Introduction
Hypersensitivity pneumonitis (HP) is a diffuse parenchymal lung disease caused by inhalation of and sensitization to an ever-expanding list of aerosolized antigens [1]. HP develops in a minority of antigenic exposure cases, and although the factors responsible for such highly variable susceptibility remain elusive, genetic predisposition and misdirected immune modulatory processes have been long suspected in the pathophysiology of HP. Animal studies utilizing in-vivo and in-vitro techniques have provided clues to some of the alterations in the function of alveolar macrophages, respiratory epithelial cells and lymphocytes which contribute to the pathogenesis of HP [2–5].
Robust epidemiological information is severely lacking in HP, in part due to non-consensus regarding diagnostic criteria as well as the complex nature of collecting data on a disease which varies with the changing seasons, and which is heavily influenced by local customs including smoking and occupational practices [6]. In 1994, Coultas et al estimated that the annual incidence of interstitial lung diseases in the Bernalillo county, New Mexico population to be approximately 30 per 100,000 per year, with HP accounting for less than 2% of these cases [7]. In 2001, these data were compared to those collected by interstitial lung disease (ILD) registries in Belgium, Germany and Italy with this aggregate analysis suggesting that HP may represent anywhere between 1.5 to 13% of all ILD cases [8].
Epidemiological data suggests that HP shows a slight female preponderance and occurs more frequently in non-smokers, with one study demonstrating a smoking rate of only 2% in the HP population [9, 10]. A large cohort study found that HP patients are less likely to be current smokers, but are equally likely to be former smokers when compared to the general population [11]. This is in opposition to idiopathic pulmonary fibrosis, which is more common in males and in smokers.
Antigens reported to cause HP are categorized by antigen subtype in table 1 [6, 12–18]. Most of the inciting antigens in HP are smaller than 5 microns in size, a diameter which permits inhalation into the tracheobronchial tree and deposition at the alveolar level [14]. Inhaled particles greater than 10 microns are retained by the oropharyngeal and nasopharyngeal mucous membranes, and conversely, particles smaller than 0.1 microns are small enough to be inhaled and subsequently exhaled without being deposited [19].
Table 1.
Antigens reported to cause HP
| Antigen Subtype | Examples | Exposure Sources | Resultant Disease |
|---|---|---|---|
| Bacteria | Saccharopolyspora rectivirgula, Thermoactinomyces vulgaris, absidia corymbifera | Moldy hay, grain, silage | Farmer’s Lung |
| Fungi, yeast | Aspergillus sp. | Moldy hay, grain | Farmer’s Lung |
| Moldy compost and mushrooms | Mushrooms Worker’s Lung | ||
| Trichosporon cutaneum | Contaminated homes | Summer-Type HP | |
| Penicillium sp. Penicillium |
Moldy cork | Suberosis | |
| Moldy cheese or cheese casings | Cheese Washer’s Lung | ||
| Dry sausage dust in salami factories | Chacinero’s Lung | ||
| Alternaria sp. | Contaminated oak, cedar and mahogany dust. Pine and spruce pulp. | Woodworker’s Lung | |
| Mycobacteria | Mycobacterium avium-intracellulare | Mold on ceiling or walls, tub water | Hot Tub Lung |
| Mist from pool water, sprays and fountains | Swimming Pool Lung | ||
| Animal Proteins | Proteins in avian droppings and serum and on feathers | Parakeets, pigeons, parrots, cockatiels, ducks, chickens, turkeys | Pigeon Breeder’s Lung Bird Fancier’s Lung |
| Animal fur dust | Animal pelts | Furrier’s Lung | |
| Rats, gerbils | Urine, serum, pelt proteins | Laboratory Worker’s Lung | |
| Avian proteins | Feather beds, pillows, duvets | Feather Duvet Lung | |
| Silkworm proteins | Dust from silkworm larvae and cocoons | Silk Production HP | |
| Chemicals | Diisocyanates, toluene, trimelletic anhydride. | Polyurethane foams, spray paints, dyes, glues, varnishes, lacquer | Chemical Worker’s Lung |
| Pyrethrum | Insecticide | Pyrethrum pneumonitis |
Normal Anatomy & Imaging Technique
Imaging Protocols
High-Resolution CT (HRCT) is the imaging modality of choice for examination of interstitial lung disease patients. Thin-section CT images (0.625-mm to 1.5-mm slice thickness) are collected with a high spatial frequency reconstruction algorithm, using multidetector volumetric acquisition with additional volumetric contiguous axial images for expiration and prone series. This has replaced the earlier method which involved acquisition of HRCT images at 10-to-20-mm intervals through the thorax, which limits evaluation of focal abnormalities [20]. The evaluation of multiple reconstructions permits thorough characterization of patchy or multifocal disease, better evaluation of the extent of disease, and improved identification of ancillary findings such as pulmonary nodules or bronchiolitis [21]. Most interstitial lung disease HRCT protocols included low-dose prone and expiratory phase imaging in order to evaluate dependent pulmonary opacities and to assess for air trapping, respectively [22].
Pathology and Imaging Findings
HP is often categorized into acute, subacute and chronic stages; however, there is considerable overlap in the presentation of patients in each stage. To meet the challenge of classifying HP patients, one study performed cluster analysis and showed that a 2-cluster solution best fit the data from a set of 199 HP patients, with one cluster of patients displaying more recurrent systemic systems and lesser abnormalities on chest radiograph when compared to the second cluster of patients who displayed more clubbing, hypoxemia, restrictive pulmonary function test (PFT) patterns and fibrosis seen on CT [23]. For the time being, however, the three-stage scheme persists among clinicians and within the literature.
Acute HP results from intermittent high-intensity antigenic exposure, with symptoms occurring 2–9 hours after antigen exposure and with resolution within 1–12 days of antigen withdrawal [14, 24, 25]. Symptoms range from myalgia, cough and dyspnea to florid pulmonary edema requiring respiratory support [25, 26]. Physical examination in acute HP reveals tachypnea, diffuse fine rales, and a characteristic high-pitched end-inspiratory wheeze known as a “squawk,” which is thought to be the manifestation of rapid oscillation during opening of small airways in deflated areas of the lung [27–29].
Subacute HP results from continuous low-intensity antigen exposure, repeated acute attacks or in some cases may be the manifestation of long-standing undiagnosed acute HP [13, 30]. Symptoms in subacute HP tend to be milder and more insidious when compared to acute HP, although associated cyanosis, fatigue, anorexia and weight loss may require hospitalization [24, 26]. Physical examination in subacute HP often reveals bibasilar inspiratory crackles and tachypnea. Wheezing is less common in subacute HP than in acute HP.
Chronic HP may occur as a progression from acute or subacute HP, or may represent a distinct etiology resulting from ongoing low-level exposure without any acute episodes. Symptoms often reported by patients with chronic HP include progressive dyspnea on exertion, fatigue and malaise, cough and weight loss. Chronic HP may evolve to pulmonary fibrosis or even emphysema in some cases, but the factors producing these two such disparate outcomes are not yet understood [14].
Pathology
Lung biopsy is not required for diagnosis of HP, but has utility in ambiguous cases [31]. In acute HP, histopathology shows nonspecific findings of acute lung injury, which include peribronchovascular fibrin deposition and interstitial accumulation of neutrophils, lymphocytes, plasma cells and macrophages [32, 33]. Alveolar spaces may contain proteinaceous exudates, edema or hemorrhage.
The histological changes of subacute HP occur at the level of the terminal bronchioles and alveoli and consist of a classic histological triad of a predominantly lymphocytic interstitial infiltrate (figure 1), cellular bronchiolitis and poorly-formed non-necrotizing granulomas (figure 1, 2) [12, 34–36]. The characteristic lymphocytic infiltrate likely develops through the interplay of heightened recruitment through upregulation of alveolar macrophage costimulatory molecules, oligoclonal expansion of lymphocyte gene segments producing local proliferation, and increased longevity of these cells through alterations in numerous apoptotic pathways [37–40]. Rarely, well-formed granulomas may be seen, but if these are numerous then alternate diagnoses should be considered [36].
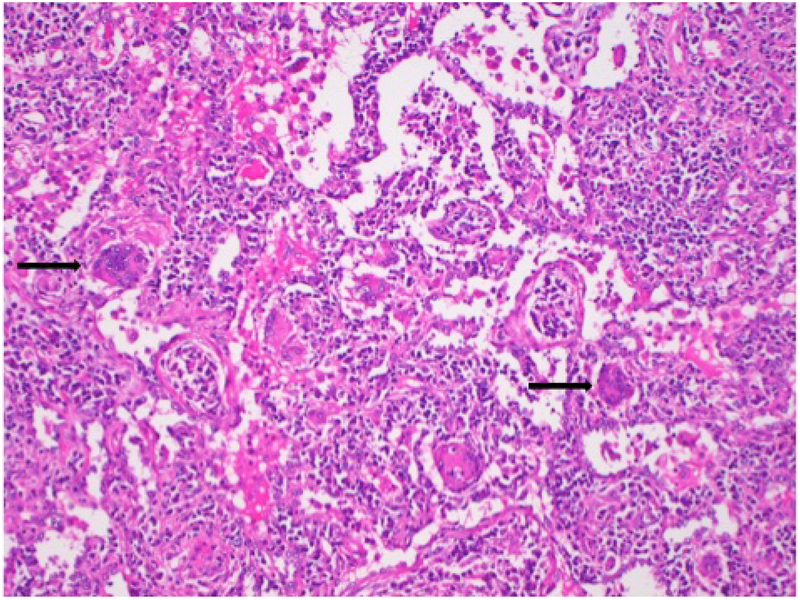
Figure 1.
