Abstract
Abnormal blood and lymphatic vessels create a hostile tumor microenvironment characterized by hypoxia, low pH, and elevated interstitial fluid pressure. These abnormalities fuel tumor progression, immunosuppression, and treatment resistance. In 2001, we proposed a novel hypothesis that the judicious use of antiangiogenesis agents—originally developed to starve tumors—could transiently normalize tumor vessels and improve the outcome of anticancer drugs administered during the window of normalization. In addition to providing preclinical and clinical evidence in support of this hypothesis, we also revealed the underlying molecular mechanisms. In parallel, we demonstrated that desmoplasia could also impair vascular function by compressing vessels, and that normalizing the extracellular matrix could improve vascular function and treatment outcome in both preclinical and clinical settings. Here, we summarize the progress made in understanding and applying the normalization concept to cancer and outline opportunities and challenges ahead to improve patient outcomes using various normalizing strategies.
Keywords: tumor microenvironment, angiogenesis, normalization, hypoxia, immunostimulation
INTRODUCTION
Blood vessels bring oxygen and nutrients to every cell in the body while removing waste and allowing immune cells to survey. These vessels do the same in cancer and other diseases (1) (Supplemental Figure 1). In most types of tumors, new vessels produced through angiogenesis have abnormal structure and function, leading to impaired perfusion that paradoxically supports malignancy (2). Specifically, hypoxia makes cancer cells more aggressive, and leaky vessels give these cells passage to distant sites to metastasize (2). Additionally, hypoxia prevents immune cells from acting on cancer cells and reduces the efficacy of radio- and chemotherapy. Through hypoxia, cancer cells promote an interlinked cycle of angiogenesis, desmoplasia, and immunosuppression, thereby creating an abnormal tumor microenvironment (TME) that results in disease progression and treatment resistance (1, 3, 4).
Because tumors rely on angiogenesis to grow and metastasize, antiangiogenesis therapies (AATs) initially were developed as a monotherapy that would starve tumors of nutrients (5, 6). However, in the initial clinical trials, bevacizumab, an antivascular endothelial growth factor (VEGF) antibody, failed to improve survival as monotherapy but improved the outcome of chemotherapy. This seemed paradoxical: How can an agent that destroys the blood vessels that bring chemotherapeutic drugs to the tumor cells improve the efficacy of chemotherapy? To this end, in 2001 we hypothesized that using AATs with the intent to normalize—not destroy—vessels would improve their function, thereby enhancing treatment outcomes by increasing oxygen and drug delivery (Figure 1) (7). We provided preclinical evidence for this hypothesis in a number of models and revealed the molecular mechanisms of vascular normalization (8). We also demonstrated that cytotoxic therapy given during the window of normalization has a better outcome than the cytotoxic therapy given before or after the window (8). Moreover, a number of other laboratories have now provided evidence in support of the normalization hypothesis (6). Although it uncovers new challenges to translation, the clinical use of AATs in the interim supports the normalization hypothesis (1–4, 9). The central lesson of these studies reinforces our prediction that judicious use of AATs is necessary to improve vessel function and thus clinical outcome. Judicious use is complicated by multiple levels of tumor heterogeneities, such as across tumor types, regions in the same lesion, and different lesions in the same patient. Other TME components, such as fibroblasts, reciprocally contribute to these heterogeneities. Thus, overarching questions remain as to how to personalize the use of AATs for patients and how to combine these AATs with other TME modulating therapies that reduce desmoplasia and stimulate antitumor immunity (4).
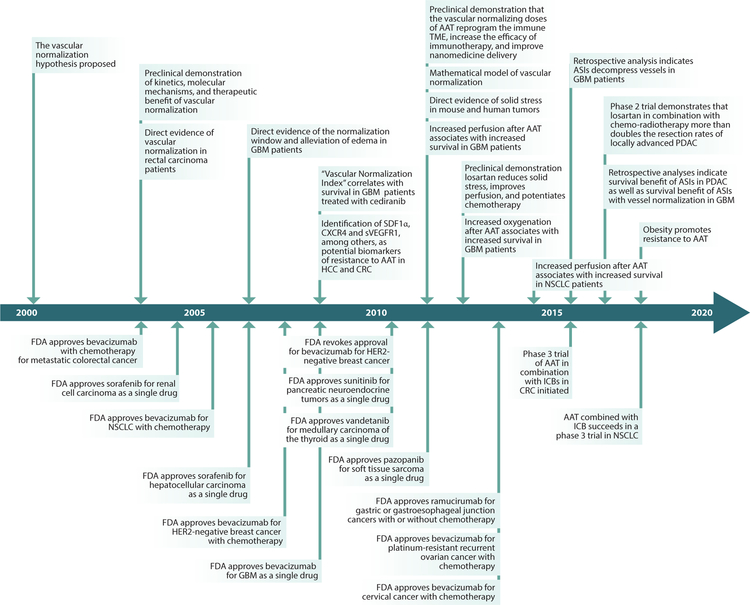
Figure 1.
Chronology of vascular normalization in preclinical and clinical studies. This time line shows the key concepts and findings related to vascular and cancer-associated fibroblast/extracellular matrix normalization from the senior author’s laboratory. References for the bottom panels can be found in Reference 4. Abbreviations: AAT, antiangiogenesis therapies; ASI, angiotensin system inhibitor; CRC, colorectal cancer; FDA, Food and Drug Administration; GBM, glioblastoma; HCC, hepatocellular carcinoma; ICB, immune checkpoint blocker; NSCLC, non-small-cell lung cancer; PDAC, pancreatic ductal adenocarcinoma; TME, tumor microenvironment.
In this article, we attempt to answer these questions by discussing vessel normalization in the context of recent hypothesis-driven clinical studies and alternative normalization targets discovered preclinically. We first summarize recent results indicating that alleviating hypoxia is the central indicator of normalization of the TME, not just blood vessels. To alleviate hypoxia, AATs must sustain oxygen delivery throughout the entire tumor. Next, we illustrate how the TME changes— angiogenesis, desmoplasia, and immunosuppression—jointly promote inefficient vessels and disease progression. In this context, we discuss promising therapeutic strategies that normalize the TME toward decreasing hypoxia, thereby breaking the vicious cycle of hypoxia-mediated tumor progression. Notably, although most normalization strategies prune inefficient vessels, here we underscore the strategies that instead increase perfusion and sustain vessel normalization without or with minimal pruning. Finally, we describe current challenges to clinical translation. Alleviating hypoxia through normalization of the TME is an emerging paradigm for improving cancer treatment using conventional (e.g., chemo- and radiotherapy) and novel therapies (e.g., immunotherapy) (1, 2, 10).
OXYGENATION IS THE CRITICAL BIOMARKER FOR PATIENT OUTCOMES AND NORMALIZATION
As a tumor begins to grow from a limited number of cells, its demand for nutrients rises. More vessels are needed to supply these nutrients, but tumor vessels become leaky (Figure 2a) and compressed (Figure 2b) in the early stages of tumor progression (11). As we describe later, cancer cells promote new vessel growth through multiple mechanisms (6, 12). These mechanisms promote chaotic vascular networks compared to physiological mechanisms of growth and repair. The result is that tumor vessels have an abnormal morphology, are leakier, and are less efficient than normal vessels (2). Furthermore, cancer cell proliferation within host tissue produces growth-induced solid stress (10, 13). As a result, a fraction of vessels in tumors are compressed, which strongly contributes to impaired perfusion (2). The leakiness and compression of tumor vessels are heterogeneous, depending on tumor type, stage, and location within the same lesion and between lesions of the same patient (3).
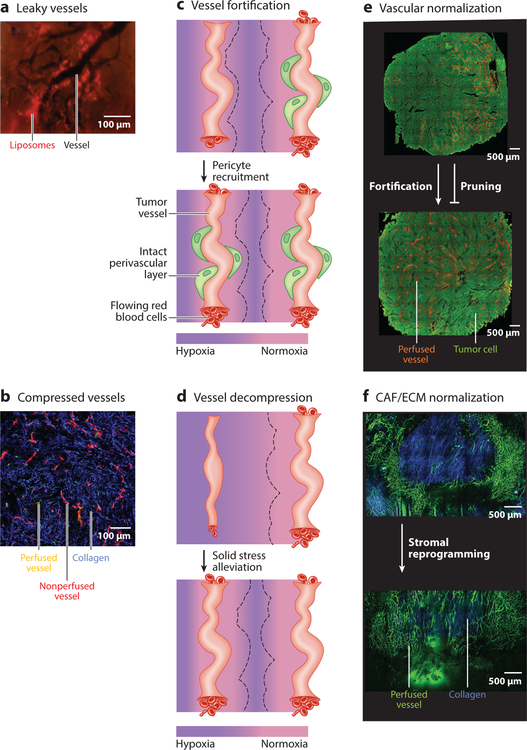
Figure 2.
(a,b) Angiogenesis, desmoplasia, and inflammation promote a cycle characterized by leaky and compressed tumor vessels. (a) Intravital microscopy image of murine tumor vessels (black; negative contrast). At 24 h postinjection, 90-nm liposomes (red) are extravasated from and accumulated around leaky tumor vessels. The liposomes’ extravasation is heterogeneous. Panel adapted from Reference 156. (b) Histological image of murine tumor vessels. Most vessels are nonperfused (red) in this collagen-rich (blue) tumor, and there is a lack of blood flow (yellow). Panel adapted from Reference 52. (c,d) Schematics of perfusion before and after normalization. (c) In the top images, the schematics depict untreated tumor vessels, with one vessel having limited flow (few red blood cells), and the other vessel is well perfused (many red blood cells). The tissue around the perfused vessel is normoxic (pink), whereas the tissue farther from the vessels is hypoxic (purple). The region of normoxia surrounding each vessel is denoted by a black dashed line. Vessel fortification occurs when pericytes are recruited and basement membrane is repaired to produce an intact perivascular layer (green), which leads to increased blood flow that produces normoxia in the surrounding tissue. (d) Vessel decompression occurs after solid stress is alleviated. The decompressed blood vessel is reperfused, and normoxia around the vessel is restored. (e,f) Normalization results in homogeneous perfusion throughout tumors. (e) In untreated tumors (green, top), perfused vessels are heterogeneous and of limited density (orange). Fortifying, not pruning, vascular normalization produces a homogeneous distribution of perfused vessels (bottom). Panel adapted from Reference 19. (f) In untreated tumors (top), collagen (blue) and other components of desmoplasia promote vessel compression, such that large regions lack perfused vessels (green). Cancer-associated fibroblast/extracellular matrix (CAF/ECM) normalization (bottom) reduces collagen and reperfuses compressed vessels. Panel adapted from Reference 52.
One would expect that cancer cells will require efficient delivery of nutrients to proliferate and grow. In fact, there are many mechanisms through which hypoxia and acidity in the TME confer a survival advantage to cancer cells. Our hypothesis is that cancer cells compromise vascular function through multiple mechanisms, thus evading the immune system and exerting a selection pressure against normal host cells and relatively benign cancer cells (2). This hypothesis is supported by the validation of hypoxia as an independent biomarker of poor prognosis and clinical data, suggesting that improved blood flow and tumor oxygenation are potential predictive biomarkers of response to AAT (3).
Hypoxia Promotes Tumor Progression
Tumor vessel leakiness and compression result in hypoxia and acidity in the TME (10), thereby promoting immune suppression (2, 4), which comprises tissue-resident and blood-borne immune cells (Supplemental Figure 1). To promote immunity against the tumor, these cells must infiltrate the TME by flowing into tumor blood vessels, adhering to the vessel wall, and transmigrating into the interstitial space (14, 15).
Recent studies clarified the numerous mechanisms through which hypoxia promotes immune suppression in tumors (16). Hypoxia directly reprograms immune cells to a protumor phenotype (17, 18). Tissue-resident macrophages in the TME (tumor-associated macrophages or TAMs) have at least two phenotypes. M1-like TAMs destroy cancer cells by stimulating cytotoxic T lymphocyte immunity, while M2-like TAMs suppress T cell immunity. TAMs are known to migrate into hypoxic and necrotic tumor areas, where hypoxia promotes polarization to the M2-like phenotype (18). As a result, M2-like TAMs predominate in hypoxic regions (18). In contrast, TAMs in well-perfused tumor regions express lower levels of M2-like genes (19). Acidity also regulates TAM polarization (18). Additionally, the TME reduces the immune effector cells’ ability to fight cancer through hypoxia-induced growth factors and cytokine signaling. In particular, transforming growth factor-β (TGF-β) and VEGF reduce T lymphocyte activity and limit the ability of dendritic cells to process and present tumor antigens to lymphocytes (17, 18). Furthermore, hypoxia-induced factor 1 (HIF-1) signaling promotes higher levels of programmed death-ligand 1 (PD-L1) expression by myeloid-derived suppressor cells, TAMs, dendritic cells, and cancer cells (17, 18). Finally, oxygen sensing in T cells limits antitumor CD8+ T cell effector function and promotes protumor CD4+-regulatory (Treg) cell induction (20). As a result, the cytolytic activity of T cells is inhibited, and cancer cell evasion of the immune system is reinforced. Besides inhibiting host immunity, tumor supportive T cells and TAMs also reduce the efficacy of radiation, indicating a protective effect on cancer cells (4, 16).
Apart from protumor immunosuppression, hypoxia selects for more malignant cancer cells, as less malignant cancer cells and normal cells in the TME will undergo apoptosis based on the physiological cues of hypoxia (21, 22). Also, hypoxia represses tumor suppressor genes, which gives cancer cells a selective advantage (23). Separately, hypoxia promotes several steps of the metastatic cascade through HIF-1. For metastasis to occur, cancer cells must migrate through the primary tumor and exit, which is facilitated by production of promigratory and extracellular matrix (ECM)-degrading proteins induced by hypoxia and acidity (24, 25). Cancer stem cell (26) and epithelialto-mesenchymal transition (27) phenotypes are controlled by hypoxia. Hypoxia confers resistance to radiation, while many, but not all, chemotherapies are less potent in hypoxia, and weak-base chemotherapies are neutralized by acidity (2, 3).
