Abstract
Background
The aim of this study was to assess the efficacy and safety of “on-demand” dapoxetine in the treatment of premature ejaculation (PE).
Material/Methods
We performed a meta-analysis of intravaginal ejaculatory latency time (IELT), patient-reported global impression of change (PGIC), perceived control over ejaculation (PCOE), and drug-related adverse effects (AEs). We searched Medline, PubMed, Embase, CNKI, Wanfang, and VIP databases up to May 30, 2018 with the following search terms: “dapoxetine” or “SSRIs” and “premature ejaculation” or “sexual dysfunction”.
Results
Our analysis included 11 RCTs (8521 cases and 4338 controls). We found that IELT, PGIC, and PCOE in PE patients with “on-demand” dapoxetine were significantly higher than in the control group, and we observed higher proportions in 60 mg vs. 30 mg dapoxetine. The AEs were mild and tolerable.
Conclusions
“On-demand” dapoxetine is effective and safe for patients with PE, and a dose of 60 mg may be more effective than 30 mg.
MeSH Keywords: Meta-Analysis, Patients, Premature Ejaculation, Safety
Background
Premature ejaculation (PE) is defined as a “male sexual dysfunction characterized by ejaculation that always or nearly always occurs prior to or within 1 min of vaginal penetration from the time of the first sexual experience (lifelong PE), or a clinically significant reduction in latency time, often to about 3 min or less (acquired PE) [1].” PE is the most common male sexual dysfunction, which affects more than 30% of males currently or in the past [2,3]. Many therapeutic measures are used, but because of the complicated patient states, ineffective treatments, and high relapse rate, it seriously influences the quality of life of patients and their partners and decreases their sexual satisfaction [4].
Recently, selective serotonin reuptake inhibitors (SSRIs) have been the mainstay of pharmacotherapy due to the delay it causes in ejaculation by inhibition of the ejaculatory reflex through inhibitory descending pathways from higher centers [5]. Dapoxetine, an SSRIs is widely used and is the first drug originally approved for the “on-demand” treatment of PE patients [6].
Many studies showed dapoxetine 30 mg or 60 mg significantly increased the intravaginal ejaculatory latency time and improved other aspects of PE patients, and most of them were generally well tolerated [7–9]. However, other studies showed contradictory results [10,11]. In recent years, some studies found that 60 mg dapoxetine was more effective for PE patients [10–12]. Hence, we conducted a study to assess the efficacy and safety of “on-demand” dapoxetine in the treatment of patients with PE, and assessed the differences in efficacy and safety for PE with either 30 mg or 60 mg dapoxetine.
Material and Methods
Study selection
We searched PubMed, Medline, EMBASE, CNKI Science Direct/Elsevier, CNKI, Wangfang, and VIP databases up to May 30, 2018 with the following search terms: “dapoxetine” or “SSRIs” and “premature ejaculation” or “sexual dysfunction”. All relevant references of studies included in our study were searched for other potential studies.
We included all published or unpublished RCTs evaluating dapoxetine interventions for PE. Studies comparing dapoxetine intervention vs. placebo or another drug intervention were eligible for this review.
Our inclusion criteria were: 1) published RCT evaluating the effect of dapoxetine on PE; 2) compared the effect of dapoxetine vs. another drug or placebo on PE; 3) patients with PE older than 18 years; 4) patients were treated with oral dapoxetine “on-demand” (1–3 h before sexual activity).
Exclusion criteria were: 1) not an RCT (e.g., review articles, case reports, animal research); 2) did not compare the effect of dapoxetine on PE; 3) patients with a mean age of less than 18 years.
Data extraction
We recorded the following information: first author name, publication year, study design, mean age, sample size, intervention method, and outcome measures.
All the above procedures were conducted by 2 independent authors, and any disagreements were resolved by discussion.
