Abstract
α-synuclein is a soluble protein that is present in abundance in the brain, though its normal function in the healthy brain is poorly defined. Intraneuronal inclusions of α-synuclein, commonly referred to as Lewy pathology, are pathological hallmarks of a spectrum of neurodegenerative disorders referred to as α-synucleinopathies. Though α-synuclein is expressed predominantly in neurons, α-synuclein aggregates in astrocytes is a common feature in these neurodegenerative diseases. How and why synuclein ends up in the astrocytes and the consequences of this dysfunctional proteostasis in immune cells is a major area of research that can have far-reaching implications for future immunobiotherapies in α-synucleinopathies. Accumulation of aggregated α-synuclein can disrupt astrocyte function in general and, more importantly, can contribute to neurodegeneration in α-synucleinopathies through various pathways. Here, we summarize our current knowledge on how astrocytic α-synucleinopathy affects CNS function in health and disease and propose a model of neuroglial connectome altered by α-synuclein proteostasis that might be amenable to immune based therapies.
Keywords: α-synuclein, Lewy body, glial cytoplasmic inclusion, neurodegeneration, exosome, tunneling nanotube, transmission, astrocyte heterogeneity, NAC domain, therapy
Introduction
Intraneuronal inclusions of α-synuclein (αSyn) protein, commonly referred to as Lewy bodies (LB) or Lewy neurites (LN), are hallmark pathologies in a group of neurodegenerative disorders collectively known as α-synucleinopathies [13, 164]. The gene coding for the αSyn protein, SNCA, is a genetic risk factor for both sporadic and familial forms of multiple α-synucleinopathies, including Parkinson’s disease (PD) and Dementia with Lewy Bodies (DLB) [70, 139]. αSyn aggregates are found in different cell types as well as in different brain regions across the spectrum of α-synucleinopathies. For example, intraneuronal LB and LN pathology are frequently observed in PD, PD with dementia (PDD), incidental Lewy body diseases (iLBD) and DLB (Figure 1a–d). In multiple system atrophy (MSA) patients, in addition to few neuronal cytoplasmic inclusions (NCI) of αSyn, αSyn inclusions are primarily found in oligodendrocytes and are referred to as glial cytoplasmic inclusions (GCIs) or Papp-Lantos bodies [75] (Figure 1e). On the other hand, astrocytic inclusions of αSyn are invariant features in PD and DLB but observed less frequently in MSA (Figure 1a–c) [145]. The clinical and neuropathological heterogeneity in the spectrum of α-synucleinopathies is believed to result from a combination of various factors, including unique conformational variants of αSyn protein that might contribute to distinct clinical phenotypes [118, 121].
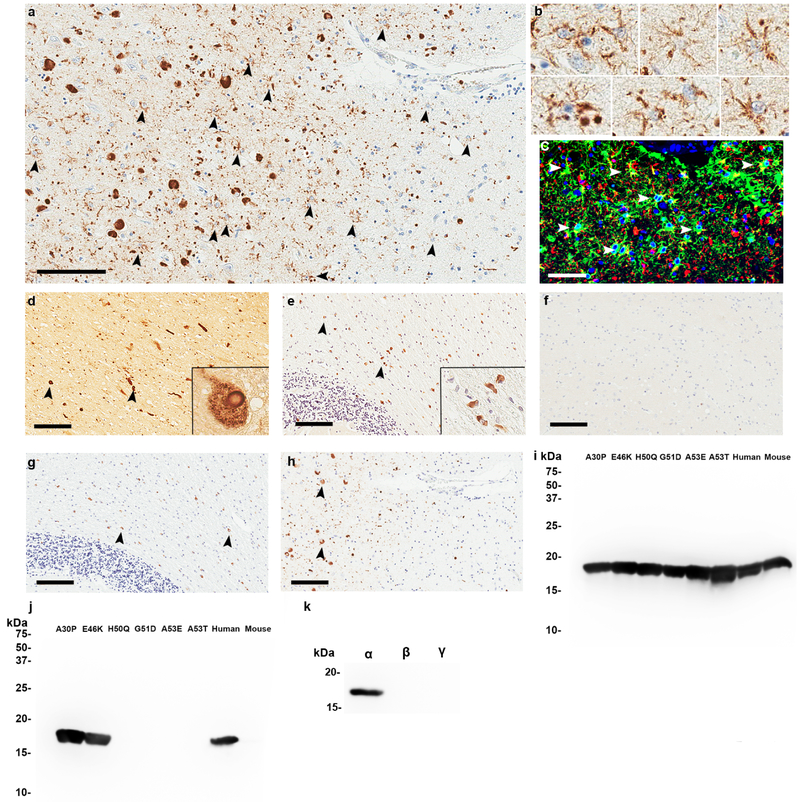
Figure 1. Astrocytic αSyn inclusions in DLB patients specifically detected by an antibody in the middle domain of αSyn.
a. Immunohistochemical staining with 3H11 antibody raised against residues 43–63 of αSyn with formic acid retrieval. αSyn is extensively detected within astrocytes in the hippocampus of a DLB patient. b. Higher magnification from Panel A showing astrocytes immunopositive for αSyn antibody 3H11. c. Immunofluorescent analysis with antibodies to GFAP (green) and αSyn 3H11 (red) demonstrating that αSyn is present in astrocytic processes in the DLB hippocampus. d. Detection of αSyn within classical LBs in the substantia nigra of a DLB patient with the 3H11 antibody. Inset shows magnified view of a typical intraneuronal LB. e. Detection of αSyn within GCI inclusions in the cerebellum of an MSA individual using the 3H11 antibody. Inset shows magnified view of typical GCI. f. αSyn antibody 3H11 fails to show any immunoreactivity in the hippocampus of an Alzheimer’s disease patient negative for pSer129 αSyn inclusions. g. Immunohistochemical staining of the DLB hippocampus from panel a using antibody EP1536Y against pSer129 αSyn; extensive astrocytic inclusions are not seen using this antibody. h. Immunohistochemical staining of the MSA cerebellum from panel e using antibody EP1536Y against pSer129 αSyn; 3H11 detects GCIs similarly to this antibody but no astrocytic staining is seen using either. i. A western blot of 200 ng recombinant αSyn proteins harboring various familial mutations probed with antibody 94–3A10 (residues 130–140) [45]. j. A western blot using the same proteins from panel G but probed with antibody 3H11; the antigenic region is residues ~47–55. k. Western blot showing that 3H11 specifically recognizes human αSyn but not βSyn or γSyn. Scale Bar: 100μm (A, D, E, F, G, H), 50μm (C).
αSyn was originally identified from an immunosera raised against purified cholinergic vesicles of the Torpedo electric organ and was also detected in the nucleus [101], resulting in the portmanteau designation of ‘synuclein’ (protein present in the synapse and nucleus). αSyn has been shown to exist as an inherently disordered monomer [161] or more controversially, as tetramers [15]. The N terminus of αSyn forms amphipathic α helix structure, whereas the highly acidic C terminus, which exists as a random coil, stabilizes the structure by transiently interacting with the N terminus [18, 55]. The non-amyloid β component (NAC) domain found in the middle of the protein is hydrophobic and is required for αSyn to polymerize into amyloid fibrils [89]. The N terminus has a unique repeated motif of seven 11 amino acid residues and all the known PD-associated mutations (A30P, E46K, H50Q, A53E, A53T, G51D) tend to cluster in this domain. This motif is key to interaction between αSyn and membranes. The physiological function of αSyn remains highly debated, though several studies have shown that it plays a highly diverse role in the CNS - synaptic vesicle trafficking [116], lipid metabolism and membrane remodeling [115, 172], synaptic plasticity [59], molecular chaperoning [129] and mitochondrial membrane remodeling [111]. The natively unfolded structure of αSyn is determined by its low hydrophobicity and high net charge (pI of 4.7 at neutral pH). Destabilization of the native structure, possibly through post-translational modification or interactions with proteins or cell membranes, induces formation of insoluble oligomers or fibrils with highly ordered β-sheet conformation [62]. Phosphorylation at Serine 129 and C terminal truncation products have both been shown to be common species in insoluble LB, LN and GCIs [5] and though controversial, some studies have shown that phosphorylation at Ser129 and C-terminal truncation can modulate αSyn fibrillization [54, 147].
Clinical research suggests that the predilection for astrocytic αSyn buildup in the temporal lobe of DLB and advanced PD patients may play a role in the symptomatic progression of these diseases and warrants further study to understand associated functional impairments [22]. It is generally believed that specific clinico-pathologic outcomes, representative of the heterogeneity observed in α-synucleinopathies, may be traced to the involvement of neuronal as well as glial αSyn pathology [119, 121, 122]. The biological mechanisms that trigger the normally soluble protein αSyn to aggregate into pathological inclusions, such as GCI, LB and LN, remains highly debated. Factors relating to the cellular environment, such as autophagic impairment, mitochondrial dysfunction, vesicular trafficking, oxidative stress, ER stress and inflammatory stress as well as factors related to αSyn metabolism, such as post translational modifications (nitrosylation or proteolytic processing) may promote αSyn aggregation in neurons [26, 60, 82, 94, 109, 137, 147]. On the other hand, the provenance of astrocyte-resident αSyn remains unclear as αSyn is expressed predominantly from neurons in both human and mice. It is thought that astrocytes accumulate αSyn by scavenging extracellular αSyn released from the neurons through an active process or following the demise of neurons containing these pathological αSyn inclusions [93, 97, 130]. In some cases, astrocytes containing αSyn inclusions are found distal to extracellular LBs or dead neurons and sometimes even outnumber LB positive neurons, which may also suggest a de novo mechanism [22]. Observations in experimental animal models have also suggested that distinct αSyn conformers formed in the neurons are capable of templating normal soluble αSyn through a prion-like process [165]. Following a prion-like seeding process, pathological αSyn conformers can potentially be passaged through inter-neuronal or even neuroastroglial connections and result in astrocytic accumulation of pathologic αSyn [1, 98]. However, overall very little mechanistic details are known regarding the role of these ubiquitous cells in the pathogenesis of α-synucleinopathies. To understand whether astrocytes play a beneficial or detrimental role, we examined the literature on interaction between pathologic αSyn and astrocytes in the context of cellular models, in vivo experimentation, and human disease. In the next sections, we will discuss how αSyn broadly affects the immune system and proceed to understanding the specific ways that αSyn accumulation alters astrocyte function and CNS homeostasis.
αSyn activates the immune system
Innate immune activation is a cardinal neuropathological feature accompanying end-stage α-synucleinopathy in patients and preclinical models (reviewed in [4, 27]). Curiously, the association of viral infection with Parkinsonism symptoms in von Economo’s and postencephalitic PD patients spurred the idea that peripheral immune activation can trigger PD type neurodegeneration [128]. One of the earliest neuropathological demonstrations of CNS immune activation in PD patients showed increased HLA-DR (human leukocyte antigen DR isotype) positive reactive microglia [102], though PD patients often do not show typical hypertrophic reactive astrocytes [145]. Interestingly, as discussed in more details in later sections, while subcortical protoplasmic astrocytes accumulate αSyn in PD, MSA patients do not typically show αSyn immunoreactive astrocytes [145]. Later studies showed that PD patients show elevated CSF levels of inflammatory cytokines such as IL-1β and IL-6 [19]. More recent studies have used cutting edge imaging and immunophenotyping studies to explore the link between inflammation and α-synucleinopathies. DLB patients with mild impairment displayed increased gliosis (using [(11)C]PK11195 PET tracer) in specific areas of the brain such as the caudate nucleus and cuneus compared to patients with more severe impairment, suggesting that inflammatory changes may occur in the early stages of disease [153]. Concomitant with CNS changes, these patients showed elevated peripheral blood inflammatory cytokines, such as MIP-3, IL-17A and IL-2 [153]. This is consistent with a recent seminal demonstration that PD patients have a unique T cell signature [151]. This study identified IL5-producing CD4+ T cells and IFN-γ producing CD8+ cytotoxic T cells in PD patients that were reactive to native as well as protofibrillar forms of αSyn. Several genome wide association studies have unearthed a link between HLA locus (human leukocyte antigen locus encoding the major histocompatibility complex class II (MHC-II)) and PD, strongly linking immune response as a risk factor in α-synucleinopathies [34]. Together these results show a functional link between activation of innate and adaptive immunity and α-synucleinopathies.
As a typical damage associated molecular pattern (DAMP) containing moiety, αSyn aggregates or conformers has been shown to directly interact with astrocytes and microglia via pattern recognition receptors, including Toll like receptor (TLR) 4 in cell culture [35, 93, 127, 179]. While αSyn aggregates have been specifically identified in hypertrophic ‘activated’ astrocytes in patient brains, whether the astrocytic inclusions themselves directly compromise these cells and trigger further cell- and non-cell autonomous damage and intercellular αSyn transmission is hotly debated. αSyn induced chronic astrocyte activation can also lead to bystander pathologies, such as increased cytokine and chemokine expression, antigen presentation, Ca2+ flux and oxidative stress that can ultimately lead to maladaptive neuronal activity and neurodegeneration by non-cell autonomous mechanisms [6, 35, 93].
Based on neuropathological findings in limited cohort of PD patients [14, 24, 63, 170] and the abundance of non-motor prodromal symptoms in PD patients [12], the gut-brain axis in PD was proposed which hypothesizes that initial αSyn aggregation can occur in the myenteric plexus of the gut (and other mucosal surfaces) which then propagates along vagal projections to the brain in a prion-like manner. There is indirect evidence of the involvement of peripheral immunity and the gut microbiome composition in induction and propagation of PD pathologies (reviewed in [80]). Indeed, the composition of PD patient gut microbiome is different from age-matched controls [77, 138] and transplantation of PD patient gut microbiota in a mouse model enhances motor deficits [135]. This latter study also showed for the first time that altering the gut microbiome composition modifies αSyn pathophysiology in the CNS of mice [135]. Since αSyn is expressed in enteric neurons [16] and can be physiologically secreted via a conventional, endoplasmic reticulum/Golgi-dependent exocytosis in a neuronal activity-regulated manner [117], it is easy to envisage that αSyn present in the gut can promote gut inflammation and contribute to αSyn pathogenesis along the gut-brain axis. However, it remains to be seen whether astrocytes (or other immune cells) in the enteric plexus play additional role in gut inflammation or the induction and spread of αSyn pathologies along the gut brain axis.
Recent advances in single cell transcriptomics and mathematical modeling have revealed regional, functional and disease state-specific heterogeneity in brain resident astrocytes [78], suggesting that astrocyte diversity in different brain regions may also affect neural circuits differentially depending on the spatio-temporal context of the underlying αSyn pathology. Interestingly, identification of MHCI in a subset of neurons also infers that the neurons may not be immune privileged after all and may dynamically interact with alterations in glial cell function [42]. Thus it is possible that a specific combination of the cellular context [121], immune milieu [176] and/or αSyn conformers [118] will determine region-specific immune response including astrocyte function [154]. This complex inter-relationship between innate immunity, peripheral immunity and dysfunctional CNS proteostasis can likely alter disease progression and the phenotypic manifestation in different α-synucleinopathies. A mechanistic knowledge of the interaction between cell autonomous (intraneuronal protein aggregation and related sequela) and non-cell autonomous (inflammation and glial response to αSyn accumulation) pathways in α-synucleinopathies can lead to potential disease modifying immunobiotherapies.