Judicious Use of Antiangiogenesis Therapy Leads to Improved Survival in Patients
Microvascular density (MVD) in areas of intense vascularization (28) and hypoxia (3, 21) are two independent, prognostic biomarkers that have been validated broadly across various types of tumors (Supplemental Table 1). In 2001, we postulated that the judicious use of AAT could normalize vascular function and thus increase oxygenation. Since then, multiple clinical trials have demonstrated that vascular normalization does occur with judicious use of AAT in cancer (1, 2, 5, 6) and other diseases (see sidebar titled Vascular Normalization Beyond Cancer). More importantly, patients that respond to AAT with increased tumor vessel function and reduced hypoxia derive the most benefit (1–3). Taken together with the mechanisms through which hypoxia drives tumor progression, these clinical results support the notion that reduction in hypoxia post-AAT is a potential predictive biomarker of response.
Direct measurements of functional biomarkers such as increased perfusion and increased oxygen delivery in responding patients supports the normalization hypothesis (Supplemental Table 1). A clinical trial in recurrent glioblastoma patients confirmed the preclinical findings that cediranib, an oral pan-VEGFR tyrosine kinase inhibitor (TKI), normalizes tumor vessels and alleviates edema, which is a consequence of abnormal vessel function (29). Indeed, improvement in progression-free survival and overall survival correlated with the extent of normalization as determined by a subset of biomarkers (30). Later studies revealed that cediranib transiently increases perfusion and oxygenation in the subset of glioblastoma patients that survive longer (31–33). Similar findings of increased vessel function and oxygenation associating with pathological response and outcome have been reported with nintedanib (34), eribulin (35), and bevacizumab (35–38) in breast cancer (BC) patients. While perfusion was reduced by bevacizumab in the overall patient cohort of non-small-cell lung cancer (NSCLC) patients, increased perfusion after bevacizumab treatment was associated with improved overall survival (39). A current clinical challenge is to prospectively verify these potential predictive physiological biomarkers of response to normalizing therapy. Nonetheless, the results of these clinical trials lead to some new hypotheses that should be tested prospectively.
First, there must be a sufficient density of vasculature before AAT treatment to avoid excessive pruning that could lead to hypoxia. This hypothesis initially appears paradoxical, because high MVD is a common, validated independent biomarker prognostic of poor outcome in a variety of tumor types (Supplemental Table 1). However, vascular normalization consists of two processes: pruning of some immature vessels and fortification of the remaining vessels by active recruitment of pericytes (8). Thus, a high MVD at the baseline ensures that an adequate number of normalized vessels will be left after pruning of immature vessels using AAT. The hypothesis that high pretreatment MVD is predictive of good response to normalizing therapy is supported by several clinical biomarker studies. The largest study was in 980 ovarian cancer patients. Importantly, in this study, there was a placebo arm for bevacizumab. In the whole population, bevacizumab increases progression-free survival but not overall survival. However, patients with higher vessel density and higher tumor VEGF-A levels had increased overall survival with bevacizumab (40). Additionally, in BC (41), NSCLC, and colorectal cancer (CRC), there is a correlation between high pretreatment vascularization and response to bevacizumab combined with chemotherapy (3). This hypothesis is further supported by the finding that BC patients with hypoxic tumors at baseline (indicating an insufficient vasculature) did not benefit from treatment with nintedanib (34) or bevacizumab (37, 38). However, in renal cancer, analysis of a surrogate marker of vessel density showed no correlation with response to AAT TKIs. As renal cancer is highly sensitive to AAT because cancer cell mutations lead to VEGF upregulation, response to treatment seems less dependent on vascular normalization (Supplemental Table 1) (42).
Second, normalization needs to favor fortification over pruning. In glioblastoma, the vascular normalization index included three biomarkers, including cerebral blood volume. In this index, reductions in cerebral blood volume, which indicate pruning, were associated with reduced survival (30). In a separate retrospective study of imaging data from a phase 3 trial comparing lomustine to lomustine with bevacizumab, post-therapy necrosis (indicating hypoxia caused by excessive vessel pruning) in both groups was associated with worse outcome. Bevacizumab reduced necrosis (43). In BC, bevacizumab-induced pericyte recruitment, as evidenced by an increase in the density of pericyte-covered vessels, was associated with better response to chemotherapy in the primary tumor (41). However, two studies do not support this hypothesis. A study in rectal cancer found fewer pericyte-covered blood vessels after bevacizumab treatment to be correlated with better response. Additionally, in renal cancer, antivascular effects of AAT TKIs were correlated with increased response (Supplemental Table 1).
Third, the extent of normalization matters only if the above two conditions are met. In other words, normalization does not affect response without a sufficient vasculature at baseline and pericyte-recruiting AAT. In the glioblastoma vascular normalization index, structural normalization of vessels (basement membrane thinning as measured by increased circulating collagen IV) and functional normalization of vessels (Ktrans or permeability) were added to changes in cerebral blood volume (30). Structural normalization of vessels alone was insufficient to predict response, as pruning could reverse the benefit to oxygen delivery. In BC, even though interstitial fluid pressure was reduced on average, increases in vessel normalization only correlated with response in those patients with high MVD at baseline. In the cohort of patients with low MVD, markers of normalization post-bevacizumab did not associate with response (41). In NSCLC, bevacizumab decreased vessel permeability, a marker of functional normalization, in the entire cohort, but this did not correlate with response. Instead, increased perfusion was associated with improved overall survival (39).
Fourth, there must be adequate density of noncompressed vessels before AAT. NSCLC (44) and BC (41) patients with a higher density of noncompressed vessels had better prognosis and response to bevacizumab combined with chemotherapy, respectively. These data suggest that if vessels could be decompressed, there would be better response to bevacizumab. There is evidence that vessel decompression occurs in patients. A retrospective analysis in glioblastoma found that angiotensin system inhibitors (ASIs), which are agents shown to decompress vessels in preclinical models, also did so in patients. Specifically, small vessels were reperfused (45). This result is supported by finding in a retrospective study that patients who took ASIs to manage hypertension either pretreatment or after bevacizumab had improved overall survival (46). These clinical results are consistent with a mathematical model predicting that in hypoperfused tumors, decompressing vessels before AAT would lead to more efficient vasculature (47) and supported by a preclinical study finding that Sonic hedgehog (Shh) signaling pathway inhibition, which decompresses vessels (48), sensitizes hypoperfused pancreatic tumors to VEGF blockade (49).
MULTIPLE STROMAL COMPONENTS PROMOTE ABNORMAL TUMOR MICROENVIRONMENT
Abnormal Tumor Vessels
Tumor vessels are irregular and chaotic in structure and are focally leaky and compressed (1, 10). Endothelial cells (ECs) that line the vessel wall are abnormal. Typically, these cells are polarized with flow and tightly connected. In tumors, they lose their polarity, detach from the vessel wall, and pile onto each other. The junctions between these cells are loosened, and the vessel becomes leaky. Also, the vessel wall is abnormal. Pericytes give structural support to vessels while filling the gaps between ECs. Pericytes support the quiescence of ECs, and during angiogenesis, pericytes detach from ECs. In tumors, pericytes are less contractile and thus unable to regulate flow and permeability. The vascular basement membrane holds ECs and pericytes in place. In tumors, the basement membrane has holes and heterogeneous thickness. Thus, irregular ECs and lack of structural integrity in the vessel wall lead to leakiness (50, 51). Vessel leakiness reduces blood flow by increasing hematocrit and thus blood viscosity, conferring resistance to flow. Also, leakiness reduces the intravascular pressure gradient, which is the driving force to flow (10). Additionally, a fraction of tumor vessels, lacking structural integrity, succumb to the elevated solid tissue pressure in tumors and are compressed to the point of lacking blood flow (52). Reductions in vessel diameter from compression reduce blood flow rates (10). In other regions, there is no flow because a vessel there, or a vessel upstream, is collapsed. Together, leakiness and compression lead to spatial and temporal heterogeneities in perfusion (53). Here, we describe how cancer cells coopt endothelial, perivascular, fibroblast, and immune cells to collaborate toward producing abnormal vessels, which produce a hypoxic and acidic microenvironment that fuels vessel abnormality, thereby creating a vicious cycle (3, 54).
Tumor Angiogenesis Leads to Vessel Leakiness and Causes Immunosuppression
Cancer cells initiate tumor vascularization through mechanisms inducing stromal cells to form new vessels or by directly participating themselves. The former includes new vessels growing from existing ones (sprouting angiogenesis), new vessels forming from recruited bone marrow–derived endothelial progenitor cells (postnatal vasculogenesis), and two vessels splitting from one when the capillary wall grows into the lumen (intussusception). Cancer cells may also migrate and grow along existing vessels (vessel co-option), transdifferentiate into cells with an endothelial phenotype (vasculogenic mimicry), and incorporate into the vessel wall (mosaic vessel formation) (6). These forms of vascularization are inefficient and result in hypoxia in tumor tissue, which causes cancer cells to produce more proangiogenic factors (3). This vicious cycle of angiogenesis leads to more hypoxia and abnormal vessels (Supplemental Figure 2c,d).
Angiogenic signaling promotes immunosuppression through at least four mechanisms (Supplemental Figure 2h) (4). First, VEGF blocks cytotoxic T lymphocyte trafficking and activity by modulating the inhibitory checkpoints of T cells (17, 55, 56). Second, VEGF limits T cell activation by inhibiting dendritic cell maturation and antigen presentation (57). Third, VEGF recruits immunosuppressive cells, including Treg cells, myeloid-derived suppressor cells, and protumor M2-like TAMs (58). Fourth, as discussed above, VEGF-induced vessel leakiness causes hypoxia that results in local and systemic immunosuppression (2, 19).
As part of this vicious cycle, protumor immune cells promote angiogenesis (Supplemental Figure 2i). Inhibiting VEGF expression from T cells induces vessel normalization, which suggests that T cells promote abnormal tumor vessel phenotypes (17). Similarly, antitumor CD4+ T cells modulate angiogenic gene expression in tumors, leading to vessel normalization (59, 60). Furthermore, the combination of the immune checkpoint blockers (ICBs) anticytotoxic T lymphocyte-4 and anti-PD-1 antibodies normalizes blood vessels in BC (59, 60). In contrast, ICBs cause edema in glioblastoma, which indicates increased vessel leakiness (61). Together, these data suggest that the effects of ICBs on vessel normalization seem to be tumor type/location dependent.
Desmoplasia Compresses Vessels and Causes Immunosuppression
In healthy organs, fibroblasts contribute to cell communication while producing and maintaining the ECM, which provides physical integrity. Like angiogenesis, desmoplasia (or fibrosis) is uncontrolled in some tumors. Cancer cells activate fibroblasts through growth factors (e.g., TGF-β), transcriptional factors (e.g., nuclear factor κ-light-chain-enhancer of activated B cells or NF-κB), interleukins, metalloproteinases (MMPs), and reactive oxygen species.
Hypoxia stimulates fibroblasts through connective tissue growth factor (CTGF), TGF-β, and Shh (Supplemental Figure 2b) (62, 63). Once activated, fibroblasts differentiate, contract, proliferate, and generate excessive ECM components. Like cancer cells, fibroblasts generate physical forces with contraction and proliferation that are stored in and transmitted by the ECM, leading to elevated solid stress that compresses vessels (Figure 2b and Supplemental Figure 2a) (10, 48). Solid stress also compresses lymphatic vessels, further elevating interstitial fluid pressure and exacerbating the reduced blood flow caused by vessel leakiness (10, 48). Besides reducing blood flow and thereby indirectly inducing disease progression by the hypoxia mediated mechanisms described above, solid stress directly promotes disease progression by making cancer cells more invasive (64) and by propagating malignancy to surrounding normal tissue (Supplemental Figure 2k) (65).
Besides contributing to hypoxia through vessel compression, cancer-associated fibroblasts (CAFs) and ECM directly promote angiogenesis and immunosuppression (Supplemental Figure 2f,j). Distinct populations of CAFs are angiogenic (66) and inflammatory (67). By secreting factors to prevent T lymphocytes from infiltrating to cancer cells (68), reduce T and natural killer cell activity, as well as promote immunosuppressive cell accumulation and inflammation, CAFs blunt antitumor immunity (69, 70). Furthermore, dense desmoplasia is a physical barrier to T cell infiltration (71, 72). Nonetheless, desmoplastic melanoma is more responsive to ICBs than nondesmoplastic melanoma (73). Completing the cycle between desmoplasia and immuno-suppression, myeloid cells can mediate fibroblast differentiation and activity, thereby contributing to desmoplasia (Supplemental Figure 2g) (74). Besides increasing solid stress levels (10), matrix stiffening from increased collagen content promotes angiogenesis (75) and tumor progression (Supplemental Figure 2j,k) (76).
NORMALIZATION—NOT DESTRUCTION—OF THE TUMOR MICROENVIRONMENT TO IMPROVE PATIENT OUTCOMES
VEGF Blockade to Normalize Vessels
Several tumor types are sensitive to VEGF blockade monotherapy (4). In some cases, in these cancers, the mechanism of action seems to be unrelated to vessel normalization but a direct effect on cancer cells (Supplemental Table 1). For example, clear cell renal carcinoma is sensitive to VEGF blockade because cancer cell mutations in this cancer directly upregulate HIF-1 and thus VEGF. Ovarian cancer is sensitive to VEGF blockade because ovulation is regulated by VEGF. Hepatocellular carcinoma is a highly angiogenic cancer type, because the liver is also highly vascularized and leaky. Thyroid cancer’s growth and invasive potential are related to concentration of VEGF. Neuroendocrine tumors, like hepatocellular carcinoma, are highly vascular and leaky. In these tumor types, clinicians found that VEGF blockade independently of vessel pruning might lead to survival benefits (Supplemental Table 1).
