Abstract
Introduction:
Risk factors for young adults with mTBI are not well understood. Improved understanding of age and sex as risk factors for impaired six-month outcomes in young adults is needed.
Methods:
Young adult mTBI subjects aged 18–39-years (18–29y; 30–39y) with six-month outcomes were extracted from the Transforming Research and Clinical Knowledge in Traumatic Brain Injury Pilot (TRACK-TBI Pilot) study. Multivariable regressions were performed for outcomes with age, sex, and the interaction factor age-group*sex as variables of interest, controlling for demographic and injury variables. Mean-differences (B) and 95% CIs are reported.
Results:
One hundred mTBI subjects (18–29y, 70%; 30–39y, 30%; male, 71%; female, 29%) met inclusion criteria. On multivariable analysis, age-group*sex was associated with six-month post-traumatic stress disorder (PTSD; PTSD Checklist-Civilian version); compared with female 30–39y, female 18–29y (B=−19.55 [−26.54, −4.45]), male 18–29y (B=−19.70 [−30.07, −9.33]), and male 30–39y (B=−15.49 [−26.54, −4.45]) were associated with decreased PTSD symptomatology. Female sex was associated with decreased six-month functional outcome (Glasgow Outcome Scale-Extended (GOSE): B=−0.6 [1.0, −0.1]). Comparatively, 30–39y scored higher on six-month nonverbal processing speed (Wechsler Adult Intelligence Scale-Processing Speed Index (WAIS-PSI); B=11.88, 95% CI [1.66, 22.09]).
Conclusions:
Following mTBI, young adults aged 18–29y and 30–39y may have different risks for impairment. Sex may interact with age for PTSD symptomatology, with females 30–39y at highest risk. These results may be attributable to cortical maturation, biological response, social modifiers, and/or differential self-report. Confirmation in larger samples is needed, however prevention and rehabilitation/counseling strategies after mTBI should likely be tailored for age and sex.
Keywords: age factors, common data elements, functional disability, mild traumatic brain injury, post-traumatic stress disorder, risk factors, sex, young adults
INTRODUCTION
Mild traumatic brain injuries (mTBI) represent 70–90% of all treated TBI cases [1]. Up to 30% of mTBI patients may experience residual cognitive, functional, and neuropsychological symptoms beyond six months [2–4] with resulting social and vocational consequences [5,6]. Heterogeneity in mTBI demographics, comorbidities, physiologies and risk factors lead to a wide range of clinical outcomes, for which consensus predictors remain elusive. While there is sizeable literature on prognostic factors for TBI, studies investigating the role of age and sex on outcome remain limited especially in mTBI.
Age is commonly stratified into two cohorts in head injury analyses, young and old, with varying age cutoffs between ages 40 to 65 years [7–9]. Aside from sports-related concussions in college athletes, there is a comparatively little focus on risk factors for poor outcome in mTBI patients aged 18 to 39 years. Younger mTBI patients experience difficulties returning to work or higher education due to cognitive and executive function deficits [10–13]. Differences in brain maturation rates and social environmental influences exist in this population [14,15]. Rarely do studies narrow age stratification further in their analyses despite preliminary studies that find increasingly poorer TBI outcomes for each advancing decade of life [16,17]. Due to known ongoing cortical development in the post-adolescent period [18,19], stratifying young adults into younger and older young adult bands could improve the assessment of risk factors for mTBI recovery and outcome.
In North American studies, males were found to have 1.4–2.1 greater risk for mTBI compared with females [1]. While females are less likely to present with TBI of any severity, they have a greater chance of mortality and poor outcomes [20,21]. Due to varying sex hormone levels, brain cortical thickening and complexity can differ between sexes [22–25]. These circulating hormones continue to exert their effect on cerebral organization and neuroplasticity into young adulthood [14,15]. Differences in physiology, societal expectations, and help-seeking preferences between males and females may also influence patient outcomes [26,27]. Much literature on the influence of sex in TBI outcomes is based on military TBI data which includes a disproportionate number of males [28–31]. When evaluating service members and veterans, multiple studies found that females were more likely to be diagnosed with depression and/or anxiety following TBI and to report greater post-concussive and PTSD symptoms compared to males. However, the effect of sex on long-term outcomes in mTBI remains understudied.
We looked at a multidimensional set of outcomes by age and sex for young adults diagnosed with mTBI. Improved understanding of how age and sex may influence recovery will better guide clinical practice by identifying those in need of early and closer monitoring and tailored intervention. We utilized the prospective Transforming Research and Clinical Knowledge in Traumatic Brain Injury Pilot (TRACK-TBI Pilot) study to characterize demographics and injury history by age group and sex, and evaluated associations with a multidimensional set of outcomes at six-months post-TBI in a cohort of young adults (aged 18–39y).
