Summary
Objective:
Examine the association of duration of therapeutic coma (TC) with seizure recurrence, morbidity, and mortality in refractory status epilepticus (RSE). Define an optimal window for TC that provides sustained seizure control and minimizes complications.
Methods:
Retrospective, observational cohort study involving patients who presented with RSE to the University of Alabama at Birmingham or the University of California at San Francisco from 2010 to 2016. Relationship of duration of TC with primary and secondary outcomes was evaluated using two-sample t tests, simple linear regression, and chi-square tests. Multivariable linear and logistic regression models were used to identify independent predictors. Predictive ability of TC for seizure recurrence was quantified using a receiver-operating characteristic curve. Youden index was used to determine an optimal cutoff value.
Results:
Multivariable analysis of clinical and treatment characteristics of 182 patients who were treated predominantly with propofol as anesthetic agent showed that longer duration of the first trial of TC (27.2 vs 15.6 hours) was independently associated with a higher chance of seizure recurrence following the first weaning attempt (P = 0.038) but not with poor functional neurologic outcome upon discharge, in-hospital complications, or mortality. Furthermore, higher doses of anesthetic utilized during the first trial of TC were independently associated with fewer in-hospital complications (P = 0.003) and associated with a shorter duration of mechanical ventilation and total length of stay. Duration of TC was identified as an independent predictor of seizure recurrence with an optimal cutoff point at 35 hours.
Significance:
This study suggests that a shorter duration yet deeper TC as treatment for RSE may be more effective and safer than the currently recommended TC duration of 24–48 hours. Prospective and randomized trials should be conducted to validate these assertions.
Keywords: anesthesia, outcome, refractory status epilepticus, seizure recurrence, therapeutic coma
1 |. INTRODUCTION
Status epilepticus (SE) is the second most common neurologic emergency and its incidence is on the rise.1,2 SE is refractory to adequate first- and second-line treatment with benzodiazepines (BZDs) and nonsedating antiseizure drugs (ASDs) in 31%−44% of cases.3 Refractory status epilepticus (RSE) is associated with higher morbidity and mortality than SE that responds to initial treatment, contributing to increased health care costs.4,5 Because RSE becomes more refractory to treatment over time, current management guidelines, based on expert opinion, suggest that the duration of an artificially induced (therapeutic) coma (TC) should last between 24 and 48 hours.3,6,7 TC carries risks related to intubation, intensive care unit (ICU) stay, and anesthetic side effects.8 There is ongoing controversy as to whether TC is an independent risk factor for prolonged hospitalizations, infection risk, poor functional outcomes, and in-hospital mortality.9–12 To date, most studies looking at TC as treatment for RSE have been retrospective, with one prospective, randomized clinical trial that was undersampled due to lack of enrollment.13–19 These studies have focused primarily on comparing the efficacy of different anesthetic agents and the depth of TC on seizure recurrence and functional outcomes. However, the duration of TC has never been studied as an independent factor for successful treatment of RSE.3,6,7,20
In this study, we examine the association between duration of the first trial of TC and the rate of in-hospital seizure recurrence after the first attempt to wean the anesthetic in patients with RSE. Furthermore, we evaluate whether duration of TC is an independent risk factor for in-hospital complications, poor functional neurologic outcome upon discharge, or death. Finally we searched for an optimal window for TC duration that maximizes the chances of sustained seizure control while minimizing the risks associated with prolonged sedation. Main hypothesis was that a profound TC of shorter duration (≤24 hours) is at least as effective in providing sustained seizure control and is associated with lower morbidity and mortality than the currently recommended duration of 24–48 hours.
2 |. METHODS
2.1 |. Study population
This retrospective, observational cohort study included adults (≥18 years) who presented with RSE to the University of Alabama at Birmingham (UAB) or the University of California at San Francisco (UCSF) Medical Center from 2010 to 2016. The UAB and UCSF patient cohorts were identified using a combination of International Classification of Diseases, Ninth Revision (ICD)-9 and ICD-10 billing codes, current procedural terminology (CPT) codes for electroencephalography (EEG) monitoring greater than one hour in duration, admission to an ICU, and individual drug identification numbers for anesthetics commonly used for treatment of RSE at both institutions (ie, propofol, midazolam, pentobarbital, and ketamine). The UCSF and UAB internal review boards approved the data collection and analysis for this study. We utilized standardized questionnaires and definitions for variables examined in this study that were developed before the initiation of data collection.
2.2 |. Inclusion and exclusion criteria
All patients who fulfilled the International League Against Epilepsy (ILAE) operational definition of status epilepticus (SE)21 were eligible for enrollment. This included all patients with generalized convulsive SE, nonconvulsive SE with coma, nonconvulsive focal SE with impaired aware-ness/confusion, absence SE, myoclonic SE unrelated to diffuse hypoxic ischemic injury, and focal SE without awareness impairment (including epilepsia partialis continua). In addition, the patients had to have failed treatment with at least one benzodiazepine (ie, lorazepam, diazepam, clonazepam, or midazolam) and one intravenous ASD (ie, fos/phenytoin, levetiracetam, valproic acid, or lacosamide) prior to intubation and treatment with either mono-therapy or combination therapy of propofol, midazolam, pentobarbital, or ketamine.
2.3 |. Study predictor and outcomes
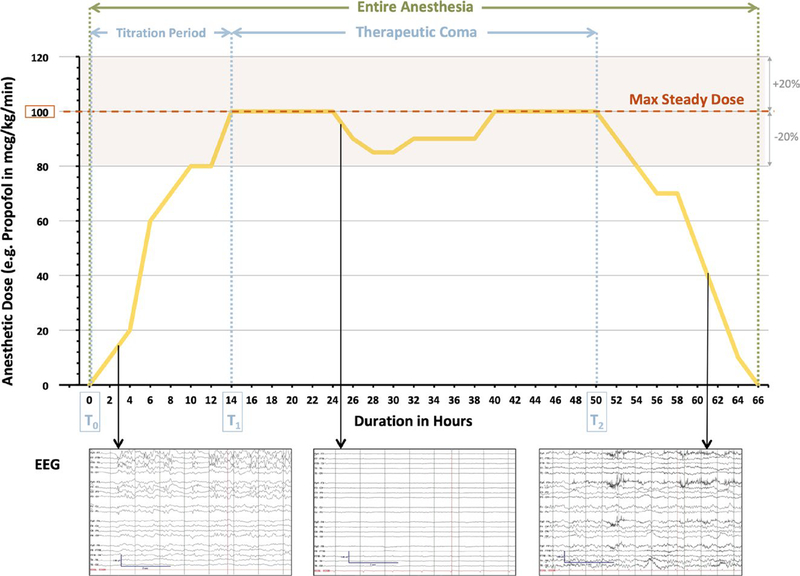
We utilized the hourly changes in infusion rates of each anesthetic as documented in the medical administration record (MAR) as a surrogate for induced coma depth and duration. The duration of TC was defined as the time frame in hours from time point T1 to T2. T1 is the very first time point at which the maximum steady dose of anesthetic was reached and maintained within a range of ±20% for at least three subsequent hours. T2 is the second time point at which the dose of anesthetic started to develop a significant downward trend, defined by a drop of at least 20% of the hourly infusion rate in three sub-sequent hours and an ultimate endpoint of 0 (Figure 1). Similar to prior studies comparing the efficacy and safety of different anesthetics, the maximum steady dose of anesthetic was converted to a time- and weight-based unit (μg/kg/min) across all anesthetic agents utilized in the first trial of TC.8,13,14,18,19
FIGURE 1.