Lung biopsy from a patient with acute exacerbation of HP shows a chronic inflammatory infiltrate composed mainly of lymphocytes in both the interstitium and the alveoli. Several multinucleated giant cells (arrows) are also present.
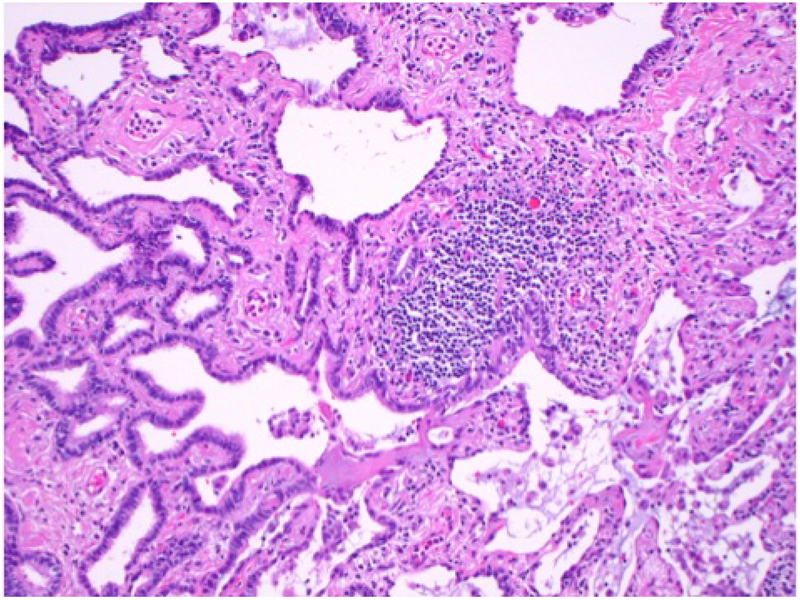
Figure 2.
Medium power photomicrograph shows an area of bronchiolar metaplasia (left) and a lymphoid aggregate (center) in the same patient.
Histopathology in chronic HP consistently shows chronic bronchiolitis, with varying degrees of patchy fibrosis, fibroblastic foci (figure 3) and occasional poorly-formed granulomas (figure 4) [31]. The classic poorly-formed granulomas are not ubiquitously present, with one study showing them in only 58% of cases and others reporting them in as few as 50% of cases [31, 41]. When present, they are most commonly seen in the peribronchiolar tissue (figure 3, 5). Giant cells are usually present in conjunction with granulomas, and often contain cholesterol clefts within their cytoplasm (figure 6) [41].
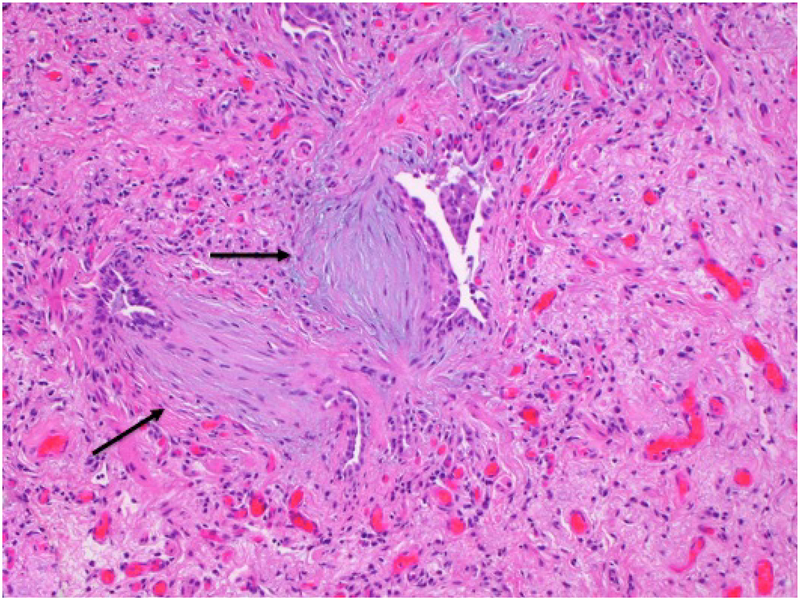
Figure 3.
Medium power photomicrograph shows a large area of fibrosis within which there are two fibroblastic foci (arrows) containing bluish myxoid stroma.
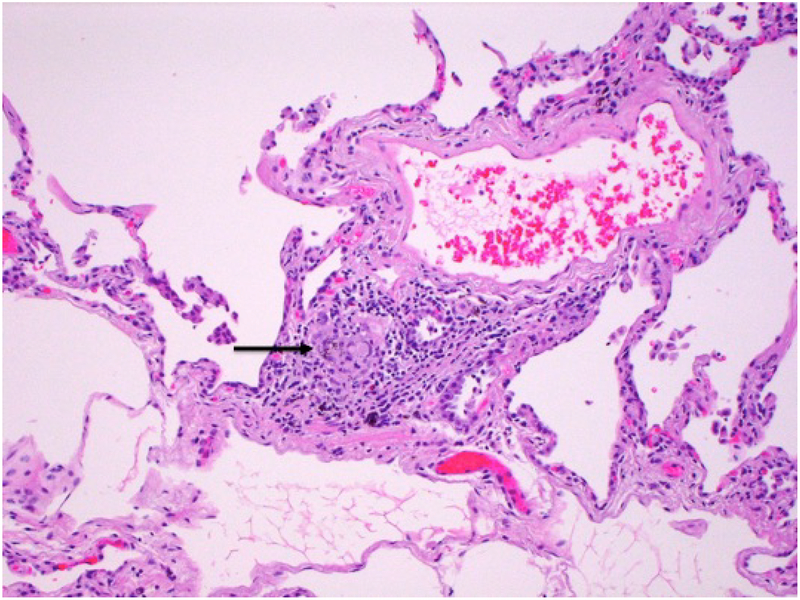
Figure 4.
Medium power micrograph shows poorly-formed granuloma in a perivascular location.
Figure 5.
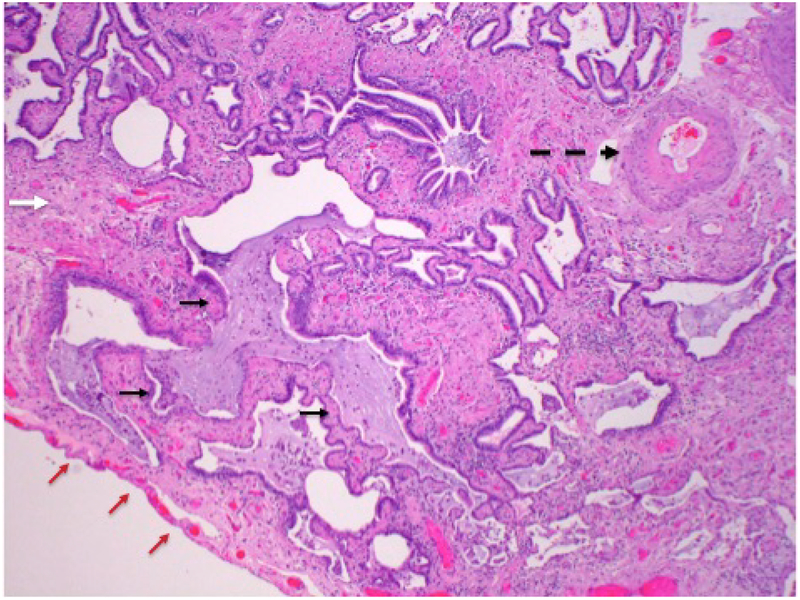
Low power photomicrograph of lung showing extensive interstitial fibrosis (solid white arrow) and honeycomb change (short black arrows) in a patient with chronic HP. Complete destruction of alveoli is seen, with only mucus-filled, dilated airspaces lined by bronchial epithelial cells. There is also secondary pulmonary hypertension, as demonstrated by the presence of a thick-walled pulmonary arterial branch (dashed black arrow). Pleural surface is indicated by red arrows.
Figure 6.
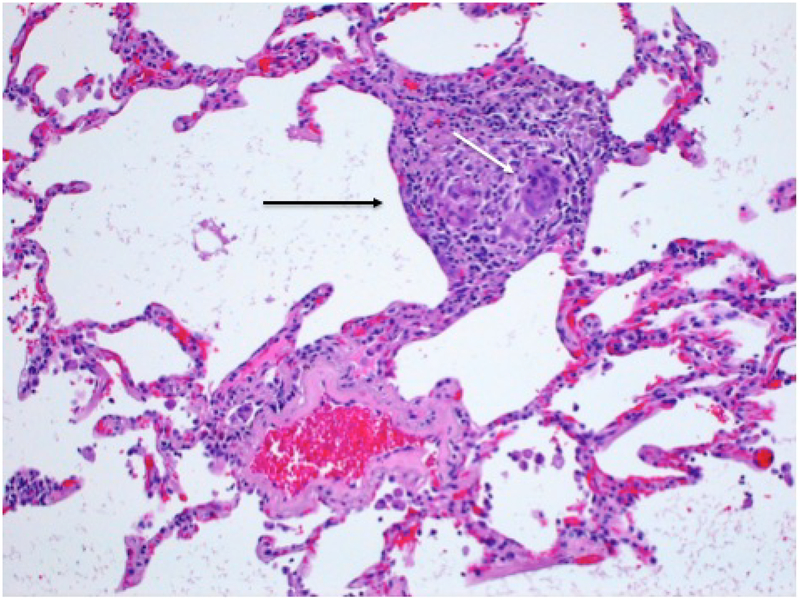
Medium power photomicrograph of lung biopsy in a patient with subacute HP showing non-necrotizing granuloma (black arrow) with multinucleated giant cells (white arrow).