METHODS
Study design
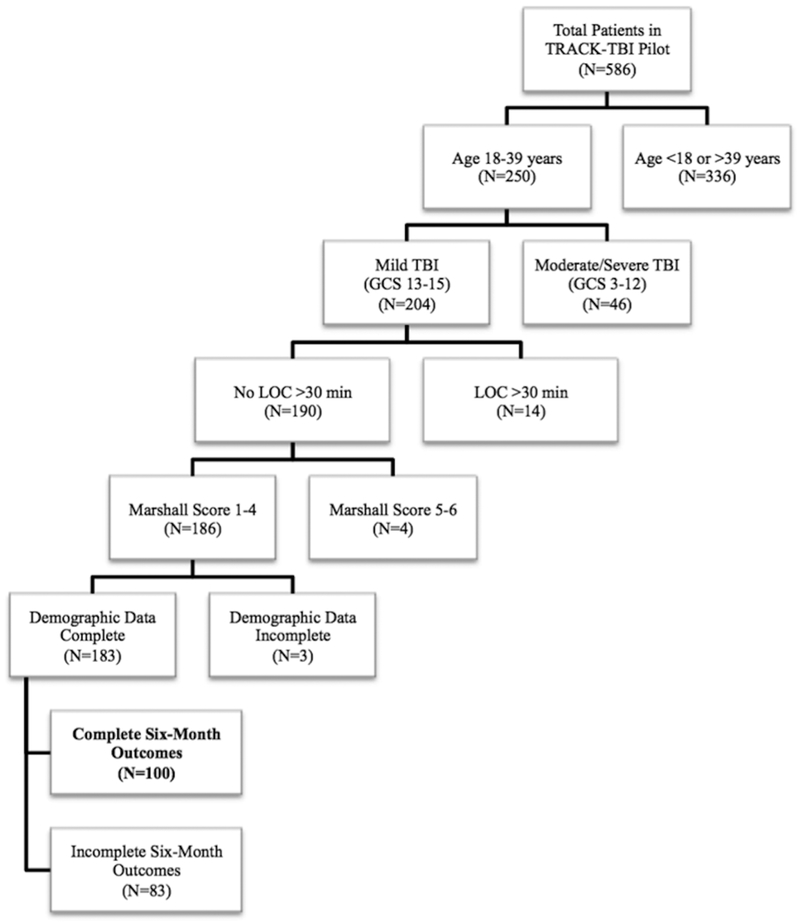
The prospective, multicenter TRACK-TBI Pilot study was conducted at three U.S. Level 1 trauma centers (Zuckerberg San Francisco General Hospital (San Francisco, CA, USA), University of Pittsburgh Medical Center (Pittsburgh, PA, USA), and University Medical Center Brackenridge (Austin, TX, USA)) using the National Institute of Neurological Disorders and Stroke (NINDS) TBI Common Data Elements (CDEs) [32–36]. Inclusion criteria for TRACK-TBI Pilot were external force trauma to the head, presentation to one of the three enrolling trauma centers, and a clinically indicated head computed tomography (CT) scan within 24 hours of injury. Exclusion criteria were pregnancy, ongoing life-threatening disease (e.g., end-stage malignancy), police custody, involuntary psychiatric hold, and non-English speakers due to multiple outcome measures being administered and/or normed only in the English language. As the goal of the current analysis was to evaluate the associations between age, sex, and injury characteristics and six-month outcomes in young adults following mTBI, subjects were included if they were age 18–39y with an emergency department (ED) admission GCS score of 13–15, loss of consciousness (LOC) <30 minutes, Marshall CT score <5 (1=no visible intracranial pathology on CT; 2=cisterns present, midline shift 0–5 mm and/or lesions present, no lesion >25 cm3; 3=cisterns compressed or absent, midline shift 0–5 mm and/or lesions present, no lesion >25 cm3; 4=midline shift >5 mm, no lesion >25 cm3; 5=surgically evacuated lesion; 6=lesion >25 cm3 not surgically evacuated) to include those without the need for surgical decompression and/or large intracranial mass lesions [37], and complete six-month outcome measures (Figure 1).
Figure 1. Flowchart of included subjects.
Flowchart of 100 mild traumatic brain injury subjects aged 18–39 years meeting inclusion criteria from the TRACK-TBI Pilot study.
In epidemiologic reporting of morbidity and mortality in TBI [38], and large prognostic studies of moderate to severe head and facial trauma [17,39–41], age has been routinely grouped by decade of life. Consistent with prior studies, in our young adult cohort we analyzed age by decade (18–29y, 30–39y) and sex (male, female) as primary variables of interest.
Eligible subjects were enrolled by convenience sampling from years 2010 to 2012. Institutional Review Board approval was obtained at each of the participating sites. Informed consent was obtained prior to study enrollment. For subjects unable to provide consent due to their injury, consent was obtained from their legally authorized representative. Subjects were then re-consented, if cognitively able, during the course of their clinical care and/or follow-up time points for continued participation in the study.
Outcome measures
Outcome measures were collected through in-person interview at six-months postinjury. Subjects completing all of the following TBI CDE outcome measures were included in the current analysis:
Glasgow Outcome Scale-Extended (GOSE):
The GOSE is a structured interview which provides an overall measure of disability based on cognition, independence, employability, and social/community participation, and has been widely used as a standard outcome measure for TBI studies [42]. Scores include: 1=dead, 2=vegetative state, 3=lower severe disability, 4=upper severe disability, 5=lower moderate disability, 6=upper moderate disability, 7=lower good recovery, and 8=upper good recovery. A GOSE score of 8 reflects recovery to baseline without new disability.
Brief Symptom Inventory 18 (BSI18):
The BSI18 is an 18-item self-report measure of distress each rated from 0=not at all to 4=extremely. These items comprise three subscales of anxiety, depression, and somatization, the sums of which yield a normed Global Severity Index (GSI) T score. Higher GSI scores reflect greater psychological distress. Normed T scores were used [43].
Rivermead Postconcussion Symptoms Questionnaire (RPQ):
The RPQ is a 16-item questionnaire in which subjects rate on a 0–4 Likert scale the extent to which symptoms have been more problematic after compared to before their injury (0=not experienced, 1=no worse than preinjury, 2=mild problem, 3=moderate problem, 4=severe problem). The current analysis examined the RPQ13, a composite of 13 items delineating later-onset symptoms after TBI. These symptoms are associated with impacts on participation, psychosocial functioning. In practice, a clinical referral for specialist assessment or treatment services is recommended if they do not resolve within three months [44].