Definition of duration of therapeutic coma (TC) and entire anesthesia. Duration of therapeutic coma is defined as the time frame in hours from time point T1 to T2. T1 is the very first time point at which the maximum steady dose of anesthetic is reached and maintained within a range of ±20% for at least three subsequent hours. T2 is the second time point at which the dose of anesthetic starts to develop a significant downward trend, defined by a drop of at least 20% of the hourly infusion rate in three subsequent hours and an ultimate endpoint of 0. In this example, TC with a maximal steady dose of 100 μg/kg/min of propofol leading to an adequate suppression of seizure activity on EEG (ie, T1) is achieved at 14 h after initiation of sedation. The end of the therapeutic coma (ie, T2) is defined by the initiation of a steady wean of the anesthetic, which starts at 50 h of sedation. This results in a calculated duration of therapeutic coma of 36 h. The time frame from time point T0 (start of anesthesia) to T1 (reach of maximum steady dose) represents the TC titration period
The primary outcome was recurrence of seizure activity either on EEG or clinical presentation within 48 hours of initiation of anesthetic taper (ie, time point T2), which has been described as withdrawal seizures in the literature.14 Secondary outcomes focused on mortality and morbidity associated with the admission for treatment of RSE. Mortality was defined as death, discharge to hospice, or transfer to comfort care at any time of the admission. Morbidity included a modified Rankin Scale (mRS) of ≥3, representing moderate to severe disability at the time of discharge and the development of any of the following in-hospital complications during the treatment for RSE: urinary tract infection, hospital-acquired/ventilator-associated pneumonia, deep vein thrombosis/pulmonary embolism, stroke, myocardial infarction, sepsis from any source, critical illness myopathy/neuropathy, or significant hypotension requiring the support of vasopressors. We also evaluated the total length of stay and the duration of ventilation in relation to duration of TC.
2.4 |. Treatment protocols for status epilepticus at UAB and UCSF
Both institutions utilized a predefined treatment protocol for status epilepticus. Treatment of SE started with administration of intravenous or intramuscular benzodiazepine (preferably lorazepam at 2–4 mg/dose and midazolam at 5–10 mg/dose) as the first-line therapy within the first 5–20 minutes of SE. If SE persists, both institutional protocols recommend treatment with intravenous fosphenytoin (20 mg/kg/dose), valproic acid (20–40 mg/kg/dose), or levetiracetam (60 mg/kg/dose) as the second-line therapy during the subsequent 20 minutes of SE. Thereafter, if SE still persists, intubation and initiation of TC is recommended. The preferred anesthetic agents at UAB and UCSF are propofol and midazolam followed by pentobarbital. At both institutions, propofol is started with a loading dose of 1–2 mg/kg with repetitive boluses of 1–2 mg/kg every 5 minutes until cessation of clinical seizure activity or a maximum dose of 10 mg/kg followed by a maintenance rate of 2 mg/kg/hour. Midazolam is started with an initial load of 0.2 mg/kg with repeated boluses of 0.2–0.4 mg/kg every 5 minutes until cessation of clinical seizure activity or up to a maximal dose of 2 mg/kg. Maintenance rate is 0.05–0.1 mg/kg/hour. Both institutions recommend continuous EEG monitoring if the patient does not awaken rapidly following first- and second-line treatment or if any form of TC is utilized. Anesthetic is titrated to suppress electrographic (nonconvulsive) seizure activity or to achieve a burst-suppression pattern with the EEG being suppressed for >50% of the time (ie, 1–2 second bursts of activity separated by 3–8 seconds of suppression). Only UAB does provide the guideline to maintain the patient in TC until a minimum of 24 hours of seizure control has been achieved.
2.5 |. Statistical analysis
Demographics, health status, details on clinical and treatment features of RSE, as well as TC were summarized using descriptive statistics. Median and interquartile range were reported for those continuous variables that showed a serious deviation from normality (such as Status Epilepticus Severity Score [STESS], Epidemiology-Based Mortality Score for Status Epilepticus [EMSE], duration of TC and entire sedation, total length of stay, and duration of ventilation). The relationships of primary and secondary out-comes (see preceding text) with demographics and clinical characteristics were initially evaluated using a two-sample t test (or Wilcoxon rank-sum test), simple linear regression, and chi-square test (or Fisher’s exact test) as appropriate. Multivariable logistic and linear regression models were used to identify independent predictors of outcomes, while adjusting for potentially confounding variables. To avoid overfitting, only variables with P < 0.2022,23 in the bivariate analysis were considered for inclusion into the final model. Multicollinearity was assessed using the Spearman correlation matrix and variance inflation factor (VIF).24 The inclusion criteria for the multivariable models were based on clinical relevance, statistical significance, VIF < 525,26 and correlation ρ < 0.40.27,28 The discrimination ability of TC with a cutoff value was quantified using the area under the curve (AUC) of a receiver-operating characteristic (ROC) curve. The Youden index (J) was used to determine the optimal cutoff time for duration of TC on seizure recurrence, where the sum of sensitivity and specificity was maximized. A P value <0.05 was considered statistically significant in two-tailed statistical tests. All analyses were conducted using SAS 9.4 (SAS Institute) and the ROC curve was generated using R 3.4.4.29
2.6 |. Selection of clinical variables for the multivariable model
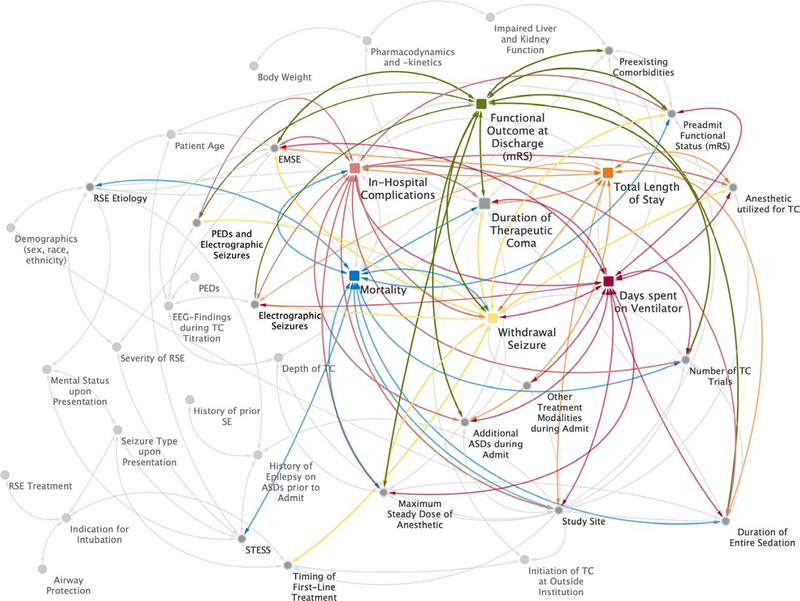
A selection of the commonly cited and examined clinical variables in outcome studies for RSE as well as their complex network of potential interactions with the main predictor,9–16,18,19,30,31 and the primary32,33 and all secondary outcomes2,9,34,35 is illustrated in the postulated causal diagram in Figure 2. The selection of variables included in the final multivariable models was based primarily on level of significance in the bivariate analysis. Yet to avoid overfitting of our models in the context of limited power with a cohort of 182 patients, we also needed to select confounding variables according to their clinical significance and overall generalizability.
FIGURE 2.
