Abstract
Objective
Pulmonary atresia with intact ventricular septum (PA/IVS) can be treated by various operative and catheter-based interventions. We aim to understand the long-term transplant-free survival of patients with PA/IVS by treatment strategy.
Methods
Cohort study from the Pediatric Cardiac Care Consortium, a multi-institutional registry with prospectively acquired outcome data after linkage with the National Death index and the Organ Procurement and Transplantation Network.
Results
Eligible patients underwent neonatal surgery or catheter-based intervention for PA/IVS between 1982 and 2003 (median follow-up of 16.7 years, IQR: 12.6–22.7). Over the study period, 616 patients with PA/IVS underwent one of three initial interventions: aortopulmonary shunt, right ventricular decompression or both. Risk factors for death at initial intervention included earlier birth era (1982–1992), chromosomal abnormality and atresia of one or both coronary ostia. Among survivors of neonatal hospitalisation (n=491), there were 99 deaths (4 post-transplant) and 10 transplants (median age of death or transplant 0.7 years, IQR: 0.3–1.8 years). Definite repair or last-stage palliation was achieved in the form of completed two-ventricle repair (n=201), one-and-a-half ventricle (n=39) or Fontan (n=96). Overall 20-year survival was 66%, but for patients discharged alive after definitive repair, it reached 97.6% for single-ventricle patients, 90.9% for those with one-and-a-half ventricle and 98.0% for those with complete two-ventricle repair (log-rank p=0.052).
Conclusions
Transplant-free survival in PA/IVS is poor due to significant infantile and interstage mortality. Survival into early adulthood is excellent for patients reaching completion of their intended path independent of type of repair.
INTRODUCTION
Pulmonary atresia with intact ventricular septum (PA/IVS) is a rare congenital heart defect with significant heterogeneity, driven primarily by varying degrees of right ventricle (RV) and tricuspid valve hypoplasia but also by coexisting coronary artery abnormalities, with important implications for therapy and prognosis.1–5
Because of this anatomic heterogeneity, patients with PA/IVS undergo a variety of interventional approaches ranging from two-ventricle (2V) repair as a neonate, to initial aortopulmonary shunting leading to single-ventricle palliation, one-and-a-half ventricle (1.5V) palliation, or 2V repair later in childhood. Finally, some require heart transplantation as a primary or secondary approach.6 Survival for patients has historically been poor.7–10 There have been few studies evaluating the long-term survival of these patients stratified by interventional strategy.11,12
Using a large, multicentre, clinical registry linked with national registries of death and transplant, we examined the long-term transplant-free survival of patients with PA/IVS.13,14 We aimed to identify patients at high risk of death or transplantation based on their interventional strategy.
METHODS
Study population
We performed a retrospective cohort study of patients with PA/IVS who underwent first cardiac intervention at an institution in the Pediatric Cardiac Care Consortium (PCCC) between 1982 and 2003. The PCCC contains information on all paediatric cardiac procedures performed at 47 US institutions, constituting 15%–30% of US annual paediatric cardiac surgeries with a similar distribution of cases and outcomes to all centres nationally.15,16
Clinical variables and follow-up data
Data collected include age and weight at initial procedure, sex, type of procedure, known chromosomal abnormalities, and catheterisation and surgical data. Coronary anatomy was determined by cardiac catheterisation during neonatal hospitalisation, if available; angiographic images were not available for independent review. Coronary anatomy was described as having atresia of one or both coronary ostia, displaying coronary fistulas±stenosis, or having no identifiable fistulous connections to the RV Information on in-hospital deaths was available from PCCC centres between 1982 and 2011. Outcome data were supplemented with linkage to the National Death Index and the Organ Procurement and Transplantation Network through 2014.13 Only patients with adequate identifiers for linkage were included in the final cohort.
Based on interventions performed during neonatal hospitalisation, patients were assigned to one of three categories for their initial repair: aortopulmonary shunt, RV decompression or shunt+RV decompression. Patients were assigned to birth eras with roughly equivalent populations: 1982–1992, 1993–1997 or 1998–2003.
All patients were assigned a physiologic end state based on status at most recent follow-up.7,10 Patients who underwent Fontan were categorised as single ventricle. Patients who underwent Glenn procedure in addition to RV decompression were categorised as 1.5V Patients who underwent RV decompression at any age with documented closure of any previously placed shunt were categorised as completed 2V repair. ‘Definite repair’ was defined as attainment of Fontan single-ventricle palliation, 1.5V palliation or completed 2V repair. Patients who did not reach definitive repair at time of last follow-up were either dead or transplanted prior to definitive repair, alive without definitive repair or underwent additional procedures outside of the PCCC.
