Abstract
Objective:
To develop and test an automated surveillance algorithm (sepsis “sniffer”) for the detection of severe sepsis and monitoring failure to recognize and treat severe sepsis in a timely manner.
Patients and Methods:
We conducted an observational diagnostic performance study using independent derivation and validation cohorts from an electronic medical record database of the medical intensive care unit (ICU) of a tertiary referral center. All patients aged 18 years and older who were admitted to the medical ICU from January 1 through March 31, 2013 (N=587), were included. The criterion standard for severe sepsis/septic shock was manual review by 2 trained reviewers with a third superreviewer for cases of interobserver disagreement. Critical appraisal of false-positive and false-negative alerts, along with recursive data partitioning, was performed for algorithm optimization.
Results:
An algorithm based on criteria for suspicion of infection, systemic inflammatory response syndrome, organ hypoperfusion and dysfunction, and shock had a sensitivity of 80% and a specificity of 96% when applied to the validation cohort. In order, low systolic blood pressure, systemic inflammatory response syndrome positivity, and suspicion of infection were determined through recursive data partitioning to be of greatest predictive value. Lastly, 117 alert-positive patients (68% of the 171 patients with severe sepsis) had a delay in recognition and treatment, defined as no lactate and central venous pressure measurement within 2 hours of the alert.
Conclusion:
The optimized sniffer accurately identified patients with severe sepsis that bedside clinicians failed to recognize and treat in a timely manner.
Sepsis is common and lethal in the United States and around the world.1–3 Septicemia was also ranked as the most expensive in-hospital condition in the United States by the US Agency for Healthcare Quality and Research, based on 2011 data.4 Current processes for sepsis management (including early goal-directed therapy [EGDT] and the data from the recent ProCESS [Protocolized Care for Early Septic Shock] and ARISE [Australasian Resuscitation in Sepsis Evaluation] trials) have been established.5–7 The Surviving Sepsis Campaign (SSC) guidelines have refined the exact criteria for advanced disease, including organ dysfunction.8 However, the fundamental process of sepsis management in these guidelines has not changed substantially, suggesting a barrier in implementation as the source of the continued sepsis problem. There is much room for improvement and optimization of existing computerized sepsis detection and alert systems. Although recent sepsis detection and alert systems have focused on clinical outcomes, these systems have failed to document improvement in clinically meaningful end points.9–12 Thus, an improved approach is necessary to develop and validate a clinically useful sepsis alert system, especially for implementation in the critical care setting.
The aim of this study was to improve on previous studies in several ways. The first was by specifically targeting severe sepsis/septic shock (referred to as severe sepsis throughout the remainder of this article for brevity) to reduce the number of false-positive alerts from isolated or nonseptic systemic inflammatory response syndrome (SIRS).13 The second was to target severe sepsis in the specific context of delay in recognition and treatment. This approach is derived from the concept of “failure to rescue” from the surgical literature, which suggests that hospital characteristics, as opposed to patient characteristics, are the primary determinant of adverse occurrences.14,15 In this context, one example of delay in recognition and treatment would be progression to severe sepsis due to failure to adhere to established sepsis response and management protocols.16 The third and final improvement was to target information overload, human error, interruption, and alert fatigue.17,18 Combined, the objective of this study was to advance, test, and refine a delay in recognition and treatment of severe sepsis detection and alert system (“sniffer”) for use in the critical care setting.
PATIENTS AND METHODS
Study Design and Setting
We conducted an observational diagnostic performance study that used independent derivation and validation cohorts for development and testing of the delay in recognition and treatment of severe sepsis sniffer. This study was performed at Mayo Clinic in Rochester, Minnesota, with Mayo Clinic Institutional Review Board approval.
Study Population and Data Collection
All patients aged 18 years and older who were admitted to the medical intensive care unit (ICU) at Mayo Clinic in Rochester, Minnesota, from January 1 through March 31, 2013, and provided research authorization were included in this study. This ICU setting has been described previously.19 The purpose of this retrospective study was development of the sepsis sniffer algorithm. Thus, no patients admitted to the ICU with research consent were excluded from this study, including those patients with goal-limiting care preferences, such as do-not-resuscitate/do-not-intubate (DNR/DNI) orders. Patients with ICU-acquired sepsis, which typically occurs several days after ICU admission, were effectively excluded from this study.20,21 It is unlikely that patient/proxy preferences, such as DNR/DNI status, would dramatically alter provisions of care, such as those related to transfer from the emergency department (ED) and/or hospital wards, in a way that would substantially confound the results of this study. At our institution, unless otherwise stated, patients with DNR/DNI orders receive central line placement when clinically indicated.