Postulated causal diagram illustration of the complex relationship between the predictor, various outcomes, and the major confounders. The colored squares represent the primary predictor (duration of TC), primary outcome (withdrawal seizure within the first 48 h of weaning the TC), and all secondary outcomes (functional outcome at time of discharge classified as modified Rankin Scale, days spent on ventilation, total length of stay, mortality, and in-hospital complications). The gray circles represent potential confounding variables for the predictor and its outcomes. The white arrows visualize these potential relationships, and the colorful arrows represent the confounding variables that showed a significant association in the bivariate analysis or have been found to be clinically significant/relevant for each outcome as a result of previous clinical studies on outcomes of refractory status epilepticus (RSE). SE, status epilepticus; TC, therapeutic coma; STESS: Status Epilepticus Severity Score; EMSE: Epidemiology-Based Mortality Score for Status Epilepticus; LTM-EEG: long-term monitoring electroencephalography; ASD: antiseizure drug; mRS: modified Rankin Scale; PEDs: pseudoperiodic epileptiform discharges
Variables that were significant on bivariate analyses but included only a small percentage of the study cohort (such as the small number of patients treated with lacosamide as a second-line therapy) or variables that were already included in other variables, were excluded (eg, age, pre-existing comorbidities, RSE etiology, and pseudoperiodic epileptiform discharges (PEDs) on EEG monitoring, all of which are already included in the EMSE score36; or age, previous history of epilepsy, presenting seizure type, and level of consciousness upon initial presentation, which are all part of the STESS37). On the other hand, variables that showed only a marginal significance on bivariate analysis but have been shown to be associated with either the primary or one of the secondary outcomes in other clinical studies were included (eg, delayed initiation of treatment >1 hour after SE onset, which has been connected previously to worse seizure control and increased risk for break-through seizures38,39). Other variables such as additional trials of TC, add-on ASDs, or other treatment modalities utilized during the admission were not included in the multivariable analysis for the primary but secondary out-comes, which were obtained either at the end or over the course of the entire admission and not just the time frame of the initial TC itself. Other variables had only weak significance on bivariate analysis but appeared clinically to be important to be included in the analysis (eg, the Charlson Comorbidity Index [CCI]).
3 |. RESULTS
3.1 |. Descriptive statistics
A total of 182 patients (105 from UAB and 77 from UCSF) with RSE were enrolled in the study. The demographics, health and functional status prior to admission, details on clinical and treatment features of RSE, as well as TC in total and within each cohort are summarized in Table 1.
TABLE 1.
Characteristics of patient cohort and bivariate analysis of primary outcome
| Seizure recurrence | |||||
|---|---|---|---|---|---|
| All (n = 182) | Missing | No (n = 138) | Yes (n = 44) | P value | |
| Demographics | |||||
| Age at admit, mean ± SD | 55.2 ± 18.5 | 0 | 55.4 ± 17.6 | 54.7 ± 21.2 | 0.84 |
| Gender, n (%) | |||||
| Male | 95 (52.2) | 0 | 72 (52.2) | 23 (52.3) | 0.99 |
| Female | 87 (47.8) | 66 (47.8) | 21 (47.7) | ||
| Race, n (%) | |||||
| White | 95 (52.2) | 0 | 73 (52.9) | 22 (50) | 0.94 |
| African American | 68 (37.4) | 51 (37) | 17 (38.6) | ||
| Others (Asian, Native American, Pacific Islander, etc.) | 19 (10.4) | 14 (10.1) | 5 (11.4) | ||
| Health and functional status prior to admit | |||||
| Weight at admit (kg), mean ± SD | 79 ± 21.1 | 51 | 80 ± 20.5 | 76.4 ± 23.1 | 0.41 |
| Previous history of epilepsy, n (%) | 102 (56) | 0 | 81 (58.7) | 21 (47.7) | 0.2 |
| Prior history of status epilepticus, n (%) | 26 (25.7) | 81 | 21 (26.3) | 5 (23.8) | 0.82 |
| Number of ASDs prior to admit, mean ± SD | 1.7 ± 1.1 | 85 | 1.6 ± 1.1 | 2 ± 1.1 | 0.12 |
| CCI, mean ± SD | 2.2 ± 2.3 | 0 | 2.2 ± 2.4 | 2.3 ± 2.1 | 0.69 |
| Modified Rankin Scale (mRS), n (%) | |||||
| No symptoms/mild disability (mRS ≤2) | 47 (25.9) | 0 | 40 (28) | 7 (15.9) | 0.05 |
| Moderate/severe disability (mRS ≥3) | 47 (25.8) | 30 (21.7) | 17 (38.6) | ||
| Unknown | 88 (48.4) | 68 (49.3) | 20 (45.5) | ||
| Clinical details of RSE | |||||
| RSE etiologies,a n (%) | |||||
| Acute | 82 (45.1) | 0 | 61 (44.2) | 21 (47.7) | 0.33 |
| Remote | 58 (31.9) | 45 (32.6) | 13 (29.6) | ||
| Progressive | 3 (1.7) | 1 (0.7) | 2 (4.6) | ||
| Unknown | 39 (21.4) | 31 (22.5) | 8 (18.2) | ||
| Presenting seizure type prior to first treatment, n (%) | |||||
| Focal onset to bilateral tonic-clonic/generalized onset tonic-clonic seizuresb | 149 (81.9) | 0 | 114 (82.6) | 35 (80) | 0.65f |
| Focal onset nonmotor seizures presenting with comac | 8 (4.4) | 7 (5.1) | 1 (2.3) | ||
| Focal onset motor/nonmotor seizures with impaired awarenessd | 4 (2.2) | 3 (2.2) | 1 (2.3) | ||
| Focal onset motor/nonmotor seizures without impaired awarenesse | 19 (10.4) | 12 (8.7) | 7 (15.9) | ||
| Unknown/unclassified | 2 (1.1) | 2 (1.5) | 0 (0) | ||
| RSE severity | 0 | ||||
| STESS, median [IQR] | 3 [2] | 3 [2] | 3 [2] | 0.78 | |
| EMSE, median [IQR] | 68 [66] | 66 [68] | 80 [71] | 0.13 | |
| Epileptiform activity on LTM-EEGg during TC titration period, n (%) | |||||
| Pseudoperiodic epileptiform discharges (PEDs) at frequency of ≤3 Hertz | 36 (25) | 0 | 25 (18.1) | 11 (25) | 0.61 |
| Electrographic seizures | 42 (29.2) | 0 | 29 (21) | 13 (30) | 0.014 |
| PEDs and electrographic seizures | 23 (16) | 0 | 16 (11.6) | 7 (15.9) | 0.55 |
| Details on initial treatment approach to RSE | |||||
| Treatment > 1 h after seizure onset, n (%) | 114 (73.6) | 27 | 84 (72.4) | 30 (76.9) | 0.58 |
| First-line treatment (BZD), n (%) | |||||
| Lorazepam (LZP) | 155 (85.2) | 0 | 116 (84.1) | 39 (88.6) | 0.46 |
| Midazolam (MDZ) | 40 (22) | 0 | 30 (21.7) | 10 (22.7) | 0.89 |
| Diazepam (DMP) | 24 (13.2) | 0 | 18 (13) | 6 (13.6) | 0.92 |
| Second-line treatment (nonsedating ASD), n (%) | |||||
| Levetiracetam (LEV) | 116 (63.7) | 0 | 81 (58.7) | 23 (52.3) | 0.15 |
| Phenytoin (PHT) | 104 (57.1) | 0 | 84 (60.9) | 32 (72.7) | 0.45 |
| Valproic acid (VPA) | 21 (11.5) | 0 | 12 (8.7) | 9 (20.5) | 0.03 |
| Lacosamide (LCM) | 12 (6.6) | 0 | 9 (6.5) | 3 (6.8) | 1.0 |