While there are excellent reviews that capture this complex interaction of immunity in α-synucleinopathies [4, 27], very little is known about how astrocytes specifically are involved in this process. In the next sections, we focus specifically on the role of astrocytes in the pathogenesis of α-synucleinopathies by examining the literature on interaction between pathologic αSyn and astrocytes.
Astrocytes: role in homeostasis and diseased brain
Astrocytes are the most numerous cell type in the brain; these “star” shaped cells use their thin processes to directly ensheath up to two million synapses each for the purpose of maintaining synaptic function [3]. At the synapse, astrocytes shuttle ions, neurotransmitters, and other species across their membranes to ensure optimal conditions for neurotransmission. Their additional functions include protection against metabolic and oxidative stress, nutrient transport across the blood brain barrier, production of a quasi-lymphatic system draining waste into the CSF, resolution of neural injury via glial scar formation, and even higher executive functions related to synaptic plasticity [47, 166]. Impairment of these processes is evident in post mortem brains of neurodegenerative proteinopathies in general, with preclinical studies further suggesting that loss of astrocytic support may play a direct causal role in the neurodegeneration in these diseases [86, 120].
Astrocytes are a heterogenous group of cells that arise from neural stem cells controlled by complex series of developmental cues, much of which is still undetermined [107]. Broadly, there are two classes of astrocytes – protoplasmic astrocytes found in grey matter and fibrous astrocytes found in the white matter. There are other populations of astrocytes with specialized functions, for example, retinal Muller glia and cerebellar Bergman glia. In the context of α-synucleinopathies, the primary role of astrocytes in relieving oxidative stress, generating neurotrophic factors, modulating inflammation, and reducing pathogenic αSyn have been demonstrated in several preclinical studies, as we will discuss in details in the next sections. Developing the concept of immunoproteostasis, where immunity and αSyn protein homeostasis pathways are continually interacting with each other, we can envision a double-edged sword scenario where this interaction can result in harmful or beneficial outcomes. Further, it can be argued that intraneuronal Lewy aggregates are relatively harmless [88] and is a simple measure of cellular response to proteostasis imbalance [39] whereas the inappropriate astrocyte response to these dysfunctional neurons is what drives the inexorable pathological sequela including synaptic failure, neuronal death and clinical symptoms. This is consistent with recently described, though controversial, paradigm called gliotransmission by which neuronal activity induces astrocytes to secrete neuroactive substances into the synaptic cleft that might ultimately regulate brain organ health. It is also tempting to speculate that the subcellular localization of αSyn aggregates within astrocytes, compared to intraneuronal αSyn found within LBs and GCIs, may result in a new form of αSyn with unique properties such as different aggregation potential, prion-like conformational templating, and toxicity. Such unique forms or αSyn ‘strains’ are increasingly being recognized as important determinants of disease pathogenesis [118, 121]. Another factor to consider is that the inherent heterogeneity in astrocytes (regional, developmental and functional; [78]) may play a role in generation of different αSyn strains, leading to specific disease progression scenarios in the brain. In the next sections, we will discuss the implications of these hypotheses and further introduce some recent paradigms that can have critical impact on therapeutic strategies.
Astrocytic αSyn inclusions are distinct from intraneuronal Lewy pathology
Aggregated αSyn have been reported in astrocytes of PD, DLB and MSA patients [79, 145] (Figure 1). On the whole, post-mortem studies show that the distribution of astrocyte αSyn pathology closely mirrors the appearance of intraneuronal LB pathology in the temporal and insular cortex in PD and DLB patients [22, 52]. In PD and DLB patients, most of the aggregated αSyn is observed in astrocytes in the white matter and in Bergman glia [110, 124] while some are noted in grey matter areas such as the temporal lobe, limbic areas, cortex and substantia nigra (Table 1). These astrocytic αSyn inclusions are characteristic of PD and DLB and rarely observed in MSA while being absent in other dementias and healthy controls (Table 1). Some studies have reported at least 60–100+ αSyn positive astrocytes within a single unilateral section from the midbrain or cortex of PD/DLB or other α-synucleinopathy patients, which was comparable to the levels of intraneuronal LB pathology within the same field of interest [66, 160, 168, 177]. The presence of αSyn positive astrocytes largely paralleled both the location and severity of neuronal LBs within the cortex or midbrain as noted by 5 studies examining LBD or PD cases [22, 66, 141, 145, 168]. In many cases, these astrocytes do not show hypertrophic morphology as would be expected in a typical reactive state, but instead are characterized as having many fine radiating processes [145, 159, 160, 168, 169]. In comparison with MSA where astrocytic αSyn is sparse but there is widespread gliosis [74], the presence of αSyn laden but non-reactive astrocytes in LBD/PD may suggest that the accumulated αSyn is impairing glial response. These astrocytic αSyn inclusions are ultrastructurally different from the typically densely packed neuronal LB pathology; astrocytic αSyn is diffusely distributed through the cell body and processes, with granular staining pattern reminiscent of vesicles or lysosomes [22]. Moreover, unlike the neighboring neurons with LBs that are readily characterized by H&E histology, silver staining or phosphorylated Serine 129 (pSer129)-αSyn immunoreactivity, astrocytic LBs do not share these histological properties. Indeed, the most critical roadblock in these post-mortem analysis appears to be accessing and identifying the specific αSyn species, especially when using formalin-fixed and paraffin-embedded human brain sections. A clear breakthrough was achieved when using antibodies to the central domain of αSyn, several groups could successfully demonstrate αSyn inclusions in astrocytes of PD/DLB patients (Table 1) [2, 7, 22, 65, 87, 110, 125, 143, 147, 159, 162, 163, 165, 171, 172, 181]. In addition, because of epitopes being potentially masked or modified in fixed brain tissues, astrocytic αSyn can be visualized only after using harsh antigen retrieval methods. Several studies used formic acid pretreatment to uncover epitopes in the central portion of αSyn (including the NAC domain) that seem to be specifically present in astrocytic αSyn inclusions in PD/DLB patients but absent in MSA patients or controls (Table 1) [22, 66, 87, 93, 141, 145, 156, 160, 177]. These same studies observed that there was little to no labeling of astrocytic αSyn when N or C-terminal antibodies against αSyn were used, or when formic acid pretreatment was excluded [22, 87, 156, 160, 177]. This pattern was not seen with neuronal αSyn aggregates where all antibodies seemed to detect LBs equally [22, 86, 156, 160, 177]. Further, while neuronal LBs are positive for Gallyas-Braak silver staining, astrocytic αSyn inclusions were reported to be reactive to Gallyas-Braak silver only in a few studies [159, 160, 168, 169] (Table 1). In addition to being unreactive to N- and C-terminal αSyn antibodies, astrocytic αSyn does not colocalize with p62/SQSTM1 or ubiquitin, as is commonly observed with neuronal LBs [22]. Collectively, these data suggest that astrocyte-resident αSyn may have unique ultrastructure and have extensive modifications, including post-translational modifications and truncations. Such unique structural properties of astrocytic αSyn can have consequences relating to αSyn aggregation and prion-like transmission properties.
Table 1.
Astrocytic αSyn inclusions: a brief review of literature.
| # subjects with astrocyte inclusions (total #) |
Location of astrocyte inclusions |
Antigen Retrieval |
αSyn antibody (astrocyte labeling) |
Comments | Ref |
|---|---|---|---|---|---|
| 24 (30) PD 0 (7) iLBD 0 (30) control |
13/24 SNpc 24/24 midbrain/pontine tegmentum |
NA | Polyclonal αSyn (114–131) (+) |
|
168 |
| 8 (8) DLB | 8/8 temporal lobe 8/8 frontal cortex 7/8 basal ganglia 6/8 midbrain/pontine tegmentum 5/8 SNpc |
FA | αSyn NAC 60–75 (+) αSyn N terminal 1–15 (−) αSyn C terminal 108–122 (↔) |
|
160 |
| x (7) PD x (5) DLB 0 (6) MSA 0 (25) other disease 0 (15) control |
PD, DLB: cerebral cortex, basal ganglia | FA | αSyn N terminal 1–15 (+) αSyn NAC 60–75 (+) αSyn 110–140 (+) |
|
141 |
| 9 (9) LBVAD 2 (2) DLB 1(1) PD 0 (17) other disease 0 (4) control |
PD, DLB, LBVAD (distinction not made): neocortex, hippocampus | FA | αSyn N terminal 1–9 (−) αSyn NAC 61–75 (+) αSyn C terminal (101–110&131–140) (−) |
|
156 |
| 10 (10) PD 0 (2) iLBD 0 (5) control |
10/10 striatum 10/10 SNpc 10/10 midbrain and pontine tegmentum 8/10 cingulate and other cortex |
HCl | Polyclonal αSyn full-length (+) |
|
66 |
| 14 (14) PD 0 (6) control |
14/14 cortex 12/14 striatum 11/14 thalamus |
FA | αSyn NAC 91–99 (+) αSyn C terminal 116–131 (−) |
|
22 |
| 0 (10) PD 0 (8) DLB 0 (9) other disease 0 (3) control |
N/A | NA | αSyn N terminal 1–15 (−) |
|
7 |
| 17 (20) PD 0 (26) other disease 0 (30) control |
8/17 SNpc 17/17 other midbrain region |
NA | No antibodies used |
|
169 |
| 6 (15) MSA 0 (20) DLB 0 (20) control |
3/6 periventricular astrocytes in cerebrum 6/6 subpial astrocytes in midbrain, brainstem 1/6 bergmann glia in cerebellum |
NA | αSyn pSer129 (+) |
|
110 |
| 1 (1) PD (fetal transplant) | Within fetal transplant | NA | αSyn pSer129 (↔) |
|
2 |
| 6 (8) DNTC 0 (6) control |
6/6 temporal lobe most densely within entorhinal cortex | FA | αSyn N terminal 1–15 (−) αSyn NAC 60–75 (+) αSyn 108–122 (−) |
|
177 |
| 4 (6) PD 5 (7) DLB 3 (7) MSA 0 (5) control |
PD, DLB, MSA: 12/12 bergmann glia molecular layer cerebellum | NA | αSyn C terminal 114–131 (+)* αSyn full-length (+)* |
|
124 |
| x (34) PD/DLB x (14) MSA 0 (x) Control |
PD,DLB: Temporal lobe, cingulate cortex | FA | αSyn N terminal 46–53 (+) |
|
87 |
| 13 (13) PD 0 (29) MSA 0 (44) other disease 0 (13) control |
13/13 subcortical grey matter | FA | αSyn 15–123 (+) αSyn full-length (+) |
|
145 |
Note: FA, Formic Acid; +, positive staining; -, negative staining; ↔, weak staining;
*, labeled Bergman Glia only; x, n number not specified; NA, information not available. Additional studies have noted glial reactivity for αSyn in PD/DLB, however the lack of differentiation between microglia, astrocytes, and oligodendrocytes led to them being omitted from this Table.
Based on the cumulative findings of the aforementioned studies, we generated a monoclonal antibody raised against the central portion of αSyn (antibody 3H11; residues 43–63 of αSyn abutting the NAC) and used it to demonstrate robust astrocyte-resident αSyn pathology in two LBD cases following antigen retrieval using formic acid (Figure 1a–c). In these LBD cases, dense astrocytic αSyn was particularly prominent in limbic structures of the temporal lobe such as the hippocampus and amygdala, where double labeling using the GFAP antibody with the 3H11 antibody confirmed the finding (Fig. 1c). This astrocytic αSyn is thought to be pathologic in nature, as no astrocytic αSyn staining was detected in the hippocampus of an Alzheimer’s case (Figure 1f). Interestingly, by immunoblotting, 3H11 antibody recognized different PD-associated αSyn mutants differentially, indicating that some of these mutations disrupts the 3H11 epitope in αSyn (Fig. 1g–i). We observed αSyn reactive astrocytes not only in the direct vicinity of cortical LBs, but even extending further into the parenchyma where the majority of αSyn reactive cells appear to be astrocytes (Figure 1a). Whether astrocytic αSyn accumulation precedes the development of cortical LBs is still unknown but knowledge about the temporal aspect of cellular vulnerability to pathological αSyn will inform us on the etiology of α-synucleinopathies, including the prion-like properties of αSyn. Whether 3H11-immunoreactive astrocyte inclusions in the enteric or central nervous system track with the Braak pathological staging [23] will need to be empirically determined to understand how astrocytes contribute to αSyn pathogenesis. One feasible theory is that astrocytes initially function as scavengers of extracellular pathologic αSyn preceding the formation of neuronal LBs. It is possible that astrocytes containing these pathologic αSyn, having processes extending to hundreds of thousands of synapses, can subsequently contribute to spreading of αSyn fibrils themselves. This will be elaborated in subsequent sections.
In rodent transgenic models of α-synucleinopathy, αSyn immunoreactivity has been readily observed in neurons as well as astrocytes [82, 132, 133, 146, 148] (Figure 2). These astrocytic inclusions even occur when αSyn is expressed under a neuronal promoter ((Thy-1)-human [A30P] αSyn), suggesting that αSyn can probably be internalized by astrocytes and subsequently accumulate [97]. An important difference between these mouse models and human disease is that full-length aggregated αSyn is detectable in mouse astrocytes even without formic acid pretreatment, although it remains to be seen whether different pre-treatment(s) would uncover additional novel pathologies in mice. Therefore, these transgenic mouse models likely represent a mix of features from both PD/DLB and MSA, but overall, they may prove useful in investigating differential evolution of inclusions in astrocytes compared with neurons.
Figure 2. Robust astrogliosis and astroglial αSyn accumulation in transgenic mouse models of α-synucleinopathy.
a. Immunofluorescent detection of GFAP in spinal sections from M83+/− transgenic mice overexpressing human [A53T] αSyn that were intramuscularly injected with preformed wild type mouse αSyn fibrils. In this model, pSer129-αSyn pathology and motor neuron death is apparent at 2 months post injection whereas increase in GFAP, representative of astrocyte activation, is observed at later time points. b. Immunoflourescent detection of GFAP (green) and pSer 129 αSyn (red) in the spine of terminal M83+/− transgenic mice described in panel a; in regions of neuronal death, astrocytes closely interact with extracellular aggregated αSyn. c. Immunoflourescent detection of GFAP (green) and pSer 129-αSyn (red) in the midbrain of M20 transgenic mice overexpressing human αSyn that were seeded with preformed αSyn fibrils in the striatum showing the presence of abundant astrocytic αSyn pathology. Scale Bar: 500μm (B), 100μm (C).