Patient data were collected using manual chart review and the METRIC (Multidisciplinary Epidemiology and Translational Research in Intensive Care) Data Mart, which has been described previously.22 The data for the output response of severe sepsis was collected through manual review and scoring of all patient records by 2 trained reviewers (A.M.H., C.T.). Interobserver variability was solved by a third superreviewer (R.K.). This data set served as the criterion standard for the cohort. The data set for the full cohort (587 patients) was then randomly divided in half into derivation (293 patients) and validation (294 patients) cohorts. The derivation cohort was used for algorithm development and testing, while the validation cohort was reserved for final algorithm validation.
Algorithm Development
Sepsis Detection Component.
For both manual review and scoring of patient records, as well as the first iteration of the severe sepsis sniffer (Algorithm 1), a standardized protocol for severe sepsis was used (Table 1). For the severe sepsis portion of this algorithm, this definition was divided into 3 components: suspicion of infection, SIRS, and organ hypoperfusion and dysfunction. A positive entry for all 3 of these components within a 6-hour window between ICU admission and ICU discharge (up to 72 hours) was required for classification as severe sepsis positive. Because of the high frequency of microbial culture orders before ICU admission, particularly in patients admitted from the ED, the suspicion of infection domain was permitted to include 72 hours before ICU admission.
TABLE 1.
| Physiologic concept | EMR representation | Rule | Additional critieria |
|---|---|---|---|
| Suspicion of infection | Any culture order | Blood or lavage, stool or urine, or fluid or sputum | ≥ 1 Event, including previous 72 h |
| Systemic inflammatory response | WBCs | <4.0 or >12.0 × 109/L | ≥ 1 (WBCs or body temperature) event and ≥ 1 additional event from any of 3 remaining categories, within a 6 h window |
| Body temperature | >38.0°C or <36.0°C | ||
| Respiratory rate | >20 breaths/min | ||
| Heart rate | >90 beats/min | ||
| Organ hypoperfusion and dysfunction | Lactate | ≥4.0 mmol/L | ≥l Event |
| SBP | <90 mm Hg | ≥l Event | |
| Shock | Vasopressors | Norepinephrine, epinephrine, dopamine, vasopressin, or phenylephrine | ≥l Event |
| Fluid resistant hypotension | SBP <90 mm Hg despite ≥30 mL/kg crystalloid and/or 18.75 mL/kg colloid fluid bolus | Within a 3 h window | |
| Delay in recognition and treatment | Lactate | >0 Measurements | Within 2 h of severe sepsis alert |
| Central venous pressure | >0 Measurements |
EMR = electronic medical record; SBP = systolic blood pressure; WBCs = white blood cells.
The physiologic concepts represent the progression from sepsis to severe sepsis to septic shock. Within each physiologic concept is at least one EMR-based element (EMR representation), as well as each element’s associated rule and additional criteria for use in the initial sepsis sniffer algorithm.
Delay in Recognition and Treatment Detection Component.
The 2012 international guidelines for management of severe sepsis and septic shock from the SSC were used as the basis for development of the delay in recognition and treatment portion of the severe sepsis sniffer.8 Specifically, the protocol portion of these guidelines emphasize the timely need for lactate measurement, appropriate antibiotic administration, adequate fluid resuscitation, and placement of a central line for measurement of central venous pressure (CVP) and central venous oxygenation. The presence or absence of central line placement (peripherally inserted central catheter or central venous catheter) was not recorded. In cases in which CVP measurement was present at the time of ICU admission, this implies central line placement and/or CVP measurement before ICU admission but was not recorded for this ICU-specific study.
To develop a sniffer to minimize inappropriate alerts, a new data set (N=171) was created by pooling all severe sepsis alerts from the optimized algorithm using both the derivation and validation data sets (N=587). Of the 171 patients who had development of severe sepsis, 123 (72%) had no computerized physician order entry (CPOE) for severe sepsis management within the time window from ICU admission to severe sepsis alert plus 2 hours (Table 2). For reference, a CPOE for severe sepsis management in the ED and ICU was implemented at Mayo Clinic in 2005 and has remained the standard of care since.23
TABLE 2.
| Variable | Cohort | ||
|---|---|---|---|
| Derivation | Validation | Total | |
| Patients with severe sepsis | 85/293 (29) | 86/294 (29) | 171/587 (29) |
| No severe sepsis CPOE | 54/85 (64) | 69/86 (80) | 123/171 (72) |
| No lactate measurement | 23/85 (27) | 19/86 (22) | 42/171 (25) |
| No CVP measurement | 51/85 (60) | 61/86 (71) | 1 12/171 (65) |
| Delay (no lactate + CVP) | 55/85 (65) | 62/86 (72) | 117/171 (68) |
| Delay + CPOE (overlap) | 46/85 (54) | 56/86 (65) | 102/171 (60) |
CPOE = computerized physician order entry; CVP = central venous pressure.
Data are presented as No. (percentage) of patients.
Algorithm Testing
Manual Chart Review.
