Abstract
Intestinal epithelial differentiation may be stimulated by diverse pathways including luminal short chain fatty acids and repetitive mechanical deformation engendered by villous motility and peristalsis. Schlafen 12 (SLFN12) is a cytosolic protein that stimulates sucrase-isomaltase (SI) expression. We hypothesized that two disparate differentiating stimuli, butyrate and repetitive deformation, would each stimulate SLFN12 expression in human Caco-2 intestinal epithelial cells and that increased SLFN12 expression would contribute to the differentiating activity of the human Caco-2 intestinal epithelial cells. We stimulated Caco-2 cells with 1–2 mM butyrate or repetitive mechanical deformation at 10 cycles/minute at an average 10% strain, and measured SLFN12 and SI expression by q-RT-PCR. Sodium butyrate enhanced SLFN12 expression at both 1 mM and 2 mM although SI expression was only significantly increased at 2 mM. Repetitive deformation induced by cyclic mechanical strain also significantly increased both SLFN12 and SI gene expression. Reducing SLFN12 by siRNA decreased basal, deformation-stimulated, and butyrate-stimulated SLFN12 levels, compared to control cells treated with non-targeting siRNA, although both deformation and butyrate were still able to stimulate SLFN12 expression in siRNA-treated cells compared to control cells treated with the same siRNA. This attenuation of the increase in SLFN12 expression in response to mechanical strain or butyrate was accompanied by parallel attenuation of SI expression. Butyrate stimulated SI-promoter activity, and reducing SLFN12 by siRNA attenuated butyrate-induced SI- promoter activity. These data suggest that SLFN12 mediates at least in part the stimulation by both butyrate and repetitive mechanical deformation of sucrase-isomaltase, a late stage differentiation marker in human intestinal epithelial cells.
Keywords: Enterocyte, intestine, Schlafen, strain, short chain fatty acid
Introduction:
The digestive and absorptive functions of the gut mucosa are determined by external factors such the luminal nutrients and microbiome to which it is exposed luminally and the blood flow that perfuses it submucosally as well as by internal factors such as the surface area of the mucosa and the phenotype of the individual intestinal epithelial cells. These are critically important issues for the management of patients with short gut syndrome, in which inadequate intestinal length can preclude oral nutrition unless diet, motility, and luminal microbiology are carefully monitored. Beyond such external factors, direct pharmacologic intervention in short gut syndrome is largely targeted to the stimulation of enterocytic proliferation to increase the remaining absorptive surface area. Both glutamine and teduglutide may be used in this fashion. However, while glutamine[1, 2] and teduglutide [3] stimulate intestinal epithelial proliferation, speeding proliferation may also decrease the time available for enterocyte maturation, reducing the individual digestive and absorptive capacity of individual enterocytes. An ideal approach would therefore target both enterocyte proliferation and differentiation.
Enterocytic differentiation is itself complex. Diverse factors, including nutrients, growth factors, and mechanical stimuli, each target different intracellular mechanisms and aspects of enterocyte phenotype. We recently reported that repetitive mechanical deformation, sodium butyrate, and TGF-beta each stimulate the differentiation of rat non-malignant IEC-6 cells by inducing the expression of Schlafen 3. In each case, Schlafen 3 is induced and reducing Schlafen 3 by siRNA prevents further differentiating effects [4]. However, humans do not express Schlafen 3, so this would not be a useful target for pharmacologic intervention.
The Schlafens are a poorly understood superfamily of proteins which in many cases are without obvious functionality [5]. Long Schlafens have a nuclear targeting sequence, and can regulate gene transcription within the nucleus, but neither short Schlafens nor intermediate Schlafens such as Schlafen 3 possess such a nuclear targeting sequence, so their actions are less clear. Schlafen 12 is a human Schlafen protein that we have previously reported to promote differentiation in human prostate cancer [6] and which promotes sucrase expression in human Caco-2 intestinal epithelial cells and non-malignant human HIEC-6 cells.[7] We now sought to determine whether two disparate differentiating stimuli, butyrate [8–10] and repetitive mechanical deformation [11, 12], stimulate Schlafen 12 expression in Caco-2 cells and, if so, whether such Schlafen 12 expression is required for the differentiating activity of these stimuli.
Material and Methods
Materials:
Dulbecco’s modified Eagle’s medium (DMEM), 0.05% Trypsin-EDTA, Lipofectamine, RNAiMAX and Plus Reagent were obtained from Thermo Fisher (Waltham, MA). Sodium butyrate and other fine reagents were purchased from Sigma (Sigma-Aldrich, St. Louis, MO). Human transferrin was obtained from Roche Applied Science. Dharmafect-Duo transfection reagent, Double-stranded short interfering RNAs (siRNAs) targeting human forms of SLFN12 and control non-targeting siRNA (NT1 siRNA) were purchased from Dharmacon (Lafayette, CO). We used at least two different sequences targeted to human SLFN12 for our initial studies of the effects of siRNA on mRNA. These yielded similar results and have been pooled for presentation
Cell Culture:
We studied Caco-2BBE intestinal epithelial cells, a subclone of the original Caco-2 cell line, selected for their ability to differentiate in culture as indicated by the formation of an apical brush border and the expression of brush-border enzymes [13, 14]. These Caco-2BBE intestinal epithelial cells were purchased from American Tissue Culture Collection (ATCC, Manassas, VA 20108 USA).
We maintained the cells at 37 C with 8% CO2 in DMEM with 4500 mg/L D-glucose, 4 mM glutamine, 1 mM sodium pyruvate, 100 U/mL penicillin, 100 μg/mL streptomycin, 10 μg/mL transferrin, 10 mM HEPES (pH 7.4), and 3.7 g/l NaHCO3, supplemented with 10% fetal bovine serum (FBS). All studies were performed on cells within 10 to 15 passages. For mechanical strain studies, cells were maintained at 37 C with 5% CO2 in DMEM supplemented with 10% heat-inactivated fetal bovine serum (FBS). A stock solution of sodium butyrate was made in sterile phosphate buffer saline (PBS) and a working concentration was made in cell culture medium.
Application of mechanical strain:
The cells were subjected to mechanical deformation using the Flexercell Strain Unit (FX-4000; Flexercell). Briefly, Caco-2 cells were plated on elastomer membranes pre-coated with collagen-I (Flexcell International corp., Hillsborough, NC) and were exposed to a continuous cycles of strain/relaxation generated by a cyclic vacuum produced by a computer-driven system (Flexcell 4000; Flexcell International corp., Hillsborough, NC). Caco-2 cells were subjected to a 20-kPa vacuum at 10 cycles/minute, with a stretch/relaxation ratio of 1:1 (3.0 second deformation alternating with 3.0 second in neutral conformation), creating an average 10% deformation that we have previously demonstrated to modulate brush-border enzyme activity in human intestinal Caco-2 cell monolayers [11] [15]. These parameters are similar in magnitude and frequency of those observed during normal intestinal peristalsis [16] and villous motility [17]. The vacuum applies negative pressure that stretches the membranes to a known percentage elongation. We have previously demonstrated that strain is transmitted to adherent cells cultured on the upper surface of the membrane, which experience similar elongation [18]. The six-well plates were maintained in a 37°C humidified incubator with 5% CO2 during the application of repetitive strain. Similar plates containing control cultures were kept in the same incubator but were not subjected to strain.
In a separate set of studies, we assessed the effects of co-stimulation with 2 mM sodium butyrate and mechanical strain with relevant controls. Forty-eight hours after transfection with non-targeting siRNA or siRNA to SLFN12, Caco-2 cells were further treated with 2 mM sodium butyrate or repetitive mechanical strain for 24 hours or the combination of both. After this additional 24 hours, the experiment was terminated and RNA was extracted for qRT-PCR.