Statistical analysis
Continuous variables were compared by Student’s t-test or Wilcoxon rank-sum test. Comparisons of categorical demographic characteristics and in-hospital deaths were performed using X2 tests or Fisher’s exact tests. Logistic regression was used to calculate adjusted ORs and 95% CIs of in-hospital death at initial intervention. Covariates assessed in this model included coronary anatomy, age at initial treatment, weight <2.5 kg at initial treatment, birth era, sex and presence of a chromosomal abnormality. Patients with coronary atresia were excluded from the expanded regression model because of their small number and high mortality. Logistic regression was used to compare in-hospital death for those who reached definitive repair by the specified repair types. Continuous transplant-free survival was calculated from hospital discharge after definitive repair. Kaplan-Meier survival was estimated up to 20 years and compared between pathways using the log-rank test. Other potential risk factors were assessed for association with long-term survival including coronary status (with or without coronary fistulas), age at definitive repair, initial intervention, birth era and sex. These comparisons were unadjusted analyses because of the limited number of events; those with coronary atresia and chromosomal abnormalities were excluded due to higher rate of mortality in these groups and unbalanced distribution between types of definitive repair. The cumulative incidence of each mutually exclusive outcome category was calculated using non-parametric methods with the alternative outcomes treated as competing risks.17 A sensitivity analysis was conducted to evaluate potential selection bias from patients excluded because of inadequate identifiers. This was done by comparing characteristics between excluded and included patients and using inverse probability weighting to estimate the potential impact of any differences (online supplementary methods). Statistical significance was set at p<0.05. Statistical analyses were performed using SAS V9.4.
RESULTS
Seven hundred and fifty-one patients with PA/IVS were identified, of which 132 were excluded (123 had inadequate identifiers for linkage, and nine underwent balloon atrial septostomy alone). Patients with inadequate identifiers were older at the time of initial surgery and had higher in-hospital mortality than those included with adequate identifiers. Distribution of initial intervention and definitive repair categories were similar between the two groups, though those with inadequate identifiers were less likely to reach definitive repair (online supplementary table 1). The comparison of the original estimates to weighted estimates to account for this potential selection bias is provided in online supplementary table 2. A total of 619 patients underwent initial surgical or catheter-based intervention during the study period, had adequate identifiers for linkage and were included in the cohort.
In-hospital neonatal mortality
Four hundred and ninety-one (79.3%) patients survived the hospitalisation associated with their neonatal procedure without transplant. Patient characteristics and risk factors for in-hospital death at initial intervention are shown in table 1.
Table 1.
Risk factors for in-hospital death at initial intervention for PA/IVS
| Total |
In-hospital death or transplant |
P value | unadjusted |
Adjusted |
|||
|---|---|---|---|---|---|---|---|
| n=616* | n=126 (20.5%) | OR | 95% CI | or | 95% CI | ||
| Initial intervention | |||||||
| Shunt only | 247 | 62 (25.1) | 0.0113† | 0.53 | 0.27 to 1.04 | 0.51 | 0.23 to 1.11 |
| Shunt+RV decompression | 273 | 41 (15.0) | 0.48 | 0.25 to 0.93 | 0.53 | 0.27 to 1.05 | |
| RV decompression | 96 | 23 (24.0) | Ref | Ref | |||
| Coronary anatomy | |||||||
| RV to coronary fistulas | 164 | 26 (15.9) | <0.0001† | 0.97 | 0.58 to 1.61 | 1.30 | 0.70 to 2.43 |
| Atresia ≥1 ostium | 17 | 12 (70.6) | – | – | – | – | |
| No abnormalities | 342 | 57 (16.7) | Ref | Ref | |||
| Missing | 93 | 31 (33.3) | – | – | |||
| Age at initial treatment (days) | |||||||
| ≤7 | 512 | 95 (18.6) | 0.0095† | 0.67 | 0.37 to 1.20 | 0.70 | 0.38 to 1.27 |
| >7 | 104 | 31 (29.8) | Ref | Ref | |||
| Weight at initial treatment | |||||||
| <2.5 kg | 117 | 31 (26.5) | 0.0718 | 1.17 | 0.65 to 2.11 | 1.35 | 0.73 to 2.49 |
| ≥2.5 kg | 494 | 94 (19.0) | Ref | Ref | |||
| Missing | 5 | 1 (20.0) | |||||
| Birth era | |||||||
| 1982–1992 | 202 | 48 (23.8) | 0.1168 | 2.79 | 1.52 to 5.13 | 2.83 | 1.51 to 5.32 |
| 1993–1997 | 206 | 45 (21.8) | 1.40 | 0.71 to 2.74 | 1.47 | 0.74 to 2.92 | |
| 1998–2003 | 208 | 33 (15.9) | Ref | Ref | |||
| Sex | |||||||
| Male | 344 | 71 (56.4) | 0.8981 | 1.01 | 0.63 to 1.62 | 1.09 | 0.67 to 1.80 |
| Female | 272 | 55 (43.7) | Ref | Ref | |||
| Chromosomal abnormality | |||||||
| Yes | 15 | 7 (46.7) | 0.0190† | 3.85 | 1.19 to 12.44 | 3.95 | 1.15 to 13.55 |
| No | 601 | 119 (19.8) | Ref | Ref | |||
Excluded two primary transplants and one primary Glenn.
Indicates statistically significant comparisons at p<0.05.
PA/IVS, pulmonary atresia with intact ventricular septum; RV, right ventricle.
