Abstract
Background:
This pilot study evaluated whether adding phentermine to liraglutide would induce further weight loss in participants who had previously lost weight with liraglutide alone.
Subjects/Methods:
Participants were 45 adults with obesity (75.6% female, 55.6% white, body mass index = 34.3±4.7 kg/m2) who had lost an average of 12.6±6.8% of initial weight during a prior 1-year randomized trial with liraglutide and intensive behavioral treatment. Participants were re-randomized, in a double-blinded fashion, to liraglutide 3.0mg plus phentermine 15.0mg (liraglutide-phentermine) or liraglutide plus placebo (liraglutide-placebo). Participants also were provided with four, 15-minute counseling sessions during the 12-week extension study.
Results:
At week 12, the liraglutide-phentermine and liraglutide-pacebo groups lost a mean (±SEM) of 1.6±0.6% and 0.1±0.5% of re-randomization weight, respectively (p=0.073). Two (9.1%) liraglutide-phentermine participants and one (4.3%) liraglutide-placebo participant lost ≥5% of re-randomization weight; 19 (86.4%) and 16 (69.9%) participants, respectively, maintained their full weight loss achieved in the prior 1-year trial (p=0.125). Liraglutide-phentermine participants generally reported larger reductions in hunger and food preoccupation than liraglutide-placebo participants during the first 8 weeks of the extension study.
Conclusions:
The combination of liraglutide and phentermine appeared to be well-tolerated but did not produce additional clinically meaningful weight loss in individuals who had already lost 12.6% of initial weight with liraglutide alone.
Keywords: weight management, lifestyle modification, anti-obesity medication
1. Introduction
Medications approved for chronic weight management are effective in inducing an average loss of 5 to 10% of initial weight when used as an adjunct to a reduced calorie diet and increased physical activity (1–3). Weight loss with pharmacotherapy, as with lifestyle modification, typically plateaus after approximately 6 to 9 months of treatment (1,4,5). Reasons for the plateau are not well understood, given that in most cases, participants remain overweight or obese and decidedly wish to lose more weight (6). Reductions in resting and non-resting energy expenditure (7), which accompany neuroendocrine changes designed to defend body weight (8,9), appear to contribute to the slowing in weight loss. Behavioral fatigue in adhering to diet and activity recommendations is another factor (10).
Combination therapies, such as phentermine and topirimate (11,12), as well as naltrexone and bupropion (13), have been shown to induce greater weight losses than either agent used alone and to be associated with more favorable side effects than higher doses of monotherapy. The effectiveness of combination therapy is thought to reside in addressing different regulatory pathways that have complementary effects on weight reduction (1,11–13). Smith et al., for example, showed that the addition of phentermine (15 mg) to lorcaserin (10 mg BID) induced a mean weight loss of 7.0 kg at 12 weeks, compared with 3.5 kg for lorcaserin with placebo (14).
The present 12-week pilot study examined whether the addition of phentermine (15.0 mg) to liraglutide 3.0 mg/d would increase weight loss in participants who had lost 12.6% of initial weight in 1 year with the combination of liraglutide 3.0 and intensive behavior therapy (IBT) (15). This addition of phentermine to liraglutide to increase large, long-term weight losses clearly represents a different clinical challenge than combining two medications to induce initial weight loss. We hypothesized that participants assigned to continued liraglutide plus phentermine 15.0 mg (liraglutide-phentermine) for 12 weeks would lose significantly more weight (approximately 3.5 percentage points) than those assigned to continued liraglutide plus placebo (liraglutide-placebo). We also examined the percentages of participants in the two groups who lost ≥5% of their re-randomization weight and who maintained their full prior 1-year weight loss (achieved with IBT and liraglutide), as well as changes in appetite and cardiometabolic risk factors.
2. Methods
2.1. Participants
Participants were 45 volunteers from a group of 91 individuals who had completed a 1-year randomized controlled trial of IBT combined with: 1) liraglutide 3.0 mg/d (IBT-liraglutide); or 2) liraglutide 3.0 mg/d plus the prescription (for 12 weeks) of a 1000–1200 kcal/d meal- replacement diet (Multi-component). These two treatment groups, as well as a third, which received IBT alone (without liraglutide or meal replacements), have been described previously (15). All participants in the original 1-year trial received 21 brief (15 minute) individual lifestyle counseling sessions during the year, which were delivered by a physician, nurse practitioners, or registered dietitians, who served as lifestyle interventionists. The structure and schedule of treatment visits were modeled on that proposed by the Centers for Medicare and Medicaid Services (16). During the original 1-year IBT program, all participants were asked to adhere to a calorie goal of 1200 – 1800 kcal per day (depending on their starting weight) and to gradually increase their physical activity to ≥225 minutes per week (15). They were instructed to use behavioral strategies such as self-monitoring of food intake and body weight.
2.2. Procedures
At their last visit (week 52) of the 1-year randomized trial, individuals who were originally randomized to liraglutide (i.e., IBT-liraglutide or Multi-component groups), took the medication at the end of the study, and met eligibility criteria (described below) were invited to participate in an extension study that would provide all participants the use of liraglutide 3.0 mg/d for an additional 21 weeks. Participants were informed that half would be randomly assigned, in a double-blind manner, to take phentermine 15.0 mg/d for 12 weeks (beginning at week 53) and the other half would receive placebo. (To maintain continuity with the prior 1-year trial, the 12-week extension study is described as extending from week 53 to week 65.) They were further informed that they would have the opportunity to remain on liraglutide 3.0 mg/d for 8 additional weeks, following completion of the 12-week extension trial. This period was for clinical purposes and was not included as part of the randomized trial. Participants originally assigned to IBT-alone in the 1-year trial were not eligible for the extension study, which was designed to assess the benefits of adding phentermine in participants previously treated by liraglutide.
Participants who wished to participate in the extension study provided their written informed consent and were scheduled, within a week of completing the original 1-year trial, for a brief medical evaluation that included an electrocardiogram (EKG) and blood draw. Participants remained on liraglutide during this assessment week. Both the original 1-year trial and the extension study were conducted at an academic medical center in Philadelphia, and participants were enrolled in the extention trial between September 2017 and May 2018. The protocol for the extension study was included as an addendum to the original 1-year protocol (ClinicalTrials.gov, NCT02911818) and was approved by the University of Pennsylvania’s Institutional Review Board. The U.S. Food and Drug Administration did not require the investigators to obtain a new drug application to conduct the trial.
Inclusion criteria for the extension study included participants having, prior to re-randomization, a normal electrocardiogram and blood chemistries, as well as a body mass index (BMI) ≥ 27.0 kg/m2. We viewed the continued use of liraglutide (for 21 weeks) as the appropriate use of a medication approved for chronic weight management. (A BMI threshold has not been stipulated at which liraglutide should be discontinued when used for weight loss maintenance.) However, we anticipated that the addition of phentermine to liraglutide would induce additional weight loss, and we did not want participants to fall substantially below the BMI threshold for normal/healthy weight (i.e., 24.9 kg/m2). We determined that a BMI eligibility criterion ≥27.0 kg/m2 would likely achieve this goal.
Exclusion criteria for the extension trial included those from the original 1-year study that were specific to the use of liraglutide (e.g., family history of medullary thyroid cancer) (15). Additional exclusion criteria, specific to the use of phentermine, included: use of monoamine oxidase inhibitors in the past 2 weeks; glaucoma; presence or history of marked agitation; history of drug abuse; known hypersensitivity to sympathomimetic amines; current use of selective serotonin re-uptake inhibitors (e.g., fluoxetine, sertraline); and current use of other weight loss medications (besides liraglutide 3.0 mg/d) (17). Participants also were excluded if they had blood pressure > 160/100 mm Hg or a heart rate > 85 beats per minute (BPM), given both liraglutide’s and phentermine’s potential to increase one or more of these values in some participants (17,18). Participants were instructed to continue to use any other medications prescribed for the control of cardiometabolic risk factors (e.g., hypercholesterolemia, hypertension) and other co-morbid conditions, with the exception of the excluded medications listed above.