Transfection with siRNA:
Below are the details of the siRNA sequences for non-targeting (NT) and SLFN12 obtained by Dharmacon (Lafayette, CO, USA) used in this study.
siGENOME Non-Targeting siRNA #1, Catalog # D-001210-01-20, control siRNA/siNT-1,
Target Sequence: UAGCGACUAAACACAUCAA
ON-TARGETplus Human SLFN12 siRNA-individual, Catalog # J-018142-10, siSLFN12-1,
SLFN12 Target Sequence: GAACAGAACUUGAUCGGAA
ON-TARGETplus Human SLFN12 siRNA-individual, Catalog # J-018142-11, siSLFN12-2,
SLFN12 Target Sequence: UCAGGAAGGAUAACGUAUA
ON-TARGETplus Human SLFN12 siRNA-individual, Catalog # J-018142-12, siSLFN12-3,
SLFN12 Target Sequence: GUGUUGAUUUGGAAACGAA
ON-TARGETplus Human SLFN12 siRNA-individual, Catalog # J-018142-19, siSLFN12-4,
SLFN12 Target Sequence: GAAAGUGUCUCACGAGCUA
All the siRNA for the SLFN12 (siSLFN12-1 to −4) tested individually for the knockdown efficiency at 100 nM concentrations each. Based on this data, siSLFN12-1 and siSLFN12-4 cocktail/mixture for SLFN12 at 50 nM concentrations for each used for further studies.
Caco-2 cells (250,000 cells/well) were plated into six-well plates one day before transfection with siNT-1 (Control, 100 nM) or siSLFN12 (100 nM) using RNAiMAX transfection reagent (7.5 ul/well) formulated in Opti-mem (serum-free medium) at a total volume of 300 (150+150) ul/well. The mixture was added gradually into the 1 ml of complete medium already present in each well. Experiments were terminated after 72 hours for RNA extraction.
RNA isolation and qRT-PCR:
Total RNA was isolated from Caco-2 cells using RNeasy Mini Kit, Qiashredders, DNase treatment and the QiaCube instrument per manufacturer’s protocols (Qiagen, Valencia, CA). cDNA synthesis was prepared from RNA samples using QuantiTect Reverse Transcription kit (Qiagen) or SMARTScribe Reverse Transcription kit (Takara Clontech, Mountain View, CA). cDNA samples were analyzed by qPCR analysis using the BioRad CFX96 Touch Real-Time PCR Detection System and the BioRad SsoAdvanced Universal SYBR Green Supermix (BioRad Laboratories, Hercules, CA). Expression levels were determined from the threshold cycle (Ct) values using the method of 2−ΔΔCt using Ribosomal Protein Lateral Stalk Subunit P1 (RPLP0) as the reference control gene. Primer design was as follows: human RPLP0 forward 5’-GCAATGTTGCCAGTGTCTG-3’, reverse 5’-GCCTTGA CC TTT TCAGCAA-3’; human Slfn12 forward 5’-ATCTGGGCTTGCAAGAGAAC-3’, reverse 5’-TTTTTGCCA GCTTCT GC TTT-3’; human sucrase-isomaltase (SI) forward 5’-GCCAGCTTATTGGGCTTTGGGTT-3’, reverse 5’-AACTGAGGAAGGTCCTGGAATGCT-3’. Primers were purchased from Integrated DNA Technology (IDT, Coralville, IA). The cycle conditions for the PCR were 1 cycle of 3 minutes at 95°C, 40 cycles of 30 seconds at 95°C, 30 seconds at the annealing temperature of 60°C and 30 seconds at 72°C, and then a melt curve of 1 cycle 65°C for 30 seconds and 60 cycles 65°C for 5 seconds + 0.5°C/cycle with a ramp of 0.5°C/s and a plate read each cycle.
Promoter activity assays:
Promoter activity assay was performed as described previously [19] with slight modifications. Briefly, 104 Caco-2BBE cells/well were seeded in 96-well (0.32 cm2) plates. On the following day, cells were transfected with 50 ng/well of the empty vector alone or human sucrase-isomaltase (SI) LightSwitch promoter reporter GoClone construct (Switchgear Genomics, Carlsbad, CA) using Dharmafect-Duo transfection reagent as per manufacturer recommendations. The mixture of DNA and transfection reagent was incubated at room temperature for 20–30 minutes and then added to the cells. After 48 hours of transfection, cells were either maintained without further treatment or treated with 2mM sodium butyrate for 24 hours and luciferase activity was measured. For siRNA studies, cells were co-transfected with siNT1 (Control, 100 nM) or siSLFN12 (100 nM) RNA with 50 ng/ml human sucrase-isomaltase (SI) LightSwitch promoter reporter goclone construct using Dharmafect-Duo transfection. This mixture was incubated at room temperature for 20–30 minutes and then added to the cells. Forty-eight hour hours after transfection, cells were incubated with or without 2 mM sodium butyrate (NaB) for additional 24 hours. The experiment was terminated by addition of luciferase substrate as per the manufacturer’s recommendations. Briefly, 100 μl/well (buffer + substrate) LightSwitch Luciferase assay reagent (Switchgear Genomics) was added and incubated for 30 minute at room temperature in the dark. Relative Luminescence (RLU) was measured using a VERITAS Microplate Luminometer (Turner Biosystems, Sunnyvale, CA).
SI-promoter activity assay with mechanical stimuli performed as described above. The transfection reaction was scaled up for the 6-well bioflex cell plates. After 48 hours of transfection, these plates were either incubated under static conditions without repetitive deformation as a control or subjected to cyclic strain as described above. The experiment was terminated by addition of luciferase substrate as per the manufacturer’s recommendations. Briefly, 500 μl/well (buffer + substrate) LightSwitch Luciferase assay reagent (Switchgear Genomics) was added and incubated for 30 minutes at room temperature in the dark. Relative Luminescence (RLU) was measured by collecting these lysed cells incubated with substrate to 96-well plate. The reading was performed using a VERITAS Microplate Luminometer (Turner Biosystems, Sunnyvale, CA).
Statistical analysis:
All experiments were done independently at least three times unless indicated otherwise. All data are expressed as mean ± standard error (X±SE). Statistical analysis was performed using paired or unpaired t tests as appropriate. A P value of <0.05 was considered significant.