Year of birth was also included in the adjusted model using a spline to assess whether more subtle changes over time were missed using the broader categories, but there was no meaningful change in results (online supplementary table 3). The higher mortality in the earliest birth era was predominantly seen in shunt+RV decompression patients (25% mortality vs 10% in latest birth era, p=0.0062) (online supplementary table 4). In patients undergoing RV decompression with or without shunt, mortality was higher in those who had surgical (19%) versus transcatheter RV decompression (8%).
Definitive repair
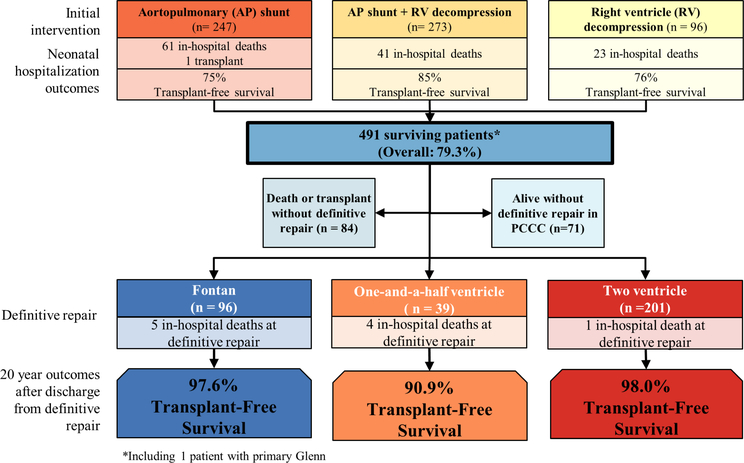
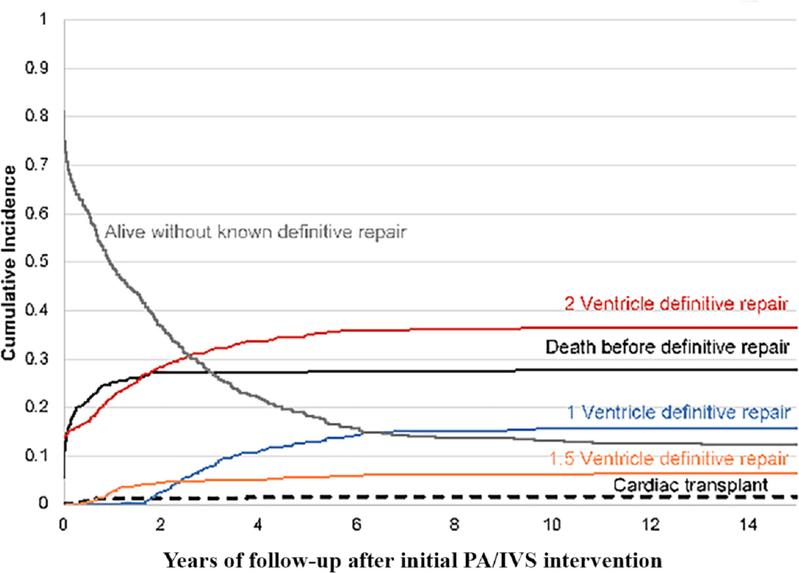
Overall, 358 patients (58%) reached definitive repair, of which 96 had definitive repair as newborns and 132 at a subsequent step (figures 1 and 2). Seventy-five deaths occurred in patients awaiting definitive repair, with a median age of death of 219 days (IQR: 82–477) (figure 3). There was a 21% rate of death or transplant in the shunt period among those not undergoing neonatal RV decompression. This decreased after successfully achieving second-stage palliation, but a 12% mortality occurred among patients with this physiology at last PCCC encounter, with median age of death of 351 days (IQR: 45–629) (figure 2B). There were an additional seven deaths during hospitalisation for definitive repair (table 2).
Figure 1.
Outcomes of all patients with PA/IVS undergoing neonatal surgery or catheter-based intervention. Transplant-free survival during neonatal hospitalisation is shown for each intervention. For survivals of neonatal hospitalisation, physiologic status at last encounter is shown, with transplant-free survival at 20 years after discharge from definitive repair. PA/IVS, pulmonary atresia with intact ventricular septum; PCCC, Pediatric Cardiac Care Consortium.
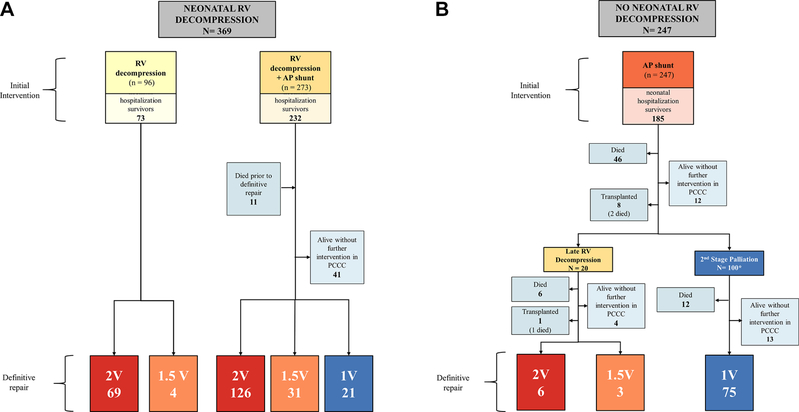
Figure 2.