2.3. Randomization
Participants who met eligibility criteria were randomly assigned in equal numbers, in double-blind fashion, to phentermine or placebo by the Investigational Drug Service at the University of Pennsylvania. Randomization was stratified based on whether participants had lost ≥10% of initial weight during the prior 1-year trial. We wished to equalize prior weight loss in the two groups, since it could affect subsequent loss with phentermine.
2.4. Medication and Medical Monitoring
Immediately following re-randomization (at week 53), participants had a medical visit with the study physician or nurse practitioner who instructed them in the continued use of liraglutide 3.0 and in taking a second medication, presented in capsule form (identical for phentermine and placebo). Participants were encouraged to take both medications in the morning upon awakening. Phentermine was provided as 8.0 mg/d for the first 2 weeks to minimize potential side effects and was increased to 15.0 mg/d thereafter. All participants had brief medical visits (5–10 minutes) at weeks 55, 57, 61, and 65 (as counted from the start of the original 1-year trial). At each visit, participant’s vital signs were measured to determine whether they exceeded predetermined upper limits for blood pressure (>160/100 mm Hg) or heart rate (>100 bpm), either of which would result in dose down-titration (or discontinuation) of phentermine/placebo if the readings persisted. Participants also were asked about any changes in their health or medication use since the prior visit, as well as about their mood and any thoughts of harming themselves.
2.5. Lifestyle Counseling
All participants received the same program of diet, physical activity, and behavior therapy during the 12-week extension study. This included four brief (15 minute), face-to-face counseling sessions, scheduled at weeks 53 (re-randomization), 57, 61, and 65. Counseling was provided by the same physician, nurse practitioners, or registered dietitians as in the original 1-year study, each of whom who continued to work with the same participants whenever possible.
Participants at re-randomization who weighed <113.6 kg (250 lb.) were prescribed a diet of 1200–1499 kcal/d, comprised of conventional foods, with approximately 15–20% kcal from protein, 20–35% from fat, and the remainder from carbohydrate. Those who weighed ≥113.6 kg were prescribed 1500–1800 kcal/d. Participants were instructed to continue to record their food and calorie intake daily. They also were instructed to continue to engage in low-to-moderate intensity physical activity (e.g., walking) 5 days per week, for at least 180 minutes per week and preferably ≥225 minutes/week.
2.6. Continued Liraglutide Monotherapy
At the conclusion of the 12-week randomized extension study, all participants discontinued the second study medication (phentermine or placebo). They continued, however, to take liraglutide 3.0 mg/d for an additional 8 weeks, at the end of which time all medications were discontinued. Participants had two monthly lifestyle counseling and medical visits during these 8 weeks. This period allowed for dose down-titration in individuals who were assigned to both liraglutide and phentermine and was not part of the randomized study design or its hypotheses.
2.7. Outcome Measures
The primary outcome was percent reduction in initial body weight, as measured from week 53 (re-randomization) to week 65 (end of the 12-week extension study). The secondary weight outcome was the proportion of participants who, at week 65, lost ≥5% or ≥10% of re-randomization body weight. Additional weight-related outcomes included change in body weight, measured in kg, and the percentage of participants who maintained their full prior 1-year weight loss (within 1 kg) at week 65.
Other pre-specified secondary outcomes included changes from week 53 to week 65 in appetite (e.g., hunger, fullness after eating, and preoccupation with food), as measured using visual analogue scales (VAS) (19), as described previously (20). Additional outcomes included changes in cardiometabolic risk factors (e.g., blood pressure, triglycerides, low-density lipoprotein [LDL] and high-density lipoprotein [HDL] cholesterol, and waist circumference), glycemic control (e.g., fasting blood glucose), mood (21), and quality of life (22), as described in the 1-year trial (15).
2.8. Safety
Safety endpoints included findings from the four brief medical visits conducted during the 12-week extension study. These included measurements of blood pressure and pulse; reported adverse events (AEs); mental health/suicidal behavior as assessed by the Columbia Suicide Severity Rating Scale (C-SSRS) (23); and blood chemistries.
2.9. Power and Statistical Analyses
Based on previous studies of phentermine combined with topirimate (12) and with lorcaserin (14), we predicted that at week 65, participants assigned to liraglutide-phentermine would lose an additional mean of 3.5% (SD=3.5) of their re-randomization weight, compared with 0.0% (SD=3.5) for those assigned to liraglutide-placebo. A planned sample size of 64 participants was estimated to provide >80% power to detect a significant difference (alpha < 0.05, two tailed test) between the two groups in percent weight change from week 53 to week 65. All secondary analyses were evaluated at an alpha level of 0.05.
Data analyses were conducted using SPSS Statistics version 25.0 (IBM). Preliminary analyses included comparisons of re-randomization characteristics between the two treatment groups. We also compared the characteristics of participants from the original 1-year trial who did and did not enroll in the extension study. Percent reduction in re-randomization weight at week 65 in the intention-to-treat (ITT) population was compared using linear mixed-effects models with residual maximum likelihood, controlling for previous weight change during the original 1-year trial. The percentages of participants who lost ≥5% and ≥10% of re-randomization weight were analyzed using logistic regression. Participants who did not complete the week 65 assessment were categorized as not having achieved the categorical losses. Linear mixed-effects models also were used to compare the groups on change in cardiometabolic risk factors and psychosocial measures from week 53 to week 65, and repeated measures analyses of variance (ANOVAs) were used to compare the groups on change in appetite.
3. Results
3.1. Participant Recruitment and Retention
Of the 91 participants who originally were randomized to the IBT-liraglutide or Multicomponent groups and completed the 1-year trial, 56 met eligibility criteria for the 12-week extension study (Figure 1). Of these, 45 (80.4%) agreed to participate and were re-randomized to liraglutide-placebo or liraglutide-phentermine. The 45 participants who participated in the extension study did not differ significantly from the 46 who did not participate, in either demographic characteristics or 1-year weight loss in the prior randomized trial (i.e., mean weight losses of 14.6% vs 11.2%, respectively, p = 0.079; Supplemental Table 1). Figure 1 shows that 44 of 45 (97.8%) extension study participants completed the 12-week end-of-study assessment (at week 65). One liraglutide-placebo participant was lost to follow-up after week 8 of treatment.
Figure 1.
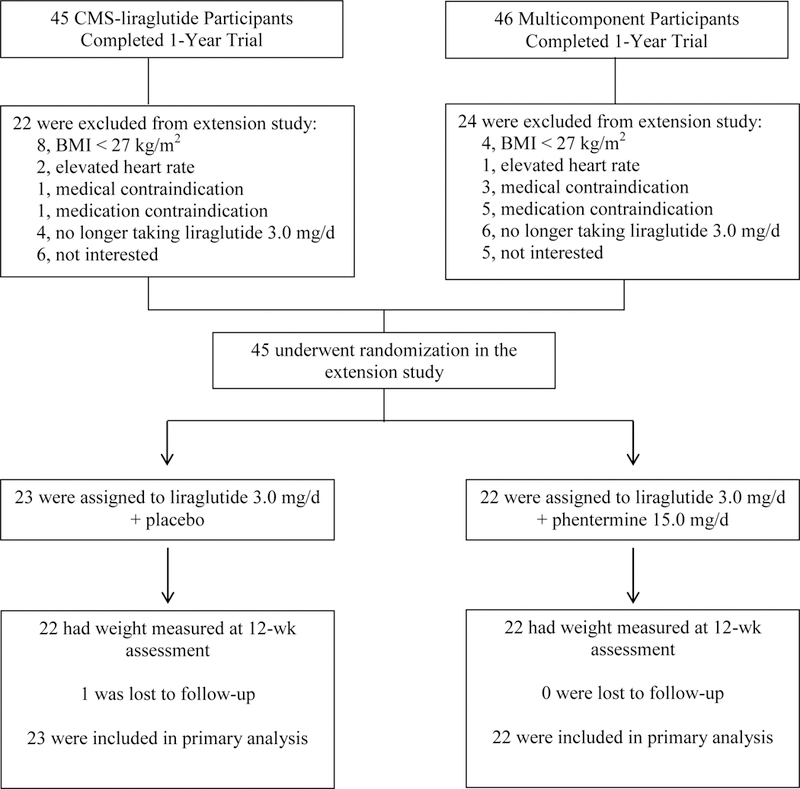
CONSORT diagram showing participant flow from completion of the 1-year trial through the 12-week extension study. Note. No participants were excluded for having a blood pressure above 160/100 mm Hg.