Results:
Sodium butyrate increases both Schlafen 12 (SLFN12) and Sucrase-Isomaltase (SI) gene expression:
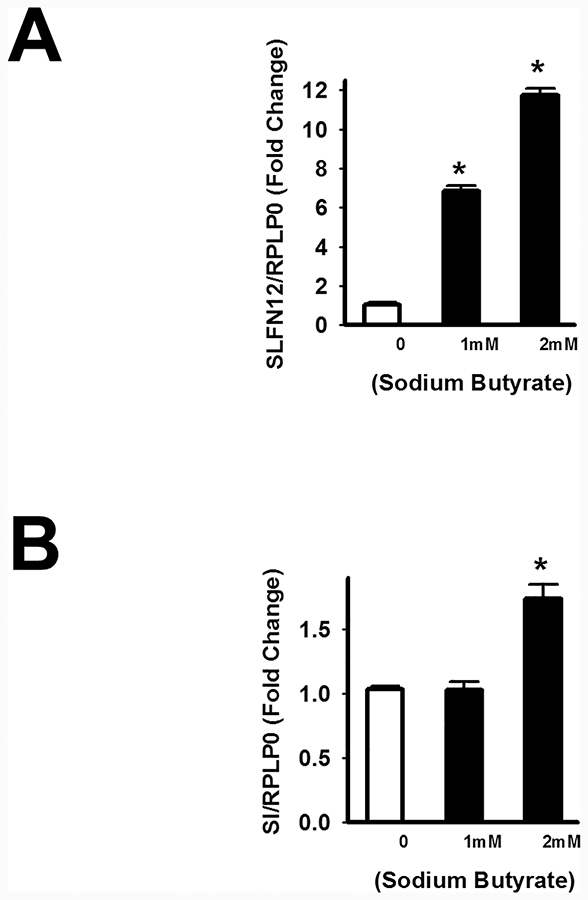
To determine whether physiologically relevant concentration (1 and 2 mM) of butyrate [20] increase the expression of SLFN12 or SI in human Caco-2 intestinal epithelial cells, we incubated confluent Caco-2 cells with 1 or 2 mM sodium butyrate for 24 hours and extracted RNA to measure the expression of SLFN12 and Sucrase-Isomaltase (SI). Butyrate significantly enhanced the expression of SLFN12 at both 1mM and 2mM (Figure 1A) while SI expression was significantly increased only at 2mM butyrate. (Figure 1B).
Figure 1: Sodium butyrate increases SLFN12 and sucrase-isomaltase (SI) gene expression in human intestinal epithelial Caco-2 cells.
Confluent Caco-2 cells were treated with vehicle control (PBS) or physiological concentrations of butyrate (1mM and 2mM NaB) for 24 hours. SLFN12 and SI gene expression were measured by qPCR. (A) Sodium butyrate significantly increases SLFN12 mRNA both at 1mM and 2mM in comparison to vehicle control cells (n=5, *p<0.05). (B) Butyrate at 2mM significantly increases SI expression in comparison to vehicle control cells (n=5, *p<0.05) while 1mM did not change the expression of SI in comparison to vehicle control cells.
Repetitive deformation increases Schlafen 12 and Sucrase-Isomaltase (SI) gene expression:
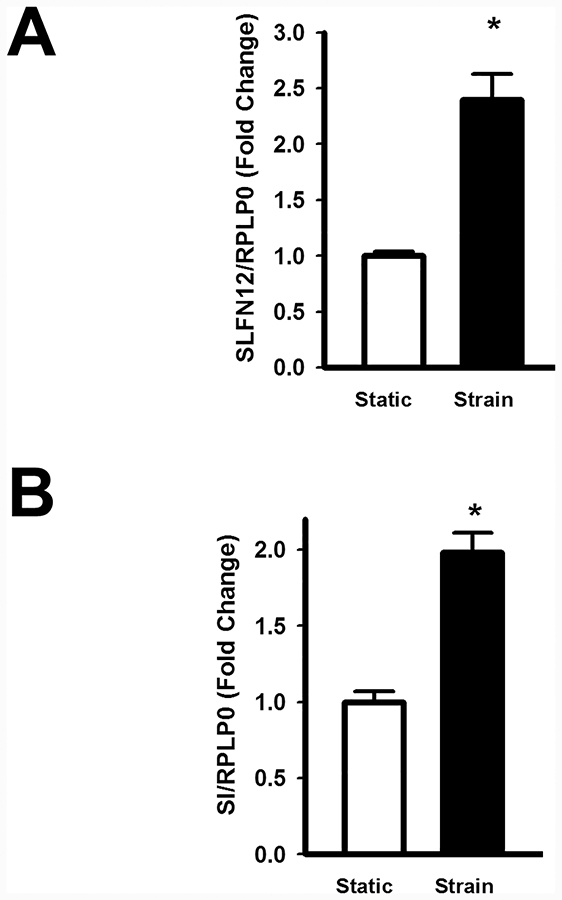
Caco-2 cells were grown to confluence on collagen-I coated flexible membranes and subjected to mechanical strain for 24 hours. Cells cultured in parallel in the absence of mechanical strain served as controls, and RNA was extracted to measure the expression of SLFN12 and SI. Repetitive deformation induced by mechanical stretch significantly increased the expression of both SLFN12 (Figure 2A) and SI in Caco-2 cells (Figure 2B).
Figure 2: Repetitive mechanical deformation increases SLFN12 and sucrase-isomaltase gene expression in human intestinal epithelial Caco-2 cells.
Caco-2 cells were grown to confluence on collagen-I coated flexible membranes and subjected to an average 10% mechanical strain at 10cycle/min for 24 hours. Cells cultured in parallel in the absence of mechanical strain served as controls and RNA was extracted to measure the expression of SLFN12 and SI. (A) Repetitive mechanical deformation significantly increases SLFN12 mRNA and (B) SI mRNA in comparison to unstimulated control cells (n=5, *p<0.05).
Schlafen 12 reduction modulates butyrate-induced Sucrase-Isomaltase (SI) promoter activity:
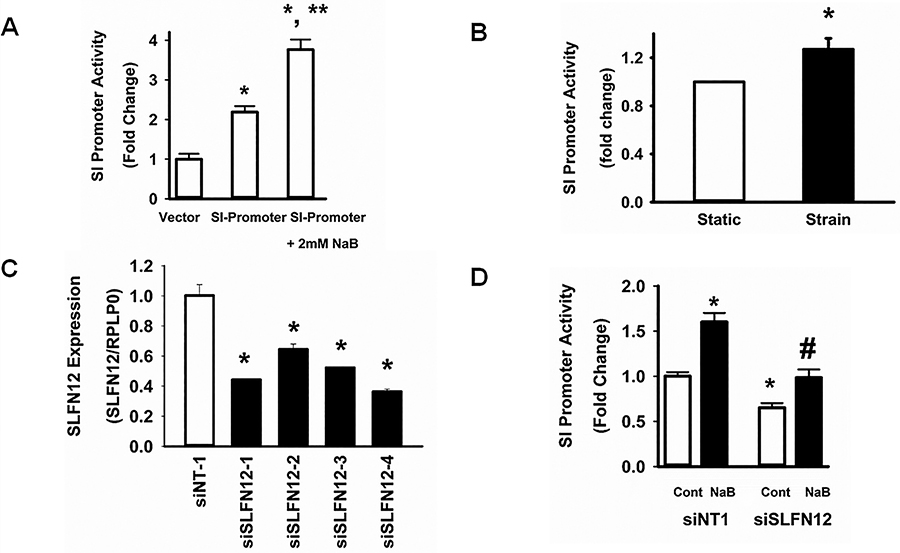
To investigate the potential role of Schlafen 12 in mediating the effects of sodium butyrate on SI-promoter activity, we first sought to confirm that butyrate treatment stimulated the SI promoter. Caco-2 cells (10,000 cell/well) were plated in ninety-six well plates. On the following day, cells were transfected with empty vector or SI-promoter GoClone construct for 48 hours followed treatment with either vehicle control (PBS) or 2 mM sodium butyrate for an additional 24 hours. Cells transfected with the SI-promoter construct displayed significantly increased basal promoter activity compared to cells transfected only with the empty vector, validating the activity of the SI promoter construct (n=5, p<0.05). SI-promoter activity was significantly further increased by the addition of 2 mM butyrate in comparison to cells transfected with the SI-promoter construct without butyrate (n=5, p<0.05) (Figure 3A).
Figure 3: SLFN12 modulates butyrate-induced sucrase-isomaltase promoter activity in human intestinal epithelial Caco-2 cells.