Strategies to normalize vessels without excessive pruning.
As discussed above, normalization of vessels (Figure 2c) without excessive pruning (Figure 2e) would result in a high enough density of fortified vessels after AAT, particularly in cancer types that are less sensitive or resistant to AAT alone (Supplemental Table 1). Less-sensitive tumor types include gastroesophageal cancer, CRC, glioma, BC, and lung cancer. Resistant cancers include pancreatic and prostate cancer (4). Excessive pruning leads to an insufficient vasculature that results in hypoxia and a shorter normalization window. Furthermore, depletion of pericytes makes blood vessels more immature and increases hypoxia, resulting in increased metastasis (2). Here, we summarize treatment strategies that interfere with angiogenesis pathways (Figure 3) toward fortifying vessels while minimizing pruning.
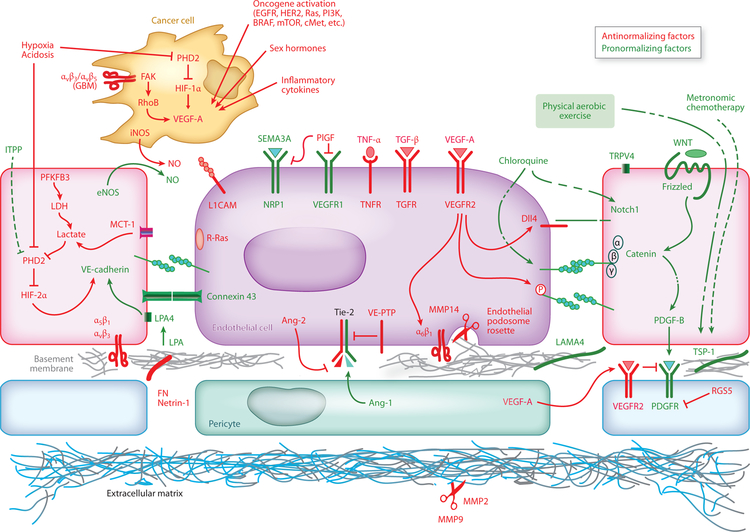
Figure 3.
Pathways that facilitate or hinder vascular normalization. Several pathways in multiple cell types can facilitate (green) or hinder (red) vessel normalization. The cancer cell is depicted near endothelial cells to save space. Figure adapted from Reference 2.
Low-dose VEGF blockade.
VEGF blockade induces vascular normalization by pruning immature blood vessels and inducing pericyte recruitment to fortify the remaining vessels (Figure 2c). The former will increase the maturity of the average remaining vessel but might induce hypoxia by making the vascular network insufficient to supply the tumor. Indeed, the normalization window closes when vessels are excessively pruned or when the tumor uses other angiogenesis pathways or methods of vessel recruitment. Furthermore, VEGF blockade may lead to increased ECM deposition via increased hypoxia. Vessel pruning, rebound vascularization, and fibrosis all lead to hypoxia through different mechanisms. These may be avoided by inhibiting other targets besides VEGF or using low doses of VEGF blockade (2, 19). In preclinical studies, between one-eighth and one-half of typical doses of VEGF blockade seem to fortify a vascular network by normalizing vessels without excessively depleting them (Figure 2e) (19, 51). These studies are supported by retrospective studies in glioblastoma indicating that low-dose bevacizumab induces a larger survival benefit than the high dose (46, 77, 78).
Tie-2/angiopoietins and VE-PTP.
Tie-2 is an EC and pericyte receptor that regulates vascular maturity. Angiopoietin 1 (Ang-1) is a ligand agonist of Tie-2. Activation of Tie-2 fortifies tumor blood vessels, whereas angiopoietin 2 (Ang-2) deactivates Tie-2 and destabilizes tumor vessels (Figure 3). Vascular endothelial protein tyrosine phosphatase (VE-PTP) inactivates Tie-2, which makes blocking VE-PTP a target to normalize vessels. Indeed, blocking this receptor tyrosine phosphatase pharmacologically fortifies vessels while increasing their density, leading to reduced metastasis and enhanced response to therapy (79). As Ang-2 promotes vessel abnormalities and mediates resistance to VEGF blockade in glioblastoma, blocking Ang-2 and VEGF together results in increased normalization and better treatment efficacy by reprogramming TAMs (80, 81). In glioblastoma, Ang-2 inhibition alone does not significantly prune vessels, and dual blockade reduces the pruning induced by VEGF blockade alone (80). In other tumors, dual inhibition promotes T cell effector function and increases efficacy of ICBs (82), in some cases despite inducing vessel regression (83).
Endothelial cell metabolism and oxygen sensors.
Prolyl-hydroxylase domain (PHD1–3) proteins sense oxygen and then target HIFs for degradation. If HIFs accumulate, angiogenesis occurs. PHD proteins function in ECs in tumors as well. Endothelial PHD2 inhibition leads to vessel maturation without pruning or affecting lumen size (Figure 3). The resulting fortification leads to increased perfusion and oxygenation and reduced metastasis (84). A pan-PHD inhibitor, molidustat, has entered clinical trials for kidney diseases, but for cancer, this drug should be targeted to ECs and CAFs. PHD1–3 proteins are also active in T cells. In lung metastases, PHD protein expression in T cells is required to permit metastasis. The hypoxia in tumors and resulting PHD protein activity promote protumor Treg cells while restricting antitumor T cell effector function (20). Pharmacological pan-inhibition of PHD proteins with dimethyloxalylglycine reduces lung metastasis and increases the efficacy of immunotherapy. PHD proteins restrain glycolysis in T cells, reducing their antitumor effect (20). This reduction is driven by mTOR, so the efficacy of mTOR inhibitors could be reduced by the restraint of T cell glycolysis.
Tumor angiogenesis depends on the metabolism of intratumor ECs, which can be more dependent on glycolysis than ECs in healthy organs and certain cancer cells. Nonetheless, cancer cell hyper-glycolysis produces excessive lactate, which is consumed by ECs and induces HIF-1α activation, thereby promoting angiogenesis. Alternatively, blocking EC metabolism reduces angiogenesis independently of proangiogenesis signaling from the TME. 6-Phosphofructo-2-kinase/fructose-2,6-bisphosphatase 3 (PFKFB3) regulates glycolysis in ECs (85). PFKFB3 can be inhibited pharmacologically using a low dose of the small molecule 3PO [3-(3-pyridinyl)-1-(4-pyridinyl)-2-propen-1-one)], which blocks pathological angiogenesis without affecting cancer cell proliferation or causing excessive vascular pruning (Figure 3) (86, 87). Blocking PFKFB3 reduces EC glycolysis levels to that of quiescent ECs (86). While extensive inhibition of glycolysis could induce systemic toxicity, a low dose of 3PO is sufficient to normalize tumor vessels without pruning them or reducing vascular area. Instead, vessel normalization occurs through pericyte activation and increased stability of the EC barrier through reduced VE-cadherin endocytosis by ECs. Thus, vessel lumen area is increased, which leads to increased drug delivery and reduced metastasis through reduced NF-κB signaling and hypoxia (87).
Beyond targeting PFKFB3, there are likely other targets of tumor EC metabolism for vessel normalization (88). Unlike other cells, ECs use fatty acid oxidation for nucleotide synthesis. Thus, blocking regulators of fatty acid oxidation could be antiangiogenic in tumors. ECs use the amino acid glutamine as a nutrient and blocking glutamine metabolism in ECs reduces angio-genesis. Thus, pharmacological inhibition of enzymes that block fatty acid oxidation or glutamine metabolism could normalize tumor vessels. Single cell profiling of EC metabolism could yield additional targets.
Other drugs might be used to inhibit PHD proteins towards vessel normalization. Inositol trispyrophosphate (ITPP) is an allosteric effector of hemoglobin and overcomes low oxygen tension. Treatment with ITPP in melanoma and BC preclinical models stably increases oxygen tension and vessel perfusion. Mechanistically, ITPP reduces HIF1–2 and PHD1–3 and activates endothelial PTEN, thus inhibiting PI3K. This treatment strongly reduces vascular leakage and tumor growth and improves drug delivery with no vessel pruning (89).
Tumor-associated macrophage metabolism.
As we discussed, protumor TAMs are angiogenic and immunosuppressive (90). Furthermore, blocking glycolysis in ECs results in vessel normalization, but glycolysis in TAMs is low (87). Glycolysis in hypoxic protumor TAMs is compromised by the upregulation of a negative regulator of mTOR. Blocking the negative regulator of mTOR enables TAMs to outcompete ECs for glucose, which results in well-organized, fortified vessels that increase oxygen delivery and reduce metastasis (91). Thus, the antitumor effect of mTOR inhibition is compromised through this inhibition of TAM metabolism.
Regulator of G protein signaling 5.
Regulator of G protein signaling 5 (Rgs5) is expressed by platelet-derived growth factor (PDGF)Rβ+ progenitor perivascular cells. Deficiency in this protein changes the phenotype of perivascular cells, which makes tumor vessels more normal in morphology, distribution, and function without reducing the density of tumor vessels. Besides reducing hypoxia, the vessel fortification can increase antitumor immunity (92).
Endothelial glycoprotein L1.
Endothelial glycoprotein L1 (L1CAM) is a neural adhesion protein expressed in many cancers. It promotes cell motility and is associated with metastasis, treatment resistance, and poor prognosis. L1CAM is expressed in tumor ECs and promotes angiogenesis. Blockade of L1CAM induces some pruning, yet dramatically increases vessel fortification and reduces vessel leakiness, with a consequent reduction of tumor growth and metastases (93).
Nitric oxide gradients.
Endothelial cells express nitric oxide (NO) synthase (eNOS), which produces NO. The NO gradient caused by high endothelial concentration and low interstitial concentration contributes to angiogenesis and vessel stabilization (6). However, tumor cells produce neuronal NO synthase (nNOS) or inducible NO synthase (iNOS), thereby increasing interstitial NO levels (Figure 3). Thus, the perivascular NO gradients are reduced. Inhibition of nNOS production of cancer cells restores the NO gradient, resulting in increased density of fortified vessels and therefore increased oxygen and drug delivery (6). This same principle could hold for lymphatic vessels. Modulating NO gradients toward restoring tumor-associated lymphatic vessel function could slow tumor progression, decrease lymphatic metastasis, and normalize the TME by reducing interstitial fluid pressure levels (1).
Semaphorin-3A.
Semaphorin-3A (SEMA3A), which signals via neuropilin-1 (NRP1)-type plexin holoreceptors, is an endogenous inhibitor of angiogenesis that is lost during progression of several cancer types (94, 95). Once SEMA3A is reintroduced, an initial pruning of the immature vessels in the tumor vasculature occurs (94), yet long-term delivery of SEMA3A dramatically and stably increases vessel maturation with an extended window of normalization where intratumor hypoxia is reduced (94). Importantly, SEMA3A markedly reduces the local invasion and metastasis induced by antiangiogenics in pancreatic neuroendocrine tumors and cervical carcinomas (95). Recently, a NRP1-independent high-affinity plexin A4-superagonist SEMA3A mutant has been shown to be an intriguing potential therapeutic strategy to normalize tumor vasculature, thus inhibiting tumor growth, improving drug delivery, and reducing metastases (96).
R-Ras.
R-Ras is a small GTPase highly expressed in quiescent vascular smooth muscle cells and ECs of normal adult vasculature. Activation or overexpression of R-Ras strongly promotes vascular normalization via maturation of tumor vessels. This in turn increases vascular perfusion and drug delivery by improving chemotherapy efficacy. Importantly, endothelial R-Ras does not induce EC death, as happens with classical antiangiogenic compounds, but it stimulates EC survival and vessel maturation (97).
Lysophosphatidic acid.
Lipid mediators also play a role in angiogenesis; one example is lysophosphatidic acid (LPA). Administration of LPA or an analog, specifically when leading to activation of the receptor LPA4, normalizes tumor vessels (98). Activation of LPA4 promotes the localization of VE-cadherin to the EC membrane, which results in increased adherent junction integrity between ECs (Figure 3). LPA4 activation does not increase pericyte coverage, but rather reduces interendothelial gaps to reduce vessel leakiness. Furthermore, rather than prune vessels, LPA4 activation promotes a normalized vessel network featuring larger, longer vessels aligned in parallel. Together, these changes lead to a higher fraction of perfused vessels, especially deep within the tumor, that results in increased oxygen and drug delivery (98).
Chloroquine.
The antimalarial drug chloroquine, independently of blocking autophagy in cancer cells or endothelial cells, normalizes vessels (99). The sustained vessel normalization results in a larger fraction vessels invested with pericytes, which leads to less hypoxia, necrosis, and increased drug delivery. Mechanistically, chloroquine induces vessel normalization through endosomal Notch1 trafficking and signaling in ECs (Figure 3).
The mechanosensitive ion channel transient receptor potential vanilloid-4.
Tumor-derived ECs (TECs), present in abnormal tumor vessels, are phenotypically different from normal ECs. One of their recently discovered alterations is reduced TEC mechanosensitivity. Specifically, transient receptor potential vanilloid-4 (TRPV4) regulates tumor angiogenesis in TECs through the modulation of mechanotransduction and Rho activity. Genetic overexpression or pharmacological activation of TRPV4 restored normal mechanosensitivity in TECs, thus normalizing vasculature and increasing drug delivery in a preclinical model of carcinoma (100).
Avoiding vascular basement membrane degradation: targeting metalloproteinases and endothelial podosome rosettes.
