Abstract
Metastasis is the primary cause of cancer morbidity and mortality. The process involves a complex interplay between intrinsic tumor cell properties as well as interactions between cancer cells and multiple microenvironments. The outcome is the development of a nearby or distant discontiguous secondary mass. To successfully disseminate, metastatic cells acquire properties in addition to those necessary to become neoplastic. Heterogeneity in mechanisms involved, routes of dissemination, redundancy of molecular pathways that can be utilized, and the ability to piggyback on the actions of surrounding stromal cells makes defining the hallmarks of metastasis extraordinarily challenging. Nonetheless, this review identifies four distinguishing features that are required: motility & invasion, ability to modulate the secondary site or local microenvironments, plasticity, and ability to colonize secondary tissues. By defining these first principles of metastasis, we provide the means for focusing efforts on the aspects of metastasis that will improve patient outcomes.
Keywords: adhesion, colonization, dissemination, extracellular matrix, extravasation, hallmark, heterogeneity, intravasation, invasion, metastasis, microenvironment, motility, plasticity, transport, tumor progression
Medical practitioners have diagnosed neoplasms for over four thousand years and have recognized that the ability to dissociate, disseminate and colonize discontinuous secondary sites (i.e., metastasize), is the most lethal attribute of neoplastic cells. In fact, cure of most cancers is probable whenever diagnosis occurs before cells have spread beyond the tissue of origin; otherwise, cancer is often referred to as incurable (1–3).
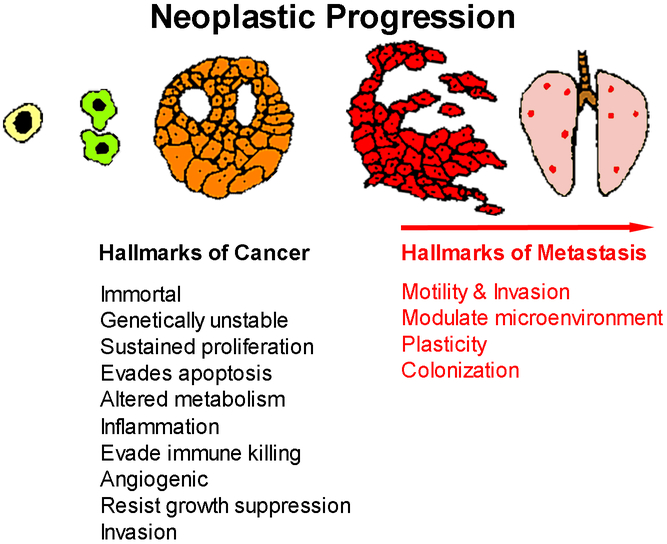
In their seminal analysis, Hanahan and Weinberg described the ‘hallmarks of cancer’ in which they identified several intrinsic characteristics of neoplastic cells – immortality, genomic instability, resisting cell death, altered metabolism and invasion/metastasis. They included several critical aspects of how cancer cells interact with the stroma – sustained angiogenesis, promote inflammation, immune evasion, resistance to growth inhibition, and relative autonomy. Together, these hallmarks and enabling characteristics define critical elements for cellular transformation (4,5). Their conceptual framework has provided clarity regarding the essential characteristics of neoplastic transformation. Yet, among those hallmarks, the only defining factor that distinguishes cancer from a mere tumor is invasion of at least one cell from the primary lump through a basement membrane (6) keeping in mind that invasion is necessary, but not sufficient, to develop metastasis. Metastasis is thought to be the ultimate manifestation of a neoplastic cell’s evolution toward becoming autonomous from the host. Upon activating the cancer hallmarks, neoplastic cells continue to evolve. Neoplastic progression is the process of evolving a normal cell into a life-threatening metastatic cancer cell (7–9) (Figure 1).
Figure 1.
Neoplastic progression is depicted as normal cells become transformed. Transformed cells can acquire additional characteristics to become neoplastic. Transition through a benign phase is depicted here; however, not all cells within a neoplasm acquire additional characteristics sequentially. The generation of a cancer/neoplasm is characterized by 10 “hallmarks of cancer” (4,5). Superimposed upon the hallmarks of cancer are four “hallmarks of metastasis” which are characteristics required for invasive neoplastic cells to establish macroscopic secondary (or higher-order) masses.
Based upon clinical and experimental observations, tumor cells acquire the hallmarks of cancer from a pre-malignant, transformed state and pass through that benign phase before acquiring invasive/malignant characteristics (10). When viewed at an organismal level, tumor progression typically follows a sequence. Before becoming tumorigenic, cells lose the ability to differentiate fully; are no longer contact inhibited; are not anchorage dependent; and are genetically unstable. Masses typically go through an expansile phase in the absence of invasion. Cells are already pleiomorphic at this stage and the mass is often encapsulated by a dense fibrous network (i.e., desmoplasia (11,12)). With successive generations, variants arise, and selection changes population composition. Subsets of the neoplastic cells acquire the ability to escape through a basement membrane, the defining hallmark of malignancy. Subsets of invasive cells then acquire the ability to detach from the primary tumor and move elsewhere to form metastases. Acquisition of traits can occur in any order, but successful transition to malignancy requires acquisition of all neoplastic traits. Similarly, the ability of cells to complete all steps in the metastatic cascade requires them to acquire certain characteristics that are superimposed upon the ‘hallmarks of cancer’ (Figure 1).
The word metastasis was first recorded in the 1580’s from a combination of the Greek prefix or preposition “meta” (change, alteration, but mostly concerned with the result of the change) and “stasis” (a state of equilibrium or standing). Thus, metastasis refers to both a process and the outcome of that process. In this review, we recognize that both are inextricably linked, and that precise use of terminology is essential to advance the field and, most importantly, clinical outcomes. While the process is important to understand, the outcome is the most critical aspect since it is the secondary mass(es) that cause clinical concern. In the end, our objective is to define the characteristics of both the process of and the eventual development of metastatic lesions. By definition, metastasis is the process of spreading to a nearby or distant, discontiguous secondary site and the establishment of macroscopic secondary foci (13). This definition provides the framework for the proposed hallmarks of metastasis discussed below and provides critical clarity with regard to patient outcomes and parameters.
An additional objective of this review is that the proposed hallmarks of metastasis will provide a conceptual framework that can be used to accelerate development of therapies designed to reduce cancer deaths (3,14). An underlying principle is that understanding the foundational biology is key to developing preventative strategies or treatments (15). So, upon defining hallmarks, we will begin to assess their tractability for diagnosis and/or prognosis.
Just as medicine has evolved toward recognition that neoplasia is a cellular disease and has further advanced to understand the molecular underpinnings of neoplastic initiation, it is now recognized that metastases represent distinct and unique subsets of cells that emigrated from the primary tumor and are behaviorally, genetically and biochemically distinct from the cells remaining at the site of tumor origin (1). Each metastatic cell must accomplish an entire series of sequential steps, termed the metastatic cascade (16). In order to define metastatic hallmarks, a detailed look at how the process of metastasis occurs is prerequisite.
At its core, metastasis requires the dissemination of cells away from the originating tumor. Since the route that results in the most widely disseminated pattern is via the bloodstream (i.e., hematogenous), many researchers and clinicians default to this being the route of metastatic spread. Nothing could be further from the truth (17). Metastatic cells enter not only the cardiovascular system. Some tumor cells migrate along nerves (17–19), along the basal side of endothelial cells (20) never entering the lumen. Others spread through the lymphatic vessels, or across coelomic cavities. Many textbooks still pay homage to the notion that carcinomas spread primarily via lymphatics, while sarcomas spread primarily through the vasculature. Importantly, lymphatics and blood vasculature are interconnected and there can be transit between the two compartments.
Critically, the defining hallmark of metastasis is development of any secondary mass that is no longer directly connected to the originating tumor, regardless of the route the cell(s) took to get there. Peritoneal metastases from ovarian carcinoma are as lethal as a brain metastasis from melanoma, though arising via different routes. Therefore, it is incumbent upon a correct understanding of the metastatic process to recognize that false oversimplifications do not accurately reflect myriad clinical situations. We will illustrate key principles involved in blood-borne metastasis as a launching point for defining the critical characteristics that are involved in any route of metastatic spread.
The process of hematogenous metastasis: the metastatic cascade
The first step in defining the characteristics of metastatic cells is to understand how they arose. Just as most tumors are clonal in origin (reviewed in (7,8,21)), almost all metastases arise from a single cell (22–26). By the time a cancer mass is clinically detectable, it is usually comprised of 1010 or 1011 cells (a cubic centimeter of tissue contains ~109 cells). To get to this size, tumors must recruit a vasculature via angiogenesis (27), co-opt already existing vasculature (28–31) or form tubes that anastomose with capillaries (32).
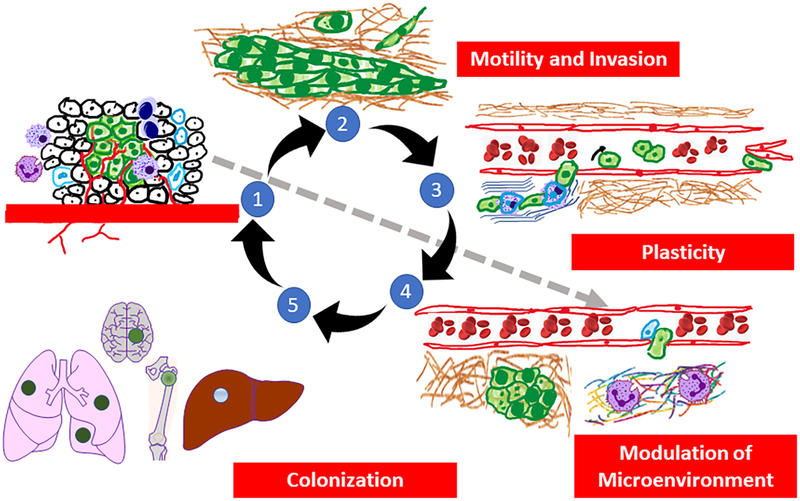
Within the tumor, cells are morphologically, biochemically and genetically heterogeneous (33–35) (Figure 2 - #1). Behavioral differences can be due to genetic, epigenetic, positional, or temporal variations (33). Genetic heterogeneity refers to the inherent properties of tumor cells themselves and is demonstrated experimentally by isolation of relatively stable single cell clones that differ from each other for a given phenotype. Epigenetic heterogeneity refers to transient chemical modifications of DNA and/or chromatin that lead to the selective spatio-temporal regulation of gene transcription for a given cell due to environmental conditions (e.g., proximity to O2, pH, growth factors, cytokines, chemokines, etc.).
Figure 2.
The pathogenesis of hematogenous metastasis. 1. The process of metastasis begins when neoplastic cells grow, recruit inflammatory cells, induce angiogenesis, and begin to initiate establishment of pre-metastatic niches (gray arrow), while generating mutant variants at high frequency. 2. Neoplastic cells then begin to invade through surrounding stroma via a variety of motility mechanisms as either single cells (via EMT) or collective migration. 3. Immune cells infiltrating the primary tumor associate with tumor cells such that neoplastic cells co-opt the invasive functions of the infiltrating immune cells to enter the vasculature (intravasation). Cells which have entered the vasculature typically roll along the endothelium but can form homotypic (tumor cell-tumor cell) or heterotypic (tumor cell-immune cell/platelet) emboli. After surviving sheer forces, tumor cells selectively adhere to endothelium or arrest when vessel diameter is too small to traverse. Note that the extracellular matrix where tumor cells invaded surrounding stroma is also reorganized and can result in the release of matrikines that affect tumor cell and/or stromal behavior. 4. Upon arrest/adhesion tumor cells exit vessels (extravasation) and interact with pre-metastatic niches which are permissive for proliferation and colonization of secondary sites. 5. Colonization is dependent upon a combination of tumor cell and tissue-specific factors. Disseminating cells selectively colonize different tissues and the process of further dissemination (i.e., metastasizing from metastases) can occur. The four proposed hallmarks of metastasis are listed in the red boxes.
The existence of heterogeneous tumor cells has been described for more than a century (33,34). Next generation sequencing and single cell analyses have provided a molecular explanation for some of the variability (36–39). But certain tenets remain consistent: most tumors and metastases are clonally derived (22,26,40,41); heterogeneity is a consistent characteristic of every tumor (7); heterogeneity exists for virtually every phenotype found in cancer (7); variants within a tumor arise independently (24) and appear to retain the capacity for self-replication and genomic instability (42,43). Isaiah “Josh” Fidler and Margaret Kripke tested whether metastatic cells arose from primary tumors using combinations of cloning and Luria-Delbrück fluctuation analysis (44). Single cell clones isolated from a single tumor varied considerably in their metastatic potentials. Continuous culture of poorly metastatic cells yielded subpopulations that were highly metastatic and vice versa. In other words, the clonal populations did not remain homogeneous. Similar results were obtained in vivo.
At the molecular level, most genetic changes that are prevalent in later stages of tumor progression are associated with tumorigenesis, invasiveness and metastasis (24). The complexity of tumor progression illustrates that a single genetic change is insufficient to render a cell metastatic. Combinations of genetic and epigenetic changes are required to progress or be used as prognostic tools (45,46). Additionally, as we discuss below, individual cells can utilize signals from surrounding cells (both tumor and stromal) to successfully progress. The challenge is sorting between driver, passenger and hitchhiker mutations.
Peter Nowell first postulated that genomic instability is the driving force for neoplastic progression, a concept which has been supported by abundant data (47). As new tumor cell subpopulations arise, selective pressures imposed by competing tumor cells as well as host response and microenvironmental conditions lead to co-evolution of tumors and stromal cells. Importantly, single cell cloning demonstrates the existence of metastatic and non-metastatic cells within the same mass. Furthermore, isolation of metastases with repeated re-injection into amenable hosts yielded increasingly metastatic subpopulations (48,49). Therefore, the existence of metastases is the result of specialized subpopulations endowed with all of the hallmarks of the process. The distinction between tumor formation and metastasis formation is most elegantly described by the discovery of a family of genes known as metastasis suppressors (50,51). This family of molecules blocks metastases while not preventing development of a primary tumor (52). Therefore, tumor formation and metastasis formation are distinguishable phenotypes. As a result, metastasis cannot be a hallmark of all cancer cells.
However, the mutation-selection theory of tumor progression is not without its detractors. Some argue that the acquisition of invasive and metastatic behaviors is more a recapitulation of a process that occurs during embryogenesis – the epithelial:mesenchymal transition (EMT) (53). The relationship between embryogenesis and metastasis is enticingly supported by studies by Illmensee and Mintz (54) and Kulesa and colleagues (55), who injected metastatic melanoma cells into embryos and found that the tumor cells differentiated into normal, non-tumorigenic tissues. Kasemeier-Kulesa and colleagues subsequently showed that for specific cells, responses to neural growth factor resulted in bipotent precursor cells (56).