Caco-2 cells were transfected with empty vector or SI-promoter GoClone construct for 48 hours followed by treatment with PBS control (Cont) or 2mM butyrate (NaB) for additional 24hr. For siRNA studies, Caco-2 cells were co-transfected with SI-promoter construct and control siNT1 or SLFN12 siRNA (siSLFN12) for 48hours followed by treatment with 2mM butyrate for additional 24 hours, then luciferase activity was measured. (A) Basal SI-promoter activity increased in comparison to empty vector (n=5, *p<0.05) and NaB further increased SI-promoter activity in comparison to empty vector or SI-promoter construct alone (n=5, *p<0.05 vs empty vector; n=5, **p<0.05 vs SI-promoter alone). (B) SI-promoter activity after mechanical stimulation increased compared to control (static) cells (n=7, *p<0.05). (C) Knockdown efficiency of each of the siRNA putatively targeting SLFN12 (siRNA-1, siRNA-2, siRNA-3 and siRNA-4) with respect to control non-targeting siRNA (siNT-1) transfected in Caco-2 cells. Each siRNA was used at 100 nM (n = 4, *, p < 0.05, siNT vs siSLFN12). (D) Sodium butyrate enhances SI promoter activity in siNT1-treated cells (n = 5, *, p < 0.05). Reduced SLFN12 expression by siSLFN12 attenuated basal (n = 5, *, p < 0.05) and butyrate-induced SI-promoter activity (n=5, #, p<0.05).
We also assessed the SI-promoter activity after mechanical stimuli. Consistent with the qRT-PCR data, the SI-promoter activity significantly increased with cyclic strain as compared to cells maintained under static conditions (Figure 3B).
We therefore next evaluated modulation of butyrate-induced SI-promoter activity by SLFN12 reduction with siSLFN12. We have previously evaluated our reagents for knockdown and overexpression of SLFN12 protein in Caco-2 cells and other cell lines [21] Immunocytochemical staining in Caco-2 cells confirmed that either adenoviral SLFN12 overexpression or knockdown using siRNA to SLFN12 results in changes in SLFN12 protein immunoreactivity that parallel SLFN12 mRNA changes by qRT-PCR. As there were no reliable and specific antibodies available to Western blot for SLFN12 at the time of writing this manuscript, we therefore chose to use qRT-PCR to confirm our SLFN12 knockdown in this work.
We initially used four different siRNAs for SLFN12 and tested the knockdown efficiency of each. Sub-confluent Caco-2 cells were transfected with non-targeted control siRNA (siNT-1) or one of four individual sequences of siRNA to Schlafen12 for 72 hours. RNA was extracted and qRT-PCR was performed. Maximum reduction of the SLFN12 expression was obtained by siSLFN12–1 and siSLFN12–4 (Figure 3C). Therefore, henceforth, a mixture of siSLFN12–1 and siSLFN12–4 at 50 nM concentrations of each was used for further studies.
For SI-promoter activity assay, Caco-2 cells (10,000 cell/well) were plated in ninety-six well plates and on the following day the cells were co-transfected with the SI-promoter construct with either non-targeting control siRNA (siNT1) or siRNA directed at SLFN12 (siSLFN12) for 48 hours. The cells were then either maintained without further treatment or treated with 2 mM sodium butyrate for an additional 24 hours prior to measurement of luciferase activity. The average knockdown efficiency obtained for all of the siSLFN12 experiments was 68.9% ±5.4. Reducing SLFN12 by siRNA substantially and statistically significantly attenuated butyrate-induced SI promoter activity (n=5, p<0.05, Figure 3D).
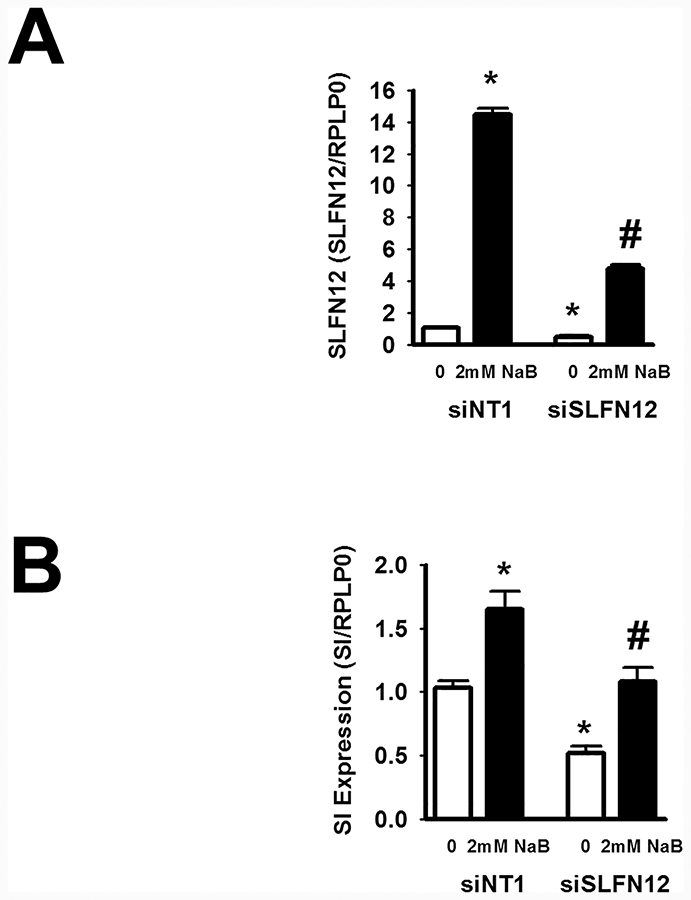
Schlafen 12 reduction modulates butyrate-induced Sucrase-Isomaltase (SI) gene expression:
Sub-confluent Caco-2 cells were next transfected with siNT1 or siRNA to Schlafen12 for 48 hours and then incubated without or with 2mM sodium butyrate for an additional 24 hours. RNA was extracted for qRT-PCR. Reducing SLFN12 by siRNA reduced basal SLFN12 expression and substantially attenuated the induction of SLFN12 expression by butyrate but did not prevent it. n=5, p<0.05, Figure 4A) SI gene expression was similarly reduced in parallel with SLFN12 and the effects of butyrate on SI expression were significantly attenuated. (n=5, p<0.05, Figure 4B).
Figure 4: SLFN12 modulates butyrate-induced sucrase-isomaltase gene expression in human intestinal epithelial Caco-2 cells.
Caco-2 cells were transfected with control siNT1 or siSLFN12 for 48 hours followed by treatment with PBS control (0 mM) or 2 mM butyrate for additional 24 h. SLFN12 and SI gene expression were measured by qPCR. (A) Sodium butyrate (NaB) enhances SLFN12 expression and (B) SI expression in siNT1-treated cells (n = 5, *, p < 0.05). Reduced SLFN12 expression by siSLFN12 attenuated butyrate-induced (A) SLFN12 (n=5, #, p<0.05) or (B) SI expression. SLFN12 siRNA inhibited basal (A) SLFN12 (n = 5, *, p < 0.05) and (B) basal SI expressions.