The angiogenic process is heavily characterized by adhesion, migration, and degradation of ECM. Almost all proangiogenic factors present in tumors induce a strong upregulation of MMPs in ECs. Indeed, in tumors the overactivation of the endothelial degradative pathways deteriorates the microanatomy of the vessels themselves, thus making them dysfunctional. The abnormal vasculature in tumors is characterized by the presence of functional podosome rosettes—ECM-degrading subcellular structures. They are precursors of de novo vessel branching points and represent a key event in the formation of new blood vessels in tumors (100). More importantly, the excessive formation of endothelial rosettes damages vascular basement membrane. The integrity of vascular basement membrane is one of the determinants of vascular normalization. A functional vascular basement membrane is crucial in controlling vessel permeability, intratumor edema, resistance to compression, bleeding, intravasation of tumor cells, and vessel perfusion. Endothelial podosome rosettes can be inhibited by targeting integrin α6 (101) that in turn reduces the engagement of MMPs devoted to degrading the vascular basement membrane.
Another strategy to avoid vascular basement membrane damage is to directly inhibit MMP14, the transmembrane MMP responsible for the endothelial podosome rosette–mediated degradation of the vascular basement membrane. Treatment with DX-2400, an anti-MMP14 inhibitory antibody, normalizes tumor vasculature with vessel perfusion increase and no vessel pruning; this reduces tumor growth and radiosensitizes BC. Mechanistically, DX-2400 treatment reduces TGF-β and increases iNOS, with a consequent increase of antitumor M1-like TAMs (102).
Thrombospondin-1.
Thrombospondin-1 (TSP-1) was recognized as the first endogenous antiangiogenic growth factor and has been studied in the treatment of multiple cancers (103). The level of TSP-1 in tumors is usually downregulated, and an increase of TSP-1 in the proximity of vessels is a marker of vascular normalization and maturation. TSP-1 mimetics have been developed for pharmacological interventions. Specifically, ABT-510 is a potent vascular normalizing compound and was tested in clinical trials in patients. The ABT-510–induced vessel normalization does not involve vessel pruning but vessel maturation and normalized function. Importantly, ABT-510 treatment improves drug delivery, thus enhancing the effect of cisplatin treatments (104). As we discuss below, trastuzumab—an oncogenic inhibitor—and metronomic chemotherapy normalize vessels by upregulating TSP-1 (105, 106).
Metronomic chemotherapy.
Metronomic chemotherapy, or the frequent administration of conventional chemotherapeutic agents at very low doses, increases TSP-1 levels (107), which are antiangiogenic (Figure 3), and leads to vascular normalization. Chemotherapy also induces cancer cell killing, and this decompresses vessels (10). Through these two mechanisms, drug and oxygen delivery is improved, leading to a positive feedback loop for the chemotherapy. The increased oxygen delivery also increases immune response, which leads to more cancer cell killing, and thus more oxygen delivery. A mathematical model describes this feedback loop (106).
Oncogenic inhibition.
Hormone withdrawal as well as inhibition of oncogenic signaling induces vascular normalization indirectly (reviewed in 6). One example of this is castration in androgen-dependent carcinoma, because hormone depletion reduces cancer cell expression of VEGF (108) (Figure 3). As mentioned above, trastuzumab in HER2+ BC upregulates expression of antiangiogenic molecules (105). CDK4/6 inhibition might induce similar effects (4). Vascular normalization through these indirect mechanisms is often more durable than direct AAT. Besides normalizing vessels through fortification, these therapies theoretically decompress vessels by depleting cancer cells and thereby reduce solid stress (48). Thus, oncogenic inhibition can reduce hypoxia through three mechanisms: vessel fortification, vessel decompression, and reduction of oxygen consumption by dying cancer cells.
Eribulin.
Eribulin is a chemotherapy that has antiangiogenic effects. In preclinical BC models, eribulin modulates expression of angiogenesis molecules related to EC-pericyte interactions (109). Similar to Tie-2 activation through VE-PTP inhibition, the result is a higher density of smaller, fortified vessels. As a result, perfusion, oxygenation, and post-eribulin chemotherapy efficacy increased (109). Moreover, in a clinical study in BC, presumably through vessel fortification and possibly decompression, eribulin treatment led to increased tumor oxygenation, whereas bevacizumab did not (35). Eribulin-induced cancer cell killing might reduce solid stress and decompresses vessels, but this has not been confirmed.
Physical aerobic exercise.
Intriguingly, vessel normalization may be also induced by lifestyle and more specifically by physical exercise (110). Indeed, emerging results show that aerobic exercise induces an enhancement of vessel perfusion, thus reducing hypoxia in preclinical models of BC, melanoma, and prostate cancer (111–113). The effects of the exercise-induced vessel normalization are (a) reduction of tumor growth, (b) improvement of drug delivery, and (c) a strong decrease of the hypoxia-mediated immunosuppressive factors in the TME. Importantly, the exercise-induced vessel normalization does not involve vessel pruning and induces significant vascular maturation (111–113). One of the possible mechanistic explanations for this is the shear stress-mediated activation of endothelial TSP-1 and calcineurin (111). Although much is still unknown about the mechanisms of action of the exercise-induced vessel normalization, incorporating physical exercise in the clinical practice appears to be a very promising therapeutic strategy for vessel normalization (110).
Strategies for Cancer-Associated Fibroblast and Extracellular Matrix Normalization
In the previous sections, we described why vascular normalization through fortification rather than pruning is important (Figure 2e). We outlined several emerging strategies to achieve this. However, these strategies cannot increase perfusion in regions that have collapsed vessels. Proangiogenesis strategies might accomplish this by making more vessels, but these strategies should be avoided, as angiogenesis is a validated, independent biomarker of worse prognosis in several tumor types. Instead, alleviating solid stress by CAF/ECM normalization decompresses vessels (Figure 2d) and reperfuses hypovascular tumor regions (Figure 2f) (10). Here, we review CAF/ECM normalization strategies (Figure 4 and Supplemental Table 2).
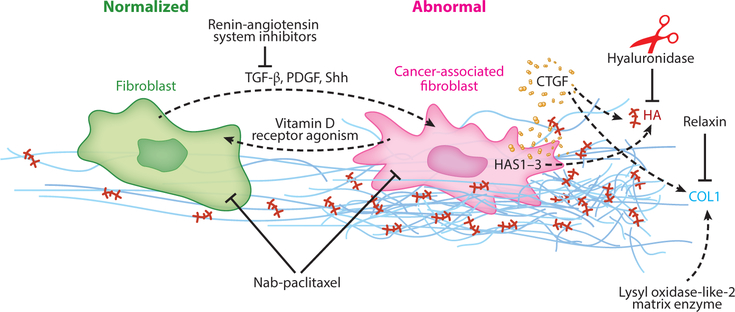
Figure 4.
Pathways that facilitate or hinder cancer-associated fibroblast/extracellular matrix (CAF/ECM) normalization. Several pathways promote activated, protumor CAFs (pink), but these cells can be reprogrammed to a quiescent phenotype (green). Abbreviations: COL1, collagen 1; CTGF, connective tissue growth factor; HA, hyaluronic acid; HAS, hyaluronan synthase; PDGF, platelet-derived growth factor; Shh, Sonic hedgehog; TGF-β, transforming growth factor-β.
Similar to vessel pruning, extensive depletion of desmoplasia, including killing CAFs, decompresses vessels (48) but likely promotes treatment resistance and disease progression (49, 114, 115). Nonetheless, like VEGF blockade, these strategies could benefit certain patients. Depletion or degradation of CAFs by Shh inhibition, collagen I by relaxin, collagen cross-linking by inhibition of lysyl oxidase-like 2, and hyaluronan by hyaluronidase has been shown to potentiate chemotherapy in preclinical studies (48, 116–118). Our hypothesis is strategies that deplete CAFs and/or ECM will have a narrow time and dose window where they will increase response to chemo-, radio-, and immunotherapies. Alternatively, reprogramming CAFs to normalize ECM could alleviate hypoxia for a longer period and break the vicious cycle of the TME (Supplemental Figure 2). Thus, we summarize such reprogramming strategies below (2, 3, 114).
TGF-β inhibition.
The potential of TGF-β inhibition in reprogramming CAFs toward normalizing ECM and decompressing vessels was realized during the repurposing of antihypertensive drugs that target the renin-angiotensin pathway for cancer treatment (52, 70). The dysregulation of this pathway is involved in a range of cardiovascular diseases, and various drugs targeting this pathway have been used safely for decades. In tumors, the main effector of the renin-angiotensin system is angiotensin II (Ang II). Although Ang II’s effects are pleiotropic, it promotes desmoplasia through the Ang II type 1 receptor (AT1R) by activating TGF-β and promoting expression of CTGF in CAFs (52). These pathways are involved in the production and maintenance of ECM. By inhibiting CAF activation and ECM production, repurposed ASIs reduce solid stress, decompress vessels, and potentiate therapy (Figure 2f) (52).
Retrospective and prospective clinical studies suggest there is a benefit in outcome with ASIs, particularly in slow-growing tumors like prostate and highly aggressive tumors like glioblastoma and pancreatic cancer (46, 70, 119, 120), with the potential to reduce side effects (121). A prospective phase 2 trial in locally advanced pancreatic ductal adenocarcinoma treated with chemoradiation combined with losartan resulted in a remarkably high fraction (61%) of patients having their primary tumor removed without microscopic disease in the margin of the surgical specimen, which suggests increased efficacy and longer survival (122). This finding needs to be confirmed in a phase 3 trial. Besides improving outcomes of chemoradiation, ASIs and direct TGF-β inhibition in some cases block angiogenesis and frequently promote antitumor immunity (70, 120). Retrospective analysis of patient samples demonstrated that ASIs reduced gene expression of ECM remodeling, increased those of T cell activity, and independently predicted prognosis (120). This study and others highlight the therapeutic potential of TGF-β inhibition through ASI use.
Supporting these clinical studies of ASI use is increasing evidence from patients of the role of TGF-β in immune suppression. Indeed, in CRC, TGF-β in the TME promotes immune evasion, while blocking TGF-β produces a potent T cell response (123). TGF-β expression and fibrosis exclude T cells from tumor parenchyma in patient samples and thus are usually associated with poor response to PD-L1 inhibition (72). In contrast, desmoplastic melanoma—more so than the nondesmoplastic form—is sensitive to ICBs. However, this sensitivity seems to be related to increased PD-L1 expression in tumor parenchyma of this phenotype, as T cells were confined by the fibrosis to the invasive margin, similar to other tumor types (73).
There are other potential strategies to reduce TGF-β signaling besides ASIs. As we describe below, obesity promotes desmoplasia and inflammation in tumors. The diabetes drug metformin can reprogram pancreatic stellate cells (PSCs), which are a large subpopulation of fibroblasts in the pancreas, by reducing TGF-β signaling, thereby reducing desmoplasia. Additionally, metformin promotes an antitumor immune TME by limiting the expression of inflammatory cytokines and infiltration and polarization of protumor M2 TAMs (124).
Another TGF-β inhibition strategy involves vitamin D receptor (VDR) agonism. Cancer-associated PSCs express high levels of VDR. Because VDR is a master regulator of fibrosis in the liver and controls some inflammatory actions, agonizing VDR was investigated in tumors. Indeed, VDR agonism blocks desmoplasia by reprogramming PSCs to a quiescent state in part by inhibiting the TGF-β pathway (115). This form of CAF normalization also attenuates inflammation. Clinical support for this strategy includes the finding that VDR expression in CAFs of CRC also is associated with better prognosis (125).
Sonic Hedgehog inhibition.
Hypoxia promotes desmoplasia in part through Shh pathway signaling in certain tumor types (63, 69). Blocking Shh signaling reduces solid stress and decompresses vessels (48). Thus, pharmacological inhibition of this pathway leads to increased delivery of chemotherapy. Furthermore, Shh inhibition increases the responsiveness of pancreatic tumors, which are AAT-resistant tumors, to VEGF blockade (49). Nonetheless, blocking Shh has not been effective clinically (118).
Nab-paclitaxel.
Nab-paclitaxel is a chemotherapy that is part of the standard care therapy in pancreatic cancer, as it increases the activity of another chemotherapy, gemcitabine (126). In addition, nab-paclitaxel has CAF/ECM-normalizing effects, as it decreases the amount CAFs in tumors (127). Nonetheless, nab-paclitaxel also depletes nonactivated fibroblasts by a similar amount (127). Therefore, whether nab-paclitaxel’s success clinically is attenuated by depleting stroma and promoting tumor progression remains unclear (49, 128).
Combination of Antiangiogenesis Therapies with Cancer-Associated Fibroblast/Extracellular Matrix Normalizing Therapies
Hypoxia drives angiogenesis and desmoplasia, thus generating leaky and compressed vessels (2). More hypoxia drives disease progression by promoting aggressive cancer cell phenotypes and immunosuppression (2). Here, we argue that by reprogramming the stromal cells that are involved in angiogenesis and desmoplasia, vessels are normalized, and the vicious cycle of hypoxia is broken (3). Still, how to best combine AATs with CAF/ECM normalizing therapies remains unclear. Based on early results, we propose two guidelines. First, the pretreatment characteristics of tumor vessels matter. Specifically, the two strategies will be best combined in tumors with hyperpermeable, hypoperfused vessels (47). Second, in most cases, CAF/ECM normalization should precede AAT, because a high density of perfused vessels pre-AAT could increase benefit. As discussed, high pretreatment decompressed (i.e., patent) MVD is a potential predictive biomarker of response to VEGF blockade (41). Furthermore, excessive vascular pruning leads to hypoxia and resistance to VEGF blockade (1, 2). Thus, we hypothesize that maximizing decompressed MVD before AAT will improve outcomes. In glioblastoma patients, ASIs increased the amount of perfused vessels by reperfusing small vessels (45). Indeed, retrospective studies of glioblastoma patients indicate that ASIs alone and in combination with bevacizumab and/or chemotherapy increase survival of patients (46). Nonetheless, sometimes the opposite order could prove optimal. Resistance to AAT can be mediated by CAF activation and ECM deposition post-therapy, so administering CAF reprogramming therapies after AAT could improve treatment outcome (74, 129, 130). Below, we describe these cases where CAF/ECM normalization strategies post-AAT are beneficial.
