Abstract
Pulmonary vasodilators and prostacyclin therapy in particular, have markedly improved the outcome of patients with pulmonary hypertension (PH). Endothelial dysfunction is a key feature of PH, and we previously reported that treprostinil therapy increases number and proliferative potential of endothelial colony forming cells (ECFC) isolated from PH patients’ blood. In the present study, objectives were to determine how treprostinil contributes to the proangiogenic functions of ECFC. We examined the effect of treprostinil on ECFC obtained from cord blood in terms of colony numbers, proliferative and clonogenic properties in vitro, as well as in vivo vasculogenic properties. Surprisingly, treprostinil inhibited viability of cultured ECFC but did not modify their clonogenic properties nor their endothelial differentiation potential from cord blood stem cells. Treprostinil treatment significantly increased the vessel-forming ability of ECFC combined with mesenchymal stem cells (MSC) in Matrigel implanted in nude mice. In vitro, ECFC proliferation was stimulated by conditioned media from treprostinil-pretreated MSC, and this effect was inhibited either by the use of VEGF-A blocking antibodies or siRNA VEGF-A in MSC. Silencing VEGF-A gene in MSC also blocked the pro-angiogenic effect of treprostinil in vivo. In conclusion, increased VEGF-A produced by MSC can account for the increased vessel formation observed during treprostinil treatment. The clinical relevance of these data was confirmed by the high level of VEGF-A detected in plasma from patients with pediatric pulmonary hypertension who had been treated with treprostinil. Moreover, our results suggest that VEGF-A level in patients could be a surrogate biomarker of treprostinil efficacy.
Keywords: Stem cells, Vascular remodelling, Vasculogenesis, endothelial progenitor cells, prostacyclin analogues
Introduction
Prostacyclin (PGI2) is a prostanoid forming part of a sub-class of the eicosanoid family generated by the arachidonic acid pathway via oxidation through the enzyme cyclooxygenase. PGI2 is produced by vascular cells and acts in an autocrine or a paracrine pathway by binding to a specific prostacyclin receptor (IP) found in megakaryocytes, in smooth muscle cells and in endothelial cells. IP is preferentially coupled to Gs subunits and PGI2 binding activates adenylate cyclase to increase cAMP levels, resulting in platelet inhibition, vasodilation, regulation of smooth muscle cell differentiation, proliferation and migration (1, 2). PGI2 thus plays important roles as endogeneous regulator of vascular homeostasis. PGI2 or prostacyclin analogues have also been involved in ischemia-reperfusion injury in particular in heart and liver transplantation (3, 4) and are important mediators of acute inflammation and inflammatory pain transmission (5, 6). Prostacyclins analogues (epoprostenol, treprostinil, iloprost) are authorized for treatment of pulmonary arterial hypertension (PAH) and are currently considered as the reference treatment.
PAH is a life threatening disease resulting from progressive pulmonary vascular disease and right heart failure. Children with idiopathic PAH have a poor prognosis with a median survival time of only 10 month after diagnosis (7). Continuous intravenous (IV) epoprostenol was the first treatment approved for adult and children’s PAH and has been shown to improve survival, functional class and quality of class (8). In children with refractory PAH, subcutaneous (SC) treprostinil (9), a prostacyclin analog that is chemically stable, is preferred to an IV prostacyclin, in particular to avoid the long term risk associated with chronic IV therapy. We previously reported that treprostinil, in addition to providing a clinical benefit, modifies the balance between circulating endothelial cells (CEC) and progenitors (10, 11) by decreasing CEC and increasing ECFC numbers, repectively.
Evidence continues to implicate endothelial progenitor cells (EPC) in angiogenesis and in particular in repairing injured endothelium in PAH. EPC have been proposed as a cell therapy product in adult and pediatric PAH (12, 13). Cells used in these trials are heterogeneous and not able to form vessels in preclinical models of vascularization (14). “Late” EPC, also called endothelial colony-forming cells (ECFC), are able to form vessel- like networks in vitro (14) and perfused vascular networks in vivo when they are associated to perivascular cells such as mesenchymal stem cells (MSC) (15). ECFC have been shown to be involved in the vascular remodeling associated with PAH (16, 17) and we previously found that ECFC isolated form PAH patients’ peripheral blood were increased in number, proliferation rate and angiogenic potential when patients were treated with SC treprostinil (11).
Given the importance of vascular dysfunction and remodeling in PAH, our aim was to explore the mechanisms by which the treprostinil acts on ECFC (or late-EPC) -induced angiogenesis to improve vascular remodeling in PAH.
Materials and Methods
ECFC and MSC isolation and culture
Human umbilical cord blood was obtained from the Brigham and Women’s Hospital in accordance with an Institutional Review Board-approved protocol. Endothelial colony forming cells (ECFC) were isolated from the adherent mononuclear cells (MNC) fraction as described (18). ECFC were expanded on fibronectin (FN)-coated plates (1 μg/cm2; Millipore, MA) using EGM-2 (without hydrocortisone; Lonza, MD) supplemented with 20 % FBS (Hyclone, UT). Mesenchymal stem cells (MSC) were isolated from the MNC fraction of human adult bone marrow as previously described (18). ECFC and MSC have a classic phenotype with positive CD31 and CD90 expression respectively, while the leukocyte marker CD45 was not detected (supplemental Figure 1).
Assays for in vitro cellular viability
To explore treprostinil effect on cell viability, ECFC or MSC (1 × 104/cm2) were seeded on fibronectin-coated 24-well plates and cultured in completed endothelial growth medium (Endothelial Basal Cell Medium EBM2 supplemented with SingleQuot Kit and 20% FBS) during 24 hours. Cells were then deprived of serum and growth factors (EBM2 medium alone) for 16 hours before adding treprostinil at three different concentrations (0.1; 1 and 10 μM) in the presence of three different medium (EBM2 with 20%FBS, 5%FBS or 1%FBS without growth factor). Indometacin and SC-560 effect were tested by using EBM-2 with 5% FBS. Viability was measured by cellular alkaline phosphatase activity using the substrate para-nitrophenol phosphate (pNPP) (Sigma) as previously described (19−21). The released pNPP was measured by spectrophotometry, OD 405 nm, after 3 days of growth.
Single cell clonogenic assays
ECFC were plated at one cell per well into 96 well plates pre-coated with Type I rat-tail collagen in 200 μl of complete EGM-2 medium. Cells were cultured at 37°C in a humidified incubator with 5% CO2. Media were changed every five days. After 14 days of culture, cells were fixed with 4% paraformaldehyde (Sigma; St. Louis, MO) in phosphate-buffered saline for 30 minutes at room temperature, then washed twice, stained with 1.5 μg/ml DAPI, and examined for ECFC growth. Those wells containing two or more cells were identified as positive for proliferation under a fluorescent microscope at 10x magnification. Wells containing fewer than 50 cells were counted by visual inspection with a fluorescent microscope at 40x magnification. For those wells with more than 50 cells, colonies were imaged and cell number quantified using an Image J1.36v program (Wayne Rasband, NIH).