Since invasive cells frequently dramatically change their cell shape to a non-polarized, motile, spindle shaped cell resembling a fibroblast, some hypothesize that neoplastic cells dedifferentiate to a more motile mesenchymal cell phenotype. Developmental EMT and cancer EMT are not equivalent at a molecular level yet share many common characteristics (53). Cancer EMT is characterized molecularly by the loss of epithelial-specific E-cadherin from the adherens junctions, and a switch from the expression of keratins as the major intermediate filament to the mesenchymal intermediate filament vimentin. Ultimately, epigenetic mechanisms and cellular plasticity may play significant roles in driving tumor progression toward malignancy than what can be explained solely by mutation and selection (57).
Establishment of a pre-metastatic niche
The process of metastasis begins long before tumors are detectable. During growth of the primary tumor, high levels of genetic and genomic instability lead to evolution of cells so that they acquire characteristics, or manifest properties, which they normally would not (40). Based upon the selection of metastatic subpopulations from mixtures of tumor cells, acquisition of traits, at least some of them, is permanent. However, the capacity of metastatic cells to regenerate non-metastatic populations suggests that some cellular properties are transient. Throughout the process, cells adapt to new environments and respond to stimuli received from other tumor cells and stroma. They must, at least temporarily, acquire the ability to survive and accomplish each selective step of the metastatic process.
Prior to exiting the mass, cells within primary tumors communicate with other parts of the body in order to establish the so-called “pre-metastatic niche” as initially described by Rosie Kaplan and David Lyden (58–60) (Figure 2 – #1). A number of soluble factors, some of which are found inside extracellular vesicles (including exosomes and exomeres (61,62)), communicate to both hematopoietic and mesenchymal stem cell populations (63–65). Stem cells are mobilized and eventually arrive in and manipulate the secondary microenvironment (sites that will eventually become metastases) by restructuring the extracellular matrices (66) and providing an environment suitable for secondary outgrowth. It is not yet certain whether the factor(s) secreted from the primary mass come from cancer cells, stromal cells or both.
Motility and Invasion
Intrinsic to the process of metastasis is the ability of tumor cells to migrate (Figure 2 – #2). As little as a decade ago, the prevailing thought was that migration was primarily a property of cytoskeletal reorganization and response to chemoattractant(s). Indeed, coordinated restructuring of the cytoplasm shifts cell shape and provides deformability for cells (67–69). Likewise, the direction of cell movement is associated with responses to attractant and repulsive stimuli (70,71). Some cells, however, can be induced to being merely hyper-motile without exhibiting directional propensity (72). Autocrine responses to motility factors [e.g., lysophospholipase D (autotaxin) cleavage of lysophosphatidylcholine to produce lysophosphatidic acid; hepatocyte growth factor/scatter factor (HGF/SF) interaction with its receptor, c-met] result in chemokinetic activity. Directionality of movement is the result of chemotaxis (following a soluble concentration gradient (73)) or haptotaxis (following an insoluble concentration gradient (74)) in response to a gradient of soluble or localized factors, respectively.
Ultimately, molecules regulating neoplastic motility are the same ones used by normal cells. Recognition of the diversity of motility mechanisms has grown in recent years. By far, the most common type of motility observed in histologic sections involves the migration of groups of cells (collective migration) (75). Analyses of the clusters indicate that the cells retain cell-cell adhesion and that there is communication from the leading edge and the trailing cells within each cluster (73,76).
Another type of cellular mobility usurps a normal embryologic developmental process (53). The temporary (or permanent) conversion arising from EMT results in the dissociation of a cell from its epithelial cousins (77,78). It is believed that, as cells acquire mesenchymal characteristics, they become endowed with the ability to migrate along with reduced cell-cell adhesion. Concomitantly, they also achieve stem-like characteristics necessary for repopulation at a secondary site (78,79). Later, upon seeding the secondary site, cells are thought to revert to the epithelial phenotype so that they can resume proliferative capacity. The reversion is termed the mesenchymal-epithelial transition (MET (80,81)). While there is abundant evidence that the EMT is associated with movement of multiple cell types, the non-bimodal continuum of epithelial and mesenchymal markers throughout the metastatic cascade is not fully consistent with EMT being a requirement for cell movement (82,83). Likewise, retention of cell-cell junctions is paradoxical to EMT being involved in some types of movement (84). Additional questions regarding the essential nature of the EMT in metastasis were presented by Tsuji and colleagues, who isolated and generated cells from a single tumor which were stable for either epithelial or mesenchymal characteristics (85,86). Evaluation of either cell’s ability to complete the metastatic cascade when isolated was nil. However, when cells were introduced together, their properties complemented the other cells’ deficiencies so that metastases resulted from both cell types. More recently, use of reporter genetic constructs were used to assess whether cells eventually colonizing secondary sites had undergone the EMT. Utilizing fluorescent reporters that turned on only after EMT-driving transcriptional regulators were activated, metastases formed from cells that had (or had not) undergone intervening EMT (87,88).
There are numerous implications of these above studies. First, each cell in a metastasis (i.e., secondary mass) is not itself necessarily endowed with every property to complete the metastatic cascade. Some cells can co-opt or complement those deficiencies with cooperating cells, either stromal or other tumor cells. Second, EMT is not essential to complete the metastatic cascade for every tumor type. This does not diminish an important role for EMT in certain tumors, but since it is not a requirement, EMT can therefore not be characterized as a hallmark of metastasis. Third, it is challenging to ascribe specific roles for EMT to the metastatic process since mesenchymal tissue derived tumors (i.e., sarcomas) are highly metastatic. Sarcomas, importantly, retain their mesenchymal characteristics after they have seeded and populated secondary tissues, frequently the lung, obviating the reverse MET process.
Invasion, the defining feature of malignancy, is the capacity for tumor cells to disrupt the basement membrane and penetrate underlying stroma. Although invasion is required for metastasis, the ability to invade is not sufficient. Some cancers are highly aggressive, forming secondary lesions with high frequency (melanoma, pancreatic ductal adenocarcinoma, small cell carcinoma of the lung), whereas others rarely metastasize despite being locally invasive (basal cell carcinomas of the skin, glioblastoma multiforme) (89). It should be emphasized that if an invasive cell cannot complete any of the subsequent steps in the metastatic cascade, it will not form a metastasis.
Invasion requires changes to cell morphology and phenotype as well as altering the surrounding environment. During invasion, three important processes are dynamically regulated that include adhesion, extracellular matrix (ECM) re-organization, and motility. Epithelial cells normally form polarized sheets that are maintained by tight junctions and desmosomes. They are anchored to a basement membrane by hemidesmosomes and intermediate filaments, integrin contacts and organized actin in the cytoskeleton. In order to invade, cells alter cell-cell and cell-matrix adhesion while, at the same time, re-organizing ECM and cellular motility (90,91). The structural and functional proteins that regulate cell adhesion and migration are key downstream targets of oncogenes and tumor suppressor-controlled signaling pathways and provide insights into how oncogenic transformation results in progression to an invasive phenotype. Many of the proteins involved in tumor invasion have also been observed to affect other processes that are part of the hallmarks of cancer including cell survival, growth, apoptosis and angiogenesis. This highlights the intricate network of interrelated pathways controlling cell behavior (92).
Normal tissues are separated by basement membranes and fascia that compartmentalize and organize physiologic functions. Extracellular matrices provide a scaffold for the organization of cells and spatial cues that dictate cell behavior (93). Each matrix is composed of proteins, primarily triple-helical collagens and glycoproteins (e.g., laminins, fibronectin, proteoglycans). Basement membranes are specialized ECM that forms a barrier separating polarized epithelial, endothelial, and muscle cells from the underlying tissues. Interstitial matrix provides the structural characteristics of connective tissues. ECM composition varies between tissues and organs; and provides important contextual information to cellular constituents (94). In addition, the ECM interacts with many secreted molecules to serve as a repository for regulatory proteins and growth factors. Thus, the interaction of cells with ECM molecules dictates their ability for survival, growth, differentiation, and migration (95). Moreover, selective proteolysis of ECM components leads to release of fragments that further regulate protein function and may be involved in cell signaling. These factors are collectively known as matrikines (96–99).
Motility is, therefore, necessary but not sufficient to transit from a tumor to the blood. Barriers must also be traversed by cells that eventually become metastases, leading Liotta to articulate the critical elements of motility and invasion (100). Specifically, cells must attach to and create passageways through extracellular matrices. Therefore, adhesion is required for cells to have sufficient traction to move forward, but not be so strongly adhered as to prohibit movement.
Adhesion occurs on the unique matrices of each tissue and occurs because of a number of molecules, primarily through transmembrane glycoproteins known as integrins (101–103). There are 18 alpha and 8 beta subunits that combine into specific heterodimers, each with specificity for certain ligands that mediate signals in both directions. Intracellular signaling pathways can modulate strength of cellular adhesion (inside-out signaling) and changes in cellular adhesion can alter cellular phenotype (outside-in signaling). Additionally, integrins cooperate with other cell surface molecules to mediate growth factor responses.
Extracellular matrices are remodeled by degradative enzymes produced directly by tumor cells or by tumor cell-associated cells. Proteolytic enzymes that contribute to matrix degradation and facilitate tumor cell invasion include, but not limited to, serine proteinases (plasmin, plasminogen activator, seprase, hepsin, and several kallikreins), cysteine proteinase (cathepsins B and K), aspartyl proteinase (cathepsins D and E), and metal-dependent proteinases of the matrix metalloproteinase (MMP) and a disintegrin and metalloproteinase (ADAM) families. Other matrix degrading enzymes such as heparanase, an endoglycosidase which cleaves heparin sulfate proteoglycans, and hyaluronidase cleavage of its substrate hyaluronic acid, have also been causally associated with tumor progression and invasion.
Matrix degrading enzymes can work either alone or by interacting with – activating or inactivating – each other. Expression of, and activity of, many of the 23 members of the MMP family of matrix-degrading metalloproteinases correlate with advanced cancer (104–106). Likewise, the plasminogen activator/plasmin system has been causally implicated in cancer invasion, and urokinase plasminogen activator (uPA) and plasminogen activator inhibitor-1 (PAI-1) are validated prognostic and predictive markers for breast cancer (107,108).
Activity cascades are important regulators of proteolytic function, protease degradation, and activation. The cascades may paradoxically also include endogenous inhibitors, including the tissue inhibitors of metalloproteinases (TIMPs), serine proteinase inhibitors (SERPINs), and cysteine protease inhibitors (CYSTATINs). As an example, pro-MMP-2 conversion to active MMP-2 requires the activity of MT1-MMP (MMP-14), a transmembrane MMP that is activated by the prohormone convertase family member furin, and TIMP-2. Stoichiometry of these molecules is critical to balance function. Other proteolytic cascades can be intertwined during the degradation of ECM, e.g., cathepsin(s) → uPA → plasmin → MMP. Importantly, the notion that proteolytic enzymes function exclusively to break down physical ECM barriers has been debunked by numerous studies demonstrating that substrates and cleavage products modulate cellular growth, differentiation, apoptosis, angiogenesis, chemotaxis, and migration (92).
Taken together, cellular movement requires coordinated cell-cell and cell-matrix adhesion, matrix degradation, and cytoskeletal activity. The type of cell migration (collective, mesenchymal, or amoeboid) is influenced by the relative levels of adhesion, cellular and nuclear deformability (109) and cytoskeletal structure (75). Modulation of any of these factors converts between motility type. During motility and invasion, cells organize adhesive, proteolytic, and motility components into specialized structures known as invadopodia (110–112).
Tissue matrices vary logarithmically in stiffness between tissues and between individuals (113–115). Differences in the relative tensegrity contribute to the strength of adhesion as well as the speed of migration (116,117). Collagen fibers are often organized as a meshwork in most matrices, but the fibers become re-aligned due to tumor cell manipulation (118–120). The migration of subsequent invading cells occurs at a faster rate than the movement of the pioneering cells. This observation leads to a sense that neoplastic cell movement may be pre-programmed. Perhaps cancer cells are attempting to recapitulate one or more embryonic processes, although there is no solid evidence to support this hypothesis.
Eventually, tumor cell migration is toward a transportation compartment (vasculature, lymphatics, coelomic cavities) (Figure 2 – #3). As mentioned above, most distant metastases arise because cells transit through the vascular system since this provides opportunities for the widest dissemination in the shortest amount of time. However, it is essential to remember the interconnectivity between transit compartments, e.g., lymphatic connection to vessels at the thoracic duct.
Migration is not accomplished only by properties of the tumor cell. In recent years, it has become increasingly apparent that inflammatory cell populations present in the primary tumor contribute to motility and invasion (Figure 2 – #1, #4). Differentially polarized innate immune cells can exert either pro-or anti-cancer affects (121–123). The immune cells which are anti-tumor are designated N1 or M1 neutrophils or macrophages, respectively. In contrast, populations of polymorphonuclear cells (PMN) or macrophages that assist tumor cell invasion and migration are designated N2 or M2, respectively. Several recent studies demonstrated that the situation is not as simplistic as a bipolar system, but from a conceptual standpoint, it is apparent that neoplastic cells engender seditious behavior of immune cells by as-yet relatively ill-defined mechanisms. Analogous scenarios with carcinoma-associated fibroblasts have also been described (124).
One of the first demonstrations of immune cell polarization to assist invasion was described by Aeed et al. (125), who demonstrated neutrophilia developed in proportion to metastatic behavior of rat mammary adenocarcinoma cell lines. Co-injection of tumor-induced PMN increased metastatic efficiency in vivo and enhanced invasive properties in vitro. The latter were associated with increased production of MMP and heparanase (125). Interestingly, only tumor-induced neutrophils promoted invasion, not artificially stimulated PMN. Subsequently, tumor cell production of granulocyte-macrophage colony stimulating factor and interleukin-3 were determined to be responsible for PMN mobilization from the bone marrow and possibly differentiation (126). At the time of those experiments, the molecular markers for the neutrophil subpopulations were not as refined as they are today, but the phenotypes are consistent with what are currently called myeloid-derived suppressor cells (127,128). Analogous situations are probably operational for macrophage or other pro-tumorigenic/pro-metastatic polarization.