CHALLENGES TO CLINICAL TRANSLATION
Resistance Mechanisms and Circulating Biomarkers
Besides sprouting angiogenesis, tumors use multiple mechanisms for recruiting vessels. Thus, the inconsistent results of VEGF blockade on tumor vessels could be expected. For example, cancer cells can migrate and grow along existing vessels to exploit their blood supply. This process, termed vessel co-option, is predominant in some metastases (as described below) or occurs in response to VEGF blockade, as in glioblastoma. Alternative mechanisms such as co-option might result from increased hypoxia after excessive vessel pruning during VEGF blockade. For example, the intrinsic tumor cell plasticity of some tumor types like glioblastoma convey intrinsic and acquired resistance to AAT by inducing the onset of Wnt7/Olig2-driven vessel co-option (131). Alternatively, tumor vessels might be quiescent or tumor cells might express endogenous antiangiogenic molecules, and thus, be resistant to VEGF blockade (132). Immune cells and fibroblasts also promote resistance to AAT (4, 69).
The ability to identify responsive patients would increase the benefit of AAT while reducing medical costs and toxicity. Unfortunately, there are no validated predictive biomarkers for VEGF blockade (2, 4). Still, there are several candidate pathways that might contribute to resistance.
An elevated level of sVEGFR1 is a potential predictive biomarker of resistance to VEGF blockade. Pretreatment levels are associated with poor outcome and less toxicity in several tumor types (2, 4). NRP1 is also associated with a poor outcome (2). These two circulating molecules bind VEGF. Thus, they could be acting as a VEGF trap so that adding exogenous VEGF blockade might not have any effect (1, 84). Indeed, in BC patients, sVEGFR1 levels are associated with pericyte coverage, indicating less VEGF signaling in these patients, with sVEGFR1 acting as an endogenous VEGF blocker (41).
The SDF1α/CXCR4 pathway is an example of evasive resistance. Circulating levels of stromal-derived factor 1α (SDF1α) increase in patients evading VEGF therapies, including CRC patients treated with bevacizumab (133). This pathway contributes to vessel co-option, fibrosis, immune cell trafficking, and cancer cell invasion, among other processes (130). Thus, it is not surprising that SDF1α mediates evasive resistance in different tumor types through varying mechanisms. In preclinical studies in CRC, experiments indicate that CXCR4 is expressed in immunosuppressive innate immune cells after VEGF blockade. Depletion of Ly6Clow monocytes through pharmacological blockade of CXCR4 using AMD3100 enhances treatment efficacy (134). CX3CL1 and CX3CR1+Ly6Clow monocytes play a similar role (135). In contrast, in hepatocellular carcinoma, the AAT sorafenib increases tumor desmoplasia by inducing SDF1α expression. The SDF1α/CXCR4 pathway promotes hepatic stellate cell differentiation and activation, thereby promoting CAF activity. Combination with the AMD3100 can help reduce this mechanism of resistance from sorafenib in hepatocellular carcinoma (74). This is an example of a treatment strategy involving CAF reprogramming after AAT. AMD3100 also increases the efficacy of ICBs (130). Finally, in glioblastoma, SDF1α stimulates vessel co-option so that the tumor may evade VEGF blockade.
Circulating Ang-2 is another potential biomarker of resistance (2, 4, 80, 81). Ang-2 regulates cell invasion and vascular structure and function (136). The effect of VEGF blockade on Ang-2 levels is tumor type dependent, as Ang-2 levels increase in some tumor types while remaining stable or decreasing in others (4). Nonetheless, Ang-2 is associated with worse outcomes in a number of tumor types, including with bevacizumab in CRC and with ICBs in melanoma (4). These associations should be tested prospectively.
Obesity
Many patients with cancer are obese, which is a condition associated with worse prognosis and treatment response (137). Obesity promotes disease progression by stimulating desmoplasia and inflammation (137) while promoting resistance to VEGF blockade (138). Obesity induces immune cells and CAFs to collaborate to stimulate desmoplasia and inflammation (137). Tumor-associated neutrophils (TANs) activate PSCs through interleukin-1β (IL-1β) secreted by adipocytes, which promotes a cycle of inflammation by inducing IL-1β secretion by PSCs and additional TAN recruitment (137). In promoting inflammation, PSCs are activated and induce desmoplasia. Fortunately, fibrosis and inflammation can be reversed by inhibiting AT1R using ASIs (137). Besides its effects on TANs, obesity also increases TAM infiltration through VEGFR-1 signaling by increasing systemic placental growth factor but not VEGF (138). These effects did not affect angiogenesis. Despite not affecting angiogenesis, adipose tissue is hypoxic and thus produces proinflammatory factors, including factors that could provide alternative angiogenic signaling during VEGF blockade. Indeed, BC patients with obesity have increased systemic IL-6 and fibroblast growth factor 2 (FGF-2) (139). Blocking these factors directly or FGF-2 pharmacologically with metformin restores sensitivity to VEGF blockade (139). Thus, obesity intensifies the abnormal TME and induces mechanisms of resistance to AAT. This resistance may be overcome through repurposing of metformin and/or ASIs such as losartan.
Metastasis
Cancer is often treated by first removing the primary tumor through surgery, if possible, and then administering systemic therapy to reduce the chance of metastasis. This is called the adjuvant treatment setting. Cancer cells metastasize through various mechanisms, including through local lymph nodes. Lymphatic cancer cells invade local blood vessels and metastasize to distant organs (140). Roughly one-third of distant metastasis in CRC patients originates from lymphatic metastases, while the remaining two-thirds come from different origins within the primary tumor (141). Nonetheless, there is no sprouting angiogenesis during the early growth of metastasis in the lymph node (142). Thus, even though these lesions are important for disease progression, they are resistant to AAT.
Vessel co-option may also confer resistance in distant metastasis to AAT. Lung (143) and liver (144) metastasis from breast, colorectal, and renal primary tumors are similar to those in the lymph node. Vascularization occurs through sprouting angiogenesis only in limited pathological sub-types of these metastases. Vessel co-option comprises the main mode of vascularization (143, 144). Nonetheless, there is evidence that vessel leakiness occurs in the earliest stages of tumor growth (11) and in clinical metastasis (145), so strategies that target both co-option and sprouting angio-genesis are warranted (131, 146). Furthermore, understanding how metastatic TME compromises oxygen delivery would help researchers design better systemic strategies to alleviate hypoxia.
Solid stress and desmoplasia also likely play a role in the resistance of metastases to treatment. Some types of liver metastases feature desmoplasia and vessel compression (144, 146). Indeed, we measured elevated solid stress in metastases (147). Additional evidence includes our observation that CAFs cotravel with metastatic cancer cells and help them grow in distant metastatic sites (148). Clinical observation of metastases of pancreatic tumors reveals desmoplasia similar to that of the primary tumors (149). In preclinical studies, we found that lesions even at the earliest stages of malignant progression feature compressed vessels (11). Although not initially desmoplastic, CRC liver metastases feature enhanced ECM deposition and vessel compression after VEGF blockade. In this case, hyaluronic acid (HA) deposition, not collagen, is increased after VEGF blockade. HA increases stiffness, and enzymatic degradation of HA increases the efficacy of combined VEGF blockade and chemotherapy (129).
Brain metastases feature a different form of resistance. These metastases are common in HER2+ BC patients, because trastuzumab is effective at treating local disease and prolonging survival. Unfortunately, HER2+ brain metastases are resistant to HER2 inhibition and their indirect vascular normalization. Combination with VEGF blockade overcomes this resistance through an enhanced antiangiogenic effect that also increases necrosis (150). Additionally, the brain microenvironment promotes resistance to HER2 and PI3K inhibition by increasing HER3 expression and phosphorylation. Thus, HER3 blockade is effective in overcoming resistance to HER2 and PI3K inhibitors in HER2+ BC brain metastases (151).
Nanomedicine
Antibodies and larger nanomedicines are used as targeted therapies and to reduce side effects (10). Anti-VEGF therapy reduces the size of pores in tumor vessel walls during normalization. Thus, the extravasation of drugs on the order of tens of nanometers is reduced (51). As a result, only the efficacy of smaller nanomedicines like 10 nm nab-paclitaxel is increased during VEGF blockade (51). In contrast, CAF/ECM normalization increases extravasation of all sizes up to 120 nm (52), because desmoplasia diminishes the transport of these nanomedicines diffusing through the interstitial space (10). How combining vessel and CAF/ECM normalizing therapies will affect extravasation of nanomedicines in a size-dependent manner is unclear.
PERSPECTIVES AND QUESTIONS
The original rationale for AAT was to starve tumors. To explain the clinical observation that AAT can improve the outcome of chemotherapy, in 2001 we proposed that, when used judiciously, AAT can improve tumor perfusion, decrease hypoxia, and enhance chemo-, radio-, and immunotherapies. Since then, small, hypothesis-driven, mechanistic, and large pivotal clinical trials have supported both the role of monotherapy AAT in VEGF-dependent tumors and the value of vascular normalization combined with cytotoxic therapies in AAT-resistant tumor types. To researchers and clinicians interested in improving outcomes with AATs, these trials raised many questions that have been addressed in additional clinical trials supported by preclinical studies. In this article, we have summarized the answers to these important questions.
Normalizing AAT benefits many patients, but it is susceptible to resistance and fails to help other patients. Potential biomarkers predictive of response or resistance such as sVEGFR1, SDF1α, and Ang-2 have not been verified prospectively. Here, as in 2001, we proposed hypoxia as the central indicator of vascular normalization. In the nearly two decades since we put forth the normalization hypothesis, the role of hypoxia in promoting a hostile TME that suppresses the immune system, promotes malignancy, and leads to treatment resistance is better understood. Mechanistic clinical studies indicate that decreased hypoxia resulting from AAT could lead to better outcomes for patients. Oxygenation as a predictive biomarker now needs to be tested prospectively.
Recently, researchers have focused on other processes in the TME, such as desmoplasia and immunosuppression, in part because of the enormous potential of immune therapies. Increasingly, the vicious cycle of hypoxia promoting angiogenesis, desmoplasia, and immunosuppression, and these three processes inducing hypoxia, is being elucidated. Here, we summarized this cycle and propose that normalizing—not destroying—the TME should be pursued. In particular, AAT should fortify rather than prune vessels to sustain normalization. Questions remain regarding how to best combine AAT with other stromal reprogramming agents. Here, we propose that reduction in hypoxia should guide the combination. Evidence thus far suggests that alleviating solid stress by reprogramming CAFs to normalize ECM should precede AAT. However, there are cases where the opposite order is beneficial, and mathematical models as well as retrospective trials indicate a benefit to simultaneous use. More importantly, no agent developed with intention to alleviate solid stress has been approved to date. Similarly, AAT combined with immune therapies are currently undergoing clinical evaluation. This review has described the studies demonstrating the mechanisms through which AAT could benefit immune therapies.
Finally, challenges identified in the clinic remain to be solved. The mechanisms of resistance and potential biomarkers vary by tumor type. The number of obese cancer patients is increasing, and this presents new challenges, as obesity promotes desmoplasia and inflammation. Fortunately, repurposing drugs used for other diseases that inhibit TGF-β and FGF-2 have potential to alleviate this problem. How to proceed in treating metastases is less clear. Metastases feature alternative mechanisms of resistance and their phenotypes are heterogeneous, even in the same patient. Targeting both vessel co-option and angiogenesis holds promise, but how either affects hypoxia in metastases is unknown. In brain metastases, the same pathways that eliminate primary tumors can have limited effect (151). AAT-induced vascular normalization affects nanomedicines, which enable targeted and safer therapies, in a size-dependent manner. Only smaller nanomedicines benefit from AAT combination, while larger nanomedicines need to be combined with CAF reprogramming strategies that normalize the ECM.
Supplementary Material
VASCULAR NORMALIZATION BEYOND CANCER.
The normalization hypothesis, originally proposed to improve the treatment of solid tumors, is successful when applied to treating various nonmalignant diseases characterized by abnormal vasculature. There are approximately 70 diseases that together afflict more 500 million people worldwide that might benefit from this strategy (1). Among these are tuberculosis (152) and neurofibromatosis-2 (NF2) (153). Based on our work, bevacizumab was approved for NF2-associated schwannomas in the United Kingdom in 2014. Additional diseases where the vascular normalization concept has been applied include age-related wet macular degeneration and atherosclerosis (154). Indeed, studies from retinal diseases revealed that leucine-rich alpha-2-glycoprotein-1 (LRG-1) promotes pathological angiogenesis in the presence of TGF-β (155) and thus is a potential target to normalize tumor vessels without excessive pruning. Other diseases that are characterized by abnormal vessels include skin psoriasis, rheumatoid arthritis, and neurodegenerative diseases (6).
ACKNOWLEDGMENTS
We would like to acknowledge the work of those that we could not cite due to space constraints. We thank Dr. Jim Baish, Dr. Vikash Chauhan, Dr. Meenal Datta, and Vinicio Melo for their critical reading of and input into this manuscript, as well as countless other current and past Edwin L. Steele Laboratories members for useful discussions. The work of R.K.J. is supported in part by US National Cancer Institute grants P01CA080124, R01-CA129371, R01-CA208205, and U01-CA 224348; Outstanding Investigator Awards R35-CA197743 and P50-CA165962; and grants from the National Foundation for Cancer Research, Jane’s Trust Foundation, and Harvard Ludwig Cancer Center. J.D.M. is supported by a fellowship from the Japan Society for the Promotion of Science (No. P16731). G.S. is supported by Institut Curie, Fondation ARC, CNRS, and INSERM (ATIP-Avenir grant).