In vivo model of microvessel formation using ECFC + MSC
Experiments were performed with 3×106 cells per implant as described (15). ECFC and/or MSC were suspended in 200 μL of Matrigel (BD Bioscience, Bedford, MA - reference 356237) and injected subcutaneously on the back of 6- to 7-week-old male athymic nu/nu mice (Massachusetts General Hospital, Boston, MA). Mice were euthanized at day 10 and Matrigel implants were removed, fixed in 10% buffered formalin overnight, embedded in paraffin, and sectioned. For the assessment of microvessel density (MVD), luminal structures containing red blood cells were counted in 4 fields from one section of mid-Matrigel hematoxylin and eosin (H&E)–stained sections from each of the animals in each group (n=5 mice per groups). MVD was expressed as vessels/mm2 +/− standard error of the mean. The protocol was approved the Institutional Animal Care and Use Committee (IACUC) of Boston Children’s Hospital. The animal protocol number is 10-11-1840R.
Cell Transfection with siRNA against VEGF-A
siRNA previously shown to silence VEGF-A (sc-29520, Santa Cruz Biotechnology, Santa Cruz, CA, USA) was mixed with the Primefect reagent (LONZA) at 10 μM to obtain the transfection complexes, which were added to 1.5×105 MSC in EGM2 medium in 6-well plates. Scrambled siRNA (Allstars Neg. control siRNA, Qiagen, Cambridge, MA, USA) was used as a control. VEGF silencing efficacy over 7 days is shown in supplemental figure 2A.
Protein Quantification
VEGF-A (reference DVE00), PDGF-BB (reference DBB00) and Angiopoietin-2 (reference DANG20) secreted in the ECFC and MSC culture medium or in patients’ plasma were quantified using the Human Quantikine kits (R&D Systems enzyme-linked immunosorbent assay) as previously described (22).
Study population
The Institutional Ethics Committee from Paris Ile de France II (protocol number: 2006–114/2007-A00765–48) approved this study and signed informed consent was obtained from patient or parents in all cases. 54 patients aged from 1 to 26 years old with pulmonary hypertension (idiopathic or associated with congenital cardiopathy) were enrolled at the Necker-Enfants Malades Hospital between February 2008 and December 2010. All clinical decisions were made by the attending physician as a part of routine care, independently of the research study.
As previously described (10), all patients had a right heart catheterization (RHC) and complete pulmonary hypertension workup including functional assessment, 6-min walk test (6MWT) (when appropriate), Brain natriuretic peptide (BNP) and echocardiography. Given the follow up along disease evolution, we analyzed 69 plasma samples from the 54 PAH patients enrolled. Fifteen patients were explored in the absence of treatment, 19 patients had oral therapy (sildenafil and/or bosentan) and 20 patients received subcutaneous (SC) treprostinil. The current therapeutic strategy in our institution consists in treating all PAH children classified with Functional Class II or higher, starting firstly with oral monotherapy. In cases of worsening or non-improvement, a second oral drug was added and, finally, prostacyclin analogues on top of oral therapy with sildenafil and bosentan have been given to 20 patients. At the time of worsening, a second oral drug was given after a repeated right heart catheterization (RHC) to confirm either an increase in pulmonary vascular resistance a decrease in cardiac output. A second oral drug was also added in case of “no improvement” but, in these circumstances, RHC was not systematically repeated. In case of no improvement or worsening under combined oral therapy, subcutaneous treprostinil was added (which is the first choice in our institution when prostanoïds are needed), as previously reported (9, 10).
Plasma samples
Peripheral venous blood was collected in tubes containing 0.105 M sodium citrate (1:9 v/v). Plasma was obtained by double centrifugation at 2,300g for 10 min and was immediately placed at −80°C until use.
Statistical Analysis
Data are expressed as mean ± SEM and were analyzed by Mann-Whitney. Differences were considered significant at P values <0.05.
RESULTS
Treprostinil decreases ECFC viability/proliferation potential
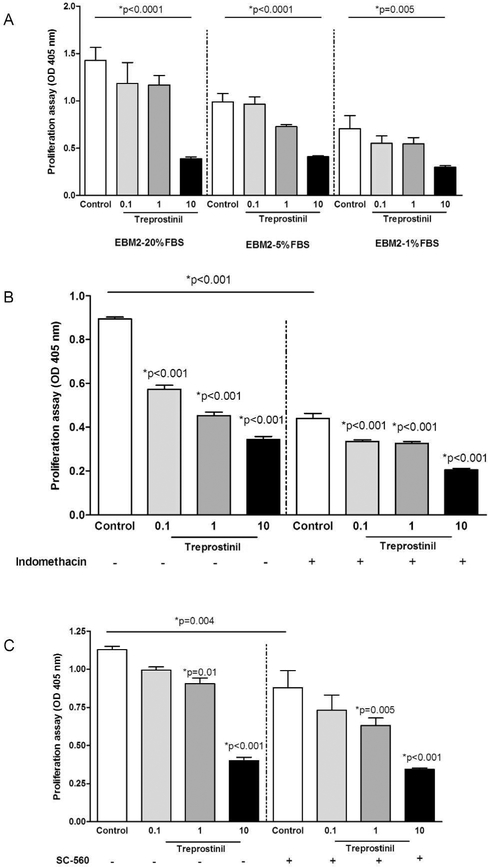
We explored the effect of treprostinil on ECFC proliferation via a viability assay consisting in measuring pNPP release. Ten μM treprostinil inhibited ECFC proliferation in media containing either high or low serum concentrations (p <0.0001, p <0.0001 and p=0.002 for EBM2 containing 20%FBS, 5%FBS and 1%FBS, respectively). Same results were obtained with treprostinil concentrations ranging from 0.1 to 1 μM (p =0.005 for EBM2 5%FBS condition). To escape autologous ECFC prostacyclin synthesis (23), ECFC were treated with either the non-selective COX inhibitor indomethacin (10 μmol/L, Figure 1B) or the selective COX-1 inhibitor SC560 (1μmol/L, Figure 1C) prior adding treprostinil. Both inhibitors halved cell proliferation and treprostinil showed an additive effect, in line with a direct antiproliferative effect similar to that of endogenous PGI2 (23, 24).
Figure 1. In vitro effect of treprostinil in ECFC viability, clonogenic properties and commitment.
A- Proliferation of ECFC cultured in EGM-2/20% FBS, EGM-2/5% FBS and EBM- 2/1% FBS in the presence or in the absence of treprostinil, evaluated after 3 days by measuring cellular phosphatase activity.
B- Effect of treprostinil on ECFC proliferation in EGM-2/5% FBS in the presence or in the absence of Indomethacin evaluated after 3 days by measuring cellular phosphatase activity.
C- Effect of treprostinil on ECFCs proliferation in EGM-2/5% FBS in the presence or in the absence of a cox1 inhibitor SC-560 evaluated after 3 days by measuring cellular phosphatase activity.