Critical appraisal of false-positive and false-negative alerts for both the sepsis detection and delay in recognition and treatment components of the sniffer began with comparison of the criterion standard against the first iteration of the improved severe sepsis sniffer (Figure 1). Critical appraisal of the first iteration of the sniffer (Algorithm 1 in the “Results” section) was performed by one reviewer (A.M.H.) manually examining all cases of disagreement between the sniffer and criterion standard for cases in which there was no interobserver disagreement. In all cases, the sources of disagreement were documented and used for generation of the second iteration of the sniffer (Algorithm 2 in the “Results” section). Critical appraisal of the second iteration of the sniffer was performed in an identical manner (Supplemental Figure, available online at http://www.mayoclinicproceedings.org), except for the inclusion of cases of disagreement between the sniffer and criterion standard in which there was interobserver disagreement (need for superreviewer and thus more complex). In all cases, the sources of disagreement were documented and used for generation of later iterations of the sniffer.
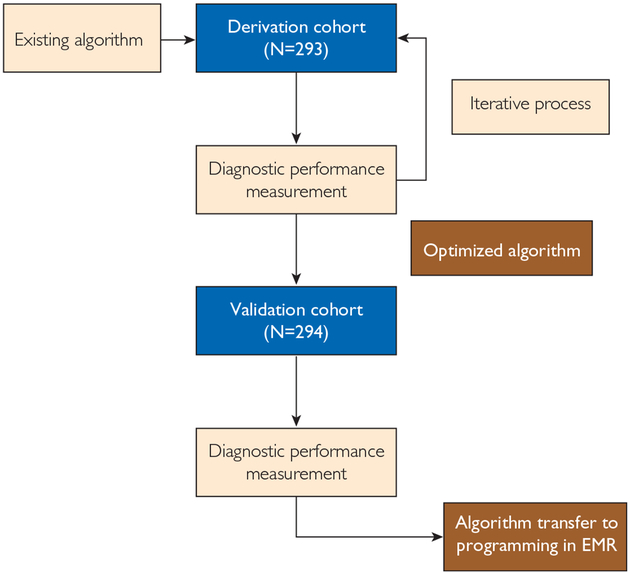
FIGURE 1.
Schematic of the derivation and validation process. EMR = electronic medical record.
Recursive Data Partitioning.
Optimization of both the sepsis detection and delay in recognition and treatment components of the sepsis sniffer algorithm was performed by recursive data partitioning using JMP statistical software (SAS Institute Inc). The recursive data partitioning feature is an advanced modeling and multivariate method that has been described in detail elsewhere.24 Recursive data partitioning was performed using all continuous and categorical variables in the existing sepsis sniffer algorithm as input factors.25 For the purpose of node splitting criterion, all additional clinically relevant ICU data, available through METRIC Data Mart, were included as input factors.22 Output response was the development of severe sepsis during the ICU stay.
Algorithm Refinement—Iterative Process
This recursive data partitioning process resulted in the generation of an optimized decision tree for the detection of severe sepsis and delay in recognition and treatment. On the basis of this result, a new sepsis sniffer was generated. Using this optimized algorithm, diagnostic performance measurements were recalculated and compared with those of the existing sepsis sniffer. In parallel, critical appraisal of false-negative and false-positive alerts was performed by manual review to determine the source of error. Both of these processes were repeated in iteration until an improved algorithm was generated. To optimize the original algorithm, these calculations were repeated following perturbation of the existing rules, as described previously. Next, this process was repeated in iteration until sufficient optimization was achieved. To validate the optimized sepsis sniffer algorithm, this process was repeated using the validation cohort. The results of these measurements were used to determine if the improved sepsis sniffer algorithm was superior to the original algorithm.
Outcome Measurement
The ability of the existing or improved severe sepsis algorithm to detect severe sepsis was evaluated using derivation and validation cohorts, which served as unique data sets. Diagnostic performance measurements—including the sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of each algorithm to detect sepsis—were then compared with the criterion standard of manual chart review. To derive an optimized sepsis sniffer algorithm, the sensitivity, specificity, PPV, and NPV of the original sepsis sniffer algorithm to detect sepsis were calculated using the validation cohort.
Statistical Analyses
In addition to recursive data partitioning, JMP statistical software (JMP Pro version 11.1.1, SAS Institute Inc) was used for all statistical analyses. For all analyses, 2-sided significance testing was performed with P<.05 considered statistically significant. Analyses performed included the Student t test, the χ2 test, and Cohen k coefficient, as appropriate.