| Additional ASDs during hospitalization, mean ± SD | 2.6 ± 1.3 | 4 | 2.3 ± 1.1 | 3.3 ± 1.4 | <0.0001 |
| Other treatment modalities (eg, steroids, IVIG, PLEX, KD, ECT), n (%) | 26 (15.3) | 0 | 16 (11.6) | 10 (22.7) | 0.07 |
| Details of therapeutic coma (TC) | |||||
| Initiation of TC at outside institution, n (%) | 56 (30.8) | 0 | 43 (31.2) | 13 (29.6) | 0.84 |
| Indication for intubation, n (%) | |||||
| Airway protection | 153 (84.1) | 0 | 115 (83.3) | 38 (86.4) | 0.63 |
| Initiation of TC | 29 (36.3) | 43 (31.2) | 6 (13.6) | ||
| Anesthetic utilized during first trial of TC, n (%) | |||||
| Propofol | 162 (89) | 0 | 125 (68.8) | 21 (47.7) | 0.1 |
| Midazolam | 20 (11.1) | 12 (8.8) | 8 (18.2) | ||
| Duration of first trial of TC in hours, median [IQR] | 17.4 [32.5] | 0 | 15.6 [25.3] | 27.2 [51.3] | 0.02 |
| Duration of first trial of entire sedation in hours, median [IQR] | 35.1 [58.5] | 0 | 31.2 [50.4] | 56.5 [68.1] | 0.11 |
| Maximal steady dose of anesthetic during first trial (μg/kg/min), mean ± SD | 42.6 ± 24.6 | 0 | 44 ± 23.2 | 38.4 ± 28.5 | 0.25 |
| Propofol (μg/kg/min), mean ± SD | 46 ± 22.6 | 46.3 ± 21.6 | 45.1 ± 26.1 | ||
| Midazolam (mg/h), mean ± SD | 3.5 ± 3.7 | 3.1 ± 2.4 | 3.9 ± 4.8 | ||
| Number of trials of TC, n (%) | |||||
| <2 Trials | 116 (63.7) | 0 | 95 (68.8) | 21 (47.7) | 0.01 |
| ≥2 Trials | 66 (36.6) | 43 (31.2) | 23 (52.3) | ||
ASDs, antiseizure drugs; CCI, Charlson Comorbidity Index; CNS, central nervous system; ECT, electroconvulsive therapy; EMSE, Epidemiology-Based Mortality Score for Status Epilepticus; ILAE, International League Against Epilepsy; IQR, interquartile range; IVIG, intravenous immunoglobulin; KD, ketogenic diet; LTM-EEG, long-term monitoring electroencephalography; mRS, modified Rankin Scale; n, number; PEDs, pseudoperiodic epileptiform discharges; PLEX, plasma exchange; RSE, re-fractory status epilepticus; SD, standard deviation; STESS, Status Epilepticus Severity Score; TC, therapeutic coma.
Etiologies classified according to the ILAE classification of SE.21
Former generalized tonic–clonic seizures.
Former nonconvulsive seizures with coma.
Former complex partial seizures.
Former simple partial seizures including epilepsia partialis continua (EPC).
For statistical analysis, all the seizure types without a tonic– clonic component as motor manifestation were grouped together.
According to the American Clinical Neurophysiology Society’s Standardized Critical Care EEG Terminology: 2012 Version.50
3.2 |. Bivariate analysis
There were no significant differences in baseline demographics, preadmission health status, and preadmission functional status (ie, mRS) between patients with and without seizure recurrence. There were also no differences in underlying etiology, clinical details, initial treatment approach, and severity of RSE as defined by STESS and EMSE scores (Table 1).
The group with seizure recurrence was treated for a longer duration during their first trial of TC than the nonrecurrence group (27.2 [51.3] vs 15.6 [25.3] hours, P = 0.02; Table 1). In addition, duration of TC was significantly associated with increased number of in-hospital complications (P = 0.002) and showed a marginal significance with worse functional neurologic outcomes on discharge (P = 0.06). There was no significant association between duration of first trial of TC and mortality (P = 0.32).
Of interest, higher maximal steady doses of anesthetic during the initial trial of TC (Figure 1) were linked to no in-hospital complications (P < 0.0001), survival (marginal P = 0.05), and better functional neurologic outcome at discharge (P = 0.013). On average, patients with sustained seizure control were treated with higher maximal steady doses of anesthetic than patients with seizure recurrence. However, the difference was not statistically significant (44 ± 23.2 vs 38.4 ± 28.5 μg/kg/min; P = 0.25). Other variables significantly associated with seizure recurrence after the initial trial of TC—mortality, poor functional neurologic outcome at time of discharge, and in-hospital complications—are summarized in Table 1 and Table S2.
The bivariate analysis between the patient cohorts at UAB and UCSF revealed some regional differences in terms of demographics, baseline health status, and prior history of SE, as well as clinical and treatment details for RSE (Table S1). Although the duration of TC was similar between both institutions, the duration of the entire sedation was significantly longer (50.1 [59] vs 19.6 [42.4] hours; P < 0.0001) and there were more trials of TC at UAB than at UCSF. The average maximal steady dose of anesthetic during the first trial of TC was significantly higher at UCSF than at UAB (38 ± 18.7 vs 48.5 ± 29.7 μg/kg/min; P = 0.008). Despite these differences, both cohorts had a seizure recurrence rate of 24% after the first trial of anesthesia. However, on average, UAB patients spent almost twice as long on mechanical ventilation (5 vs 3 days; P = 0.002), had 24% more in-hospital complications (P = 0.002), and had more than twice the mortality rate compared to their UCSF counterparts (20% vs 9.1%; P = 0.04; Table S1).
We also determined whether the presence of electrographic seizures and pseudoperiodic epileptiform discharges (PEDs) on EEG during the TC titration period (time epoch T0 to T1 in Figure 1) influenced the duration of the first trial of TC and the maximal steady dose of anesthetic utilized for that trial. The analysis showed that the presence of seizures on EEG during the titration period was significantly associated with a prolonged duration of TC (28.6 [38.2] vs 11.2 [17.1] hours; P = 0.001) and with a lower maximal steady dose of anesthetic utilized for that trial (36.5 ± 21.2 vs 46 ± 21.8 μg/kg/min; P = 0.028). However, PEDs on EEG during the titration period were not significantly associated with either maximal steady dose of anesthetic (P = 0.27) or TC duration (P = 0.07).
3.3 |. Multivariable analysis
After adjusting for potential confounding variables, duration of TC remained significantly associated with seizure recurrence (OR 1.2, 95% CI 1.01–1.43; P = 0.038) but not for in-hospital complications (Table 2). In this patient cohort, for every additional 12 hours of TC, there was a 20% greater chance of seizure recurrence following the first weaning attempt. Duration of TC was also positively associated with total length of stay (β = 0.13; SE = 0.06; P = 0.003) and days spent on ventilation (β = 0.14; SE = 0.03; P < 0.0001; Table S3).
TABLE 2.