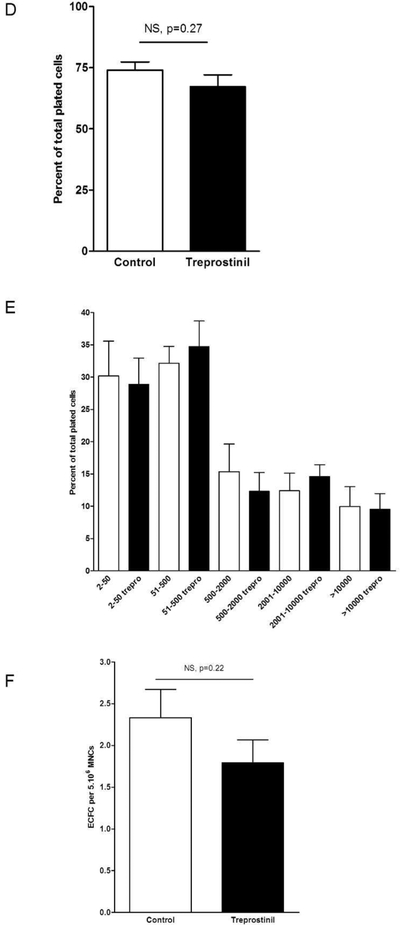
D- The percentage of ECFC undergoing at least 1 cell division after 14 days of culture in the presence or in the absence of treprostinil. Results represent the mean ± SEM of 3 independent experiments using single endothelial cells derived from different donors.
E- Number of cell progeny derived from a single ECFC in an individual well after 14 days of culture. Results represent the mean ± SEM of 3 independent experiments.
F- Number of ECFC isolated from umbilical cord blood (CB). Results represent the mean ± SEM ECFC of 5 independent experiments for cord blood samples.
A single-cell clonogenic assay was performed to further explore treprostinil effect on ECFC. After single ECFC were plated in culture, some cells did not divide, while other divided and formed colonies of different sizes composed of variable cell numbers. The frequency of single cells undergoing division was similar between untreated ECFC and treprostinil-treated ECFC (74 ± 6.9 vs 67 ± 9.2, respectively, p=0.27, Figure 1D). Moreover, the same hierarchy of ECFC, composed of high proliferative (HPP)-, low proliferative (LPP)-ECFC, endothelial- cluster and non-dividing mature EC, was found in untreated-ECFC and treprostinil-treated ECFC (Figure 1E). We also examined the possible involvement of treprostinil in cord blood MNC commitment to ECFC. Treprostinil was added to EGM2–5% FBS from the first day of culture of cord blood MNC. Thereafter the number and timing of ECFC colonies that appeared on the culture dishes was recorded. As shown in Figure 1F, treprostinil did not modify the number of colonies (2.3 ± 1.4 vs 1.8 ± 1.1 colonies per 5.106 MNC, mean ± SEM, p = 0.22) nor the timing of colony emergence (not shown).
Treprostinil increases vessel density in ECFC + MSC implants by a VEGF-A dependant mechanism
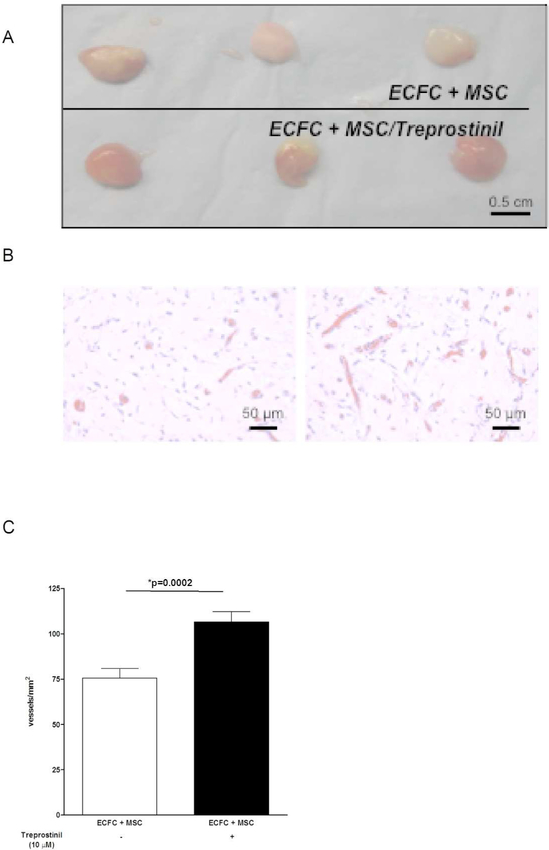
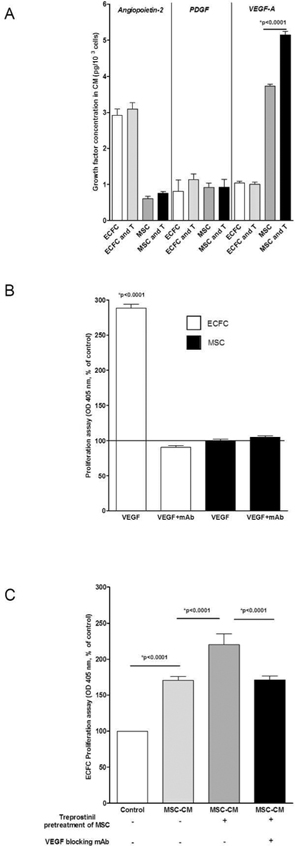
We previously described that ECFC isolated from children treated with treprostinil have an increased proliferation potential (11). However, results presented in Figure 1 are not in accordance with a direct proliferative effect on ECFC. We then analysed the effect of treprostinil by pre-treating cells 48 hours before and also by adding treprostinil inside implants. ECFC/Matrigel implants in nude mice form very few to no vessels (15), and adding treprostinil to ECFC alone did not improve this (data not shown). When ECFC were combined with MSC, in vivo vessel formation occurred and was measured after 10 days. Treprostinil significantly stimulated ECFC+MSC induced vessel formation (Figure 2A and 2B) with a significant increase of microvessel density (+35%, p=0.0002, Figure 2C). This result is in favour of a cooperative effect between both cell types in the presence of treprostinil and prompted us to explore the effect of treprostinil on secretion of angiogenic molecules by ECFC and MSC. Three molecules known to be involved in PAH vessel remodelling were tested, namely angiopoietin-2 (Ang2), VEGF-A and PDGF-BB. Incubation of ECFC and MSC with treprostinil did not modify Ang2 (p=0.43 and p=0.54 for ECFC and MSC, respectively) nor PDGF-BB levels (p=0.13 and p=0.96 for ECFC and MSC, respectively). Although treprostinil did not affect VEGF-A secreted from ECFC (p=0.8), it significantly increased VEGF-A level secreted by MSC (Figure 3A, p<0.0001).
Figure 2. Treprostinil increases in vivo vasculogenic potential of ECFC and MSC combination.
Experiments were performed with 3×106 cells per implant. ECFC and/or MSC were suspended in 200 μL of Matrigel and injected subcutaneously on the back of 6- to 7-week-old male athymic nu/nu mice. Mice were euthanized at day 10 and Matrigel implants were removed, fixed in 10% buffered formalin overnight, embedded in paraffin, and sectioned. For the assessment of microvessel density (MVD), luminal structures containing red blood cells were counted in 4 fields from one section of mid-Matrigel hematoxylin and eosin (H&E)– stained sections from each of the animals in each group (n=5 mice per groups).