Statistical analysis
In our study, all statistical analyses were performed using RevMan 5.3 (Cochrane Collaboration, Oxford, UK), and if the P value was less than 0.05, it was considered as statistically significant. The Mantel-Haenszel χ2 test and I2 statistic were used to assess heterogeneity [13]. Based on a fixed-effects model or random-effects model, OR and corresponding 95%CI were used to assess dichotomous variables such as PGIC, PCOE and AEs, while SMD and corresponding 95%CI were used to assess continuous variable IELT.
Results
Study selection process and characteristics of the included studies
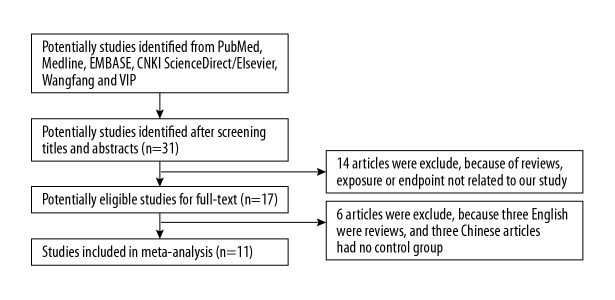
After scanning Medline, PubMed, Embase, CNKI, Wanfang, and VIP databases, 215 potential articles were found, but most of them were excluded because they were reviews, they did not have a control group, or the contents were not relevant to our analysis. Finally, only 11 articles were included from the literature search [7–12,14–18]. Figure 1 presents the process of study selection.
Figure 1.
Flow chart of study selection.
Table 1 presents the main characteristics of the included studies. They were published from 2006 to 2016, and all were RCTS. Of them, 6 reported the difference in efficacy and safety between dapoxetine and placebo in the treatment of patients with PE [7–9,12,14,15], 2 reported the difference between 30 mg dapoxetine and 60 mg dapoxetine [10,16], 2 reported the difference between 30 mg dapoxetine and sertraline [17,18], and 1 reported the difference between dapoxetine and fluoxetine [11]. Of them, 3 were performed in China [11,17,18]. The main outcome measures were intravaginal ejaculatory latency time (IELT), patient-reported global impression of change (PGIC), perceived control over ejaculation (PCOE), and drug-related adverse effects (AEs).
Table 1.
The main characteristics of the 11 included studies.
| Study | Year | Population | Invention | Mean age | Patients(n) | TD (w) | Outcomes measures |
|---|---|---|---|---|---|---|---|
| Pryor et al. | 2006 | American | D 30, 60 mg vs. P | 40.3, 40.9 vs. 40.3 | 878, 870 vs. 870 | 12 | IELT, PGIC, PCOE, AEs |
| Shabsigh et al. | 2008 | American | D 30,60 mg vs. P | NA | 800, 769 vs. 772 | 12 | IELT, PGIC, AEs |
| Kaufman et al. | 2009 | American, Canadian | D 60 mg vs. P | 40.9 vs. 41.8 | 491 vs. 245 | 9 | PGIC, PCOE, AEs |
| Buvat et al. | 2009 | 22 countries | D 30, 60 mg vs. P | 39.6, 40.5 vs. 40.1 | 388, 389 vs. 385 | 24 | IELT, PGIC, PCOE, AEs |
| McMahon et al. | 2010 | Asia-Pacific region | D 30, 60 mg vs. P | 41.2, 41.0 vs. 40.6 | 354, 356 vs. 357 | 12 | IELT, PGIC, PCOE, AEs |
| McMahon et al. | 2011 | 25 countries | D 30, 60 mg vs. P | NA | 1449, 1497 vs. 1468 | 12 | IELT, PCOE |
| Pastore et al. | 2012 | Italian | D 30 vs. 60 mg | 31 vs. 31 | 8 vs. 7 | 12 | IELT |
| Simsek et al. | 2014 | Turk | D 30 vs. 60 mg | 33.5 vs. 32.4 | 50 vs. 50 | 4 | IELT, AEs |
| Yang et al. | 2015 | Chinese | D 30 vs. S | 31.72 vs. 32.24 | 63 vs. 32 | 4 | IELT, PGIC, AEs |
| Shi et al. | 2016 | Chinese | D 30 vs. S | 32.73 vs. 31.54 | 99 vs. 96 | 4 | IELT, AEs |
| Chen et al. | 2016 | Chinese | D 30 vs. F | 33.1 vs. 35.0 | 60 vs. 56 | 2 | IELT, PGIC, AEs |
D – dapoxetine; P – placebo; S – sertraline; F – fluoxetine; NA – no advice; TD – treat duration; IELT – intravaginal ejaculatory latency time; PGIC – patient-reported global impression of change; PCOE – perceived control over ejaculation; AEs – drug-related adverse effects.
