Abstract
Rapid diagnosis of active Mycobacterium tuberculosis (Mtb) infection remains a clinical and laboratory challenge. We have analyzed the cytokine profile (interferon-γ (IFN-γ), tumor necrosis factor-α (TNF-α) and interleukin-2 (IL-2)) of Mtb-specific T cells by polychromatic flow cytometry. We studied Mtb-specific CD4+ T cell responses in subjects with latent Mtb infection and active tuberculosis disease. The results showed substantial increase in the proportion of single-positive TNF-α Mtb-specific CD4+ T cells in subjects with active disease, and this parameter was the strongest predictor of diagnosis of active disease versus latent infection. We validated the use of this parameter in a cohort of 101 subjects with tuberculosis diagnosis unknown to the investigator. The sensitivity and specificity of the flow cytometry–based assay were 67% and 92%, respectively, the positive predictive value was 80% and the negative predictive value was 92.4%. Therefore, the proportion of single-positive TNF-α Mtb-specific CD4+ T cells is a new tool for the rapid diagnosis of active tuberculosis disease.
Cellular immunity, particularly of CD4+ T cells, IFN-γ and TNF-α, has a central role in the control of and protection against Mycobacterium tuberculosis (Mtb) infection1,2. Diagnosis of Mtb infection remains complex and requires several clinical, radiological, histopathological, bacteriological and molecular parameters. IFN-γ release assays measure responses to antigens (for example, 6-kDa early secretory antigenic target (ESAT-6) or 10-kDa culture filtrate antigen (CFP-10)) expressed by Mtb itself and discriminate between infection by Mtb and immunity induced by vaccination with Bacille Calmette-Guérin (BCG)3,4 but not between active disease and latent infection5,6.
Studies in the field of antiviral immunity have shown that polyfunctional (IFN-γ+IL-2+TNF-α+) profiles of virus-specific T cell responses, and not IFN-γ production alone, correlated with disease activity7–10.
Therefore, we have used the same strategy, polychromatic flow cytometry, to functionally characterize Mtb-specific T cells in subjects with latent Mtb infection or active tuberculosis disease and tested the hypothesis that different cytokine profiles of pathogen-specific T cells may discriminate between active tuberculosis disease and latent Mtb infection.
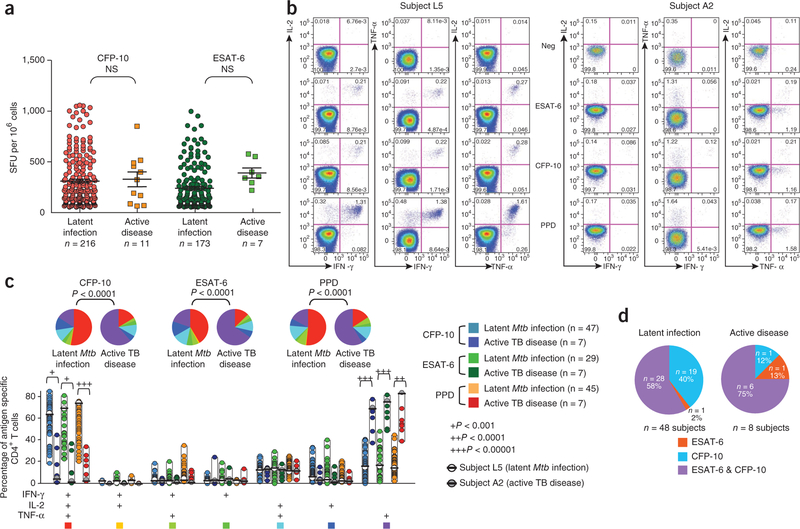
We enrolled an initial cohort of 283 individuals with known diagnosis of Mtb infection in Switzerland and termed it the ‘test cohort’ (Supplementary Fig. 1). Subjects were selected on the basis of positive IFN-γ ELISPOT responses against CFP-10, ESAT-6 or both. Among the 283 subjects, active tuberculosis disease was diagnosed in 11 subjects on the basis of clinical signs (for example, cough, weight loss and lymphadenopathy), sputum stain for acid-fast bacilli (AFB), culture and PCR for Mtb and chest radiography6 (the Online Methods and Supplementary Table 1 contain detailed clinical parameters). The remaining 272 participants were diagnosed with latent Mtb infection. We first assessed the magnitude of Mtb-specific T cell responses by IFN-γ ELISPOT after stimulation with pools of peptides encompassing CFP-10 or ESAT-6 proteins. In agreement with previous studies11,12, Mtb-specific T cell responses were similar in subjects with latent infection and active disease (Fig. 1a).