3.2. Participants’ Characteristics at Re-randomization
Participants in the extension study included 34 (75.6%) women and 11 men, who volunteered in approximately equal numbers from the prior IBT-liraglutide (N = 23) and Multicomponent (N = 22) groups. At re-randomization (week 53), the 45 participants had a mean (±SD) age of 48.6±12.3 years, weight of 98.5±16.4 kg, and BMI of 34.3±4.7 kg/m2; 55.6% self-identified as white, 42.2% as black, and 6.7% as Hispanic. As shown in Table 1, the two groups differed significantly at re-randomization in waist circumference and symptoms of depression, which were controlled for in relevant analyses. There were no other significant differences between groups.
Table 1.
Participants’ mean (±SD) characteristics at re-randomization (week 53) to liraglutide-placebo or liraglutide-phentermine for the 12-week extension study.
| Characteristic | Liraglutide- placebo, N = 23 |
Liraglutide- phentermine, N = 22 |
Total, N = 45 |
|---|---|---|---|
| Sex (female), n (%) | 18 (78.3%) | 16 (72.7%) | 34 (75.6%) |
| Race, n (%) | |||
| Black | 8 (34.8%) | 11 (50.0%) | 19 (42.2%) |
| White | 15 (65.2%) | 10 (45.5%) | 25 (55.6%) |
| Pacific Islander | 0 (0%) | 1 (4.5%) | 1 (2.2%) |
| Ethnicity (Hispanic), n (%) | 0 (0%) | 3 (13.6%) | 3 (6.7%) |
| Age (years) | 47.3 ± 13.0 | 49.9 ± 11.7 | 48.6 ± 12.3 |
| Weight (kg) | 95.3 ± 15.7 | 101.8 ± 16.8 | 98.5 ± 16.4 |
| Height (cm) | 169.9 ± 9.9 | 168.9 ± 9.2 | 169.4 ± 9.5 |
| BMI (kg/m2) | 33.0 ± 4.3 | 35.6 ± 4.7 | 34.3 ± 4.7 |
| Treatment group in previous 1-year trial | 13 (56.6%) | 9 (40.9%) | 22 (48.9%) |
| (Multi-component), n (%) | |||
| Week-53 weight loss (% lost) from | 14.0 ± 6.4 | 11.2 ± 7.1 | 12.6 ± 6.8 |
| week 1 | |||
| Week-53 weight loss (kg) from week 1 | 15.5 ± 7.6 | 12.8 ± 8.2 | 14.2 ± 7.9 |
| Systolic BP (mm Hg) | 118.0 ± 11.8 | 120.9 ± 10.8 | 119.4 ± 11.3 |
| Diastolic BP (mm Hg) | 71.3 ± 8.9 | 71.3 ± 6.2 | 71.3 ± 7.6 |
| Heart rate (bpm) | 74.4 ± 8.7 | 71.5 ± 8.7 | 73.0 ± 8.7 |
| Waist circumference (cm) | 104.6 ± 13.1* | 112.5 ± 12.1* | 108.3 ± 13.1 |
| Total cholesterol (mg/dL) | 171.6 ± 20.8 | 182.3 ± 33.4 | 176.8 ± 27.9 |
| HDL cholesterol (mg/dL) | 58.6 ± 14.4 | 58.6 ± 11.3 | 58.6 ± 12.8 |
| LDL cholesterol (mg/dL) | 95.9 ± 16.6 | 106.5 ± 32.4 | 101.1 ± 25.8 |
| Triglycerides (mg/dL) | 80.4 ± 37.8 | 79.1 ± 31.3 | 79.8 ± 34.4 |
| C-reactive protein (mg/L) | 3.5 ± 4.3 | 4.0 ± 4.1 | 3.7 ± 4.1 |
| HbA1C | 5.1 ± 0.3 | 5.1 ± 0.3 | 5.1 ± 0.3 |
| Fasting glucose (mg/dL) | 80.8 ± 4.4 | 81.7 ± 6.8 | 81.2 ± 5.7 |
| Fasting insulin (μU/mL) | 6.5 ± 5.5 | 7.3 ± 6.6 | 6.9 ± 6.0 |
| HOMA-IR | 1.3 ± 1.1 | 1.5 ± 1.3 | 1.4 ± 1.2 |
| Depression symptoms (PHQ-9) | 2.4 ± 2.5* | 0.9 ± 1.2* | 1.6 ± 2.1 |
| SF-36 Physical Health | 52.5 ± 7.4 | 53.3 ± 3.8 | 52.9 ± 5.9 |
| SF-36 Mental Health | 54.2 ± 9.5 | 57.3 ± 4.1 | 55.7 ± 7.4 |
Values are means ± SD unless otherwise noted. BP = blood pressure; HDL = high-density lipoprotein; LDL = low-density lipoprotein; Hb = hemoglobin; HOMA-IR = homeostatic model assessment of insulin resistance; PHQ-9 = Patient Health Questionnaire, 9-item; SF-36 = Short-Form Health Survey – 36 Item; IWQOL = Impact of Weight on Quality of Life. When conducting analyses, values for triglycerides, C- reactive protein, fasting insulin, and HOMA-IR were log transformed and values for depression symptoms (PHQ-9), SF-36 Physical Health and SF-36 and Mental Health were square root transformed.
Groups differed significantly in waist circumference (p = 0.049) and depression symptoms (p = 0.012). There were no other significant differences between the groups at re-randomization.
3.3. Weight Loss
At the end of the 12-week extension study (week 65), the liraglutide-placebo and liraglutide-phentermine groups lost a mean (±SEM) of 0.1±0.5% and 1.6±0.6% of re-randomization weight, respectively (Table 2). The difference between groups at week 65 was not statistically significant (MD=1.4%; 95% CI: −0.1 to 3.0%; p=0.073). Table 3 and Figure 2 show the mean percentage reduction in weight at each medical visit during the extension study. At weeks 55, 57, and 61, the liraglutide-phentermine participants lost a significantly greater percentage of re-randomization weight than participants assigned to liraglutide-placebo. However, the absolute differences in weight loss between groups were modest at these times (i.e., 0.7% to 1.6%).
Table 2.