Multivariable linear regression analysis for primary and secondary outcomes
| Seizure recurrence | |||
|---|---|---|---|
| Variable | Adjusted OR | 95% CI | P value |
| Duration of first trial of TC (12 h) | 1.2 | (1.01, 1.43) | 0.038 |
| Modified Rankin Scale (mRS) prior to admit | |||
| No symptoms/mild disability (mRS ≤2) | Reference | Reference | 0.11 |
| Moderate/severe disability (mRS ≥3) | 2.53 | (0.81, 7.92) | |
| Unknown | 0.06 | (0.001, 2.82) | |
| Maximal steady dose of anesthetic during first trial (40 μg/kg/min) | 0.89 | (0.41, 1.97) | 0.78 |
| Anesthetic utilized during first trial of TC (propofol vs midazolam) | 1.88 | (0.32, 11.1) | 0.49 |
| Study site (UCSF vs UAB) | 0.07 | (0.002, 2.92) | 0.16 |
| Treatment > 1 h after seizure onset | 1.11 | (0.38, 3.23) | 0.86 |
| EMSE (64 Units) | 1.14 | (0.55, 2.37) | 0.74 |
| Electrographic seizures during TC titration period | 2.8 | (0.64, 12.2) | 0.005 |
| PEDs and electrographic seizures during TC titration period | 0.81 | (0.11, 5.92) | 0.02 |
| In-hospital complications | |||
| Adjusted OR | 95% CI | P value | |
| Duration of first trial of TC (12 h) | 1.07 | (0.9, 1.27) | 0.46 |
| Maximum steady dose of anesthetic during first trial (40 μg/kg/min) | 0.32 | (0.15, 0.67) | 0.003 |
| Number of trials of TC (≥2 vs <2) | 1.97 | (0.77, 5.07) | 0.16 |
| Additional ASDs during hospitalization | 1.07 | (0.75, 1.52) | 0.71 |
| Modified Rankin Scale (mRS) prior to admit | |||
| No symptoms/mild disability (mRS ≤2) | Reference | Reference | 0.19 |
| Moderate/severe disability (mRS ≥3) | 1.51 | (0.54, 4.27) | |
| Unknown | 14.7 | (0.72, 301.8) | |
| Levetiracetam (LEV) as second-line treatment | 1.17 | (0.53, 2.59) | 0.71 |
| EMSE (64 Units) | 1.27 | (0.65, 2.47) | 0.49 |
| Seizure recurrence after first trial of TC | 2.26 | (0.83, 6.13) | 0.11 |
| Study site (UCSF vs UAB) | 15.9 | (0.77, 325.6) | 0.07 |
| Anesthetic utilized during first trial of TC (propofol vs midazolam) | 0.98 | (0.12, 7.81) | 0.99 |
| Electrographic seizures during titration period | 1.2 | (0.33, 4.38) | 0.96 |
| PEDs and electrographic seizures during TC titration period | 1.55 | (0.28, 8.54) | 0.77 |
| Functional outcome of mRS ≥3 upon discharge | |||
| Adjusted OR | 95% CI | P value | |
| Duration of TC (12 h) | 1.02 | (0.85, 1.22) | 0.84 |
| Modified Rankin Scale (mRS) prior to admit | |||
| No symptoms/mild disability (mRS ≤2) | Reference | Reference | 0.036 |
| Moderate/severe disability (mRS ≥3) | 5.74 | (1.52, 21.7) | |
| Unknown | 3.29 | (0.24, 45.8) | |
| EMSE (64 Units) | 0.93 | (0.38, 2.25) | 0.86 |
| Lorazepam (LZP) as first-line treatment | 0.75 | (0.24, 2.38) | 0.63 |
| CCI | 1.11 | (0.91, 1.35) | 0.3 |
| Anesthetic utilized during first trial of TC (propofol vs midazolam) | 1.44 | (0.17, 12.2) | 0.74 |
| Maximum steady dose of anesthetic during first trial (40 μg/kg/min) | 0.45 | (0.19, 1.08) | 0.07 |
| Initiation of TC at home vs outside institution | 2.23 | (0.8, 6.22) | 0.13 |
| In-hospital complications | 1.13 | (0.45, 2.83) | 0.79 |
| Study site (UCSF vs UAB) | 1.05 | (0.09, 11.8) | 0.97 |
| Number of trials of TC (≥2 vs <2) | 1.83 | (0.62, 5.46) | 0.28 |
| Seizure recurrence after first trial of TC | 3.44 | (1.03, 11.4) | 0.044 |
| Electrographic seizures during titration period | 0.08 | (0.01, 0.97) | 0.13 |
| PEDs and electrographic seizures during TC titration period | 23.9 | (1.59, 359) | 0.07 |
| Mortality | |||
| Adjusted OR | 95% CI | P value | |
| Modified Rankin Scale (mRS) prior to admit | |||
| No symptoms/mild disability (mRS ≤2) | Reference | Reference | 0.08 |
| Mod/severe disability (mRS ≥3) | 3.65 | (0.97, 13.7) | |
| Unknown | 6.77 | (0.99, 46.6) | |
| Duration of first trial of TC in hours (12 h) | 1.02 | (0.93, 1.13) | 0.19 |
| Duration of first trial of entire sedation in hours (12 h) | 0.87 | (0.7, 1.08) | 0.65 |
| RSE etiology | |||
| Acute | Reference | Reference | 0.13 |
| Remote | 0.2 | (0.05, 0.86) | |
| Progressive | 3.84 | (0.23, 64.3) | |
| Unknown | 0.56 | (0.15, 2.09) | |
| In-hospital complications | 4.44 | (1.01, 19.5) | 0.048 |
| STESS | 1.58 | (1.07, 2.33) | 0.02 |
| Seizure recurrence after first trial of TC | 1.35 | (0.48, 3.83) | 0.57 |
| Maximum steady dose of anesthetic during first trial (40 μg/kg/min) | 0.59 | (0.24, 1.46) | 0.26 |
| Study site (UCSF vs UAB) | 6.46 | (0.92, 45.4) | 0.06 |
| Number of trials of TC (≥2 vs <2) | 3.01 | (1.1, 8.25) | 0.03 |
ASD, antiseizure drug; CCI, Charlson Comorbidity Index; CI, confidence interval; EMSE, Epidemiology-Based Mortality Score for Status Epilepticus; LTM-EEG, long-term monitoring electroencephalography; mRS, modified Rankin Scale; OR, odds ratio; PEDs, pseudoperiodic epileptiform discharges; RSE, refractory status epilepticus; SE, standard error; TC, therapeutic coma; UAB, University of Alabama at Birmingham; UCSF, University of California San Francisco.
Maximal steady dose of anesthetic during the first trial of TC remained an independent predictor for in-hospital complications. With every 40 μg/kg/min (ie, achieving a deeper TC) increase of anesthetic dose, the patients were 68% less likely to experience complications during hospitalization (OR 0.32; 95% CI 0.15–0.67; P = 0.003). Maximal steady dose of anesthetic was also negatively correlated with duration of ventilation (P = 0.012) and length of stay (P = 0.015). This means that higher doses of anesthetic and therefore a deeper TC resulted in shorter time spent on mechanical ventilation and shorter length of stays.
Other factors that remained independent predictors for poor functional neurologic outcome upon discharge, in-hospital complications, duration of mechanical ventilation, and mortality after adjusting for clinically important confounders are summarized in Table 2 and Table S3.
3.4 |. ROC curve and Youden index
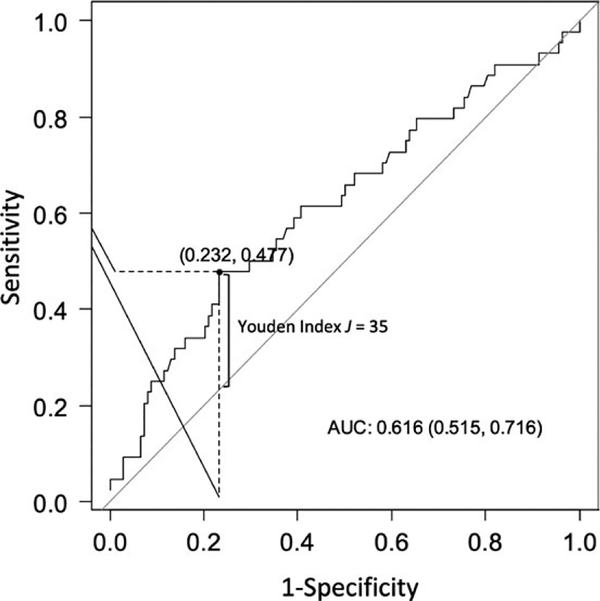
The ROC curve analysis and Youden index suggested 35 hours as the time point for TC duration with the maximal sum of sensitivity and specificity for the prediction of seizure recurrence, providing a sensitivity of 48% and a specificity of 77% (AUC 0.62; 95% CI 0.52–0.72; Figure 3). Beyond this time point, almost half of the patients had seizure recurrence, whereas 141 of 182 patients (77%) with ≤35 hours of TC had no recurrence after the anesthetic was weaned.