Glossary
- Desmoplasia
abnormal growth of fibrous tissue characterized by activation of fibroblasts and excessive extracellular matrix components
- Immunosuppression
the processes that limit the innate anticancer immunity of both tissue-resident and blood-borne, tumor-infiltrating cells
- TME
tumor microenvironment comprises components of the tumor that are not cancer cells called the stroma
- AAT
antiangiogenesis therapies that block proangiogenic or promote antiangiogenic signaling; in cancer patients, AATs are used to prune and/or fortify vessels
- VEGF
vascular endothelial growth factor is a critical proangiogenic growth factor overexpressed in tumors and the most common target of clinically used AATs
- Solid stress
the force generated by cells and stored in the resulting deformations of the other cells and matrix components
- Predictive biomarker
observable data that can be used to predict whether a particular patient is likely to benefit from a particular therapy
- ECM
extracellular matrix is a noncellular, non-necrotic tissue supporting the organ’s structure that includes collagen and hyaluronic acid
- Prognostic biomarker
observable data that can be used to predict the outcome of a patient regardless of the therapy used
- Pericyte
a perivascular cell that lies within the basement membrane, structurally stabilizes the endothelium, and interacts with ECs
- Permeability
also known as leakiness, it reflects how readily solutes may cross the vessel wall
- Interstitial fluid pressure
(hydrostatic pressure) in tumor tissue indicates abnormal vessel function and is not directly related to solid stress
- EC
endothelial cells line the inside of the vessel wall; they are organized in normal tissues but disorganized and poorly connected in tumors
- CAF
cancer-associated fibroblasts are nonmalignant mesenchymal cells conscripted by cancer cells to contribute to the abnormal physiology of tumors
- Stiffness
a material property of the tissue that measures how much it deforms when under a given force
Footnotes
DISCLOSURE STATEMENT
R.K.J. received an honorarium from Amgen and consultancy fees from Merck, Ophthotech, Pfizer, SPARC, SynDevRx, and XTuit. He has equity in Enlight, Ophthotech, and SynDevRx and serves on the boards of trustees of Tekla Healthcare Investors, Tekla Life Sciences Investors, Tekla Healthcare Opportunities Fund, and Tekla World Healthcare Fund. No reagent or any funding from these agencies was used in the studies discussed here. J.D.M. has a patent application EP2866791A4 “Novel compositions and uses of anti-hypertensives for cancer therapy.” G.S. has no conflicts of interest to declare.
LITERATURE CITED
- 1.Jain RK. 2013. Normalizing tumor microenvironment to treat cancer: bench to bedside to biomarkers. J. Clin. Oncol 31:2205–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jain RK. 2014. Antiangiogenesis strategies revisited: from starving tumors to alleviating hypoxia. Cancer Cell 26:605–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martin JD, Fukumura D, Duda DG, Boucher Y, Jain RK. 2016. Reengineering the tumor microenvironment to alleviate hypoxia and overcome cancer heterogeneity. Cold Spring Harb. Perspect. Med 6:a027094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fukumura D, Kloepper J, Amoozgar Z, Duda DG, Jain RK. 2018. Enhancing cancer immunotherapy using antiangiogenics: opportunities and challenges. Nat. Rev. Clin. Oncol 15:325–40 [DOI] [PMC free article] [PubMed] [Google Scholar]; Provides a current and comprehensive perspective on using AAT to improve immunotherapy.
- 5.Carmeliet P, Jain RK. 2011. Principles and mechanisms of vessel normalization for cancer and other angiogenic diseases. Nat. Rev. Drug Discov 10:417–27 [DOI] [PubMed] [Google Scholar]
- 6.Goel S, Duda DG, Xu L, Munn LL, Boucher Y, et al. 2011. Normalization of the vasculature for treatment of cancer and other diseases. Physiol. Rev 91:1071–121 [DOI] [PMC free article] [PubMed] [Google Scholar]; Represents the most comprehensive review of studies on tumor vessel normalization until 2011.
- 7.Jain RK. 2001. Normalizing tumor vasculature with anti-angiogenic therapy: a new paradigm for combination therapy. Nat. Med 7:987–89 [DOI] [PubMed] [Google Scholar]; Introduces the normalization hypothesis.
- 8.Winkler F, Kozin SV, Tong RT, Chae SS, Booth MF, et al. 2004. Kinetics of vascular normalization by VEGFR2 blockade governs brain tumor response to radiation: role of oxygenation, angiopoietin-1, and matrix metalloproteinases. Cancer Cell 6:553–63 [DOI] [PubMed] [Google Scholar]
- 9.Lu-Emerson C, Duda DG, Emblem KE, Taylor JW, Gerstner ER, et al. 2015. Lessons from anti-vascular endothelial growth factor and anti-vascular endothelial growth factor receptor trials in patients with glioblastoma. J. Clin. Oncol 33:1197–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stylianopoulos T, Munn LL, Jain RK. 2018. Reengineering the physical microenvironment of tumors to improve drug delivery and efficacy: from mathematical modeling to bench to bedside. Trends Cancer 4:292–319 [DOI] [PMC free article] [PubMed] [Google Scholar]; Reviews the mechanical microenvironment of tumors, including solid stress and abnormal vessels.
- 11.Hagendoorn J, Tong R, Fukumura D, Lin Q, Lobo J, et al. 2006. Onset of abnormal blood and lymphatic vessel function and interstitial hypertension in early stages of carcinogenesis. Cancer Res 66:3360–64 [DOI] [PubMed] [Google Scholar]
- 12.Carmeliet P, Jain RK. 2011. Molecular mechanisms and clinical applications of angiogenesis. Nature 473:298–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Helmlinger G, Netti PA, Lichtenbeld HC, Melder RJ, Jain RK. 1997. Solid stress inhibits the growth of multicellular tumor spheroids. Nat. Biotechnol 15:778–83 [DOI] [PubMed] [Google Scholar]
- 14.Motz GT, Santoro SP, Wang L-P, Garrabrant T, Lastra RR, et al. 2014. Tumor endothelium FasL establishes a selective immune barrier promoting tolerance in tumors. Nat. Med 20:607–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jain RK, Koenig GC, Dellian M, Fukumura D, Munn LL, Melder RJ. 1996. Leukocyte-endothelial adhesion and angiogenesis in tumors. Cancer Metastasis Rev 15:195–204 [DOI] [PubMed] [Google Scholar]
- 16.Noman MZ, Hasmim M, Messai Y, Terry S, Kieda C, et al. 2015. Hypoxia: a key player in antitumor immune response. A review in the theme: cellular responses to hypoxia. Am. J. Physiol. Cell Physiol 309:C569–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Palazon A, Tyrakis PA, Macias D, Veliça P, Rundqvist H, et al. 2017. An HIF-1α/VEGF-A axis in cytotoxic T cells regulates tumor progression. Cancer Cell 32:669–83.e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Engblom C, Pfirschke C, Pittet MJ. 2016. The role of myeloid cells in cancer therapies. Nat. Rev. Cancer 16:447. [DOI] [PubMed] [Google Scholar]
- 19.Huang Y, Yuan J, Righi E, Kamoun WS, Ancukiewicz M, et al. 2012. Vascular normalizing doses of antiangiogenic treatment reprogram the immunosuppressive tumor microenvironment and enhance immunotherapy. PNAS 109:17561–66 [DOI] [PMC free article] [PubMed] [Google Scholar]; Shows the beneficial effect of tumor vessel normalization on immunotherapy.
- 20.Clever D, Roychoudhuri R, Constantinides MG, Askenase MH, Sukumar M, et al. 2016. Oxygen sensing by T cells establishes an immunologically tolerant metastatic niche. Cell 166:1117–31.e14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wilson WR, Hay MP. 2011. Targeting hypoxia in cancer therapy. Nat. Rev. Cancer 11:393–410 [DOI] [PubMed] [Google Scholar]
- 22.Carmeliet P, Dor Y, Herbert JM, Fukumura D, Brusselmans K, et al. 1998. Role of HIF-1α in hypoxia-mediated apoptosis, cell proliferation and tumour angiogenesis. Nature 394:485–90 [DOI] [PubMed] [Google Scholar]
- 23.Thienpont B, Steinbacher J, Zhao H, D’Anna F, Kuchnio A, et al. 2016. Tumour hypoxia causes DNA hypermethylation by reducing TET activity. Nature 537:63–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Estrella V, Chen T, Lloyd M, Wojtkowiak J, Cornnell HH, et al. 2013. Acidity generated by the tumor microenvironment drives local invasion. Cancer Res 73:1524–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schito L, Semenza GL. 2016. Hypoxia-inducible factors: master regulators of cancer progression. Trends Cancer 2:758–70 [DOI] [PubMed] [Google Scholar]
- 26.Semenza GL. 2016. Dynamic regulation of stem cell specification and maintenance by hypoxia-inducible factors. Mol. Aspects Med 47–48:15–23 [DOI] [PubMed] [Google Scholar]
- 27.Philip B, Ito K, Moreno-Sánchez R, Ralph SJ. 2013. HIF expression and the role of hypoxic microenvironments within primary tumours as protective sites driving cancer stem cell renewal and metastatic progression. Carcinogenesis 34:1699–707 [DOI] [PubMed] [Google Scholar]
- 28.Weidner N, Folkman J, Pozza F, Bevilacqua P, Allred EN, et al. 1992. Tumor angiogenesis: a new significant and independent prognostic indicator in early-stage breast carcinoma. J. Natl. Cancer Inst 84:1875–87 [DOI] [PubMed] [Google Scholar]
- 29.Batchelor TT, Sorensen AG, di Tomaso E, Zhang WT, Duda DG, et al. 2007. AZD2171, a pan-VEGF receptor tyrosine kinase inhibitor, normalizes tumor vasculature and alleviates edema in glioblastoma patients. Cancer Cell 11:83–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sorensen AG, Batchelor TT, Zhang WT, Chen PJ, Yeo P, et al. 2009. A “vascular normalization index” as potential mechanistic biomarker to predict survival after a single dose of cediranib in recurrent glioblastoma patients. Cancer Res 69:5296–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Batchelor TT, Gerstner ER, Emblem KE, Duda DG, Kalpathy-Cramer J, et al. 2013. Improved tumor oxygenation and survival in glioblastoma patients who show increased blood perfusion after cediranib and chemoradiation. PNAS 110:19059–64 [DOI] [PMC free article] [PubMed] [Google Scholar]; Demonstrates that improved oxygenation relates to outcome in glioblastoma patients treated with AAT.
- 32.Emblem KE, Mouridsen K, Bjornerud A, Farrar CT, Jennings D, et al. 2013. Vessel architectural imaging identifies cancer patient responders to anti-angiogenic therapy. Nat. Med 19:1178–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sorensen AG, Emblem KE, Polaskova P, Jennings D, Kim H, et al. 2012. Increased survival of glioblastoma patients who respond to antiangiogenic therapy with elevated blood perfusion. Cancer Res 72:402–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Quintela-Fandino M, Lluch A, Manso LM, Calvo I, Cortes J, et al. 2016. 18F-fluoromisonidazole PET and activity of neoadjuvant nintedanib in early HER2-negative breast cancer: a window-of-opportunity randomized trial. Am. Assoc. Cancer Res 23:1432–41 [DOI] [PubMed] [Google Scholar]
- 35.Ueda S, Saeki T, Takeuchi H, Shigekawa T, Yamane T, et al. 2016. In vivo imaging of eribulin-induced reoxygenation in advanced breast cancer patients: a comparison to bevacizumab. Br. J. Cancer 114:1212–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Garcia-Foncillas J, Martinez P, Lahuerta A, Cussac AL, Gonzalez MG, et al. 2012. Dynamic contrast-enhanced MRI versus 18F-misonidazol-PET/CT to predict pathologic response in bevacizumab-based neoadjuvant therapy in breast cancer. J. Clin. Oncol 30:10512 (Abstr.) [Google Scholar]
- 37.Ueda S, Kuji I, Shigekawa T, Takeuchi H, Sano H, et al. 2014. Optical imaging for monitoring tumor oxygenation response after initiation of single-agent bevacizumab followed by cytotoxic chemotherapy in breast cancer patients. PLOS ONE 9:e98715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ueda S, Saeki T, Osaki A, Yamane T, Kuji I. 2017. Bevacizumab induces acute hypoxia and cancer progression in patients with refractory breast cancer: multimodal functional imaging and multiplex cytokine analysis. Clin. Cancer Res 23:5769–78 [DOI] [PubMed] [Google Scholar]
- 39.Heist RS, Duda DG, Sahani DV, Ancukiewicz M, Fidias P, et al. 2015. Improved tumor vascularization after anti-VEGF therapy with carboplatin and nab-paclitaxel associates with survival in lung cancer. PNAS 112:1547–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bais C, Mueller B, Brady MF, Mannel RS, Burger RA, et al. 2017. Tumor microvessel density as a potential predictive marker for bevacizumab benefit: GOG-0218 biomarker analyses. J. Natl. Cancer Inst 109:djx066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tolaney SM, Boucher Y, Duda DG, Martin JD, Seano G, et al. 2015. Role of vascular density and normalization in response to neoadjuvant bevacizumab and chemotherapy in breast cancer patients. PNAS 112:14325–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jayson GC, Kerbel R, Ellis LM, Harris AL. 2016. Antiangiogenic therapy in oncology: current status and future directions. Lancet 388:518–29 [DOI] [PubMed] [Google Scholar]
- 43.Nowosielski M, Gorlia T, Bromberg J, Sahm F, Harting I, et al. 2017. Nimg-75. Necrosis during treatment is associated with worse survival in recurrent glioblastoma patients—post hoc image analysis of EORTC 26101. Neuro-Oncology 19:vi159 [Google Scholar]
- 44.Fang L, He Y, Tong Y, Hu L, Xin W, et al. 2017. Flattened microvessel independently predicts poor prognosis of patients with non-small cell lung cancer. Oncotarget 8:30092–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Emblem KE, Gerstner ER, Sorensen G, Rosen BR, Wen PY, et al. 2016. Matrix-depleting antihypertensives decompress tumor blood vessels and improve perfusion in patients with glioblastomas receiving anti-angiogenic therapy. Cancer Res 76:3975 [Google Scholar]
- 46.Levin VA, Chan J, Datta M, Yee JL, Jain RK. 2017. Effect of angiotensin system inhibitors on survival in newly diagnosed glioma patients and recurrent glioblastoma patients receiving chemotherapy and/or bevacizumab. J. Neuro-Oncol 134:325–30 [DOI] [PubMed] [Google Scholar]; Demonstrates the potential of ASIs in combination with bevacizumab through a retrospective analysis of glioblastoma patients.