Intravasation
Following local invasion, cellular dispersion requires entry into a transit compartment (Figure 2 – #3), in the case of blood-borne metastasis, the process is termed intravasation. John Condeelis, Jeff Pollard, and colleagues (129–131) have characterized a number of metastasis-promoting aspects of macrophages, particularly at the step of intravasation (i.e., entry into a vascular compartment). Interestingly, alternating tumor cell and macrophages into a “conga line” suggests that the macrophages may be induced to secrete proteinases which the tumor cells do not then need to produce on their own.
Intravasation requires, at least, partial degradation of the ECM and basement membrane underlying endothelial cells. The invasion processes described above continue so that tumor cells squeeze between endothelial cells in order to extend filopodia into the lumen. During this process, in vitro studies suggest that the integrity of the endothelial barrier (as measured by electrical resistance) is not significantly disrupted (132,133). In other words, tumor cells, by mechanisms which are still not fully understood, do not fully disrupt the tight junctions and yield overt leakage from capillary intima. This point does not undermine what is known about angiogenesis-induced vasculature and tumors. Specifically, the vasculature in tumors is notoriously tortuous and ill-formed (134,135). Tumor cell production of vascular endothelial growth factor (VEGF) is also associated with increased vascular permeability. Indeed, VEGF was originally called VPF or vascular permeability factor (136). Critically, tumor cells enter the vasculature or lymphatics or body cavities most often by wedging between cells at the junctions between them (137). Hence, the abnormal vasculature resulting from tumor angiogenesis represents an easier entry point for tumor cells.
The above observations highlight other mechanisms of motility as well. Overholtzer proposed another mechanism by which tumors could transit endothelial linings, a process they termed entosis (138). In essence, tumor cells can pass through vessels and emerge on the other side. However, most cells fail to survive this non-apoptotic cell invasion process. The frequency of entosis is open to debate since it is not widely observed in pathologic sections. But the frequency of trans-endothelial migration in vitro does appear to occasionally involve entosis.
More recently, a mechanism of intravasation reminiscent of passive invasive mechanisms characterized by Dale Coman, Irving Zeidman and colleagues has been described (118,139,140). Dissociated tumor cells at the leading edge are ‘pushed’ into the circulation by the division – and natural expansion – of the cells behind them (141). As blood passes by cells in a vessel lumen, neoplastic cells can enter the blood by passive pushing and pulling forces. The more passive nature of cellular invasion and intravasation highlighted by this recent study were first described using quick setting polymers in the early days of metastatic research. Tumor cells frequently take the line of least resistance during the metastatic process. As alluded above, pioneering cells will restructure matrices or open channels through which subsequent cells may more efficiently (and quickly) traverse.
Dissemination and Transport
Upon entry into a transport compartment, tumor cells can then disseminate wherever that compartment goes (Figure 2 – #3). Movement within vessels or cavities can be either active or passive. Although the efficiency of entry of cells into the vascular compartment varies widely, the frequency of tumor cell entry into the vasculature is amazingly common – between 1–4 × 106 cells per gram of tumor enter the vasculature per day (142,143). The early steps of metastasis are therefore not as infrequent as the successful colonization of secondary sites. The process of successfully metastasizing is highly inefficient (142,144). Thus, it is critical to distinguish between mere dissemination or spreading of cells and development of overt metastases.
A clinical study exemplifies this critical distinction elegantly. Palliative treatment of patients with advanced ovarian carcinoma using peritoneovenous (LeVeen) shunts to reduce ascites burden showed that, despite continuous entry of billions of viable tumor cells into the circulation, metastases to the lung (i.e., the first capillary bed encountered) were rare (145).
During dissemination, characteristics of the circulating tumor cells (CTC) vary widely. For example, a full continuum of markers associated with epithelial or mesenchymal characteristics are found in the vasculature. Of the millions of cells that enter the vasculature daily, the overwhelming majority (<<0.01%) fail to successfully colonize secondary sites. The efficiency of metastasis can increase if tumor cells maintain structural emboli (both homotypic or heterotypic) or if they become encased within fibrin clots (146). Larger emboli are more efficiently trapped as vessel diameters decrease (144,147,148). The latter situation, in part, explains how the vast majority of metastases tend to occur at the first capillary bed encountered (149,150). Still, the location of successful colonization of secondary tissues is not entirely determined by non-specific arrest. In addition to the structural impact of emboli, CTC exhibit altered gene expression patterns, in part, due to changes in epigenetic marks (151).
Once tumor cells enter any circulatory compartment, they move via active motility mechanisms or more passively (i.e., pushed along with fluid flow). Injection of radiolabeled cells directly into circulation reveals that a substantial proportion are lost during the transport phase of the metastatic cascade. In the bloodstream, tumor cells are in intimate contact with leukocytes and other immune components. Throughout transit, tumor cells evade immune insults. They do this by down-regulating antigens, secretion of factors that trick the immune system into recognizing tumor as “normal,” or direct killing of immune cells. Others are killed by exposure to hemodynamic sheer forces (152). The average tumor cell (20–30 μm diameter) must squeeze through capillaries significantly smaller (6–7 μm). Even when tumor cells can deform, they encounter significant hydrostatic pressures. Cell origin and biophysical parameters (i.e., membrane fluidity, cellular elasticity and cytoskeletal organization) determine whether cells remain intact or are broken by sheer. Experimental measurement of these parameters in vivo is complicated by the time it takes to remove lungs, liver, heart and muscle post-injection of tumor cells (2–3 minutes) since most cells are already dead due to mechanical trauma.
Deformability is also impacted by the pressures found within various tissues. For example, blood flow in osseous sinusoids is sluggish compared to capillaries and post-capillary venules (~30-fold lower). By analogy flow in bone is like the ebb and flow of the everglades compared to the river rapids found in capillaries. During transport, tumor cell behavior is determined by their existence as either single cells or as emboli (153–155). Embolization can either be homotypic (tumor cell-tumor cell) or heterotypic (tumor cell-leukocyte, -platelet, -fibrin). Embolus size contributes to where cells adhere but also protects cells from shear forces and immune attack (156).
Intravital videomicroscopy of cells in circulation reveals that most roll rather than float, just like leukocytes (157,158). During this time, tumor cells remain weakly adherent and subject to anoikis (a specialized type of apoptosis in which cells which are anchorage-dependent are induced to die). Note: anchorage independence is a misnomer since the type of substrate to which cells are attached also plays a critical role. Cells growing in soft agar are, in fact, attached to carbohydrate moieties via lectins, for example adherence to galactose by galectins. Some tumor cells will induce apoptosis even if firmly attached to a substrate if that substrate is not the preferred one for that cell type (159).
Cellular arrest, vascular adhesion and extravasation
Following circulation, tumor cells either arrest due to physical constraints such as microvascular diameter or adhere to the intimal layers of a vessel (Figure 2 – #3). Endothelial cells lining blood vessels are the first barrier to exiting vessels, although cells can adhere to exposed basement membrane underlying the endothelium. There are three types of vessel intimal structures found in higher vertebrates – continuous, discontinuous and fenestrated. Most endothelial cells form tight junctions and have a continuous, unbroken basement membrane beneath them. In certain organs, such as liver and spleen, endothelial cells and the basement membrane are dis-continuous. In the kidney, although there are gaps between endothelial cells, a membrane-like structure connects them. The entire structure overlaps in a continuous basement membrane. These endothelial/basement membrane barriers contribute to the normal function of the tissues and form additional barriers through which tumor cells must pass.
Endothelial cells in each tissue express unique (combinations of) markers (160–163). Those markers represent tissue ‘addresses’ which tumor cells can recognize and adhere to in a selective manner (164,165). This selectivity is thought to be among the mechanisms leading to selective organotropism of metastasis (see below). Both in vivo and in vitro analyses indicate that initial attachment of cancer cells occurs preferentially at endothelial cell junctions, followed by endothelial retraction and adhesion of cancer cells to the underlying basement membrane. Even more efficiently, tumor cells adhere at sites where inflammation is taking place (166–168), perhaps explaining why metastases often arise at sites of tissue injury (169–171). During inflammation, endothelial surface markers change so that leukocytes more readily adhere and eventually traverse the site of tissue injury (158,172). Following transendothelial migration, cancer cells encounter the basement membrane, begin to secrete proteinases, deform as they squeeze between cells and through holes in the matrix and begin the process of colonization. The molecular mechanisms of extravasation are thought to be similar to those involved during intravasation but have not been exhaustively studied.
Extravasation is exiting a vessel and entry into an organ parenchyma. Previously, extravasation was viewed as a key rate limiting step for metastasis formation, but intravital videomicroscopy studies indicated that extravasation can be remarkably efficient. For example, Ann Chambers and colleagues showed that nearly 90% of B16-F1 murine melanoma cells injected into the mesenteric vein arrested in the liver 90 minutes after injection. Of the injected cells, 83% were in the liver parenchyma three days later (i.e., >95% of the arrested cells extravasated) (173).
The time between detachment of cells from the primary tumor mass to re-adhesion or arrest of cells at secondary sites is minutes, far shorter than the time required for transcription, translation and re-expression of adhesion molecules. Exactly how cells lose cohesion yet regain it during such a short interval is perplexing. Whether it involves a different cache of adhesion molecules, the involvement of other cells or different associations between adhesion molecule regulators will await experimental testing.
It is important to note that there is debate whether extravasation is required for metastasis. In the case of some pulmonary metastases, there is evidence that tumor cells can attach to the lung endothelium, survive and grow intravascularly (174). Extravasation occurs in this model only after intravascular foci expand to destroy vessel integrity.
Colonization
Colonization of secondary tissues requires the same elements as growth of the primary tumor (Figure 2 – #4). There must be sufficient oxygenation and nutrients to divide. Initial growth of the metastases can occur in the absence of angiogenesis, but growth beyond a certain size (~1 mm) requires co-option of existing vessels, development of new vessels or the formation of vascular channels by tumor cells themselves (27,175,176).
The establishment of a supportive metastatic environment occurs prior to the arrival of any carcinoma cell, the so-called pre-metastatic niche (Figure 2 – #4). Elements of the supportive environment include VEGFR+ bone marrow progenitors (177), MDSC (58), and neutrophils (178,179). Like what has been observed in primary tumors, polarization of some immune cells at the pre-metastatic niche creates an environment that will be more amenable to tumor growth. Relatively recently, a process in which neutrophils extrude DNA that can be used to kill bacteria has been observed at sites where tumor cells more efficiently colonize. The extruded DNA is termed a neutrophil extracellular trap in a process known as NETosis (180,181). The underlying mechanisms for this immune change are not fully defined but are consistent with a profound manipulation of host responses to tumor cells necessary for successful completion of the metastatic process.
The patterns of metastases are non-random (49,182,183). Using autopsy data, Leonard Weiss determined that most metastases formed where disseminated cells encountered the first capillary bed or lymph node (149). Yet, despite receiving only approximately 15% of cardiac output, bone is the most common site of metastases across all tumor types. Specifically, bone is the most common site for breast and prostate cancer metastasis. Stephen Paget, in a seminal study in 1889 evaluated the patterns of metastases for breast cancer and determined that the bone is disproportionally involved (183). Through this, he posited what is now widely recognized as the “seed and soil” hypothesis. Abundant evidence has been collected to support the key tenets of this hypothesis (reviewed in (49,182)) (Table 1, Figure 2 – #5). First, tumor cells are endowed with certain characteristics that enable them to survive the multiple steps of the metastatic cascade. Second, tumor cells selectively respond to select signals from host tissues so that the distribution of metastases is not due to chance, but due to the combination of properties between the cell <seed> and the organ <soil> in which it will develop. Recent data have added a third element to the seed and soil hypothesis, that we analogize to the climate (184–187). The latter represents the overall health of the individual and certain inherent background genetic components that lead to alterations of immune function, metabolism, etc.
Table 1:
Organotropism of metastasis
| Primary Tumor Site | Common site(s) of metastasis | Sites not explained by circulatory pattern |
|---|---|---|
| Breast | bone, lung (pleura), liver, brain, adrenal, axillary lymph nodes, contralateral breast, ovary | bone, adrenal glands, contralateral breast, ovary |
| Colon | liver, lymph node, lung, direct extension into urinary bladder or stomach | |
| Kidney | lung, liver, bone | bone |
| Krukenberg adenocarcinoma | Liver, ovary | ovary |
| Lung | bone, brain, lymph nodes, pleura, diaphragm (by direct extension), liver, kidney, adrenal, thyroid, spleen | bone, adrenal, thyroid, spleen |
| Ocular (uveal) melanoma | liver | liver |
| Ovary | diaphragm, peritoneal surfaces, lymph nodes | |
| Pancreas | liver, stomach (by direct extension), colon, peritoneum | |
| Prostate | bone (particularly vertebrae and pelvis), lymph nodes | bone |
| Stomach | liver, lymph nodes, lung, bone | bone |
| Testes | lymph nodes, lung, liver | |
| Urinary bladder | lung, rectum (by direct extension), colon, prostate, ureter, vagina, bone, lymph nodes, peritoneum, pleura, liver, brain | |
| Uterine endometrium | lung, lymph nodes, liver, ovary |
Since Otto Warburg posited that cell metabolism was key to the transformation of cells (188), exploration of the roles of metabolism in metastasis has been studied extensively (reviewed in (189–192)). Both pro- and anti-metastatic impact of mitochondrial content (193,194) and function (195) as well as location (196–199) have been reported. Additionally, metabolism-related functions, such as autophagy and mitophagy, have been associated with invasive and metastatic activities of cancer cells (200,201).
Importantly, the mechanical or anatomic theory and the seed and soil hypothesis are not mutually exclusive. Even if tumor cells are directly injected into certain organs, only those able to respond to the local environment by proliferating will form metastases. Experimental data supporting the “seed and soil” hypothesis include organ-selective adhesion (49,202), invasion (203), and growth (204–208).
What mechanisms are responsible for differential growth of tumor cells in various tissues? This question has been extensively studied; yet, the answer still remains largely unknown. It is clear that coordinated expression of many genes is required (46,209–212). It would seem that there is a hierarchy of gene expression responsible for metastatic propensity. We propose that there are genes that endow neoplastic cells with the ability to metastasize upon which there are additional genes that determine where metastases will develop. Multiple investigations have attempted to isolate the factor(s) in various tissues that promote the growth of organ-specific metastasis. While crude lysates or conditioned media do show patterns of organ-selective growth promotion or inhibition, to the best of our knowledge no one has yet fully succeeded in identifying and purifying the combination of factors responsible for organ-specific colonization. Nonetheless, some metastasis regulatory factors have been identified. Although natural to focus upon growth promoting factors, there is also evidence that some tissues are hostile to tumor cells (204,213). As is the case for growth-promoting factors, the identity of the growth inhibitory factors is also largely ill-defined (210,214,215). Evidence that Wnt pathways might be interact with TGF-beta signaling to regulate bone metastasis development have been identified (215). Likewise, the immune and inflammatory systems vary by tissue and could contribute to differential growth (216–218). For example, Boire and colleagues recently identified complement component 3 as a factor contributing to the development of leptomeningeal metastases (210). Conversely, Ku et al. determined that factors secreted from myeloid derived suppressor cell populations can impact L-selectin-dependent adaptive immunity in lymph nodes (219) which could, in turn, influence the efficiency of metastasis to draining lymph nodes. Additionally, physical forces such as the strong hemodynamic vortices in the heart are not amenable to arrest, survival and growth for most cells.