Main analysis
The effect on intravaginal ejaculatory latency time (IELT)
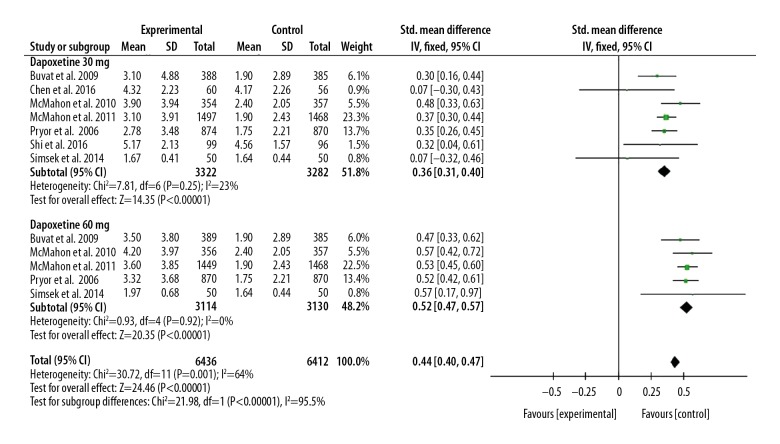
Seven included studies assessed the IELT between the dapoxetine group and control group [7,10–12,14,15,18]. Our study showed that patients with PE had a longer IELT after dapoxetine intervention than with placebo or another drug intervention, and the SMD (95%CI) was 0.44 (0.40, 0.57), P<0.05.
In subgroup analysis, we found the IELT was significantly different in the group treated with 30 mg dapoxetine compared with the placebo or another drug-treated group. That result was also found in the group treated with 60 mg dapoxetine compared with the placebo or another drug-treated group, and the SMD (95%CI) was 0.36 (0.31, 0.40) and 0.52 (0.47, 0.57), P<0.05, respectively.
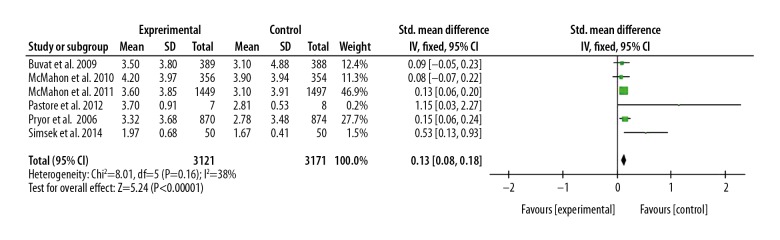
We also compared the IELT between the 30 mg dapoxetine group and 60 mg dapoxetine groups, showing that patients with PE had a longer IELT after 60 mg dapoxetine treatment than with 30 mg dapoxetine intervention, and the SMD (95%CI) was 0.13 (0.08, 0.18), P<0.05. These results are presented in Figures 2 and 3.
Figure 2.
Forest plot of IELT comparing the dapoxetine (30 mg and 60 mg subgroups) and control group.
Figure 3.
Forest plot of IELT comparing the 60 mg and 30 mg dapoxetine groups.