- 47.Stylianopoulos T, Jain RK. 2013. Combining two strategies to improve perfusion and drug delivery in solid tumors. PNAS 110:18632–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stylianopoulos T, Martin JD, Chauhan VP, Jain SR, Diop-Frimpong B, et al. 2012. Causes, consequences, and remedies for growth-induced solid stress in murine and human tumors. PNAS 109:15101–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rhim AD, Oberstein PE, Thomas DH, Mirek ET, Palermo CF, et al. 2014. Stromal elements act to restrain, rather than support, pancreatic ductal adenocarcinoma. Cancer Cell 25:735–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jain RK. 1987. Transport of molecules across tumor vasculature. Cancer Metastasis Rev 6:559–93 [DOI] [PubMed] [Google Scholar]
- 51.Chauhan VP, Stylianopoulos T, Martin JD, Popovic Z, Chen O, et al. 2012. Normalization of tumour blood vessels improves the delivery of nanomedicines in a size-dependent manner. Nat. Nanotechnol 7:383–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chauhan VP, Martin JD, Liu H, Lacorre DA, Jain SR, et al. 2013. Angiotensin inhibition enhances drug delivery and potentiates chemotherapy by decompressing tumour blood vessels. Nat. Commun 4:2516. [DOI] [PMC free article] [PubMed] [Google Scholar]; Illustrates the potential of ASIs in reprogramming CAFs to improve perfusion and drug delivery.
- 53.Jain RK. 1988. Determinants of tumor blood flow: a review. Cancer Res 48:2641–58 [PubMed] [Google Scholar]
- 54.Jain RK. 2005. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science 307:58–62 [DOI] [PubMed] [Google Scholar]
- 55.Voron T, Colussi O, Marcheteau E, Pernot S, Nizard M, et al. 2015. VEGF-A modulates expression of inhibitory checkpoints on CD8+ T cells in tumors. J. Exp. Med 212:139–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wallin JJ, Bendell JC, Funke R, Sznol M, Korski K, et al. 2016. Atezolizumab in combination with bevacizumab enhances antigen-specific T-cell migration in metastatic renal cell carcinoma. Nat. Commun 7:12624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gabrilovich D, Ishida T, Oyama T, Ran S, Kravtsov V, et al. 1998. Vascular endothelial growth factor inhibits the development of dendritic cells and dramatically affects the differentiation of multiple hematopoietic lineages in vivo. Blood 92:4150–66 [PubMed] [Google Scholar]
- 58.Maenhout SK, Thielemans K, Aerts JL. 2014. Location, location, location: functional and phenotypic heterogeneity between tumor-infiltrating and non-infiltrating myeloid-derived suppressor cells. Oncoimmunology 3:e956579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tian L, Goldstein A, Wang H, Lo HC, Kim IS, et al. 2017. Mutual regulation of tumour vessel normalization and immunostimulatory reprogramming. Nature 544:250–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.De Palma M, Jain RK. 2017. CD4+ T cell activation and vascular normalization: Two sides of the same coin? Immunity 46:773–75 [DOI] [PubMed] [Google Scholar]
- 61.Qin L, Li X, Stroiney A, Qu J, Helgager J, et al. 2017. Advanced MRI assessment to predict benefit of anti-programmed cell death 1 protein immunotherapy response in patients with recurrent glioblastoma. Neuroradiology 59:135–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Eguchi D, Ikenaga N, Ohuchida K, Kozono S, Cui L, et al. 2013. Hypoxia enhances the interaction between pancreatic stellate cells and cancer cells via increased secretion of connective tissue growth factor. J. Surg. Res 181:225–33 [DOI] [PubMed] [Google Scholar]
- 63.Spivak-Kroizman TR, Hostetter G, Posner R, Aziz M, Hu C, et al. 2013. Hypoxia triggers hedgehogmediated tumor–stromal interactions in pancreatic cancer. Cancer Res 73:3235–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tse JM, Cheng G, Tyrrell JA, Wilcox-Adelman SA, Boucher Y, et al. 2012. Mechanical compression drives cancer cells toward invasive phenotype. PNAS 109:911–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fernández-Sánchez ME, Barbier S, Whitehead J, Béalle G, Michel A, et al. 2015. Mechanical induction of the tumorigenic β-catenin pathway by tumour growth pressure. Nature 523:92–95 [DOI] [PubMed] [Google Scholar]
- 66.Fukumura D, Xavier R, Sugiura T, Chen Y, Park EC, et al. 1998. Tumor induction of VEGF promoter activity in stromal cells. Cell 94:715–25 [DOI] [PubMed] [Google Scholar]
- 67.Öhlund D, Handly-Santana A, Biffi G, Elyada E, Almeida AS, et al. 2017. Distinct populations of inflammatory fibroblasts and myofibroblasts in pancreatic cancer. J. Exp. Med 214:579–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Feig C, Jones JO, Kraman M, Wells RJ, Deonarine A, et al. 2013. Targeting CXCL12 from FAP-expressing carcinoma-associated fibroblasts synergizes with anti–PD-L1 immunotherapy in pancreatic cancer. PNAS 110:20212–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Öhlund D, Elyada E, Tuveson D. 2014. Fibroblast heterogeneity in the cancer wound. J. Exp. Med 211:1503–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pinter M, Jain RK. 2017. Targeting the renin-angiotensin system to improve cancer treatment: implications for immunotherapy. Sci. Transl. Med 9:eaan5616. [DOI] [PMC free article] [PubMed] [Google Scholar]; Reviews ASIs’ clinical potential, with an emphasis on immunotherapy.
- 71.Salmon H, Franciszkiewicz K, Damotte D, Dieu-Nosjean M-C, Validire P, et al. 2012. Matrix architecture defines the preferential localization and migration of T cells into the stroma of human lung tumors. J. Clin. Investig 122:899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mariathasan S, Turley SJ, Nickles D, Castiglioni A, Yuen K, et al. 2018. TGFβ attenuates tumour response to PD-L1 blockade by contributing to exclusion of T cells. Nature 554:544–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Eroglu Z, Zaretsky JM, Hu-Lieskovan S, Kim DW, Algazi A, et al. 2018. High response rate to PD-1 blockade in desmoplastic melanomas. Nature 553:347–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chen Y, Huang Y, Reiberger T, Duyverman AM, Huang P, et al. 2014. Differential effects of sorafenib on liver versus tumor fibrosis mediated by stromal-derived factor 1 alpha/C-X-C receptor type 4 axis and myeloid differentiation antigen-positive myeloid cell infiltration in mice. Hepatology 59:1435–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bordeleau F, Mason BN, Lollis EM, Mazzola M, Zanotelli MR, et al. 2017. Matrix stiffening promotes a tumor vasculature phenotype. PNAS 114:492–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mouw JK, Yui Y, Damiano L, Bainer RO, Lakins JN, et al. 2014. Tissue mechanics modulate microRNA-dependent PTEN expression to regulate malignant progression. Nat. Med 20:360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lorgis V, Maura G, Coppa G, Hassani K, Taillandier L, et al. 2012. Relation between bevacizumab dose intensity and high-grade glioma survival: a retrospective study in two large cohorts. J. Neuro-Oncol 107:351–58 [DOI] [PubMed] [Google Scholar]
- 78.Kreisl TN, Smith P, Sul J, Salgado C, Iwamoto FM, et al. 2013. Continuous daily sunitinib for recurrent glioblastoma. J. Neuro-Oncol 111:41–48 [DOI] [PubMed] [Google Scholar]
- 79.Goel S, Gupta N, Walcott BP, Snuderl M, Kesler CT, et al. 2013. Effects of vascular-endothelial protein tyrosine phosphatase inhibition on breast cancer vasculature and metastatic progression. J. Natl. Cancer Inst 105:1188–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Peterson TE, Kirkpatrick ND, Huang Y, Farrar CT, Marijt KA, et al. 2016. Dual inhibition of Ang-2 and VEGF receptors normalizes tumor vasculature and prolongs survival in glioblastoma by altering macrophages. PNAS 113:4470–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kloepper J, Riedemann L, Amoozgar Z, Seano G, Susek K, et al. 2016. Ang-2/VEGF bispecific antibody reprograms macrophages and resident microglia to anti-tumor phenotype and prolongs glioblastoma survival. PNAS 113:4476–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wu FTH, Man S, Xu P, Chow A, Paez-Ribes M, et al. 2016. Efficacy of cotargeting angiopoietin-2 and the VEGF pathway in the adjuvant postsurgical setting for early breast, colorectal, and renal cancers. Cancer Res 76:6988–7000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Schmittnaegel M, Rigamonti N, Kadioglu E, Cassará A, Rmili CW, et al. 2017. Dual angiopoietin-2 and VEGFA inhibition elicits antitumor immunity that is enhanced by PD-1 checkpoint blockade. Sci. Transl. Med 9:eaak9670. [DOI] [PubMed] [Google Scholar]
- 84.Mazzone M, Dettori D, de Oliveira RL, Loges S, Schmidt T, et al. 2009. Heterozygous deficiency of PHD2 restores tumor oxygenation and inhibits metastasis via endothelial normalization. Cell 136:839–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.De Bock K, Georgiadou M, Schoors S, Kuchnio A, Wong BW, et al. 2013. Role of PFKFB3-driven glycolysis in vessel sprouting. Cell 154:651–63 [DOI] [PubMed] [Google Scholar]
- 86.Schoors S, De Bock K, Cantelmo AR, Georgiadou M, Ghesquière B, et al. 2014. Partial and transient reduction of glycolysis by PFKFB3 blockade reduces pathological angiogenesis. Cell Metab 19:37–48 [DOI] [PubMed] [Google Scholar]
- 87.Cantelmo AR, Conradi L-C, Brajic A, Goveia J, Kalucka J, et al. 2016. Inhibition of the glycolytic activator PFKFB3 in endothelium induces tumor vessel normalization, impairs metastasis, and improves chemotherapy. Cancer Cell 30:968–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Li X, Carmeliet P. 2018. Targeting angiogenic metabolism in disease. Science 359:1335–56 [DOI] [PubMed] [Google Scholar]
- 89.Kieda C, El Hafny-Rahbi B, Collet G, Lamerant-Fayel N, Grillon C, et al. 2013. Stable tumor vessel normalization with pO2 increase and endothelial PTEN activation by inositol trispyrophosphate brings novel tumor treatment. J. Mol. Med 91:883–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Casazza A, Laoui D, Wenes M, Rizzolio S, Bassani N, et al. 2013. Impeding macrophage entry into hypoxic tumor areas by Sema3A/Nrp1 signaling blockade inhibits angiogenesis and restores antitumor immunity. Cancer Cell 24:695–709 [DOI] [PubMed] [Google Scholar]
- 91.Wenes M, Shang M, Di Matteo M, Goveia J, Martín-Pérez R, et al. 2016. Macrophage metabolism controls tumor blood vessel morphogenesis and metastasis. Cell Metab 24:701–15 [DOI] [PubMed] [Google Scholar]
- 92.Hamzah J, Jugold M, Kiessling F, Rigby P, Manzur M, et al. 2008. Vascular normalization in Rgs5-deficient tumours promotes immune destruction. Nature 453:410–14 [DOI] [PubMed] [Google Scholar]
- 93.Magrini E, Villa A, Angiolini F, Doni A, Mazzarol G, et al. 2014. Endothelial deficiency of L1 reduces tumor angiogenesis and promotes vessel normalization. J. Clin. Investig 124:4335–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Maione F, Molla F, Meda C, Latini R, Zentilin L, et al. 2009. Semaphorin 3A is an endogenous angiogenesis inhibitor that blocks tumor growth and normalizes tumor vasculature in transgenic mouse models. J. Clin. Investig 119:3356–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Maione F, Capano S, Regano D, Zentilin L, Giacca M, et al. 2012. Semaphorin 3A overcomes cancer hypoxia and metastatic dissemination induced by antiangiogenic treatment in mice. J. Clin. Investig 122:1832–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gioelli N, Maione F, Camillo C, Ghitti M, Valdembri D, et al. 2018. A rationally-designed NRP1-independent superagonist SEMA3A mutant is an effective anticancer agent. Sci. Transl. Med 10:eaah4807. [DOI] [PubMed] [Google Scholar]
- 97.Sawada J, Urakami T, Li F, Urakami A, Zhu W, et al. 2012. Small GTPase R-Ras regulates integrity and functionality of tumor blood vessels. Cancer Cell 22:235–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Takara K, Eino D, Ando K, Yasuda D, Naito H, et al. 2017. Lysophosphatidic acid receptor 4 activation augments drug delivery in tumors by tightening endothelial cell-cell contact. Cell Rep 20:2072–86 [DOI] [PubMed] [Google Scholar]
- 99.Maes H, Kuchnio A, Peric A, Moens S, Nys K, et al. 2014. Tumor vessel normalization by chloroquine independent of autophagy. Cancer Cell 26:190–206 [DOI] [PubMed] [Google Scholar]
- 100.Adapala RK, Thoppil RJ, Ghosh K, Cappelli H, Dudley AC, et al. 2016. Activation of mechanosensitive ion channel TRPV4 normalizes tumor vasculature and improves cancer therapy. Oncogene 35:314–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Primo L, Seano G, Roca C, Maione F, Gagliardi PA, et al. 2010. Increased expression of α6 integrin in endothelial cells unveils a proangiogenic role for basement membrane. Cancer Res 70:5759–69 [DOI] [PubMed] [Google Scholar]