Figure 1.
Quantitative and qualitative analysis of Mtb-specific T cell responses in the test cohort. (a) IFN-γ ELISPOT responses after stimulation with ESAT-6 or CFP-10 peptide pools in a cohort of 283 participants with latent Mtb infection (n = 272) or active tuberculosis disease (n = 11, Supplementary Fig. 1). Shown are the numbers of spot-forming units (SFU) per 106 mononuclear cells. Statistical significance (P values) of the results was calculated by unpaired two-tailed Student’s t test using GraphPad Prism 5. Bonferroni’s correction for multiples analyses was applied. (b) Qualitative analysis of Mtb-specific CD4+ T cell responses by polychromatic flow cytometry. Shown are representative flow cytometry analyses of the functional profile of Mtb-specific CD4+ T cell responses in participants with either latent Mtb infection (Subject L5, left) or active tuberculosis disease (Subject A2, right). Profiles are gated on live CD3+CD4+ T cells, and the various combinations of IFN-γ, IL-2 and TNF-α are shown following stimulation with ESAT-6 and CFP-10 peptide pools or PPD. NS, not significant; Neg, negative control (unstimulated). (c) Simultaneous analysis of the functional profile of Mtb-specific CD4+ T cells on the basis of IFN-γ, IL-2 or TNF-α production. ESAT-6–, CFP-10– and PPD-specific CD4+ T cell responses are shown (as indicated by the six colored boxes to the right of the panel) from 48 participants with latent Mtb infection and eight participants with active tuberculosis (TB) disease. Representative examples from subject L5 and subject A2 are also identified. All the possible combinations of the various functions are shown on the x axis, whereas the percentages of the distinct cytokine-producing cell subsets within Mtb-specific CD4+ T cells are shown on the y axis. The pie charts summarize the data, and each slice corresponds to the proportion of Mtb-specific CD4+ T cells positive for a certain combination of functions. Colors in the pie charts are indicated by the seven colored boxes at the bottom of the panel. (d) Distribution of CFP-10– and/or ESAT-6–specific CD4+ T cell responses among subjects with latent Mtb infection or active tuberculosis disease.
We then assessed the functional profile of Mtb-specific T cell responses by polychromatic flow cytometry and a panel of markers including a viability marker and antibodies specific for CD3, CD4, CD8, IL-2, TNF-α and IFN-γ. Owing to blood specimen availability or quality (see flowchart in Supplementary Fig. 1), this analysis was performed in 48 subjects with latent infection and eight subjects with active disease (Supplementary Table 1). Within the group with latent infection, five were investigated for suspected tuberculosis disease but had negative sputum AFB staining and culture and PCR for Mtb. Twenty-three were health-care workers screened for Mtb infection as part of routine surveillance at the Centre Hospitalier Universitaire Vaudois (CHUV; Supplementary Fig. 1). Twenty were investigated for Mtb infection before the initiation of anti-TNF-α antibody treatment and had negative chest radiographs (Supplementary Fig. 1). In agreement with previous studies11,12, Mtb-specific CD4+ T cell responses in a representative subject with latent Mtb infection (subject L5) were mostly (>70%) polyfunctional (Fig. 1b), that is, producing IFN-γ, IL-2 and TNF-α. In contrast, a representative subject with active tuberculosis disease (subject A2) (Fig. 1b) showed a dominant (>70% of CD4+ T cells) TNF-α–only response. In these two participants, the functional profile of Mtb-specific CD4+ T cells was similar regardless of the stimulus, that is, ESAT-6 or CFP-10 peptide pools or tuberculin purified protein derivative (PPD). Of note, Mtb-specific T cell responses (analyzed by either IFN-γ ELISPOT or flow cytometry) from the 20 subjects analyzed before the initiation of TNF-α–specific antibody treatment were not different from T cell responses in the remaining 28 subjects also diagnosed with latent infection (Supplementary Fig. 2). The marked difference between the functional profile of Mtb-specific CD4+ T cell responses in latent infection versus active disease was confirmed in a total of 142 Mtb-specific CD4+ T cell responses (all P < 0.0001) (Fig. 1c) from the 56 subjects (48 with latent infection