Astrocytic αSyn pathology: questions on its origin and fate
The etiology and functional consequence of astrocytic αSyn remains unresolved. The field is generally constrained by a lack of systems level insights into neuronal function and cell type specific gene expression patterns that can shed light into astrocyte-mediated functional impairment, neurodegeneration and symptomatic progression of PD/DLB. A critical question in this context is whether astrocytes facilitate removal of toxic αSyn by phagocytosis [53] or do astrocytes directly contribute to the increasing neuronal proteostasis via uptake and subsequent prion-like propagation of the phagocytosed pathologic material? The latter premise that astrocytes can facilitate αSyn transmission comes from a transgenic mouse line that overexpresses human A53T αSyn from the astrocyte-specific GFAP promoter (GFAP-tTA/tetO-α-syn mice). In these mice, diffuse intraneuronal LBs are observed throughout the CNS suggesting that astrocyte to neuronal spread of pathologic αSyn had taken place [64]. Some plausible theories behind the origin of astrocytic αSyn pathology are 1) phagocytic or pinocytotic uptake of extracellular αSyn, 2) direct transfer from neurons through an active process, such as tunneling nanotubes or exosomal transfer or 3) de novo induction of αSyn pathology during pathogenesis. In this section, we will discuss the literal yin-yang of astrocyte scavenger function vis-à-vis its role in propagation of αSyn pathology.
Several in vitro studies have reported that astrocytes can rapidly ingest extracellular αSyn, within minutes of being exposed [25, 33, 83, 97]. All of these studies concluded that astrocytes internalize pathologic αSyn more readily than neurons, suggesting that astrocytes are primed to scavenge DAMPs, such as αSyn conformers. In vivo evidence of this phenomenon was reported in mice where overexpression of αSyn under neuronal Thy-1 or PDGFβ promoters (A30P αSyn and wild type αSyn respectively) resulted in increased astrocytic αSyn, presumably following endocytosis from extracellular sources [93, 97]. Following endocytosis, the αSyn can be subsequently trafficked to the lysosomes [93, 97]. The process of αSyn internalization and cellular trafficking is thought to be different between neurons, microglia and astrocytes, which suggests that this might potentially lead to formation of unique αSyn conformers within different cell types. Neurons can internalize αSyn by interacting with cell surface heparin sulphates [67] or specific receptors such as Lag3 [100] or Na+/K+ transporting ATPase subunit α3 [142], following which it can be either transported along microtubules as naked proteins or inside vesicles into the lysosomes. Microglia can engulf αSyn using TLR4 [53], resulting in NFκB activation, release of pro-inflammatory cytokines and production of reactive oxygen species. Astrocytes, however, do not seem to require TLR4 for αSyn phagocytosis [53]. Further, astrocytes seem to process endocytosed αSyn differentially than neurons, as shown by a unique interactome revealed by mass spectrometry [143] and presence of partial breakdown products of αSyn that are reactive specifically to αSyn central domain antibodies (Figure 1, Table 1). It is possible that extracellular αSyn is cleaved by matrix metalloprotease MMP3 before astrocytic phagocytosis or processed by lysosomal cathepsins after ingestion, leading to generation of truncated species [125, 152]. In vitro studies have shown αSyn aggregation follows different kinetics at normal pH (7.4) and at acidic pH (typically found in lysosomes and endosomes). At pH values below 6, αSyn aggregation is predominantly triggered via secondary nucleation changes (such as fragmentation and surface-assisted nucleation) rather than a simple elongation process [30]. Such secondary nucleation processes can lead to faster multiplication of aggregates and presumably aggravate disease pathogenesis. Therefore, endosomal trafficking of αSyn may result in an exacerbated neurotoxic phenotype since C-terminal truncated αSyn is known to fibrillize more rapidly than full length variants and cause more cytotoxicity [147].
Astrocytes and neurons are capable of undertaking bidirectional or unidirectional inter-cellular transport of αSyn through various mechanisms [167]. Both in vitro and in vivo studies have shown the presence of tunneling nanotubes (TNT) that can transfer αSyn between neurons and astrocytes [1, 46, 130]. TNTs are thin (50 – 200 nm in diameter), actin-rich membranous channels that can connect the cytoplasms of cells as far apart as 100 μm. Lysosomal resident αSyn can traverse between neurons [1] or more intriguingly, stressed astrocytes with defective lysosomal function can barter ‘undigested’ αSyn with a healthy astrocyte in exchange for healthy functional mitochondria [130]. This could result in a double-edged sword scenario: infection of healthy astrocyte with potentially toxic αSyn conformers leading to prion-like transmission and functional recuperation of αSyn-damaged astrocytes with fresh mitochondria. Another mode of intercellular communication is through exosomes. Exosomes are small (40–100nm) vesicles that originate from multivesicular bodies of the endocytic pathway of neurons, astrocytes and microglia and are thought to be involved in clearance of cell-derived debris [29]. αSyn carrying exosomes can readily cause neuronal death in vitro [50]. Recent data also shows that brain-derived exosomes from DLB patients contain pathologic αSyn that can be internalized by both neurons and astrocytes leading to increased pathogenicity in mouse brains [112]. Interestingly, exosomal αSyn release is not reduced by blocking ER-Golgi transport mechanism, suggesting that αSyn containing exosomes can be extruded through non-conventional pathways [65, 71], such as exosome-associated exocytosis and/or exophagy [49]. Thus, so far, the data suggests that uptake of exosomes or vesicular αSyn by astrocytes can potentially have detrimental effects on disease pathogenesis in α-synucleinopathies by allowing transcellular propagation of pathologic αSyn and altering astrocyte homeostasis.
Among these theories, the last one, i.e., reactive de novo aggregation of αSyn is not fully supported by direct experimental evidence in vivo. αSyn immunoreactive protoplasmic astrocytes have been found in abundance in human brain regions typically devoid of LB, such as striatum and dorsal thalamus [22] which anecdotally supports this theory. However, the amount of endogenous αSyn protein that is typically expressed by glia is far less than what would be expected to be needed for successful de novo templated aggregation and transmission of pathology. In the healthy brain, αSyn is detectable in white matter astrocytes following immunohistochemical staining; however, this occurs only at very low levels and the tissue must be extensively pre-treated [108]. Immuno-EM studies have shown that the subcellular distribution of αSyn in these astrocytes tend to be diffuse throughout the cell body and processes and remain in association with mitochondrial membranes, ribosomes, and small vesicles [108]. In vitro evidence from astrocytic cell lines showed that only small amounts of αSyn can be detected by both mRNA and protein assays [158]. Interestingly, later studies have shown a clear association between upregulation of synuclein levels and astrocyte activation status, at least in vitro. For example, αSyn is upregulated in cultured astrocytes when exposed to inflammatory cytokines [158] or during oxidative stress [37]. Therefore, although upregulation of endogenous astrocytic αSyn may contribute to disease, it is more likely that the majority of αSyn found within astrocytes in the diseased brain is mostly derived from neuronal origins.
Functional consequences of astrocytic αSyn: lessons from in vitro studies and transgenic animal models
The belief that astrogliosis is a passive bystander response to increasing intraneuronal αSyn proteostasis and the neurodegenerative cascade is now under debate. Recent data showing that astrocytes can actively communicate with neurons and alter neuronal plasticity and homeostasis has been critical in establishing the hypothesis that astrocytes may be more directly involved in αSyn pathogenesis [61].
The function of endogenous αSyn within normal astrocytes is unclear, although there is some evidence that αSyn is involved in astrocytic fatty acid metabolism [32]. However, later studies have shown a clear association between upregulation of synuclein levels and astrocyte activation status, at least in vitro [161]. Although upregulation of endogenous astrocytic αSyn may contribute to disease, it is more likely that the majority of αSyn found within astrocytes in the diseased brain is derived from neuronal origins, as has been discussed in the previous section. Studies in PD brains have shown that midbrain astrocytes undergo apoptosis in addition to nigral neurodegeneration [73, 85, 106], suggesting that non-cell autonomous events have a profound effect on the neurodegenerative cascade. In cultures treated with pathologic αSyn or human brain-derived LBs, astrocytes display morphological changes consistent with activation, as well as mitochondrial fragmentation, autophagic impairment, impaired Ca2+ flux, sensitization to oxidative stress and death [6, 25, 33, 64, 83, 97, 104, 149]. The mechanism by which aggregated αSyn induces toxicity in astrocytes appears to be through a combination of mitochondrial dysfunction and impaired autophagy and mitophagy leading to oxidative stress and apoptosis, as demonstrated by in-vitro studies (Figure 3) [25, 33, 51, 97]. It is possible that several familial PD risk genes that lead to Parkinsonism without necessarily causing αSyn accumulation have important roles in mitochondrial function and oxidative stress, suggestive of commonalities in etiology of Parkinsonian syndromes (Figure 3).
Figure 3. αSyn mediated alterations in neuro-glial homeostasis in health and disease.
Accumulation of αSyn leads to Lewy pathology through a variety of cellular processes. In neurons and astrocytes, αSyn has been implicated in dysfunction of lysosomes (LYS) and mitochondria (MITO), which enhances ROS production and toxic sequela. Neuronal αSyn can be transported into neighboring astrocytes via tunneling nanotubes (TNT), receptors such as Toll like receptors (TLR), or several indirect methods such as internalization of exocytosed material from the synaptic space. We hypothesize that astrocytic lysosomes preferentially cleave full length αSyn into smaller toxic truncation products that might have additional pathological functions with astrocytes. Toxic accumulation of αSyn within astrocytes may damage their normal functions and have adverse results such as synaptic accumulation of neurotransmitters including glutamate and dopamine (DA) that are in part cleared by astrocytes through EAAT2 and DAT/NET respectively. Astrocytes normally protect against oxidative stress in the CNS through production of multiple products including ARE factors, KEAP1/NFR2, Glutathione, NURR1 and NQO1; loss of these factors due to toxic accumulation of αSyn may contribute to neurodegeneration. Astrocytic uptake of αSyn and oxidative stress may impinge on the NF-κB pathways that can result in increased inflammatory cytokines, such as IL-6 or TNFα that will affect the function of neighboring microglia or peripheral immune cells which represents another modality by which astrocytes may contribute to neurodegeneration. Additionally, common risk factor genes such as DJ-1, Parkin and Pink1 are highly expressed in astrocytes and their dysfunction may affect mitophagy in a parallel pathway to that of toxic αSyn resulting in similar degenerative features. Red arrows denote pathological processes, green arrows denote putative therapies or beneficial pathways and black arrows denote normal physiological processes.
In animal models, neuronal overexpression of human αSyn or injection of human brain materials leads to astrocyte activation, as evident by increased GFAP, S100β or vimentin immunoreactivity (reviewed in [84]). In a peripheral to central transmission model of αSyn pathology expressing human [A53T] αSyn from prion promoter (Line M83; B6;C3-Tg(Prnp-SNCA*A53T)83Vle/J), we observed motor neuron death and pSer129-αSyn pathology prior to the appearance of astrogliosis (Figure 2a) [132, 148] as in human PD where astrocytic hyperactivation usually follows nigral degeneration [163]. Intriguingly, in Line M83 mice, we observed widespread nigral astrocytic pSer129-αSyn pathology while sparing the dopaminergic neurons following intracranial seeding of αSyn aggregates (Figure 2c). Therefore, the relationship between astrocytic αSyn toxicity and neuronal dysfunction remains unclear. This aspect was also investigated in transgenic mice expressing αSyn exclusively from astrocytes. In an inducible transgenic mouse model expressing human [A53T] αSyn specifically from astrocytes (GFAP-tTA/tetO-α-syn line), extensive astrocytic death, mitochondrial dysfunction and dopaminergic neurodegeneration was observed [64]. However, another transgenic model with dramatic levels of astrocytic expression of αSyn (human [A30P+A53T] αSyn driven by chicken β actin promoter; BAsyn/PaKO line) did not show overt neurodegeneration in the substantia nigra but had profound motor deficits, suggesting that impairment of astrocytes can lead to neuronal dysfunction even without overt cell death [104]. Interestingly, a specific subtype of astrocyte, A1 astrocyte, has been identified in the nigra of PD patients [95] and a prion promoter driven human [A53T] αSyn transgenic mouse model (C57BL/6; Prnp-SNCA*A53T [182]) that has been postulated to drive neurodegenerative cascade in α-synucleinopathies. These A1 astrocytes are devoid of their usual ability to promote neuronal outgrowth, synaptogenesis and phagocytosis, and further can lead to death of neurons and oligodendrocytes. In addition, blocking the formation of these reactive astrocytes leads to elongation of life in the human [A53T] αSyn mouse model of α-synucleinopathy [182]. Overall, though the A1/A2 characterization of astrocyte functionality has provided a simplistic and convenient platform for initiating our understanding into astrocyte involvement in α-synucleinopathies, future transcriptomic and functional studies should be conducted to reveal physiologically-relevant disease-associated profiles that can shed light on astrocyte heterogeneity and selective vulnerability in α-synucleinopathies.
Recent work has illuminated that one of the pathways underlying the stereotypical spread of αSyn pathology is by prion-like transmission of αSyn in transgenic mouse models of α-synucleinopathy injected with αSyn fibrils or disease associated brain extracts (reviewed in [165]). While some groups reported mostly intraneuronal αSyn pathology during this transmission process in human [A53T] αSyn transgenic mice [99], other groups including ours reported robust astrocytic αSyn pathology in these mice suggesting that astrocytes can also contribute to αSyn transmission [131, 146, 148]. Interestingly, we observed that the majority of the pathological αSyn in the substantia nigra of these mice were localized to astrocytes, and none to dopaminergic neurons (Figure 2C). This might imply that astrocytes can modify disease pathogenesis in this transmission model either by scavenging and having a neuroprotective function or as an unwitting accomplice in αSyn pathogenesis. Other groups have shown that astrocytes can take up these αSyn assemblies and direct these to lysosomes for degradation in organotypic slice cultures from wild type mice [98], though chronic uptake may also lead to cellular damage and inclusion formation in primary cultures [97]. Importantly, whether astrocyte resident pathological αSyn has unique strain-like properties distinct from neuronal αSyn inclusions, as observed in primary astrocytes in vitro [131], remains a matter of debate [103].
Astrocytes can protect dopaminergic neurons by improving mitochondrial function [48] and simultaneously can also regulate αSyn pathogenesis in the synaptic clefts [97]. Additionally, astrocytes participate in dopamine metabolism through uptake from the synaptic cleft [157] and impairment of this process may lead to increased cellular stress through buildup of reactive dopamine products [105, 173]. However, whether astrocytic processes can potentially modulate clinical symptoms by regulating formation and clearance of dopamine-αSyn adducts or αSyn induced dopamine oxidation in the extrasynaptic space, remains to be investigated.
Cumulatively, the current evidence suggests that various pathologic forms of αSyn are toxic to astrocytes through a pathway incorporating impaired autophagy and resulting mitochondrial dysfunction from what may be mitophagy dysregulation [51]. Overall, an idea that is supported by clinical and preclinical evidence is that pathologically modified forms of αSyn may slowly accumulate in astrocytes following passive uptake or through some form of extrusion from neurons. Ultimately, this might reach a threshold where astrocytes are too impaired to respond to toxic build-up of LB pathology. One might speculate that αSyn overload of astrocytes would theoretically result in the loss of the preferential uptake of αSyn by astrocytes causing a shift towards trans-synaptic propagation and microglial involvement which would lead to dysfunctional immune function and worsening of disease.