FIGURE 3.
Receiver-operating characteristic (ROC) curve for duration of therapeutic coma and seizure recurrence. Area under the curve (AUC) represents the predictive ability of therapeutic coma duration for seizure recurrence after the first trial of therapeutic coma. Youden index J is the point on the ROC curve that is farthest from the line of equality (diagonal line)
4 |. DISCUSSION
This retrospective, observational study systematically analyzed the association between duration of TC and various outcomes in RSE and used clinical data to suggest an efficient and safe window for the duration of TC as treatment for RSE.
4.1 |. TC duration and outcome of RSE
Our analysis suggests an association between prolonged duration of TC, seizure recurrence after the first trial of TC, prolonged total length of stay, and days spent on ventilation, but not with in-hospital complications, poor functional neurologic outcome upon discharge, or death. The association between prolonged ventilation and increased length of stay and extended duration of TC seems self-explanatory. However, the relationship between duration of TC and seizure recurrence is not intuitive and requires further deliberation.
One could argue that patients with worse clinical presentations, or more severe or acute etiologies are at higher risk for seizure recurrence and, therefore, more likely to be maintained in prolonged TC. However, none of these factors were significantly different between the recurrence and no-recurrence group. The presence of electrographic seizure activity on EEG during the TC titration period was the only other variable independently associated with seizure recurrence. Seizure activity was also associated with duration of TC in the bivariate analysis. Yet with correcting for this potential confounder, duration of TC remained independently associated with seizure recurrence, which raises the following questions: Is prolonged duration of TC any better for sustained seizure control than shorter coma periods, and does prolonged TC contribute to recurrence of seizure activity?
Two retrospective studies including a recent, multicenter study looking at 362 episodes of SE from two major academic medical centers showed a similar association between the use of TC and these outcomes without an increased risk for mortality.9,31 Several other studies suggested that TC increases the risk for infections, new disability upon discharge, and mortality.10,11,13,40,41 Similar to our cohort, the duration of TC in all these studies had a relatively wide range of treatment duration anywhere from 24 to 96 hours. The rate of seizure recurrence following treatment with TC was found to be be-tween 10% and 20%, but none of these studies looked at seizure recurrence in association with duration of TC.10,11,13,40,41 Only one study analyzed the association between duration of TC and various clinical outcomes, and found that treatment duration for greater than 20 hours was associated with poor functional outcome and death.19 However, none of these studies had as strict of a definition for TC as our study and they most likely included the titration and weaning period for anesthetics in those time frames, which overestimates the actual duration of TC. Moreover, variable and nonstandardized protocols for TC at each institution make it difficult to compare these findings across studies.
One neurobiologic explanation as to how prolonged TC may be associated with an increased risk of withdrawal seizures is the homeostatic plasticity hypothesis that was originally proposed by Turrigiano et al.42 According to their hypothesis, neuronal networks are optimized to maintain firing rates within a small range. One of these adaptive mechanisms is synaptic scaling, which allows neurons to detect changes in their own firing rates through a set of calcium-dependent sensors that then regulate receptor trafficking to increase or decrease excitability of the neuronal network by modifying the number of glutamate receptors available at synaptic sites.42 As such, the prolonged exposure to anesthetic agents that are inhibitory and decrease neuronal firing rates might lead to a compensatory upregulation of excitatory receptors such as α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) and N-methyl-D-asparate (NMDA) receptors, which puts the brain at an increased risk for withdrawal seizures, once that chronic inhibition is being removed (ie, TC is weaned).
4.2 |. TC depth and outcome in RSE
Maximal steady dose of anesthetic during the first trial of TC (Figure 1) was significantly associated with fewer in-hospital complications and negatively correlated with time spent on ventilation and total length of stay in this cohort. Anesthetics (such as propofol and midazolam) are well known to suppress EEG activity in a dose-dependent fashion, that is, higher doses of anesthetic result in more profound burst-suppression pattern on EEG or a deeper TC.43,44 Patients who underwent a deeper TC (ie, received higher doses of anesthetic) were 68% less likely to experience complications during their hospitalization than patients maintained under lighter sedation. This might be in part related to the fact that higher maximal steady doses of anesthetic were significantly associated with lower prevalence of electrographic seizures on EEG during the TC titration period, which is an independent predictor for seizure recurrence after the first trial of TC. This association is also supported by the bivariate analysis comparing the primary and secondary outcomes in the UAB and UCSF patient cohorts. Patients at UCSF were on average treated with the same duration but higher maximal steady doses of anesthetic during their first trial of TC and had significantly fewer in-hospital complications, a shorter duration of ventilation, and a lower mortality rate compared to their UAB counterparts. This is somewhat in contrast to a previous retrospective study, which suggested that a more profound TC was associated with a higher incidence of in-hospital complications including significant hypotension requiring the support of vasopressors and failure to wean from ventilator support.14 However, the majority of patients in that study were treated with pentobarbital rather than midazolam or propofol as in our cohort. Other studies found sustained seizure control and even lower mortality rates in RSE patients treated with high doses of anesthetic, even though those studies looked primarily at midazolam and pentobarbital rather than propofol as the main anesthetic agent. All studies considered early and sustained seizure control, which might be in part related to cumulative effects of redistributed high-dose anesthetic as the main reason for lower mortality rates.15,30,45,46 Even though in our study cohort patients with sustained seizure control were on average treated with higher doses of anesthetic than patients with seizure recurrence, we were not powered to reveal a significant association between these two variables.
4.3 |. A safe and effective window for TC in RSE
Our study suggests that an initial trial of TC with a duration of greater than 35 hours can be associated with an increased risk for seizure recurrence following the anesthetic wean. This cutoff lies within the currently recommended time window of 24–48 hours.3,6,7,47 Furthermore, our study suggests that higher doses of anesthetic (ie, deeper TC) are not only safe to use but also associated with fewer in-hospital complications and shorter duration of ventilation and total length of stay. Our findings suggest that clinicians should consider the duration and depth of TC when managing patients with RSE and should try to expose the patient for the shortest time possible with an adequately deep TC throughout the entire duration of treatment.
4.4 |. Study limitations
This is a retrospective, observational cohort study, which always carries the risk of extraction, allocation, and recall bias, and commonly encounters the challenge of an incomplete dataset. As such, a retrospective study can only reveal associations and assist with hypothesis generation for future clinical research questions but cannot establish clear causation between the variables and outcomes examined.
Although including patients from two large academic medical centers ensures some degree of diversity and generalizability of our study population, and there were similar standardized treatment protocols for RSE at both institutions, the strict adherence to these protocols with regard to titration, maintenance, and weaning of TC as well as the supervision of proper coma depth was not supervised. Unfortunately, the continuous video-EEG files were routinely clipped and pruned at both institutions, which made it impossible to verify the diagnosis of electrographic SE, and the proper burst-suppression and the actual duration of TC on EEG.
In addition, our analysis included patients who were intubated and placed on anesthesia at outside facilities prior to transfer to our study sites. This means that in some RSE patients the duration of TC was possibly longer than reported—even though not necessarily maintained at an adequate coma depth due to lack of EEG monitoring. We also included patients who were intubated for profound sedation and airway protection following first- and second-line treatment for SE, rather than just for the initiation of TC. Some of these patients did not have evidence of ongoing seizure activity on continuous video-EEG monitoring a couple of hours after intubation, which most likely meant an interval resolution of RSE even before initiation of TC. Fortunately the proportion of patients falling into either of the above cohorts was not significantly different for any of the outcomes analyzed in our study other than the functional neurologic outcome on discharge.