Outcomes by interventional strategy with and without neonatal RV decompression. Definitive repair status at time of last PCCC is shown for all survivors of neonatal hospitalisation for (A) those who underwent neonatal RV decompression and (B) those who underwent shunt without neonatal RV decompression. 1V, single-ventricle palliation; 1.5V, one-and-a-half ventricle palliation; 2V, complete two-ventricle repair; AP, aortopulmonary; PCCC, Pediatric Cardiac Care Consortium; RV, right ventricle.
Figure 3.
Non-parametric cumulative incidence of each competing-risk outcome after initial PA/IVS intervention. Cumulative incidence of the following exclusive end states is shown: alive without known definitive repair, death before definitive repair, complete two-ventricle repair (2V), one- and-a-half ventricle palliation repair (1.5V), single-ventricle palliation repair (1V). Deaths occurring after definitive repair are not represented here. PA/IVS, pulmonary atresia with intact ventricular septum.
Table 2.
Risk factors for death at definitive repair and after discharge
| Total procedures |
In-hospital death |
20-year survival after hospital discharge |
|||
|---|---|---|---|---|---|
| n=347 | n (%) | P value | % | P value | |
| Definitive repair | |||||
| Single ventricle | 89 | 4 (4.5) | 0.1416 | 97.6 | 0.0555 |
| One-and-a-half ventricle | 36 | 3 (8.3) | 90.9 | ||
| Two-ventricle | 222 | 26 (11.7) | 98.0 | ||
| Coronary status | |||||
| RV to coronary fistulas | 76 | 5 (6.6) | 0.0241 † | 97.0 | 0.3526 |
| Atresia of ≥1 ostium | 3 | 2 (66.7) | * | ||
| Normal coronaries | 219 | 21 (9.6) | 95.7 | ||
| Missing | 49 | 5 (10.2) | 100 | ||
| Age at definitive repair (years) | |||||
| <1 | 148 | 29 (19.6) | <0.0001† | 95.8 | 0.0167 |
| 1–5 | 171 | 4 (2.3) | 99.4 | ||
| >5 | 28 | 0 (0.0) | 88.8 | ||
| Initial intervention | |||||
| Shunt | 78 | 4 (5.1) | <0.0001† | 98.6 | 0.6532 |
| Shunt+RV decompression | 173 | 4 (2.3) | 95.8 | ||
| RV decompression | 96 | 25 (26.0) | 97 | ||
| Birth era | |||||
| 1982–1992 | 106 | 17 (16.0) | 0.0043† | 94.3 | 0.1847 |
| 1993–1997 | 118 | 12 (10.2) | 98.1 | ||
| 1998–2003 | 123 | 4 (3.3) | – | ||
| Sex | |||||
| Male | 194 | 18 (9.3) | 0.8684 | 97.2 | 0.6849 |
| Female | 153 | 15 (9.8) | 97.0 | ||
| Chromosomal abnormality | |||||
| No | 340 | 28 (8.2) | <0.0001† | 97.1 | – |
| Yes | 7 | 5 (71.4) | * | – | |
The number with chromosomal abnormality or coronary atresia is too small to estimate meaningful survival.
Indicates statistically significant comparisons at p<0.05. I
RV, right ventricle.
The end-state physiology at last follow-up is shown in figure 1. The decision for or against RV decompression in the neonatal period was strongly associated with the likelihood of reaching biventricular repair, 2.4% without vs 59.4% with RV decompression (p<0.01) (figure 2). Decompression of the RV done after the neonatal hospitalisation was associated with 35% risk of death or transplant. Closure of atrial septal defect or patent foramen ovale was performed in 17 (47%) 1.5V patients and 118 (53%) 2V patients, either at the time of definitive repair or in a subsequent procedure.
Long-term transplant-free survival
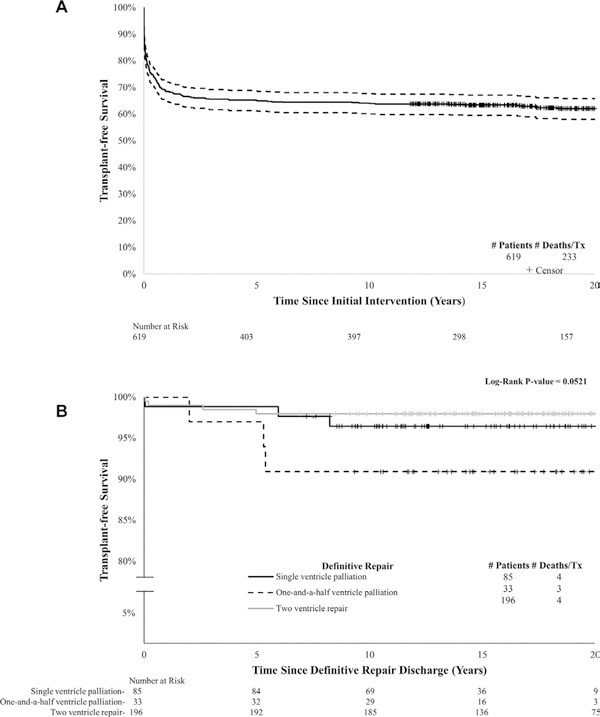
Cumulative 1-year, 5-year and 20-year transplant-free survival for the cohort was 69% (95% CI 65% to 72%), 65% (95% CI 61% to 68%) and 62% (95% CI 58% to 66%), respectively (figure 4A), with a median follow-up time of 16.7 years (IQR: 12.6–22.7).