Gliotransmission in α-synucleinopathies: possible co-morbid factor in αSyn pathogenesis
Metabolic exchanges across the astrocyte-neuron synapses or tripartite synapses are critical in maintaining CNS homeostasis [123]. Astrocytes are responsible for scavenging glutamate from the synaptic cleft as well as supplying lactate for neuronal activity through a process called gliotransmission. αSyn, being an abundant pre-synaptic protein, can potentially alter the function of the tripartite synapse formed between astrocytes and the pre- and post-synaptic densities. Recent studies have reported toxic gain of function of αSyn at the synapse by redistribution of SNARE protein, affecting dopamine transporter function and increasing glutamate excitotoxicity [17, 58, 136]. Overexpression of human [A53T] αSyn in the GFAP-tTA/tetO-α-syn transgenic mice led to reduced glutamate transporter expression from the astrocytes, suggesting a direct role of αSyn in neurometabolic coupling to astroglia [64]. Whether this altered gliotransmission plays a role in αSyn pathogenesis remains an intriguing premise that needs to be validated in preclinical mouse models, but could be a modality by which impaired astrocytes potentially contribute to clinical symptoms (Figure 3).
Astrocyte function can be altered by PD-associated genetic risk factors
Aside from SNCA, several other genes have been identified to be key risk factors in PD (reviewed in [21]). Among these, PARK2, PARK7, GBA, LRRK2, PINK1 and NR4A2 are expressed from mature astrocytes and unsurprisingly, most of these have been linked to neuroinflammatory response in preclinical models [21]. Therefore, it is possible that aside from disrupting neuronal function, these αSyn variants also compromise astrocyte homeostasis. Some of these gene variants do not necessarily cause αSyn pathology in all patients; however, by regulating mitophagy or redox function, these genes may cause generalized proteostasis imbalance or dysfunctional autophagic response in astrocytes leading to clinical symptoms reminiscent of Parkinsonism [21].
A common major cellular pathways affected by these genes is induction of oxidative stress causing widespread damage to cellular lipids, proteins, and DNA [41, 96, 114]. Indeed, accumulation of reactive oxygen species (ROS) can induce formation of TNTs [182], which has been shown by various groups to be conduits for αSyn propagation between astrocytes and neurons [1, 132, 146]. A homeostatic response of astrocytes to mitigate ROS is by a redox-sensitive decrease in KEAP1 mediated degradation of a transcriptional regulator, Nrf2. This induces expression of anti-oxidant response elements (ARE) leading to expression of detoxification and antioxidant enzymes such as glutathione, metallothioneins, NAD(P)H Quinone Dehydrogenase 1 (NQO1) and heme oxygenase. Several preclinical studies have shown a protective effect of Nrf2 on α-synucleinopathy [36, 57, 69, 72, 90, 171]. Interestingly, astrocytic Nrf2 overexpression rescues the phenotype and extends life span of human [A53T] αSyn transgenic mice [57]. This has spurred multiple studies testing the neuroprotective effects of Nrf2 activators as potential PD therapeutics [44, 56, 76]. Impairment of these protective functions of astrocytes by αSyn accumulation may contribute to neuronal demise, and restoration of them could represent avenues for therapeutic development (Figure 3).
DJ-1 (encoded by PARK7) that has been implicated in oxidative stress in PD [20, 155] as well as modulating αSyn aggregation and toxicity in preclinical models [140, 183]. DJ-1 is redox sensor of the cell and upon identifying ROS, it utilizes multiple defense mechanisms including direct neutralization of ROS, stabilization of Nrf2 and increased mitochondrial association [31, 40, 81]. Upregulating DJ-1 reduces αSyn pathology and rescues motor symptoms [181]. Additionally, DJ-1 can physically interact with αSyn to reduce its aggregation propensity [183]. Disease-associated variants of other astrocytic specific genes, such as PARK2, LRRK2 and GBA1, lead to increased αSyn pathology in patient-derived induced pluripotent stem cells [68, 113, 174], suggesting a close association between astrocyte homeostasis and αSyn pathogenesis. In spite of reports that PINK1 is absolutely critical for astrogliogenesis [38] and that it phenocopies PARKIN (encoded by PARK2) function [8], there are no direct reports associating PINK1 with αSyn pathogenesis.
NURR1/NR4A2, enriched in mouse astrocytes and human microglia, is essential for dopaminergic neuronal development. Mutations in this gene has been linked to familial late-onset PD [92]. The primary function of Nurr1 is to suppress NF-κB, leading to neuroprotection of dopaminergic neurons [134]. A second, more controversial, study showed that Nurr1 can also remediate αSyn toxicity in dopaminergic neurons by stimulating GDNF signaling [43]. Interestingly, Nurr1 was also shown to negatively regulate αSyn transcription [175], suggesting a common link between glial function and αSyn levels.
In PD and LBD, evidence of oxidative stress in areas of neuronal loss is seen in post mortem samples with increased lipid and protein peroxidation products, and altered expression of antioxidants within astrocytes (reviewed in [126]). Inadequate astrocytic production of glutathione, metallothionein 1/2, NQO1, DJ-1 and other ROS neutralizing factors in times of dopaminergic neuronal stress may contribute to their selective neurodegeneration in α-synucleinopathies. Coupled with the possibility that αSyn can induce astrocyte toxicity, the impairment of supportive functions may represent a new dimension for αSyn toxicity. Many of the PD risk genes discussed here function in ROS regulatory pathways within astrocytes, and their loss may herald the onset of Parkinsonism either synergistically with αSyn pathology or in a parallel pathway by causing functional impairment of astrocytes (Figure 3).
Immune based biotherapies in α-synucleinopathies
A major unmet need in α-synucleinopathies is the lack of disease modifying therapies. Clinical and neuropathological heterogeneity in the spectrum of α-synucleinopathies, unclear etiologies, as well as the absence of validated biomarkers presents critical challenges regarding the development of effective therapeutics. The two major broad therapeutic approaches that are being currently tested in patients are anti-αSyn immunotherapy (passive as well as active) and small molecule drugs that inhibit αSyn accumulation [28], neither of which directly target astrocyte or innate immune mediators. The fact that astrocytes accumulate αSyn aggregates and may be involved in transmission of α-synucleinopathy opens up possibilities that targeting these specific pathologic inclusions or attenuating their consequences through astroglia targeted therapies can have disease modifying effects. However, it is not yet clear how best to harness the immune system towards a beneficial outcome on astrocyte function. In this section, we will first describe the trophic factor therapies that have been used in patient trials, which among many factors, alters neuroinflammation but does not necessarily modulate astrocyte function directly.
In previous clinical trials, several neurotrophic factors (that are produced by astrocytes) were tested as neuroprotective pharmacotherapeutics [150]. Of these, glial cell line-derived neurotrophic factor (GDNF) and Neurturin (NRTN) were well-tolerated in patients but, unfortunately, did not meet predetermined end-points in efficacy. More recently, other neurotrophic factors that can be produced by astrocytes, such as cerebral dopamine neurotrophic factor (CDNF) [91] and mesencephalic astrocyte-derived neurotrophic factor (MANF) [180], have shown promise in preclinical models of α-synucleinopathy. Since the receptors and cellular signaling pathways for many of these neurotrophic factors are relatively unknown, more work needs to be done to forward these into the clinical realm.
Several preclinical strategies that work partly by targeting astrocytes have been recently described. Glucagon-like peptide-1 receptor (GLP1R) agonists have shown efficacy in multiple models of neurodegenerative proteinopathies by modulating mitochondrial biogenesis, suppressing microglial activation and inflammation, enhancing autophagy, and clearance of aggregated proteins. One such GLP1R agonist, NLY01, was shown to be neuroprotective in PD models by specifically regulating NF-κB mediated inflammatory signaling which is downstream of the GLP-1R/PI3K/AKT pathway [9]. More recently, NLY01 was effective in blocking microglia-mediated conversion of astrocytes to the neurotoxic A1 phenotype in a rodent model of α-synucleinopathy [178]. Indeed, a limited open-label trial and a subsequent double blind trial of the GLP1R agonist exenatide appeared to improve motor and cognitive function in PD patients [10, 11].
A direct line of preclinical research targeting astrocyte function was done by using midbrain derived astrocyte grafts into a rat model of PD. These astrocytes, following grafting into the striatum, resulted in remarkable recovery of dopaminergic neurons, especially in the presence of Nurr1 and Foxa2 co-expression. Through a paracrine pathway, these grafted astrocytes were found to exert marked trophic action on the glutamatergic and GABAergic neurons and stimulate endogenous dopaminergic neurons to secrete dopamine [144]. Such therapies have the potential to be the most effective, as instead of isolating one single astrocytic product for supplementation, this would generate the entire spectrum of trophic factors, anti-oxidant compounds, and other astrocytic protective functions needed to assist neurons for overcoming cellular stresses induced by pathologic αSyn.
Conclusion
Astrocytes are the most predominant glial cells in the brain and play critical role in neuronal development, neural activity, synaptic transmission and learning and memory. However, their role in the etiology of neurodegenerative α-synucleinopathies is still mostly sheathed in mystery (Box 1). Several lines of evidence, neuropathological, functional and genetic, indicate that astrocytes are functionally involved in α-synucleinopathies (Figure 3). How and why these astrocytes synergize with the evolution of proteostasis failure and heterogeneous clinical symptoms need to be charted out, along with the contribution that regional astrocyte heterogeneity may play a role in selective neurodegeneration. Interestingly, the underlying mechanisms of astrocytic modulation of α-synucleinopathy may be divergent – in some cases, astrocytic activity may need to be enhanced whereas in some cases, its pathological function will need to be attenuated based on the context and timing of intervention. The fact that astrocytes are a heterogeneous group of cells, in terms of functionality and region-wide distribution, remains under-appreciated in the field of neurodegenerative α-synucleinopathies. Whether this physiological heterogeneity has a role to play in selective vulnerability of neurons along the spectrum of α-synucleinopathy (PD, DLB, MSA) that might ultimately be informative of the spectrum of clinical heterogeneity seen in the patients also need to be examined. Genetic predisposing factors that alter risk for α-synucleinopathies such as PARK7, GBA, LRRK2, PINK1 and NR4A2 may also affect astrocyte function and alter disease etiology through altering redox stress or other pathways (Figure 3). Whether mining such genetic and risk factor data can enable stratification of patients for personalizing treatment options, based on their unique clinical presentation, should be examined in future therapeutic studies. Current strategies of harnessing immunoproteostasis by either using small molecule drugs or astrocyte-derived neurotrophic molecules or astrocyte transplantation in combination with immunotherapy remain intriguing and will have to be tested in multiple models of α-synucleinopathies (Figure 3). Overall, bilateral interactions or non-cell autonomous interactions between astrocytes and neurons or other glia in specific regions of the brain may provide us insights into neuronal dysfunction and death (Figure 3). Deeper knowledge of astrocyte biology and its functional alterations will be necessary before we can successfully embark on such disease modifying therapies in α-synucleinopathies.
BOX 1: CRITICAL OUTSTANDING QUESTIONS.
What is the source of astrocytic α-synuclein?
Does a bilateral communication between neurons and astrocytes lead to exacerbated disease?
What is the mechanism of bilateral transmission of αSyn – direct physical contact or passive uptake?
How do the astrocyte-resident PD risk genes lead to proteostasis failure in glia – through direct or non cell autonomous pathways?
Does astrocytic dysfunction precede αSyn proteinopathy or is the dysfunction a result of neuronal αSyn proteinopathy?
Does astrocytic metabolism of internalized αSyn result in toxic by-products that contribute to disease pathogenesis?
Is restoring astrocytic function sufficient to rebalance proteostasis as a monotherapy?
Acknowledgement
This work was supported by NIH grant NS099738 (PC) and NS089622 (BIG). We acknowledge the University of Florida Neuromedicine Brain Bank for access to human tissue samples.