Posttraumatic Stress Disorder (PTSD) Checklist for Civilians (PCL-C):
The PCL-C is a 17-item self-report measure for symptoms of re-experiencing, avoidance, and hypervigilance, and assesses for PTSD symptomatology per the Diagnostic and Statistical Manual for Psychological Disorders, Fourth Edition (DSM-IV) criteria. Patients rate the extent to which they have been bothered by each symptom on a 1–5 scale (1=not at all; 5=extremely) to yield a total score of 17–85. Higher values in the civilian population ≥36 are suggestive of PTSD [45].
Satisfaction with Life Scale (SWLS):
The SWLS is a 5-item measure of life satisfaction. Subjects are asked to rate their agreement with each item on a 7-point scale (1=strongly disagree; 7=strongly agree), with higher scores indicating greater satisfaction. A score of 20 is considered the “neutral” point below which participants are considered “unsatisfied” with life to varying degrees [46].
Trail Making Test (TMT):
The TMT is a two-part timed test (TMT-A and TMT-B). TMT-A assesses visual processing and TMT-B assesses mental flexibility and processing speed. Lower scores suggest better performance. In order to increase the accuracy of the score with respect to the flexibility and processing speed without accounting for visual processing, the first trial is subtracted from the second trial to yield the TMT B minus A score (TMT B-A) [47]
Wechsler Adult Intelligence Scale Fourth Edition, Processing Speed Index (WAIS-PSI):
The WAIS-PSI includes the Symbol Search and Coding tasks, which require visual attention and motor speed. The processing speed index (PSI) score is normed by age as part of the scoring process with percentile scores shown [48].
California Verbal Learning Test, Second Edition (CVLT-II):
The CVLT-II is a verbal learning and memory task in which five learning trials, an interference trial, an immediate recall trial and a post-20 minute recall trial are performed. The CVLT-II Trials 1–5 standard score (CVLT Trials 1–5) provides an age-normed global index of verbal learning ability and was used in the current analysis [49].
Statistical analysis
Subjects were grouped as young adults aged 18–29y and 30–39y [50], and by male/female sex as variables of interest. Descriptive statistics are presented as means and standard deviations (SD) for continuous variables and proportions for categorical variables. Group differences were assessed using analysis of variance for continuous variables and Pearson’s chi-squared test for categorical variables, except in cases with cell counts ≤5 when Fisher’s Exact Test was used. Multivariable regression was performed for each outcome measure with age group and sex as target variables, controlling for race (Caucasian, African American/African, other races), years of education, psychiatric history (no/yes), mechanism of injury of assault (no/yes), LOC (none; <30 minutes; unknown), GCS (15 vs. <15), acute intracranial lesion on CT (no/yes), and polytrauma (Abbreviated Injury Scale (AIS) score of >2 in any extracranial region) which are validated predictors for outcome following mTBI [51]. The interaction factor age-group*sex was analyzed in multivariable outcome models, and if not statistically significant, was removed from the regression. Mean differences (B) and their associated 95% confidence intervals (CI) are reported for each predictor in the regression analyses. Statistical significance was assessed at p<0.05 for descriptive variables. To account for multiple comparisons, the Benjamini-Hochberg Procedure was utilized for the 24 main comparisons [three variables (age group, sex, and age group*sex) for each of eight outcome measures) and 64 regression control comparisons [eight variables (race, education, psychiatric history, mechanism of injury of assault, LOC, GCS, intracranial lesion on CT, polytrauma) for each of eight outcome measures] to decrease the false discovery rate (FDR; q threshold 0.10) [52]. Subsequently, the p-value threshold was determined to be p<0.025 for main comparisons and p<0.023 for regression control comparisons.
To evaluate for the possibility of bias between subjects who did and did not return for six-month outcomes, chi-squared tests and t-tests were performed for demographic and clinical variables between the 100 included subjects vs. the 83 excluded subjects who did not have complete six-month outcomes. No statistically significant differences were found between included vs. excluded subjects for age, sex, education, race, psychiatric history, injury mechanism, LOC, GCS, CT findings, or polytrauma. We further evaluated demographic and clinical variables between included and excluded subjects within each age*sex cohort (male 18–29y, male 30–39y, female 18–29y, female 30–39y). With the exception of the included male 18–29y subjects having a higher incidence of unknown LOC (37% vs. 12%), no statistically significant differences were found between included and excluded subjects by age*sex cohort (data not shown).
All analyses were performed using Statistical Package for the Social Sciences, version 25 (IBM Corporation, Chicago, IL).
RESULTS
Demographic and clinical characteristics
Overall, 100 TBI subjects met inclusion criteria. The full cohort was, on average, 26.9±6.1 years of age and 72% were Caucasian. By age group, 70% were 18–29y and 30% were 30–39y. Seventy-one percent were male. Thirty-five percent had or reported a psychiatric disorder at baseline. By mechanism of injury, 22% were motor vehicle accident or motorcycle crash, 14% were pedestrian vs. auto, 41% were falls, 21% were assaults, and 2% were other. LOC was negative in 24%, positive in 45%, and unknown in 31% of subjects. ED GCS was predominantly 15 (78%). Intracranial lesions on CT were observed in 23% of subjects. Thirteen percent of subjects had polytrauma. No differences were observed in demographic and clinical variables between by age group (Table 1). Female subjects had higher education level (15.1±2.8 vs. 13.9±2.3 years, p=0.029) compared with male subjects (Table 1).