Estimated mean (± SEM) percent reduction in body weight from re-randomization (week 53) to week 65 in the intention-to-treat population
|
Liraglutide-placebo, N = 23 |
Liraglutide- phentermine, N = 22 |
p value | |
|---|---|---|---|
|
Change from week 53 to week 65 |
|||
| Body weight (% change from re- | −0.1 ± 0.5 | −1.6 ± 0.6 | 0.073 |
| randomization) | (−1.2, 1.0) | (−2.7, −0.4) | |
| Body weight (kg) | 0.0 ± 0.5 | −1.4 ± 0.5 | 0.063 |
| (−1.0, 1.0) | (−2.4, −0.4) | ||
| Proportion with weight loss ≥ 5% of | 1 (4.3%) | 2 (9.1%) | 0.146 |
| randomization weight, n (%) | |||
| Proportion who maintained previous | 16 (69.6%) | 19 (86.4%) | 0.125 |
| weight loss (< 1 kg gain from | |||
| randomization), n (%) | |||
|
Change from week 1 (start of 1-year trial) to week 65 |
|||
| Body weight (% change from week 1) | −14.2 ± 1.6 | −12.4 ± 1.6 | 0.444 |
| (−17.4, −10.9) | (−15.7, −9.0) | ||
| Body weight (kg) | −15.8 ± 1.8 | −14.1 ± 1.9 | 0.539 |
| (−19.5, −12.1) | (−17.9, −10.3) | ||
Data for body weight change (% change and kg) from re-randomization (week 53) and from week 1 (start of the 1-year trial) to week 65 are estimated marginal means (± SEM) for the intention-to-treat population (N = 45) with the 95% confidence interval in parenthesis. Analyses of body weight change from rerandomization control for weight change during the initial 1-year trial. Participants re-randomized to liraglutide-placebo lost a mean ± SD of 14.0 ± 6.4% of initial weight (15.5 ± 7.6 kg) at the end of the original 52-week trial, compared with 11.2 ± 7.1% (12.8 ± 8.2 kg) for those re-randomized to liraglutide- phentermine (p = 0.180 and p = 0.263, respectively).
Table 3.
Estimated mean changes in body weight from re-randomization (week 53) at each medical visit in the intention-to-treat population.
| Liraglutide-placebo | Liraglutide- phentermine |
Statistical comparison (p) |
|
|---|---|---|---|
| Body weight (% change from re-randomization) | |||
| Week 55 | 0.0 ± 0.2 (−0.4, 0.4) | −0.7 ± 0.2 (−1.1, −0.3) | 0.010 |
| Week 57 | +0.2 ± 0.4 (−0.6, 0.9) | −1.4 ± 0.4 (−2.2, −0.7) | 0.004 |
| Week 61 | 0.0 ± 0.4 (−0.8, 0.8) | −1.5 ± 0.4 (−2.3, −0.7) | 0.012 |
| Week 65 | −0.1 ± 0.5 (−1.2, 1.0) | −1.6 ± 0.6 (−2.7, −0.4) | 0.073 |
| Body weight (kg) | |||
| Week 55 | +0.1 ± 0.2 (−0.2, 0.5) | −0.7 ± 0.2 (−1.0, −0.3) | 0.002 |
| Week 57 | +0.3 ± 0.3 (−0.4, 1.0) | −1.3 ± 0.4 (−2.1, −0.6) | 0.002 |
| Week 61 | +0.1 ± 0.4 (−0.6, 0.9) | −1.4 ± 0.4 (−2.1, −0.6) | 0.007 |
| Week 65 | 0.0 ± 0.5 (−1.0, 1.0) | −1.4 ± 0.5 (−2.4, −0.4) | 0.063 |
Note. Data for body weight change (% change and kg) from re-randomization (week 53) are estimated marginal means (± SE) for the intention-to-treat population (N = 45) with the 95% confidence interval in parenthesis. These analyses controlled for weight change during the initial 1-year trial. The statistically significant differences between groups at these earlier times are due, in part, to the lower variability in the SEMs, as compared with the SEMs at week 65.
Figure 2.
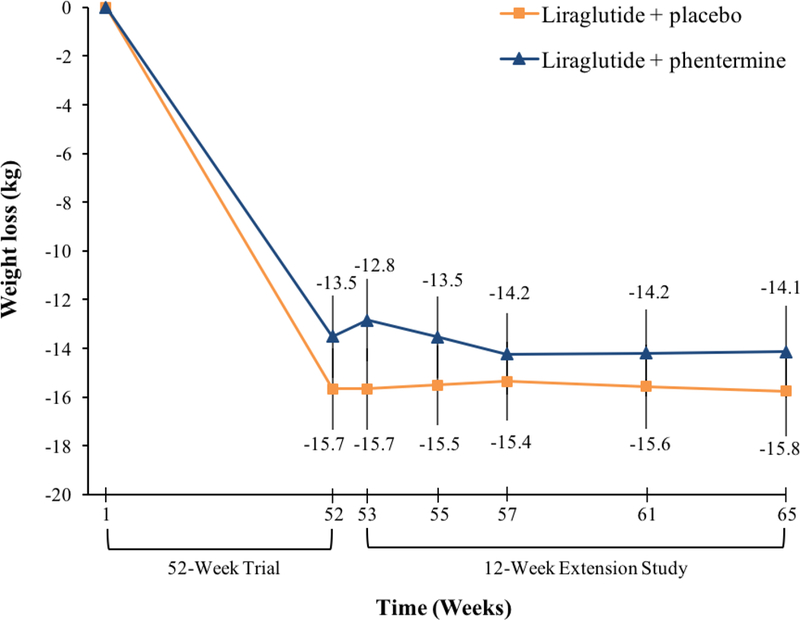
Change in body weight during the 12-week extension study for participants assigned to liraglutide-placebo (N=23) or liraglutide-phentermine (N = 22).
At week 65, one (4.3%) liraglutide-placebo participant and two (9.1%) liraglutide-phentermine participants lost ≥5% of re-randomization weight (Table 2). No participants in either group lost ≥10% of re-randomization weight. A total of 16 (69.9%) liraglutide-placebo and 19 (86.4%) liraglutide-phentermine participants maintained their full weight loss achieved in the prior 1-year trial (p=0.125).
3.4. Changes in Appetite
Table 4 shows mean changes in VAS ratings of appetite for the two groups at each medical visit during the 12-week extension study. At weeks 55, 57, and 61, liraglutide-phentermine participants reported larger reductions in hunger and food preoccupation, as well as larger increases in fullness after meals, than liraglutide-placebo participants; however, not all comparisons were statistically significant. At weeks 55 and 57, for example, liraglutide- phentermine participants reported reductions in hunger of −13.6±4.4 mm and −8.9±5.3 mm, respectively, compared with increases of +4.1±4.2 mm and +6.2±5.0 mm, respectively, in liraglutide-placebo participants (both ps <0.05). Improvements in appetite in the phentermine-treated participants declined over time, and the two groups did not differ on any measure at week 65.
Table 4.
Changes in appetite from re-randomization (week 53) at each treatment visit.