Abbreviations
- αSyn
α-synuclein
- AKT
‘AK’ thymoma
- ARE
Anti-oxidant response element
- CNS
Central Nervous System
- CDNF
Cerebral dopamine neurotrophic factor
- CSF
Cerebrospinal fluid
- DAMP
Damage associated molecular pattern
- DLB
Dementia with Lewy Bodies
- EM
Electron Microscope
- FA
Formic Acid
- Foxa1
Forkhead Box A1
- GABA
Gamma-Amino Butyric acid
- GDNF
Glial cell line-derived neurotrophic factor
- GCI
Glial cytoplasmic inclusions
- GFAP
Glial Fibrillary Acidic Protein
- GLP1R
Glucagon-like peptide-1 receptor
- GBA
Glucosidase beta acid
- H&E
Hematoxylin & Eosin staining
- HLA-DR
Human leukocyte antigen DR isotype
- MHC-II
Major histocompatibility complex class II
- MMP
Matrix metalloproteinase
- MANF
Mesencephalic astrocyte-derived neurotrophic factor
- HLA-DR
Human Leukocyte Antigen – DR isotype
- iLBD
Incidental Lewy body diseases
- Keap1
Kelch-like ech-associated protein 1
- LRRK2
Leucine-rich repeat kinase 2
- LB
Lewy bodies
- LN
Lewy neurites
- MSA
Multiple System Atrophy
- NQO1
NAD(P)H Quinone Dehydrogenase 1
- NCI
Neuronal cytoplasmic inclusions
- NRTN
Neurturin
- NAC
Non-amyloid β component
- Nrf2
Nuclear factor erythroid 2-like 2
- NFκB
Nuclear factor κ-light-chain-enhancer of activated B cells
- Nurr1/NR4A2
Nuclear receptor subfamily 4, group A, member 2
- PD
Parkinson’s disease
- PARK
Parkinson’s disease associated gene
- PDD
PD with dementia
- PI3K
Phosphatidylinositol 3-kinase
- PDGF β
Platelet-derived growth factor β
- PET
Positron-emission tomography
- pSer129
Phosphorylated Serine 129
- PINK1
Pten-induced putative kinase 1
- ROS
Reactive oxygen species
- SQSTM1
Sequestosome 1
- Thy-1
Thymocyte differentiation antigen 1
- TLR
Toll-like receptor
- TNT
Tunneling nanotube
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
References
- 1.Abounit S, Bousset L, Loria F, Zhu S, de Chaumont F, Pieri L, Olivo-Marin J-C, Melki R, Zurzolo C (2016) Tunneling nanotubes spread fibrillar α-synuclein by intercellular trafficking of lysosomes. EMBO J 35:2120–2138. doi: 10.15252/embj.201593411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahn T-B, Langston JW, Aachi VR, Dickson DW (2012) Relationship of neighboring tissue and gliosis to alpha-synuclein pathology in a fetal transplant for Parkinson’s disease. Am J Neurodegener Dis 1:49–59 [PMC free article] [PubMed] [Google Scholar]
- 3.Allen NJ, Eroglu C (2017) Cell Biology of Astrocyte-Synapse Interactions. Neuron 96:697–708. doi: 10.1016/j.neuron.2017.09.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Allen Reish HE, Standaert DG (2015) Role of α-synuclein in inducing innate and adaptive immunity in Parkinson disease. J Parkinsons Dis 5:1–19. doi: 10.3233/JPD-140491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anderson JP, Walker DE, Goldstein JM, de Laat R, Banducci K, Caccavello RJ, Barbour R, Huang J, Kling K, Lee M, Diep L, Keim PS, Shen X, Chataway T, Schlossmacher MG, Seubert P, Schenk D, Sinha S, Gai WP, Chilcote TJ (2006) Phosphorylation of Ser-129 Is the Dominant Pathological Modification of α-Synuclein in Familial and Sporadic Lewy Body Disease. J Biol Chem 281:29739–29752. doi: 10.1074/jbc.M600933200 [DOI] [PubMed] [Google Scholar]
- 6.Angelova PR, Ludtmann MHR, Horrocks MH, Negoda A, Cremades N, Klenerman D, Dobson CM, Wood NW, Pavlov EV, Gandhi S, Abramov AY (2016) Ca2+ is a key factor in alpha-synuclein-induced neurotoxicity. J Cell Sci 129:1792–1801. doi: 10.1242/jcs.180737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arai T, Uéda K, Ikeda K, Akiyama H, Haga C, Kondo H, Kuroki N, Niizato K, Iritani S, Tsuchiya K (1999) Argyrophilic glial inclusions in the midbrain of patients with Parkinson’s disease and diffuse Lewy body disease are immunopositive for NACP/alpha-synuclein. Neurosci Lett 259:83–6 [DOI] [PubMed] [Google Scholar]
- 8.Ashrafi G, Schlehe JS, LaVoie MJ, Schwarz TL (2014) Mitophagy of damaged mitochondria occurs locally in distal neuronal axons and requires PINK1 and Parkin. J Cell Biol 206:655–70. doi: 10.1083/jcb.201401070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Athauda D, Foltynie T (2016) The glucagon-like peptide 1 (GLP) receptor as a therapeutic target in Parkinson’s disease: mechanisms of action. Drug Discov Today 21:802–818. doi: 10.1016/j.drudis.2016.01.013 [DOI] [PubMed] [Google Scholar]
- 10.Athauda D, Maclagan K, Skene SS, Bajwa-Joseph M, Letchford D, Chowdhury K, Hibbert S, Budnik N, Zampedri L, Dickson J, Li Y, Aviles-Olmos I, Warner TT, Limousin P, Lees AJ, Greig NH, Tebbs S, Foltynie T (2017) Exenatide once weekly versus placebo in Parkinson’s disease: a randomised, double-blind, placebo-controlled trial. Lancet 390:1664–1675. doi: 10.1016/S0140-6736(17)31585-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aviles-Olmos I, Dickson J, Kefalopoulou Z, Djamshidian A, Kahan J, Ell P, Whitton P, Wyse R, Isaacs T, Lees A, Limousin P, Foltynie T (2015) Motor and Cognitive Advantages Persist 12 Months After Exenatide Exposure in Parkinson’s Disease. J Parkinsons Dis 4:337–344. doi: 10.3233/JPD-140364 [DOI] [PubMed] [Google Scholar]
- 12.Baig F, Lawton M, Rolinski M, Ruffmann C, Nithi K, Evetts SG, Fernandes HR, Ben-Shlomo Y, Hu MTM (2015) Delineating nonmotor symptoms in early Parkinson’s disease and first-degree relatives. Mov Disord 30:1759–66. doi: 10.1002/mds.26281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barker RA, Williams-Gray CH (2016) Review: The spectrum of clinical features seen with alpha synuclein pathology. Neuropathol Appl Neurobiol 42:6–19. doi: 10.1111/nan.12303 [DOI] [PubMed] [Google Scholar]
- 14.Barrenschee M, Zorenkov D, Böttner M, Lange C, Cossais F, Scharf AB, Deuschl G, Schneider SA, Ellrichmann M, Fritscher-Ravens A, Wedel T (2017) Distinct pattern of enteric phospho-alpha-synuclein aggregates and gene expression profiles in patients with Parkinson’s disease. Acta Neuropathol Commun 5:1. doi: 10.1186/s40478-016-0408-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bartels T, Choi JG, Selkoe DJ (2011) α-Synuclein occurs physiologically as a helically folded tetramer that resists aggregation. Nature 477:107–110. doi: 10.1038/nature10324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beach TG, Adler CH, Sue LI, Vedders L, Lue L, White Iii CL, Akiyama H, Caviness JN, Shill HA, Sabbagh MN, Walker DG, Arizona Parkinson’s Disease Consortium (2010) Multi-organ distribution of phosphorylated alpha-synuclein histopathology in subjects with Lewy body disorders. Acta Neuropathol 119:689–702. doi: 10.1007/s00401-010-0664-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bellucci A, Collo G, Sarnico I, Battistin L, Missale C, Spano P (2008) Alpha-synuclein aggregation and cell death triggered by energy deprivation and dopamine overload are counteracted by D 2 D 3 receptor activation. J Neurochem 106:560–577. doi: 10.1111/j.1471-4159.2008.05406.x [DOI] [PubMed] [Google Scholar]
- 18.Bertoncini CW, Jung Y-S, Fernandez CO, Hoyer W, Griesinger C, Jovin TM, Zweckstetter M (2005) Release of long-range tertiary interactions potentiates aggregation of natively unstructured alpha-synuclein. Proc Natl Acad Sci U S A 102:1430–5. doi: 10.1073/pnas.0407146102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blum-Degen D, Müller T, Kuhn W, Gerlach M, Przuntek H, Riederer P (1995) Interleukin-1 beta and interleukin-6 are elevated in the cerebrospinal fluid of Alzheimer’s and de novo Parkinson’s disease patients. Neurosci Lett 202:17–20 [DOI] [PubMed] [Google Scholar]
- 20.Bonifati V, Rizzu P, Squitieri F, Krieger E, Vanacore N, van Swieten JC, Brice A, van Duijn CM, Oostra B, Meco G, Heutink P (2003) DJ-1(PARK7), a novel gene for autosomal recessive, early onset parkinsonism. Neurol Sci 24:159–160. doi: 10.1007/s10072-003-0108-0 [DOI] [PubMed] [Google Scholar]
- 21.Booth HDE, Hirst WD, Wade-Martins R (2017) The Role of Astrocyte Dysfunction in Parkinson’s Disease Pathogenesis. Trends Neurosci 40:358–370. doi: 10.1016/j.tins.2017.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Braak H, Sastre M, Del Tredici K (2007) Development of alpha-synuclein immunoreactive astrocytes in the forebrain parallels stages of intraneuronal pathology in sporadic Parkinson’s disease. Acta Neuropathol 114:231–241. doi: 10.1007/s00401-007-0244-3 [DOI] [PubMed] [Google Scholar]
- 23.Braak H, Del Tredici K, Rüb U, de Vos RAI, Jansen Steur ENH, Braak E Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging 24:197–211 [DOI] [PubMed] [Google Scholar]
- 24.Braak H, de Vos RAI, Bohl J, Del Tredici K (2006) Gastric α-synuclein immunoreactive inclusions in Meissner’s and Auerbach’s plexuses in cases staged for Parkinson’s disease-related brain pathology. Neurosci Lett 396:67–72. doi: 10.1016/j.neulet.2005.11.012 [DOI] [PubMed] [Google Scholar]
- 25.Braidy N, Gai W-P, Xu YH, Sachdev P, Guillemin GJ, Jiang X-M, Ballard JWO, Horan MP, Fang ZM, Chong BH, Chan DKY (2013) Uptake and mitochondrial dysfunction of alpha-synuclein in human astrocytes, cortical neurons and fibroblasts. Transl Neurodegener 2:20. doi: 10.1186/2047-9158-2-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Breydo L, Wu JW, Uversky VN (2012) α-Synuclein misfolding and Parkinson’s disease. Biochim Biophys Acta - Mol Basis Dis 1822:261–285. doi: 10.1016/j.bbadis.2011.10.002 [DOI] [PubMed] [Google Scholar]
- 27.Brück D, Wenning GK, Stefanova N, Fellner L (2016) Glia and alpha-synuclein in neurodegeneration: A complex interaction. Neurobiol Dis 85:262–274. doi: 10.1016/j.nbd.2015.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brundin P, Dave KD, Kordower JH (2017) Therapeutic approaches to target alpha-synuclein pathology. Exp Neurol 298:225–235. doi: 10.1016/j.expneurol.2017.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Budnik V, Ruiz-Cañada C, Wendler F (2016) Extracellular vesicles round off communication in the nervous system. Nat Rev Neurosci 17:160–72. doi: 10.1038/nrn.2015.29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Buell AK, Galvagnion C, Gaspar R, Sparr E, Vendruscolo M, Knowles TPJ, Linse S, Dobson CM (2014) Solution conditions determine the relative importance of nucleation and growth processes in α-synuclein aggregation. Proc Natl Acad Sci U S A 111:7671–6. doi: 10.1073/pnas.1315346111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Canet-Aviles RM, Wilson MA, Miller DW, Ahmad R, McLendon C, Bandyopadhyay S, Baptista MJ, Ringe D, Petsko GA, Cookson MR (2004) The Parkinson’s disease protein DJ-1 is neuroprotective due to cysteine-sulfinic acid-driven mitochondrial localization. Proc Natl Acad Sci 101:9103–9108. doi: 10.1073/pnas.0402959101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Castagnet PI, Golovko MY, Barceló-Coblijn GC, Nussbaum RL, Murphy EJ (2005) Fatty acid incorporation is decreased in astrocytes cultured from alpha-synuclein gene-ablated mice. J Neurochem 94:839–49. doi: 10.1111/j.1471-4159.2005.03247.x [DOI] [PubMed] [Google Scholar]
- 33.Cavaliere F, Cerf L, Dehay B, Ramos-Gonzalez P, De Giorgi F, Bourdenx M, Bessede A, Obeso JA, Matute C, Ichas F, Bezard E (2017) In vitro alpha-synuclein neurotoxicity and spreading among neurons and astrocytes using Lewy body extracts from Parkinson disease brains. Neurobiol Dis 103:101–112. doi: 10.1016/j.nbd.2017.04.011 [DOI] [PubMed] [Google Scholar]
- 34.Chang D, Nalls MA, Hallgrímsdóttir IB, Hunkapiller J, van der Brug M, Cai F, Kerchner GA, Ayalon G, Bingol B, Sheng M, Hinds D, Behrens TW, Singleton AB, Bhangale TR, Graham RR, Bhangale TR, Graham RR (2017) A meta-analysis of genome-wide association studies identifies 17 new Parkinson’s disease risk loci. Nat Genet 49:1511–1516. doi: 10.1038/ng.3955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chavarría C, Rodríguez-Bottero S, Quijano C, Cassina P, Souza JM (2018) Impact of monomeric, oligomeric and fibrillar alpha-synuclein on astrocyte reactivity and toxicity to neurons. Biochem J 475:3153–3169. doi: 10.1042/BCJ20180297 [DOI] [PubMed] [Google Scholar]
- 36.Chen P-C, Vargas MR, Pani AK, Smeyne RJ, Johnson DA, Kan YW, Johnson JA (2009) Nrf2-mediated neuroprotection in the MPTP mouse model of Parkinson’s disease: Critical role for the astrocyte. Proc Natl Acad Sci U S A 106:2933–8. doi: 10.1073/pnas.0813361106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cheng SY, Trombetta LD (2004) The induction of amyloid precursor protein and alpha-synuclein in rat hippocampal astrocytes by diethyldithiocarbamate and copper with or without glutathione. Toxicol Lett 146:139–49 [DOI] [PubMed] [Google Scholar]
- 38.Choi I, Kim J, Jeong H-K, Kim B, Jou I, Park SM, Chen L, Kang U-J, Zhuang X, Joe E (2013) Pink1 deficiency attenuates astrocyte proliferation through mitochondrial dysfunction, reduced akt and increased p38 mapk activation, and downregulation of egfr. Glia 61:800–812. doi: 10.1002/glia.22475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chu CT, Zhu J, Dagda R Beclin 1-independent pathway of damage-induced mitophagy and autophagic stress: implications for neurodegeneration and cell death. Autophagy 3:663–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Clements CM, McNally RS, Conti BJ, Mak TW, Ting JP-Y (2006) DJ-1, a cancer- and Parkinson’s disease-associated protein, stabilizes the antioxidant transcriptional master regulator Nrf2. Proc Natl Acad Sci 103:15091–15096. doi: 10.1073/pnas.0607260103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cobb CA, Cole MP (2015) Oxidative and nitrative stress in neurodegeneration. Neurobiol Dis 84:4–21. doi: 10.1016/j.nbd.2015.04.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Darmanis S, Sloan SA, Zhang Y, Enge M, Caneda C, Shuer LM, Hayden Gephart MG, Barres BA, Quake SR (2015) A survey of human brain transcriptome diversity at the single cell level. Proc Natl Acad Sci U S A 112:7285–90. doi: 10.1073/pnas.1507125112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Decressac M, Kadkhodaei B, Mattsson B, Laguna A, Perlmann T, Bjorklund A (2012) - Synuclein-Induced Down-Regulation of Nurr1 Disrupts GDNF Signaling in Nigral Dopamine Neurons. Sci Transl Med 4:163ra156–163ra156. doi: 10.1126/scitranslmed.3004676 [DOI] [PubMed] [Google Scholar]