Table 1A.
Demographic and clinical variables by age-group and sex
| Variable | Overall (N=100) |
18–29 years (N=70) |
30–39 years (N=30) |
Sig. (p) | Variable | Male (N=71) |
Female (N=29) |
Sig. (p) |
|---|---|---|---|---|---|---|---|---|
| Sex | 0.736 | Age Group | 0.736 | |||||
| Male | 71 (71.0%) | 49 (70.0%) | 22 (73.3%) | 18–9 years | 49 (69.0%) | 21 (72.4%) | ||
| Female | 29 (29.0%) | 21 (30.0%) | 8 (26.7%) | 30–39 years | 22 (31.0%) | 8 (27.6%) | ||
| Education | 0.182 | Education | 0.029 | |||||
| Years (mean, SD) | 14.3 (2.5) | 14.1 (2.1) | 14.8 (3.2) | Years (mean, SD) | 13.9 (2.3) | 15.1 (2.8) | ||
| Race | 0.129 | Race | 0.999 | |||||
| Caucasian | 72 (72.0%) | 46 (65.7%) | 26 (86.7%) | Caucasian | 51 (71.8%) | 21 (72.4%) | ||
| African-American/African | 9 (9.0%) | 8 (11.4%) | 1 (3.3%) | African-American/African | 6 (8.5%) | 3 (10.3%) | ||
| Other Races | 19 (19.0%) | 16 (22.9%) | 3 (10.0%) | Other Races | 14 (19.7%) | 5 (17.2%) | ||
| Psychiatric History | 0.819 | Psychiatric History | 0.188 | |||||
| No | 65 (65.0%) | 45 (64.3%) | 20 (66.7%) | No | 49 (69.0%) | 20 (55.2%) | ||
| Yes | 35 (35.0%) | 25 (35.7%) | 10 (33.3%) | Yes | 22 (31.0%) | 13 (44.8%) | ||
| Mechanism of Injury | 0.311 | Mechanism of Injury | 0.089 | |||||
| MVA/MCC | 22 (22.0%) | 12 (17.2%) | 10 (33.4%) | MVA/MCC | 18 (25.3%) | 4 (13.8%) | ||
| PVA | 14 (14.0%) | 11 (15.7%) | 3 (10.0%) | VA | 7 (9.9%) | 7 (24.1%) | ||
| Fall | 41 (41.0%) | 29 (41.4%) | 12 (40.0%) | Fall | 27 (38.0%) | 14 (48.4%) | ||
| Assault | 21 (21.0%) | 17 (24.3%) | 4 (13.3%) | Assault | 18 (25.4%) | 3 (10.3%) | ||
| Other | 2 (2.0%) | 1 (1.4%) | 1 (3.3%) | Other | 1 (1.4%) | 1 (3.4%) | ||
| Non-Assault | 85 (80.2%) | 53 (75.7%) | 26 (86.7%) | 0.288 | Non-Assault | 53 (74.6%) | 26 (89.7%) | 0.112 |
| Assault | 21 (19.8%) | 17 (24.3%) | 4 (13.3%) | Assault | 18 (25.4%) | 3 (10.3%) | ||
| Loss of Consciousness | 0.614 | Loss of Consciousness | 0.310 | |||||
| None | 24 (24.0%) | 18 (25.7%) | 6 (20.0%) | None | 15 (21.1%) | 9 (31.0%) | ||
| <0.5 hrs | 45 (45.0%) | 29 (41.4%) | 16 (53.3%) | <0.5 hrs | 31 (43.7%) | 14 (48.3%) | ||
| Unknown | 31 (31.0%) | 23 (32.9%) | 8 (26.7%) | Unknown | 25 (35.2%) | 6 (20.7%) | ||
| ED GCS | 0.811 | ED GCS | 0.409 | |||||
| 13 | 3 (3.0%) | 3 (4.3%) | 0 (0.0%) | 0.999* | 13 | 2 (2.8%) | 1 (3.4%) | 0.289* |
| 14 | 19 (19.0%) | 13 (18.6%) | 6 (20.0%) | (<15 vs. =15) | 14 | 16 (22.5%) | 3 (10.3%) | (<15 vs. =15) |
| 15 | 78 (78.0%) | 54 (77.1%) | 24 (80.0%) | 15 | 53 (74.6%) | 25 (86.2%) | ||
| Marshall Score | 0.701 | Marshall Score | 0.213 | |||||
| 1 | 77 (77.0%) | 53 (75.7%) | 24 (80.0%) | 1 | 51 (71.8%) | 26 (89.7%) | ||
| 2 | 18 (18.0%) | 12 (17.1%) | 6 (20.0%) | 2 | 16 (22.5%) | 2 (6.9%) | ||
| 3 | 4 (4.0%) | 4 (5.7%) | 0 (0.0%) | 3 | 3 (4.2%) | 1 (3.4%) | ||
| 4 | 1 (1.0%) | 1 (1.4%) | 0 (0.0%) | 4 | 1 (1.4%) | 0 (0.0%) | ||
| CT Intracranial Lesion | 0.641 | CT Intracranial Lesion | 0.055 | |||||
| No | 77 (77.0%) | 53 (75.7%) | 24 (80.0%) | No | 51 (71.8%) | 26 (89.7%) | ||
| Yes | 23 (23.0%) | 17 (24.3%) | 6 (20.0%) | Yes | 20 (28.2%) | 3 (10.3%) | ||
| Polytrauma | 0.749 | Polytrauma | 0.336 | |||||
| No | 87 (87.0%) | 60 (85.7%) | 27 (90.0%) | No | 60 (84.5%) | 27 (93.1%) | ||
| Yes | 13 (13.0%) | 10 (14.3%) | 3 (10.0%) | Yes | 11 (15.5%) | 2 (6.9%) |