and eight with active disease). Among the 56 subjects (active disease plus latent infection), most responded to both ESAT-6 and CFP-10 (Fig. 1d). However, two out of eight (25%) of the subjects with active disease and 19 out of 48 (40%) of subjects with latent Mtb infection responded to one peptide pool. Furthermore, most subjects (>90%) also responded to PPD. Of the 142 responses, 21 were detected in subjects with active disease and 121 in subjects with latent infection (Fig. 1c). Of note, we confirmed the differences in the profile of cytokines between active disease and latent infection when we expressed the data as absolute frequency of cytokine-producing CD4+ T cells (Supplementary Fig. 3). The frequency of single-positive TNF-α–producing CD4+ T cells was higher in individuals with active disease (Supplementary Fig. 3).
In summary, in an opportunistically selected (on the basis of a sufficient number of mononuclear cells; Supplementary Data 1) subgroup of individuals subjected to detailed intracellular cytokine staining (ICS) studies, a functional profile (single-positive TNF-α Mtb-specific CD4+ T cells) was associated with disease activity and so might be helpful for rapid diagnosis of active disease as compare to the conventional culture tuberculosis tests, which require up to several weeks.
We then calculated which parameter (that is, functional T cell subset) was the strongest predictive measure of discrimination between active disease and latent infection. For these purposes, because CFP-10 was more frequently recognized than ESAT-6 (Fig. 1d), we focused the analysis on CFP-10–specific CD4+ T cell responses and included ESAT-6–specific CD4+ T cell responses only when CFP-10 responses were negative. We observed the latter scenario in only one individual with active disease and one individual with latent infection (Fig. 1d).
On the basis of the logistic regression analysis of the multiple functionally distinct T cell subsets, the proportion of TNF-α single-positive Mtb-specific CD4+ T cells was the strongest predictive measure of discrimination between active disease and latent infection (area under the curve (AUC) = 0.995 (95% confidence interval: 0.984–1); odds ratio = 1.45; Supplementary Fig. 4). In addition, a cutoff of 37.4% of single-positive TNF-α–producing CD4+ T cells was calculated as the value allowing the best (sensitivity of 100% and specificity of 96%) separation between latent infection and active disease (Supplementary Fig. 4).
A limitation of these results was that the laboratory investigators were not blinded to the diagnosis of latent infection or active disease. We therefore examined peripheral blood mononuclear cells from a second independent cohort termed the ‘validation cohort’, whose clinical status was unknown to the investigators. We tested whether the proportion of TNF-α single-positive Mtb-specific CD4+ T cells, and particularly the cutoff at 37.4%, could discriminate between latent infection and active disease.
One hundred and fourteen participants from both Switzerland (n = 72) and South Africa (n = 42) were enrolled between 2009 and 2010 to confirm the functional profile also in persons from a setting (South Africa) where tuberculosis is prevalent (Supplementary Fig. 5). Participants from South Africa were enrolled from clinics in the public health sector in Cape Town and Worcester, both in the Western Cape province of South Africa. Participants from Switzerland in the validation cohort were not included in the test cohort described above. Subjects were selected on the basis of the following criteria: positive Mtb-specific IFN-γ ELISPOT responses, absence of Mtb-specific treatment, seronegative for HIV and good general health status (the Online Methods and Supplementary Fig. 5 contain a full description of the subjects). Active tuberculosis disease diagnosis in subjects from both Switzerland and South Africa was based on clinical signs (for example, cough, weight loss and lymphadenopathy), sputum stain for AFB, culture and PCR for Mtb and chest radiography6 (the Online Methods and Supplementary Table 2 contain detailed clinical parameters). Flow cytometry analyses were performed on the 101 subjects from the validation cohort with positive Mtb-specific CD4+ T cell responses (Supplementary Fig. 5).