Organ selectivity of metastasis has led many to speculate that there are “homing” mechanisms such as chemotaxis or haptotaxis. Evidence supporting the notion of chemotactic gradients was first provided by Zlotnik laboratory (220). Using microarrays, they found that tumor cells expressing CXCR4, a chemokine receptor, preferentially metastasized to tissues expressing the ligand, SDF1/CXCL12. While the data strongly support the notion that there are soluble factors produced in different tissues to which tumor cells can respond, homing has never been observed. Strictly speaking, homing would require directed movement throughout the transit of tumor cells as they leave the primary tumor. Rather, tumor cells distributed according to circulatory patterns initially but may “home” once they are more proximate to a site of eventual colonization. Many of the homing mechanisms utilized by lymphocytes for peripheral lymph nodes or sites of inflammation are apparently shared by tumor cells as well.
Colonization of secondary (or higher-order) sites does not require immediate cell division. Dormancy refers to an interlude during a progressive process. In the case of metastasis, disseminated cells recur as macroscopic lesions months to years post-seeding (205,221,222). During the interim, they are below the limit of detection and, except for a handful of experiments, their status during that time is largely a black box with regard to understanding. Several mechanisms leading to observed dormancy have been proposed and recently reviewed (205,223). Briefly, Hadfield first proposed that cells may have undergone a temporary mitotic arrest or a prolonged time in the G0/G1 phases of cell cycle (224). This theory is supported by observations that labeling CTC or disseminated tumor cells (DTC) with the proliferation marker, Ki67, suggests that a high proportion are non-dividing (225). Judah Folkman proposed that observations of dormancy were due to lack of sufficient blood supply, keeping tumor masses below the limits of detection (i.e., angiogenic dormancy) (226). Similarly, DTC may be held in check by immune mechanisms, sometimes referred to as immune editing (227). Also, stromal changes in metabolism (223,228) or other aging-associated phenotypes (229) could be responsible for escape from dormancy. At this juncture, however, whether other populations of cells have remained quiescent or have undergone balanced cell division/apoptosis during the interim is not yet known. Among the most critical insights necessary for improving therapeutic outcomes related to metastasis will be understanding the mechanisms responsible for escape from dormancy in order to colonize secondary sites.
Metastases can subsequently metastasize (230). Once a tumor cell colonizes a secondary site, genetic instability inherent in neoplastic cells continues to operate at each cell division. It is observed that cells from metastases can recapitulate the multiple steps above and move to other sites in the body. A clinical manifestation occurs with the observance of DTC in the bone marrow of tumors. Klaus Pantel and colleagues have elegantly shown in a variety of tumor types that DTC are often associated with worse clinical outcome (231,232). Even for cancers that rarely metastasize to the bone (i.e., colon) the presence of DTC in bone marrow biopsies is often a predictor of development of liver metastases.
Questions that influenced our defining the hallmarks of metastasis
When do cancer cells metastasize?
With the advent of next generation sequencing, exploration of the processes of metastasis and the genetic relationship of primary tumor to metastases has been explored in greater detail. The long-held notion that metastatic cells are the ultimate stage of tumor progression (i.e., a late event in tumor progression) has been challenged (206,233,234). While clinical observations clearly suggest that earlier diagnosis results in better prognosis, re-emergence of dormant cells decades following initial diagnosis of early stage tumors questions that assumption (235–237). Obviously, cells have disseminated prior to diagnosis.
There is debate regarding whether metastases arise following parallel or sequential evolution (35,238). Both mechanisms have been observed and are entirely consistent with the metastatic cascade outlined above. As populations of tumor cells arise at the primary tumor, some disseminate early, others disseminate later, and still others acquire mutations after they have already seeded the secondary sites. Single cell analyses confirm that >90% of metastases are clonally derived and possess the same driver mutations that led to tumorigenesis.
What induces cells to disseminate?
Neoplastic cells are dependent, but not entirely so, on the stromal microenvironment. They do not require exogenous growth inducers to the same extent as their normal counterparts nor are they as sensitive to growth inhibitory signals. If the primary tumor has limited nutrients or oxygen, cells may be “motivated” to move elsewhere. Several studies demonstrate that transient hypoxic conditions alter gene expression rendering cells more metastatic (239,240). The primary mechanism thought to engender the metastatic capability is through a gene expression cassette controlled by hypoxia inducible factor (HIF 1) (241,242), which includes several pro-metastatic genes such as Ras, EGFR, Her2/neu, VEGF, IL8, MMP, uPA, PIK3CA, etc.
When is a metastasis not a metastasis?
Since metastasis requires accomplishing several different steps, the questions arise: what is the clinical relevance of disseminated cells? And are single disseminated cells metastases? These questions have been debated for more than a century and are even more relevant today.
The presence of undetectable single ectopic cells was not an issue until techniques capable of detecting them (or small emboli) were developed. Happenstance sections in autopsy tissues often detect disseminated neoplastic cells, but their clinical importance was negligible since they were not affecting organ or tissue function. This observation does not belittle the potential clinical importance of disseminated cells, since they have accomplished antecedent steps of metastasis. They may retain potential to become a bona fide metastasis, even after years in ectopic sites (231,243). This fact emphasizes the critical aspects of disseminated cell dormancy before colonization.
The clinical challenge is to discriminate between cells merely leaning towards metastatic behavior from those destined to become actual secondary masses. Mere presence of tumor cells in the peripheral circulation, lymph nodes or bone marrow are indicators of poor prognosis (244,245); however, correlations are imperfect (246). Recent staging criteria incorporate parameters of single cells or microscopic metastases. Are they metastases? We would argue not since it is not known whether those cells are fully capable of colonizing a secondary site.
The distinction between disseminated (or disseminating) cells from macroscopic metastases has important clinical implications. Presently it is not known whether persistent cells are dormant (i.e., nondividing) or of limited replicative ability. Dormancy would explain resistance to treatments that, for the most part, target proliferating cells. By contrast, limited replicative ability would provide a potential mechanism for emerging new mutations being passed on to progeny. Failure to distinguish bona fide metastases from inert disseminated cells has important implications. Current medical practice is to eliminate or diminish all risk to the patient. If cells have already spread, then aggressive treatments are advocated. The extent and location of spread determines a treatment plan. However, patients might be subjected to more cytotoxic agents than necessary since most disseminated cells fail to form secondary tumors. Findings that cancer cells may emigrate when tumors are microscopic may warrant aggressive treatments regardless.
The studies above also highlight limitations regarding recent studies analyzing single cells. While it is certainly possible to demonstrate heterogeneity between cells, it is impossible to determine which cells, if any, would eventually develop metastasis. Similarly, which of the biomarkers identified (or combinations of biomarkers) is functionally relevant will require further validation.
Defining the Hallmarks of Metastasis
When we embarked upon writing this review, we recognized that there have been many articles written on topics related to metastasis, but none in recent history that deals with the specific properties of a metastatic cell. We also recognized that a plethora of “hallmarks” papers have been published in recent years (more than 700 titles in PubMed). Many have helped refine the discussion, but others missed the key point regarding a hallmark, defined by Merriam-Webster as a distinctive or distinguishing feature or trait.
Ascribing specific hallmarks to either the metastatic process or the development of metastatic lesions has been challenging because tumor cells utilize cellular processes, signaling cascades and molecules that are shared by normal cells. Except for mutated driver mutations, cancer cells use the same cellular machinery as their normal counterparts. This fact represents one of the major reasons that cancer has been so difficult to prevent or treat effectively without significant toxicities. Cancer cells largely do normal things at inappropriate times and at incorrect places.
In describing what takes place during the metastatic cascade, it is wholly clear that there is redundancy allowing cells to overcome one deficiency yet complete individual steps using alternative pathways. Furthermore, at many steps in the metastatic cascade neoplastic cells piggyback on the actions of surrounding stroma. Thus, the heterogeneity present within any tumor makes ascribing a hallmark extraordinarily challenging (247). The situation is even more complex when dealing with the process of metastasis since any hallmarks of metastasis are superimposed upon the hallmarks of cancer itself.
Notwithstanding these obvious limitations, we propose that there are four capabilities or essential hallmarks for the metastatic cascade and formation a secondary tumor focus: motility & invasion; ability to modulate the secondary site or local microenvironment(s); plasticity; and, ability to colonize secondary tissues. These hallmarks are discussed extensively above but are summarized below in the context of refining key definitions and implications.
Motility & Invasion
To emancipate themselves from a primary tumor, cells must possess the ability to move. There are many ways in which cancer cells can escape, but the capacity to migrate is the sine qua non of metastasis. While cellular movement can be aided by other cells, metastatic cells must inherently possess the ability to move, either as an individual cell or as a community of cells.
By definition, malignancy requires cells to penetrate a basement membrane. Therefore, metastatic cells must somehow possess the capacity to invade. However, this capacity need not be intrinsic but can be the result of usurping the invasive capabilities of infiltrating immune cells or altering the behavior of adjacent stromal populations. Also critical is recognition that motility is necessary, but not sufficient, for invasion.
Modulation of the microenvironment
A striking characteristic of all metastases is their ability to restructure the local tissue by: recruiting new cells into the local microenvironment, eliciting mobilization of immune/inflammatory cells, telegraphing a restructuring of other tissues, altering metabolism of surrounding stroma, negating anti-tumor actions of the immune system, manipulating the behavior of other cancer cells, altering the extracellular matrix, or co-opting normal behaviors of other cells to accomplish one or more steps of the metastatic cascade.
Plasticity
All cancers are heterogeneous (248–250). Furthermore, measurement of tumor composition at any time is merely a snapshot because selective pressures alter the composition and cellular behavior. Tumor cells communicate with each other as well as with normal stroma. Neoplastic cells can alter growth rate of other cells (43,251), drug resistance (252–255) and metastatic capability (85,256). Communication can be directly to another tumor cell or via an intermediary cell. Regardless, communication between tumor cells and between tumor cells and host cells results in changed behavior and plasticity. Therefore, heterogeneity is heterogeneous both spatially and temporally (33).
From an evolutionary perspective, cellular plasticity provides a selective advantage (257). This is especially true when one considers the variety of microenvironments through which disseminating cells must transit during the metastatic cascade (257). Likewise, the capacity to grow in more than one soil requires the capacity to adapt.
Throughout the metastatic cascade, cells must respond to continuously changing matrices, environmental conditions (e.g., pO2, nutrients, sheer, etc.), and insults. The redundancy of mechanisms capable of accomplishing any step in the metastatic process provides a clear competitive advantage to cells which have the greatest number of tools in their toolbox. Rather than using a single integrin heterodimer for adhesion, cells that can use alternative adhesion molecules or recognize another matrix component, have a selective advantage. Likewise, the ability to adapt by using a different protease to invade represents another way in which disseminating cells can achieve metastasis.
It is important to consider that the formation of a metastatic locus is not necessarily a binary process. Obviously, if a cell cannot complete one step of the metastatic cascade, it cannot successfully accomplish subsequent ones. However, if a cell possesses even a modicum of capability for each step, efficiency is low but capacity to metastasize still exists. This point is highlighted by the continuum of states between epithelial and mesenchymal. Neoplastic cells appear to reside anywhere between the extremes in these phenotypes. One does not yet know whether the rate of transition between states is critical. However, the ability to adapt to ever-changing conditions is necessary transiting from one part of the body to another. Indeed, recent evidence suggests that loss of the ability to adapt (i.e., terminal differentiation into adipocytes) can block the ability to metastasize (258).
Colonization
Among the most critical distinctions when defining the hallmarks of metastasis is between the words dissemination and metastasis. Confusion often arises because metastasis refers to both a process as well as an outcome. From a clinical perspective, it is the outcome which is most critical. For if a cell begins the process, but cannot complete it (i.e., establish a macroscopic lesion), then it is irrelevant since the patient will not succumb to cancer as long as the primary tumor is removed. However, if a disseminated cell successfully colonizes a tissue, then survivability plummets. As a result, we strongly recommend distinguishing the mere dissemination of cells from the word metastasis. Equating CTC or DTC to metastases is incorrect. Most CTC and DTC never proliferate to form a macroscopic lesion. Each has the potential to do so, but it is not yet realized. As with these other hallmarks of metastasis, the ability to colonize at a discontiguous site incorporates the ability to manipulate the microenvironment as well as adapt to growth elsewhere.
Implications and opportunities
Far and away, the most lethal attribute of cancer cells is their ability to metastasize. The development of metastasis is currently considered incurable. Patients, their families and their physicians ride a roller coaster of responses and non-responses, remissions and recurrences, as well as hope and despair. Since the introduction of (neo)adjuvant therapies into oncologists’ armamentaria, long-term survival rates have increased significantly for many cancers. Such statistics are encouraging beginnings but are certainly not adequate. The “Sword of Damocles” still hangs over patients’ heads. When tumors recur, the clinical response is like a game of whack-a-mole that appears to prolong the inevitable, non-responsive recurrence.
Several misconceptions associated with metastasis can be dispelled by disciplined use of these hallmarks. While earlier detection of tumors has contributed to increased 5-year survival rates, the inference is that tumor size determines whether metastases will develop. Unfortunately, recent data demonstrate that cells can disseminate when primary tumors are still undetectable (206,234).
When discussing neoplasms, many combine the terms invasion and metastasis as if they are identical. As illustrated above, invasion is necessary, but not sufficient, for metastasis development. While detection of invasion in a biopsy provides important information regarding likelihood of developing metastasis (259), the two terms cannot be equated.
Another misconception is that all metastases are equal. Certainly, location of metastatic lesions determines risk to patient survivability (i.e., brain metastases are certainly more immediately concerning than subcutaneous metastases). However, sequencing and single cell analysis data clearly show that some metastases arise from distinct lineages from others (35,88,260–263) and metastases can seed other metastases (35,230).