Imaging
The chest radiograph is of limited utility in confirming a diagnosis of HP, and is felt to have greater clinical significance in ruling out other diagnoses in question [26]. Up to 20% of HP patients will have no abnormalities on chest radiograph [42]. When abnormalities are noted, the most common pattern of findings is nodular or reticulonodular ground glass nodules with sparing of the lung bases is the most common pattern of findings [43]. Chronic HP often manifests as nonspecific upper lung zone predominant fibrotic changes such as honeycombing and reticular opacities.
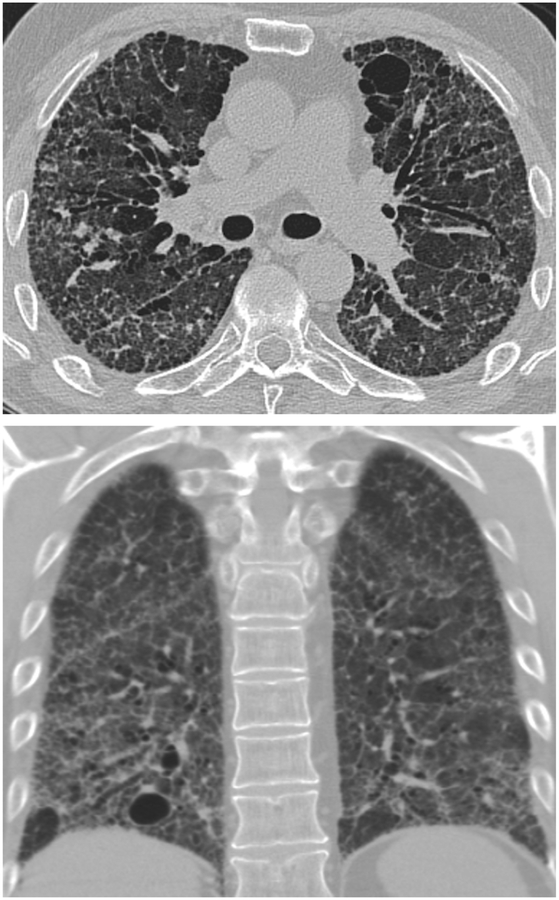
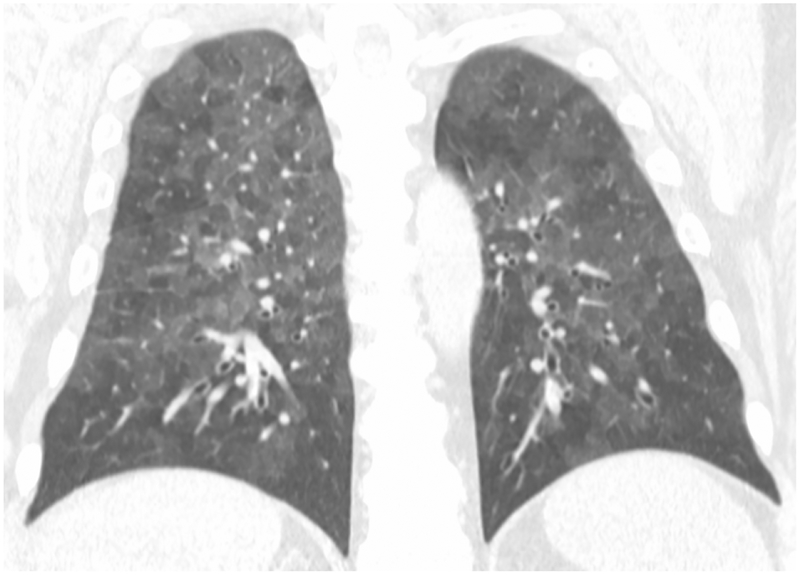
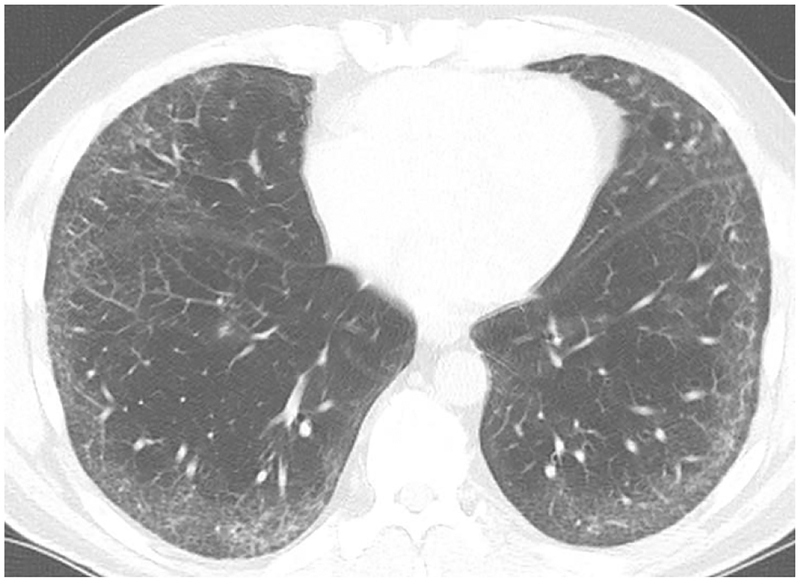
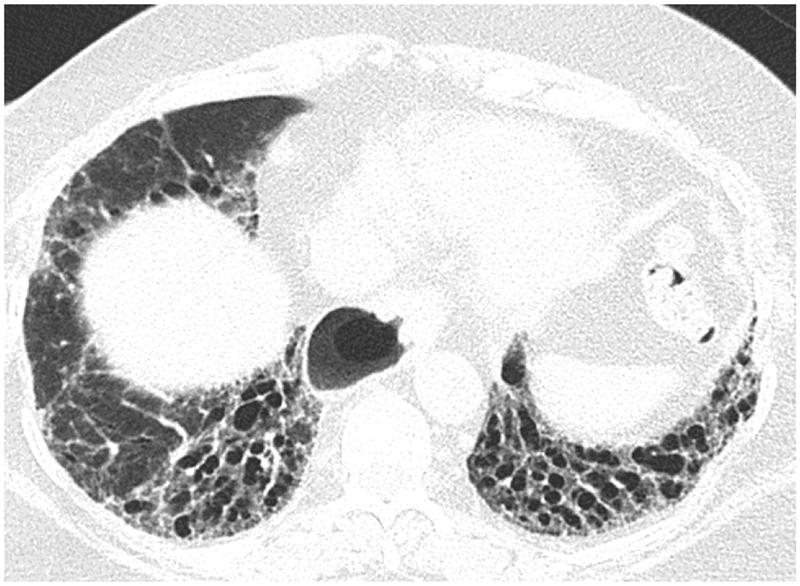
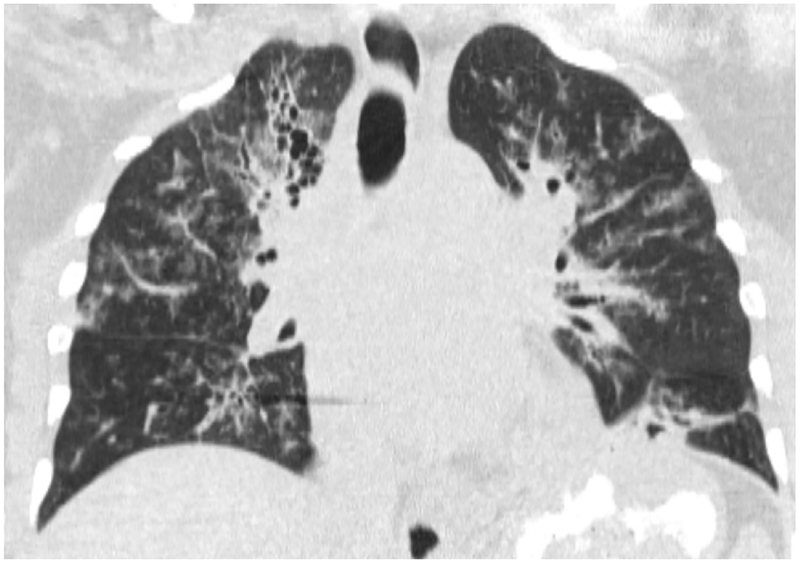
High-Resolution CT (HRCT) is preferred in the radiologic evaluation of HP. In acute HP, HRCT may be normal or may show diffuse ground glass or centrilobular ground glass nodules (figure 7) [44]. Subacute HP often features ground glass opacification (figure 8), poorly defined centrilobular nodules, and areas of air trapping at the level of the secondary pulmonary lobule with mid- and upper-lung zone predominance, although a diffuse pattern is also not uncommon [45]. Expiratory phase imaging in subacute HP often features lobular areas of decreased attenuation representing air trapping (figure 9). Air-trapping may be seen in up to 75% of HP patients, and has been shown to correlate with obstructive pattern findings on PFTs, including reduced FEV1/FVC ratio and reduced FEV25–75 [46]. One study found that in up to 13% of subacute HP cases, thin-walled cysts with diameter less than 15mm are present (figure 10) ([47].