| Characteristic | Liraglutide-placebo | Liraglutide- phentermine |
Statistical comparison (p) |
|---|---|---|---|
| Hunger (mm) | |||
| Week 55 (n = 42) | +4.1 ± 4.0 (−3.9, 12.2) | −13.6 ± 4.4 (−22.4, −4.7) | 0.005 |
| Week 57 (n = 40) | +6.2 ± 5.0 (−4.0, 16.5) | −8.9 ± 5.3 (−19.6, 1.8) | 0.046 |
| Week 61 (n = 41) | +0.5 ± 5.4 (−10.4, 11.5) | −5.9 ± 5.5 (−17.1, 5.3) | 0.412 |
| Week 65 (n = 40) | +0.1 ± 5.4 (−10.8, 11.0) | −3.1 ± 5.4 (−14.0, 7.9) | 0.683 |
| Fullness after meals (mm) | |||
| Week 55 (n = 42) | −5.6 ± 3.3 (−12.2, 1.1) | +3.9 ± 3.6 (−3.3, 11.2) | 0.058 |
| Week 57 (n = 44) | −1.2 ± 3.9 (−9.0, 6.7) | +1.5 ± 4.1 (−6.7, 9.7) | 0.634 |
| Week 61 (n = 42) | −7.5 ± 2.7 (−12.9, −2.1) | +0.8 ± 2.5 (−4.3, 6.0) | 0.030 |
| Week 65 (n = 40) | 0.0 ± 3.4 (−6.9, 7.0) | −2.2 ± 3.6 (−9.4, 5.1) | 0.659 |
| Food preoccupation (mm) | |||
| Week 55 (n = 42) | +2.3 ± 4.3 (−6.3, 10.9) | −14.5 ± 4.5 (−23.5, −5.4) | 0.010 |
| Week 57 (n = 41) | −0.5 ± 4.1 (−8.9, 7.9) | −12.0 ± 4.4 (−21.0, −3.0) | 0.065 |
| Week 61 (n = 40) | +0.1 ± 4.5 (−9.0, 9.2) | −10.9 ± 4.7 (−20.5, −1.4) | 0.097 |
| Week 65 (n = 40) | −0.6 ± 4.6 (−9.9, 8.8) | −8.6 ± 4.6 (−17.9, 0.8) | 0.226 |
| Craving frequency (mm) | |||
| Week 55 (n = 41) | −3.4 ± 5.0 (−13.6, 6.8) | −10.1 ± 5.7 (−21.6, 1.4) | 0.381 |
| Week 57 (n = 44) | +0.5 ± 5.0 (−9.6, 10.7) | −11.6 ± 5.3 (−22.2, −1.0) | 0.104 |
| Week 61 (n = 42) | −9.3 ± 4.9 (−19.2, 0.7) | −7.3 ± 4.7 (−16.8, 2.2) | 0.778 |
| Week 65 (n = 41) | −1.5 ± 5.0 (−11.5, 8.5) | +0.2 ± 5.1 (−10.1, 10.4) | 0.820 |
| Liking of food (mm) | |||
| Week 55 (n = 42) | −3.8 ± 2.6 (−9.1, 1.5) | −2.0 ± 2.9 (−7.8, 3.8) | 0.642 |
| Week 57 (n = 43) | −8.7 ± 4.1 (−17.0, −0.4) | −3.1 ± 4.4 (−12.0, 5.9) | 0.355 |
| Week 61 (n = 42) | −11.8 ± 5.8 (−23.5, −0.1) | −3.1 ± 5.5 (−14.3, 8.1) | 0.283 |
| Week 65 (n = 41) | −8.4 ± 5.8 (−20.0, 3.3) | −8.0 ± 5.9 (−19.9, 4.0) | 0.959 |
Note. Values shown are mean (± SE) changes in appetite measures for the participants who completed VAS ratings at that treatment visit, with the 95% confidence interval in parenthesis.
3.5. Changes in Cardiometabolic and Psychosocial Outcomes
Changes in cardiometabolic risk factors and psychosocial outcomes from re-randomization to week 65 were generally small, and differences between groups were not statistically significant (with one exception) (Table 5). Systolic blood pressure increased from week 53 to week 65 by 1.2±2.1 mm Hg and 2.0±2.1 mm Hg in the liraglutide-placebo and liraglutide-phentermine groups (p=0.769), respectively, and diastolic blood pressure increased by 0.2±1.6 and 1.3±1.6 mm Hg (p=0.625), respectively. Heart rate increased in the two groups by 0.0±1.4 and 2.1±1.4 BPM (p=0.326), respectively. Table 5 shows that at week 65, all of these values remained substantially below those observed at the beginning of the original 1-year trial (i.e., week 1). For example, week-65 systolic blood pressure was 15.5 mm Hg below week 1 values in liraglutide-placebo participants and was reduced by 14.8 mm Hg in liraglutide-phentermine participants.
Table 5.
Estimated mean (± SEM) changes at week 65 in weight, cardiometabolic risk factors, and quality of life, as measured from re-randomization (week 53) and from the start of the original 1-year trial (week 1).
| Characteristic | Liraglutide-placebo N = 23 |
Liraglutide- phentermine N = 22 |
Total N = 45 |
Comparison between groups (p) |
|---|---|---|---|---|
| Systolic BP (mm Hg) | ||||
| From week 53 | +1.2 ± 2.1 (−3.1, 5.5) | +2.0 ± 2.1 (−2.3, 6.4) | +1.6 ± 1.7 (−1.4, 4.6) | 0.769 |
| From week 1 | −15.5 ± 3.3 (−22.1, −8.9) | −14.8 ± 3.3 (−21.4, −8.1) | −15.1 ± 2.3 (−19.7, −10.5)*** | |
| Diastolic BP (mm Hg) | ||||
| From week 53 | +0.2 ± 1.6 (−2.9, 3.4) | +1.3 ± 1.6 (−1.9, 4.5) | +0.8 ± 1.1 (−1.5, 3.0) | 0.625 |
| From week 1 | −4.7 ± 1.9 (−8.6, −0.8) | −3.8 ± 2.0 (−7.8, 0.1) | −4.2 ± 1.4 (−7.0, −1.5)** | |
| Heart rate (bpm) | ||||
| From week 53 | 0.0 ± 1.4 (−2.9, 2.9) | +2.1 ± 1.4 (−0.8, 5.0) | +1.0 ± 1.0 (−1.0, 3.1) | 0.326 |
| From week 1 | −6.6 ± 2.2 (−11.0, −2.2) | −5.1 ± 2.2 (−9.5, −0.7) | −5.8 ± 1.5 (−8.9, −2.7)*** | |
| Waist circumference (cm) ‡ | ||||
| From week 53† | −0.6 ± 0.8 (−2.2, 1.1) | −0.4 ± 0.8 (−2.2, 1.3) | −0.5 ± 0.6 (−1.6, 0.6) | 0.923 |
| From week 1 | −13.8 ± 2.0 (−17.9, −9.7) | −11.1 ± 2.0 (−15.2, −7.0) | −12.4 ± 1.4 (−15.3, −9.5)*** | |
| Total Cholesterol (mg/dL) | ||||
| From week 53† | +3.4 ± 3.3 (−3.3, 10.0) | +0.4 ± 3.3 (−6.2, 7.0) | +1.9 ± 2.3 (−2.8, 6.5) | 0.528 |
| From week 1 | −7.4 ± 4.4 (−16.3, 1.5) | −7.2 ± 4.4 (−16.2, 1.7) | −7.3 ± 3.1 (−13.5, −1.1)* | |
| HDL Cholesterol (mg/dL) | ||||
| From week 53† | +0.6 ± 1.4 (−2.2, 3.4) | +2.0 ± 1.4 (−0.8, 4.8) | +1.3 ± 1.0 (−0.7, 3.3) | 0.483 |
| From week 1 | +4.0 ± 1.8 (0.3, 7.7) | +6.7 ± 1.8 (3.0, 10.4) | +5.3 ± 1.3 (2.7, 7.9)*** | |
| LDL Cholesterol (mg/dL) | ||||
| From week 53† | +2.3 ± 2.8 (−3.3, 7.9) | −2.4 ± 2.8 (−7.9, 3.2) | 0.0 ± 2.0 (−4.0, 3.9) | 0.241 |
| From week 1 | −8.1 ± 4.1 (−16.3, 0.2) | −10.7 ± 4.1 (−19.0, −2.5) | −9.4 ± 2.9 (−15.2, −3.6)** | |
| Triglycerides (mg/dL) | ||||
| From week 53† | +4.1 ± 4.7 (−5.3, 13.5) | +6.6 ± 4.7 (−2.8, 16.0) | +5.3 ± 3.3 (−1.2, 11.9) | 0.740 |
| From week 1 | −19.3 ± 7.2 (−33.8, −4.8) | −20.1 ± 7.2 (−34.7, −5.6) | −19.7 ± 5.0 (−29.9, −9.6)*** | |
| C-Reactive Protein (mg/L) | ||||
| From week 53† | −0.8 ± 0.6 (−1.9, 0.4) | −0.6 ± 0.6 (−1.7, 0.6) | −0.7 ± 0.4 (−1.5, 0.2) | 0.831 |
| From week 1 | −3.9 ± 1.0 (−6.0, −1.8) | −3.2 ± 1.1 (−5.3, −1.1) | −3.5 ± 0.7 (−5.0, −2.1)*** | |
| HbA1C | ||||
| From week 53† | 0.0 ± 0.0 (−0.1, 0.01) | 0.0 ± 0.0 (−0.04, 0.1) | 0.0 ± 0.0 (−0.1, 0.02) | 0.198 |
| From week 1 | −0.6 ± 0.1 (−0.7, −0.5) | −0.7 ± 0.1 (−0.8, −0.5) | −0.6 ±0.0 (−0.7, −0.6)*** | |
| Fasting Glucose (mg/dL) | ||||
| From week 53† | +1.4 ± 1.6 (−1.8, 4.6) | +6.3 ± 1.6 (3.1, 9.6) | +3.9 ± 1.2 (1.5, 6.2) | 0.034 |
| From week 1 | −6.9 ± 1.5 (−10.0, −3.8) | −4.1 ± 1.5 (−7.2, −1.0) | −5.5 ± 1.1 (−7.7, −3.3)*** | |
| Fasting Insulin (μU/mL) | ||||
| From week 53† | +0.2 ± 1.0 (−1.8, 2.2) | +0.5 ± 1.0 (−1.5, 2.5) | +0.4 ± 0.7 (−1.1, 1.8)* | 0.421 |