A common misconception regarding metastasis is that they are universally therapy resistant. This notion has gained popularity with the acceptance of the so-called cancer stem cell hypothesis. Cancer stem cells are thought to be those from which metastases arise. In separate studies, stem cells are generally therapy resistant due to efflux pumps and reduced rates of cell division (264). However, comparison of metastatic and non-metastatic cell responses to radiation, chemotherapy and immunotherapies reveals that individual cell clones are both sensitive and resistant (7,265).
Finally, many refer to metastasis as incurable (238). We choose to say they are currently incurable, thereby retaining hope that metastasis may one day be controlled or cured. There are some essential differences between normal cells and metastatic cells. Importantly, all properties necessary for metastasis must co-exist within a single cell or co-opted from ancillary cells (including the properties necessary to recruit another cell to complement a specific defect). Conceptually, this offers opportunities for cancer patients in the future.
Defining the hallmarks of metastasis has been complicated by both the heterogeneity amongst tumor cells as well as their myriad interactions with other molecules and cells throughout the process. Our attempts to identify the underlying first principles of the metastatic process hopefully provide a means for simplifying the processes that are essential for all metastases to develop. As insights into the molecular circuitry involved are uncovered, the phenotypes identified as hallmarks will be more amenable to therapeutic intervention and/or prevention. To achieve this end, focused research into the fundamental processes of each hallmark will be needed. Direct comparisons between primary tumors, CTC, DTC and metastases in the same patient will be necessary. And disavowing the notion that primary tumors are equivalent to metastases will be required.
Acknowledgments:
DRW has been supported by grants from the National Cancer Institute, Department of Defense, Susan G. Komen for the Cure, National Foundation for Cancer Research, METAvivor Research and Support Inc., Theresa’s Research Foundation and the Hall Family Professorship in Molecular Medicine. DRH has been supported by grants from American Cancer Society, METAvivor Research and Support Inc., and the Elsa U. Pardee Foundation. We are grateful to many colleagues and collaborators for fruitful discussions and apologize to authors whose work was not cited due to space limitations.
Footnotes
Conflict of interest: The authors declare no conflicts of interest.
References
- 1.Eccles SA, Welch DR. Metastasis: recent discoveries and novel treatment strategies. Lancet 2007;369:1742–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Klein CA. Framework models of tumor dormancy from patient-derived observations. Curr Opin Genet Dev 2011;21:42–9 [DOI] [PubMed] [Google Scholar]
- 3.Steeg PS, Theodorescu D. Metastasis: A therapeutic target for cancer. Nat Clin Pract Oncol 2007;5:206–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell 2000;100:57–70 [DOI] [PubMed] [Google Scholar]
- 5.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011;144:646–74 [DOI] [PubMed] [Google Scholar]
- 6.Lazebnik Y What are the hallmarks of cancer? Nat Rev Cancer 2010;10:232–3 [DOI] [PubMed] [Google Scholar]
- 7.Welch DR, Tomasovic SP. Implications of tumor progression on clinical oncology. Clin Exp Metastasis 1985;3:151–88 [DOI] [PubMed] [Google Scholar]
- 8.Heppner GH, Miller FR. The cellular basis of tumor progression. Int Rev Cytol 1998;177:1–56 [DOI] [PubMed] [Google Scholar]
- 9.Foulds L Tumor progression. Cancer Res 1957;17:355–6 [PubMed] [Google Scholar]
- 10.Vogelstein B, Fearon ER, Hamilton SR, Kern SE, Preisinger AC, Leppert M, et al. Genetic alterations during colorectal-tumor development. N Engl J Med 1988;319:525–32 [DOI] [PubMed] [Google Scholar]
- 11.Kunz-Schughart LA, Knuechel R. Tumor-associated fibroblasts (part I): Active stromal participants in tumor development and progression? Histol Histopathol 2002;17:599–621 [DOI] [PubMed] [Google Scholar]
- 12.Dvorak HF, Senger DR, Dvorak AM. Fibrin as a Component of the Tumor Stroma - Origins and Biological Significance. Cancer Metastasis Rev 1983;2:41–73 [DOI] [PubMed] [Google Scholar]
- 13.Welch DR. Defining a cancer metastasis In: Research AAfC, editor. AACR Education Book 2006. Philadelphia: AACR; 2006. p 111–5. [Google Scholar]
- 14.Steeg PS. Targeting metastasis. Nature Reviews Cancer 2016;16:201–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fidler IJ. The Biology of Cancer Metastasis or, You Cannot Fix It If You Do Not Know How It Works. Bioessays 1991;13:551–4 [DOI] [PubMed] [Google Scholar]
- 16.Fidler IJ. Timeline - The pathogenesis of cancer metastasis: the ‘seed and soil’ hypothesis revisited. Nature Reviews Cancer 2003;3:453–8 [DOI] [PubMed] [Google Scholar]
- 17.Sleeman JP, Nazarenko I, Thiele W. Do all roads lead to Rome? Routes to metastasis development. Int J Cancer 2011;128:2511–26 [DOI] [PubMed] [Google Scholar]
- 18.Roh J, Muelleman T, Tawfik O, Thomas SM. Perineural growth in head and neck squamous cell carcinoma: a review. Oral Oncol 2015;51:16–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marchesi F, Piemonti L, Mantovani A, Allavena P. Molecular mechanisms of perineural invasion, a forgotten pathway of dissemination and metastasis. Cytokine Growth Factor Rev 2010;21:77–82 [DOI] [PubMed] [Google Scholar]
- 20.Lugassy C, Kleinman HK, Engbring JA, Welch DR, Harms JF, Rufner R, et al. Pericyte-like location of GFP-tagged melanoma cells: ex vivo and in vivo studies of extravascular migratory metastasis. Am J Pathol 2004;164:1191–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blanpain C Tracing the cellular origin of cancer. Nat Cell Biol 2013;15:126–34 [DOI] [PubMed] [Google Scholar]
- 22.Yamamoto N, Yang M, Jiang P, Xu M, Tsuchiya H, Tomita K, et al. Determination of clonality of metastasis by cell-specific color-coded fluorescent-protein imaging. Cancer Res 2003;63:7785–90 [PubMed] [Google Scholar]
- 23.Talmadge JE, Wolman SR, Fidler IJ. Evidence for the clonal origin of spontaneous metastases. Science 1982;217:361–3 [DOI] [PubMed] [Google Scholar]
- 24.Navin N, Kendall J, Troge J, Andrews P, Rodgers L, McIndoo J, et al. Tumour evolution inferred by single-cell sequencing. Nature 2011;472:90–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McGranahan N, Swanton C. Cancer Evolution Constrained by the Immune Microenvironment. Cell 2017;170:825–7 [DOI] [PubMed] [Google Scholar]
- 26.McGranahan N, Swanton C. Clonal Heterogeneity and Tumor Evolution: Past, Present, and the Future. Cell 2017;168:613–28 [DOI] [PubMed] [Google Scholar]
- 27.Angiogenesis Folkman J.. Annu Rev Med 2006;57:1–18 [DOI] [PubMed] [Google Scholar]
- 28.Paulis YW, Soetekouw PM, Verheul HM, Tjan-Heijnen VC, Griffioen AW. Signalling pathways in vasculogenic mimicry. Biochim Biophys Acta 2010;1806:18–28 [DOI] [PubMed] [Google Scholar]
- 29.Sleeman JP, Thiele W. Tumor metastasis and the lymphatic vasculature. Int J Cancer 2009;125:2747–56 [DOI] [PubMed] [Google Scholar]
- 30.Folberg R, Maniotis AJ. Vasculogenic mimicry. APMIS 2004;112:508–25 [DOI] [PubMed] [Google Scholar]
- 31.Bentolila LA, Prakash R, Mihic-Probst D, Wadehra M, Kleinman HK, Carmichael TS, et al. Imaging of Angiotropism/Vascular Co-Option in a Murine Model of Brain Melanoma: Implications for Melanoma Progression along Extravascular Pathways. Sci Rep 2016;6:23834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maniotis AJ, Folberg R, Hess A, Seftor EA, Gardner LM, Pe’er J, et al. Vascular channel formation by human melanoma cells in vivo and in vitro: vasculogenic mimicry. Am J Pathol 1999;155:739–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Welch DR. Tumor Heterogeneity--A ‘Contemporary Concept’ Founded on Historical Insights and Predictions. Cancer Res 2016;76:4–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Heppner GH. Cancer cell societies and tumor progression. Stem Cells 1993;11:199–203 [DOI] [PubMed] [Google Scholar]
- 35.Hunter KW, Amin R, Deasy S, Ha NH, Wakefield L. Genetic insights into the morass of metastatic heterogeneity. Nat Rev Cancer 2018;18:211–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.El-Kebir M, Satas G, Raphael BJ. Inferring parsimonious migration histories for metastatic cancers. Nat Genet 2018;50:718–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Baslan T, Hicks J. Unravelling biology and shifting paradigms in cancer with single-cell sequencing. Nat Rev Cancer 2017;17:557–69 [DOI] [PubMed] [Google Scholar]
- 38.Abbosh C, Birkbak NJ, Wilson GA, Jamal-Hanjani M, Constantin T, Salari R, et al. Phylogenetic ctDNA analysis depicts early-stage lung cancer evolution. Nature 2017;545:446–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gundem G, Van Loo P, Kremeyer B, Alexandrov LB, Tubio JMC, Papaemmanuil E, et al. The evolutionary history of lethal metastatic prostate cancer. Nature 2015;520:353–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fidler IJ, Talmadge JE. Evidence that intraveously derived murine pulmonary melanoma metastases can originate from the expansion of a single tumor cell. Cancer Res 1986;46:5167–71 [PubMed] [Google Scholar]
- 41.Wolman SR, McMorrow LE, Fidler IJ, Talmadge JE. Development and progression of karyotypic variability in melanoma K1735 following X-irradiation. Cancer Res 1985;45:1839–44 [PubMed] [Google Scholar]
- 42.Poste G, Doll J, Fidler IJ. Interactions among clonal subpopulations affect stability of the metastatic phenotype in polyclonal populations of B16 melanoma cells. Proc Natl Acad Sci 1981;78:6226–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Poste G, Tzeng J, Doll J, Greig R, Rieman D, Zeidman I. Evolution of tumor cell heterogeneity during progressive growth of individual lung metastases. Proc Natl Acad Sci U S A 1982;79:6574–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fidler IJ, Kripke ML. Metastasis results from pre-existing variant cells within a malignant tumor. Science 1977;197:893–5 [DOI] [PubMed] [Google Scholar]
- 45.Liu R, Wang X, Chen GY, Dalerba P, Gurney A, Hoey T, et al. The prognostic role of a gene signature from tumorigenic breast-cancer cells. N Engl J Med 2007;356:217–26 [DOI] [PubMed] [Google Scholar]
- 46.Kang Y, Siegel PM, Shu W, Drobnjak M, Kakonen SM, Cordon-Cardo C, et al. A multigenic program mediating breast cancer metastasis to bone. Cancer Cell 2003;3:537–49 [DOI] [PubMed] [Google Scholar]
- 47.Nowell PC. The clonal evolution of tumor cell populations. Science 1976;194:23–8 [DOI] [PubMed] [Google Scholar]
- 48.Fidler IJ. Selection of successive tumor lines for metastasis. Nature New Biology 1973;242:148–9 [DOI] [PubMed] [Google Scholar]
- 49.Nicolson GL. Organ Specificity of Tumor-Metastasis - Role of Preferential Adhesion, Invasion and Growth of Malignant-Cells at Specific Secondary Sites. Cancer Metastasis Rev 1988;7:143–88 [DOI] [PubMed] [Google Scholar]
- 50.Bohl CR, Harihar S, Denning WL, Sharma R, Welch DR. Metastasis suppressors in breast cancers: mechanistic insights and clinical potential. J Mol Med (Berl) 2014;92:13–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Welch DR. Preface. Metastasis genes. Cancer Metastasis Rev 2012;31:417–8 [DOI] [PubMed] [Google Scholar]
- 52.Welch DR. Metastsis suppressors: Discovery, mechanisms and translation In: Research AAfC, editor. AACR Education Book 2009. Philadelphia: AACR; 2009. p 191–6. [Google Scholar]
- 53.Thiery JP. Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer 2002;2:442–54 [DOI] [PubMed] [Google Scholar]
- 54.Illmensee K, Mintz B. Totipotency and Normal Differentiation of Single Teratocarcinoma Cells Cloned by Injection into Blastocysts. Proc Natl Acad Sci USA 1976;73:549–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kulesa PM, Kasemeier-Kulesa JC, Teddy JM, Margaryan NV, Seftor EA, Seftor RE, et al. Reprogramming metastatic melanoma cells to assume a neural crest cell-like phenotype in an embryonic microenvironment. Proc Natl Acad Sci U S A 2006;103:3752–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kasemeier-Kulesa JC, Romine MH, Morrison JA, Bailey CM, Welch DR, Kulesa PM. NGF reprograms metastatic melanoma to a bipotent glial-melanocyte neural crest-like precursor. Biol Open 2018;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.van der Horst G, Bos L, van der Pluijm G. Epithelial plasticity, cancer stem cells, and the tumor-supportive stroma in bladder carcinoma. Mol Cancer Res 2012;10:995–1009 [DOI] [PubMed] [Google Scholar]
- 58.Psaila B, Lyden D. The metastatic niche: adapting the foreign soil. Nat Rev Cancer 2009;9:285–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kaplan RN, Rafii S, Lyden D. Preparing the “soil”: the premetastatic niche. Cancer Res 2006;66:11089–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Peinado H, Zhang H, Matei IR, Costa-Silva B, Hoshino A, Rodrigues G, et al. Pre-metastatic niches: organ-specific homes for metastases. Nat Rev Cancer 2017;17:302–17 [DOI] [PubMed] [Google Scholar]