RESULTS
For all study results, there was no statistically significant difference between the deviation and validation cohorts with respect to age (P=.79), sex (P=.54), hospital length of stay (LOS) (P=.44), ICU LOS (P=.70), Sequential Organ Failure Assessment score (P=.72), and Acute Physiology and Chronic Health Evaluation III score (P=.23) (Supplemental Table 1, available online at http://www.mayoclinicproceedings. org). The k value for interrater agreement for severe sepsis was 0.74 (substantial agreement, 0.61–0.80).26,27 However, agreement between the criterion standard and the first iteration of the sepsis sniffer (Algorithm 1) was not as good. In particular, the sensitivity and NPV of Algorithm 1 were relatively low (Table 3). As a result, critical appraisal of false-positive and false-negative alerts was performed on the derivation cohort (N=293) for cases in which there was no interobserver disagreement (N=26). These results were used to perform optimization of the initial severe sepsis sniffer algorithm. At this point, critical appraisal for cases in which there was interobserver disagreement (need for superreviewer and thus more complex) was not performed (N=18). For all other cases (N=249), there was agreement between the criterion standard and Algorithm 1.
TABLE 3.
All Iterations Performed for Sepsis Sniffer Optimization
| Algorithm | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) |
|---|---|---|---|---|
| Algorithm 1 (initial) | 59 | 97 | 92 | 83 |
| Algorithm 2 (debugging) | 82 | 97 | 93 | 92 |
| Algorithm 3 (MAP) | 82 | 95 | 90 | 92 |
| Algorithm 4 (bilirubin) | 82 | 96 | 92 | 92 |
| Algorithm 5 (platelets) | 82 | 95 | 89 | 92 |
| Algorithm 6 (INR) | 82 | 94 | 87 | 92 |
| Algorithm 7 (mechanical ventilation) | 82 | 93 | 86 | 92 |
| Algorithm 8 (creatinine)) | 82 | 97 | 93 | 92 |
| Algorithm 9 (PaO2/FlO2) | 82 | 91 | 81 | 92 |
| Algorithm 10 (urine output) | 82 | 89 | 79 | 91 |
| Algorithm 11 (GCS score) | 82 | 92 | 83 | 91 |
| Validation (using Algorithm 2) | 80 | 96 | 92 | 91 |
GCS = Glasgow Coma Scale; INR = international normalized ratio; MAP = mean arterial pressure; NPV = negative predictive value; PPV = positive predictive value.
After optimization and debugging, the sensitivity and NPV of the second iteration of the sepsis sniffer (Algorithm 2) was considerably improved compared with Algorithm 1 (Table 3). The number of cases of disagreement between the criterion standard and Algorithm 2, for which there was no interobserver disagreement, was reduced from 26 to 9 (Supplemental Figure). The number of cases of disagreement between the criterion standard and Algorithm 2, for which there was interobserver disagreement, was also reduced from 18 to 14. Although fundamentally more complex (because of the need for the superreviewer), critical appraisal of all of these cases was performed. Most of these cases were likely either severe sepsis of short duration or failure of Algorithm 2 to detect suspicion of infection and/or identify positive SIRS criteria. However, the number of cases of agreement between the criterion standard and Algorithm 2, as compared with Algorithm 1, was increased from 249 to 270. Thus, this optimized and debugged sniffer was used to begin introducing and testing new variables as markers of organ dysfunction in subsequent sniffer iterations.
To test whether additional markers of organ dysfunction beyond high lactate level and low systolic blood pressure have the potential to be useful in detecting severe sepsis with the sepsis sniffer, 9 additional variables (Supplemental Table 2, available online at http://www.mayoclinicproceedings.org) were identified on the basis of physiologic rationale and existing guidelines.8 To test this hypothesis, each variable was introduced individually into the organ hypoperfusion and dysfunction domain of the second iteration of the sepsis sniffer (Algorithm 2) with the derivation data set. As with high lactate level and low systolic blood pressure, only 1 of 3 possible positive alerts in this category was necessary to trigger a positive alert in this domain. However, introduction of these variables into Algorithm 2 (debugged sepsis sniffer) did not increase the sensitivity, specificity, PPV, or NPV of the sniffer (Algorithms 3 through 11, Table 3). Thus, the approach of recursive data partitioning was also used for sepsis sniffer optimization.