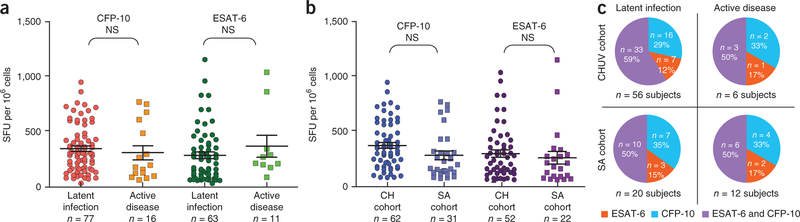
IFN-γ ELISPOT and CD4+ T cell specific cytokine expression in response to CFP-10, ESAT-6 or both were evaluated, and the data were provided to the biostatistics facility of CHUV. Later, unblinding of the Mtb clinical status allowed us to confirm that IFN-γ ELISPOT responses were not significantly different between latent infection and active disease (Fig. 2a). Of note, the magnitude of Mtb-specific IFN-γ ELISPOT responses (Fig. 2b) and the distribution of CFP-10– and/or ESAT-6–specific CD4+ T cell responses among subjects with latent Mtb infection or active disease were similar between subjects from Switzerland and South Africa (Fig. 2c).
Figure 2.
Analysis of Mtb-specific T cell responses in the validation cohort after unblinding of the clinical status. (a) IFN-γ ELISPOT responses after stimulation with ESAT-6 or CFP-10 peptide pools. Shown are the numbers of SFU per 106 mononuclear cells. Statistical significance (P values) of the results was calculated by unpaired two-tailed Student’s t test in GraphPad Prism 5. Bonferroni’s correction for multiples analyses was applied. (b) Analysis of Mtb-specific IFN-γ ELISPOT T cell responses in subjects enrolled in Switzerland (CH) and SA. (c) Distribution of CFP-10– and/or ESAT-6–specific CD4+ T cell responses among subjects from the validation cohort with positive and concordant Mtb-specific CD4+ T cell responses (Supplementary Fig. 5).
With regard to the polychromatic flow cytometric cytokine profile, 15 participants had a dominant TNF-α single-positive Mtb-specific CD4+ T cell response, that is, >37.4%, considered predictive of active disease in the test cohort (Supplementary Fig. 4). After unblinding, active disease was confirmed in 12 of these 15 participants (Fig. 3a). Along the same line, 79 participants had polyfunctional Mtb-specific CD4+ T cells, including a TNF-α single-positive proportion of <37.4%, which we considered predictive of latent infection in the test cohort (Supplementary Fig. 4). After unblinding, 73 out of these 79 participants had latent infection (Fig. 3a). The distribution of subjects from Switzerland and South Africa is also shown in Figure 3b. Notably, among the 94 aforementioned subjects (that is, 15 with a profile of active disease and 79 with a profile of latent infection), CFP-10– and ESAT-6–specific CD4+ T cell responses, when both positive, were concordant (that is, both either above or below the cut-off of 37.4% TNF-α single-positive cells). In these 94 concordant cases, the data of CFP-10–specific CD4+ T cell responses were considered for the analyses, and ESAT-6–specific CD4+ T cell responses were only included when CFP-10 responses were negative (Fig. 3a,b). Seven out of 101 (6.9%) participants showed discordant CD4+ T cell responses to ESAT-6 and CFP-10 peptide pools (one response >37.4% and the other response <37.4%) and were therefore excluded from the analysis (Supplementary Fig. 6). The performance of the test on the cohorts from Switzerland and South Africa was not significantly different (P > 0.05 for positive predictive value (PPV), negative predictive value (NPV), sensitivity and specificity), thus providing evidence that the combined analysis of Swiss and South African cohorts is valid. On the basis of the analysis on the combined cohorts, the global performance of the assay was as follows: PPV = 80%, NPV = 92.4%, sensitivity = 66.67% and specificity = 92.41% (Supplementary Fig. 7). Overall, the cytokine profile predicted the clinical diagnosis in 90% of cases. Of note, these values apply to subjects with detectable ICS responses. When subjects with discordant ESAT-6 and CFP-10 responses were also included in the analysis, the correct clinical diagnosis was made in 84% of subjects.