CT = computed tomography; ED = emergency department; GCS = Glasgow Coma Scale; LOC = loss of consciousness; MCC = motorcycle crash; MVA = motor vehicle accident; PVA = pedestrian vs. auto; SD = standard deviation
Age group and sex interact and predict post-traumatic stress disorder
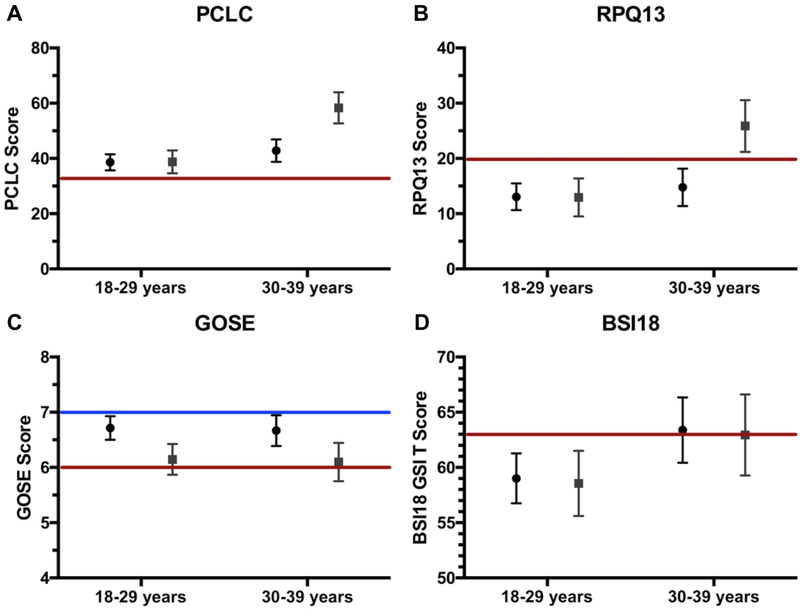
On multivariable analysis of six-month PTSD symptoms using the PCLC, both age group (p<0.001) and sex (p=0.021) emerged as independent predictors of outcome (Table 2). When considering the interaction factor age group*sex, compared to female 30–39y, male 18–29y (B=−19.70 (95% CI [−30.07, −9.33], p<0.001), male 30–39y (B=15.49, 95% CI [−26.54, −4.45], p=0.007), and female 30–39y showed decreased symptom burden (B=−19.55, 95% CI [−26.54, −4.45], p=0.001) (Table 2). Adjusted means for PCLC by age-group and sex are shown in Figure 2A.
Table 2.
Multivariable regression of outcomes (PCLC, RPQ13), with significant age group*sex interaction
| PCLC | RPQ13 | |||
|---|---|---|---|---|
| Variable | B [95% CI] | Sig. (p) | B [95% CI] | Sig. (p) |
| Age Group*Sex | 0.022* | 0.044 | ||
| 18–29 years * Male | −19.70 [−30.07, −9.33] | <0.001 | −12.81 [−21.43, −4.19] | 0.004 |
| 18–29 years * Female | −19.55 [−30.64, −8.47] | 0.001 | −12.92 [−22.14, −3.71] | 0.007 |
| 30–39 years * Male | −15.49 [−26.54, −4.45] | 0.007 | −11.10 [−20.27, −1.92] | 0.018 |
| 30–39 years * Female | reference | --- | reference | --- |
| Age Group | <0.001* | 0.007* | ||
| Sex | 0.021* | 0.050 | ||
| Race | 0.097 | 0.118 | ||
| African-American/African | reference | --- | reference | --- |
| Caucasian | −5.16 [−14.52, 4.19] | 0.276 | −3.29 [−11.06, 4.49] | 0.403 |
| Other races | 1.68 [−8.93, 12.29] | 0.754 | 2.35 [−6.47, 11.17] | 0.598 |
| Education Years | 0.002* | 0.004* | ||
| Per-Year | −1.79 [−2.93, −0.66] | −1.40 [−2.35, −0.46] | ||
| PMH Psychiatric | 0.007* | 0.014* | ||
| No | reference | --- | reference | --- |
| Yes | 8.37 [2.34, 14.41] | 6.31 [1.30, 11.32] | ||
| Mechanism of Assault | 0.006* | 0.014* | ||
| No | reference | --- | reference | --- |
| Yes | 10.45 [3.13, 17.77] | 7.65 [1.57, 13.73] | ||
| LOC Duration | 0.896 | 0.917 | ||
| Unknown | reference | --- | reference | --- |
| None | 1.33 [−6.57, 9.23] | 0.738 | 1.26 [−5.31, 7.82] | 0.704 |
| <30 min | −0.20 [−6.83, 6.44] | 0.954 | 1.01 [−4.51, 6.52] | 0.718 |
| ED GCS | 0.074 | 0.260 | ||
| <15 | reference | --- | reference | --- |
| =15 | −6.39 [−13.40, 0.62] | −3.32 [−9.15, 2.51] | ||
| CT Intracranial Lesion | 0.496 | 0.264 | ||
| No | reference | --- | reference | --- |
| Yes | −2.19 [−8.57, 4.19] | −3.00 [−8.29, 2.31] | ||
| Polytrauma | 0.313 | 0.217 | ||
| No | reference | --- | reference | --- |
| Yes | −4.10 [−12.14, 3.94] | −4.18 [−10.86, 2.50] | ||
delineates statistical significance at Benjamini-Hochberg correction threshold of p<0.025 for main comparisons (age group*sex, age group, sex) and p<0.023 for regression control comparisons (race, education, PMH psychiatric, mechanism, LOC, GCS, CT, polytrauma). B = mean difference; CI = confidence interval; CT = computed tomography; ED = emergency department; GCS = Glasgow Coma Scale; LOC = loss of consciousness; PCLC = Post-Traumatic Stress Disorder Checklist-Civilian Version. PMH = prior medical history; RPQ13 = Rivermead Postconcussional Symptoms Questionnaire-13 Item
Figure 2. Six-month outcomes by age-group and sex.