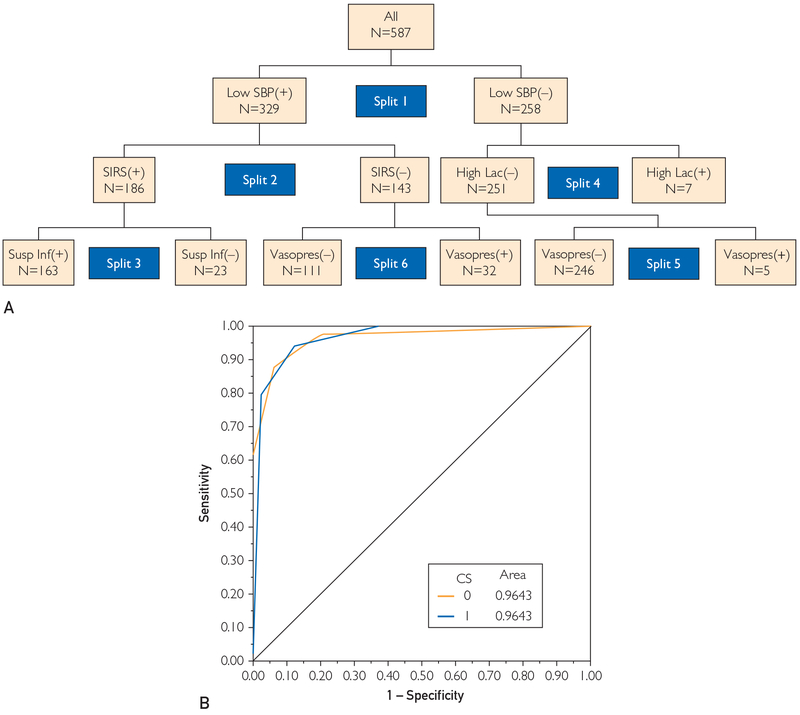
For recursive data partitioning, all initial binary (positive or negative) variable results from Algorithm 2 and all 9 additional binary variables (Supplemental Table 2) were included in the analysis with the derivation data set. The binary criterion standard of severe sepsis or no severe sepsis was used as the output for this analysis. Node splitting was performed in an unbiased fashion, with all splits determined by the statistical software. Maximization of the receiver operating characteristic curve value was used as the stopping criterion for node splitting. In this case, 6 node splits were required to maximize the receiver operating characteristic curve value (0.95). In order, these 6 splits were systolic blood pressure, SIRS criteria, suspicion of infection, high lactate level, and vasopressor use (twice) (Figure 2). On the basis of these results, Algorithm 2 was determined to be the best severe sepsis detection algorithm. To confirm this result, Algorithm 2 was applied to the validation cohort (N=294) and found to be in good agreement with the derivation cohort results (Table 3). This knowledge was then used to develop the delay in recognition and treatment portion of the sniffer.
FIGURE 2.
Recursive data partitioning. A, Results represented as a decision tree with the order of the 6 splits indicated. B, Receiver operating characteristic curve result using this optimized decision tree. CS = criterion standard; High Lac = high lactate level; SBP = systolic blood pressure; SIRS = systemic inflammatory response syndrome; Susp Inf = suspicion of infection; Vasopres = vasopressor use; (+) = present; (−) = absent.
When the delay in recognition and treatment component of the algorithm was applied, 42 of the 171 patients with severe sepsis (25%) had no lactate measurement from ICU admission to alert time plus 2 hours. Impressively, 112 of the 171 patients (65%) had no CVP measurement within this same time window. Combined, 117 patients (68%) had neither lactate level nor CVP measurement. Of the 171 patients with severe sepsis, 60% had both delay and no CPOE. Combined, 117 patients (68%) had neither lactate level or CVP measurement (delay in recognition and treatment). Thus, it was determined that the absence of both a lactate and CVP measurement within 2 hours of severe sepsis alert is sufficient criteria to serve as an alert trigger for delay in recognition and treatment (Table 1).
DISCUSSION
Iterative optimization of an improved severe sepsis algorithm has led from a sensitivity and specificity of 59% and 97% (derivation cohort, Algorithm 1) to 80% and 96% (validation cohort, Algorithm 2), respectively, based on criteria of suspicion of infection, SIRS, organ hypoperfusion and dysfunction, and shock when applied to a validation cohort of medical ICU patients. In those patients with severe sepsis, the optimized delay in recognition and treatment component of the sniffer identified that 68% of patients did not have a lactate level and CVP measurement in a timely manner. In order, low systolic blood pressure, SIRS positivity, and suspicion of infection were determined through recursive data partitioning to be of greatest predictive value.
There have been several prospective studies of various forms of sepsis detection and alert systems in recent years. In 2011, Sawyer et al9 reported that the implementation of a real-time computerized sepsis alert in non-ICU patients was able to increase early therapeutic and diagnostic interventions among non-ICU patients at risk for sepsis. This study was a prospective, observational pilot study at a single academic center. Also in 2011, Nelson et al10 reported that an automated algorithm for detecting potential sepsis increased the frequency and timeliness of some ED intervetions for severe sepsis. This prospective, before-and-after study was also performed at a single academic center. In 2012, LaRosa et al11 reported that a combined screening tool and alert system could improve compliance with sepsis bundle elements and improve survival from severe sepsis. This prospective study was also performed at a single academic center but in the ICU environment. Also in 2012, Hooper et al12 performed a randomized trial of an automated modified SIRS monitoring system to facilitate early detection of sepsis in the ICU setting. Once again, this was a single academic center study, which failed to document improvement in outcomes such as time to first new antibiotic, time to adequate fluid resuscitation, ICU LOS, hospital LOS, and mortality. However, it is known that SSC guideline compliance is poor, even after implementation with educational interventions.28 These pioneering studies have greatly advanced the state of automated sepsis detection and alert. However, there is still an important need for an optimized system.