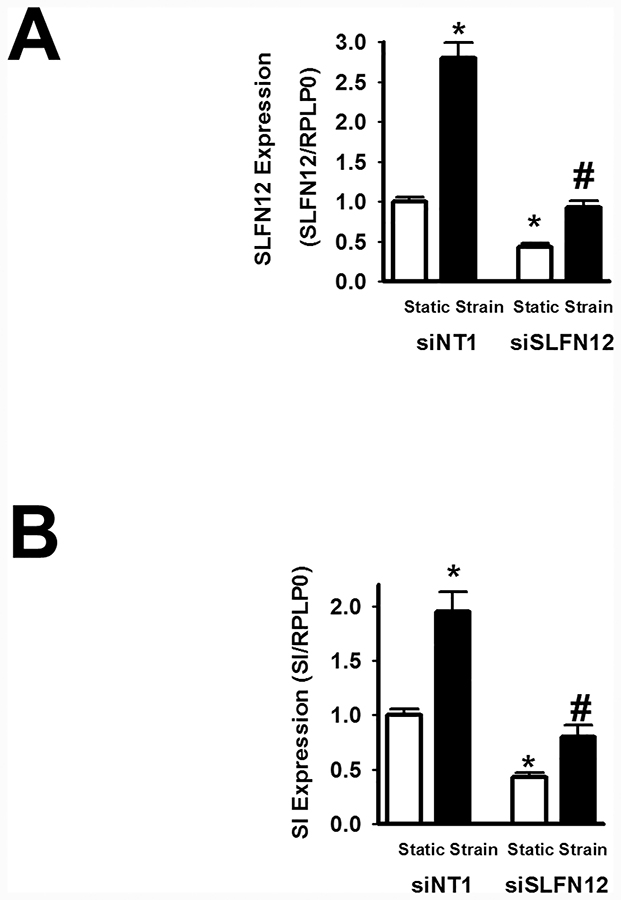
Schlafen 12 modulates mechanical strain-induced Sucrase-Isomaltase (SI) gene expression:
Finally, we sought to determine whether Schlafen 12 might similarly be involved in the effects of repetitive mechanical deformation on Caco-2 SI gene expression. Caco-2 cells were plated on collagen-I-coated flexible membranes. On the following day, the cells were transfected with control siNT1 or siRNA to Schlafen12 for 48 hours. Cells were then repetitively exposed to an average 10% mechanical strain at 10 cycles/minute for an additional 24 hours. In parallel, cells were transfected but not subjected to mechanical strain as controls. RNA was extracted for qRT-PCR. Reducing SLFN12 by siRNA substantially attenuated but did not prevent the deformation-induced increase in SLFN12 expression (n=5, p<0.05, Figure 5A). Again, SI gene expression paralleled SLFN12 expression and the stimulation of SI expression by repetitive deformation was attenuated by reducing SLFN12 (n=5, p<0.05, Figure 5B).
Figure 5: SLFN12 modulates repetitive mechanical deformation-induced sucrase-isomaltase gene expression in human intestinal epithelial Caco-2 cells.
Caco-2 cells were plated on collagen-I coated flexible membranes and next day cells were transfected with control siNT1 or siSLFN12 for 48 hours followed by mechanical strain at 10cycle/min for 24 hours. Cells cultured in parallel in the absence of mechanical strain served as controls and SLFN12 and SI gene expression were measured by qPCR. Repetitive mechanical deformation enhances (A) SLFN12 and (B) SI expression in siNT1-treated cells (n = 5, *, p < 0.05). Reduced SLFN12 expression by siSLFN12 attenuated deformation-induced (A) SLFN12 and (B) SI expression (n=5, #, p<0.05). SLFN12 siRNA inhibited basal (A) SLFN12 and (B) basal SI expression (n = 5, *, p < 0.05).
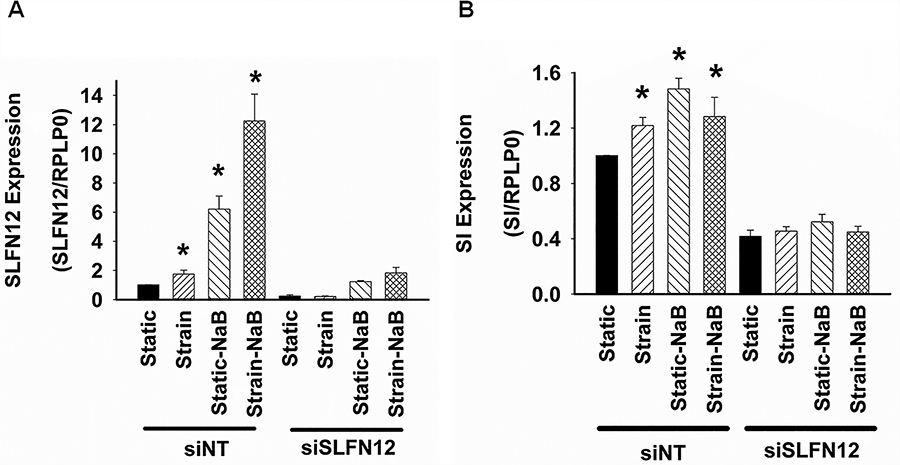
Finally, we assessed the co-stimulation effect of 2 mM sodium butyrate and mechanical strain on SI expression in SLFN12 knockdown Caco-2 cells. As expected, mechanical strain or sodium butyrate alone promoted SLFN12 expression while knockdown of SLFN12 reduced SLFN12 expression. Co-stimulation further increased SLFN12 expression as compared to treatment with either mechanical strain or butyrate alone (Figure 6A), while knockdown of SLFN12 hampered this co-stimulatory effect on SLFN12. Similarly, SI expression was increased in response to cyclic strain or butyrate alone as compared to its respective controls while silencing SLFN12 blocked the effect. Surprisingly, although co-stimulation with both mechanical strain and butyrate stimulated SI expression compared to controls, there was no further increase in SI expression compared with either cyclic strain or butyrate alone (Figure 6B). Reduction of SLFN12 by siRNA prevented the stimulation of SI expression by co-stimulation with strain and butyrate.
Figure 6: Co-stimulatory effects of repetitive mechanical deformation and butyrate on SLFN12 and SI-expression in the absence and presence of siRNA to SLFN12.
(A) SLFN12 and (B) SI expression analyzed by qTR-PCT in Caco-2 cells transfected with siNT-1 control siRNA or the siRNA-1/siRNA-4 mixture to reduce SLFN12. Forty-eight hours after transfection, these cells were maintained for an additional 24 hours under control conditions (black bars), with cyclic strain (shaded bars from lower left to upper right), 2 mM butyrate (shaded bars from upper right to lower left), or in combination for co-stimulation (cross-hatched bars). Cells not exposed to cyclic strain were cultured under static conditions and cells not treated with butyrate were treated with an equal volume of PBS as a vehicle control (n=4, *p<0.05, siNT static vs either strain or static-NaB or strain-NaB).
Discussion:
Intestinal epithelial differentiation is a complex process, regulated by diverse extracellular stimuli, including luminal nutrients, growth factors, and mechanical stimulation. Sucrase-isomaltase expression is a late stage marker of intestinal epithelial differentiation [22] stimulated by short chain fatty acids such as sodium butyrate at physiologically relevant concentrations [20]. Repetitive mechanical deformation has previously been demonstrated to stimulate the expression of DPP-IV and reduce intestinal alkaline phosphatase in Caco-2 cells [4], but its effects on sucrase-isomaltase (SI) expression have not previously been studied. This study suggests that repetitive deformation stimulates sucrase-isomaltase expression as well. Moreover, SLFN12 appears to mediate, at least in part, the phenotypic effects of both butyrate and repetitive deformation.