A- ECFC co-implanted with MSC, with or without treprostinil. Representative photographs of Matrigel explants at day10 after injection
B- ECFC co-implanted with MSC, with or without treprostinil. Representative photographs of histological sections stained with H&E. Scale bar = 50 μm
C- Quantification of microvessel density as microvessels/mm2. Data are mean ± SEM.
Figure 3. Treprostinil induces VEGF-A secretion from MSC.
A- ECFC and MSC were incubated with treprostinil for 72 hours. Conditioned media from each cell type was tested for VEGF-A, PDGF-BB and angiopoietin-2 by ELISA (R&D systems).
B- Recombinant human VEGF-A induced ECFC proliferation while no proliferative effect was observed in MSC.
C- Conditioned media from MSC pretreated with treprostinil induced ECFC proliferation, which was abolished by a blocking mAb against VEGF-A.
ECFC and MSC were then treated with human recombinant VEGF-A (50 ng/ml), and, as expected, an increased proliferative effect on ECFC was observed that was abrogated by a blocking mAb (MAB293, 0.1 μg/ml, Figure 3B). Conversely, VEGF-A had no effect on MSC proliferation (Figure 3B). ECFC were then exposed for 72h to conditioned media (CM) from MSC pretreated with treprostinil or not (Figure 3C). MSC-CM significantly increased ECFC proliferation (p<0.0001), this effect being significantly higher when MSC were treated with treprostinil (p<0.0001), and fully abrogated by VEGF-A blocking mAb (p<0.0001).
Reversal of treprostinil-induced angiogenic properties by VEGF-A gene silencing in MSC
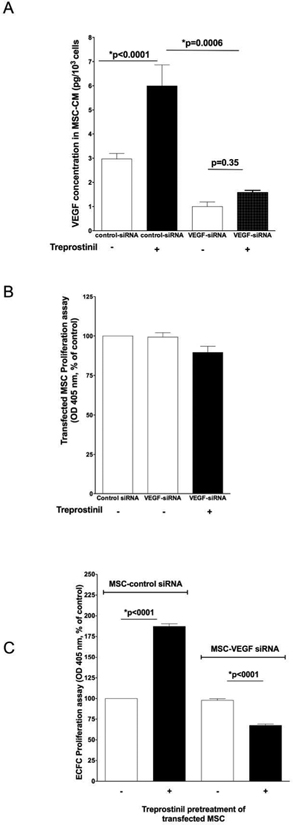
A 48 hour-transfection of MSC with VEGF-A siRNAs (10 μM) resulted in strong inhibition of VEGF-A secretion by MSC, as shown at the protein level (Figure 4A, open columns). Moreover, the stimulation of VEGF-A secretion induced by treprostinil in MSC transfected with control scrambled siRNA was completely abolished in MSC transfected with VEGF-A-siRNA. VEGF-A silencing did not affect MSC viability (Figure 4B), either in the presence or in the absence of treprostinil.
Figure 4. VEGF-A gene silencing in MSC abolishes the treprostinil-mediated increase in ECFC- proliferation.
A- Analysis of VEGF-A secretion by ELISA on MSC supernatants, after transfection with si-control (all-star negative control, Qiagen®) and siVEGF-A (Santacruz Biotechnologies®) with Primefect® (LONZA). VEGF-A gene silencing in MSC resulted in a 75% inhibition of VEGF-A secretion (*p=0.0006). MSC were incubated with treprostinil for 72 hours. Mean and S.E.M. of three experiments is shown.
B- Inhibition of VEGF-A in MSC did not modify their proliferation ability.
C- ECFC proliferation mediated by conditioned media from siVEGF-A transfected versus si control MSC. The mean and S.E.M. of three experiments are shown. *P < 0.05
In a second set of experiments, ECFC were incubated in the presence of CM from transfected MSC. When ECFC were exposed for 72h to CM from MSC transfected with scrambled-siRNA pretreated with treprostinil, we found, as expected, a significant increase in ECFC proliferation. On the contrary, when ECFC were exposed for 72h to CM from MSC transfected with VEGF-A-siRNA pretreated or not with treprostinil, no effect on proliferation was observed (Figure 4C).
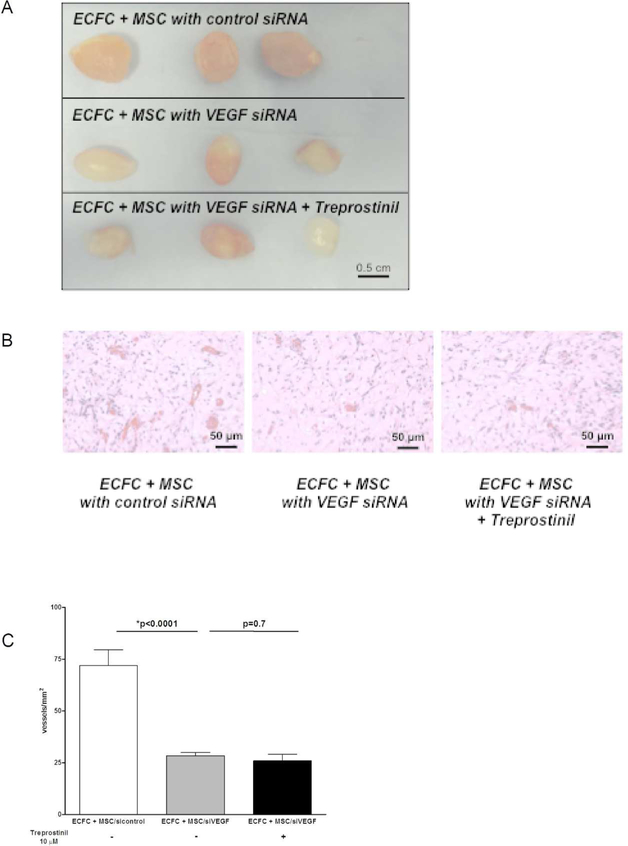
Finally, we tested the effect of treprostinil on the vessel-forming ability of ECFC combined with MSC transfected with VEGF-A-siRNA. ECFC combined with MSC transfected with VEGF-A-siRNA formed fewer vessels in vivo compared to ECFC combined with MSC transfected with control scrambled siRNA (Figure 5A and 5B) with a significant decrease of microvessel density (p<0.0001; Figure 5C). Treprostinil did not alter microvessel density in the Matrigel implants containing ECFC and MSC transfected with VEGF-A-siRNA (Figure 5C). As a whole, these results indicate that treprostinil exerts an unexpected effect on MSC – it increases VEGF-A secretion which in turn can stimulate proliferation and vessel-forming activities in ECFC.
Figure 5. VEGF-A silencing in MSCpotential abolishes treprostinil-mediated vasculogenic.
Experiments were performed with 3×106 cells per implant. ECFC and transfected MSC were suspended in 200 μL of Matrigel and injected subcutaneously on the back of 6- to 7-week-old male athymic nu/nu mice.