- 44.Denzer I, Münch G, Friedland K (2016) Modulation of mitochondrial dysfunction in neurodegenerative diseases via activation of nuclear factor erythroid-2-related factor 2 by food-derived compounds. Pharmacol Res 103:80–94. doi: 10.1016/j.phrs.2015.11.019 [DOI] [PubMed] [Google Scholar]
- 45.Dhillon J-KS, Riffe C, Moore BD, Ran Y, Chakrabarty P, Golde TE, Giasson BI (2017) A novel panel of α-synuclein antibodies reveal distinctive staining profiles in synucleinopathies. PLoS One 12:e0184731. doi: 10.1371/journal.pone.0184731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dieriks BV, Park TI-H, Fourie C, Faull RLM, Dragunow M, Curtis MA (2017) α-synuclein transfer through tunneling nanotubes occurs in SH-SY5Y cells and primary brain pericytes from Parkinson’s disease patients. Sci Rep 7:42984. doi: 10.1038/srep42984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dossi E, Vasile F, Rouach N (2017) Human astrocytes in the diseased brain. Brain Res Bull. doi: 10.1016/j.brainresbull.2017.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Du F, Yu Q, Chen A, Chen D, Yan SS (2018) Astrocytes Attenuate Mitochondrial Dysfunctions in Human Dopaminergic Neurons Derived from iPSC. Stem cell reports 10:366–374. doi: 10.1016/j.stemcr.2017.12.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ejlerskov P, Rasmussen I, Nielsen TT, Bergström A-L, Tohyama Y, Jensen PH, Vilhardt F (2013) Tubulin Polymerization-promoting Protein (TPPP/p25α) Promotes Unconventional Secretion of α-Synuclein through Exophagy by Impairing Autophagosome-Lysosome Fusion. J Biol Chem 288:17313–17335. doi: 10.1074/jbc.M112.401174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Emmanouilidou E, Melachroinou K, Roumeliotis T, Garbis SD, Ntzouni M, Margaritis LH, Stefanis L, Vekrellis K (2010) Cell-produced alpha-synuclein is secreted in a calcium-dependent manner by exosomes and impacts neuronal survival. J Neurosci 30:6838–51. doi: 10.1523/JNEUROSCI.5699-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Erustes AG, Stefani FY, Terashima JY, Stilhano RS, Monteforte PT, da Silva Pereira GJ, Han SW, Calgarotto AK, Hsu Y-T, Ureshino RP, Bincoletto C, Smaili SS (2018) Overexpression of α-synuclein in an astrocyte cell line promotes autophagy inhibition and apoptosis. J Neurosci Res 96:160–171. doi: 10.1002/jnr.24092 [DOI] [PubMed] [Google Scholar]
- 52.Fathy YY, Jonker AJ, Oudejans E, de Jong FJJ, van Dam A-MW, Rozemuller AJM, van de Berg WDJ (2018) Differential insular cortex subregional vulnerability to α-synuclein pathology in Parkinson’s disease and dementia with Lewy bodies. Neuropathol Appl Neurobiol. doi: 10.1111/nan.12501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fellner L, Irschick R, Schanda K, Reindl M, Klimaschewski L, Poewe W, Wenning GK, Stefanova N (2013) Toll-like receptor 4 is required for alpha-synuclein dependent activation of microglia and astroglia. Glia 61:349–360. doi: 10.1002/glia.22437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fujiwara H, Hasegawa M, Dohmae N, Kawashima A, Masliah E, Goldberg MS, Shen J, Takio K, Iwatsubo T (2002) α-Synuclein is phosphorylated in synucleinopathy lesions. Nat Cell Biol 4:160–164. doi: 10.1038/ncb748 [DOI] [PubMed] [Google Scholar]
- 55.Fusco G, De Simone A, Gopinath T, Vostrikov V, Vendruscolo M, Dobson CM, Veglia G (2014) Direct observation of the three regions in α-synuclein that determine its membrane-bound behaviour. Nat Commun 5:3827. doi: 10.1038/ncomms4827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gan L, Johnson DA, Johnson JA (2010) Keap1-Nrf2 activation in the presence and absence of DJ-1. Eur J Neurosci 31:967–977. doi: 10.1111/j.1460-9568.2010.07138.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gan L, Vargas MR, Johnson DA, Johnson JA (2012) Astrocyte-specific overexpression of Nrf2 delays motor pathology and synuclein aggregation throughout the CNS in the alpha-synuclein mutant (A53T) mouse model. J Neurosci 32:17775–17787. doi: 10.1523/JNEUROSCI.3049-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Garcia-Reitböck P, Anichtchik O, Bellucci A, Iovino M, Ballini C, Fineberg E, Ghetti B, Della Corte L, Spano P, Tofaris GK, Goedert M, Spillantini MG (2010) SNARE protein redistribution and synaptic failure in a transgenic mouse model of Parkinson’s disease. Brain 133:2032–44. doi: 10.1093/brain/awq132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.George JM, Jin H, Woods WS, Clayton DF (1995) Characterization of a novel protein regulated during the critical period for song learning in the zebra finch. Neuron 15:361–72 [DOI] [PubMed] [Google Scholar]
- 60.Giasson BI, Duda JE, Murray IV, Chen Q, Souza JM, Hurtig HI, Ischiropoulos H, Trojanowski JQ, Lee VM (2000) Oxidative damage linked to neurodegeneration by selective alpha-synuclein nitration in synucleinopathy lesions. Science 290:985–9 [DOI] [PubMed] [Google Scholar]
- 61.Gittis AH, Brasier DJ (2015) Astrocytes tell neurons when to listen up. Science (80-) 349:690–691. doi: 10.1126/science.aad0678 [DOI] [PubMed] [Google Scholar]
- 62.Goedert M, Masuda-Suzukake M, Falcon B (2017) Like prions: the propagation of aggregated tau and α-synuclein in neurodegeneration. Brain 140:266–278. doi: 10.1093/brain/aww230 [DOI] [PubMed] [Google Scholar]
- 63.Gray MT, Munoz DG, Gray DA, Schlossmacher MG, Woulfe JM (2014) Alpha-synuclein in the appendiceal mucosa of neurologically intact subjects. Mov Disord 29:991–8. doi: 10.1002/mds.25779 [DOI] [PubMed] [Google Scholar]
- 64.Gu X-L, Long C-X, Sun L, Xie C, Lin X, Cai H (2010) Astrocytic expression of Parkinson’s disease-related A53T alpha-synuclein causes neurodegeneration in mice. Mol Brain 3:12. doi: 10.1186/1756-6606-3-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hasegawa T, Konno M, Baba T, Sugeno N, Kikuchi A, Kobayashi M, Miura E, Tanaka N, Tamai K, Furukawa K, Arai H, Mori F, Wakabayashi K, Aoki M, Itoyama Y, Takeda A (2011) The AAA-ATPase VPS4 Regulates Extracellular Secretion and Lysosomal Targeting of α-Synuclein. PLoS One 6:e29460. doi: 10.1371/journal.pone.0029460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hishikawa N, Hashizume Y, Yoshida M, Sobue G (2001) Widespread occurrence of argyrophilic glial inclusions in Parkinson’s disease. Neuropathol Appl Neurobiol 27:362–72 [DOI] [PubMed] [Google Scholar]
- 67.Ihse E, Yamakado H, van Wijk XM, Lawrence R, Esko JD, Masliah E (2017) Cellular internalization of alpha-synuclein aggregates by cell surface heparan sulfate depends on aggregate conformation and cell type. Sci Rep 7:9008. doi: 10.1038/s41598-017-08720-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Imaizumi Y, Okada Y, Akamatsu W, Koike M, Kuzumaki N, Hayakawa H, Nihira T, Kobayashi T, Ohyama M, Sato S, Takanashi M, Funayama M, Hirayama A, Soga T, Hishiki T, Suematsu M, Yagi T, Ito D, Kosakai A, Hayashi K, Shouji M, Nakanishi A, Suzuki N, Mizuno Y, Mizushima N, Amagai M, Uchiyama Y, Mochizuki H, Hattori N, Okano H (2012) Mitochondrial dysfunction associated with increased oxidative stress and α-synuclein accumulation in PARK2 iPSC-derived neurons and postmortem brain tissue. Mol Brain 5:35. doi: 10.1186/1756-6606-5-35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Innamorato NG, Jazwa A, Rojo AI, García C, Fernández-Ruiz J, Grochot-Przeczek A, Stachurska A, Jozkowicz A, Dulak J, Cuadrado A (2010) Different susceptibility to the Parkinson’s toxin MPTP in mice lacking the redox master regulator Nrf2 or its target gene heme oxygenase-1. PLoS One 5:e11838. doi: 10.1371/journal.pone.0011838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.International Parkinson Disease Genomics Consortium, Nalls MA, Plagnol V, Hernandez DG, Sharma M, Sheerin U-M, Saad M, Simón-Sánchez J, Schulte C, Lesage S, Sveinbjörnsdóttir S, Stefánsson K, Martinez M, Hardy J, Heutink P, Brice A, Gasser T, Singleton AB, Wood NW (2011) Imputation of sequence variants for identification of genetic risks for Parkinson’s disease: a meta-analysis of genome-wide association studies. Lancet 377:641–649. doi: 10.1016/S0140-6736(10)62345-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jang A, Lee H-J, Suk J-E, Jung J-W, Kim K-P, Lee S-J (2010) Non-classical exocytosis of alpha-synuclein is sensitive to folding states and promoted under stress conditions. J Neurochem 113:1263–74. doi: 10.1111/j.1471-4159.2010.06695.x [DOI] [PubMed] [Google Scholar]
- 72.Jazwa A, Rojo AI, Innamorato NG, Hesse M, Fernández-Ruiz J, Cuadrado A (2011) Pharmacological targeting of the transcription factor Nrf2 at the basal ganglia provides disease modifying therapy for experimental parkinsonism. Antioxid Redox Signal 14:2347–60. doi: 10.1089/ars.2010.3731 [DOI] [PubMed] [Google Scholar]
- 73.Jellinger KA (2000) Cell death mechanisms in Parkinson’s disease. J Neural Transm 107:1–29. doi: 10.1007/s007020050001 [DOI] [PubMed] [Google Scholar]
- 74.Jellinger KA (2018) Multiple System Atrophy: An Oligodendroglioneural Synucleinopathy1. J Alzheimers Dis 62:1141–1179. doi: 10.3233/JAD-170397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jellinger KA, Lantos PL (2010) Papp-Lantos inclusions and the pathogenesis of multiple system atrophy: an update. Acta Neuropathol 119:657–67. doi: 10.1007/s00401-010-0672-3 [DOI] [PubMed] [Google Scholar]
- 76.Johnson DA, Johnson JA (2015) Nrf2—a therapeutic target for the treatment of neurodegenerative diseases. Free Radic Biol Med 88:253–267. doi: 10.1016/j.freeradbiomed.2015.07.147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Keshavarzian A, Green SJ, Engen PA, Voigt RM, Naqib A, Forsyth CB, Mutlu E, Shannon KM (2015) Colonic bacterial composition in Parkinson’s disease. Mov Disord 30:1351–60. doi: 10.1002/mds.26307 [DOI] [PubMed] [Google Scholar]
- 78.Khakh BS, Sofroniew MV (2015) Diversity of astrocyte functions and phenotypes in neural circuits. Nat Neurosci 18:942–952. doi: 10.1038/nn.4043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kim WS, Kågedal K, Halliday GM (2014) Alpha-synuclein biology in Lewy body diseases. Alzheimers Res Ther 6:73. doi: 10.1186/s13195-014-0073-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Klingelhoefer L, Reichmann H (2015) Pathogenesis of Parkinson disease--the gut-brain axis and environmental factors. Nat Rev Neurol 11:625–36. doi: 10.1038/nrneurol.2015.197 [DOI] [PubMed] [Google Scholar]
- 81.Kojima W, Kujuro Y, Okatsu K, Bruno Q, Koyano F, Kimura M, Yamano K, Tanaka K, Matsuda N (2016) Unexpected mitochondrial matrix localization of Parkinson’s disease-related DJ-1 mutants but not wild-type DJ-1. Genes to Cells 21:772–788. doi: 10.1111/gtc.12382 [DOI] [PubMed] [Google Scholar]
- 82.Koller EJ, Brooks MMT, Golde TE, Giasson BI, Chakrabarty P (2017) Inflammatory preconditioning restricts the seeded induction of α-synuclein pathology in wild type mice. Mol Neurodegener 12:1. doi: 10.1186/s13024-016-0142-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Koob AO, Paulino AD, Masliah E (2010) GFAP reactivity, apolipoprotein E redistribution and cholesterol reduction in human astrocytes treated with alpha-synuclein. Neurosci Lett 469:11–14. doi: 10.1016/j.neulet.2009.11.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Koprich JB, Kalia LV, Brotchie JM (2017) Animal models of α-synucleinopathy for Parkinson disease drug development. Nat Rev Neurosci 18:515–529. doi: 10.1038/nrn.2017.75 [DOI] [PubMed] [Google Scholar]
- 85.Kosel S, Egensperger R, von Eitzen U, Mehraein P, Graeber MB (1997) On the question of apoptosis in the parkinsonian substantia nigra. Acta Neuropathol 93:105–108 [DOI] [PubMed] [Google Scholar]
- 86.Kovacs GG, Lee VM, Trojanowski JQ (2017) Protein astrogliopathies in human neurodegenerative diseases and aging. Brain Pathol 27:675–690. doi: 10.1111/bpa.12536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kovacs GG, Wagner U, Dumont B, Pikkarainen M, Osman AA, Streichenberger N, Leisser I, Verchère J, Baron T, Alafuzoff I, Budka H, Perret-Liaudet A, Lachmann I (2012) An antibody with high reactivity for disease-associated α-synuclein reveals extensive brain pathology. Acta Neuropathol 124:37–50. doi: 10.1007/s00401-012-0964-x [DOI] [PubMed] [Google Scholar]
- 88.Kramer ML, Schulz-Schaeffer WJ (2007) Presynaptic -Synuclein Aggregates, Not Lewy Bodies, Cause Neurodegeneration in Dementia with Lewy Bodies. J Neurosci 27:1405–1410. doi: 10.1523/JNEUROSCI.4564-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lashuel HA, Overk CR, Oueslati A, Masliah E (2013) The many faces of α-synuclein: from structure and toxicity to therapeutic target. Nat Rev Neurosci 14:38–48. doi: 10.1038/nrn3406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lastres-Becker I, Ulusoy A, Innamorato NG, Sahin G, Rábano A, Kirik D, Cuadrado A (2012) α-Synuclein expression and Nrf2 deficiency cooperate to aggravate protein aggregation, neuronal death and inflammation in early-stage Parkinson’s disease. Hum Mol Genet 21:3173–92. doi: 10.1093/hmg/dds143 [DOI] [PubMed] [Google Scholar]