Figure 4.
Kaplan-Meier transplant-free survival for (A) all patients with PA/IVS who underwent initial surgical or catheter-based intervention in the PCCC from time of initial intervention, and (B) patients discharged alive from definitive repair hospitalisation stratified by repair type from time of discharge. PA/IVS, pulmonary atresia with intact ventricular septum; PCCC, Pediatric Cardiac Care Consortium
For patients discharged alive after definitive repair, 20-year survival reached 98% (95% CI 89% to 99%) for single-ventricle patients, 91% (95% CI 74% to 97%) for those with 1.5V and 98% (95% CI 95% to 99%) for those with complete 2V repair (log-rank p=0.052) (figure 4B). Neither cumulative survival from initial intervention nor survival after discharge from definitive repair meaningfully changed after applying the inverse probability weights, suggesting no meaningful selection bias related to exclusion of patients without full identifiers (online supplementary table 2). Thirteen patients died after definitive repair, with median age of death of 2.74 years (IQR: 0.64–9.67). Median age at last follow-up was 19 years (IQR: 15–23). Cause of death was ascertained for all patients who died after surviving neonatal hospitalisation (n=99). The majority (88%) of deaths were attributed to the underlying congenital heart disease or related cardiovascular diseases. Cardiac arrest, heart failure and ischaemic heart disease were the most frequently cited contributing modes of death in our cohort (table 3).
Table 3.
Frequency of CHD and CVD codes as contributing causes of death
| Contributing cause of death* | Overall n=99 |
|---|---|
| Congenital heart diseases | 86 (87%) |
| Cardiovascular diseases | 57 (58%) |
| Cardiac arrest | 17 (17%) |
| Heart failure | 20 (20%) |
| Ischaemic heart disease | 10 (10%) |
| Other heart disease | 7 (7%) |
| Arrhythmias | 7 (7%) |
| Cerebrovascular conditions | 5 (5%) |
| Pulmonary heart disease | 3 (3%) |
Contributing causes of death are non-exclusive.
CHD, congenital heart disease; CVD, cardiovascular disease.
A total of 13 heart transplants occurred over the study period. Two were primary transplants and one occurred during the neonatal hospitalisation after initial shunt; all were alive at latest follow-up. Nine transplants occurred in patients after shunt palliation prior to definitive repair and resulted in three post-transplant deaths. One late transplant occurred after 1.5 V repair.
DISCUSSION
Due to the heterogeneity of PA/IVS, management strategies vary substantially. This study is the largest to date to evaluate the long-term outcomes of patients with PA/IVS focusing on the effect of different interventional strategies. By using surgical and catheterisation data from the PCCC and linkage with National Death Index and Organ Procurement and Transplantation Network databases, we were able to track the outcomes of this heterogeneous group from initial intervention through definitive repair and into early adulthood.
Our cohort had significant neonatal in-hospital mortality across intervention categories. Transplant-free survival to hospital discharge was 80%, comparable to the 77% 1 month survival reported by the Congenital Heart Surgeon’s Society (CHSS) study, the largest multicentre study to date on this lesion.10 This improved in the later era (1993–2003), particularly among patients undergoing RV decompression, consistent with other studies.10,18,19 There was a trend towards less frequent neonatal RV decompression and more shunt palliation over the most recent years, with growing recognition of the risk of RV decompression in patients with possible RV-dependent coronary circulation, which may account for some of this improvement.20 Additionally, we saw a transition away from surgical RV decompression in favour of transcatheter approaches, which has been reported to have significantly lower mortality.20–22 These improved outcomes with more conservative RV decompression point to the importance of proper selection of initial intervention for these patients and corroborate previous reports of high mortality seen at institutions favouring early 2V repair regardless of RV size.10 We saw the lowest mortality in the most recent cohort in patients undergoing RV decompression plus shunt; this strategy shows promise for borderline cases where there are no coronary contraindications to RV decompression. Addition of a shunt may help avoid early morbidity and mortality related to significant cyanosis while awaiting RV growth and did not preclude eventual 2V repair in most of the patients.