A- ECFC co-implanted with transfected MSC (si control all-star negative control from Qiagen® or siVEGF-A from Santa Cruz Biotechnologies®) with or without treprostinil. Representative photographs of Matrigel explants at day 10 after injection
B- ECFC co-implanted with transfected MSC with or without treprostinil. Representative photographs of histological sections stained with H&E. Scale bar = 50 μm
C- Quantification of microvessel density as microvessels/mm2. Data are mean ± SEM.
VEGF-A is increased in plasma from PAH children treated with treprostinil.
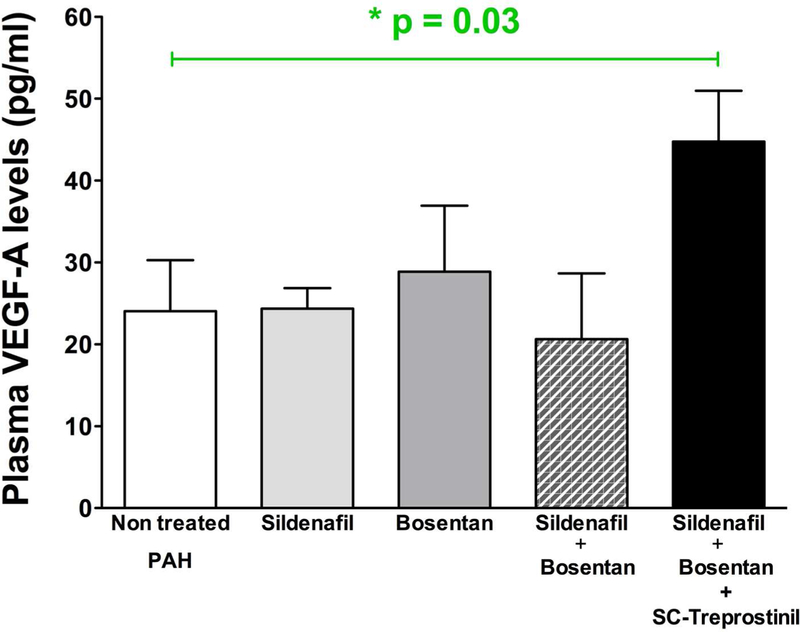
Compared to untreated PAH patients, VEGF-A levels were significantly higher in plasma from PAH patients requiring prostacyclin analogue treatment with SC-treprostinil (n=20) (respectively p=0.03 vs non-treated PAH patients), whereas no modification was noticed in patients receiving either oral sildenafil and/or bosentan (p=0.8 versus untreated patients), in line with a potential involvement of VEGF-A in the pharmacology of treprostinil. To be sure this VEGF increase is not related to hypoxia, we thus compared, as described in supplemental Figure 3, hemoglobin (Hb) levels in different patient groups. Compared to untreated PAH patients, no differences were observed in Hb level after subcutaneous treprostinil treatment (respectively with a p=0.1 vs none treated PAH patients and p=0.35 vs oral sildenafil and bosentan treated patients).
DISCUSSION
The current study demonstrates that the prostacyclin analogue treprostinil stimulates ECFC+MSC-induced angiogenesis by a VEGF-A dependent mechanism. Specifically, we show that treprostinil alone has an in vitro inhibitory effect on ECFC angiogenic potential. However it increases the capacity of MSC to secrete VEGF-A, and this in turn increases the proliferation of ECFC in vitro and vessel formation of ECFC + MSC in vivo. Finally, we show elevated plasma VEGF-A level in a cohort of PAH pediatric patients receiving treprostinil therapy.
During past years, it has been documented that endothelium is a highly dynamic tissue in equilibrium with circulating cells of various types, in particular circulating endothelial cells (CEC) and endothelial progenitor cells (EPC), offering opportunities for a better understanding of vascular dysfunction, especially in pulmonary arterial hypertension (PAH). On one hand, CEC are mature cells detached from injured, proliferative and/or activated vessels, and represent rare events in peripheral blood of healthy controls. We have reported that CEC are increased in PAH, and that is associated with a strong vessel remodeling (25, 26). On the other hand, EPC are derived from bone marrow and are characterized by their ability to participate in endothelial repair. Impaired function of EPC has been related to cardiovascular and pulmonary diseases, but their real involvement in PAH remains unclear. Prostacyclin therapy has markedly improved the outcome of patients with PAH and we previously described that treprostinil induces an imbalance between EPC and CEC. Indeed, we observed that an increase of EPC number and function (11) together with a decrease of CEC was correlated to clinical efficacy (10). Our data suggest that treprostinil modifies the balance between endothelial activation or injury (CEC) and repair (EPC). The present objectives were to determine how treprostinil contributes to the proangiogenic functions of ECFC.
Several EPC phenotypes have been described in literature, the true vasculogenic cells able to build vessels being ECFC (endothelial colony forming cells). ECFC are proliferative and clonogenic vasculogenic cells that are very rare in adult circulation (14, 27). They have been isolated from cord and adult blood, from human umbilical vein and aorta, and from the pulmonary arteries and microvessels (28, 29). ECFC has been largely shown to participate in vascular repair in mouse models of hind limb ischemia (19, 30), ischemic myocardium (31) but also in pulmonary hypertension associated to bronchopulmonary dysplasia (17). Moreover, circulating ECFC dysfunction has been demonstrated in diseases with dysregulated angiogenesis, such as diabetes mellitus (32), idiopathic pulmonary fibrosis (33) and PAH (16, 34). There is thus a growing interest to elucidate the physiopathological role of ECFC in PAH. Because of their vasculogenic properties and capacity to repair dysfunctional vessels, ECFC may partly mediate the clinical benefits of prostanoids in PAH.
Prostacyclin and its mimetic have been shown to decrease smooth muscle cell proliferation (35). It has been shown that treprostinil mediates antiproliferative mechanisms of resident lung fibroblasts (36) and reduces circulating fibrocytes, their adhesion and differentiation potential in parallel to an overall reduction of pulmonary vascular remodelling (37, 38). Prostacyclin and its mimetics have also been shown to improve EPC function (11, 23, 24, 39) and to mobilize different EPC subtypes in patients (11, 40). Moreover, a recent study found a correlation between EPC number and plasma prostacyclin concentration in PAH (41). Our present results suggest 2 apparent opposite mechanisms for treprostinil effect on ECFC-induced vasculogenesis: 1) an increased vessel formation mediated by VEGF-A when ECFC are mixed with MSC, and 2) a dose-dependent anti-proliferative action on ECFC.