5 |. CONCLUSION AND FUTURE DIRECTIONS
This retrospective analysis of the association between duration of TC and seizure recurrence, mortality, and morbidity in patients with RSE treated with anesthetics (mainly propofol and midazolam) suggests that prolonged duration of TC (>35 hours) was associated with increased risk for withdrawal seizures, prolonged length of hospital stay, and days spent on ventilation. Furthermore, a deeper coma appears to be an important factor for a decrease rate of in-hospital complications, shorter duration of ventilation, and total length of stay, but an association between coma depth and sustained seizure control after the first trial of TC could not be established.
In the future, EEG-based scores that help with prediction of seizure recurrence48 could be utilized to determine more patient- and etiology-specific time points for TC, whereas quantitative analysis of the EEG signal49 could help the medical staff with titrating the anesthetic to an adequate coma depth. Finally, despite all efforts to obtain a comprehensive dataset, our cohort study cannot replace the longstanding need for a prospective, randomized clinical trial to determine the safest and most efficient duration of TC and to better characterize the short- and long-term functional and cognitive outcomes in patients undergoing TC as treatment for RSE.
Supplementary Material
Key Points.
Therapeutic coma (TC) duration is independently associated with seizure recurrence after weaning attempt, prolonged hospitalization, and mechanical ventilation.
Deeper TC is independently associated with fewer in-hospital complications, and shorter duration of mechanical ventilation and length of stay.
Shorter duration (<35 hours) yet more profound TC for refractory status epilepticus (RSE) might be as effective and safer than the current guideline of 24–48 hours.
Clinicians should try to expose RSE patients to TC for the shortest time possible while ensuring an adequate depth of coma.
ACKNOWLEDGMENTS
Statistical analysis of the research reported in this publication was partially supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under award number UL1TR001417.
Funding information
Statistical analysis of research reported in this publication was partially supported by National Center for Advancing Translational Sciences of the National Institutes of Health under award number UL1TR001417.
Footnotes
CONFLICTS OF INTEREST
Dr. Muhlhofer receives funding from Eisai Inc. in the last 24 months. Dr. Szaflarski received funding from the NIH, the National Science Foundation (NSF), Shor Foundation for Epilepsy Research, EFA, Department of Defense, UCB Biosciences, US Food and Drug Administration (FDA), AES, SAGE Therapeutics Inc., GW Pharmaceuticals, Biogen, and Eisai Inc.; served as a Consultant for SK LifeScience Inc., GW Pharmaceuticals Inc., NeuroPace, Inc., Upsher-Smith Laboratories, Inc., Medical Association of the state of Alabama, Serina Therapeutics Inc., LivaNova Inc., and Elite Medical Experts LLC (legal); and served on editorial boards for Epilepsy & Behavior, Journal of Epileptology (associate editor), Restorative Neurology and Neuroscience (associate editor), Journal of Medical Science, Epilepsy Currents (contributing editor), and Folia Medica Copernicana. Stephen Layfield, Daniel Lowenstein, Chee Paul Lin, Robert D. Johnson, and Shalini Saini report no disclosures. We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section at the end of the article.
REFERENCES
- 1.Towne AR. Epidemiology and outcomes of status epilepticus in the elderly. Int Rev Neurobiol. 2007;81:111–27. [DOI] [PubMed] [Google Scholar]
- 2.Dham BS, Hunter K, Rincon F. The epidemiology of status epilepticus in the United States. Neurocrit Care. 2014;20:476–83. [DOI] [PubMed] [Google Scholar]
- 3.Brophy GM, Bell R, Claassen J, Alldredge B, Bleck TP, Glauser T, et al. Guidelines for the evaluation and management of status epilepticus. Neurocrit Care. 2012;17:3–23. [DOI] [PubMed] [Google Scholar]
- 4.Beg JM, Anderson TD, Francis K, Meckley LM, Fitzhenry D, Foster T, et al. Burden of illness for super-refractory status epilepticus patients. J Med Econ. 2017;20:45–53. [DOI] [PubMed] [Google Scholar]
- 5.Kortland LM, Alfter A, Bahr O, Carl B, Dodel R, Freiman TM, et al. Costs and cost-driving factors for acute treatment of adults with status epilepticus: a multicenter cohort study from Germany. Epilepsia. 2016;57:2056–66. [DOI] [PubMed] [Google Scholar]
- 6.Glauser T, Shinnar S, Gloss D, Alldredge B, Arya R, Bainbridge J, et al. Evidence-based guideline: treatment of convulsive status epilepticus in children and adults: report of the guideline committee of the American Epilepsy Society. Epilepsy Curr. 2016;16:48–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meierkord H, Boon P, Engelsen B, Göcke K, Shorvon S, Tinuper P, et al. EFNS guideline on the management of status epilepticus in adults. Eur J Neurol. 2010;17:348–55. [DOI] [PubMed] [Google Scholar]
- 8.Reznik ME, Berger K, Claassen J. Comparison of intravenous anesthetic agents for the treatment of refractory status epilepticus. J Clin Med. 2016;5:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alvarez V, Lee JW, Westover MB, Drislane FW, Novy J, Faouzi M. Therapeutic coma for status epilepticus: differing practices in a prospective multicenter study. Neurology. 2016;87:1650–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kowalski RG, Ziai WC, Rees RN, Werner JK Jr, Kim G, Goodwin H, et al. Third-line antiepileptic therapy and outcome in status epilepticus: the impact of vasopressor use and prolonged mechanical ventilation. Crit Care Med. 2012;40:2677–84. [DOI] [PubMed] [Google Scholar]
- 11.Marchi NA, Novy J, Faouzi M, Stähli C, Burnand B, Rossetti AO. Status epilepticus: impact of therapeutic coma on outcome. Crit Care Med. 2015;43:1003–9. [DOI] [PubMed] [Google Scholar]
- 12.Sutter R, Marsch S, Fuhr P, Kaplan PW, Rüegg S. Anesthetic drugs in status epilepticus: risk or rescue? A 6-year cohort study. Neurology. 2014;82:656–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bellante F, Legros B, Depondt C, Créteur J, Taccone FS, Gaspard N. Midazolam and thiopental for the treatment of refractory status epilepticus: a retrospective comparison of efficacy and safety. J Neurol. 2016;263:799–806. [DOI] [PubMed] [Google Scholar]
- 14.Claassen J, Hirsch LJ, Emerson RG, Mayer SA. Treatment of refractory status epilepticus with pentobarbital, propofol, or midazolam: a systematic review. Epilepsia. 2002;43:146–53. [DOI] [PubMed] [Google Scholar]
- 15.Krishnamurthy KB, Drislane FW. Depth of EEG suppression and outcome in barbiturate anesthetic treatment for refractory status epilepticus. Epilepsia. 1999;40:759–62. [DOI] [PubMed] [Google Scholar]
- 16.Parviainen I, Uusaro A, Kalviainen R, Mervaala E, Ruokonen E. Propofol in the treatment of refractory status epilepticus. Intensive Care Med. 2006;32:1075–9. [DOI] [PubMed] [Google Scholar]