- 61.Zhang H, Freitas D, Kim HS, Fabijanic K, Li Z, Chen H, et al. Identification of distinct nanoparticles and subsets of extracellular vesicles by asymmetric flow field-flow fractionation. Nat Cell Biol 2018;20:332–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Costa-Silva B, Aiello NM, Ocean AJ, Singh S, Zhang H, Thakur BK, et al. Pancreatic cancer exosomes initiate pre-metastatic niche formation in the liver. Nat Cell Biol 2015;17:816–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sansone P, Savini C, Kurelac I, Chang Q, Amato LB, Strillacci A, et al. Packaging and transfer of mitochondrial DNA via exosomes regulate escape from dormancy in hormonal therapy-resistant breast cancer. Proc Natl Acad Sci U S A 2017;114:E9066–E75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Matei I, Kim HS, Lyden D. Unshielding Exosomal RNA Unleashes Tumor Growth And Metastasis. Cell 2017;170:223–5 [DOI] [PubMed] [Google Scholar]
- 65.Hoshino A, Costa-Silva B, Shen TL, Rodrigues G, Hashimoto A, Tesic Mark M, et al. Tumour exosome integrins determine organotropic metastasis. Nature 2015;527:329–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cox TR, Rumney RMH, Schoof EM, Perryman L, Hoye AM, Agrawal A, et al. The hypoxic cancer secretome induces pre-metastatic bone lesions through lysyl oxidase. Nature 2015;522:106–U279 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 67.Hou HW, Li QS, Lee GY, Kumar AP, Ong CN, Lim CT. Deformability study of breast cancer cells using microfluidics. Biomed Microdevices 2009;11:557–64 [DOI] [PubMed] [Google Scholar]
- 68.Ochalek T, Nordt FJ, Tullberg K, Burger MM. Correlation between Cell Deformability and Metastatic Potential in B16-F1 Melanoma Cell Variants. Cancer Res 1988;48:5124–8 [PubMed] [Google Scholar]
- 69.Skau CT, Fischer RS, Gurel P, Thiam HR, Tubbs A, Baird MA, et al. FMN2 Makes Perinuclear Actin to Protect Nuclei during Confined Migration and Promote Metastasis. Cell 2016;167:1571–85 e18 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 70.Hou ST, Jiang SX, Smith RA. Permissive and repulsive cues and signalling pathways of axonal outgrowth and regeneration. Int Rev Cell Mol Biol 2008;267:125–81 [DOI] [PubMed] [Google Scholar]
- 71.Kruger RP, Aurandt J, Guan KL. Semaphorins command cells to move. Nat Rev Mol Cell Biol 2005;6:789–800 [DOI] [PubMed] [Google Scholar]
- 72.Seiki M, Sato H, Liotta LA, Schiffmann E. Comparison of autocrine mechanisms promoting motility in two metastatic cell lines: human melanoma and ras-transfected NIH3T3 cells. Int J Cancer 1991;49:717–20 [DOI] [PubMed] [Google Scholar]
- 73.Stuelten CH, Parent CA, Montell DJ. Cell motility in cancer invasion and metastasis: insights from simple model organisms. Nat Rev Cancer 2018;18:296–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.McCarthy JB, Furcht LT. Laminin and fibronectin promote the haptotactic migration of B16 mouse melanoma cells in vitro. J Cell Biol 1984;98:1474–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Te Boekhorst V, Preziosi L, Friedl P. Plasticity of Cell Migration In Vivo and In Silico. Annu Rev Cell Dev Biol 2016;32:491–526 [DOI] [PubMed] [Google Scholar]
- 76.van Helvert S, Storm C, Friedl P. Mechanoreciprocity in cell migration. Nat Cell Biol 2018;20:8–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chaffer CL, San Juan BP, Lim E, Weinberg RA. EMT, cell plasticity and metastasis. Cancer Metastasis Rev 2016;35:645–54 [DOI] [PubMed] [Google Scholar]
- 78.Lambert AW, Pattabiraman DR, Weinberg RA. Emerging Biological Principles of Metastasis. Cell 2017;168:670–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell 2008;133:704–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chaffer CL, Brennan JP, Slavin JL, Blick T, Thompson EW, Williams ED. Mesenchymal-to-epithelial transition facilitates bladder cancer metastasis: role of fibroblast growth factor receptor-2. Cancer Res 2006;66:11271–8 [DOI] [PubMed] [Google Scholar]
- 81.Tsai JH, Donaher JL, Murphy DA, Chau S, Yang J. Spatiotemporal Regulation of Epithelial-Mesenchymal Transition Is Essential for Squamous Cell Carcinoma Metastasis. Cancer Cell 2012;22:725–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yu M, Bardia A, Wittner BS, Stott SL, Smas ME, Ting DT, et al. Circulating breast tumor cells exhibit dynamic changes in epithelial and mesenchymal composition. Science 2013;339:580–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nieto MA. Epithelial plasticity: a common theme in embryonic and cancer cells. Science 2013;342:1234850. [DOI] [PubMed] [Google Scholar]
- 84.Saitoh M Involvement of partial EMT in cancer progression. J Biochem 2018;164:257–64 [DOI] [PubMed] [Google Scholar]
- 85.Tsuji T, Ibaragi S, Hu GF. Epithelial-mesenchymal transition and cell cooperativity in metastasis. Cancer Res 2009;69:7135–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tsuji T, Ibaragi S, Shima K, Hu MG, Katsurano M, Sasaki A, et al. Epithelial-mesenchymal transition induced by growth suppressor p12CDK2-AP1 promotes tumor cell local invasion but suppresses distant colony growth. Cancer Res 2008;68:10377–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zheng X, Carstens JL, Kim J, Scheible M, Kaye J, Sugimoto H, et al. Epithelial-to-mesenchymal transition is dispensable for metastasis but induces chemoresistance in pancreatic cancer. Nature 2015;527:525–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fischer KR, Durrans A, Lee S, Sheng J, Li F, Wong ST, et al. Epithelial-to-mesenchymal transition is not required for lung metastasis but contributes to chemoresistance. Nature 2015;527:472–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Esserman LJ, Varma M. Should we rename low risk cancers? BMJ 2019;364:k4699. [DOI] [PubMed] [Google Scholar]
- 90.Liotta LA. Tumor Invasion and Metastases Role of the Extracellular-Matrix - Rhoads Memorial Award Lecture. Cancer Res 1986;46:1–7 [PubMed] [Google Scholar]
- 91.Friedl P, Locker J, Sahai E, Segall JE. Classifying collective cancer cell invasion. Nat Cell Biol 2012;14:777–83 [DOI] [PubMed] [Google Scholar]
- 92.Chambers AF, Matrisian LM. Changing views of the role of matrix metalloproteinases in metastasis. J Natl Cancer Inst 1997;89:1260–70 [DOI] [PubMed] [Google Scholar]
- 93.Naba A, Clauser KR, Lamar JM, Carr SA, Hynes RO. Extracellular matrix signatures of human mammary carcinoma identify novel metastasis promoters. Elife 2014;3:e01308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Salvador F, Martin A, Lopez-Menendez C, Moreno-Bueno G, Santos V, Vazquez-Naharro A, et al. Lysyl Oxidase-like Protein LOXL2 Promotes Lung Metastasis of Breast Cancer. Cancer Res 2017;77:5846–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Moon HJ, Finney J, Xu L, Moore D, Welch DR, Mure M. MCF-7 cells expressing nuclear associated lysyl oxidase-like 2 (LOXL2) exhibit an epithelial-to-mesenchymal transition (EMT) phenotype and are highly invasive in vitro. J Biol Chem 2013;288:30000–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Patel DF, Peiro T, Shoemark A, Akthar S, Walker SA, Grabiec AM, et al. An extracellular matrix fragment drives epithelial remodeling and airway hyperresponsiveness. Sci Transl Med 2018;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Arroyo AG, Iruela-Arispe ML. Extracellular matrix, inflammation, and the angiogenic response. Cardiovasc Res 2010;86:226–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Duca L, Floquet N, Alix AJ, Haye B, Debelle L. Elastin as a matrikine. Crit Rev Oncol Hematol 2004;49:235–44 [DOI] [PubMed] [Google Scholar]
- 99.Tran KT, Griffith L, Wells A. Extracellular matrix signaling through growth factor receptors during wound healing. Wound Repair Regen 2004;12:262–8 [DOI] [PubMed] [Google Scholar]
- 100.Liotta LA. Cancer cell invasion and metastasis. Sci Am 1992;266:54–9, 62–3 [DOI] [PubMed] [Google Scholar]
- 101.Hehlgans S, Haase M, Cordes N. Signalling via integrins: implications for cell survival and anticancer strategies. Biochim Biophys Acta 2007;1775:163–80 [DOI] [PubMed] [Google Scholar]
- 102.Schwartz MA, Ginsberg MH. Networks and crosstalk: integrin signalling spreads. Nat Cell Biol 2002;4:E65–8 [DOI] [PubMed] [Google Scholar]
- 103.Hamidi H, Ivaska J. Every step of the way: integrins in cancer progression and metastasis. Nat Rev Cancer 2018;18:533–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Fingleton B Matrix metalloproteinases: roles in cancer and metastasis. Front Biosci 2006;11:479–91 [DOI] [PubMed] [Google Scholar]
- 105.Coussens LM, Fingleton B, Matrisian LM. Cancer therapy - Matrix metalloproteinase inhibitors and cancer: Trials and tribulations. Science 2002;295:2387–92 [DOI] [PubMed] [Google Scholar]
- 106.Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer 2002;2:161–74 [DOI] [PubMed] [Google Scholar]
- 107.Mason SD, Joyce JA. Proteolytic networks in cancer. Trends Cell Biol 2011;21:228–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Allgayer H Translational research on u-PAR. Eur J Cancer 2010;46:1241–51 [DOI] [PubMed] [Google Scholar]
- 109.Denais CM, Gilbert RM, Isermann P, McGregor AL, te Lindert M, Weigelin B, et al. Nuclear envelope rupture and repair during cancer cell migration. Science 2016;352:353–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Weaver AM. Invadopodia: specialized cell structures for cancer invasion. Clin Exp Metastasis 2006;23:97–105 [DOI] [PubMed] [Google Scholar]
- 111.Revach OY, Geiger B. The interplay between the proteolytic, invasive, and adhesive domains of invadopodia and their roles in cancer invasion. Cell Adh Migr 2013;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Murphy DA, Courtneidge SA. The ‘ins’ and ‘outs’ of podosomes and invadopodia: characteristics, formation and function. Nat Rev Mol Cell Biol 2011;12:413–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Charras G, Sahai E. Physical influences of the extracellular environment on cell migration. Nat Rev Mol Cell Biol 2014;15:813–24 [DOI] [PubMed] [Google Scholar]
- 114.Bonnans C, Chou J, Werb Z. Remodelling the extracellular matrix in development and disease. Nat Rev Mol Cell Biol 2014;15:786–801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Huang S, Ingber DE. Cell tension, matrix mechanics, and cancer development. Cancer Cell 2005;8:175–6 [DOI] [PubMed] [Google Scholar]
- 116.Mouw JK, Ou G, Weaver VM. Extracellular matrix assembly: a multiscale deconstruction. Nat Rev Mol Cell Biol 2014;15:771–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kumar S, Weaver VM. Mechanics, malignancy, and metastasis: the force journey of a tumor cell. Cancer Metastasis Rev 2009;28:113–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Erdogan B, Ao M, White LM, Means AL, Brewer BM, Yang L, et al. Cancer-associated fibroblasts promote directional cancer cell migration by aligning fibronectin. J Cell Biol 2017;216:3799–816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Friedl P, Wolf K. Proteolytic interstitial cell migration: a five-step process. Cancer Metastasis Rev 2009;28:129–35 [DOI] [PubMed] [Google Scholar]
- 120.Friedl P, Wolf K. Tube travel: the role of proteases in individual and collective cancer cell invasion. Cancer Res 2008;68:7247–9 [DOI] [PubMed] [Google Scholar]
- 121.Allavena P, Sica A, Garlanda C, Mantovani A. The Yin-Yang of tumor-associated macrophages in neoplastic progression and immune surveillance. Immunol Rev 2008;222:155–61 [DOI] [PubMed] [Google Scholar]
- 122.Carron EC, Homra S, Rosenberg J, Coffelt SB, Kittrell F, Zhang Y, et al. Macrophages promote the progression of premalignant mammary lesions to invasive cancer. Oncotarget 2017;8:50731–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Piccard H, Muschel RJ, Opdenakker G. On the dual roles and polarized phenotypes of neutrophils in tumor development and progression. Crit Rev Oncol Hematol 2012;82:296–309 [DOI] [PubMed] [Google Scholar]
- 124.Attieh Y, Vignjevic DM. The hallmarks of CAFs in cancer invasion. Eur J Cell Biol 2016;95:493–502 [DOI] [PubMed] [Google Scholar]
- 125.Aeed PA, Nakajima M, Welch DR. The role of polymorphonuclear leukocytes (PMN) on the growth and metastatic potential of 13762NF mammary adenocarcinoma cells. Int J Cancer 1988;42:748–59 [DOI] [PubMed] [Google Scholar]
- 126.McGary CT, Miele ME, Welch DR. Highly metastatic 13762NF rat mammary adenocarcinoma cell clones stimulate bone marrow by secretion of granulocyte-macrophage colony-stimulating factor/interleukin-3 activity. Am J Pathol 1995;147:1668–81 [PMC free article] [PubMed] [Google Scholar]
- 127.Safari E, Ghorghanlu S, Ahmadi-Khiavi H, Mehranfar S, Rezaei R, Motallebnezhad M. Myeloid-derived suppressor cells and tumor: Current knowledge and future perspectives. J Cell Physiol 2018;0. [DOI] [PubMed] [Google Scholar]
- 128.Talmadge JE, Donkor M, Scholar E. Inflammatory cell infiltration of tumors: Jekyll or Hyde. Cancer Metastasis Rev 2007;26:373–400 [DOI] [PubMed] [Google Scholar]
- 129.Wyckoff JB, Wang Y, Lin EY, Li JF, Goswami S, Stanley ER, et al. Direct visualization of macrophage-assisted tumor cell intravasation in mammary tumors. Cancer Res 2007;67:2649–56 [DOI] [PubMed] [Google Scholar]
- 130.Yamaguchi H, Wyckoff J, Condeelis J. Cell migration in tumors. Curr Opin Cell Biol 2005;17:559–64 [DOI] [PubMed] [Google Scholar]
- 131.Condeelis J, Pollard JW. Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell 2006;124:263–6 [DOI] [PubMed] [Google Scholar]
- 132.Huber AR, Kunkel SL, Todd RF 3rd, Weiss SJ. Regulation of transendothelial neutrophil migration by endogenous interleukin-8. Science 1991;254:99–102 [DOI] [PubMed] [Google Scholar]