Also consistent with existing reports, we found high rates of early mortality and transplant in those with the most severe form of coronary disease (atresia of one or both coronary ostia).5,6,8,20,23 This group had by far the highest mortality with only one patient reaching Fontan stage. However, patients with less severe coronary disease (fistulas±stenosis but no atresia) had similar mortality as those with no known coronary abnormalities, both in the neonatal period and after definitive repair. Our study corroborates previous reports that found coronary abnormalities apart from the most severe forms to be less important in predicting mortality outside of the neonatal period and extends the observation of very low risk of death or transplant in patients reaching definitive repair by any path over a median of 16.2 years after definitive repair (maximum follow-up time 32.2 years).7,23,24
In the PCCC cohort, at least 58% of patients achieved definitive repair or last-stage palliation, slightly higher to the 43% success rate by 5 years of age reported for the neonatal survivors of the CHSS study; the mortality without documented repair was similar at 36%.10 Those not deemed candidates for RV decompression as neonates in our cohort continued to be at elevated risk on shunt and Glenn physiology. High interstage mortality while on shunt physiology has been reported by previous studies, both for PA/IVS and other shunted lesions.6,25,26 The high risk of patients with PA/IVS at the Glenn stage has been attributed at least partially to coexisting coronary abnormalities; until Fontan palliation, any RV-dependent coronary circulation is obligatorily fed by deoxygenated blood.24 Decompression of the RV outside of the neonatal hospitalisation had generally poor outcomes in our series. It is possible that in these patients, the opportunity for growth of the RV had passed; alternatively, whatever factor had led to their initial interventional decision not to decompress the RV continued to be an important factor in their pathophysiology. A recent study noted similar results with late RV decompressions in a population where later presentation to care is more common,27 but other previous large studies have not analysed survival by type of repair.7,8,10
After definitive repair or last-stage palliation, our cohort had excellent survival even when 2V repair was not feasible. Neither initial intervention nor type of definitive repair affected long-term survival among those discharged alive from definitive repair. Surprisingly, single-ventricle patients had similar survival to those with 1.5V and with complete 2V repair. It is likely that as these patients get older, outside of the 20-year time frame of this study, they may show divergence of outcomes as the known complications of Fontan physiology become more prominent. Our data suggest that many risk factors for mortality, such as severe coronary abnormalities, chromosomal abnormalities and additional unknown patient-specific factors, result in death either in the neonatal period or before achieving definitive repair. Patients able to survive these challenges are therefore a self-selected group with more favourable characteristics and are at low risk for death or transplant independent of the pathway they followed to that point. This suggests that coronary abnormalities do not affect long-term survival once proven to provide safe perfusion with or without RV decompression. In contrast to our results, a recent multi-institution study from Australia and New Zealand analysed long-term survival in their Fontan cohort and found higher rates of late death in patients with PA/IVS, particularly in those reported as having RV-dependent coronaries.12 This may explain the discrepancy since our Fontan subcohort had only one patient with documented coronary ostia atresia. Our study suggests that with proper selection of patients both for initial intervention and definitive repair, good long-term survival can be achieved,10,28 but further investigation is warranted to evaluate whether with age the less significant coronary abnormalities in this selected group of patients put patients at risk for late ischaemia and death.
The finding of a trend towards worse survival in our 1.5V patients is intriguing and has not been previously reported. Several studies have found functional capacity and health-related quality-of-life scores similar or worse than those of Fontan patients in teens and young adults with 1.5V palliation for PA/IVS.29,30 This morbidity and mortality may be related to adverse effects on their coronary perfusion at the time of RV decompression or unfavourable consequences of this unusual physiology that may lead to pulmonary regurgitation or right atrial hypertension. More detailed analysis of coronary perfusion and haemodynamics in these patients is warranted to assist with identification of patients that would be better served without RV decompression. Neither our data nor previous reports on functional capacity have demonstrated superior outcomes as compared with Fontan, which does bring into question the utility of this strategy in this patient population. However, it will be important to follow these patients further into adulthood to determine if freedom from known Fontan complications gives the 1.5V patients a long-term survival advantage.
This study is limited by its retrospective nature. Detailed analysis of individual patient anatomy was limited, as neither echocardiograms nor angiograms were available for review. However, diagnostic criteria for determining RV hypoplasia and coronary dependency have been variable and to some degree subjective. By including all cases falling under this diagnosis, we are capturing the whole spectrum of this condition as reported in the literature. This is reflected by the similar distribution of interventions and shorter term outcomes in our cohort compared with published single or multicentre studies. Additionally, patients with no reported chromosomal abnormalities were assumed to be unaffected. Given the changing recognition of chromosomal abnormalities over the study period, some cases may have been missed particularly in the earlier eras.
CONCLUSIONS
PA/IVS remains a challenging lesion with significant neonatal mortality regardless of type of intervention. Neonatal mortality has improved over time, particularly in patients with RVs amenable to decompression, though significant risk of mortality remains up to the stage of definite repair. This was especially true of patients with chromosomal abnormalities and coronary atresia, of whom very few reached definitive repair. Patients with atresia of one or both coronary ostia have very high early mortality and should be considered for primary heart transplant. However, conditioning on the ability to reach definitive repair or palliation, survival is excellent and reaches 95% independent of the type of their last procedure, though there was a trend towards worse outcomes in the 1.5V group.