Given the beneficial effect of treprostinil on ECFC+MSC-induced vessel formation, we explored growth factors secreted by MSC with a focus on vascular endothelial growth factor A (VEGF-A), previously involved in ECFC proliferation and PAH pathophysiology (42–46). VEGF-A is an endothelial-cell-specific-angiogenic mitogen acting via two high-affinity tyrosine kinase receptors, VEGFR-1 and VEGFR-2, both present on ECFC (47, 48). Although the physiological role of the abundantly expressed VEGF-A in the lung is unknown, it has been proposed that VEGF-A supports pulmonary endothelial cell maintenance and survival. In PAH, VEGF-A expression is increased within the pulmonary vasculature, including the plexiform lesions and endothelial cells from irreversible PAH associated to congenital heart disease (25). The SU5416, a VEGFR2 inhibitor, has been used to induce PAH in vivo (49). Prostacyclin analogue has been shown to increase VEGF-A level in lung fibroblasts (50), in line with our results on MSC. Indeed, our present data suggest that treprostinil proliferative properties previously observed in ECFC derived from PAH patients (11) are VEGF-A dependent, resulting from an increase in VEGF-A secretion from stromal cells. VEGF-A increase in plasma of treprostinil treated patients supports this hypothesis. Mechanisms by which treprostinil increases VEGF in MSC are still to explore. Prostacyclin (PGI2) and PGI2 analog biological activities were thought to be exclusively mediated by cell surface receptors named IP. Recent studies have instead identified a novel pathway of PGI2 signaling, occurring through activation of peroxisome proliferator-activated receptors (PPARs) located in the nucleus. Both type of receptor interaction have been described in several cell types to modulate VEGF synthesis (51–53). Treprostinil involvement on IP receptor or a PPAR- mediated pathway in VEGF mediated synthesis still needs to be explored.
We evaluated VEGF levels in plasma after a double centrifugation to completely eliminate platelets and in the first two hours after blood sampling to avoid any platelet activation. Thus, platelet aggregation and growth factor release are not supposed to influence VEGF levels quantified in plasma. In contrast, VEGF quantified in serum is higher than in plasma because of the VEGF content in alpha granules released upon activation. Moreover, previous work (43) demonstrated that increased VEGF levels found in serum of PAH patients was the result of platelet aggregation and correlated with VEGF release from platelets (while no correlation with hypoxia or platelet number was found). Thus, since we evaluated VEGF in plasma with strict respect of pre-analytical conditions, we hypothesize that platelet VEGF does not interfere with our results. Moreover, prostacyclin was first identified because of its ability to inhibit platelet aggregation (54) and this is probably one of the mechanisms by which prostacyclin favorably influences pulmonary circulation. Thus, if treprostinil was able to modify platelet VEGF secretion, we should observe a decrease in VEGF levels. The increased VEGF levels observed here do not match this hypothesis. However, during PAH, an increase of mean platelet volume has been described. MPV is an indirect reflection of platelet production and stimulation, because larger platelets are generally younger, contain more granules, and have been reported to be more functional (55). Thus, any platelet activation is likely to influence VEGF level. Moreover, we cannot exclude an indirect effect of platelets related to the presence of microparticules in plasma. Platelet microparticles (PMP) have been found increased during PAH (56). Although we currently do not know if vasodilator therapy modifies PMP, these PMP can convey VEGF and contribute to to plasma VEGF levels.
In PAH patients, we previously reported that treprostinil treatment induces a decrease of CEC count (10), a biomarker of endothelial dysfunction. In idiopathic PAH and PAH associated with congenital heart diseases, CEC also reflect endothelial remodeling (25, 57). We show here that direct treatment of ECFC with treprostinil decreases proliferation. Prostacyclin has been previously shown to have antimitogenic properties (58) and thus may be relevant with decreased ECFC proliferation induced by treprostinil observed here.
ECFCs used in our work were cord blood-derived. However, ECFC derived from adult and cord blood share a similar phenotype and we previously reported that they were both able to form blood vessels in a preclinical model of vascularization (15). We do not think that developmental difference can occur therefore we anticipate adult ECFC will respond similarly. However, this is a limitation of our study.
Thus, our present finding may be reconciled with previous findings, suggesting a loss of proliferative signal of resident endothelial cells inside lung that results in an decreased CEC number. It in vivo has been suggested that endothelial proliferation observed in PAH lesions may be a marker of a fundamental endothelial abnormality, possibly playing a key role in PAH pathogenesis. CEC level is also correlated to clinical worsening (10). In this context, increasing treprostinil dose decreased CEC count while improving patient hemodynamic parameters (10), could be the consequence of treprostinil local antiproliferative effect.
In conclusion, in vitro and in vivo data presented here show that VEGF-A plays a functional role in angiogenic properties balance upon treprostinil treatment. VEGF-A secretion from stromal cells is involved in ECFC proliferation and their ability to form blood vessels in vivo. A better understanding of this VEGF-A/ECFC pathway in PAH pathophysiology could lead to new strategies for prostacyclin analogue therapeutic indication. Thus, angiogenesis could be one of the targets for relapse PAH when treated with treprostinil.
Supplementary Material
Figure 6. Treprostinil treatment increases plasma VEGF-A in pediatric pulmonary hypertension.
Treprostinil treatment was associated with increased VEGF-A levels. Compared to untreated PAH patients, no differences were observed in VEGF-A level after oral mono and/or bitherapy while a significant higher VEGF-A plasma level was observed during subcutaneous treprostinil treatment (respectively with a *p=0.03 vs non treated PAH patients).
What is known about this topic?
Pulmonary vasodilators in general and prostacyclin therapy in particular, have markedly improved the outcome of patients with pulmonary hypertension.
Endothelial dysfunction is a key feature of PH and endothelial progenitor cells are the main cell involved in repairing injured endothelium.
Prostacyclin analogues and in particular Treprostinil therapy has been described to increase number and proliferative potential of endothelial progenitors in PAH patients.
What does this paper add?
We described that Treprostinil increases vessel forming ability of endothelial progenitors angiogenic and. mesenchymal stem cells.
VEGF-A blocking antibodies or VEGF-A silencing in MSC blocks the pro-angiogenic effect of treprostinil in vitro and vivo
The clinical relevance of these data was confirmed by the high level of VEGF-A detected in plasma from patients with pediatric pulmonary hypertension who had been treated with treprostinil. Our results indicate that VEGF-A level in patients could be a surrogate biomarker of treprostinil efficacy.
Acknowledgments:
Research reported in this manuscript was supported by a grant from United Therapeutics, Inc.
David M. Smadja is supported by grants from Région Ile de France-CORDDIM (Domaine d’intérêt majeur Cardiovasculaire Obésité Rein Diabète) and Conny-Maeva Charitable Foundation.
Elisa Rossi is supported by Conny-Maeva Charitable Foundation.
Joyce Bischoff is supported by the National Heart Lung and Blood Institute, part of the National Institutes of Health, under Award Number R01 HL096384 and by the National Institute of Arthritis and Musculoskeletal and Skin Diseases, part of the National Institutes of Health, under Award Number P01 AR048564.
The content is solely the responsibility of the authors and does not represent the official views of United Therapeutics, National Institutes of Health, CORDDIM or Conny-Maeva Charitable Foundation.
Footnotes
Conflicts of interest:
Authors have no conflicts of interest to declare.