- 91.Latge C, Cabral KMS, de Oliveira GAP, Raymundo DP, Freitas JA, Johanson L, Romão LF, Palhano FL, Herrmann T, Almeida MS, Foguel D (2015) The Solution Structure and Dynamics of Full-length Human Cerebral Dopamine Neurotrophic Factor and Its Neuroprotective Role against α-Synuclein Oligomers. J Biol Chem 290:20527–40. doi: 10.1074/jbc.M115.662254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Le W-D, Xu P, Jankovic J, Jiang H, Appel SH, Smith RG, Vassilatis DK (2003) Mutations in NR4A2 associated with familial Parkinson disease. Nat Genet 33:85–9. doi: 10.1038/ng1066 [DOI] [PubMed] [Google Scholar]
- 93.Lee H-J, Suk J-E, Patrick C, Bae E-J, Cho J-H, Rho S, Hwang D, Masliah E, Lee S-J (2010) Direct transfer of alpha-synuclein from neuron to astroglia causes inflammatory responses in synucleinopathies. J Biol Chem 285:9262–9272. doi: 10.1074/jbc.M109.081125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Li W, West N, Colla E, Pletnikova O, Troncoso JC, Marsh L, Dawson TM, Jäkälä P, Hartmann T, Price DL, Lee MK (2005) Aggregation promoting C-terminal truncation of alpha-synuclein is a normal cellular process and is enhanced by the familial Parkinson’s disease-linked mutations. Proc Natl Acad Sci U S A 102:2162–7. doi: 10.1073/pnas.0406976102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Liddelow SA, Guttenplan KA, Clarke LE, Bennett FC, Bohlen CJ, Schirmer L, Bennett ML, Münch AE, Chung W-S, Peterson TC, Wilton DK, Frouin A, Napier BA, Panicker N, Kumar M, Buckwalter MS, Rowitch DH, Dawson VL, Dawson TM, Stevens B, Barres BA (2017) Neurotoxic reactive astrocytes are induced by activated microglia. Nature 541:481–487. doi: 10.1038/nature21029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lin MT, Beal MF (2006) Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature 443:787–795. doi: 10.1038/nature05292 [DOI] [PubMed] [Google Scholar]
- 97.Lindstrom V, Gustafsson G, Sanders LH, Howlett EH, Sigvardson J, Kasrayan A, Ingelsson M, Bergstrom J, Erlandsson A (2017) Extensive uptake of alpha-synuclein oligomers in astrocytes results in sustained intracellular deposits and mitochondrial damage. Mol Cell Neurosci. doi: 10.1016/j.mcn.2017.04.009 [DOI] [PubMed] [Google Scholar]
- 98.Loria F, Vargas JY, Bousset L, Syan S, Salles A, Melki R, Zurzolo C (2017) α-Synuclein transfer between neurons and astrocytes indicates that astrocytes play a role in degradation rather than in spreading. Acta Neuropathol 134:789–808. doi: 10.1007/s00401-017-1746-2 [DOI] [PubMed] [Google Scholar]
- 99.Luk KC, Kehm VM, Zhang B, O’Brien P, Trojanowski JQ, Lee VMY (2012) Intracerebral inoculation of pathological α-synuclein initiates a rapidly progressive neurodegenerative α-synucleinopathy in mice. J Exp Med 209:975–86. doi: 10.1084/jem.20112457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mao X, Ou MT, Karuppagounder SS, Kam T-I, Yin X, Xiong Y, Ge P, Umanah GE, Brahmachari S, Shin J-H, Kang HC, Zhang J, Xu J, Chen R, Park H, Andrabi SA, Kang SU, Gonçalves RA, Liang Y, Zhang S, Qi C, Lam S, Keiler JA, Tyson J, Kim D, Panicker N, Yun SP, Workman CJ, Vignali DAA, Dawson VL, Ko HS, Dawson TM (2016) Pathological α-synuclein transmission initiated by binding lymphocyte-activation gene 3. Science 353:aah3374–aah3374. doi: 10.1126/science.aah3374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Maroteaux L, Campanelli JT, Scheller RH (1988) Synuclein: a neuron-specific protein localized to the nucleus and presynaptic nerve terminal. J Neurosci 8:2804–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.McGeer PL, Itagaki S, Boyes BE, McGeer EG (1988) Reactive microglia are positive for HLA-DR in the substantia nigra of Parkinson’s and Alzheimer’s disease brains. Neurology 38:1285–91 [DOI] [PubMed] [Google Scholar]
- 103.Melki R (2015) Role of Different Alpha-Synuclein Strains in Synucleinopathies, Similarities with other Neurodegenerative Diseases. J Parkinsons Dis 5:217–227. doi: 10.3233/JPD-150543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mendritzki S, Schmidt S, Sczepan T, Zhu X-R, Segelcke D, Lübbert H (2010) Spinal Cord Pathology in Alpha-Synuclein Transgenic Mice. Parkinsons Dis 2010:1–9. doi: 10.4061/2010/375462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Miyazaki I, Asanuma M (2008) Dopaminergic neuron-specific oxidative stress caused by dopamine itself. Acta Med Okayama 62:141–50 [DOI] [PubMed] [Google Scholar]
- 106.Mochizuki H, Goto K, Mori H, Mizuno Y (1996) Histochemical detection of apoptosis in Parkinson’s disease. J Neurol Sci 137:120–123 [DOI] [PubMed] [Google Scholar]
- 107.Molofsky AV, Deneen B (2015) Astrocyte development: A Guide for the Perplexed. Glia 63:1320–1329. doi: 10.1002/glia.22836 [DOI] [PubMed] [Google Scholar]
- 108.Mori F, Tanji K, Yoshimoto M, Takahashi H, Wakabayashi K (2002) Demonstration of alpha-synuclein immunoreactivity in neuronal and glial cytoplasm in normal human brain tissue using proteinase K and formic acid pretreatment. Exp Neurol 176:98–104 [DOI] [PubMed] [Google Scholar]
- 109.Murray IVJ, Giasson BI, Quinn SM, Koppaka V, Axelsen PH, Ischiropoulos H, Trojanowski JQ, Lee VM-Y (2003) Role of α-Synuclein Carboxy-Terminus on Fibril Formation in Vitro †. Biochemistry 42:8530–8540. doi: 10.1021/bi027363r [DOI] [PubMed] [Google Scholar]
- 110.Nakamura K, Mori F, Kon T, Tanji K, Miki Y, Tomiyama M, Kurotaki H, Toyoshima Y, Kakita A, Takahashi H, Yamada M, Wakabayashi K (2016) Accumulation of phosphorylated α-synuclein in subpial and periventricular astrocytes in multiple system atrophy of long duration. Neuropathology 36:157–67. doi: 10.1111/neup.12243 [DOI] [PubMed] [Google Scholar]
- 111.Nakamura K, Nemani VM, Azarbal F, Skibinski G, Levy JM, Egami K, Munishkina L, Zhang J, Gardner B, Wakabayashi J, Sesaki H, Cheng Y, Finkbeiner S, Nussbaum RL, Masliah E, Edwards RH (2011) Direct membrane association drives mitochondrial fission by the Parkinson disease-associated protein alpha-synuclein. J Biol Chem 286:20710–26. doi: 10.1074/jbc.M110.213538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ngolab J, Trinh I, Rockenstein E, Mante M, Florio J, Trejo M, Masliah D, Adame A, Masliah E, Rissman RA (2017) Brain-derived exosomes from dementia with Lewy bodies propagate α-synuclein pathology. Acta Neuropathol Commun 5:46. doi: 10.1186/s40478-017-0445-5 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 113.Nguyen HN, Byers B, Cord B, Shcheglovitov A, Byrne J, Gujar P, Kee K, Schüle B, Dolmetsch RE, Langston W, Palmer TD, Pera RR (2011) LRRK2 Mutant iPSC-Derived DA Neurons Demonstrate Increased Susceptibility to Oxidative Stress. Cell Stem Cell 8:267–280. doi: 10.1016/j.stem.2011.01.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Nunomura A, Perry G, Aliev G, Hirai K, Takeda A, Balraj EK, Jones PK, Ghanbari H, Wataya T, Shimohama S, Chiba S, Atwood CS, Petersen RB, Smith MA (2001) Oxidative damage is the earliest event in Alzheimer disease. J Neuropathol Exp Neurol 60:759–67 [DOI] [PubMed] [Google Scholar]
- 115.Ouberai MM, Wang J, Swann MJ, Galvagnion C, Guilliams T, Dobson CM, Welland ME (2013) α-Synuclein senses lipid packing defects and induces lateral expansion of lipids leading to membrane remodeling. J Biol Chem 288:20883–95. doi: 10.1074/jbc.M113.478297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Outeiro TF, Lindquist S (2003) Yeast cells provide insight into alpha-synuclein biology and pathobiology. Science 302:1772–5. doi: 10.1126/science.1090439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Paillusson S, Clairembault T, Biraud M, Neunlist M, Derkinderen P (2013) Activity-dependent secretion of alpha-synuclein by enteric neurons. J Neurochem 125:512–7. doi: 10.1111/jnc.12131 [DOI] [PubMed] [Google Scholar]
- 118.Peelaerts W, Bousset L, Baekelandt V, Melki R (2018) ɑ-Synuclein strains and seeding in Parkinson’s disease, incidental Lewy body disease, dementia with Lewy bodies and multiple system atrophy: similarities and differences. Cell Tissue Res 373:195–212. doi: 10.1007/s00441-018-2839-5 [DOI] [PubMed] [Google Scholar]
- 119.Peelaerts W, Bousset L, Van der Perren A, Moskalyuk A, Pulizzi R, Giugliano M, Van den Haute C, Melki R, Baekelandt V (2015) α-Synuclein strains cause distinct synucleinopathies after local and systemic administration. Nature 522:340–4. doi: 10.1038/nature14547 [DOI] [PubMed] [Google Scholar]
- 120.Pekny M, Pekna M, Messing A, Steinhauser C, Lee J-M, Parpura V, Hol EM, Sofroniew MV, Verkhratsky A (2016) Astrocytes: a central element in neurological diseases. Acta Neuropathol 131:323–345. doi: 10.1007/s00401-015-1513-1 [DOI] [PubMed] [Google Scholar]
- 121.Peng C, Gathagan RJ, Covell DJ, Medellin C, Stieber A, Robinson JL, Zhang B, Pitkin RM, Olufemi MF, Luk KC, Trojanowski JQ, Lee VM-Y (2018) Cellular milieu imparts distinct pathological α-synuclein strains in α-synucleinopathies. Nature 557:558–563. doi: 10.1038/s41586-018-0104-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Peng C, Gathagan RJ, Lee VM-Y (2018) Distinct α-Synuclein strains and implications for heterogeneity among α-Synucleinopathies. Neurobiol Dis 109:209–218. doi: 10.1016/j.nbd.2017.07.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Perea G, Navarrete M, Araque A (2009) Tripartite synapses: astrocytes process and control synaptic information. Trends Neurosci 32:421–431. doi: 10.1016/j.tins.2009.05.001 [DOI] [PubMed] [Google Scholar]
- 124.Piao Y-S, Mori F, Hayashi S, Tanji K, Yoshimoto M, Kakita A, Wakabayashi K, Takahashi H (2003) Alpha-synuclein pathology affecting Bergmann glia of the cerebellum in patients with alpha-synucleinopathies. Acta Neuropathol 105:403–9. doi: 10.1007/s00401-002-0655-0 [DOI] [PubMed] [Google Scholar]
- 125.Pieri L, Chafey P, Le Gall M, Clary G, Melki R, Redeker V (2016) Cellular response of human neuroblastoma cells to α-synuclein fibrils, the main constituent of Lewy bodies. Biochim Biophys Acta 1860:8–19. doi: 10.1016/j.bbagen.2015.10.007 [DOI] [PubMed] [Google Scholar]
- 126.Poewe W, Seppi K, Tanner CM, Halliday GM, Brundin P, Volkmann J, Schrag A-E, Lang AE (2017) Parkinson disease. Nat Rev Dis Prim 3:17013. doi: 10.1038/nrdp.2017.13 [DOI] [PubMed] [Google Scholar]
- 127.Rannikko EH, Weber SS, Kahle PJ (2015) Exogenous α-synuclein induces toll-like receptor 4 dependent inflammatory responses in astrocytes. BMC Neurosci 16:57. doi: 10.1186/s12868-015-0192-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ravenholt RT, Foege WH (1982) 1918 influenza, encephalitis lethargica, parkinsonism. Lancet (London, England) 2:860–4 [DOI] [PubMed] [Google Scholar]
- 129.Rekas A, Ahn KJ, Kim J, Carver JA (2012) The chaperone activity of α-synuclein: Utilizing deletion mutants to map its interaction with target proteins. Proteins 80:1316–25. doi: 10.1002/prot.24028 [DOI] [PubMed] [Google Scholar]
- 130.Rostami J, Holmqvist S, Lindström V, Sigvardson J, Westermark GT, Ingelsson M, Bergström J, Roybon L, Erlandsson A (2017) Human Astrocytes Transfer Aggregated Alpha-Synuclein via Tunneling Nanotubes. J Neurosci 37:11835–11853. doi: 10.1523/JNEUROSCI.0983-17.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Sacino AN, Brooks M, McKinney AB, Thomas MA, Shaw G, Golde TE, Giasson BI (2014) Brain injection of alpha-synuclein induces multiple proteinopathies, gliosis, and a neuronal injury marker. J Neurosci 34:12368–12378. doi: 10.1523/JNEUROSCI.2102-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Sacino AN, Brooks M, Thomas MA, McKinney AB, Lee S, Regenhardt RW, McGarvey NH, Ayers JI, Notterpek L, Borchelt DR, Golde TE, Giasson BI (2014) Intramuscular injection of -synuclein induces CNS -synuclein pathology and a rapid-onset motor phenotype in transgenic mice. Proc Natl Acad Sci 111:10732–10737. doi: 10.1073/pnas.1321785111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Sacino AN, Thomas MA, Ceballos-Diaz C, Cruz PE, Rosario AM, Lewis J, Giasson BI, Golde TE (2013) Conformational templating of α-synuclein aggregates in neuronal-glial cultures. Mol Neurodegener 8:17. doi: 10.1186/1750-1326-8-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Saijo K, Winner B, Carson CT, Collier JG, Boyer L, Rosenfeld MG, Gage FH, Glass CK (2009) A Nurr1/CoREST pathway in microglia and astrocytes protects dopaminergic neurons from inflammation-induced death. Cell 137:47–59. doi: 10.1016/j.cell.2009.01.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Sampson TR, Debelius JW, Thron T, Janssen S, Shastri GG, Ilhan ZE, Challis C, Schretter CE, Rocha S, Gradinaru V, Chesselet M-F, Keshavarzian A, Shannon KM, Krajmalnik-Brown R, Wittung-Stafshede P, Knight R, Mazmanian SK (2016) Gut Microbiota Regulate Motor Deficits and Neuroinflammation in a Model of Parkinson’s Disease. Cell 167:1469–1480.e12. doi: 10.1016/j.cell.2016.11.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Sarafian TA, Littlejohn K, Yuan S, Fernandez C, Cilluffo M, Koo B-K, Whitelegge JP, Watson JB (2017) Stimulation of synaptoneurosome glutamate release by monomeric and fibrillated α-synuclein. J Neurosci Res 95:1871–1887. doi: 10.1002/jnr.24024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Sato H, Kato T, Arawaka S (2013) The role of Ser129 phosphorylation of α-synuclein in neurodegeneration of Parkinson’s disease: a review of in vivo models. Rev Neurosci 24:115–23. doi: 10.1515/revneuro-2012-0071 [DOI] [PubMed] [Google Scholar]
- 138.Scheperjans F, Aho V, Pereira PAB, Koskinen K, Paulin L, Pekkonen E, Haapaniemi E, Kaakkola S, Eerola-Rautio J, Pohja M, Kinnunen E, Murros K, Auvinen P (2015) Gut microbiota are related to Parkinson’s disease and clinical phenotype. Mov Disord 30:350–8. doi: 10.1002/mds.26069 [DOI] [PubMed] [Google Scholar]
