’ contributions
The first two authors (AC and RM) contributed equally to the search, systematic review and writing of this paper. WJC (last author) co-ordinated the systematic review as per the PRISMA guidelines and checked and re-ran all searches, as well as contributing to the Background section. DA contributed to fact-checking of the background and discussion section. KR contributed to editing the background and discussion section. KP contributed to the presentation and content of the results section. NY assisted in checking the reference data base for the review and editing of the entire paper. MM provided sections of the discussion section. The remaining authors contributed equally to editing and fact-checking of the manuscript. All authors read and approved the final manuscript. All listed co-authors have consented to publication.
Funding
Nil
Availability of data and materials
Not applicable, as no data was used. The EndNote reference library used in this study can be provided via email if required by the BMC journal (please contact last author WJC). The search strategies used in this systematic review have been included and are reproducible on public databases.
Ethics approval and consent to participate
Human Research Ethics Committee approval was not required for the submitted work at our institution.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Ananya Chakravorty, Email: ananya.chakravorty@gmail.com.
Ralph J. Mobbs, Phone: +61 2 9650 4766, Email: r.mobbs@unsw.edu.au
David B. Anderson, Email: dand3231@uni.sydney.edu.au
Kaitlin Rooke, Email: nurse@neurospineclinic.com.au.
Kevin Phan, Email: kpha5955@uni.sydney.edu.au.
Nicole Yoong, Email: n.yoong@student.unsw.edu.au.
Monish Maharaj, Email: monish.maharaj@gmail.com.
Wen Jie Choy, Email: timanchoy@gmail.com.
References
- 1.Genevay S, Atlas SJ. Lumbar spinal stenosis. Best Pract Res Clin Rheumatol. 2010;24(2):253–265. doi: 10.1016/j.berh.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahmed SI, Javed G, Bareeqa SB, et al. Comparison of decompression alone versus decompression with fusion for stenotic lumbar spine: a systematic review and meta-analysis. Cureus. 2018;10(8):e3135. doi: 10.7759/cureus.3135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Genevay Stephane, Atlas Steven J. Lumbar Spinal Stenosis. Best Practice & Research Clinical Rheumatology. 2010;24(2):253–265. doi: 10.1016/j.berh.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mobbs R, Phan KJJEST. Minimally Invasive Unilateral Laminectomy for Bilateral Decompression. JBJS Essent Surg Tech. 2017;7(1):e9. doi: 10.2106/JBJS.ST.16.00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mobbs RJ, Li J, Sivabalan P, Raley D, PJJJoNS R. Outcomes after decompressive laminectomy for lumbar spinal stenosis: comparison between minimally invasive unilateral laminectomy for bilateral decompression and open laminectomy. J Neurosurg Spine. 2014;21(2):179–186. doi: 10.3171/2014.4.SPINE13420. [DOI] [PubMed] [Google Scholar]
- 6.Försth P, Ólafsson G, Carlsson T, et al. A randomized, controlled trial of fusion surgery for lumbar spinal stenosis. N Engl J Med. 2016;374(15):1413–1423. doi: 10.1056/NEJMoa1513721. [DOI] [PubMed] [Google Scholar]
- 7.Ghogawala Z, Dziura J, Butler WE, et al. Laminectomy plus fusion versus laminectomy alone for lumbar spondylolisthesis. N Engl J Med. 2016;374(15):1424–1434. doi: 10.1056/NEJMoa1508788. [DOI] [PubMed] [Google Scholar]
- 8.Weinstein JN, Tosteson TD, Lurie JD, et al. Surgical vs nonoperative treatment for lumbar disk herniation: the Spine Patient Outcomes Research Trial (SPORT): a randomized trial. JAMA. 2006;296(20):2441–2450. doi: 10.1001/jama.296.20.2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khodadadeh S, Eisenstein SM. Gait analysis of patients with low back pain before and after surgery. Spine. 1993;18(11):1451–1455. doi: 10.1097/00007632-199318110-00008. [DOI] [PubMed] [Google Scholar]
- 10.Mobbs RJ, Katsinas CJ, Choy WJ, Rooke K, JoSS MMJ. Objective monitoring of activity and Gait Velocity using wearable accelerometer following lumbar microdiscectomy to detect recurrent disc herniation. J Spine Surg. 2018;4(4):792–797. doi: 10.21037/jss.2018.12.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Papadakis NC, Christakis DG, Tzagarakis GN, et al. Gait variability measurements in lumbar spinal stenosis patients: part a. comparison with healthy subjects. Physiol Meas. 2009;30(11):1171. doi: 10.1088/0967-3334/30/11/003. [DOI] [PubMed] [Google Scholar]
- 12.Papadakis NC, Christakis DG, Tzagarakis GN, et al. Gait variability measurements in lumbar spinal stenosis patients: part B. preoperative versus postoperative gait variability. Physiol Meas. 2009;30(11):1187. doi: 10.1088/0967-3334/30/11/004. [DOI] [PubMed] [Google Scholar]