Six-month adjusted scores on outcome measures displayed by age group on the x-axis (18–29y, 30–39y) and sex (male: mean displayed as circle; female: mean displayed as square). Colored lines indicated clinical cutoffs for screening and/or classification. All scores are adjusted for race, education, psychiatric history, mechanism of injury, loss of consciousness, Glasgow Coma Scale, intracranial lesion on computed tomography, and polytrauma. Bars represent standard errors.
Panel A: Posttraumatic stress disorder (PTSD) symptomatology measured by the PTSD Checklist-Civilian Version (PCLC); higher scores indicate worse severity, and the red line at PCLC=32 indicates the clinical cutoff for PTSD screening in the civilian population.
Panel B: Postconcussional symptomatology (PCS) measured by the Rivermead Postconcussional Symptoms Questionnaire-13 item score (RPQ13); higher scores indicate worse severity, and the red line at RPQ13=20 indicates the clinical cutoff for PCS screening.
Panel C: Functional recovery measured by the Glasgow Outcome Scale-Extended (GOSE); higher scores indicate better functional recovery, and a score of 8 indicates full recovery to baseline function. The blue line at GOSE 7 indicates the lower limit of “good recovery”, while the red line at GOSE 6 indicates the upper limit of “moderate disability”.
Panel D: Global psychiatric burden measured by the Brief Symptom Inventory-18 Global Severity Index (BSI18 GSI), an overall measure of somatization, depression, and anxiety symptoms; higher scores indicate worse severity, and the red line at BSI18 GSI=63 indicates the clinical cutoff for screening for psychiatric symptoms.
Baseline psychiatric history (B=8.37, 95% CI [2.34, 14.41], p=0.007 and mechanism of assault (B=10.45, 95% CI [3.13, 17.77], p=0.006) were associated with greater six-month PTSD symptoms, while higher education level was protective (B=−1.79 per year, 95% CI [−2.93, −0.66], p=0.002).
Age group and sex interact and marginally predict postconcussion symptoms
On multivariable analysis of six-month PCS using the RPQ13, age group (p=0.007) was an independent predictor of outcome while sex was marginal (p=0.050) (Table 2). The interaction factor age-group*sex showed marginal statistical significance below our FDR threshold (p=0.044); compared with female 30–39y, female 18–29y B=−12.92 (95% CI [−22.14, −3.71]; p=0.007), male 18–29y B=−12.81 (95% CI [−21.43, −4.19], p=0.004) and male 30–39y B=−11.10 (95% CI [−20.27, −1.92], p=0.018) (Table 2). Adjusted means for RPQ13 by age-group and sex are shown in Figure 2B.
Similar to the results for the PCLC, baseline psychiatric history (B=6.31, 95% CI [1.30, 11.32], p=0.014) and the injury mechanism of assault (B=7.65, 95% CI [1.57, 13.73], p=0.014) were associated with six-month PCS, while higher education level was protective (B=−1.40 per year, 95% CI [−2.35, −0.46], p=0.004).Age group and sex did not interact for the remaining six-month outcome measures.
Six-month functional, other psychiatric, and quality-of-life outcomes
On multivariable analysis of six-month functional outcome using the GOSE, female sex was associated with a mean decrease of −0.57 ([−1.02, 0.12], p=0.013) compared with male sex. Mechanism of assault was associated with decreased GOSE (B=−0.73 [−1.26, −0.20], p=0.007) and higher educational attainment was again protective (B=0.13 per year, 95% CI [0.05, 0.21], p=0.002) (Table 3). Adjusted means for GOSE by age group and sex are shown in Figure 2C.
Table 3.