The effect on patient-reported global impression of change (PGIC)
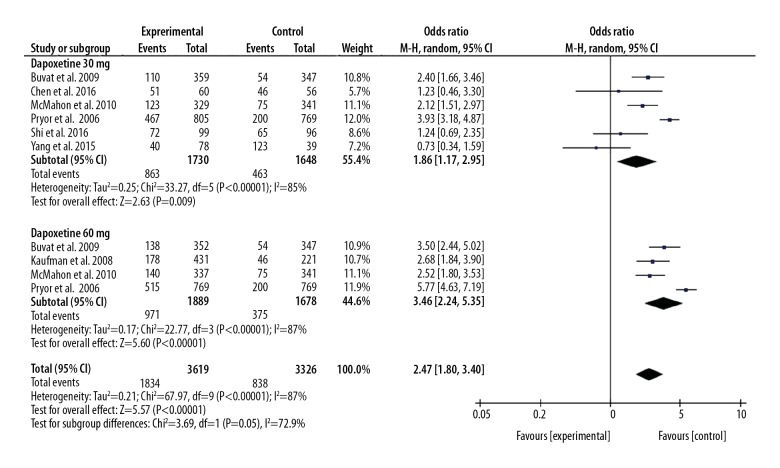
Six included studies compared the PGIC between the dapoxetine 30 mg group and the control group [7,11,14,15,17,18], and 4 included studies reported the PGIC between the dapoxetine 60 mg group and the control group [7,9,14,15]. The pooled result suggested that patients with PE had a higher PGIC rate in the dapoxetine intervention group than in the placebo or another drugs intervention group, and the OR (95%CI) was 2.47(1.80, 3.40), P<0.05.
This result was not changed by subgroup analysis, and we also found a higher PGIC rate in the dapoxetine 30 mg group and 60 mg group than in the control group, and the OR (95%CI) was 1.86 (1.17, 2.95) and 3.46 (2.24, 5.35), P<0.05, respectively.
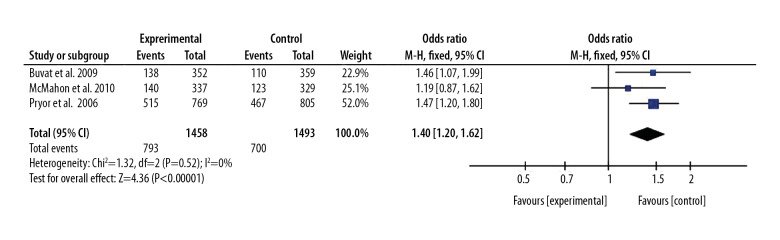
We also compared the PGIC rate between the 30 mg dapoxetine group and 60 mg dapoxetine group, finding that patients with PE had a higher r PGIC rate after 60 mg dapoxetine intervention than after 30 mg dapoxetine intervention, and the OR (95%CI) was 1.40(1.20, 1.62), P<0.05. These results are presented in Figures 4 and 5.
Figure 4.
Forest plot of PGIC comparing the dapoxetine (30 mg and 60 mg subgroups) and control group.
Figure 5.
Forest plot of PGIC comparing the 60 mg and 30 mg dapoxetine groups.