- 133.Nicolson GL. Metastatic tumor cell attachment and invasion assay utilizing vascular endothelial cell monolayers. J Histochem Cytochem 1982;30:214–20 [DOI] [PubMed] [Google Scholar]
- 134.DP M, Biziato D, Petrova TV. Microenvironmental regulation of tumour angiogenesis. Nat Rev Cancer 2017;17:457–74 [DOI] [PubMed] [Google Scholar]
- 135.Stacker SA, Williams SP, Karnezis T, Shayan R, Fox SB, Achen MG. Lymphangiogenesis and lymphatic vessel remodelling in cancer. Nat Rev Cancer 2014;14:159–72 [DOI] [PubMed] [Google Scholar]
- 136.Keck PJ, Hauser SD, Krivi G, Sanzo K, Warren T, Feder J, et al. Vasular permeability factor, an endothelial cell mitogen related to PDGF. Science 1989;246:1309–12 [DOI] [PubMed] [Google Scholar]
- 137.Kramer RH, Nicolson GL. Interactions of tumor cells with vascular endothelial cell monolayers: a model for metastatic invasion. Proc Natl Acad Sci U S A 1979;76:5704–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Overholtzer M, Mailleux AA, Mouneimne G, Normand G, Schnitt SJ, King RW, et al. A nonapoptotic cell death process, entosis, that occurs by cell-in-cell invasion. Cell 2007;131:966–79 [DOI] [PubMed] [Google Scholar]
- 139.Coman DR. Decreased mutual adhesiveness, a property of cells from squamous cell carcinomas. Cancer Res 1944;4:625–9 [Google Scholar]
- 140.Zeidman I, Mc CM, Coman DR. Factors affecting the number of tumor metastases; experiments with a transplantable mouse tumor. Cancer Res 1950;10:357–9 [PubMed] [Google Scholar]
- 141.Wong AD, Searson PC. Mitosis-Mediated Intravasation in a Tissue-Engineered Tumor-Microvessel Platform. Cancer Res 2017;77:6453–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Butler TP, Gullino PM. Quantitation of Cell Shedding into Efferent Blood of Mammary Adenocarcinoma. Cancer Res 1975;35:512–6 [PubMed] [Google Scholar]
- 143.Jain RK. Transport of molecules accross tumor vasculature. Cancer Metastasis Rev 1987:559–93 [DOI] [PubMed] [Google Scholar]
- 144.Fidler IJ. Metastasis: quantitative analysis of distribution and fate of tumor emboli labeled with 125I-5-iodo-2’-deoxyuridine. J Natl Cancer Inst 1970;45:773–82 [PubMed] [Google Scholar]
- 145.Tarin D, Price JE, Kettlewell MG, Souter RG, Vass AC, Crossley B. Mechanisms of human tumor metastasis studied in patients with peritoneovenous shunts. Cancer Res 1984;44:3584–92 [PubMed] [Google Scholar]
- 146.Ao Z, Shah SH, Machlin LM, Parajuli R, Miller PC, Rawal S, et al. Identification of Cancer-Associated Fibroblasts in Circulating Blood from Patients with Metastatic Breast Cancer. Cancer Res 2015;75:4681–7 [DOI] [PubMed] [Google Scholar]
- 147.Fidler IJ. The relationship of embolic heterogeneity, number size and viability to the incidence of experimental metastasis. Eur J Cancer 1973;9:223–7 [DOI] [PubMed] [Google Scholar]
- 148.Updyke TV, Nicolson GL. Malignant melanoma cell lines selected in vitro for increased homotypic adhesion properties have increased experimental metastasis potential. CEM 1986;4:273–84 [DOI] [PubMed] [Google Scholar]
- 149.Weiss L Comments on Hematogenous Metastatic Patterns in Humans as Revealed by Autopsy. Clin Exp Metastasis 1992;10:191–9 [DOI] [PubMed] [Google Scholar]
- 150.Ewing J Neoplastic disease. Philadelphia: W.B. Saunders; 1919. [Google Scholar]
- 151.Gkountela S, Castro-Giner F, Szczerba BM, Vetter M, Landin J, Scherrer R, et al. Circulating Tumor Cell Clustering Shapes DNA Methylation to Enable Metastasis Seeding. Cell 2019;176:98–112 e14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Krog BL, Henry MD. Biomechanics of the Circulating Tumor Cell Microenvironment. Adv Exp Med Biol 2018;1092:209–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Paoletti C, Li YF, Muniz MC, Kidwell KM, Aung K, Thomas DG, et al. Significance of Circulating Tumor Cells in Metastatic Triple-Negative Breast Cancer Patients within a Randomized, Phase II Trial: TBCRC 019. Clin Cancer Res 2015;21:2771–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Aceto N, Toner M, Maheswaran S, Haber DA. En Route to Metastasis: Circulating Tumor Cell Clusters and Epithelial-to-Mesenchymal Transition Trends in Cancer. Volume 1: Elsevier; 2015. p 44–52. [DOI] [PubMed] [Google Scholar]
- 155.Aceto N, Bardia A, Miyamoto DT, Donaldson MC, Wittner BS, Spencer JA, et al. Circulating tumor cell clusters are oligoclonal precursors of breast cancer metastasis. Cell 2014;158:1110–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Crissman JD, Hatfield J, Schaldenbrand M, Sloane BF, Honn KV. Arrest and extravasation of B16 amelanotic melanoma in murine lungs: A light and EM study. Lab Invest 1985;53:470–8 [PubMed] [Google Scholar]
- 157.Ito S, Nakanishi H, Ikehara Y, Kato T, Kasai Y, Ito K, et al. Real-time observation of micrometastasis formation in the living mouse liver using a green fluorescent protein gene-tagged rat tongue carcinoma cell line. Int J Cancer 2001;93:212–7 [DOI] [PubMed] [Google Scholar]
- 158.Springer TA. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell 1994;76:301–14 [DOI] [PubMed] [Google Scholar]
- 159.Stupack DG, Puente XS, Boutsaboualoy S, Storgard CM, Cheresh DA. Apoptosis of adherent cells by recruitment of caspase-8 to unligated integrins. J Cell Biol 2001;155:459–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Alby L, Auerbach R. Differential adhesion of tumor cells to capillary endothelial cells in vitro. Proc Natl Acad Sci U S A 1984;81:5739–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Auerbach R, Lu WC, Pardon E, Gumkowski F, Kaminska G, Kaminski MS. Specificity of adhesion between murine tumor cells and capillary endothelium: an in vitro correlate of preferential metastasis in vivo. Cancer Res 1987;47:1492–6 [PubMed] [Google Scholar]
- 162.Belloni PN, Tressler RJ. Microvascular endothelial cell heterogeneity: Interactions with leukocytes and tumor cells. Cancer Metastasis Rev 1989;8:353–89 [DOI] [PubMed] [Google Scholar]
- 163.Belloni PN, Nicolson GL. Differential Expression of Cell-Surface Glycoproteins on Various Organ-Derived Microvascular Endothelia and Endothelial-Cell Cultures. J Cell Physiol 1988;136:398–410 [DOI] [PubMed] [Google Scholar]
- 164.Pasqualini R, Arap W, McDonald DM. Probing the structural and molecular diversity of tumor vasculature. Trends Mol Med 2002;8:563–71 [DOI] [PubMed] [Google Scholar]
- 165.Pasqualini R, Ruoslahti E. Organ targeting in vivo using phage display peptide libraries. Nature 1996;380:364–6 [DOI] [PubMed] [Google Scholar]
- 166.Lala PK, Orucevic A. Role of nitric oxide in tumor progression: lessons from experimental tumors. Cancer Metastasis Rev 1998;17:91–106 [DOI] [PubMed] [Google Scholar]
- 167.Orr FW, Warner DJ. Effects of neutrophil-mediated pulmonary endothelial injury on the localization and metastasis of circulating Walker carcinosarcoma cells. Invasion Metastasis 1987;7:183–96 [PubMed] [Google Scholar]
- 168.Orr FW, Adamson IYR, Young L. Promotion of Pulmonary Metastasis in Mice by Bleomycin-Induced Endothelial Injury. Cancer Res 1986;46:891–7 [PubMed] [Google Scholar]
- 169.Sugarbaker EV, Ketcham AS. Mechanisms and prevention of cancer dissemination: an overview. Semin Oncol 1977;4:19–32 [PubMed] [Google Scholar]
- 170.Cho EJ, Kim MH, Cha SH, Hyun S, Jeong S, Lee JD. Breast Cancer Cutaneous Metastasis at Core Needle Biopsy Site. Annals of Dermatology 2010;22:238–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Retsky MW, Demicheli R, Hrushesky WJ, Baum M, Gukas ID. Dormancy and surgery-driven escape from dormancy help explain some clinical features of breast cancer. APMIS 2008;116:730–41 [DOI] [PubMed] [Google Scholar]
- 172.Osborne L Leukocyte adhesion to endothelium in inflammation. Cell 1990;62:3–6 [DOI] [PubMed] [Google Scholar]
- 173.Chambers AF, Groom AC, MacDonald IC. Dissemination and growth of cancer cells in metastatic sites. Nat Rev Cancer 2002;2:563–72 [DOI] [PubMed] [Google Scholar]
- 174.Al-Mehdi AB, Tozawa K, Fisher AB, Shientag L, Lee A, Muschel RJ. Intravascular origin of metastasis from the proliferation of endothelium-attached tumor cells: a new model for metastasis. Nat Med 2000;6:100–2 [DOI] [PubMed] [Google Scholar]
- 175.Naumov GN, Folkman J, Straume O, Akslen LA. Tumor-vascular interactions and tumor dormancy. APMIS 2008;116:569–85 [DOI] [PubMed] [Google Scholar]
- 176.Hendrix MJ, Seftor EA, Hess AR, Seftor RE. Vasculogenic mimicry and tumour-cell plasticity: lessons from melanoma. Nat Rev Cancer 2003;3:411–21 [DOI] [PubMed] [Google Scholar]
- 177.Kaplan RN, Riba RD, Zacharoulis S, Bramley AH, Vincent L, Costa C, et al. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature 2005;438:820–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Wculek SK, Malanchi I. Neutrophils support lung colonization of metastasis-initiating breast cancer cells. Nature 2015;528:413–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Albrengues J, Shields MA, Ng D, Park CG, Ambrico A, Poindexter ME, et al. Neutrophil extracellular traps produced during inflammation awaken dormant cancer cells in mice. Science 2018;361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Cedervall J, Zhang YY, Olsson AK. Tumor-Induced NETosis as a Risk Factor for Metastasis and Organ Failure. Cancer Res 2016;76:4311–5 [DOI] [PubMed] [Google Scholar]
- 181.Demers M, Krause DS, Schatzberg D, Martinod K, Voorhees JR, Fuchs TA, et al. Cancers predispose neutrophils to release extracellular DNA traps that contribute to cancer-associated thrombosis. Proc Natl Acad Sci USA 2012;109:13076–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Langley RR, Fidler IJ. The seed and soil hypothesis revisited--the role of tumor-stroma interactions in metastasis to different organs. Int J Cancer 2011;128:2527–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Paget S The distribution of secondary growths in cancer of the breast. Lancet 1889;1:571–3 [PubMed] [Google Scholar]
- 184.Beadnell TC, Scheid AD, Vivian CJ, Welch DR. Roles of the mitochondrial genetics in cancer metastasis: not to be ignored any longer. Cancer Metastasis Rev 2018;37:615–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Brinker AE, Vivian CJ, Koestler DC, Tsue TT, Jensen RA, Welch DR. Mitochondrial Haplotype Alters Mammary Cancer Tumorigenicity and Metastasis in an Oncogenic Driver-Dependent Manner. Cancer Res 2017;77:6941–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Feeley KP, Bray AW, Westbrook DG, Johnson LW, Kesterson RA, Ballinger SW, et al. Mitochondrial Genetics Regulate Breast Cancer Tumorigenicity and Metastatic Potential. Cancer Res 2015;75:4429–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Telonis AG, Rigoutsos I. Race Disparities in the Contribution of miRNA Isoforms and tRNA-Derived Fragments to Triple-Negative Breast Cancer. Cancer Res 2018;78:1140–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Warburg O On the origin of cancer cells. Science 1956;123:309–14 [DOI] [PubMed] [Google Scholar]
- 189.Weber GF. Metabolism in cancer metastasis. Int J Cancer 2016;138:2061–6 [DOI] [PubMed] [Google Scholar]
- 190.Valcarcel-Jimenez L, Gaude E, Torrano V, Frezza C, Carracedo A. Mitochondrial Metabolism: Yin and Yang for Tumor Progression. Trends Endocrinol Metab 2017;28:748–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Tan AS, Baty JW, Dong LF, Bezawork-Geleta A, Endaya B, Goodwin J, et al. Mitochondrial Genome Acquisition Restores Respiratory Function and Tumorigenic Potential of Cancer Cells without Mitochondrial DNA. Cell Metab 2015;21:81–94 [DOI] [PubMed] [Google Scholar]
- 192.Porporato PE, Sonveaux P. Paving the way for therapeutic prevention of tumor metastasis with agents targeting mitochondrial superoxide. Mol Cell Oncol 2015;2:e968043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Liu W, Beck BH, Vaidya KS, Nash KT, Feeley KP, Ballinger SW, et al. Metastasis suppressor KISS1 appears to reverse the Warburg effect by enhancing mitochondrial biogenesis. Cancer Res 2013;74:954–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.LeBleu VS, O’Connell JT, Gonzalez Herrera KN, Wikman H, Pantel K, Haigis MC, et al. PGC-1alpha mediates mitochondrial biogenesis and oxidative phosphorylation in cancer cells to promote metastasis. Nat Cell Biol 2014;16:992–1003, 1–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Santidrian AF, Matsuno-Yagi A, Ritland M, Seo BB, LeBoeuf SE, Gay LJ, et al. Mitochondrial complex I activity and NAD+/NADH balance regulate breast cancer progression. J Clin Invest 2013;123:1068–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Seo JH, Agarwal E, Bryant KG, Caino MC, Kim ET, Kossenkov AV, et al. Syntaphilin Ubiquitination Regulates Mitochondrial Dynamics and Tumor Cell Movements. Cancer Res 2018;78:4215–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Altieri DC. Mitochondrial dynamics and metastasis. Cell Mol Life Sci 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Lisanti S, Garlick DS, Bryant KG, Tavecchio M, Mills GB, Lu Y, et al. Transgenic Expression of the Mitochondrial Chaperone TNFR-associated Protein 1 (TRAP1) Accelerates Prostate Cancer Development. J Biol Chem 2016;291:25247–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Caino MC, Seo JH, Aguinaldo A, Wait E, Bryant KG, Kossenkov AV, et al. A neuronal network of mitochondrial dynamics regulates metastasis. Nat Commun 2016;7:13730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Mowers EE, Sharifi MN, Macleod KF. Autophagy in cancer metastasis. Oncogene 2017;36:1619–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Vlahakis A, Debnath J. The Interconnections between Autophagy and Integrin-Mediated Cell Adhesion. J Mol Biol 2017;429:515–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Pauli BU, Augustin-Voss HG, el-Sabban ME, Johnson RC, Hammer DA. Organ-preference of metastasis. The role of endothelial cell adhesion molecules. Cancer Metastasis Rev 1990;9:175–89 [DOI] [PubMed] [Google Scholar]