| From week 1 | −2.5 ± 0.9 (−4.2, −0.7) | −1.0 ± 0.9 (−2.8, 0.7) | −1.8 ± 0.6 (−3.0, −0.5)** | |
| HOMA-IR | ||||
| From week 53† | +0.1 ± 0.2 (−0.3, 0.5) | +0.3 ± 0.2 (−0.2, 0.7) | +0.2± 0.2 (−0.1, 0.5)* | 0.260 |
| From week 1 | −0.6 ± 0.2 (−1.0, −0.2) | −0.3 ± 0.2 (−0.7, 0.1) | −0.5 ± 0.1 (−0.8, −0.2)** | |
| Depression Symptoms | ||||
| (PHQ-9) ‡ | ||||
| From week 53† | +0.2 ± 0.4 (−0.6, 1.0) | 0.0 ± 0.4 (−0.8, 0.8) | +0.1 ± 0.3 (−0.5, 0.6) | 0.854 |
| From week 1 | −1.9 ± 0.7 (−3.3, −0.5) | −2.7 ± 0.7 (−4.2, −1.3) | −2.3 ± 0.5 (−3.3, −1.3)*** | |
| SF-36 Physical Health | ||||
| From week 53† | +0.3 ± 1.0 (−1.7, 2.4) | −1.2 ± 1.0 (−3.2, 0.9) | −0.4 ± 0.7 (−1.9, 1.1) | 0.488 |
| From week 1 | +3.3 ± 1.6 (−0.01, 6.6) | +1.9 ± 1.7 (−1.5, 5.3) | +2.6 ± 1.2 (0.3, 4.9)* | |
| SF-36 Mental Health | ||||
| From week 53† | −0.1 ± 1.1 (−2.4, 2.2) | +0.2 ± 1.1 (−2.1, 2.5) | +0.1 ± 0.8 (−1.6, 1.7) | 0.561 |
| From week 1 | +4.1 ± 1.6 (0.8, 7.4) | +6.2 ± 1.7 (2.9, 9.6) | +5.1 ± 1.2 (2.8, 7.5)*** | |
Note. Values shown are means (± SEM) of the intention-to-treat population (N = 45) with the 95% confidence interval in parenthesis. When conducting analyses, values for triglycerides, C-reactive protein, fasting insulin, and HOMA-IR were log transformed and values for depression symptoms (PHQ-9), SF-36 Physical Health and SF-36 and Mental Health were square root transformed. The untransformed means and confidence intervals are presented in the table. The final column lists the p value for between-group comparisons of change from randomization (week 53). In cases where there was no significant difference between the groups, changes over time in the total sample (N=45) that were statistically significant are indicated using
p <0.05
p <0.01
p < 0.001.
BP = blood pressure; HDL = high-density lipoprotein; LDL = low-density lipoprotein; Hb = hemoglobin; HOMA-IR = homeostatic model assessment of insulin resistance; PHQ-9 = Patient Health Questionnaire, 9-item; SF-36 = Short-Form Health Survey – 36 Item.
Re-randomization measurements of blood assays and psychosocial outcomes were completed at week 52 (end of the original 1-year trial).
Analyses of change from week 53 to week 65 in waist circumference and depression symptoms controlled for differences in these measures at re-randomization.
Fasting glucose was the only cardiometabolic outcome that differed between the two groups in change from week 53 to week 65, with an increase of 1.4±1.6 mg/dL in the liraglutide-placebo group, compared with an increase of 6.3±1.6 mg/dL in liraglutide-phentermine participants (p=0.034). As measured from the start of the 1-year trial (week 1), participants in both groups maintained substantial improvements in waist circumference, triglycerides, high- density-lipoprotein (HDL) cholesterol, and c-reactive protein, with smaller improvements in several other outcomes including fasting glucose and low-density-lipoprotein (LDL) cholesterol.
There were no significant differences between the two groups in changes from week 53 to week 65 in physical and mental health or depression (Table 5). Both groups maintained improvements in these outcomes relative to the start of the 1-year trial.
3.6. Safety and Adverse Events
During the 12-week extension trial, one liraglutide-placebo participant had a measured systolic blood pressure above 160 mm Hg, but values fell below this level with repeated assessment. No liraglutide-phentermine participant had a measured systolic blood pressure >160 mm Hg. However, at week 61, one participant had an increase to 158 mm Hg, from a re-randomization value of 132 mm Hg; phentermine was discontinued, but the patient remained on liraglutide. No participant had a measured diastolic blood pressure >100 mm Hg or heart rate >100 bpm, and none reported suicidal ideation, as assessed by the C-SSRS (23).
Similar percentages of participants in the liraglutide-placebo and liraglutide-phentermine groups reported experiencing an AE during the 12-week extension study (65.2 and 54.5%, respectively). Table 6 summarizes events that occurred in 5% or more of participants in either treatment group. The most common AEs were musculoskeletal injury and gastroesophageal reflux disorder. Heart palpitations were reported by two placebo-treated participants and no phentermine-treated participants. A hypertensive AE was recorded for one phentermine-treated participant, as described previously. All AEs were resolved upon subsequent assessment. There were no serious adverse events during the 12-week extension study.
Table 6.
Adverse events with an incidence of 5% or more of participants in any treatment group occurring between re-randomization (week 53) and week 65.
| Event | Liraglutide-placebo, N = 23 |
Liraglutide-phentermine, N =22 |
||
|---|---|---|---|---|
| N (%) | Events, N | N (%) | Events, N | |
| All adverse events | 15 (65.2%) | 23 | 12 (54.5%) | 16 |
| Adverse events ≥5% in any | 12 (52.2%) | 16 | 11 (50.0%) | 14 |
| treatment group | ||||
| Musculoskeletal injury | 5 (21.7%) | 6 | 1 (4.5%) | 1 |
| Gastroesophageal reflux disorder | 1 (4.3%) | 1 | 3 (13.6%) | 3 |
| Diarrhea | 1 (4.3%) | 2 | 2 (9.1%) | 2 |
| Dry mouth | 2 (8.7%) | 2 | 1 (4.5%) | 1 |
| Gastroenteritis | 1 (4.3%) | 1 | 2 (9.1%) | 2 |
| Nausea | 1 (4.3%) | 1 | 2 (9.1%) | 2 |
| Upper respiratory infection | 2 (8.7%) | 2 | 1 (4.5%) | 1 |
| Heart palpitations | 2 (8.7%) | 2 | 0 | 0 |
| Restlessness | 0 | 0 | 2 (9.1%) | 2 |
| Urinary tract infection | 2 (8.7%) | 2 | 0 | 0 |
3.7. Continued Liraglutide Monotherapy
With the discontinuation of phentermine/placebo at week 65 (but with the continued use of liraglutide for 8 more weeks), participants who had been assigned to liraglutide-placebo gained 0.3±0.4% of their re-randomization weight (i.e., 0.3 kg) from week 65 to week 73, compared with a significantly greater gain of 1.5±0.4% (1.6 kg) for those assigned to liraglutide-phentermine (p=0.021). Thus, the discontinuation of phentermine resulted in these participants’ returning to their re-randomization weight. One participant in each group maintained a weight loss ≥5% of re-randomization weight at week 73 (Supplemental Table 2).