Figure 7.
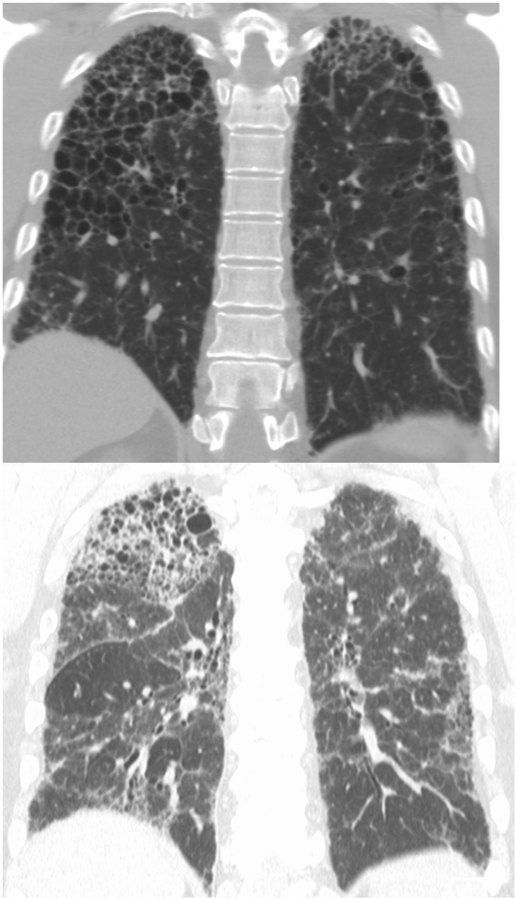
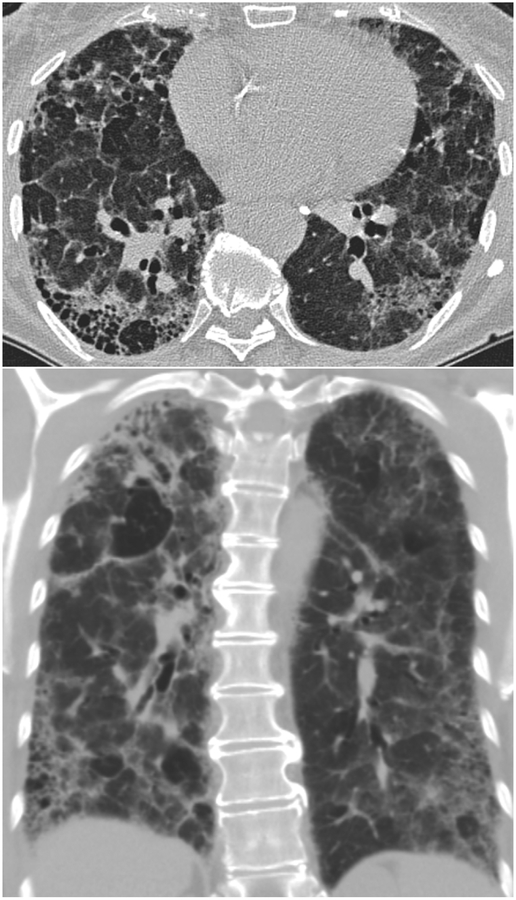
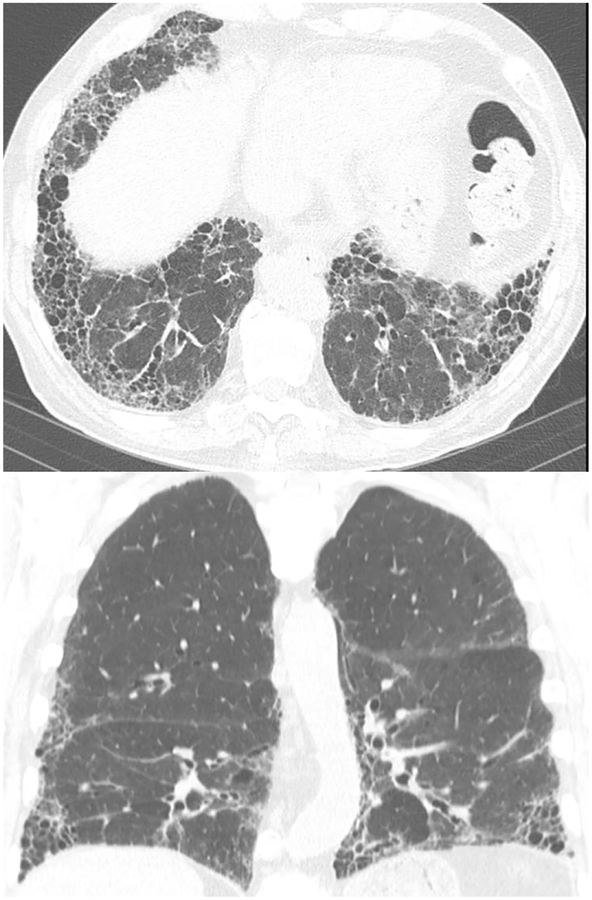
Coronal CT images (a and b) from two separate patients with HP show upper lung zone-predominant reticular fibrosis, subpleural honeycombing and traction bronchiectasis. The 2nd image (b) shows mosaic attenuation consistent with concomitant air-trapping.
Figure 8.
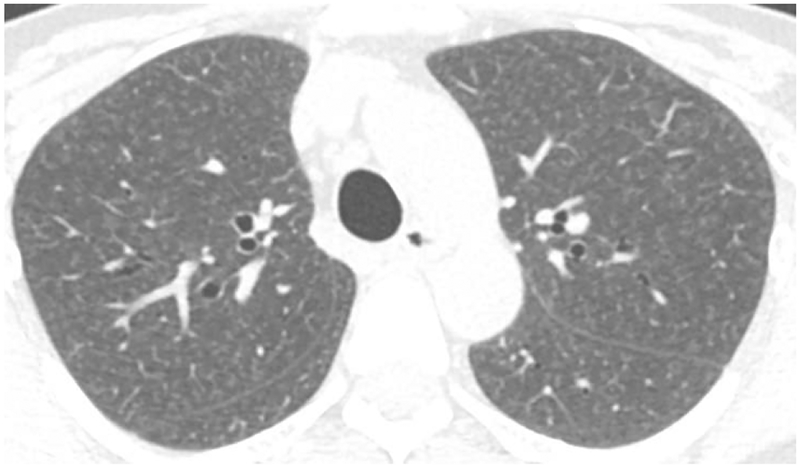
Axial CT in a patient with Bird Fancier’s Disease shows diffuse centrilobular ground glass opacities within the lungs.
Figure 9.
Axial (9a) and coronal (9b) expiratory phase CT showing fibrotic changes of HP with superimposed finding of air-trapping in this patient with known HP.
Figure 10.
Axial (10a) and coronal (10b) CT show predominantly subpleural fibrotic changes including honeycombing and traction bronchiectasis typical of fibrotic HP with superimposed focal cystic regions.