The effect on perceived control over ejaculation (PCOE)
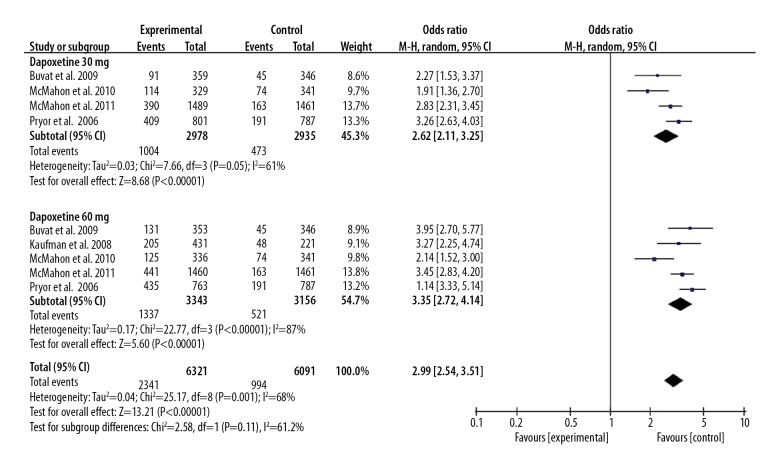
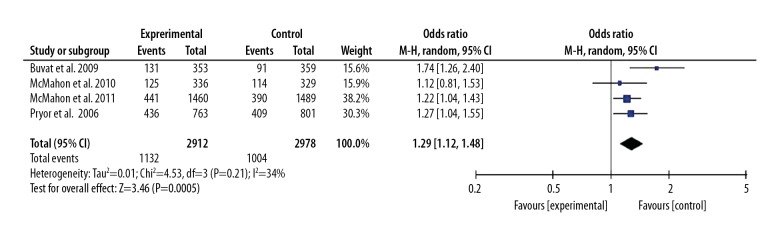
All included studies showed 30 mg or 60 mg dapoxetine intervention contributed to improved PCOE for patients with PE. The pooled OR (95%CI) was 2.99 (2.54, 3.51), P<0.05. We also found that the 60 mg dose was more effective than the 30 mg dose, and the OR (95%CI) was 1.29 (1.12, 1.48), P<0.05. These results are presented in Figures 6 and 7.
Figure 6.
Forest plot of PCOE comparing the dapoxetine (30 mg and 60 mg subgroups) and control group.
Figure 7.
Forest plot of PCOE comparing the 60 mg and 30 mg dapoxetine groups.
Drug-related adverse effects (AEs)
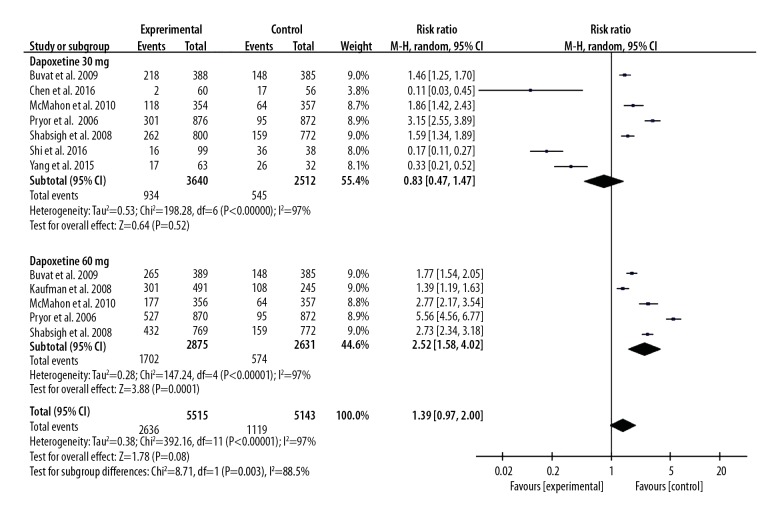
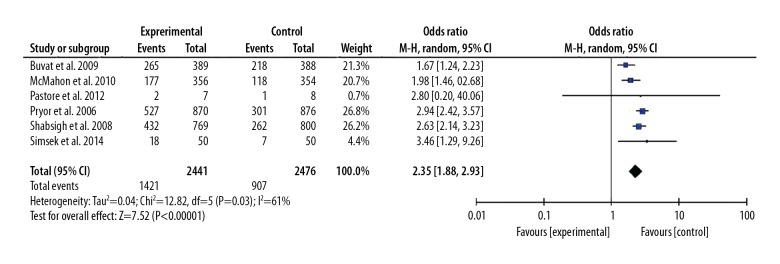
Seven included studies assessed the AEs between the 30 mg dapoxetine group and control group [7,8,11,14,15,17,18]. Five included studies assessed the AEs between the 60 mg dapoxetine group and the control group [7–9,14,15]. However, we did not find a significant difference in the incidence of AEs between the dapoxetine intervention group and the placebo or another drug intervention group, and the pooled OR (95%CI) was 1.39 (0.97, 2.00), P>0.05. However, we found a significant difference in the incidence of AEs between the 60 mg and 30 mg group, and the OR (95%CI) was 2.35 (1.88, 2.93), P<0.05. These results are presented in Figures 8 and 9.