Multivariable regression of outcomes (GOSE, BSI18, SWLS), without age group*sex interaction
| GOSE | BSI18 GSI | SWLS | ||||
|---|---|---|---|---|---|---|
| Variable | B [95% CI] | Sig. (p) | B [95% CI] | Sig. (p) | B [95% CI] | Sig. (p) |
| Age Group*Sex | (0.478) | (0.369) | (0.942) | |||
| Interaction factor | (ns, not included) | (ns, not included) | (ns, not included) | |||
| Age Group | 0.821 | 0.052 | 0.052 | |||
| 18–29 years | reference | --- | reference | --- | reference | --- |
| 30–39 years | −0.05 [−0.46, 0.37] | 4.38 [−0.03, 8.79] | −2.99 [−6.00, 0.02] | |||
| Sex | 0.013* | 0.850 | 0.197 | |||
| Male | reference | --- | reference | --- | reference | --- |
| Female | −0.57 [−1.02, 0.12] | −0.45 [−5.16, 4.27] | 2.11 [−1.11, 5.33] | |||
| Race | 0.519 | 0.386 | 0.083 | |||
| African-American/African | reference | --- | reference | --- | reference | --- |
| Caucasian | −0.09 [−0.77, 0.60] | 0.803 | −1.76 [−9.01, 5.48] | 0.630 | 1.58 [−3.37, 6.53] | 0.528 |
| Other races | −0.35 [−1.11, 0.42] | 0.372 | 1.79 [−6.34, 9.92] | 0.663 | −2.42 [−7.97, 3.13] | 0.389 |
| Education Years | 0.002* | 0.008* | 0.030 | |||
| Per-Year | 0.13 [0.05, 0.21] | −1.18 [−2.05, −0.32] | 0.66 [0.06, 1.25] | |||
| PMH Psychiatric | 0.603 | 0.021* | 0.014* | |||
| No | reference | --- | reference | reference | --- | |
| Yes | 0.11 [−0.32, 0.54] | 5.39 [0.84, 9.95] | −3.94 [−7.05, −0.83] | |||
| Mechanism of Assault | 0.007* | 0.050 | 0.049 | |||
| No | reference | --- | reference | --- | reference | --- |
| Yes | −0.73 [−1.26, −0.20] | 5.61 [0.01, 11.21] | −3.85 [−7.67, −0.02] | |||
| LOC Duration | 0.992 | 0.552 | 0.233 | |||
| Unknown | reference | --- | reference | --- | reference | --- |
| None | 0.03 [−0.56, 0.61] | 0.924 | 1.58 [−4.60, 7.77] | 0.612 | −2.17 [−6.39, 2.05] | 0.310 |
| <30 min | 0.03 [−0.46, 0.52] | 0.899 | −1.21 [−6.40, 3.97] | 0.643 | 0.87 [−2.67, 4.41] | 0.625 |
| ED GCS | 0.127 | 0.106 | 0.083 | |||
| <15 | reference | --- | reference | --- | reference | --- |
| =15 | 0.40 [−0.12, 0.92] | −4.51 [−10.00, 0.97] | 3.31 [−0.44, 7.05] | |||
| CT Intracranial Lesion | 0.547 | 0.297 | 0.107 | |||
| No | reference | --- | reference | --- | reference | --- |
| Yes | −0.14 [−0.62, 0.33] | −2.64 [−7.63, 2.36] | 2.80 [−0.61, 6.20] | |||
| Polytrauma | 0.785 | 0.479 | 0.672 | |||
| No | reference | --- | reference | --- | reference | --- |
| Yes | −0.08 [−0.67, 0.51] | −2.25 [−8.53, 4.03] | 0.92 [−3.37, 5.20] | |||
delineates statistical significance at Benjamini-Hochberg correction threshold of p<0.025 for main comparisons (age group*sex, age group, sex) and p<0.023 for regression control comparisons (race, education, PMH psychiatric, mechanism, LOC, GCS, CT, polytrauma). B = mean difference; BSI18 GSI = Brief Symptom Inventory 18 Global Severity Index; CI = confidence interval; CT = computed tomography; ED = emergency department; GCS = Glasgow Coma Scale; GOSE = Glasgow Outcome Scale-Extended; LOC = loss of consciousness; PMH = prior medical history; SWLS = Satisfaction With Life Scale
For six-month psychiatric outcomes using the BSI18 GSI, those with ages 30–39y showed a marginal association with increased psychiatric symptom severity (B=4.38, 95% CI [−0.03, 8.79], p=0.052) compared with 18–29y. Sex did not associate with BSI18. Baseline psychiatric history (B=5.39, 95% CI [0.84, 9.95], p=0.021) was associated with increased six-month psychiatric symptom severity, while higher education level was protective (B=−1.18 per year, 95% CI [−2.05, −0.32], p=0.008) (Table 3). Adjusted means for BSI18 GSI by age-group and sex are shown in Figure 2D.
For six-month quality-of-life outcomes using the SWLS, subjects aged 30–39y showed a marginal association with decreased life satisfaction (B=−2.99, 95% CI [−6.00, 0.02], p=0.052) compared with subjects aged 18–29y. Sex did not associate with BSI18. Baseline psychiatric history (B=−3.94, 95% CI [−7.05, −0.83], p=0.014) was associated with decreased life satisfaction (Table 3).
Six-month cognitive outcomes
On multivariable analysis of six-month nonverbal processing speed using the WAIS-PSI, the 30–39y group associated with increased processing speed (B=11.88 percentile increase, 95% CI [1.66, 22.09], p=0.023) compared with the 18–29y group. Sex did not associate with WAIS-PSI. Mechanism of assault (B=−22.73, 95% CI [−35.71, −9.75], p=0.001) associated with decreased performance, while higher education level was protective [B=2.62 percentile increase per year, 95% CI [0.62, 4.63], p=0.011) (Table 4).
Table 4.