As knowledge of sepsis management—as well as information overload, human error, interruption, and alert fatigue—improves, so will the accuracy of the sepsis sniffer. The delay in recognition and treatment component of the sniffer is one example. In one study of the SIRS criteria (only one component of this sepsis sniffer), the sensitivity of the SIRS criteria to detect infection compared with that of both the clinical and microbiological criterion standards of infection detection was 69%, while the specificity compared with that of both criterion standards was 35% and 32%, respectively.29 Likewise, in perhaps the best prospective study of sepsis detection and alert in the ICU setting, the PPV of the automated system in the randomized trial by Hooper et al12 was 41%. If the accuracy of the sepsis sniffer were improved in a prospective study, more widespread implementation could be performed through existing electronic medical record (EMR) systems to test for improvement in hard outcomes, such as improved ICU LOS and decreased mortality. Despite these challenges, there has been a decrease in mortality in patients with severe sepsis enrolled in “usual care” arms of multicenter randomized trials for the past 15 years.30,31 However, there is considerable variability among reports of the degree of decrease, which depends largely on the methodology used in individual studies.32 Importantly, to further reduce mortality it will be necessary to combine novel pathobiological findings with increasingly sophisticated technology for a true clinical revolution in sepsis management.33
Recently, results of the ProCESS6 and ARISE7 trials were published. Both of these US and Australia/New Zealand multicenter trials, respectively, call into question the need for elements of EGDT and the SSC guidelines/bundles, such as CVP measurement. Because the current EGDT-based SSC guidelines and bundles have existed as the standard of care for septic shock for over a decade, it is too soon to know precisely how the results of these trials will alter future standards of care. For example, a recent multicenter crosssectional study found that 21.2% of clinicians were unaware of the presence or absence of central venous access (peripherally inserted central catheter or central venous catheter) in their patients.34 Smaller studies also continue to document a correlation between CVP measurement and potential reduction in mortality.35 Thus, the true value of central venous access and/or CVP measurement as a risk stratification tool in patients with sepsis remains to be determined. This is particularly the case in the setting of clinical decision making in the context of vasopressors and abnormal lactate values, which are other components of the sepsis surveillance algorithm used in this study.
There are several limitations to this study. Like all the other studies referenced in this article, our study was conducted at a single academic medical center, which limits the potential transferability of these results to other medical centers. The single-center study design can also introduce bias into the study outcomes. In addition, this was an observational diagnostic performance study. Although this design was also used by several cited studies (and independent derivation and validation cohorts were used in this study), the observational study design has the potential to introduce more error in clinical data availability compared with the prospective study design. Our study was conducted using only adult patients in the medical ICU of an academic medical center, and thus the delay in recognition and treatment of sepsis sniffer may not be generalizable to all septic patients, especially those outside the ICU setting such as hospital wards and EDs. Although current sepsis guidelines and clinical knowledge were used to design the sniffer, it is still possible there is room for additional improvement. For example, some of the clinical variables found to not improve the sniffer may in fact be valuable for severe sepsis diagnosis in the clinical setting. Alternatively, the inclusion of other variables not analyzed in this study because of electronic infrastructure limitations, such as timely and appropriate antibiotic administration, must be examined. Thus, a prospective (and eventual multicenter) study is necessary to further validate the sniffer by examining hard clinical end points such as ICU LOS, hospital LOS, mortality, and longterm outcomes. The process described in defining delay in recognition and treatment has not been widely reported outside the surgical and nursing literature, where it is termed failure to rescue. Thus, the novel application of this concept to sepsis and the critical care setting requires further exploration by other research groups. More complex methods of recursive data portioning, such as continuous variable analysis and random forests, have the potential to improve algorithm accuracy but were not employed in our study because the relatively small size of our data set limits the interpretability of such approaches.
CONCLUSION
A severe sepsis sniffer was able to correctly identify delay in recognition and treatment, which is necessary for implementation into existing EMR systems. Likewise, an algorithm for delay in recognition and treatment of severe sepsis was successfully developed. This component of the sepsis sniffer is important to decrease information overload, human error, interruption, and alert fatigue in intelligent EMR alerting systems. Combined, such a sniffer has the potential for implementation in the ICU setting.
Supplementary Material
ACKNOWLEDGMENTS
The authors gratefully acknowledge the entire METRIC research group for their support, which made this project possible. We also thank Mayo Clinic’s Center for Translational Science Activities for their support. The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
Grant Support: This work was supported by grant 1C1CMS330964-01-00 from the Center for Medicare and Medicaid Innovation (Health Care Innovation Award “Patient Centered Cloud-based Electronic System: Ambient Warning and Response Evaluation [ProCCESs AWARE])”, grant UL1 TR 000135 from the National Center for Advancing Translational Sciences to the Mayo Clinic Center for Translational Science Activities, and doctoral dissertation grant 1 R36 HS 022799–01 from the Agency for Healthcare Research and Quality (A.M.H.).