Figure 8.
Forest plot of AEs comparing the dapoxetine (30 mg and 60 mg subgroups) and control group.
Figure 9.
Forest plot of AEs comparing the 60 mg and 30 mg dapoxetine groups.
Discussion
In 2006, dapoxetine compared with placebo was used for treatment of patients with PE, showing that dapoxetine “on-demand” was helpful to improve the ejaculation time and symptoms of PE patients [7]. Since then, many studies have assessed the efficacy and safety of “on-demand” dapoxetine in the treatment of patients with premature ejaculation vs. placebo and other drugs [8–12,14–18]. Most of them suggested the efficacy of “on-demand” dapoxetine was significantly higher than that of placebo or other drugs, but a small number of results were inconsistent. Furthermore, there was no strong statistical evidence of the difference between 60 mg vs. 30 mg dapoxetine for patients with PE. Hence, we conducted a meta-analysis by combining 11 articles to assess the efficacy and safety of “on-demand” dapoxetine in the treatment of patients with PE. We found that IELT, PGIC, and PCOE in PE patients with “on-demand” dapoxetine 30 mg or 60 mg were significantly higher in patients treated with placebo or other drugs, and we observed higher proportions in 60 mg vs. 30 mg of dapoxetine. However, the AEs were mild and tolerable.
The efficacy of IELT is the primary focus for patients. Ejaculation reflex activity is mainly regulated by spinal nerve, motor central nervous system, 5-hydroxytryptamine system, and brain catecholamine system; the latter is the ejaculation activation system and the former is the ejaculation control system [19]. IELT is regulated by the ejaculation control system; hence, if we want to extend IELT, we must regulate the ejaculation control system, that is, adjust the 5-hydroxytryptamine system. Dapoxetine, a selective serotonin reuptake inhibitor (SSRIs), prolongs ejaculation time by highly selective inhibition of serotonin receptors [20]. In our study, we found that the IELT of patients with dapoxetine was longer than in patients treated with placebo or other drugs, and 60 mg dapoxetine was more effective than 30 mg dapoxetine. Hence, these results suggest that dapoxetine helps to prolong IELT.
Scholars consider that PGIC and PCOE are informative study endpoints with respect to men’s perception of minor detectable changes in IELT [7]; hence, PGIC and PCOE are positively associated with the change in IELT. Our results showed that patients with PE had a higher PGIC and PCOE rate in the dapoxetine intervention group than that in the placebo or another drugs intervention group, and we also observed that patients with 60 mg dapoxetine had higher PGIC and PCOE rates than in patients with 30 mg dapoxetine. These results prove that PGIC and PCOE were positively consistent with the change in IELT. This also was consistent with previous studies [21,22].
AEs are important in determining whether a drug can be clinically used. Many studies showed that the incidence of AEs with dapoxetine was higher than with placebo [7,8,12,14], but lower than with other drugs used to treated PE (e.g., sertraline and fluoxetine) [11,17,18]. In our study, we found 52.1% total AEs in dapoxetine (2875/5515), and with placebo or other drugs it was 51.2% (2631/5143); however, the result was not statistically significant. We also found that the incidence of total AEs was higher in patients with 60 mg dapoxetine than in patients with 30 mg dapoxetine. However, the most frequently reported AEs of dapoxetine were headache, nausea, dizziness, insomnia, and diarrhea [23], and AEs of dapoxetine are generally mild or moderate, transient, and tolerable.