REFERENCES
- 1.Moncada S, Vane JR. Pharmacology and endogenous roles of prostaglandin endoperoxides, thromboxane A2, and prostacyclin. Pharmacol Rev. 1978; 30: 293–331. [PubMed] [Google Scholar]
- 2.Fetalvero KM, Martin KA, Hwa J. Cardioprotective prostacyclin signaling in vascular smooth muscle. Prostaglandins Other Lipid Mediat. 2007; 82: 109–18. [DOI] [PubMed] [Google Scholar]
- 3.Xiao CY, Hara A, Yuhki K, et al. Roles of prostaglandin I(2) and thromboxane A(2) in cardiac ischemia-reperfusion injury: a study using mice lacking their respective receptors. Circulation. 2001; 104: 2210–5. [DOI] [PubMed] [Google Scholar]
- 4.Ghonem N, Yoshida J, Stolz DB, et al. Treprostinil, a prostacyclin analog, ameliorates ischemia-reperfusion injury in rat orthotopic liver transplantation. Am J Transplant. 2011; 11: 2508–16. [DOI] [PubMed] [Google Scholar]
- 5.Murata T, Ushikubi F, Matsuoka T, et al. Altered pain perception and inflammatory response in mice lacking prostacyclin receptor. Nature. 1997; 388: 678–82. [DOI] [PubMed] [Google Scholar]
- 6.Doi Y, Minami T, Nishizawa M, et al. Central nociceptive role of prostacyclin (IP) receptor induced by peripheral inflammation. Neuroreport. 2002; 13: 93–6. [DOI] [PubMed] [Google Scholar]
- 7.Yung D, Widlitz AC, Rosenzweig EB, et al. Outcomes in children with idiopathic pulmonary arterial hypertension. Circulation. 2004; 110: 660–5. [DOI] [PubMed] [Google Scholar]
- 8.Barst RJ, Maislin G, Fishman AP. Vasodilator therapy for primary pulmonary hypertension in children. Circulation. 1999; 99: 1197–208. [DOI] [PubMed] [Google Scholar]
- 9.Levy M, Celermajer DS, Bourges-Petit E, et al. Add-on therapy with subcutaneous treprostinil for refractory pediatric pulmonary hypertension. J Pediatr. 2011; 158: 584–8. [DOI] [PubMed] [Google Scholar]
- 10.Levy M, Bonnet D, Mauge L, et al. Circulating endothelial cells in refractory pulmonary hypertension in children: markers of treatment efficacy and clinical worsening. PLoS One. 2013; 8: e65114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smadja DM, Mauge L, Gaussem P, et al. Treprostinil increases the number and angiogenic potential of endothelial progenitor cells in children with pulmonary hypertension. Angiogenesis. 2011; 14: 17–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang XX, Zhang FR, Shang YP, et al. Transplantation of autologous endothelial progenitor cells may be beneficial in patients with idiopathic pulmonary arterial hypertension: a pilot randomized controlled trial. J Am Coll Cardiol. 2007; 49: 1566–71. [DOI] [PubMed] [Google Scholar]
- 13.Zhu JH, Wang XX, Zhang FR, et al. Safety and efficacy of autologous endothelial progenitor cells transplantation in children with idiopathic pulmonary arterial hypertension: open-label pilot study. Pediatr Transplant. 2008; 12: 650–5. [DOI] [PubMed] [Google Scholar]
- 14.Yoder MC, Mead LE, Prater D, et al. Redefining endothelial progenitor cells via clonal analysis and hematopoietic stem/progenitor cell principals. Blood. 2007; 109: 1801–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Melero-Martin JM, De Obaldia ME, Kang SY, et al. Engineering robust and functional vascular networks in vivo with human adult and cord blood-derived progenitor cells. Circ Res. 2008; 103: 194–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Toshner M, Voswinckel R, Southwood M, et al. Evidence of dysfunction of endothelial progenitors in pulmonary arterial hypertension. Am J Respir Crit Care Med. 2009; 180: 780–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baker CD, Seedorf GJ, Wisniewski BL, et al. Endothelial colony-forming cell conditioned media promote angiogenesis in vitro and prevent pulmonary hypertension in experimental bronchopulmonary dysplasia. Am J Physiol Lung Cell Mol Physiol. 2013; 305: L73–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Melero-Martin JM, Bischoff J. Chapter 13. An in vivo experimental model for postnatal vasculogenesis. Methods Enzymol. 2008; 445: 303–29. [DOI] [PubMed] [Google Scholar]
- 19.Smadja DM, d’Audigier C, Bieche I, et al. Thrombospondin-1 is a plasmatic marker of peripheral arterial disease that modulates endothelial progenitor cell angiogenic properties. Arterioscler Thromb Vasc Biol. 2011; 31: 551–9. [DOI] [PubMed] [Google Scholar]
- 20.Smadja DM, Mulliken JB, Bischoff J. E-selectin mediates stem cell adhesion and formation of blood vessels in a murine model of infantile hemangioma. Am J Pathol. 2012; 181: 2239–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smadja DM, Guerin CL, Boscolo E, et al. alpha6-Integrin is required for the adhesion and vasculogenic potential of hemangioma stem cells. Stem Cells. 2014; 32: 684–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smadja D, Gaussem P, Roncal C, et al. Arterial and venous thrombosis is associated with different angiogenic cytokine patterns in patients with antiphospholipid syndrome. Lupus. 2010; 19: 837–43. [DOI] [PubMed] [Google Scholar]
- 23.He T, Lu T, d’Uscio LV, et al. Angiogenic function of prostacyclin biosynthesis in human endothelial progenitor cells. Circ Res. 2008; 103: 80–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kawabe J, Yuhki K, Okada M, et al. Prostaglandin I2 promotes recruitment of endothelial progenitor cells and limits vascular remodeling. Arterioscler Thromb Vasc Biol. 2010; 30: 464–70. [DOI] [PubMed] [Google Scholar]
- 25.Smadja DM, Gaussem P, Mauge L, et al. Circulating endothelial cells: a new candidate biomarker of irreversible pulmonary hypertension secondary to congenital heart disease. Circulation. 2009; 119: 374–81. [DOI] [PubMed] [Google Scholar]
- 26.Smadja DM, Mauge L, Sanchez O, et al. Distinct patterns of circulating endothelial cells in pulmonary hypertension. Eur Respir J. 2010; 36: 1284–93. [DOI] [PubMed] [Google Scholar]
- 27.Mauge L, Sabatier F, Boutouyrie P, et al. Forearm ischemia decreases endothelial colony-forming cell angiogenic potential. Cytotherapy. 2014; 16: 213–24. [DOI] [PubMed] [Google Scholar]
- 28.Ingram DA, Mead LE, Moore DB, et al. Vessel wall-derived endothelial cells rapidly proliferate because they contain a complete hierarchy of endothelial progenitor cells. Blood. 2005; 105: 2783–6. [DOI] [PubMed] [Google Scholar]
- 29.Alvarez DF, Huang L, King JA, et al. Lung microvascular endothelium is enriched with progenitor cells that exhibit vasculogenic capacity. Am J Physiol Lung Cell Mol Physiol. 2008; 294: L419–30. [DOI] [PubMed] [Google Scholar]
- 30.Hur J, Yoon CH, Kim HS, et al. Characterization of two types of endothelial progenitor cells and their different contributions to neovasculogenesis. Arterioscler Thromb Vasc Biol. 2004; 24: 288–93. [DOI] [PubMed] [Google Scholar]