Taken together, our data within the context of previous reports suggest that coronary abnormalities are important but difficult to assess in patients with PA/IVS. The raised threshold for concern for RV-dependent coronary perfusion and the trend towards more conservative approach in RV decompression may be related to improved early outcomes observed in more recent years. In addition, the data show that coronary perfusion variations with fistulas proven adequate at whatever path deemed appropriate do not increase risk of late cardiac death within the period studied.
Supplementary Material
Key messages.
What is already known on this subject?
It is known that pulmonary atresia with intact ventricular septum has significant neonatal mortality, with coronary abnormalities contributing to this early mortality. Outcomes of children surviving the neonatal period are better, but longer term outcomes into adolescence and adulthood are less clear.
What might this study add?
This study adds the previously unreported finding that patients able to be discharged alive from definitive repair have excellent survival (>90% at 20 years) that is not affected by either initial or definitive repair strategy.
How might this impact on clinical practice?
Our study may help inform the decision-making process when choosing between the available intervention strategies for these patients as neonates and young children, and will provide important prognostic information as they grow into adulthood.
Acknowledgements
We thank the programme directors and data collection coordinators from the participating PCCC centres; without their effort and dedication, this work could not have been completed.
Funding This study was supported by the National Heart, Lung, and Blood institute (R01 HL122392).
Footnotes
Competing interests None declared.
Disclaimer The data reported here have been supplied by the United Network for Organ Sharing (UNOS) as the contractor for the Organ Procurement and Transplantation Network (OPTN). The interpretation and reporting of these data are the responsibility of the author(s) and in no way should be seen as an official policy of or interpretation by the OPTN or the US Government.
Patient consent for publication Not required.
Ethics approval The study was approved by the institutional review board at Emory University, the National Death index and the United Network for Organ Sharing that maintains the Organ Procurement and Transplantation Network.
Provenance and peer review Not commissioned; externally peer reviewed.
Data sharing statement A database of included patients was created from the larger PCCC database and is available in spreadsheet form. Additional unpublished data include additional cardiac surgeries or catheter-based procedures undergone in a PCCC centre. This can be shared by contacting the corresponding author.
REFERENCES
- 1.Daubeney PE, Delany DJ, Anderson RH, et al. Pulmonary atresia with intact ventricular septum: range of morphology in a population-based study. J Am Coll Cardiol 2002;39:1670–9. [DOI] [PubMed] [Google Scholar]
- 2.Daubeney PE, Sharland GK, Cook AC, et al. Pulmonary atresia with intact ventricular septum: impact of fetal echocardiography on incidence at birth and postnatal outcome. UK and Eire Collaborative Study of Pulmonary Atresia with intact Ventricular Septum. Circulation 1998;98:562–6. [DOI] [PubMed] [Google Scholar]
- 3.Zuberbuhler JR, Anderson RH. Morphological variations in pulmonary atresia with intact ventricular septum. Br Heart J 1979;41:281–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Calder AL, Co EE, Sage MD. Coronary arterial abnormalities in pulmonary atresia with intact ventricular septum. Am J Cardiol 1987;59:436–42. [DOI] [PubMed] [Google Scholar]
- 5.Calder AL, Peebles CR, Occleshaw CJ. The prevalence of coronary arterial abnormalities in pulmonary atresia with intact ventricular septum and their influence on surgical results. Cardiol Young 2007;17:387–96. [DOI] [PubMed] [Google Scholar]
- 6.Cheung EW, Richmond ME, Turner ME, et al. Pulmonary atresia/intact ventricular septum: influence of coronary anatomy on single-ventricle outcome. Ann Thorac Surg 2014;98:1371–7. [DOI] [PubMed] [Google Scholar]
- 7.Daubeney PE, Wang D, Delany DJ, et al. Pulmonary atresia with intact ventricular septum: predictors of early and medium-term outcome in a population-based study. J Thorac Cardiovasc Surg 2005;130:1071e1–9. [DOI] [PubMed] [Google Scholar]
- 8.Hanley FL, Sade RM, Blackstone EH, et al. Outcomes in neonatal pulmonary atresia with intact ventricular septum. A multiinstitutional study. J Thorac Cardiovasc Surg 1993;105:406–23 24–7; discussion 23–4. [PubMed] [Google Scholar]
- 9.Dyamenahalli U, McCrindle BW, McDonald C, et al. Pulmonary atresia with intact ventricular septum: management of, and outcomes for, a cohort of 210 consecutive patients. Cardiol Young 2004;14:299–308. [DOI] [PubMed] [Google Scholar]
- 10.Ashburn DA, Blackstone EH, Wells WJ, et al. Determinants of mortality and type of repair in neonates with pulmonary atresia and intact ventricular septum. J Thorac Cardiovasc Surg 2004;127:1000–8. discussion 7–8. [DOI] [PubMed] [Google Scholar]