During the 8-week period of liraglutide monotherapy, no blood pressure or pulse values exceeded pre-determined upper limits. Supplemental Table 3 provides a summary of AEs reported between weeks 65 and 73. There was one serious adverse event (SAE) during this time. A participant originally assigned to liraglutide-phentermine experienced quadriplegic paralysis following elective surgery for a cervical spine disorder; the participant died shortly after surgery.
4. Discussion
In adults with obesity who had lost 12.6% of initial weight (14.2 kg) with 1 year of intensive behavioral therapy and liraglutide 3.0 mg, the addition for 12 weeks of phentermine 15.0 mg (to liraglutide) reduced body weight by an additional 1.6% (1.4 kg). This reduction was not significantly larger than the 0.1% loss (0.1 kg) in participants assigned to liraglutide-placebo. The 1.6% reduction in re-randomization weight was approximately half the amount we had expected, based on prior studies that combined phentermine with topiramate (12) or with lorcaserin (14). Weight reduction of this magnitude does not meet conventional criteria for clinically meaningful weight loss of at least 2–5% of initial weight (24–25), although two liraglutide-phentermine participants did lose ≥5% of their re-randomization weight (as did one liraglutide-placebo participant). Phentermine-treated participants regained their additional weight loss (i.e., 1.5%) in the 8 weeks following phentermine’s discontinuation.
The combination of liraglutide and phentermine appeared to be well tolerated. There was only one clinically significant elevation in blood pressure (which resulted in discontinuation) and no significant elevations in pulse. At 12 weeks, liraglutide-phentermine participants had relative mean increases in systolic and diastolic blood pressure of 0.8 and 1.1 mm Hg more, respectively, than placebo; heart rate increased by 2.1 BPM more. The absence of greater increases in blood pressure and pulse with this combination therapy is noteworthy given that each medication may increase one or more of these values in some patients (17,18). However, the sample size (N=45) of the present pilot study is too small to provide a robust assessment of the potential cardiovascular and other risks associated with this combination therapy. In addition, we note that all patients had been established (and closely monitored) on liraglutide for 1 year prior to their taking phentermine. Only participants who had acceptable blood pressure and pulse levels after 1 year on liraglutide were allowed to participate in the extension study. Clinically significant elevations in blood pressure or pulse potentially could be observed if the two medications were introduced concurrently to induce weight loss. Because both groups were provided with at least one active medication (liraglutide), we also are not able to determine how the observed rates of adverse events would compare to a no-treatment or placebo-only control group.
In our original 1-year randomized controlled trial, participants who received the combination of IBT plus liraglutide 3.0 mg reported significantly greater reductions in hunger and preoccupation with food than participants treated with IBT alone (20). These improvements in appetite, however, declined after week 24 and were not statistically different from IBT-alone at weeks 40 or 52. A similar pattern was observed in the 12-week extension study. Participants assigned to liraglutide-phentermine reported marked reductions in hunger and food preoccupation during the first 4 to 8 weeks of the extension trial, compared to generally small increases reported by liraglutide-placebo participants. Phentermine-treated participants lost 1.4% of their re-randomization weight in these first 4 weeks, after which their self-reported improvements in appetite declined, and they lost only an additional 0.2% of initial weight in the remaining 8 weeks of the trial. If replicated, the present findings suggest that patients are likely to experience noticeable improvements in appetite (and weight) in only the first 1–2 months of combined liraglutide-phentermine treatment, with limited additional benefit thereafter (alhough ongoing treatment may help to maintain the additional weight loss.) From a clinical perspective, such improved appetite control potentially could be useful in coping with overeating during the winter holidays.
We are not able to explain why the liraglutide-phentermine group had a 4.9 mg/dL larger increase in fasting glucose than the liraglutide-placebo group during the extension study. Previous studies that evaluated the effects of phentermine alone and in combination with other medications did not find that phentermine increased glucose when compared to placebo or to other weight loss medication alone (26, 27). In the present study, both groups maintained significant decreases in fasting glucose relative to the start of the original 52-week trial. Because 23 secondary outcomes were compared in the extension study, it is possible that at least one significant difference could have been attributable to chance. We believe that this finding will need to be replicated in order to determine whether the combination of lirglutide and phentermine produces a true small increase in blood glucose.
The present study design, in which phentermine was used to induce further weight loss after participants had already lost 12.6% of initial weight with liraglutide alone, clearly represents a different paradigm than the use of combination therapy to induce initial weight loss. No present behavioral or pharmacologic weight loss therapy consistently reduces and maintains body weight, on average, by more than 10–12% below baseline levels (5). This finding has been interpreted as evidence of the defense of body weight, which is supported by studies of changes in appetite-related hormones (8,9) and energy expenditure (7). From this perspective, phentermine could be expected to add to mean weight loss when combined with liraglutide (or topiramate or lorcaserin) to induce an initial weight loss of up to 10–12% of body weight, but not to add substantially to a pre-existing weight loss of that amount. Using a similar study design, we found that patients who had lost an average of 11.6% in 1 year with sibutramine and behavior therapy lost no additional weight when sibutramine was combined for an additional 16 weeks with orlistat (+0.1 kg gain) or placebo (+0.5 kg gain) (23). Thus, the addition of a second weight loss medication could potentially facilitate the maintenance of a prior 10–12% reduction in weight but not the induction of further clinically meaningful weight loss. The large long-term weight losses of 20–25% of initial weight achieved with bariatric surgery (e.g., Roux-en-Y bypass) appear attributable, in part, to the effects of these procedures on appetite-related hormones, in addition to the robust effects of gastric restriction (28, 29).
Strengths of our 12-week extension study include its use of a randomized, double blind design with a group of participants who had achieved a mean 12.6% reduction in weight in a prior 1-year trial. The study’s small sample size is a major limitation that prevented us from having optimal statistical power to test differences between the groups. The addition of only a handful of participants likely would have resulted in a statistically significant difference at week 12 between the liraglutide-phentermine and liraglutide-placebo groups, a difference that was observed at all other weeks of the extension trial. We do not, however, believe that a larger sample size would have significantly increased the size of the added weight loss achieved with liraglutide-phentermine, to approach a clinically meaningful reduction. A second limitation is that we only prescribed phentermine for 12 weeks; use of phentermine for 6 to 12 months may have resulted in greater weight loss (although the present data do not suggest such a likelihood). We limited phentermine’s use to 12 weeks in order to minimize potential safety concerns in this first pilot investigation of its combination with liraglutide 3.0 mg.
In summary, the addition of phentermine to liraglutide for 12 weeks, in participants with obesity who had previously lost 12.6% of initial weight with liraglutide alone, induced a further 1.5% reduction in body weight relative to placebo. The combination of liraglutide and phentermine appeared to have an acceptable safety profile, but a substantially larger sample of participants is needed to definitively assess potential cardiovascular and other risks of this treatment combination.