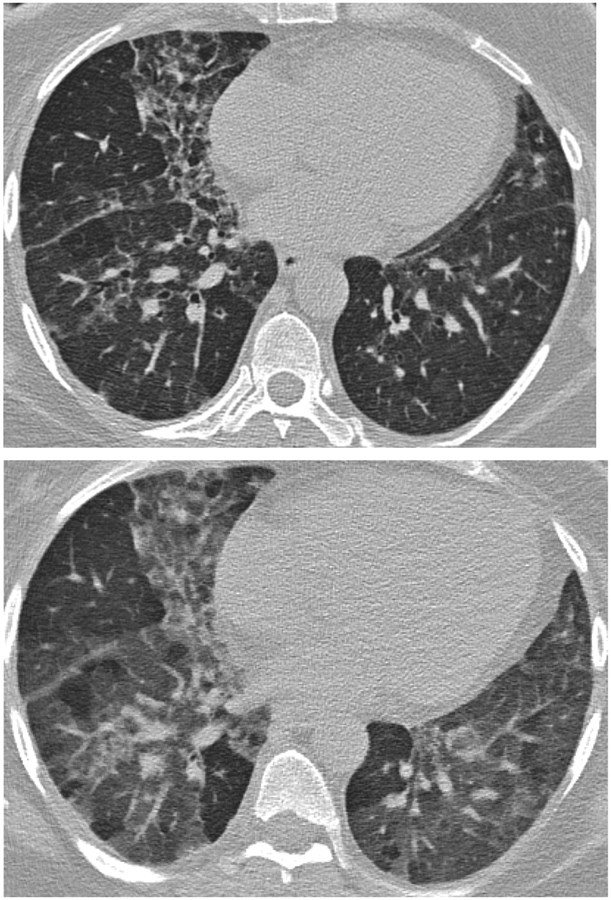
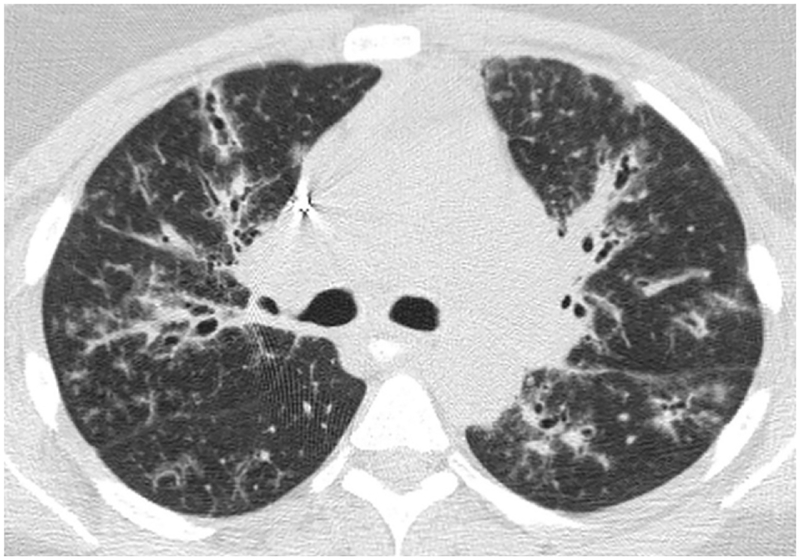
Given the importance of air trapping in the diagnosis of HP, identifying probable air trapping on nonexpiratory CT scans is a useful skill. Obviously, the most accurate way to assess air trapping is to include expiratory CT to the protocol such that it can be compared to inspiratory CT scans. Focal areas of lung which do not increase in attenuation with expiration are representative of air trapping. However, many chest CT scans are not performed with expiratory phase CT and, therefore, possible or probable air trapping must be inferred based on inspiratory images. On inspiratory imaging, significant air trapping usually manifests as mosaic attenuation (well demarcated areas of variable lung attenuation) (figure 11a). Mosaic attenuation may be due to air trapping in the setting of small airway disease, vascular diseases such pulmonary hypertension, and especially pulmonary arterial hypertension, and parenchymal infiltration including pulmonary edema, infection, hemorrhage, and pulmonary fibrosis. CT findings suggestive of air trapping as a cause of mosaic attenuation include bronchial wall thickening, bronchiectasis, mucous plugging which suggest large airway disease, and in contrast include direct signs of small airway disease including tree-in-bud as well as centrilobular nodules (figure 11b).
Figure 11.
Inspiratory phase imaging (11a) in this patient with HP demonstrates mosaic attenuation and patchy pulmonary fibrosis. Expiratory phase imaging (11b) demonstrates superimposed air trapping.
Mosaic attenuation is not exclusive to HP, and must be considered carefully when present on HRCT in a patient with suspected HP. This pattern of attenuation can be seen in patients with small airway disease such as bronchiolitis, in large airway disease such as bronchiectasis or asthma, and in vascular pathologies including chronic thromboembolic pulmonary hypertension (Kligerman 2015).
In chronic HP, fibrotic changes such as septal thickening, traction bronchiectasis and honeycombing are seen, classically, in a peribronchovascular distribution with a mid- and upper-lung zone predominance, although lower and peripheral lung distribution may also be seen [14, 48]. The presence and the extent of fibrotic changes are of strong clinical interest, as the extent of pulmonary fibrosis has been shown to positively correlate with increased mortality in patients with HP [49]. Several studies have shown that honeycombing and traction bronchiectasis associated with fibrotic changes are better able to predict mortality than PFTs [50–52]. Airspace consolidation may be seen in chronic HP, but is felt to represent superimposed infection, and is not intrinsically related to HP pathophysiology.
The headcheese sign is relatively specific for HP, and consists of a constellation of ground glass opacities, air trapping and normal intervening lung with geographic (abrupt) margination (figure 12). The headcheese sign reflects both the infiltrative (ground glass opacities) and obstructive (air trapping) elements of HP. The abrupt changes in lung parenchymal density are caused by the margins of secondary pulmonary lobules. The headcheese pattern can on occasion be seen in sarcoidosis, respiratory bronchiolitis, mycoplasma pneumonia and desquamative interstitial pneumonitis [53].
Figure 12.
Coronal CT reformatted image shows the headcheese sign, featuring sharp geographic margination along the edges of the secondary pulmonary lobules with three distinct levels of attenuation representing normal, ground glass and hyperinflated regions of lung. This sign in the subacute or chronic setting is highly suggesting of HP.
The prognostic utility of radiologic findings in HP, particularly those indicating parenchymal fibrosis, is often critical in assessment of patients who cannot tolerate a lung biopsy [44, 49]. In histological evaluation, the fibroblastic focus is thought to represent active lung injury in the setting of fibrotic lung disease, and profusion of fibroblastic foci is felt to be a valid marker of continued antigenic exposure and physiologic decline [54, 55]. The extent of traction bronchiectasis in patients with chronic HP has been recently shown by multivariate regression analysis to be a significant independent predictor of increased fibroblastic foci, providing a valuable imaging correlate for this histopathologic marker of active lung injury [56].
Diagnostic Criteria
The diagnostic criteria for HP are not widely agreed upon. In 1989 Richerson proposed that the constellation of pulmonary function test findings, a known exposure with the presence of a corresponding antibody, and chest radiographic abnormalities have diagnostic utility in identifying cases of HP [16]. In 1997, Schuyler proposed a set of major and minor criteria, stating that a patient who fulfills four major criteria and two minor criteria may have HP assuming similar diseases have been excluded. Major criteria consist of appropriate symptoms, evidence of exposure, compatible radiologic findings, lymphocytosis on bronchoalveolar lavage, compatible histological findings, and reproduction of symptoms following exposure. Minor criteria consisted of bibasilar rales, decreased diffusing capacity, and hypoxemia, either at rest or on exertion [57].
In 2003, the HP Study, a prospective multi-center cohort study, developed a clinical prediction rule for HP through evaluation of 661 patients. Six statistically significant predictors of active HP (exposure to a known offending antigen, symptoms 4–8 hours after exposure, positive precipitating antibodies, inspiratory crackles, recurrent symptoms, and weight loss, listed in order of descending positive predictive value) were identified through regression analyses. This clinical model concluded that the presence of all 6 predictors confers a 98% probability of HP [15].
In the absence of firm diagnostic criteria, the diagnosis of HP is reached through the collection of supporting data. A multidisciplinary approach, involving clinical, radiographic and pathologic perspectives, is essential in navigating the often broad differential considerations which must be considered in a patient with suspected HP. [58]. Testing for serum precipitins to common antigens, specific inhalation challenge, pulmonary function testing, bronchoalveolar lavage and lung biopsy can be used in diagnostic evaluation of a patient with suspected HP.
There is debate regarding the clinical utility of testing for specific antibodies in the work-up on HP. The HP study showed that the presence of serum precipitins has utility as a predictor of HP, with an odds ratio of 5.3, but numerous studies have demonstrated positive precipitins in asymptomatic patients who have had incidental or ongoing exposure to common HP-inciting antigens. For instance, 54% of asymptomatic dairy farmers assessed in one study tested positive for precipitins to mycropolyspora faeni antigens. [15, 31, 59–61]. Testing for precipitins is widely regarded as only a confirmatory test.
Specific inhalation challenge (SIC) is controlled exposure of a patient to a nebulized antigenic inhalation under medical supervision. A positive result requires a 15% decrease in FVC, a 20% decrease in DLCO, or a 10–15% decrease in FVC accompanied by a 0.5 degrees Celsius increase in core body temperature within 24 hours of antigenic inhalation. One study found that with these criteria, SIC had a sensitivity of 73% and a specificity of 84% in diagnosing HP, but notes frequent false negatives and advises that it be used as only a confirmatory test [62, 63].
Pulmonary function testing (PFT) in HP often shows resting hypoxemia and restrictive-pattern findings with reduced diffusion capacity for carbon monoxide (DLCO) [12, 35]. Findings are useful in guiding treatment, particularly in indicating whether or not a patient may benefit from corticosteroid therapy, but have limited utility in distinguishing HP from other pulmonary diseases [26]. PFT is useful in monitoring changes in the pulmonary functioning of HP patients over time, with significant decreases in total lung capacity and DLCO seen in acute exacerbations [64]. Not surprisingly, PFT findings have been shown to correlate with changes in the appearance of patients’ chest CT examination. The presence of ground glass opacities has been shown to correlate positively with FEV1/FVC ratio. The presence of a pulmonary reticular pattern correlates negatively with residual volume, and correlates positively with FEV1/FVC ratio. And decreased attenuation on CT shows strong positive correlation to RV and the RV/TLC ratio, consistent with an explanation of air trapping for both findings [65].
The American Thoracic Society guidelines define lymphocytosis in bronchoalveolar lavage (BAL) fluid as exceeding 15% lymphocytes [66]. Bronchoalveolar lavage in HP patients shows an increase in overall cellularity as well as a characteristic lymphocytosis [55, 67]. However, during an acute HP exacerbation, BAL fluid may show a greater proportion of neutrophils relative to lymphocytes [64].