- 11.John AS, Warnes CA. Clinical outcomes of adult survivors of pulmonary atresia with intact ventricular septum. Int J Cardiol 2012;161:13–17. [DOI] [PubMed] [Google Scholar]
- 12.Elias P, Poh CL, du Plessis K, et al. Long-term outcomes of single-ventricle palliation for pulmonary atresia with intact ventricular septum: Fontan survivors remain at risk of late myocardial ischaemia and death. Eur J Cardiothorac Surg 2018;53:1230–6. [DOI] [PubMed] [Google Scholar]
- 13.Spector LG, Menk JS, Vinocur JM, et al. In-Hospital Vital Status and Heart Transplants After intervention for Congenital Heart Disease in the Pediatric Cardiac Care Consortium: Completeness of Ascertainment Using the National Death index and United Network for Organ Sharing Datasets. J Am Heart Assoc 2016;5:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Spector LG, Menk JS, Knight JH, et al. Trends in Long-Term Mortality After Congenital Heart Surgery. J Am Coll Cardiol 2018;71:2434–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vinocur JM, Moller JH, Kochilas LK. Putting the Pediatric Cardiac Care Consortium in context: evaluation of scope and case mix compared with other reported surgical datasets. Circ Cardiovasc Qual Outcomes 2012;5:577–9. [DOI] [PubMed] [Google Scholar]
- 16.Moller JH, Hills CB, Pyles LA. A multi-center cardiac registry. A method to assess outcome of catheterization intervention or surgery. Prog Pediatr Cardiol 2005;20:7–12. [Google Scholar]
- 17.Lin G, So Y, Johnson G. Analyzing Survival Data with Competing Risks Using SAS Software. SAS institute inc 2012 Proceedings of the SAS®Global Forum 2012 Conference, Cary, NC:SAS institute inc, 2012:344. [Google Scholar]
- 18.Grant S, Faraoni D, DiNardo J, et al. Predictors of Mortality in Children with Pulmonary Atresia with intact Ventricular Septum. Pediatr Cardiol 2017;38:1627–32. [DOI] [PubMed] [Google Scholar]
- 19.Jahangiri M, Zurakowski D, Bichell D, et al. Improved results with selective management in pulmonary atresia with intact ventricular septum. J Thorac Cardiovasc Surg 1999;118:1046–52. [DOI] [PubMed] [Google Scholar]
- 20.Giglia TM, Mandell VS, Connor AR, et al. Diagnosis and management of right ventricle-dependent coronary circulation in pulmonary atresia with intact ventricular septum. Circulation 1992;86:1516–28. [DOI] [PubMed] [Google Scholar]
- 21.Alwi M, Geetha K, Bilkis AA, et al. Pulmonary atresia with intact ventricular septum percutaneous radiofrequency-assisted valvotomy and balloon dilation versus surgical valvotomy and Blalock Taussig shunt. J Am Coll Cardiol 2000;35:468–76. [DOI] [PubMed] [Google Scholar]
- 22.Justo RN, Nykanen DG, Williams WG, et al. Transcatheter perforation of the right ventricular outflow tract as initial therapy for pulmonary valve atresia and intact ventricular septum in the newborn. Cathet Cardiovasc Diagn 1997;40:408–13. [DOI] [PubMed] [Google Scholar]
- 23.Guleserian KJ, Armsby LB, Thiagarajan RR, et al. Natural history of pulmonary atresia with intact ventricular septum and right-ventricle-dependent coronary circulation managed by the single-ventricle approach. Ann Thorac Surg 2006;81:2250–8. discussion 8. [DOI] [PubMed] [Google Scholar]
- 24.Rychik J, Levy H, Gaynor JW, et al. Outcome after operations for pulmonary atresia with intact ventricular septum. J Thorac Cardiovasc Surg 1998;116:924–31. [DOI] [PubMed] [Google Scholar]
- 25.Bove T, Vandekerckhove K, Panzer J, et al. Disease-specific outcome analysis of palliation with the modified Blalock-Taussig shunt. World J Pediatr Congenit Heart Surg 2015;6:67–74. [DOI] [PubMed] [Google Scholar]
- 26.Ghanayem NS, Allen KR, Tabbutt S, et al. Interstage mortality after the Norwood procedure: results of the multicenter Single Ventricle Reconstruction trial. J Thorac Cardiovasc Surg 2012;144:896–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zheng J, Gao B, Zhu Z, et al. Surgical results for pulmonary atresia with intact ventricular septum: a single-centre 15-year experience and medium-term follow-up. Eur J Cardiothorac Surg 2016;50:1083–8. [DOI] [PubMed] [Google Scholar]
- 28.Odim J, Laks H, Plunkett MD, et al. Successful management of patients with pulmonary atresia with intact ventricular septum using a three tier grading system for right ventricular hypoplasia. Ann Thorac Surg 2006;81:678–84. [DOI] [PubMed] [Google Scholar]
- 29.Numata S, Uemura H, Yagihara T, et al. Long-term functional results of the one and one half ventricular repair for the spectrum of patients with pulmonary atresia/stenosis with intact ventricular septum. Eur J Cardiothorac Surg 2003;24:516–20. [DOI] [PubMed] [Google Scholar]
- 30.Karamlou T, Poynter JA, Walters HL, et al. Long-term functional health status and exercise test variables for patients with pulmonary atresia with intact ventricular septum: a Congenital Heart Surgeons Society study. J Thorac Cardiovasc Surg 2013;145:1018–27. discussion 25–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.