Abbreviations and Acronyms:
- CPOE
computerized physician order entry
- CVP
central venous pressure
- DNR/DNI
do-not-resuscitate/do-not-intubate
- ED
emergency department
- EGDT
early goal-directed therapy
- EMR
electronic medical record
- ICU
intensive care unit
- LOS
length of stay
- NPV
negative predictive value
- PPV
positive predictive value
- SIRS
systemic inflammatory response syndrome
- SSC
Surviving Sepsis Campaign
Footnotes
Potential Competing Interests: AWARE is patent pending (US 2010/0198622, 12/697861, PCT/US2010/022750). Sepsis sniffer is patent number 20110137852. Drs Herasevich, Gajic, and Pickering and Mayo Clinic have a financial interest relating to licensed technology described in this article. This research has been reviewed by the Mayo Clinic Conflict of Interest Review Board and is being conducted in compliance with Mayo Clinic Conflict of Interest Policies.
SUPPLEMENTAL ONLINE MATERIAL
Supplemental material can be found online at http://www.mayoclinicproceedings.org.
REFERENCES
- 1.Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N EnglJ Med. 2003;348(16):1546–1554. [DOI] [PubMed] [Google Scholar]
- 2.Poeze M, Ramsay G, Gerlach H, Rubulotta F, Levy M. An international sepsis survey: a study of doctors’ knowledge and perception about sepsis. Crit Care. 2004;8(6): R409–R413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miller RR III, Dong L, Nelson NC, et al. ; Intermountain Healthcare Intensive Medicine Clinical Program. Multicenter implementation of a severe sepsis and septic shock treatment bundle. Am J Respir Crit Care Med. 2013;188(1):77–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Torio CM, Andrews RM. National Inpatient Hospital Costs: The Most Expensive Conditions by Payer, 2011. Rockville, MD: Agency for Healthcare Research and Quality; 2013: HCUP Statistical Brief 160. [PubMed] [Google Scholar]
- 5.Rivers E, Nguyen B, Havstad S, et al. ; Early Goal-Directed Therapy Collaborative Group. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345(19):1368–1377. [DOI] [PubMed] [Google Scholar]
- 6.ProCESS Investigators. A randomized trial of protocol-based care for early septic shock. N Engl J Med. 2014;370 (18): 1683–1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.ARISE Investigators ANZICS Clinical Trials Group. Goal-directed resuscitation for patients with early septic shock. N Engl J Med. 2014;371(16):1496–1506. [DOI] [PubMed] [Google Scholar]
- 8.Dellinger RP, Levy MM, Rhodes A, et al. ; Surviving Sepsis Campaign Guidelines Committee includingthe Pediatric Subgroup. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med. 2013;41: 580–637. [DOI] [PubMed] [Google Scholar]
- 9.Sawyer AM, Deal EN, Labelle AJ, et al. Implementation of a real-time computerized sepsis alert in nonintensive care unit patients. Crit Care Med. 2011;39(3):469–473. [DOI] [PubMed] [Google Scholar]
- 10.Nelson JL, Smith BL, Jared JD, Younger JG. Prospective trial of real-time electronic surveillance to expedite early care of severe sepsis. Ann Emerg Med. 2011;57(5):500–504. [DOI] [PubMed] [Google Scholar]
- 11.LaRosa JA, Ahmad N, Feinberg M, Shah M, Dibrienza R, Studer S. The use of an early alert system to improve compliance with sepsis bundles and to assess impact on mortality. Crit Care Res Pract. 2012; 2012:980369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hooper MH, Weavind L, Wheeler AP, et al. Randomized trial of automated, electronic monitoring to facilitate early detection of sepsis in the intensive care unit. Crit Care Med. 2012;40(7): 2096–2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klein Klouwenberg PM, Ong DS, Bonten MJ, CremerOL. Classification of sepsis, severe sepsis and septic shock: the impact of minor variations in data capture and definition of SIRS criteria. Intensive Care Med. 2012;38(5):811–819. [DOI] [PubMed] [Google Scholar]
- 14.Silber JH, Williams SV, Krakauer H, Schwartz JS. Hospital and patient characteristics associated with death after surgery: a study of adverse occurrence and failure to rescue. Med Care. 1992;30(7):615–629. [DOI] [PubMed] [Google Scholar]
- 15.Silber JH, Rosenbaum PR, Schwartz JS, Ross RN, Williams SV. Evaluation of the complication rate as a measure of quality of care in coronary artery bypass graft surgery. JAMA. 1995. 274(4):317–323. [PubMed] [Google Scholar]