Differential Diagnosis
Subacute HP
The differential diagnosis for subacute HP includes respiratory bronchiolitis, atypical (viral) infection, pulmonary hemorrhage, aspiration, and rarely, metastatic calcification. Of these, the most common alternative consideration is respiratory bronchiolitis given the often indolent presentation of both conditions. The imaging appearance of HP and respiratory bronchiolitis are nearly identical, especially when presenting with centrilobular groundglass nodularity. Presence of associated air trapping is more suggestive of HP than respiratory bronchiolitis; however, smoking may also lead to small airway disease which would manifest as air trapping on chest CT. That being said, a large degree of air trapping with concomitant centrilobular groundglass nodularity is highly suggestive of HP. One of the most helpful differentiators is the clinical history of smoking. As aforementioned, smoking is relatively protective in the setting of HP while being the causative agent in almost all patients with a respiratory bronchiolitis.
Respiratory bronchiolitis is extremely common in smokers, and by definition, is asymptomatic. Patients who have clinical and/or physiological abnormalities with respiratory bronchiolitis are considered to have respiratory bronchiolitis interstitial lung disease. Most patients who smoke do not develop respiratory bronchiolitis interstitial lung disease suggesting that other factors play a role in the development of this more severe condition. Patient’s with respiratory bronchiolitis interstitial lung disease usually present in middle age with indolent symptoms of mild cough and/or dyspnea. Pulmonary function tests usually demonstrate a mixed obstructive restrictive pattern (though often with more restriction) with a reduction in diffusion capacity.
Chronic HP
The differential diagnosis for fibrotic fibrotic HP includes idiopathic pulmonary fibrosis (IPF), nonspecific intersitial pneumonia (NSIP) and systemic etiologies such as Sarcoidosis.
IPF is a chronic fibrosing idiopathic interstitial lung disease which occurs more commonly in the 6th and 7th decades of life, is common among smokers and shows male predominance [68]. Notably, environmental exposures including metal dusts, wood dust, avian antigens and vegetable and animal dusts are associated with an increased risk of developing IPF [69]. IPF is characterized by radiologic and pathologic features known as the usual interstitial pneumonitis (UIP) pattern. IPF is indistinguishable from HP in terms of nonspecific clinical presentation and pulmonary function testing, which shows restrictive pattern and decreased DLCO [35]. The usual interstitial pneumonia (UIP) pattern on HRCT is characterized by a basilar or peripheral distribution of reticular opacities with subpleural fibrosis, traction bronchiectasis, and honeycombing (figure 13). Up to 70% of IPF patients will have mediastinal lymphadenopathy[70]. Both UIP and HP may feature honeycombing, traction bronchiectasis and irregular reticulations on CT. The presence of micronodules, a peribronchovascular distribution of findings, multilobar decreased attenuation and sparing of the lung bases favor HP over UIP [41, 58, 69].
Figure 13.
Axial (13a) CT in a case of UIP shows peripheral reticular abnormality, traction bronchiectasis and honeycombing. Coronal (13b) CT reformat shows honeycombing and reticular opacities in a subpleural and basilar distribution.
NSIP is a chronic inflammatory infiltrative process, which most commonly presents with cough and progressive dyspnea, and occurs most commonly in nonsmokers. NSIP shows a female predominance. PFTs in NSIP show a restrictive pattern. Like HP, NSIP may show HRCT findings of patchy ground glass opacities with reticular opacities and honeycombing present to a highly variable extent (figure 14) [71]. Unlike HP, however, there is a lower lung zone predominance in NSIP of up to 94% (figure 15) [21, 48].
Figure 14.
Axial CT in a patient with NSIP shows bilateral lower lung predominant fine reticular abnormality and ground glass opacity with subpleural sparing.
Figure 15.
Axial CT in a case of NSIP shows symmetric lower lung predominant reticular abnormality, exuberant traction bronchiectasis and ground glass opacity as well as a dilated esophagus in this patient with underlying collagen vascular disease.
HP may mimic UIP and NSIP on imaging. Upper lung zone predominance is more common in chronic HP than in UIP or NSIP. Lower lung zone predominance was found in one study in 83% of UIP/IPF patients and 94% of NSIP patients, and was seen in 31% of chronic HP patients, demonstrating that although often upper lung predominant, HP is not uncommonly basilar predominant [48]. This study also demonstrated that peripheral predominance of abnormalities was less common in HP than in UIP/IPF and NSIP. HP, IPF and NSIP are characterized by a peribronchovascular distribution of abnormalities on HRCT [48]. The features which best differentiate chronic HP from IPF and NSIP are the presence of lobular areas of decreased attenuation, centrilobular and peribronchovascular distribution of nodules, and mid- and upper-lung zone predominance [48].
Sarcoidosis is a multisystem granulomatous disease, which features a nonspecific clinical presentation and a female predominance. PFTs can show obstruction, restriction or both. HRCT features common to both HP and sarcoidosis include ground glass opacities, mosaic perfusion and upper-lobe predominant fibrosis (figure 16). Architectural distortion with traction bronchiectasis and honeycombing are commonly observed with fibrotic progression of the disease [72]. Bilateral hilar lymphadenopathy with a symmetric perilymphatic micronodular pattern is highly specific for sarcoidosis [58, 73]. The presence of well-formed non-necrotic granulomas on pathology strongly favors sarcoidosis over HP.
Figure 16.
Axial (16a) and Coronal (16b) CT in a patient with sarcoidosis show bilateral upper and middle lung zone reticulonodular opacities and changes of pulmonary fibrosis.
Pearls & Pitfalls
HP is a complex syndrome of diffuse parenchymal lung disease caused by inhalation of and sensitization to an ever-expanding list of aerosolized antigens[1]. Notably, environmental exposures including metal dusts, wood dust, avian antigens and vegetable and animal dusts are also associated with an increased risk of developing IPF.
Lower rates of HP occur in smokers as compared to matched non-smokers.
Histopathologically, HP features a triad of predominantly lymphocytic interstitial infiltrate, cellular bronchiolitis and poorly-formed non-necrotizing granulomas.
There is a lack of consensus regarding diagnostic criteria for HP, and diagnosis requires a multidisciplinary approach involving clinicians, radiologists and pathologists.
The classic HRCT appearance of HP features upper lung predominant pulmonary changes including ground glass opacities, poorly-defined centrilobular nodules and lobular areas of decreased attenuation representing air trapping. The characteristic constellation of findings is termed the headcheese sign.
What the Referring Physician Needs to Know
Because of the nonspecific clinical presentation, laboratory findings and radiologic findings in HP, a high degree of clinical suspicion is essential in avoiding delays in diagnosis. Early diagnosis of HP and prompt intervention can slow the progression of irreversible parenchymal damage, and can preserve patients’ quality of life. A study using a regression model to compare health-related quality of life between patients with HP and IPF found that HP patients experienced poorer quality of life in 8 out of 8 evaluated domains, including physical functioning, emotional functioning, mental health and vitality [68].
Mortality data are thought to grossly under-report the reality of HP, as these data are largely collected from death certificates, which frequently code cause of death as acute causes, some of which may be related to ILD, such as respiratory failure. A study evaluating all-cause mortality in patients with HP found 5-year survival for HP patients was 82%, as compared to 93% in the demographically-matched control group. The hazard ratio of 2.17 increased to 2.98 when adjustments were made for age, sex and smoking [11]. Another study revealed through univariate analysis that older age, male gender, presence of crackles on auscultation, higher FEV1/FEV ratio, desaturation during exercise, and presence of fibrosis on high-resolution CT were statistically significant predictors of mortality in HP. Multivariate analysis revealed that age, oxygen saturation during exercise, and radiologic fibrosis remained significant [74].
Although HP occurs less frequently in smokers, it can take a more severe clinical course in this subset of patients. Cigarette smoke exposes the lung parenchyma to high concentrations of reactive oxygen species, and rodent studies have demonstrated that the oxidative stress induced on alveolar macrophages induces macrophage death in a dose-dependent and time-dependent manner [75]. A study evaluating BAL fluid results in HP patients has shown a near-complete lack of macrophage co-stimulatory molecule expression in smokers, and a decreased percentage of lymphocytes constituting BAL fluid cellularity in smokers when compared to nonsmokers, suggesting active inhibition of alveolar macrophage activity in smokers [37].
Primary prevention of HP has practical limitations, as educating healthy individuals in high-risk groups, such as farmers, to the hazards of antigenic exposures and recommended avoidance techniques is inherently difficult. Secondary prevention, consisting of elimination and avoidance of the offending antigen, is more practically implemented. Avoidance of antigenic triggers can halt progression of the disease process, as demonstrated by a 2-week in-hospital avoidance trial, which showed statistically significant improvements in vital capacity and leukocytosis after short-duration antigen avoidance[76]. Antigen determination requires thoughtful history-taking with consideration of the patient’s environmental and occupational experiences. Although the temporal relationship between exposure and development of symptoms is highly variable, which complicates the physician’s task of correlating the two, the endeavor is worthwhile as the ability to identify an inciting antigen has been shown to be independently associated with improved survival in HP [77]. Use of corticosteroids is appropriate for acute symptomatic relief or in patients who are non-candidates for life-saving transplant, but it should be noted that long-term outcome does not seem to be impacted by corticosteroid use. Lung or heart/lung transplant is the only life-saving measure in HP, with one retrospective cohort study reporting 5-year post-operative survival rates of 89%. Unfortunately, recurrence of HP occurs in up to 6% of patients post-transplant [78]. The pathophysiology of this recurrence is not yet understood, but components of environmental exposure and continued antigenic stimulation are felt to be critical.