There are some limitations in the present study that should be considered. First, although all included studies all had a follow-up, the duration of follow-up varied; importantly, the duration of follow-up of the 4 Chinese studies was shorter, and this may have influenced the findings of therapeutic efficacy. Secondly, the most of the research subjects were Europeans and Americans, and it is unclear if these results apply to other populations. Thirdly, although the present study included more research subjects than any previous single study, the sample size was also small. Fourth, there was strong evidence of heterogeneity in the included studies, primarily because they were performed in different populations, had various durations of follow-up, and used different drug research, which may have been sources of heterogeneity. Hence, these limitations should be taken into consideration in future studies.
Conclusions
In summary, “on-demand” dapoxetine in the treatment of patients with PE had a longer or higher IELT, PGIC, and PCOE, and 60 mg had a better efficacy than 30 mg dapoxetine “on-demand”. Most of the patients had mild and tolerable AEs. Hence, we conclude that “on-demand” dapoxetine (30 mg or 60 mg, particularly the 60 mg) may be considered as an effective and safe drug for treatment of patients with PE.
Footnotes
Source of support: The present study was supported by the National Science Foundation of China (grant No. 81372736)
Competing interests
None.
References
- 1.Althof SE, McMahon CG, Waldinger MD, et al. An update of the International Society of Sexual Medicine’s guidelines for the diagnosis and treatment of premature ejaculation (PE) J Sex Med. 2014;11:1392–422. doi: 10.1111/jsm.12504. [DOI] [PubMed] [Google Scholar]
- 2.Park HJ, Park NC, Kim TN, et al. Discontinuation of dapoxetine treatment in patients with premature ejaculation: A 2-year prospective observational study. Sex Med. 2017;5:e99–105. doi: 10.1016/j.esxm.2017.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Masciovecchio S, Saldutto P, Del Rosso A, et al. The daily therapy with silodosin can have a role in the treatment of ‘life-long’ premature ejaculation. J Androl Sci. 2011;18:48–51. [Google Scholar]
- 4.Rowland D, Perelman M, Althof S, et al. Self-reported premature ejaculation and aspects of sexual functioning and satisfaction. J Sex Med. 2004;1:225–32. doi: 10.1111/j.1743-6109.2004.04033.x. [DOI] [PubMed] [Google Scholar]
- 5.Giuliano F, Clement P. Serotonin and premature ejaculation: From physiology to patient management. Eur Urol. 2006;50:454–66. doi: 10.1016/j.eururo.2006.05.055. [DOI] [PubMed] [Google Scholar]
- 6.De Hong C, Ren LL, Yu H, et al. The role of dapoxetine hydrochloride on-demand for the treatment of men with premature ejaculation. Sci Rep. 2014;4:7269. doi: 10.1038/srep07269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pryor JL, Althof SE, Steidle C, et al. Efficacy and tolerability of dapoxetine in treatment of premature ejaculation: An integrated analysis of two double-blind, randomized controlled trials. Lancet. 2006;368:929–37. doi: 10.1016/S0140-6736(06)69373-2. [DOI] [PubMed] [Google Scholar]
- 8.Shabsigh R, Patrick DL, Rowland DL, et al. Perceived control over ejaculation is central to treatment benefit in men with premature ejaculation: Results from phase III trials with dapoxetine. BJU Int. 2008;102:824–28. doi: 10.1111/j.1464-410X.2008.07845.x. [DOI] [PubMed] [Google Scholar]