- 16.Levy MM, Dellinger RP, Townsend SR, et al. ; Surviving Sepsis Campaign. The Surviving Sepsis Campaign: results of an international guideline-based performance improvement program targeting severe sepsis. Crit Care Med. 2010;38(2):367v374. [DOI] [PubMed] [Google Scholar]
- 17.Donchin Y, Gopher D, Olin M, et al. A look into the nature and causes of human errors in the intensive care unit. Crit Care Med. 1995;23(2):294–300. [DOI] [PubMed] [Google Scholar]
- 18.Herasevich V, Kor DJ, Subramanian A, Pickering BW. Connecting the dots: rule-based decision support systems in the modern EMR era. J Clin Monit Comput. 2013;27(4):443–448. [DOI] [PubMed] [Google Scholar]
- 19.Afessa B, Keegan MT, Hubmayr RD, et al. Evaluating the performance of an institution using an intensive care unit benchmark. Mayo Clin Proc. 2005;80(2):174–180. [DOI] [PubMed] [Google Scholar]
- 20.Alberti C, Brun-Buisson C, Burchardi H, et al. Epidemiology of sepsis and infection in ICU patients from an international multicentre cohort study. Intensive Care Med. 2002;28(2):108–121. [DOI] [PubMed] [Google Scholar]
- 21.Annane D, Aegerter P, Jars-Guincestre MC, Guidet B; CUB-Réa Network. Current epidemiology of septic shock: the CUB-Réa Network. Am J Respir Crit Care Med. 2003;168(2):165–172. [DOI] [PubMed] [Google Scholar]
- 22.Herasevich V, Pickering BW, Dong Y, Peters SG, Gajic O. Informatics infrastructure for syndrome surveillance, decision support, reporting, and modeling of critical illness. Mayo Clin Proc. 2010;85(3):247–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schramm GE, Kashyap R, Mullon JJ, Gajic O, Afessa B. Septic shock: a multidisciplinary response team and weekly feedback to clinicians improve the process of care and mortality. Crit Care Med. 2011;39(2):252–258. [DOI] [PubMed] [Google Scholar]
- 24.Gaudard M, Ramsey P, Stephens M. Interactive Data Mining and Design of Experiments: The JMP® Partition and Custom Design Platforms. Brookline, NH: North Haven Group, LLC; 2006. [Google Scholar]
- 25.Herasevich V, Pieper MS, Pulido J, Gajic O. Enrollment into a time sensitive clinical study in the critical care setting: results from computerized septic shock sniffer implementation. J Am Med Inform Assoc. 2011;18(5):639–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Viera AJ, Garrett JM. Understanding interobserver agreement: the kappa statistic. Fam Med. 2005;37(5):360–363. [PubMed] [Google Scholar]
- 27.Sim J, Wright CC. The kappa statistic in reliability studies: use, interpretation, and sample size requirements. Phys Ther. 2005; 85(3):257–268. [PubMed] [Google Scholar]
- 28.Ferrer R, Artigas A, Levy MM, et al. ; Edusepsis Study Group. Improvement in process of care and outcome after a multicenter severe sepsis educational program in Spain. JAMA. 2008;299(19):2294–2303. [DOI] [PubMed] [Google Scholar]
- 29.Jaimes F, Garcés J, Cuervo J, et al. The systemic inflammatory response syndrome (SIRS) to identify infected patients in the emergency room. Intensive Care Med. 2003;29(8):1368–1371. [DOI] [PubMed] [Google Scholar]
- 30.Kaukonen KM, Bailey M, Suzuki S, PilcherD, Bellomo R. Mortality related to severe sepsis and septic shock among critically ill patients in Australia and New Zealand, 2000–2012. JAMA. 2014;311(13): 1308–1316. [DOI] [PubMed] [Google Scholar]
- 31.Stevenson EK, Rubenstein AR, Radin GT, Wiener RS, Walkey AJ. Two decades of mortality trends among patients with severe sepsis: a comparative meta-analysis. Crit Care Med. 2014;42(3):625–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gaieski DF, Edwards JM, Kallan MJ, Carr BG. Benchmarking the incidence and mortality of evere sepsis in the United States. Crit Care Med. 2013;41(5):11 67–74. [DOI] [PubMed] [Google Scholar]
- 33.Artenstein AW, Higgins TL, Opal SM. Sepsis and scientific revolutions. Crit Care Med. 2013;41(12):2770–2772. [DOI] [PubMed] [Google Scholar]
- 34.Chopra V, Govindan S, Kuhn L, et al. Do clinicians know which of their patients have central venous catheters? a multicenter observational study. Ann Intern Med. 2014;161(8):562–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nguyen L, Patrick H. Unexpected relationship between central venous pressure (CVP) and mortality in patients with severe sepsis [abstract]. Chest. 2014;146(4):231A. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.