- 203.Nicolson GL, Belloni PN, Tressler RJ, Dulski K, Inoue T, Cavanaugh PG. Adhesive, invasive, and growth properties of selected metastatic variants of a murine large-cell lymphoma. Invasion Metastasis 1989;9:102–16 [PubMed] [Google Scholar]
- 204.Nicolson GL, Dulski K, Basson C, Welch DR. Preferential organ attachment and invasion in vitro by B16 melanoma cells selected for differing metastatic colonization and invasive properties. Invasion Metastasis 1985;5:144–58 [PubMed] [Google Scholar]
- 205.Aguirre-Ghiso JA, Sosa MS. Emerging Topics on Disseminated Cancer Cell Dormancy and the Paradigm of Metastasis. Annual Review of Cancer Biology 2018;2:377–93 [Google Scholar]
- 206.Hosseini H, Obradovic MM, Hoffmann M, Harper KL, Sosa MS, Werner-Klein M, et al. Early dissemination seeds metastasis in breast cancer. Nature 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Hoffman RM. Patient-derived orthotopic xenografts: better mimic of metastasis than subcutaneous xenografts. Nature Reviews Cancer 2015;15:451–2 [DOI] [PubMed] [Google Scholar]
- 208.Ghinassi B, Martelli F, Verrucci M, D’Amore E, Migliaccio G, Vannucchi AM, et al. Evidence for organ-specific stem cell microenvironments. J Cell Physiol 2010;223:460–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Minn AJ, Kang Y, Serganova I, Gupta GP, Giri DD, Doubrovin M, et al. Distinct organ-specific metastatic potential of individual breast cancer cells and primary tumors. J Clin Invest 2005;115:44–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Boire A, Zou Y, Shieh J, Macalinao DG, Pentsova E, Massague J. Complement Component 3 Adapts the Cerebrospinal Fluid for Leptomeningeal Metastasis. Cell 2017;168:1101–13 e13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Bos PD, Zhang XH, Nadal C, Shu W, Gomis RR, Nguyen DX, et al. Genes that mediate breast cancer metastasis to the brain. Nature 2009;459:1005–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.van der Weyden L, Arends MJ, Campbell AD, Bald T, Wardle-Jones H, Griggs N, et al. Genome-wide in vivo screen identifies novel host regulators of metastatic colonization. Nature 2017;541:233–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Nicolson GL, Dulski KM. Organ Specificity of Metastatic Tumor Colonization Is Related to Organ-Selective Growth-Properties of Malignant-Cells. Int J Cancer 1986;38:289–94 [DOI] [PubMed] [Google Scholar]
- 214.Altorki NK, Markowitz GJ, Gao D, Port JL, Saxena A, Stiles B, et al. The lung microenvironment: an important regulator of tumour growth and metastasis. Nat Rev Cancer 2019;19:9–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Zhuang X, Zhang H, Li X, Li X, Cong M, Peng F, et al. Differential effects on lung and bone metastasis of breast cancer by Wnt signalling inhibitor DKK1. Nat Cell Biol 2017;19:1274–85 [DOI] [PubMed] [Google Scholar]
- 216.Bowman RL, Klemm F, Akkari L, Pyonteck SM, Sevenich L, Quail DF, et al. Macrophage Ontogeny Underlies Differences in Tumor-Specific Education in Brain Malignancies. Cell Rep 2016;17:2445–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Kim SJ, Kim JS, Park ES, Lee JS, Lin Q, Langley RR, et al. Astrocytes upregulate survival genes in tumor cells and induce protection from chemotherapy. Neoplasia 2011;13:286–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Jackson W 3rd, Sosnoski DM, Ohanessian SE, Chandler P, Mobley A, Meisel KD, et al. Role of Megakaryocytes in Breast Cancer Metastasis to Bone. Cancer Res 2017;77:1942–54 [DOI] [PubMed] [Google Scholar]
- 219.Ku AW, Muhitch JB, Powers CA, Diehl M, Kim M, Fisher DT, et al. Tumor-induced MDSC act via remote control to inhibit L-selectin-dependent adaptive immunity in lymph nodes. Elife 2016;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Muller A, Homey B, Soto H, Ge N, Catron D, Buchanan ME, et al. Involvement of chemokine receptors in breast cancer metastasis. Nature 2001;410:50–6 [DOI] [PubMed] [Google Scholar]
- 221.Gay LJ, Malanchi I. The sleeping ugly: Tumour microenvironment’s act to make or break the spell of dormancy. Biochim Biophys Acta Rev Cancer 2017;1868:231–8 [DOI] [PubMed] [Google Scholar]
- 222.Yeh AC, Ramaswamy S. Mechanisms of Cancer Cell Dormancy-Another Hallmark of Cancer? Cancer Res 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Sosa MS, Bragado P, Aguirre-Ghiso JA. Mechanisms of disseminated cancer cell dormancy: an awakening field. Nat Rev Cancer 2014;14:611–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Hadfield G The dormant cancer cell. Br Med J 1954;2:607–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Vishnoi M, Peddibhotla S, Yin W, A TS, George GC, Hong DS, et al. The isolation and characterization of CTC subsets related to breast cancer dormancy. Sci Rep 2015;5:17533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Naumov GN, Bender E, Zurakowski D, Kang SY, Sampson D, Flynn E, et al. A model of human tumor dormancy: an angiogenic switch from the nonangiogenic phenotype. J Natl Cancer Inst 2006;98:316–25 [DOI] [PubMed] [Google Scholar]
- 227.Manjili MH. A Theoretical Basis for the Efficacy of Cancer Immunotherapy and Immunogenic Tumor Dormancy: The Adaptation Model of Immunity. Adv Cancer Res 2018;137:17–36 [DOI] [PubMed] [Google Scholar]
- 228.Yumoto K, Eber MR, Berry JE, Taichman RS, Shiozawa Y. Molecular pathways: niches in metastatic dormancy. Clin Cancer Res 2014;20:3384–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Kaur A, Ecker BL, Douglass SM, Kugel CH 3rd,Webster MR, Almeida FV , et al. Remodeling of the Collagen Matrix in Aging Skin Promotes Melanoma Metastasis and Affects Immune Cell Motility. Cancer Discov 2019;9:64–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Fidler IJ, Nicolson GL. Fate of recirculating B16 melanoma metastatic variant cells in parabiotic syngeneic recipients. J Natl Cancer Inst 1977;58:1867–72 [DOI] [PubMed] [Google Scholar]
- 231.Bartkowiak K, Kwiatkowski M, Buck F, Gorges TM, Nilse L, Assmann V, et al. Disseminated Tumor Cells Persist in the Bone Marrow of Breast Cancer Patients through Sustained Activation of the Unfolded Protein Response. Cancer Res 2015;75:5367–77 [DOI] [PubMed] [Google Scholar]
- 232.Pantel K, Cote RJ, Fodstad O. Detection and clinical importance of micrometastatic disease. J Natl Cancer Inst 1999;91:1113–24 [DOI] [PubMed] [Google Scholar]
- 233.Friberg S, Nystrom A Cancer Metastases: Early Dissemination and Late Recurrences. Cancer Growth Metastasis 2015;8:43–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Harper KL, Sosa MS, Entenberg D, Hosseini H, Cheung JF, Nobre R, et al. Mechanism of early dissemination and metastasis in Her2(+) mammary cancer. Nature 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Leonard GD, Swain SM. Ductal carcinoma in situ, complexities and challenges. J Natl Cancer Inst 2004;96:906–20 [DOI] [PubMed] [Google Scholar]
- 236.Silver SA, Tavassoli FA. Mammary ductal carcinoma in situ with microinvasion. Cancer 1998;82:2382–90 [DOI] [PubMed] [Google Scholar]
- 237.Narod SA, Iqbal J, Giannakeas V, Sopik V, Sun P. Breast Cancer Mortality After a Diagnosis of Ductal Carcinoma In Situ. JAMA Oncol 2015;1:888–96 [DOI] [PubMed] [Google Scholar]
- 238.Turajlic S, Swanton C. Metastasis as an evolutionary process. Science 2016;352:169–75 [DOI] [PubMed] [Google Scholar]
- 239.Subarsky P, Hill RP. The hypoxic tumour microenvironment and metastatic progression. Clin Exp Metastasis 2003;20:237–50 [DOI] [PubMed] [Google Scholar]
- 240.De Jaeger K, Kavanagh MC, Hill RP. Relationship of hypoxia to metastatic ability in rodent tumours. Br J Cancer 2001;84:1280–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Gilkes DM, Semenza GL, Wirtz D. Hypoxia and the extracellular matrix: drivers of tumour metastasis. Nat Rev Cancer 2014;14:430–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Gilkes DM, Xiang L, Lee SJ, Chaturvedi P, Hubbi ME, Wirtz D, et al. Hypoxia-inducible factors mediate coordinated RhoA-ROCK1 expression and signaling in breast cancer cells. Proc Natl Acad Sci U S A 2014;111:E384–93 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 243.Castro-Vega LJ, Jouravleva K, Ortiz-Montero P, Liu WY, Galeano JL, Romero M, et al. The senescent microenvironment promotes the emergence of heterogeneous cancer stem-like cells. Carcinogenesis 2015;36:1180–92 [DOI] [PubMed] [Google Scholar]
- 244.Pan H, Gray R, Braybrooke J, Davies C, Taylor C, McGale P, et al. 20-Year Risks of Breast-Cancer Recurrence after Stopping Endocrine Therapy at 5 Years. N Engl J Med 2017;377:1836–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Alix-Panabieres C, Pantel K. Clinical Applications of Circulating Tumor Cells and Circulating Tumor DNA as Liquid Biopsy. Cancer Discov 2016;6:479–91 [DOI] [PubMed] [Google Scholar]
- 246.Christiano AP, Yoshida BA, Dubauskas Z, Sokoloff M, Rinker-Schaeffer CW. Development of markers of prostate cancer metastasis. Review and perspective. Urol Oncol 2000;5:217–23 [DOI] [PubMed] [Google Scholar]
- 247.Floor SL, Dumont JE, Maenhaut C, Raspe E. Hallmarks of cancer: of all cancer cells, all the time? Trends Mol Med 2012;18:509–15 [DOI] [PubMed] [Google Scholar]
- 248.Lawson DA, Kessenbrock K, Davis RT, Pervolarakis N, Werb Z. Tumour heterogeneity and metastasis at single-cell resolution. Nat Cell Biol 2018;20:1349–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Welch DR. Biologic considerations for drug targeting in cancer patients. Cancer Treat Rev 1987;14:351–8 [DOI] [PubMed] [Google Scholar]
- 250.Heppner GH. Tumor heterogeneity. Cancer Res 1984;44:2259–65 [PubMed] [Google Scholar]
- 251.Rak JW, Kerbel RS. Growth advantage (“clonal dominance”) of metastatically competent tumor cell variants expressed under selective two- or three-dimensional tissue culture conditions. In Vitro Cell and Developmental Biology: Animal Cells 1993;29A:742–8 [DOI] [PubMed] [Google Scholar]
- 252.Miller BE, McInerney D, Jackson D, Miller FR. Metabolic cooperation between mouse mammary tumor subpopulations in three-dimensional collagen gel cultures. Cancer Res 1986;46:89–93 [PubMed] [Google Scholar]
- 253.Miller FR, Miller BE, Heppner GH. Subpopulation interactions in drug resistance, metastasis and tumor progression In: Jacquillat C, Weil M, Khayat D, editors. Neo-adjuvant chemotherapy. London: John Libbey & Co.; 1986. p 69–74. [Google Scholar]
- 254.Miller BE, Miller FR, Heppner GH. Factors affecting growth and drug sensitivity of mouse mammary tumor lines in collagen gel cultures. Cancer Res 1985;45:4200–5 [PubMed] [Google Scholar]
- 255.Miller BE, Miller FR, Wilburn D, Heppner GH. Dominance of a tumor subpopulation line in mixed heterogeneous mouse mammary tumors. Cancer Res 1988;48:5747–53 [PubMed] [Google Scholar]
- 256.Miller FR, Heppner GH. Cellular interactions in metastasis. Cancer Metastasis Rev 1990;9:21–34 [DOI] [PubMed] [Google Scholar]
- 257.Somarelli JA, Schaeffer D, Marengo MS, Bepler T, Rouse D, Ware KE, et al. Distinct routes to metastasis: plasticity-dependent and plasticity-independent pathways. Oncogene 2016;35:4302–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Ishay-Ronen D, Diepenbruck M, Kalathur RKR, Sugiyama N, Tiede S, Ivanek R, et al. Gain Fat-Lose Metastasis: Converting Invasive Breast Cancer Cells into Adipocytes Inhibits Cancer Metastasis. Cancer Cell 2019;35:17–32 e6 [DOI] [PubMed] [Google Scholar]
- 259.The Molecular Basis of Cancer Philadelphia: Elsevier - Saunders; 2015. 888 p. [Google Scholar]
- 260.Boral D, Vishnoi M, Liu HN, Yin W, Sprouse ML, Scamardo A, et al. Molecular characterization of breast cancer CTCs associated with brain metastasis. Nat Commun 2017;8:196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Robinson DR, Wu YM, Lonigro RJ, Vats P, Cobain E, Everett J, et al. Integrative clinical genomics of metastatic cancer. Nature 2017;548:297–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Naxerova K, Reiter JG, Brachtel E, Lennerz JK, van de Wetering M, Rowan A, et al. Origins of lymphatic and distant metastases in human colorectal cancer. Science 2017;357:55–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Naxerova K, Jain RK. Using tumour phylogenetics to identify the roots of metastasis in humans. Nat Rev Clin Oncol 2015;12:258–72 [DOI] [PubMed] [Google Scholar]
- 264.Lytle NK, Barber AG, Reya T. Stem cell fate in cancer growth, progression and therapy resistance. Nat Rev Cancer 2018;18:669–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Welch DR, Milas L, Tomasovic SP, Nicolson GL. Heterogeneous response and clonal drift of sensitivities of metastatic 13762NF mammary adenocarcinoma clones to gamma-radiation in vitro. Cancer Res 1983;43:6–10 [PubMed] [Google Scholar]
- 266.Welch DR, Hurst DR. Beyond the Primary Tumor: Progression, Invasion, and Metastasis In: Coleman WB, Tsongalis GJ, editors. The Molecular Basis of Human Cancer. New York: Springer Science+Business Media; 2017. p 203–16. [Google Scholar]