- 31.Kang KT, Coggins M, Xiao C, et al. Human vasculogenic cells form functional blood vessels and mitigate adverse remodeling after ischemia reperfusion injury in rats. Angiogenesis. 2013; 16: 773–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tan K, Lessieur E, Cutler A, et al. Impaired function of circulating CD34(+) CD45(−) cells in patients with proliferative diabetic retinopathy. Exp Eye Res. 2010; 91: 229–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smadja DM, Mauge L, Nunes H, et al. Imbalance of circulating endothelial cells and progenitors in idiopathic pulmonary fibrosis. Angiogenesis. 2013; 16: 147–57. [DOI] [PubMed] [Google Scholar]
- 34.Duong HT, Comhair SA, Aldred MA, et al. Pulmonary artery endothelium resident endothelial colony-forming cells in pulmonary arterial hypertension. Pulm Circ. 2011; 1: 475–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Clapp LH, Finney P, Turcato S, et al. Differential effects of stable prostacyclin analogs on smooth muscle proliferation and cyclic AMP generation in human pulmonary artery. Am J Respir Cell Mol Biol. 2002; 26: 194–201. [DOI] [PubMed] [Google Scholar]
- 36.Ali FY, Egan K, FitzGerald GA, et al. Role of prostacyclin versus peroxisome proliferator-activated receptor beta receptors in prostacyclin sensing by lung fibroblasts. Am J Respir Cell Mol Biol. 2006; 34: 242–6. [DOI] [PubMed] [Google Scholar]
- 37.Nikam VS, Schermuly RT, Dumitrascu R, et al. Treprostinil inhibits the recruitment of bone marrow-derived circulating fibrocytes in chronic hypoxic pulmonary hypertension. Eur Respir J. 2010; 36: 1302–14. [DOI] [PubMed] [Google Scholar]
- 38.Nikam VS, Wecker G, Schermuly R, et al. Treprostinil Inhibits the Adhesion and Differentiation of Fibrocytes via the Cyclic Adenosine Monophosphate-Dependent and Ras-Proximate Protein-Dependent Inactivation of Extracellular Regulated Kinase. Am J Respir Cell Mol Biol. 2011; 45: 692–703. [DOI] [PubMed] [Google Scholar]
- 39.Liu Q, Xi Y, Terry T, et al. Engineered endothelial progenitor cells that overexpress prostacyclin protect vascular cells. J Cell Physiol. 2012; 227: 2907–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Di Stefano R, Barsotti MC, Melillo E, et al. The prostacyclin analogue iloprost increases circulating endothelial progenitor cells in patients with critical limb ischemia. Thromb Haemost. 2008; 100: 871–7. [PubMed] [Google Scholar]
- 41.Song Y Association study of circulating endothelial progenitor cells and plasma prostacyclin levels in pulmonary hypertension rats. Genet Mol Res. 2014; 13: 438–44. [DOI] [PubMed] [Google Scholar]
- 42.Asahara T, Takahashi T, Masuda H, et al. VEGF contributes to postnatal neovascularization by mobilizing bone marrow-derived endothelial progenitor cells. Embo J. 1999; 18: 3964–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Eddahibi S, Humbert M, Sediame S, et al. Imbalance between platelet vascular endothelial growth factor and platelet-derived growth factor in pulmonary hypertension. Effect of prostacyclin therapy. Am J Respir Crit Care Med. 2000; 162: 1493–9. [DOI] [PubMed] [Google Scholar]
- 44.Smadja DM, Laurendeau I, Avignon C, et al. The angiopoietin pathway is modulated by PAR-1 activation on human endothelial progenitor cells. J Thromb Haemost. 2006; 4: 2051–8. [DOI] [PubMed] [Google Scholar]
- 45.Mata-Greenwood E, Meyrick B, Soifer SJ, et al. Expression of VEGF and its receptors Flt-1 and Flk-1/KDR is altered in lambs with increased pulmonary blood flow and pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol. 2003; 285: L222–31. [DOI] [PubMed] [Google Scholar]
- 46.Barst RJ. PDGF signaling in pulmonary arterial hypertension. J Clin Invest. 2005; 115: 2691–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.d’Audigier C, Gautier B, Yon A, et al. Targeting VEGFR1 on endothelial progenitors modulates their differentiation potential. Angiogenesis. 2014; 17: 603–16. [DOI] [PubMed] [Google Scholar]
- 48.Smadja DM, Bieche I, Helley D, et al. Increased VEGFR2 expression during human late endothelial progenitor cells expansion enhances in vitro angiogenesis with up-regulation of integrin alpha(6). J Cell Mol Med. 2007; 11: 1149–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sakao S, Tatsumi K. The effects of antiangiogenic compound SU5416 in a rat model of pulmonary arterial hypertension. Respiration. 2010; 81: 253–61. [DOI] [PubMed] [Google Scholar]
- 50.Kamio K, Sato T, Liu X, et al. Prostacyclin analogs stimulate VEGF production from human lung fibroblasts in culture. Am J Physiol Lung Cell Mol Physiol. 2008; 294: L1226–32. [DOI] [PubMed] [Google Scholar]
- 51.Pola R, Gaetani E, Flex A, et al. Comparative analysis of the in vivo angiogenic properties of stable prostacyclin analogs: a possible role for peroxisome proliferator-activated receptors. J Mol Cell Cardiol. 2004; 36: 363–70. [DOI] [PubMed] [Google Scholar]
- 52.Park DW, Baek K, Lee JG, et al. Activation of toll-like receptor 4 modulates vascular endothelial growth factor synthesis through prostacyclin-IP signaling. Biochem Biophys Res Commun. 2007; 362: 1090–5. [DOI] [PubMed] [Google Scholar]
- 53.Biscetti F, Gaetani E, Flex A, et al. Peroxisome proliferator-activated receptor alpha is crucial for iloprost-induced in vivo angiogenesis and vascular endothelial growth factor upregulation. J Vasc Res. 2009; 46: 103–8. [DOI] [PubMed] [Google Scholar]
- 54.Moncada S, Gryglewski R, Bunting S, et al. An enzyme isolated from arteries transforms prostaglandin endoperoxides to an unstable substance that inhibits platelet aggregation. Nature. 1976; 263: 663–5. [DOI] [PubMed] [Google Scholar]
- 55.Varol E, Uysal BA, Ozaydin M. Platelet indices in patients with pulmonary arterial hypertension. Clin Appl Thromb Hemost. 2011; 17: E171–4. [DOI] [PubMed] [Google Scholar]
- 56.Nadaud S, Poirier O, Girerd B, et al. Small platelet microparticle levels are increased in pulmonary arterial hypertension. Eur J Clin Invest. 2013; 43: 64–71. [DOI] [PubMed] [Google Scholar]
- 57.Levy M, Maurey C, Celermajer DS, et al. Impaired apoptosis of pulmonary endothelial cells is associated with intimal proliferation and irreversibility of pulmonary hypertension in congenital heart disease. J Am Coll Cardiol. 2007; 49: 803–10. [DOI] [PubMed] [Google Scholar]
- 58.Nana-Sinkam SP, Lee JD, Sotto-Santiago S, et al. Prostacyclin prevents pulmonary endothelial cell apoptosis induced by cigarette smoke. Am J Respir Crit Care Med. 2007; 175: 676–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.