Although we have previously reported that both butyrate and cyclic strain stimulate expression of a different differentiation marker, DPPIV, in rat IEC-6 cells via induction of Schlafen 3, it was by no means obvious from this that strain and/or butyrate would induce SI in human cells via Schlafen 12. First, not all differentiation markers vary in parallel. For instance, repetitive differentiation stimulates DPPIV expression in human Caco-2 cells cultured on type I collagen but inhibits alkaline phosphatase expression in these same cells in these same circumstances[11] even though both alkaline phosphatase and DPPIV are stimulated in Caco-2 cells by culture on inserts that allow basal nutrition [23]. Similarly, short chain fatty acids such as butyrate may induce expression of some genes and reduce the expression of others [24, 8]. Indeed, the induction of SI by cyclic strain in human intestinal epithelial cells has not previously been described. Second, Schlafen 3 is a rodent protein with about 40% homology to human Schlafen 12, and so it was important to confirm that Schlafen 12 might have the same effects in human intestinal epithelial cells that Schlafen 3 does in rodent cells. The failure of pathways and drug targets to cross species lines is unfortunately common in drug development. [25–27].
Third, to our knowledge, the effects of repetitive mechanical strain and butyrate have not been studied in combination as we did here. Thus, our present results, while consistent with previous observations in the literature, are novel and relevant.
Although originally derived from a colon cancer, Caco-2BBE cells are a good model for the study of enterocytic differentiation. Caco-2 cells upon differentiation express several morphological and biochemical characteristics of small intestine enterocytes [28]. These cells grow in monolayers and evince a columnar polarized morphology, with microvilli on the apical side, tight junctions between adjacent cells, and small intestinal hydrolase (sucrase-isomaltase, lactase aminopeptidase N, dipeptidylpeptidase-IV) enzyme activities on the apical membrane. These cells also differentiate spontaneously in long term culture, with increased expression of typical enterocytic differentiation markers such as sucrase-isomaltase and glucose transporter GLUT-5 as passage number increases [29]. Yang and colleagues (2013) have also observed increased sucrase-isomaltase protein after four days of post-confluent culture in Caco-2 cells [30]. Caco-2 cells thus represent a good model to study the regulation of endogenous sucrase-isomaltase expression [31]. Although the magnitude of the stimulation of sucrase-isomaltase expression by strain and butyrate described here might seem modest, these are comparable to that studied by others previously, consistent with physiological changes, and likely to be potentially biologically important because this is an enzyme whose effects will be amplified [32–38] [39]. An increase of sucrase-isomaltase expression/activity following butyrate treatment in Caco-2 cells [40, 41] [42] and HT-29 cells[43], has been reported previously with similar results to our present findings. The effect of butyrate treatment on sucrase-isomaltase promoter activity has not been investigated previously.
The Schlafens are a poorly understood superfamily of proteins that were first described in the early 1990’s [44]. Although their function and mechanism of action is unclear, some other Schlafen proteins have previously been reported to contribute to the differentiation of monocytes to dendritic cells [45], monocytes to macrophages [46], and immature to mature enterocytes [47] [48]. We have previously reported that butyrate and repetitive deformation induce Schlafen 3 expression in rat IEC-6 cells [4], that Schlafen 3 overexpression or reduction modulates enterocytic differentiation in rats in vivo [47] and that Slfn3 acts from within the cytosol to induce enterocytic differentiation in Caco-2 cells by a mechanism involving its P-loop [19]. Schlafen 3 expression correlates with other enterocytic differentiation markers in rat small intestinal mucosa in vivo [47] and increases in the rat gut mucosa after birth in parallel with other differentiation markers while decreasing postnatally in the lung and liver. [49]. Indeed, Schlafen 3 is required for the induction of villin expression by the differentiating transcription factor Cdx2 in rat IEC-6-Cdx2-L1 cells. [50]. Thus, there may be good reason to link Schlafen 3 to rodent enterocyte differentiation. However, Slfn3 is not expressed in humans. SLFN12 is a human protein approximately 40% homologous to Slfn3 and which contains a structure similar to the Slfn3 P-loop. Viral SLFN12 overexpression appears to stimulate Caco-2 sucrase-isomaltase expression by itself [7], but whether SLFN12 contributes to the effects of butyrate or repetitive deformation in human cells was not previously known.
Butyrate induces intestinal epithelial differentiation [10, 9, 24], at least in part, by inhibiting histone deacetylation [51] while repetitive deformation acts in a matrix-dependent and integrin-dependent manner to stimulate a complex intracellular signaling web that has both cytoskeletally-dependent and cytoskeletally-independent components [52, 53, 4, 54–56]. Surprisingly, these two very disparate stimuli each appear to converge upon SLFN12 in their stimulation of SLFN12 expression. The stimulation of SLFN12 expression by each stimulus, together with the attenuation of their effects on SI expression by siRNA targeting SLFN12, suggests that both butyrate and repetitive deformation act at least in part through their promotion of SLFN12 expression. Because both stimuli still increase SLFN12 (albeit in an attenuated fashion) even in siRNA-treated cells, it is not possible to determine from these studies whether the stimulation of SLFN12 completely explains the effects of butyrate and repetitive deformation on SI expression or whether either of these stimuli may induce SI via both SLFN12 expression and some other pathway. This remains a subject for future study.
Although co-stimulation with both butyrate and mechanical strain increased SLFN12 expression more than either stimulus alone, a parallel further increase in SI was not observed with co-stimulation. This could be explained by the expression of the SI is already at maximum level or both the treatment interferes with each other for regulation of SI expression as opposed to SLFN12 regulation and the last possibility could be the hyper-activation of the SLFN12 by co-stimulation results in activation of other possible negative feedback regulation of SI expression.
Differences between the effects of butyrate alone in the co-stimulation experiment and the effects of butyrate in the experiment studying only butyrate effects could be explained by the required methodology for the former. The repetitive deformation studies were conducted in cell culture medium containing 10% heat- inactivated fetal bovine serum previously [11] because serum fibronectin in medium that is not heat inactivated blocks and in some cases reverses the response to cyclic strain [18]. In contrast, the butyrate only experiment was conducted in culture medium in which the serum was not heat-inactivated. It is likely that heat inactivation also interferes with other cytokines and growth factors in the serum that could interact with butyrate effects.
In summary, taken together with our previous observations, the present study demonstrates that human Schlafen 12 and its murine ortholog Schlafen 3 are likely to mediate, at least in part, the stimulation of sucrase-isomaltase expression by both butyrate and repetitive mechanical deformation in intestinal epithelial cells. Further elucidation of how SLFN12 functions may guide the development of new interventions to stimulate enterocyte differentiation that could be useful to promote intestinal adaptation in patients with mucosal atrophy after prolonged fasting or patients with short gut syndrome.
Acknowledgments
Supported in part by NIH RO1 DK096137 (MDB)
Footnotes
Declaration on conflict of interest:
All the authors in the present manuscript declare that they have no potential conflict of interest relevant to this study.