Figure 3.
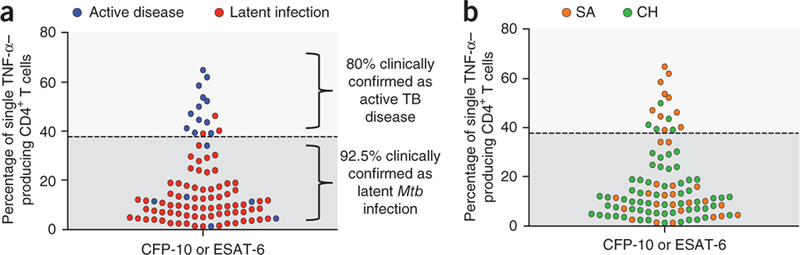
Percentages of CFP-10– or ESAT-6–specific single-positive TNF-α–producing CD4+ T cells of the 94 subjects from the validation cohort with concordant responses against CFP-10 and ESAT-6. Dashed line represents the cutoff of 37.4% of single-positive TNF-α. (a) Subjects with active disease or latent infection are identified with blue and red dots, respectively. (b) Subjects from South Africa (SA) or Switzerland (CH) are identified with orange and green dots, respectively.
We then investigated whether the percentage of Mtb-specific TNF-α–producing CD4+ T cells was the parameter with the strongest predictive value of the clinical status in the validation cohort. On the basis of the logistic regression analysis of the multiple functionally distinct T cell subsets, the proportion of TNF-α single-positive Mtb-specific CD4+ T cells was indeed the strongest predictive measure of discrimination between active disease and latent infection (AUC = 0.825 (95% confidence interval: 0.683–0.968); odds ratio = 1.10; Supplementary Fig. 7). In addition, a cutoff of 38.8% (as compared to 37.4% obtained in the test cohort) of single-positive TNF-α–producing CD4+ T cells was calculated as the value allowing the best separation between latent infection and active disease (Supplementary Fig. 7).
We also had the opportunity to analyze T cell cytokine profiles in five participants during untreated active tuberculosis disease and then after tuberculosis treatment (Fig. 4). In agreement with the above data, the percentage of single-positive TNF-α–producing CD4+ T cells was >37.4% in individuals with active tuberculosis disease. We observed a shift to a polyfunctional profile (single-positive TNF-α–producing CD4+ T cells < 37.4%) of Mtb-specific CD4+ T cell responses after therapy and transition to latent infection in all five participants (Fig. 4).
Figure 4.

Longitudinal analysis of the functional profile of Mtb-specific CD4+ T cells from five subjects analyzed during untreated active tuberculosis disease and then after tuberculosis treatment. Shown is the full functional profile on the basis of IFN-γ, IL-2 and TNF-α production of a total of 7 Mtb-specific CD4+ T cell responses. All the possible combinations of the different functions are shown on the x axis, whereas the percentages of the distinct cytokine-producing cell subsets within Mtb-specific CD4+ T cells are shown on the y axis. The pie charts summarize the data, and each slice corresponds to the proportion of Mtb-specific CD4+ T cells positive for a certain combination of functions.
The association between different functional profiles of T cell responses and disease activity is consistent with the current paradigm in antiviral immunity9,10, where virus-specific T cell responses endowed with only effector functions such as production of IFN-γ, TNF-α or both have been found in individuals with active virus replication and active disease. In contrast, polyfunctional responses, comprising cells producing IL-2 in addition to effector and inflammatory cytokines, have been found to be present in individuals with controlled virus replication and no signs of clinical disease7–10.
The fundamental role of TNF-α in the control of Mtb infection in both humans and mice is well established1,2,13, and this is also supported by the increased risk of Mtb reactivation in patients with rheumatoid arthritis receiving anti–TNF-α therapy14,15. However, the dominant TNF-α single-positive CD4+ T cell response observed during active tuberculosis disease may rather reflect the elevated degree of inflammation rather than of protection.