- 13.Nagai K, Aoyama T, Yamada M, et al. Quantification of Changes in Gait Characteristics Associated With Intermittent Claudication in Patients With Lumbar Spinal Stenosis. J Spinal Disord Tech. 2014;27(4):E136–E142. doi: 10.1097/BSD.0b013e3182a2656b. [DOI] [PubMed] [Google Scholar]
- 14.Whittle MW. Clinical gait analysis: a review. Hum Mov Sci. 1996;15(3):369–387. doi: 10.1016/0167-9457(96)00006-1. [DOI] [Google Scholar]
- 15.Hanlon M, Anderson R. Real-time gait event detection using wearable sensors. Gait Posture. 2009;30(4):523–527. doi: 10.1016/j.gaitpost.2009.07.128. [DOI] [PubMed] [Google Scholar]
- 16.Maffiuletti NA, Gorelick M, Kramers-de Quervain I, et al. Concurrent validity and intrasession reliability of the IDEEA accelerometry system for the quantification of spatiotemporal gait parameters. Gait Posture. 2008;27(1):160–163. doi: 10.1016/j.gaitpost.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 17.Gonzalez RC, Lopez AM, Rodriguez-Uria J, Alvarez D, Alvarez JC. Real-time gait event detection for normal subjects from lower trunk accelerations. Gait Posture. 2010;31(3):322–325. doi: 10.1016/j.gaitpost.2009.11.014. [DOI] [PubMed] [Google Scholar]
- 18.Kim SC, Kim JY, Lee HN, et al. A quantitative analysis of gait patterns in vestibular neuritis patients using gyroscope sensor and a continuous walking protocol. J Neuroeng Rehabil. 2014;11:58. doi: 10.1186/1743-0003-11-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mobbs RJ, Phan K, Maharaj M, Rao PJ. Physical activity measured with accelerometer and self-rated disability in lumbar spine surgery: a prospective study. Global Spine J. 2015;6(5):459–464. doi: 10.1055/s-0035-1565259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Phan K, RJJJoss M. Systematic reviews and meta-analyses in spine surgery, neurosurgery and orthopedics: guidelines for the surgeon scientist. J Spine Surg. 2015;1(1):19. doi: 10.3978/j.issn.2414-469X.2015.06.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sun J, Liu YC, Yan SH, et al. Clinical gait evaluation of patients with lumbar spine stenosis. Orthop Surg. 2018;10(1):32–39. doi: 10.1111/os.12367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee SI, Campion A, Huang A, et al. Identifying predictors for postoperative clinical outcome in lumbar spinal stenosis patients using smart-shoe technology. J Neuroeng Rehabil. 2017;14(1):77. doi: 10.1186/s12984-017-0288-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Loske S, Nuesch C, Byrnes KS, et al. Decompression surgery improves gait quality in patients with symptomatic lumbar spinal stenosis. Spine J : official journal of the North American Spine Society. 2018;18(12):2195–2204. doi: 10.1016/j.spinee.2018.04.016. [DOI] [PubMed] [Google Scholar]
- 24.Kmet LM, Cook LS, Lee RC. Standard quality assessment criteria for evaluating primary research papers from a variety of fields. 2004. [Google Scholar]
- 25.Downs SH, NJJoE B, Health C. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health. 1998;52(6):377–384. doi: 10.1136/jech.52.6.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moher D., Liberati A., Tetzlaff J., Altman D. G. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339(jul21 1):b2535–b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carragee EJ, Cheng IJTSJ. Minimum acceptable outcomes after lumbar spinal fusion. Spine J. 2010;10(4):313–320. doi: 10.1016/j.spinee.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 28.Carragee EJJTSJ. The rise and fall of the “minimum clinically important difference”. Spine J. 2010;10(4):283–284. doi: 10.1016/j.spinee.2010.02.013. [DOI] [PubMed] [Google Scholar]
- 29.Rao PJ, Phan K, Maharaj MM, Pelletier MH, Walsh WR, RJJJoCN M. Accelerometers for objective evaluation of physical activity following spine surgery. J Clin Neurosci. 2016;26:14–18. doi: 10.1016/j.jocn.2015.05.064. [DOI] [PubMed] [Google Scholar]
- 30.Bohannon RW. Comfortable and maximum walking speed of adults aged 20—79 years: reference values and determinants. Age Ageing. 1997;26(1):15–19. doi: 10.1093/ageing/26.1.15. [DOI] [PubMed] [Google Scholar]
- 31.Conrad BP, Shokat MS, Abbasi AZ, et al. Associations of self-report measures with gait, range of motion and proprioception in patients with lumbar spinal stenosis. Gait Posture. 2013;38(4):987–992. doi: 10.1016/j.gaitpost.2013.05.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable, as no data was used. The EndNote reference library used in this study can be provided via email if required by the BMC journal (please contact last author WJC). The search strategies used in this systematic review have been included and are reproducible on public databases.