Summary/Conclusions
The management of HP depends on early identification of the disease process, which is complicated by its nonspecific clinical presentation in addition to variable and diverse laboratory and radiologic findings. HP is the result of exposure and sensitization to myriad aerosolized antigens. HP develops in the minority of antigenic exposures, and conversely has been documented in patients with no identifiable exposure, complicating the diagnostic algorithm significantly. Physicians must have a high degree of clinical suspicion; alas many cases of HP go misdiagnosed or undiagnosed. Key imaging findings including a constellation of changes seen on HRCT known as the headcheese sign can contribute greatly to the diagnostic challenge, but a multidisciplinary approach is essential in this endeavor. Prompt diagnosis and early intervention are critical in slowing the progression of irreversible parenchymal damage, and additionally in preserving the quality of life of affected patients.
References
- 1.Demedts M, et al. , Interstitial lung diseases: an epidemiological overview. Eur Respir J Suppl, 2001. 32: p. 2s–16s. [PubMed] [Google Scholar]
- 2.Keskinen P, et al. , Regulation of HLA class I and II expression by interferons and influenza A virus in human peripheral blood mononuclear cells. Immunology, 1997. 91(3): p. 421–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dakhama A, et al. , Common respiratory viruses in lower airways of patients with acute hypersensitivity pneumonitis. Am J Respir Crit Care Med, 1999. 159(4 Pt 1): p. 1316–22. [DOI] [PubMed] [Google Scholar]
- 4.Wang SZ, et al. , Adhesion molecule expression on epithelial cells infected with respiratory syncytial virus. Eur Respir J, 2000. 15(2): p. 358–66. [DOI] [PubMed] [Google Scholar]
- 5.Cormier Y and Israel-Assayag E, The role of viruses in the pathogenesis of hypersensitivity pneumonitis. Curr Opin Pulm Med, 2000. 6(5): p. 420–3. [DOI] [PubMed] [Google Scholar]
- 6.Bourke SJ, et al. , Hypersensitivity pneumonitis: current concepts. Eur Respir J Suppl, 2001. 32: p. 81s–92s. [PubMed] [Google Scholar]
- 7.Coultas DB, et al. , The epidemiology of interstitial lung diseases. Am J Respir Crit Care Med, 1994. 150(4): p. 967–72. [DOI] [PubMed] [Google Scholar]
- 8.Thomeer MJ, et al. , Comparison of registries of interstitial lung diseases in three European countries. Eur Respir J Suppl, 2001. 32: p. 114s–118s. [PubMed] [Google Scholar]
- 9.Hanak V, Golbin JM, and Ryu JH, Causes and presenting features in 85 consecutive patients with hypersensitivity pneumonitis. Mayo Clin Proc, 2007. 82(7): p. 812–6. [DOI] [PubMed] [Google Scholar]
- 10.Mooney JJ, et al. , Radiographic fibrosis score predicts survival in hypersensitivity pneumonitis. Chest, 2013. 144(2): p. 586–92. [DOI] [PubMed] [Google Scholar]
- 11.Solaymani-Dodaran M, et al. , Extrinsic allergic alveolitis: incidence and mortality in the general population. Qjm, 2007. 100(4): p. 233–7. [DOI] [PubMed] [Google Scholar]
- 12.Spagnolo P, et al. , Hypersensitivity Pneumonitis: A Comprehensive Review. J Investig Allergol Clin Immunol, 2015. 25(4): p. 237–50; quiz follow 250. [PubMed] [Google Scholar]
- 13.Selman M and Buendia-Roldan I, Immunopathology, diagnosis, and management of hypersensitivity pneumonitis. Semin Respir Crit Care Med, 2012. 33(5): p. 543–54. [DOI] [PubMed] [Google Scholar]
- 14.Selman M, Hypersensitivity pneumonitis: a multifaceted deceiving disorder. Clin Chest Med, 2004. 25(3): p. 531–47, vi. [DOI] [PubMed] [Google Scholar]
- 15.Lacasse Y, et al. , Clinical diagnosis of hypersensitivity pneumonitis. Am J Respir Crit Care Med, 2003. 168(8): p. 952–8. [DOI] [PubMed] [Google Scholar]
- 16.Richerson HB, et al. , Guidelines for the clinical evaluation of hypersensitivity pneumonitis. Report of the Subcommittee on Hypersensitivity Pneumonitis. J Allergy Clin Immunol, 1989. 84(5 Pt 2): p. 839–44. [DOI] [PubMed] [Google Scholar]
- 17.Morell F, et al. , Chacinero’s lung - hypersensitivity pneumonitis due to dry sausage dust. Scand J Work Environ Health, 2011. 37(4): p. 349–56. [DOI] [PubMed] [Google Scholar]
- 18.Guillot M, et al. , [Dry sausage mould hypersensitivity pneumonitis: three cases]. Rev Mal Respir, 2008. 25(5): p. 596–600. [DOI] [PubMed] [Google Scholar]
- 19.Akira M and Suganuma N, Acute and subacute chemical-induced lung injuries: HRCT findings. Eur J Radiol, 2014. 83(8): p. 1461–9. [DOI] [PubMed] [Google Scholar]
- 20.Remy-Jardin M, et al. , Usefulness of coronal reformations in the diagnostic evaluation of infiltrative lung disease. J Comput Assist Tomogr, 2003. 27(2): p. 266–73. [DOI] [PubMed] [Google Scholar]
- 21.Sverzellati N, et al. , American Thoracic Society-European Respiratory Society Classification of the Idiopathic Interstitial Pneumonias: Advances in Knowledge since 2002. Radiographics, 2015: p. 140334. [DOI] [PubMed] [Google Scholar]
- 22.Small JH, et al. , Air-trapping in extrinsic allergic alveolitis on computed tomography. Clin Radiol, 1996. 51(10): p. 684–8. [DOI] [PubMed] [Google Scholar]
- 23.Lacasse Y, et al. , Classification of hypersensitivity pneumonitis: a hypothesis. Int Arch Allergy Immunol, 2009. 149(2): p. 161–6. [DOI] [PubMed] [Google Scholar]
- 24.Girard M, Lacasse Y, and Cormier Y, Hypersensitivity pneumonitis. Allergy, 2009. 64(3): p. 322–34. [DOI] [PubMed] [Google Scholar]
- 25.Agostini C, et al. , New aspects of hypersensitivity pneumonitis. Curr Opin Pulm Med, 2004. 10(5): p. 378–82. [DOI] [PubMed] [Google Scholar]
- 26.Lacasse Y and Cormier Y, Hypersensitivity pneumonitis. Orphanet J Rare Dis, 2006. 1: p. 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Earis JE, et al. , The inspiratory “squawk” in extrinsic allergic alveolitis and other pulmonary fibroses. Thorax, 1982. 37(12): p. 923–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Forgacs P, The functional basis of pulmonary sounds. Chest, 1978. 73(3): p. 399–405. [DOI] [PubMed] [Google Scholar]
- 29.Forgacs P, Crackles and wheezes. Lancet, 1967. 2(7508): p. 203–5. [DOI] [PubMed] [Google Scholar]
- 30.Selman M, Pardo A, and King TE Jr., Hypersensitivity pneumonitis: insights in diagnosis and pathobiology. Am J Respir Crit Care Med, 2012. 186(4): p. 314–24. [DOI] [PubMed] [Google Scholar]
- 31.Trahan S, et al. , Role of surgical lung biopsy in separating chronic hypersensitivity pneumonia from usual interstitial pneumonia/idiopathic pulmonary fibrosis: analysis of 31 biopsies from 15 patients. Chest, 2008. 134(1): p. 126–32. [DOI] [PubMed] [Google Scholar]
- 32.Cordier JF, Challenges in pulmonary fibrosis. 2: Bronchiolocentric fibrosis. Thorax, 2007. 62(7): p. 638–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hariri LP, et al. , Distinct histopathology of acute onset or abrupt exacerbation of hypersensitivity pneumonitis. Hum Pathol, 2012. 43(5): p. 660–8. [DOI] [PubMed] [Google Scholar]
- 34.Costabel U, Bonella F, and Guzman J, Chronic hypersensitivity pneumonitis. Clin Chest Med, 2012. 33(1): p. 151–63. [DOI] [PubMed] [Google Scholar]
- 35.Jeong YJ, et al. , Chronic hypersensitivity pneumonitis and pulmonary sarcoidosis: differentiation from usual interstitial pneumonia using high-resolution computed tomography. Semin Ultrasound CT MR, 2014. 35(1): p. 47–58. [DOI] [PubMed] [Google Scholar]
- 36.Castonguay MC, et al. , Granulomas and giant cells in hypersensitivity pneumonitis. Hum Pathol, 2015. 46(4): p. 607–13. [DOI] [PubMed] [Google Scholar]
- 37.Israel-Assayag E, et al. , Expression of costimulatory molecules on alveolar macrophages in hypersensitivity pneumonitis. Am J Respir Crit Care Med, 1999. 159(6): p. 1830–4. [DOI] [PubMed] [Google Scholar]
- 38.Facco M, et al. , T cells in the lung of patients with hypersensitivity pneumonitis accumulate in a clonal manner. J Leukoc Biol, 2004. 75(5): p. 798–804. [DOI] [PubMed] [Google Scholar]