A recent study showed that a neutrophil-driven interferon-inducible gene profile correlated with active tuberculosis disease, and it was also found in about 10% of people with latent infection16. As about 10% of subjects with latent infection go on to develop active disease, it was suggested that this biomarker may be useful in both prognosis and diagnosis16. There was no evidence in that study of a T cell–driven TNF-α–inducible gene profile correlated with active tuberculosis disease. However, the transcriptional profile was assessed on total unstimulated blood cell populations. It was therefore not suitable for evaluating the transcriptional profile in T cell populations, an assay that needs to be performed on stimulated T cells16.
Our results indicate that the analysis of cytokine profiles in Mtb-specific CD4+ T cells by polychromatic flow cytometry is a major immunological measure discriminating between active and latent Mtb infection. Therefore, polychromatic flow cytometry may be a reliable laboratory tool for the timely diagnosis of active Mtb infection.
ONLINE METHODS
Study groups.
Participants (n = 283) from the test cohort were all recruited at CHUV. These samples were selected based on positive Mtb-specific IFN-γ ELISPOT responses routinely performed in the context of the diagnosis for Mtb infection at CHUV. Individuals with active tuberculosis disease had a diagnosis based on laboratory isolation of Mtb in mycobacterial culture from sputum, broncho alveolar lavage fluid or biopsies and/or tuberculin skin test and/or PCR (Supplementary Table 1 contains a full clinical description of each subject). The final diagnosis was given by a clinician after validation of these criteria associated with clinical symptoms. The selection of subjects tested by flow cytometry was based on the availability of cryopreserved material. In addition, samples with low (<70%) cell recovery and viability upon thawing were discarded from the analyses, in concordance with the current guidelines in the field of intracellular flow cytometric analyses17. Furthermore, none of these subjects was undergoing antimycobacterium treatment at the time of the present analyses (Supplementary Fig. 1). Participants from the validation cohort (n = 114) were obtained from two clinical sites (Supplementary Fig. 5); subjects from Switzerland were all recruited from the CHUV, subjects from South Africa were recruited from clinics in the public health sectors in Cape Town and Worcester and subjects with latent infection were recruited from the South African Tuberculosis Vaccine Initiative clinical trials field site in Worcester. Inclusion criteria included positive Mtb-specific IFN-γ ELISPOT responses, age between 18 and 80 years old, body weight ≥50 kg, hemoglobin ≥100 g per liter, leukocyte count ≥3,000 cells mm3 per liter, platelet count ≥75,000 cells mm3 per liter and negative for HIV-specific antibody on the basis of a routine rapid HIV test. Individuals with active tuberculosis had a diagnosis based on laboratory isolation of Mtb in mycobacterial culture from sputum, broncho alveolar lavage fluid or biopsies and/or tuberculin skin test and/or PCR (Supplementary Table 2 contains full clinical descriptions of each subject). The final diagnosis was given by a clinician after validation of these criteria associated with clinical symptoms such as cough or weight loss. Furthermore, none of these subjects was undergoing antimycobacterium treatment at the time of the present analyses. All participants gave written informed consent.The study was approved by the Institutional Review Board of the Centre Hospitalier Universitaire Vaudois, University of Lausanne.
Peptides.
Stimulations were performed with Mtb-derived peptide pools covering ESAT-6 and CFP-10. CFP-10 and ESAT-6 peptides pools are composed of 15-mers overlapping by 11 amino acids, and all peptides were HPLC purified (>80% purity). Tuberculin Purified Protein Derivative (PPD RT 23) was purchased from Statens Serum Institute.
IFN-γ ELISPOT assays.