- 139.Scholz SW, Houlden H, Schulte C, Sharma M, Li A, Berg D, Melchers A, Paudel R, Gibbs JR, Simon-Sanchez J, Paisan-Ruiz C, Bras J, Ding J, Chen H, Traynor BJ, Arepalli S, Zonozi RR, Revesz T, Holton J, Wood N, Lees A, Oertel W, Wüllner U, Goldwurm S, Pellecchia MT, Illig T, Riess O, Fernandez HH, Rodriguez RL, Okun MS, Poewe W, Wenning GK, Hardy JA, Singleton AB, Gasser T, Schneider S, Bhatia KP, Gasser T (2009) SNCA variants are associated with increased risk for multiple system atrophy. Ann Neurol 65:610–614. doi: 10.1002/ana.21685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Shendelman S, Jonason A, Martinat C, Leete T, Abeliovich A (2004) DJ-1 Is a Redox-Dependent Molecular Chaperone That Inhibits α-Synuclein Aggregate Formation. PLoS Biol 2:e362. doi: 10.1371/journal.pbio.0020362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Shoji M, Harigaya Y, Sasaki A, Uéda K, Ishiguro K, Matsubara E, Watanabe M, Ikeda M, Kanai M, Tomidokoro Y, Shizuka M, Amari M, Kosaka K, Nakazato Y, Okamoto K, Hirai S (2000) Accumulation of NACP/alpha-synuclein in lewy body disease and multiple system atrophy. J Neurol Neurosurg Psychiatry 68:605–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Shrivastava AN, Redeker V, Fritz N, Pieri L, Almeida LG, Spolidoro M, Liebmann T, Bousset L, Renner M, Lena C, Aperia A, Melki R, Triller A (2015) -synuclein assemblies sequester neuronal 3-Na+/K+-ATPase and impair Na+ gradient. EMBO J 34:2408–2423. doi: 10.15252/embj.201591397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Shrivastava AN, Redeker V, Fritz N, Pieri L, Almeida LG, Spolidoro M, Liebmann T, Bousset L, Renner M, Lena C, Aperia A, Melki R, Triller A (2016) Data in support of the identification of neuronal and astrocyte proteins interacting with extracellularly applied oligomeric and fibrillar alpha-synuclein assemblies by mass spectrometry. Data Br 7:221–228. doi: 10.1016/j.dib.2016.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Song J-J, Oh S-M, Kwon O-C, Wulansari N, Lee H-S, Chang M-Y, Lee E, Sun W, Lee SE, Chang S, An H, Lee CJ, Lee S-H (2017) Cografting astrocytes improves cell therapeutic outcomes in a Parkinson’s disease model. J Clin Invest 128:463–482. doi: 10.1172/JCI93924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Song YJC, Halliday GM, Holton JL, Lashley T, O’Sullivan SS, McCann H, Lees AJ, Ozawa T, Williams DR, Lockhart PJ, Revesz TR (2009) Degeneration in different parkinsonian syndromes relates to astrocyte type and astrocyte protein expression. J Neuropathol Exp Neurol 68:1073–1083. doi: 10.1097/NEN.0b013e3181b66f1b [DOI] [PubMed] [Google Scholar]
- 146.Sorrentino ZA, Brooks MMT, Hudson V, Rutherford NJ, Golde TE, Giasson BI, Chakrabarty P (2017) Intrastriatal injection of α-synuclein can lead to widespread synucleinopathy independent of neuroanatomic connectivity. Mol Neurodegener 12:40. doi: 10.1186/s13024-017-0182-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Sorrentino ZA, Vijayaraghavan N, Gorion K-M, Riffe CJ, Strang KH, Caldwell J, Giasson BI (2018) Physiological carboxy-truncation of α-synuclein potentiates the prion-like formation of pathological inclusions. J Biol Chem jbc.RA118.005603. doi: 10.1074/jbc.RA118.005603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Sorrentino ZA, Xia Y, Funk C, Riffe CJ, Rutherford NJ, Ceballos Diaz C, Sacino AN, Price ND, Golde TE, Giasson BI, Chakrabarty P (2018) Motor neuron loss and neuroinflammation in a model of α-synuclein-induced neurodegeneration. Neurobiol Dis 120:98–106. doi: 10.1016/j.nbd.2018.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Stefanova N, Klimaschewski L, Poewe W, Wenning GK, Reindl M (2001) Glial cell death induced by overexpression of alpha-synuclein. J Neurosci Res 65:432–438. doi: 10.1002/jnr.1171 [DOI] [PubMed] [Google Scholar]
- 150.Sullivan A, O′Keeffe G (2016) Neurotrophic factor therapy for Parkinson′s disease: past, present and future. Neural Regen Res 11:205. doi: 10.4103/1673-5374.177710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Sulzer D, Alcalay RN, Garretti F, Cote L, Kanter E, Agin-Liebes J, Liong C, McMurtrey C, Hildebrand WH, Mao X, Dawson VL, Dawson TM, Oseroff C, Pham J, Sidney J, Dillon MB, Carpenter C, Weiskopf D, Phillips E, Mallal S, Peters B, Frazier A, Lindestam Arlehamn CS, Sette A (2017) T cells from patients with Parkinson’s disease recognize α-synuclein peptides. Nature 546:656–661. doi: 10.1038/nature22815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Sung JY, Park SM, Lee C-H, Um JW, Lee HJ, Kim J, Oh YJ, Lee S-T, Paik SR, Chung KC (2005) Proteolytic cleavage of extracellular secreted {alpha}-synuclein via matrix metalloproteinases. J Biol Chem 280:25216–24. doi: 10.1074/jbc.M503341200 [DOI] [PubMed] [Google Scholar]
- 153.Surendranathan A, Su L, Mak E, Passamonti L, Hong YT, Arnold R, Vázquez Rodríguez P, Bevan-Jones WR, Brain SAE, Fryer TD, Aigbirhio FI, Rowe JB, O’Brien JT (2018) Early microglial activation and peripheral inflammation in dementia with Lewy bodies. Brain 141:3415–3427. doi: 10.1093/brain/awy265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Surmeier DJ, Obeso JA, Halliday GM (2017) Selective neuronal vulnerability in Parkinson disease. Nat Rev Neurosci 18:101–113. doi: 10.1038/nrn.2016.178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Taipa R, Pereira C, Reis I, Alonso I, Bastos-Lima A, Melo-Pires M, Magalhães M (2016) DJ-1 linked parkinsonism (PARK7) is associated with Lewy body pathology. Brain 139:1680–1687. doi: 10.1093/brain/aww080 [DOI] [PubMed] [Google Scholar]
- 156.Takeda A, Hashimoto M, Mallory M, Sundsumo M, Hansen L, Masliah E (2000) C-terminal alpha-synuclein immunoreactivity in structures other than Lewy bodies in neurodegenerative disorders. Acta Neuropathol 99:296–304 [DOI] [PubMed] [Google Scholar]
- 157.Takeda H, Inazu M, Matsumiya T (2002) Astroglial dopamine transport is mediated by norepinephrine transporter. Naunyn Schmiedebergs Arch Pharmacol 366:620–623. doi: 10.1007/s00210-002-0640-0 [DOI] [PubMed] [Google Scholar]
- 158.Tanji K, Imaizumi T, Yoshida H, Mori F, Yoshimoto M, Satoh K, Wakabayashi K (2001) Expression of alpha-synuclein in a human glioma cell line and its up-regulation by interleukin-1beta. Neuroreport 12:1909–1912 [DOI] [PubMed] [Google Scholar]
- 159.Terada S, Ishizu H, Haraguchi T, Takehisa Y, Tanabe Y, Kawai K, Kuroda S (2000) Tau-negative astrocytic star-like inclusions and coiled bodies in dementia with Lewy bodies. Acta Neuropathol 100:464–8 [DOI] [PubMed] [Google Scholar]
- 160.Terada S, Ishizu H, Yokota O, Tsuchiya K, Nakashima H, Ishihara T, Fujita D, Ueda K, Ikeda K, Kuroda S (2003) Glial involvement in diffuse Lewy body disease. Acta Neuropathol 105:163–169. doi: 10.1007/s00401-002-0622-9 [DOI] [PubMed] [Google Scholar]
- 161.Theillet F-X, Binolfi A, Bekei B, Martorana A, Rose HM, Stuiver M, Verzini S, Lorenz D, van Rossum M, Goldfarb D, Selenko P (2016) Structural disorder of monomeric α-synuclein persists in mammalian cells. Nature 530:45–50. doi: 10.1038/nature16531 [DOI] [PubMed] [Google Scholar]
- 162.Togo T, Iseki E, Marui W, Akiyama H, Uéda K, Kosaka K (2001) Glial involvement in the degeneration process of Lewy body-bearing neurons and the degradation process of Lewy bodies in brains of dementia with Lewy bodies. J Neurol Sci 184:71–5 [DOI] [PubMed] [Google Scholar]
- 163.Tong J, Ang L-C, Williams B, Furukawa Y, Fitzmaurice P, Guttman M, Boileau I, Hornykiewicz O, Kish SJ (2015) Low levels of astroglial markers in Parkinson’s disease: relationship to alpha-synuclein accumulation. Neurobiol Dis 82:243–253. doi: 10.1016/j.nbd.2015.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Tong J, Wong H, Guttman M, Ang LC, Forno LS, Shimadzu M, Rajput AH, Muenter MD, Kish SJ, Hornykiewicz O, Furukawa Y (2010) Brain alpha-synuclein accumulation in multiple system atrophy, Parkinson’s disease and progressive supranuclear palsy: a comparative investigation. Brain 133:172–188. doi: 10.1093/brain/awp282 [DOI] [PubMed] [Google Scholar]
- 165.Uchihara T, Giasson BI (2016) Propagation of alpha-synuclein pathology: hypotheses, discoveries, and yet unresolved questions from experimental and human brain studies. Acta Neuropathol 131:49–73. doi: 10.1007/s00401-015-1485-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Vasile F, Dossi E, Rouach N (2017) Human astrocytes: structure and functions in the healthy brain. Brain Struct Funct. doi: 10.1007/s00429-017-1383-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Victoria GS, Zurzolo C (2017) The spread of prion-like proteins by lysosomes and tunneling nanotubes: Implications for neurodegenerative diseases. J Cell Biol 216:2633–2644. doi: 10.1083/jcb.201701047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Wakabayashi K, Hayashi S, Yoshimoto M, Kudo H, Takahashi H (2000) NACP/alpha-synuclein-positive filamentous inclusions in astrocytes and oligodendrocytes of Parkinson’s disease brains. Acta Neuropathol 99:14–20 [DOI] [PubMed] [Google Scholar]
- 169.Wakabayashi K, Takahashi H (1996) Gallyas-positive, tau-negative glial inclusions in Parkinson’s disease midbrain. Neurosci Lett 217:133–6 [PubMed] [Google Scholar]
- 170.Wakabayashi K, Takahashi H, Takeda S, Ohama E, Ikuta F (1988) Parkinson’s disease: the presence of Lewy bodies in Auerbach’s and Meissner’s plexuses. Acta Neuropathol 76:217–221 [DOI] [PubMed] [Google Scholar]
- 171.Williamson TP, Johnson DA, Johnson JA (2012) Activation of the Nrf2-ARE pathway by siRNA knockdown of Keap1 reduces oxidative stress and provides partial protection from MPTP-mediated neurotoxicity. Neurotoxicology 33:272–279. doi: 10.1016/j.neuro.2012.01.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Willingham S, Outeiro TF, DeVit MJ, Lindquist SL, Muchowski PJ (2003) Yeast genes that enhance the toxicity of a mutant huntingtin fragment or alpha-synuclein. Science 302:1769–72. doi: 10.1126/science.1090389 [DOI] [PubMed] [Google Scholar]
- 173.Winner BM, Zhang H, Farthing MM, Karchalla LM, Lookingland KJ, Goudreau JL (2017) Metabolism of Dopamine in Nucleus Accumbens Astrocytes Is Preserved in Aged Mice Exposed to MPTP. Front Aging Neurosci 9:410. doi: 10.3389/fnagi.2017.00410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Woodard CM, Campos BA, Kuo S-H, Nirenberg MJ, Nestor MW, Zimmer M, Mosharov EV, Sulzer D, Zhou H, Paull D, Clark L, Schadt EE, Sardi SP, Rubin L, Eggan K, Brock M, Lipnick S, Rao M, Chang S, Li A, Noggle SA (2014) iPSC-derived dopamine neurons reveal differences between monozygotic twins discordant for Parkinson’s disease. Cell Rep 9:1173–82. doi: 10.1016/j.celrep.2014.10.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Yang YX, Latchman DS (2008) Nurr1 transcriptionally regulates the expression of ??-synuclein. Neuroreport 19:867–871. doi: 10.1097/WNR.0b013e3282ffda48 [DOI] [PubMed] [Google Scholar]
- 176.Yeh T-H, Lee DY, Gianino SM, Gutmann DH (2009) Microarray analyses reveal regional astrocyte heterogeneity with implications for neurofibromatosis type 1 (NF1)-regulated glial proliferation. Glia 57:1239–1249. doi: 10.1002/glia.20845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Yokota O, Terada S, Ishizu H, Tsuchiya K, Kitamura Y, Ikeda K, Uéda K, Kuroda S (2002) NACP/alpha-synuclein immunoreactivity in diffuse neurofibrillary tangles with calcification (DNTC). Acta Neuropathol 104:333–41. doi: 10.1007/s00401-002-0545-5 [DOI] [PubMed] [Google Scholar]
- 178.Yun SP, Kam T-I, Panicker N, Kim S, Oh Y, Park J-S, Kwon S-H, Park YJ, Karuppagounder SS, Park H, Kim S, Oh N, Kim NA, Lee S, Brahmachari S, Mao X, Lee JH, Kumar M, An D, Kang S-U, Lee Y, Lee KC, Na DH, Kim D, Lee SH, Roschke VV, Liddelow SA, Mari Z, Barres BA, Dawson VL, Lee S, Dawson TM, Ko HS (2018) Block of A1 astrocyte conversion by microglia is neuroprotective in models of Parkinson’s disease. Nat Med 24:931–938. doi: 10.1038/s41591-018-0051-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Zhang W, Wang T, Pei Z, Miller DS, Wu X, Block ML, Wilson B, Zhang W, Zhou Y, Hong J-S, Zhang J (2005) Aggregated alpha-synuclein activates microglia: a process leading to disease progression in Parkinson’s disease. FASEB J 19:533–42. doi: 10.1096/fj.04-2751com [DOI] [PubMed] [Google Scholar]
- 180.Zhang Z, Shen Y, Luo H, Zhang F, Peng D, Jing L, Wu Y, Xia X, Song Y, Li W, Jin L (2018) MANF protects dopamine neurons and locomotion defects from a human α-synuclein induced Parkinson’s disease model in C. elegans by regulating ER stress and autophagy pathways. Exp Neurol 308:59–71. doi: 10.1016/j.expneurol.2018.06.016 [DOI] [PubMed] [Google Scholar]
- 181.Zhou W, Bercury K, Cummiskey J, Luong N, Lebin J, Freed CR (2011) Phenylbutyrate up-regulates the DJ-1 protein and protects neurons in cell culture and in animal models of Parkinson disease. J Biol Chem 286:14941–51. doi: 10.1074/jbc.M110.211029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Zhu D, Tan KS, Zhang X, Sun AY, Sun GY, Lee JC-M (2005) Hydrogen peroxide alters membrane and cytoskeleton properties and increases intercellular connections in astrocytes. J Cell Sci 118:3695–3703. doi: 10.1242/jcs.02507 [DOI] [PubMed] [Google Scholar]
- 183.Zondler L, Miller-Fleming L, Repici M, Gonçalves S, Tenreiro S, Rosado-Ramos R, Betzer C, Straatman KR, Jensen PH, Giorgini F, Outeiro TF (2014) DJ-1 interactions with α-synuclein attenuate aggregation and cellular toxicity in models of Parkinson’s disease. Cell Death Dis 5:e1350. doi: 10.1038/cddis.2014.307 [DOI] [PMC free article] [PubMed] [Google Scholar]