References
- 1.Turowski GA, Rashid Z, Hong F, Madri JA, Basson MD. Glutamine modulates phenotype and stimulates proliferation in human colon cancer cell lines. Cancer Res. 1994;54(22):5974–80. [PubMed] [Google Scholar]
- 2.Rhoads JM, Argenzio RA, Chen W, Rippe RA, Westwick JK, Cox AD et al. L-glutamine stimulates intestinal cell proliferation and activates mitogen-activated protein kinases. Am J Physiol. 1997;272(5 Pt 1):G943–53. [DOI] [PubMed] [Google Scholar]
- 3.Chaturvedi LS, Basson MD. Glucagonlike peptide 2 analogue teduglutide: stimulation of proliferation but reduction of differentiation in human Caco-2 intestinal epithelial cells. JAMA Surg. 2013;148(11):1037–42. doi: 10.1001/jamasurg.2013.3731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yuan L, Yu Y, Sanders MA, Majumdar AP, Basson MD. Schlafen 3 induction by cyclic strain regulates intestinal epithelial differentiation. Am J Physiol Gastrointest Liver Physiol. 2010;298(6):G994–G1003. doi: 10.1152/ajpgi.00517.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu F, Zhou P, Wang Q, Zhang M, Li D. The Schlafen family: complex roles in different cell types and virus replication. Cell Biol Int. 2017. doi: 10.1002/cbin.10778. [DOI] [PubMed] [Google Scholar]
- 6.Kovalenko PL, Basson MD. Schlafen 12 expression modulates prostate cancer cell differentiation. J Surg Res. 2014;190(1):177–84. doi: 10.1016/j.jss.2014.03.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Basson MD, Wang Q, Chaturvedi LS, More S, Vomhof-DeKrey EE, Al-Marsoummi S et al. Schlafen12 interaction with SerpinB12 and deubiquitylases drives human enterocyte differentiation. Cellular Physiology and Biochemistry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Emenaker NJ, Basson MD. Short chain fatty acids inhibit human (SW1116) colon cancer cell invasion by reducing urokinase plasminogen activator activity and stimulating TIMP-1 and TIMP-2 activities, rather than via MMP modulation. J Surg Res. 1998;76(1):41–6. doi: 10.1006/jsre.1998.5279. [DOI] [PubMed] [Google Scholar]
- 9.Basson MD, Emenaker NJ, Hong F. Differential modulation of human (Caco-2) colon cancer cell line phenotype by short chain fatty acids. Proc Soc Exp Biol Med. 1998;217(4):476–83. [DOI] [PubMed] [Google Scholar]
- 10.Basson MD, Sgambati SA. Effects of short-chain fatty acids on human rectosigmoid mucosal colonocyte brush-border enzymes. Metabolism. 1998;47(2):133–4. [DOI] [PubMed] [Google Scholar]
- 11.Basson MD, Li GD, Hong F, Han O, Sumpio BE. Amplitude-dependent modulation of brush border enzymes and proliferation by cyclic strain in human intestinal Caco-2 monolayers. J Cell Physiol. 1996;168(2):476–88. doi:. [DOI] [PubMed] [Google Scholar]
- 12.Gayer CP, Basson MD. The effects of mechanical forces on intestinal physiology and pathology. Cell Signal. 2009;21(8):1237–44. doi: 10.1016/j.cellsig.2009.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peterson MD, Mooseker MS. Characterization of the enterocyte-like brush border cytoskeleton of the C2BBe clones of the human intestinal cell line, Caco-2. J Cell Sci. 1992;102 (Pt 3):581–600. [DOI] [PubMed] [Google Scholar]
- 14.Peterson MD, Bement WM, Mooseker MS. An in vitro model for the analysis of intestinal brush border assembly. II. Changes in expression and localization of brush border proteins during cell contact-induced brush border assembly in Caco-2BBe cells. J Cell Sci. 1993;105 (Pt 2):461–72. [DOI] [PubMed] [Google Scholar]
- 15.Han O, Li GD, Sumpio BE, Basson MD. Strain induces Caco-2 intestinal epithelial proliferation and differentiation via PKC and tyrosine kinase signals. Am J Physiol. 1998;275(3 Pt 1):G534–41. [DOI] [PubMed] [Google Scholar]
- 16.Froehlich JM, Patak MA, von Weymarn C, Juli CF, Zollikofer CL, Wentz KU. Small bowel motility assessment with magnetic resonance imaging. J Magn Reson Imaging. 2005;21(4):370–5. doi: 10.1002/jmri.20284. [DOI] [PubMed] [Google Scholar]
- 17.Womack WA, Barrowman JA, Graham WH, Benoit JN, Kvietys PR, Granger DN. Quantitative assessment of villous motility. Am J Physiol. 1987;252(2 Pt 1):G250–6. [DOI] [PubMed] [Google Scholar]
- 18.Zhang J, Li W, Sanders MA, Sumpio BE, Panja A, Basson MD. Regulation of the intestinal epithelial response to cyclic strain by extracellular matrix proteins. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2003;17(8):926–8. doi: 10.1096/fj.02-0663fje. [DOI] [PubMed] [Google Scholar]
- 19.Chaturvedi L, Sun K, Walsh MF, Kuhn LA, Basson MD. The P-loop region of Schlafen 3 acts within the cytosol to induce differentiation of human Caco-2 intestinal epithelial cells. Biochim Biophys Acta. 2014;1843(12):3029–37. doi: 10.1016/j.bbamcr.2014.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Orchel A, Dzierzewicz Z, Parfiniewicz B, Weglarz L, Wilczok T. Butyrate-induced differentiation of colon cancer cells is PKC and JNK dependent. Dig Dis Sci. 2005;50(3):490–8. [DOI] [PubMed] [Google Scholar]
- 21.Basson MD, Wang Q, Chaturvedi LS, More S, Vomhof-DeKrey EE, Al-Marsoummi S et al. Schlafen 12 Interaction with SerpinB12 and Deubiquitylases Drives Human Enterocyte Differentiation. Cell Physiol Biochem. 2018;48(3):1274–90. doi: 10.1159/000492019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tung J, Markowitz AJ, Silberg DG, Traber PG. Developmental expression of SI is regulated in transgenic mice by an evolutionarily conserved promoter. Am J Physiol. 1997;273(1 Pt 1):G83–92. [DOI] [PubMed] [Google Scholar]
- 23.Perdikis DA, Basson MD. Basal nutrition promotes human intestinal epithelial (Caco-2) proliferation, brush border enzyme activity, and motility. Crit Care Med. 1997;25(1):159–65. [DOI] [PubMed] [Google Scholar]
- 24.Basson MD, Liu YW, Hanly AM, Emenaker NJ, Shenoy SG, Gould Rothberg BE. Identification and comparative analysis of human colonocyte short-chain fatty acid response genes. J Gastrointest Surg. 2000;4(5):501–12. [DOI] [PubMed] [Google Scholar]
- 25.Lawrence J, Cameron D, Argyle D. Species differences in tumour responses to cancer chemotherapy. Philos Trans R Soc Lond B Biol Sci. 2015;370(1673). doi: 10.1098/rstb.2014.0233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yamasaki K, Enokida T, Taguchi K, Miyamura S, Kawai A, Miyamoto S et al. Species Differences in the Binding of Sodium 4-Phenylbutyrate to Serum Albumin. J Pharm Sci. 2017;106(9):2860–7. doi: 10.1016/j.xphs.2017.04.025. [DOI] [PubMed] [Google Scholar]
- 27.Moggs J, Moulin P, Pognan F, Brees D, Leonard M, Busch S et al. Investigative safety science as a competitive advantage for Pharma. Expert Opin Drug Metab Toxicol. 2012;8(9):1071–82. doi: 10.1517/17425255.2012.693914. [DOI] [PubMed] [Google Scholar]
- 28.Zweibaum A, Triadou N, Kedinger M, Augeron C, Robine-Leon S, Pinto M et al. Sucrase-isomaltase: a marker of foetal and malignant epithelial cells of the human colon. Int J Cancer. 1983;32(4):407–12. [DOI] [PubMed] [Google Scholar]
- 29.Chantret I, Rodolosse A, Barbat A, Dussaulx E, Brot-Laroche E, Zweibaum A et al. Differential expression of sucrase-isomaltase in clones isolated from early and late passages of the cell line Caco-2: evidence for glucose-dependent negative regulation. J Cell Sci. 1994;107 (Pt 1):213–25. [DOI] [PubMed] [Google Scholar]