ELISPOT assays were performed per the manufacturer’s instructions (Becton Dickinson). Briefly, cryopreserved blood mono-nuclear cells were rested for 8 h at 37 °C, and then 2 × 105 cells were stimulated with peptide pools (1 μg of each single peptide) in 100 μl of complete medium (RPMI + 10%FBS) in quadruplicate conditions as previously described18. Medium only was used as negative control. Staphylococcal enterotoxin B (SEB; Sigma; 200 ng ml−1) was used as a positive control on 50,000 cells. Results are expressed as the mean number of SFU per 106 cells from quadruplicate assays. Only cell samples with >80% viability after thawing were analyzed, and only assays with <50 SFU per 106 cells for the negative control and >500 SFU per 106 cells after SEB stimulation were considered as valid. An ELISPOT result was defined as positive if the number of SFUs was ≥55 SFU per 106 cells and more than fourfold higher than the negative control.
Flow cytometry analysis.
For ICS, cryopreserved blood mononuclear cells (1–2 × 106) were rested for 6–8 h and then stimulated overnight in 1 ml of complete medium containing Golgiplug (1 μl ml−1, Becton Dickinson) and CD28-specific antibodies (0.5 μg ml−1, Becton Dickinson) as previously described19. For stimulation of blood mononuclear cells, peptide pools were used at 1 μl ml−1 for each peptide. SEB stimulation (200 ng ml−1) served as positive control. At the end of the stimulation period, cells were stained for dead cells (LIVE/DEAD kit, Invitrogen), permeabilized (Cytofix/Cytoperm, Becton Dickinson) and then stained with antibodies specific for CD3, CD4, CD8, IFN-γ, TNF-α and IL-2. All antibodies but those specific for CD3 (Invitrogen) and CD4 and CD19 (VWR International) were purchased from Becton Dickinson. Cells were then fixed, acquired on an LSRII SORP (four lasers) and analyzed with FlowJo 8.8.2 and SPICE 4.2.3 (developed by M. Roederer, Vaccine Research Center, National Institute of Allergy and Infectious Diseases, US National Institutes of Health) as previously described18. The number of lymphocyte-gated events ranged between 105 and 106 in the flow cytometry experiments shown.
Statistical analyses.
Comparisons of categorical variables were made with Fisher’s exact test. Statistical significance (P values) of the magnitude of ELISPOT responses was calculated by unpaired two-tailed Student’s t test using GraphPad Prism 5. Bonferroni’s correction for multiples analyses was applied. The selection of the optimal parameters to discriminate between cases of latent infection and cases of active disease was performed using a logistic regression analysis followed by a receiver operating characteristic (ROC) curve analysis20–22 to evaluate the diagnostic performance of each parameter. Results for the optimal parameter (single-positive TNF-α) are summarized as a contingency table giving sensitivity, specificity and positive and negative predictive values (PPV and NPV). Analyses provided include a ROC-curve graph and a sensitivity and specificity graph as a function of the probability cutoff.
Supplementary Material
ACKNOWLEDGMENTS
This research was partially conducted as part of the Vaccine Immune Monitoring Consortium under the Collaboration for AIDS Vaccine Discovery with support from the Bill & Melinda Gates Foundation. Furthermore, we thank N. Rettby, D. Bonnet and K. Ellefsen Lavoie for logistic coordination. We also thank many additional members of the South African Tuberculosis Vaccine Initiative team who helped with enrollment and evaluation of participants and, finally, the participants themselves.
Footnotes
METHODS
Methods and any associated references are available in the online version of the paper at http://www.nature.com/naturemedicine/.
Note: Supplementary information is available on the Nature Medicine website.
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests.