- 17.Power KN, Gramstad A, Gilhus NE, Engelsen BA. Prognostic factors of status epilepticus in adults. Epileptic Disord. 2016;18:297–304. [DOI] [PubMed] [Google Scholar]
- 18.Prasad A, Worrall BB, Bertram EH, Bleck TP. Propofol and midazolam in the treatment of refractory status epilepticus. Epilepsia. 2001;42:380–6. [DOI] [PubMed] [Google Scholar]
- 19.Rossetti AO, Milligan TA, Vulliemoz S, Michaelides C, Bertschi M, Lee JW. A randomized trial for the treatment of refractory status epilepticus. Neurocrit Care. 2011;14:4–10. [DOI] [PubMed] [Google Scholar]
- 20.Claassen J, Hirsch LJ, Mayer SA. Treatment of status epilepticus: a survey of neurologists. J Neurol Sci. 2003;211:37–41. [DOI] [PubMed] [Google Scholar]
- 21.Trinka E, Cock H, Hesdorffer D, Rossetti AO, Scheffer IE, Shinnar S, et al. A definition and classification of status epilepticus–Report of the ILAE task force on classification of status epilepticus. Epilepsia. 2015;56:1515–23. [DOI] [PubMed] [Google Scholar]
- 22.Maldonado G, Greenland S. Simulation study of confounder-selection strategies. Am J Epidemiol. 1993;138:923–36. [DOI] [PubMed] [Google Scholar]
- 23.Vittinghoff E, Glidden DV, Shiboski SC, McCulloch CE. Statistics for biology and health Regression methods in biostatistics: Linear, logistic, survival, and repeated measures models. New York, NY: Springer Publishing Co, 2005. [Google Scholar]
- 24.Allison PD. Logistic regression using SAS®: theory and application. Cary, NC: SAS Institute Inc.; 2012. [Google Scholar]
- 25.O’Brien RM. A caution regarding rules of thumb for variance inflation factors. Qual Quant. 2007;41:673–90. [Google Scholar]
- 26.Kutner MH, Nachtsheim C, Neter J. Applied linear regression models. New York, NY: McGraw–Hill Irwin, 2004. [Google Scholar]
- 27.Methods development for assessing air pollution control benefits Volume 1 Office of Health and Ecological Effects; United States Environmental Protection Agency. Office of Health and Ecological Effects; EPA-600/5-79-001a. 1979; 44. [Google Scholar]
- 28.Dormann CF. Collinearity: a review of methods to deal with it and a simulation study evaluating their performance. Ecography (Cop). 2013;36:027–46. [Google Scholar]
- 29.López-Ratón M, Rodríguez-Álvarez MX, Cadarso-Suárez C, Sampedro FG. Optimalcutpoints: an R package for selecting optimal cutpoints in diagnostic tests. J Stat Softw. 2014;61:9–18. [Google Scholar]
- 30.Fernandez A, Lantigua H, Lesch C, Shao B, Foreman B, Schmidt JM. High-dose midazolam infusion for refractory status epilepticus. Neurology. 2014;82:359–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kang BS, Jung KH, Shin JW, Moon JS, Byun JI, Lim JA, et al. Induction of burst suppression or coma using intravenous anesthetics in refractory status epilepticus. J Clin Neurosci. 2015;22:854–8. [DOI] [PubMed] [Google Scholar]
- 32.Johnson EL, Martinez NC, Ritzl EK. EEG characteristics of successful burst suppression for refractory status epilepticus. Neurocrit Care. 2016;25:407–14. [DOI] [PubMed] [Google Scholar]
- 33.Thompson SA, Hantus S. Highly epileptiform bursts are associated with seizure recurrence. J Clin Neurophysiol. 2016;33:66–71. [DOI] [PubMed] [Google Scholar]
- 34.Mesraoua B, Deleu D, Al Hail H, Ibrahim F, Melikyan G, Al Hussein H, et al. Clinical presentation, epidemiology, neurophys-iological findings, treatment and outcome of nonconvulsive status epilepticus: a 3-year prospective, hospital-based study. J Drug Assess. 2017;6:18–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rossetti AO, Hurwitz S, Logroscino G, Bromfield EB. Prognosis of status epilepticus: role of aetiology, age, and consciousness impairment at presentation. J Neurol Neurosurg Psychiatry. 2006;77:611–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leitinger M, Holler Y, Kalss G, Rohracher A, Novak HF, Höfler J, et al. Epidemiology-based mortality score in status epilepticus (EMSE). Neurocrit Care. 2015;22:273–82. [DOI] [PubMed] [Google Scholar]
- 37.Rossetti AO, Logroscino G, Milligan TA, Michaelides C, Ruffieux C, Bromfield EB. Status Epilepticus Severity Score (STESS): a tool to orient early treatment strategy. J Neurol. 2008;255:1561–6. [DOI] [PubMed] [Google Scholar]
- 38.Giovannini G, Monti G, Tondelli M, Marudi A, Valzania F, Leitinger M, et al. Mortality, morbidity and refractoriness prediction in status epilepticus: comparison of STESS and EMSE scores. Seizure. 2017;46:31–7. [DOI] [PubMed] [Google Scholar]
- 39.Kamppi L, Mustonen H, Kotisaari K, Soinila S. The essence of the first 2.5 h in the treatment of generalized convulsive status epilepticus. Seizure 2018;55:9–16. [DOI] [PubMed] [Google Scholar]
- 40.Sutter R, Dittrich T, Semmlack S, Rüegg S, Marsch S, Kaplan PW. Acute systemic complications of convulsive status epilepticus-a systematic review. Crit Care Med. 2018;46:138–5. [DOI] [PubMed] [Google Scholar]
- 41.Lin JJ, Chou CC, Lan SY, Hsiao HJ, Wang Y, Chan OW, et al. Therapeutic burst-suppression coma in pediatric febrile refractory status epilepticus. Brain Dev. 2017;39:693–702. [DOI] [PubMed] [Google Scholar]
- 42.Turrigiano G Homeostatic synaptic plasticity: local and global mechanisms for stabilizing neuronal function. Cold Spring Harb Perspect Biol. 2012;4:a005736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Greenblatt DJ, Ehrenberg BL, Gunderman J, Locniskar A, Scavone JM, Harmatz JS, et al. Pharmacokinetic and electroencephalographic study of intravenous diazepam, midazolam, and placebo. Clin Pharmacol Ther. 1989;45:356–65. [DOI] [PubMed] [Google Scholar]
- 44.Kanto J, Gepts E. Pharmacokinetic implications for the clinical use of propofol. Clin Pharmacokinet. 1989;17:308–26. [DOI] [PubMed] [Google Scholar]
- 45.Gulati S, Sondhi V, Chakrabarty B, Jauhari P, Lodha R, Sankar J. High dose phenobarbitone coma in pediatric refractory status epilepticus; a retrospective case record analysis, a proposed protocol and review of literature. Brain Dev. 2018;40:316–24. [DOI] [PubMed] [Google Scholar]
- 46.Morrison G, Gibbons E, Whitehouse WP. High-dose midazolam therapy for refractory status epilepticus in children. Intensive Care Med. 2006;32:2070–6. [DOI] [PubMed] [Google Scholar]
- 47.Datar S New Developments in refractory status epilepticus. Neurosurg Clin N Am. 2018;29:273–9. [DOI] [PubMed] [Google Scholar]
- 48.Struck AF, Ustun B, Ruiz AR, Lee JW, LaRoche SM, Hirsch LJ, et al. Association of an electroencephalography-based risk score with seizure probability in hospitalized patients. JAMA Neurol. 2017;74:1419–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Punjasawadwong Y, Phongchiewboon A, Bunchungmongkol N. Bispectral index for improving anaesthetic delivery and postoperative recovery. Cochrane Database Syst Rev 2014;4:CD003843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hirsch LJ, LaRoche SM, Gaspard N, Gerard E, Svoronos A, Herman ST, et al. American clinical neurophysiology society’s standardized critical care EEG terminology: 2012 version. J Clin Neurophysiol. 2013;30:1–27. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.