- 102.Ager EI, Kozin SV, Kirkpatrick ND, Seano G, Kodack DP, et al. 2015. Blockade of MMP14 activity in murine breast carcinomas: implications for macrophages, vessels, and radiotherapy. J. Natl. Cancer Inst 107:djv017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Adams JC, Lawler J. 2004. The thrombospondins. Int. J. Biochem. Cell Biol 36:961–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Campbell NE, Greenaway J, Henkin J, Moorehead RA, Petrik J. 2010. The thrombospondin-1 mimetic ABT-510 increases the uptake and effectiveness of cisplatin and paclitaxel in a mouse model of epithelial ovarian cancer. Neoplasia 12:275–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Izumi Y, Xu L, di Tomaso E, Fukumura D, Jain RK. 2002. Tumour biology: herceptin acts as an antiangiogenic cocktail. Nature 416:279–80 [DOI] [PubMed] [Google Scholar]
- 106.Mpekris F, Baish JW, Stylianopoulos T, Jain RK. 2017. Role of vascular normalization in benefit from metronomic chemotherapy. PNAS 114:1994–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Bocci G, Francia G, Man S, Lawler J, Kerbel RS. 2003. Thrombospondin 1, a mediator of the antiangiogenic effects of low-dose metronomic chemotherapy. PNAS 100:12917–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Jain RK, Safabakhsh N, Sckell A, Chen Y, Jiang P, et al. 1998. Endothelial cell death, angiogenesis, and microvascular function after castration in an androgen-dependent tumor: role of vascular endothelial growth factor. PNAS 95:10820–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Funahashi Y, Okamoto K, Adachi Y, Semba T, Uesugi M, et al. 2014. Eribulin mesylate reduces tumor microenvironment abnormality by vascular remodeling in preclinical human breast cancer models. Cancer Sci 105:1334–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ruiz-Casado A, Martín-Ruiz A, Pérez LM, Provencio M, Fiuza-Luces C, Lucia A. 2017. Exercise and the hallmarks of cancer. Trends Cancer 3:423–41 [DOI] [PubMed] [Google Scholar]
- 111.Schadler KL, Thomas NJ, Galie PA, Bhang DH, Roby KC, et al. 2016. Tumor vessel normalization after aerobic exercise enhances chemotherapeutic efficacy. Oncotarget 7:65429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Betof AS, Lascola CD, Weitzel D, Landon C, Scarbrough PM, et al. 2015. Modulation of murine breast tumor vascularity, hypoxia, and chemotherapeutic response by exercise. J. Natl. Cancer Inst 107:djv040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.McCullough DJ, Stabley JN, Siemann DW, Behnke BJ. 2014. Modulation of blood flow, hypoxia, and vascular function in orthotopic prostate tumors during exercise. J. Natl. Cancer Inst 106:dju036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Whatcott CJ, Han H, Von Hoff DD. 2015. Orchestrating the tumor microenvironment to improve survival for patients with pancreatic cancer: normalization, not destruction. Cancer J 21:299–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sherman MH, Yu RT, Engle DD, Ding N, Atkins AR, et al. 2014. Vitamin D receptor-mediated stromal reprogramming suppresses pancreatitis and enhances pancreatic cancer therapy. Cell 159:80–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Jacobetz MA, Chan DS, Neesse A, Bapiro TE, Cook N, et al. 2013. Hyaluronan impairs vascular function and drug delivery in a mouse model of pancreatic cancer. Gut 62:112–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Chauhan VP, Boucher Y, Ferrone CR, Roberge S, Martin JD, et al. 2014. Compression of pancreatic tumor blood vessels by hyaluronan is caused by solid stress and not interstitial fluid pressure. Cancer Cell 26:14–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Mpekris F, Papageorgis P, Polydorou C, Voutouri C, Kalli M, et al. 2017. Sonic-hedgehog pathway inhibition normalizes desmoplastic tumor microenvironment to improve chemo- and nanotherapy. J. Control. Release 261:105–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Pinter M, Weinmann A, Wörns M-A, Hucke F, Bota S, et al. 2017. Use of inhibitors of the renin-angiotensin system is associated with longer survival in patients with hepatocellular carcinoma. United Eur. Gastroenterol. J 5:987–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Liu H, Naxerova K, Pinter M, Incio J, Lee H, et al. 2017. Use of angiotensin system inhibitors is associated with immune activation and longer survival in nonmetastatic pancreatic ductal adenocarcinoma. Clin. Cancer Res 23:5959–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Pinter M, Kwanten WJ, Jain RK. 2018. Renin-angiotensin system inhibitors to mitigate cancer treatment-related adverse events. Clin. Cancer Res 24:3803–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Murphy JE, Wo JY-L, Ryan DP, Jiang W, Yeap BY, et al. 2018. Potentially curative combination of TGF-β1 inhibitor losartan and FOLFIRINOX (FFX) for locally advanced pancreatic cancer (LAPC): R0 resection rates and preliminary survival data from a prospective phase II study Paper presented at the Annual Meeting of the American Society of Clinical Oncology, Chicago, IL, June 3 [Google Scholar]
- 123.Tauriello DV, Palomo-Ponce S, Stork D, Berenguer-Llergo A, Badia-Ramentol J, et al. 2018. TGFβ drives immune evasion in genetically reconstituted colon cancer metastasis. Nature 554:538–43 [DOI] [PubMed] [Google Scholar]
- 124.Incio J, Suboj P, Chin SM, Vardam-Kaur T, Liu H, et al. 2015. Metformin reduces desmoplasia in pancreatic cancer by reprogramming stellate cells and tumor-associated macrophages. PLOS ONE 10:e0141392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ferrer-Mayorga G, Gómez-López G, Barbáchano A, Fernández-Barral A, Peña C, et al. 2016. Vitamin D receptor expression and associated gene signature in tumour stromal fibroblasts predict clinical outcome in colorectal cancer. Gut 66:1449–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Frese KK, Neesse A, Cook N, Bapiro TE, Lolkema MP, et al. 2012. Nab-paclitaxel potentiates gemcitabine activity by reducing cytidine deaminase levels in a mouse model of pancreatic cancer. Cancer Discov 2:260–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Alvarez R, Musteanu M, Garcia-Garcia E, Lopez-Casas PP, Megias D, et al. 2013. Stromal disrupting effects of nab-paclitaxel in pancreatic cancer. Br. J. Cancer 109:926–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Cooke VG, LeBleu VS, Keskin D, Khan Z, O’Connell JT, et al. 2012. Pericyte depletion results in hypoxia-associated epithelial-to-mesenchymal transition and metastasis mediated by met signaling pathway. Cancer Cell 21:66–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Rahbari NN, Kedrin D, Incio J, Liu H, Ho WW, et al. 2016. Anti-VEGF therapy induces ECM remodeling and mechanical barriers to therapy in colorectal cancer liver metastases. Sci. Transl. Med 8:360ra135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Chen Y, Ramjiawan RR, Reiberger T, Ng MR, Hato T, et al. 2015. CXCR4 inhibition in tumor microenvironment facilitates anti-programmed death receptor-1 immunotherapy in sorafenib-treated hepatocellular carcinoma in mice. Hepatology 61:1591–602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Griveau A, Seano G, Shelton SJ, Kupp R, Jahangiri A, et al. 2018. A glial signature and Wnt7 signaling regulate glioma-vascular interactions and tumor microenvironment. Cancer Cell 33:874–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Duda DG, Willett CG, Ancukiewicz M, di Tomaso E, Shah M, et al. 2010. Plasma soluble VEGFR-1 is a potential dual biomarker of response and toxicity for bevacizumab with chemoradiation in locally advanced rectal cancer. Oncologist 15:577–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Duda DG, Kozin SV, Kirkpatrick ND, Xu L, Fukumura D, Jain RK. 2011. CXCL12 (SDF1α)-CXCR4/CXCR7 pathway inhibition: an emerging sensitizer for anticancer therapies? Clin. Cancer Res 17:2074–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Jung K, Heishi T, Incio J, Huang Y, Beech EY, et al. 2017. Targeting CXCR4-dependent immunosuppressive Ly6Clow monocytes improves antiangiogenic therapy in colorectal cancer. PNAS 114:10455–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Jung K, Heishi T, Khan OF, Kowalski PS, Incio J, et al. 2017. Ly6Clo monocytes drive immunosuppression and confer resistance to anti-VEGFR2 cancer therapy. J. Clin. Investig 127:3039–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Vasudev NS, Reynolds AR. 2014. Anti-angiogenic therapy for cancer: current progress, unresolved questions and future directions. Angiogenesis 17:471–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Incio J, Liu H, Suboj P, Chin SM, Chen IX, et al. 2016. Obesity-induced inflammation and desmoplasia promote pancreatic cancer progression and resistance to chemotherapy. Cancer Discov 6:852–69 [DOI] [PMC free article] [PubMed] [Google Scholar]; Demonstrates the role of obesity in desmoplasia and immunosuppression while highlighting the potential of ASIs.
- 138.Incio J, Tam J, Rahbari NN, Suboj P, McManus DT, et al. 2016. PlGF/VEGFR-1 signaling promotes macrophage polarization and accelerated tumor progression in obesity. Clin. Cancer Res 22:2993–3004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Incio J, Ligibel JA, McManus DT, Suboj P, Jung K, et al. 2018. Obesity promotes resistance to anti-VEGF therapy in breast cancer by up-regulating IL-6 and potentially FGF-2. Sci. Transl. Med 10:eaag0945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Pereira ER, Kedrin D, Seano G, Gautier O, Meijer EF, et al. 2018. Lymph node metastases can invade local blood vessels, exit the node, and colonize distant organs in mice. Science 359:1403–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Naxerova K, Reiter JG, Brachtel E, Lennerz JK, Van De Wetering M, et al. 2017. Origins of lymphatic and distant metastases in human colorectal cancer. Science 357:55–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Jeong HS, Jones D, Liao S, Wattson DA, Cui CH, et al. 2015. Investigation of the lack of angiogenesis in the formation of lymph node metastases. J. Natl. Cancer Inst 107:djv155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Bridgeman VL, Vermeulen PB, Foo S, Bilecz A, Daley F, et al. 2016. Vessel co-option is common in human lung metastases and mediates resistance to anti-angiogenic therapy in preclinical lung metastasis models. J. Pathol 241:362–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Frentzas S, Simoneau E, Bridgeman VL, Vermeulen PB, Foo S, et al. 2016. Vessel co-option mediates resistance to anti-angiogenic therapy in liver metastases. Nat. Med 22:1294–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Lee H, Shields AF, Siegel BA, Miller KD, Krop I, et al. 2017. 64Cu-MM-302 positron emission tomography quantifies variability of enhanced permeability and retention of nanoparticles in relation to treatment response in patients with metastatic breast cancer. Clin. Cancer Res 23:4190–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Emblem KE, Jain RK. 2016. Improving treatment of liver metastases by targeting nonangiogenic mechanisms. Nat. Med 22:1209. [DOI] [PubMed] [Google Scholar]
- 147.Nia HT, Liu H, Seano G, Datta M, Jones D, et al. 2016. Solid stress and elastic energy as measures of tumour mechanopathology. Nat. Biomed. Eng 1:0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Duda DG, Duyverman AM, Kohno M, Snuderl M, Steller EJ, et al. 2010. Malignant cells facilitate lung metastasis by bringing their own soil. PNAS 107:21677–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Whatcott CJ, Diep CH, Jiang P, Watanabe A, LoBello J, et al. 2015. Desmoplasia in primary tumors and metastatic lesions of pancreatic cancer. Clin. Cancer Res 21:3561–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Kodack DP, Chung E, Yamashita H, Incio J, Duyverman AM, et al. 2012. Combined targeting of HER2 and VEGFR2 for effective treatment of HER2-amplified breast cancer brain metastases. PNAS 109:E3119–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Kodack DP, Askoxylakis V, Ferraro GB, Sheng Q, Badeaux M, et al. 2017. The brain microenvironment mediates resistance in luminal breast cancer to PI3K inhibition through HER3 activation. Sci. Transl. Med 9:eaal4682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Datta M, Via LE, Kamoun WS, Liu C, Chen W, et al. 2015. Anti-vascular endothelial growth factor treatment normalizes tuberculosis granuloma vasculature and improves small molecule delivery. PNAS 112:1827–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Plotkin SR, Stemmer-Rachamimov AO, Barker FG 2nd, Halpin C, Padera TP, et al. 2009. Hearing improvement after bevacizumab in patients with neurofibromatosis type 2. N. Engl. J. Med 361:358–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Jain RK, Finn AV, Kolodgie FD, Gold HK, Virmani R. 2007. Antiangiogenic therapy for normalization of atherosclerotic plaque vasculature: a potential strategy for plaque stabilization. Nat. Rev. Cardiol 4:491. [DOI] [PubMed] [Google Scholar]
- 155.Wang X, Abraham S, McKenzie JAG, Jeffs N, Swire M, et al. 2013. LRG1 promotes angiogenesis by modulating endothelial TGFβ signaling. Nature 499:306–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Yuan F, Leunig M, Huang SK, Berk DA, Papahadjopoulos D, et al. 1994. Microvascular permeability and interstitial penetration of sterically stabilized (stealth) liposomes in a human tumor xenograft. Cancer Res 54:3352–56 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.