References
- 1.Kaufmann SH How can immunology contribute to the control of tuberculosis? Nat. Rev. Immunol 1, 20–30 (2001). [DOI] [PubMed] [Google Scholar]
- 2.Flynn JL & Chan J Immunology of tuberculosis. Annu. Rev. Immunol 19, 93–129 (2001). [DOI] [PubMed] [Google Scholar]
- 3.Lalvani A et al. Enhanced contact tracing and spatial tracking of Mycobacterium tuberculosis infection by enumeration of antigen-specific T cells. Lancet 357, 2017–2021 (2001). [DOI] [PubMed] [Google Scholar]
- 4.Ewer K et al. Comparison of T cell-based assay with tuberculin skin test for diagnosis of Mycobacterium tuberculosis infection in a school tuberculosis outbreak. Lancet 361, 1168–1173 (2003). [DOI] [PubMed] [Google Scholar]
- 5.Meier T, Eulenbruch HP, Wrighton-Smith P, Enders G & Regnath T Sensitivity of a new commercial enzyme-linked immunospot assay (T SPOT-TB) for diagnosis of tuberculosis in clinical practice. Eur. J. Clin. Microbiol. Infect. Dis 24, 529–536 (2005). [DOI] [PubMed] [Google Scholar]
- 6.Jasmer RM, Nahid P & Hopewell PC Clinical practice. Latent tuberculosis infection. N. Engl. J. Med 347, 1860–1866 (2002). [DOI] [PubMed] [Google Scholar]
- 7.Betts MR et al. HIV nonprogressors preferentially maintain highly functional HIV-specific CD8+ T cells. Blood 107, 4781–4789 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harari A et al. Functional signatures of protective antiviral T cell immunity in human virus infections. Immunol. Rev 211, 236–254 (2006). [DOI] [PubMed] [Google Scholar]
- 9.Pantaleo G & Harari A Functional signatures in antiviral T cell immunity for monitoring virus-associated diseases. Nat. Rev. Immunol 6, 417–423 (2006). [DOI] [PubMed] [Google Scholar]
- 10.Pantaleo G & Koup RA Correlates of immune protection in HIV-1 infection: what we know, what we don’t know, what we should know. Nat. Med 10, 806–810 (2004). [DOI] [PubMed] [Google Scholar]
- 11.Day CL et al. Detection of polyfunctional Mycobacterium tuberculosis-specific T cells and association with viral load in HIV-1-infected persons. J. Infect. Dis 197, 990–999 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sutherland JS, Adetifa IM, Hill PC, Adegbola RA & Ota MO Pattern and diversity of cytokine production differentiates between Mycobacterium tuberculosis infection and disease. Eur. J. Immunol 39, 723–729 (2009). [DOI] [PubMed] [Google Scholar]
- 13.Flynn JL et al. Tumor necrosis factor-? is required in the protective immune response against Mycobacterium tuberculosis in mice. Immunity 2, 561–572 (1995). [DOI] [PubMed] [Google Scholar]
- 14.Feldmann M & Maini RN Anti-TNF ? therapy of rheumatoid arthritis: what have we learned? Annu. Rev. Immunol 19, 163–196 (2001). [DOI] [PubMed] [Google Scholar]
- 15.Maini R et al. Infliximab (chimeric anti-tumour necrosis factor ? monoclonal antibody) versus placebo in rheumatoid arthritis patients receiving concomitant methotrexate: a randomised phase III trial. ATTRACT Study Group. Lancet 354, 1932–1939 (1999). [DOI] [PubMed] [Google Scholar]
- 16.Berry MP et al. An interferon-inducible neutrophil-driven blood transcriptional signature in human tuberculosis. Nature 466, 973–977 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lamoreaux L, Roederer M & Koup R Intracellular cytokine optimization and standard operating procedure. Nat. Protoc 1, 1507–1516 (2006). [DOI] [PubMed] [Google Scholar]
- 18.Harari A et al. An HIV-1 clade C DNA prime, NYVAC boost vaccine regimen induces reliable, polyfunctional and long-lasting T cell responses. J. Exp. Med 205, 63–77 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zimmerli SC et al. HIV-1-specific IFN-?/IL-2-secreting CD8 T cells support CD4-independent proliferation of HIV-1-specific CD8 T cells. Proc. Natl. Acad. Sci. USA 102, 7239–7244 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Griner PF, Mayewski RJ, Mushlin AI & Greenland P Selection and interpretation of diagnostic tests and procedures. Principles and applications. Ann. Intern. Med 94, 557–592 (1981). [PubMed] [Google Scholar]
- 21.Metz CE Basic principles of ROC analysis. Semin. Nucl. Med 8, 283–298 (1978). [DOI] [PubMed] [Google Scholar]
- 22.Zweig MH & Campbell G Receiver-operating characteristic (ROC) plots: a fundamental evaluation tool in clinical medicine. Clin. Chem 39, 561–577 (1993). [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.