Supplementary Material
Highlights.
Phentermine did not improve on large weight losses achieved with liraglutide alone.
The addition of phentermine to liraglutide led to short-term improvements in appetite.
The combination of liraglutide and phentermine appeared to be well-tolerated.
6 Acknowledgements
6.2 Funding: This study was supported, in part, by an Investigator-Sponsored Study award from Novo Nordisk to the University of Pennsylvania on behalf of Dr. Wadden. Dr. Tronieri’s effort was supported, in part, by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health (K23DK116935). Dr. Chao was supported by the National Institute of Nursing Research of the National Institutes of Health (K23NR017209).
6.3 Role of the funding source: Novo Nordisk did not have any role in the study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the article for publication.
Footnotes
Declaration of Interests: Dr. Tronieri, Ms. Walsh, and Dr. Alamuddin report having served as consultants to Novo Nordisk. Dr. Wadden reports serving on advisory boards for Novo Nordisk and Weight Watchers Inc, and Dr. Berkowitz serves as a consultant to Eisai Pharmaceutical. Dr. Chao has consulted with and received grant support from Shire Pharmaceutical, outside the submitted work.
Trial Registration: ClinicalTrials.gov number, NCT02911818
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Apovian CM, Aronne LJ, Bessesen DH, et al. Pharmacological management of obesity: An Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2015;100:342–362. [DOI] [PubMed] [Google Scholar]
- 2.Khera R, Murad MH, Chandar AK, et al. Association of pharmacological treatments for obesity with weight loss and adverse events: a systematic review and meta-analysis. JAMA 2016;315:2424–2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yanovski SZ, Yanovski JA Long-term drug treatment for obesity: a systematic and clinical review. JAMA. 2014;311:74–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jensen MD, Ryan DH, Donato SM, et al. Executive summary: guidelines (2013) for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Obesity Society published by the Obesity Society and American College of Cardiology/American Heart Association Task Force on practice guidelines. Based on a systematic review from The Obesity Expert Panel, 2013. Obesity (Silver Spring) 2014;22(suppl 2):S5–S39. [DOI] [PubMed] [Google Scholar]
- 5.Heymsfield SB, Wadden TA. Mechanisms, pathophysiology, and management of obesity. N Engl J Med 2017;376:254–266. [DOI] [PubMed] [Google Scholar]
- 6.Wadden TA, Womble LG, Sarwer DB, Berkowitz RI, Clark VL, Foster GD. Great expectations: “I’m losing 25% of my weight no matter what you say”. J Consult Clin Psychol. 2003;71:1084–1089. [DOI] [PubMed] [Google Scholar]
- 7.Rosenbaum M, Goldsmith R, Bloomfield D, et al. Low-dose leptin reverses skeletal muscle, autonomic, and neuroendocrine adaptations to maintenance of reduced weight. J Clin Invest. 2005;115:3579–3586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sumithran P1, Prendergast LA, Delbridge E, Purcell K, Shulkes A, Kriketos A, Proietto J. Long-term persistence of hormonal adaptations to weight loss. N Engl J Med. 2011. 27;365:1597–604. [DOI] [PubMed] [Google Scholar]
- 9.Schwartz MW, Seeley RJ, Zeltser LM, et al. Obesity pathogenesis: an Endocrine Society scientific statement. Endocr Rev. 2017;38:267–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wadden TA, Butryn ML, Byrne KJ. Efficacy of lifestyle modification for long-term weight control. Obes Res. 2004;12(suppl):151S–162S. [DOI] [PubMed] [Google Scholar]
- 11.Gadde KM, Allison DB, Ryan DH, et al. Effects of low-dose, controlled-release, phentermine plus topiramate combination on weight and associated comorbidities in overweight and obese adults (CONQUER): a randomised, placebo-controlled, phase 3 trial. Lancet 2011;377:1341–1352. [DOI] [PubMed] [Google Scholar]
- 12.Aronne LJ, Wadden TA, Peterson C, Winslow D, Odeh S, Gadde KM. Evaluation of phentermine and topiramate versus phentermine/topiramate extended-release in obese adults. Obesity (Silver Spring) 2013;21:2163–2171. [DOI] [PubMed] [Google Scholar]
- 13.Greenway FL, Fujioka K, Plodowski RA, et al. Effect of naltrexone plus buproprion on weight loss in overweight and obese adults (COR-I): a multi-centre, randomised, double-blind, placebo-controlled, phase 3 trial [abstract]. Lancet 2010; 376:595–605. [DOI] [PubMed] [Google Scholar]
- 14.Smith SR, Garvey WT, Greenway FL, et al. Coadministration of lorcaserin and phentermine for weight management: A 12-week, randomized, pilot safety study. Obesity (Silver Spring) 2017;25:857–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wadden TA, Walsh OA, Berkowitz RI, et al. Intensive behavioral therapy for obesity combined with liraglutide 3.0 mg: a randomized controlled trial. Obesity (Silver Spring). 2019, 27:75–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Centers for Medicare & Medicaid Services. Decision Memo for Intensive Behavioral Therapy for Obesity (CAG-00423N). November 29, 2011. [Google Scholar]
- 17.Adipex-p (phentermine hydrochloride USP)[package insert]. Sellersville, PA: Teva Pharmaceuticals; 2012. [Google Scholar]
- 18.Liraglutide (rDNA origin) injection [package insert]. Plainsboro, NJ: Novo Nordisk, Inc; 2017. [Google Scholar]
- 19.Womble LG, Wadden TA, Chandler JM, Martin AR. Agreement between weekly vs. daily assessment of appetite. Appetite. 2003;40:131–135. [DOI] [PubMed] [Google Scholar]
- 20.Tronieri JS, Wadden TA, Berkowitz RI, et al. Effect of liraglutide on appetite, food preoccupation, and food liking: Results of a randomized controlled trial. International Journal of Obesity. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med 2001;16:606–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ware J, Sherbourne C. The MOS 36-item Short Form Health Survey (SF-36): conceptual framework and item selection. Med Care 1992;30:473–483. [PubMed] [Google Scholar]
- 23.Posner K, Brent D, Lucas C, et al. Columbia-Suicide Severity Rating Scale (C-SSRS). New York, NY: Columbia University Medical Center; 2008. [Google Scholar]
- 24.Wing RR, Lang W, Wadden TA, et al. Benefits of modest weight loss in improving cardiovascular risk factors in overweight and obese individuals with type 2 diabetes. Diabetes Care. 2011;34:1481–1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wadden TA, Berkowitz RI, Womble LG, Sarwer DB, Arnold ME, Steinberg CM. Effects of sibutramine plus orlistat in obese women following 1 year of treatment by sibutramine alone: a placebo-controlled trial. Obes Res. 2000;8:431–437. [DOI] [PubMed] [Google Scholar]
- 26.Allison DB, Gadde KM, Garvey WT, et al. Controlled-release phentermine/topiramate in severely obese adults: A randomized controlled trial (EQUIP). Obesity. 2012;20:330–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aronne LJ, Wadden TA, Peterson C, Winslow D, Odeh S, Gadde KM. Evaluation of phentermine and topiramate versus phentermine/topiramate extended-release in obese adults. Obesity. 2013;21:2163–2171. [DOI] [PubMed] [Google Scholar]
- 28.Miras AD, Le Roux CW. Mechanisms underlying weight loss after bariatric surgery. Nature Reviews Gastroenterology and Hepatology. 2013;10(10):575–84. [DOI] [PubMed] [Google Scholar]
- 29.Alamuddin N, Vetter ML, Ahima RS, et al. Changes in Fasting and Prandial Gut and Adiposity Hormones Following Vertical Sleeve Gastrectomy or Roux-en-Y-Gastric Bypass: an 18-Month Prospective Study. Obesity Surgery. 2017;27:1563–1572. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