- 39.Laflamme C, Israel-Assayag E, and Cormier Y, Apoptosis of bronchoalveolar lavage lymphocytes in hypersensitivity pneumonitis. Eur Respir J, 2003. 21(2): p. 225–31. [DOI] [PubMed] [Google Scholar]
- 40.Semenzato G, et al. , Lung T cells in hypersensitivity pneumonitis: phenotypic and functional analyses. J Immunol, 1986. 137(4): p. 1164–72. [PubMed] [Google Scholar]
- 41.Takemura T, et al. , Pathological differentiation of chronic hypersensitivity pneumonitis from idiopathic pulmonary fibrosis/usual interstitial pneumonia. Histopathology, 2012. 61(6): p. 1026–35. [DOI] [PubMed] [Google Scholar]
- 42.Hodgson MJ, Parkinson DK, and Karpf M, Chest X-rays in hypersensitivity pneumonitis: a metaanalysis of secular trend. Am J Ind Med, 1989. 16(1): p. 45–53. [DOI] [PubMed] [Google Scholar]
- 43.Monkare S, Ikonen M, and Haahtela T, Radiologic findings in farmer’s lung. Prognosis and correlation to lung function. Chest, 1985. 87(4): p. 460–6. [DOI] [PubMed] [Google Scholar]
- 44.Tateishi T, et al. , Serial high-resolution computed tomography findings of acute and chronic hypersensitivity pneumonitis induced by avian antigen. J Comput Assist Tomogr, 2011. 35(2): p. 272–9. [DOI] [PubMed] [Google Scholar]
- 45.Remy-Jardin M, et al. , Subacute and chronic bird breeder hypersensitivity pneumonitis: sequential evaluation with CT and correlation with lung function tests and bronchoalveolar lavage. Radiology, 1993. 189(1): p. 111–8. [DOI] [PubMed] [Google Scholar]
- 46.Chung MH, et al. , Mixed infiltrative and obstructive disease on high-resolution CT: differential diagnosis and functional correlates in a consecutive series. J Thorac Imaging, 2001. 16(2): p. 69–75. [DOI] [PubMed] [Google Scholar]
- 47.Franquet T, et al. , Lung cysts in subacute hypersensitivity pneumonitis. J Comput Assist Tomogr, 2003. 27(4): p. 475–8. [DOI] [PubMed] [Google Scholar]
- 48.Silva CI, et al. , Chronic hypersensitivity pneumonitis: differentiation from idiopathic pulmonary fibrosis and nonspecific interstitial pneumonia by using thin-section CT. Radiology, 2008. 246(1): p. 288–97. [DOI] [PubMed] [Google Scholar]
- 49.Hanak V, et al. , High-resolution CT findings of parenchymal fibrosis correlate with prognosis in hypersensitivity pneumonitis. Chest, 2008. 134(1): p. 133–8. [DOI] [PubMed] [Google Scholar]
- 50.Walsh SL, et al. , Connective tissue disease related fibrotic lung disease: high resolution computed tomographic and pulmonary function indices as prognostic determinants. Thorax, 2014. 69(3): p. 216–22. [DOI] [PubMed] [Google Scholar]
- 51.Walsh SL, et al. , Chronic hypersensitivity pneumonitis: high resolution computed tomography patterns and pulmonary function indices as prognostic determinants. Eur Radiol, 2012. 22(8): p. 1672–9. [DOI] [PubMed] [Google Scholar]
- 52.Siemienowicz ML, et al. , Agreement and mortality prediction in high-resolution CT of diffuse fibrotic lung disease. J Med Imaging Radiat Oncol, 2015. 59(5): p. 555–63. [DOI] [PubMed] [Google Scholar]
- 53.Chong BJ, Kanne JP, and Chung JH, Headcheese sign. J Thorac Imaging, 2014. 29(1): p. W13. [DOI] [PubMed] [Google Scholar]
- 54.Churg A, et al. , Pathologic patterns and survival in chronic hypersensitivity pneumonitis. Am J Surg Pathol, 2009. 33(12): p. 1765–70. [DOI] [PubMed] [Google Scholar]
- 55.Takemura T, et al. , Pathology of hypersensitivity pneumonitis. Curr Opin Pulm Med, 2008. 14(5): p. 440–54. [DOI] [PubMed] [Google Scholar]
- 56.Walsh SL, et al. , Relationship between fibroblastic foci profusion and high resolution CT morphology in fibrotic lung disease. BMC Med, 2015. 13: p. 241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schuyler M and Cormier Y, The diagnosis of hypersensitivity pneumonitis. Chest, 1997. 111(3): p. 534–6. [DOI] [PubMed] [Google Scholar]
- 58.Elicker BM, et al. , Multidisciplinary Approach to Hypersensitivity Pneumonitis. J Thorac Imaging, 2015. [DOI] [PubMed] [Google Scholar]
- 59.Costabel U, et al. , T-lymphocytosis in bronchoalveolar lavage fluid of hypersensitivity pneumonitis. Changes in profile of T-cell subsets during the course of disease. Chest, 1984. 85(4): p. 514–22. [DOI] [PubMed] [Google Scholar]
- 60.Cormier Y, et al. , Abnormal bronchoalveolar lavage in asymptomatic dairy farmers. Study of lymphocytes. Am Rev Respir Dis, 1984. 130(6): p. 1046–9. [DOI] [PubMed] [Google Scholar]
- 61.Fenoglio CM, et al. , Diagnostic value of serum precipitins to mould antigens in active hypersensitivity pneumonitis. Eur Respir J, 2007. 29(4): p. 706–12. [DOI] [PubMed] [Google Scholar]
- 62.Munoz X, Morell F, and Cruz MJ, The use of specific inhalation challenge in hypersensitivity pneumonitis. Curr Opin Allergy Clin Immunol, 2013. 13(2): p. 151–8. [DOI] [PubMed] [Google Scholar]
- 63.Munoz X, et al. , Diagnostic yield of specific inhalation challenge in hypersensitivity pneumonitis. Eur Respir J, 2014. 44(6): p. 1658–65. [DOI] [PubMed] [Google Scholar]
- 64.Miyazaki Y, et al. , Clinical predictors and histologic appearance of acute exacerbations in chronic hypersensitivity pneumonitis. Chest, 2008. 134(6): p. 1265–70. [DOI] [PubMed] [Google Scholar]
- 65.Hansell DM, et al. , Hypersensitivity pneumonitis: correlation of individual CT patterns with functional abnormalities. Radiology, 1996. 199(1): p. 123–8. [DOI] [PubMed] [Google Scholar]
- 66.Meyer KC, et al. , An official American Thoracic Society clinical practice guideline: the clinical utility of bronchoalveolar lavage cellular analysis in interstitial lung disease. Am J Respir Crit Care Med, 2012. 185(9): p. 1004–14. [DOI] [PubMed] [Google Scholar]
- 67.D’Ippolito R, et al. , Induced sputum and bronchoalveolar lavage from patients with hypersensitivity pneumonitis. Respir Med, 2004. 98(10): p. 977–83. [DOI] [PubMed] [Google Scholar]
- 68.Lubin M, et al. , A comparison of health-related quality of life in idiopathic pulmonary fibrosis and chronic hypersensitivity pneumonitis. Chest, 2014. 145(6): p. 1333–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Raghu G, Idiopathic pulmonary fibrosis: guidelines for diagnosis and clinical management have advanced from consensus-based in 2000 to evidence-based in 2011. Eur Respir J, 2011. 37(4): p. 743–6. [DOI] [PubMed] [Google Scholar]
- 70.Souza CA, et al. , Idiopathic interstitial pneumonias: prevalence of mediastinal lymph node enlargement in 206 patients. AJR Am J Roentgenol, 2006. 186(4): p. 995–9. [DOI] [PubMed] [Google Scholar]
- 71.Kligerman SJ, et al. , Nonspecific interstitial pneumonia: radiologic, clinical, and pathologic considerations. Radiographics, 2009. 29(1): p. 73–87. [DOI] [PubMed] [Google Scholar]
- 72.Patterson KC and Strek ME, Pulmonary fibrosis in sarcoidosis. Clinical features and outcomes. Ann Am Thorac Soc, 2013. 10(4): p. 362–70. [DOI] [PubMed] [Google Scholar]
- 73.Nunes H, et al. , Imaging of sarcoidosis of the airways and lung parenchyma and correlation with lung function. Eur Respir J, 2012. 40(3): p. 750–65. [DOI] [PubMed] [Google Scholar]
- 74.Lima MS, et al. , Subacute and chronic hypersensitivity pneumonitis: histopathological patterns and survival. Respir Med, 2009. 103(4): p. 508–15. [DOI] [PubMed] [Google Scholar]
- 75.Aoshiba K, Tamaoki J, and Nagai A, Acute cigarette smoke exposure induces apoptosis of alveolar macrophages. Am J Physiol Lung Cell Mol Physiol, 2001. 281(6): p. L1392–401. [DOI] [PubMed] [Google Scholar]
- 76.Tsutsui T, et al. , Antigen avoidance tests for diagnosis of chronic hypersensitivity pneumonitis. Respir Investig, 2015. 53(5): p. 217–24. [DOI] [PubMed] [Google Scholar]
- 77.Fernandez Perez ER, et al. , Identifying an inciting antigen is associated with improved survival in patients with chronic hypersensitivity pneumonitis. Chest, 2013. 144(5): p. 1644–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kern RM, et al. , Lung transplantation for hypersensitivity pneumonitis. Chest, 2015. 147(6): p. 1558–65. [DOI] [PMC free article] [PubMed] [Google Scholar]