- 9.Kaufman JM, Rosen RC, Mudumbi RV, et al. Treatment benefit of dapoxetine for premature ejaculation: Results from a placebo-controlled phase III trial. BJU Int. 2009;103:651–58. doi: 10.1111/j.1464-410X.2008.08165.x. [DOI] [PubMed] [Google Scholar]
- 10.Simsek A, Kirecci SL, Kucuktopcu O, et al. Comparison of paroxetine and dapoxetine, a novel selective serotonin reuptake inhibitor in the treatment of premature ejaculation. Asian J Androl. 2014;16:725–27. doi: 10.4103/1008-682X.128467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen XY, Que YW, Wang SG. Efficacy and safety of dapoxetine in the treatment of premature ejaculation. Natl J Androl. 2016;5:411–14. [PubMed] [Google Scholar]
- 12.McMahon CG, Althof SE, Kaufman JM, et al. Efficacy and safety of dapoxetine for the treatment of premature ejaculation: Integrated analysis of results from five phase 3 trials. J Sex Med. 2011;8:524–39. doi: 10.1111/j.1743-6109.2010.02097.x. [DOI] [PubMed] [Google Scholar]
- 13.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–58. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 14.Buvat J, Tesfaye F, Rothman M, et al. Dapoxetine for the treatment of prematureejaculation: Results from a randomized, double-blind, placebo-controlled phase 3 trialin 22 countries. Eur Urol. 2009;55:957–67. doi: 10.1016/j.eururo.2009.01.025. [DOI] [PubMed] [Google Scholar]
- 15.McMahon C, Kim SW, Park NC, et al. Treatment of premature ejaculation in the Asia-Pacific region: Results from a phase III double-blind, parallel-group study of dapoxetine. J Sex Med. 2010;7:256–68. doi: 10.1111/j.1743-6109.2009.01560.x. [DOI] [PubMed] [Google Scholar]
- 16.Pastore AL, Palleschi G, Leto A, et al. A prospective randomized study to compare pelvic floor rehabilitation and dapoxetine for treatment of lifelong premature ejaculation. Int J Androl. 2012;35:528–33. doi: 10.1111/j.1365-2605.2011.01243.x. [DOI] [PubMed] [Google Scholar]
- 17.Yang L, Luo L, Chen XH, et al. [Efficacy and tolerability of dapoxetine in the treatment of premature ejaculation]. Zhonghua Nan Ke Xue. 2015;21:892–95. [in Chinese] [PubMed] [Google Scholar]
- 18.Shi CL, Wang X, Tu MQ, et al. [Efficacy and tolerability of dapoxetine in the treatment of male premature ejaculation and female sexual dissatisfaction]. Zhonghua Nan Ke Xue. 2016;11:41–44. [in Chinese] [Google Scholar]
- 19.La Pera G. Awareness of the role of the pelvic floor muscles in controlling the ejaculatory reflex: Preliminary results. Arch Ital Urol Androl. 2012;84:74–78. [PubMed] [Google Scholar]
- 20.Akhvlediani ND, Matyukhov IP. The evidence for the efficiency and safety of dapoxetine in treating premature ejaculation. Urologiia. 2017;5:106–10. doi: 10.18565/urology.2017.5.106-110. [DOI] [PubMed] [Google Scholar]
- 21.Mirone V, Arcaniolo D, Rivas D, et al. Results from a prospective observational study of men with premature ejaculation treated with dapoxetine or alternative care: The pause study. Euro Urol. 2014;65:733–39. doi: 10.1016/j.eururo.2013.08.018. [DOI] [PubMed] [Google Scholar]
- 22.Safarinejad MR. Comparison of dapoxetine versus paroxetine in patients with premature ejaculation: A double-blind, placebo-controlled, fixed-dose, randomized study. Clin Neuropharmacol. 2006;29:243–52. doi: 10.1097/01.WNF.0000228210.12194.46. [DOI] [PubMed] [Google Scholar]
- 23.Mohee A, Eardley I. Medical therapy for premature ejaculation. Ther Adv Urol. 2011;3:211–22. doi: 10.1177/1756287211424172. [DOI] [PMC free article] [PubMed] [Google Scholar]