- 30.Yang B, Cao L, Liu B, McCaig CD, Pu J. The transition from proliferation to differentiation in colorectal cancer is regulated by the calcium activated chloride channel A1. PLoS One. 2013;8(4):e60861. doi: 10.1371/journal.pone.0060861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hauri HP, Sterchi EE, Bienz D, Fransen JA, Marxer A. Expression and intracellular transport of microvillus membrane hydrolases in human intestinal epithelial cells. J Cell Biol. 1985;101(3):838–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Traber PG, Wu GD, Wang W. Novel DNA-binding proteins regulate intestine-specific transcription of the sucrase-isomaltase gene. Mol Cell Biol. 1992;12(8):3614–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Traber PG, Yu L, Wu GD, Judge TA. Sucrase-isomaltase gene expression along crypt-villus axis of human small intestine is regulated at level of mRNA abundance. Am J Physiol. 1992;262(1 Pt 1):G123–30. [DOI] [PubMed] [Google Scholar]
- 34.Markowitz AJ, Wu GD, Bader A, Cui Z, Chen L, Traber PG. Regulation of lineage-specific transcription of the sucrase-isomaltase gene in transgenic mice and cell lines. Am J Physiol. 1995;269(6 Pt 1):G925–39. [DOI] [PubMed] [Google Scholar]
- 35.Hecht A, Torbey CF, Korsmo HA, Olsen WA. Regulation of sucrase and lactase in developing rats: role of nuclear factors that bind to two gene regulatory elements. Gastroenterology. 1997;112(3):803–12. [DOI] [PubMed] [Google Scholar]
- 36.Kishi K, Takase S, Goda T. Enhancement of sucrase-isomaltase gene expression induced by luminally administered fructose in rat jejunum. J Nutr Biochem. 1999;10(1):8–12. [DOI] [PubMed] [Google Scholar]
- 37.Petersen YM, Elnif J, Schmidt M, Sangild PT. Glucagon-like peptide 2 enhances maltase-glucoamylase and sucrase-isomaltase gene expression and activity in parenterally fed premature neonatal piglets. Pediatr Res. 2002;52(4):498–503. doi: 10.1203/00006450-200210000-00007. [DOI] [PubMed] [Google Scholar]
- 38.Pacheco II, Macleod RJ. CaSR stimulates secretion of Wnt5a from colonic myofibroblasts to stimulate CDX2 and sucrase-isomaltase using Ror2 on intestinal epithelia. Am J Physiol Gastrointest Liver Physiol. 2008;295(4):G748–59. doi: 10.1152/ajpgi.00560.2007. [DOI] [PubMed] [Google Scholar]
- 39.Roostaee A, Guezguez A, Beausejour M, Simoneau A, Vachon PH, Levy E et al. Histone deacetylase inhibition impairs normal intestinal cell proliferation and promotes specific gene expression. J Cell Biochem. 2015;116(11):2695–708. doi: 10.1002/jcb.25274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang Q, Wang X, Hernandez A, Kim S, Evers BM. Inhibition of the phosphatidylinositol 3-kinase pathway contributes to HT29 and Caco-2 intestinal cell differentiation. Gastroenterology. 2001;120(6):1381–92. [DOI] [PubMed] [Google Scholar]
- 41.Malago JJ, Koninkx JF, Douma PM, Dirkzwager A, Veldman A, Hendriks HG et al. Differential modulation of enterocyte-like Caco-2 cells after exposure to short-chain fatty acids. Food Addit Contam. 2003;20(5):427–37. doi: 10.1080/0265203031000137728. [DOI] [PubMed] [Google Scholar]
- 42.Takahashi J, Ogihara K, Naya Y, Kimura F, Itoh M, Iwama Y et al. An in vitro assay system for antihyperlipidemic agents by evaluating lipoprotein profiles from human intestinal epithelium-like cells. 3 Biotech. 2013;3(3):213–8. doi: 10.1007/s13205-012-0085-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Graz CJ, Cowley HM. Energy state in HT-29 cells is linked to differentiation. In Vitro Cell Dev Biol Anim. 1997;33(4):277–81. [DOI] [PubMed] [Google Scholar]
- 44.Schwarz DA, Katayama CD, Hedrick SM. Schlafen, a new family of growth regulatory genes that affect thymocyte development. Immunity. 1998;9(5):657–68. [DOI] [PubMed] [Google Scholar]
- 45.Puck A, Aigner R, Modak M, Cejka P, Blaas D, Stockl J. Expression and regulation of Schlafen (SLFN) family members in primary human monocytes, monocyte-derived dendritic cells and T cells. Results Immunol. 2015;5:23–32. doi: 10.1016/j.rinim.2015.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.van Zuylen WJ, Garceau V, Idris A, Schroder K, Irvine KM, Lattin JE et al. Macrophage activation and differentiation signals regulate schlafen-4 gene expression: evidence for Schlafen-4 as a modulator of myelopoiesis. PLoS One. 2011;6(1):e15723. doi: 10.1371/journal.pone.0015723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kovalenko PL, Basson MD. The correlation between the expression of differentiation markers in rat small intestinal mucosa and the transcript levels of schlafen 3. JAMA Surg. 2013;148(11):1013–9. doi: 10.1001/jamasurg.2013.3572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Patel VB, Yu Y, Das JK, Patel BB, Majumdar AP. Schlafen-3: a novel regulator of intestinal differentiation. Biochem Biophys Res Commun. 2009;388(4):752–6. doi: 10.1016/j.bbrc.2009.08.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Walsh MF, Hermann R, Sun K, Basson MD. Schlafen 3 changes during rat intestinal maturation. Am J Surg. 2012;204(5):598–601. doi: 10.1016/j.amjsurg.2012.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Walsh MF, Hermann R, Lee JH, Chaturvedi L, Basson MD. Schlafen 3 Mediates the Differentiating Effects of Cdx2 in Rat IEC-Cdx2L1 Enterocytes. J Invest Surg. 2015;28(4):202–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Marks PA, Richon VM, Rifkind RA. Histone deacetylase inhibitors: inducers of differentiation or apoptosis of transformed cells. J Natl Cancer Inst. 2000;92(15):1210–6. [DOI] [PubMed] [Google Scholar]
- 52.Owen CR, Yuan L, Basson MD. Smad3 knockout mice exhibit impaired intestinal mucosal healing. Lab Invest. 2008;88(10):1101–9. doi: 10.1038/labinvest.2008.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chaturvedi LS, Marsh HM, Basson MD. Role of RhoA and its effectors ROCK and mDia1 in the modulation of deformation-induced FAK, ERK, p38, and MLC motogenic signals in human Caco-2 intestinal epithelial cells. Am J Physiol Cell Physiol. 2011;301(5):C1224–38. doi: 10.1152/ajpcell.00518.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Craig DH, Zhang J, Basson MD. Cytoskeletal signaling by way of alpha-actinin-1 mediates ERK1/2 activation by repetitive deformation in human Caco2 intestinal epithelial cells. Am J Surg. 2007;194(5):618–22. doi: 10.1016/j.amjsurg.2007.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gayer CP, Chaturvedi LS, Wang S, Alston B, Flanigan TL, Basson MD. Delineating the signals by which repetitive deformation stimulates intestinal epithelial migration across fibronectin. Am J Physiol Gastrointest Liver Physiol. 2009;296(4):G876–85. doi: 10.1152/ajpgi.90648.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gayer CP, Chaturvedi LS, Wang S, Craig DH, Flanigan T, Basson MD. Strain-induced proliferation requires the phosphatidylinositol 3-kinase/AKT/glycogen synthase kinase pathway. J Biol Chem. 2009;284(4):2001–11. doi: 10.1074/jbc.M804576200. [DOI] [PMC free article] [PubMed] [Google Scholar]