Multivariable regression of cognitive outcomes, without age group*sex interaction
| WAIS-PSI | CVLT Trials 1–5 | TMT B-A | ||||
|---|---|---|---|---|---|---|
| Variable | B [95% CI] | Sig. (p) | B [95% CI] | Sig. (p) | B [95% CI] | Sig. (p) |
| Age Group*Sex | 0.058 | 0.839 | 0.896 | |||
| Interaction factor | (ns, not included) | (ns, not included) | (ns, not included) | |||
| Age Group | 0.023* | 0.151 | 0.990 | |||
| 18–29 years | reference | --- | reference | --- | reference | --- |
| 30–39 years | 11.88 [1.66, 22.09] | −3.88 [−9.21, 1.44] | −0.05 [−8.99, 8.88] | |||
| Sex | 0.448 | 0.194 | 0.397 | |||
| Male | reference | --- | reference | --- | reference | --- |
| Female | 4.19 [−6.73, 15.11] | −3.74 [−9.43, 1.95] | 4.11 [−5.47, 13.69] | |||
| Race | 0.043 | 0.067 | 0.336 | |||
| African-American/African | reference | --- | reference | --- | reference | --- |
| Caucasian | 4.60 [−12.18, 21.39] | 0.587 | 9.38 [0.63, 18.12] | 0.036 | 9.68 [−4.99, 24.36] | 0.193 |
| Other races | 18.85 [0.01, 37.68] | 0.050 | 5.06 [−4.75, 14.88] | 0.308 | 12.08 [−4.37, 28.52] | 0.148 |
| Education Years | 0.011* | 0.011* | 0.013* | |||
| Per-Year | 2.62 [0.62, 4.63] | 1.37 [0.32, 2.42] | −2.23 [−3.98, −0.48] | |||
| PMH Psychiatric | 0.761 | 0.064 | 0.817 | |||
| No | reference | --- | reference | --- | reference | --- |
| Yes | 1.62 [−8.93, 12.18] | 5.19 [−0.31, 10.69] | 1.09 [−8.24, 10.42] | |||
| Mechanism of Assault | 0.001* | 0.071 | 0.531 | |||
| No | reference | --- | reference | --- | reference | --- |
| Yes | −22.73 [−35.71, −9.75] | −6.22 [−12.98, 0.54] | 3.59 [−7.77, 14.95] | |||
| LOC Duration | 0.378 | 0.928 | 0.827 | |||
| Unknown | reference | --- | reference | --- | reference | --- |
| None | 8.59 [−5.72, 22.92] | 0.236 | 0.04 [−7.42, 7.50] | 0.992 | −3.86 [−16.33, 8.60] | 0.540 |
| <30 min | 0.80 [−11.21, 12.80] | 0.895 | 0.99 [−5.27, 7.25] | 0.754 | −1.83 [−12.30, 8.64] | 0.728 |
| ED GCS | 0.251 | 0.855 | 0.220 | |||
| <15 | reference | --- | reference | --- | reference | --- |
| =15 | 7.39 [−5.31, 20.10] | −0.61 [−7.23, 6.01] | −6.89 [−17.98, 4.20] | |||
| CT Intracranial Lesion | 0.144 | 0.396 | 0.259 | |||
| No | reference | --- | reference | --- | reference | --- |
| Yes | 8.58 [−2.98, 20.14] | −2.59 [−8.61, 3.44] | 5.93 [−4.44, 16.29] | |||
| Polytrauma | 0.030 | 0.522 | 0.679 | |||
| No | reference | --- | reference | --- | reference | --- |
| Yes | −16.16 [−30.70, −1.61] | −2.45 [−10.03, 5.13] | −2.66 [−15.38, 10.06] | |||
delineates statistical significance at Benjamini-Hochberg correction threshold of p<0.025 for main comparisons (age group*sex, age group, sex) and p<0.023 for regression control comparisons (race, education, PMH psychiatric, mechanism, LOC, GCS, CT, polytrauma). B = mean difference; CI = confidence interval; CT = computed tomography; CVLT = California Verbal Learning Test; ED = emergency department; GCS = Glasgow Coma Scale; LOC = loss of consciousness; PMH = prior medical history; TMT B-A = Trailmaking Test Trial B minus Trial A; WAIS-PSI = Wechsler Adult Intelligence Scale-Processing Speed Index
For six-month verbal memory using the CVLT1–5SS, age group and sex did not associate with outcome. Education level was associated with improved outcome (B=1.37 per year, 95% CI [0.32, 2.42], p=0.011) (Table 4).
For six-month executive function and mental flexibility using the TMT B-A score, age group and sex did not associate with outcome. Education level was associated with improved outcome (B=−2.23 [−3.98, −0.48], p=0.013) (Table 4).
DISCUSSION
While age by decade has been routinely evaluated in epidemiologic reporting and as a demographic risk factor [17,38–40], literature has been sparse in the context of sex differences in young adults with mTBI. In the first study to evaluate the independent associations of age and sex, and the interaction between age and sex, with multidimensional outcome measures in young adults diagnosed with mTBI, we found effects of age and sex interaction for six-month PTSD, and a marginal effect of interaction for six-month PCS. Females aged 30–39y carried a risk of increased symptomatology compared with the other three groups (males 18–29y, males 30–39y, females 18–29y). We also found female sex to be independently associated with decreased functional outcome, and young adults 30–39y to have increased nonverbal processing speed performance. These results suggest that clinicians should carefully surveil and assess at-risk young adult patients after mTBI who might otherwise be missed if considering age and sex factors separately.
Sex-specific differences in brain organization, adaptation and outcomes
Sex-specific differences in brain morphology are well established in neurological research, specifically in functional brain organization and cortical thickness [18,21,24,53]. While men tend to have larger brains, on average, with increased white matter volume, women tend to have thicker cortical gray matter specifically in the right parietal and temporal lobes [18,24,25]. This variance in brain morphology may be attributable to sex hormones, as both animal and human research have shown increased gray matter volume in female brain regions with higher quantities of sex steroid receptors [23,24,53]. The result of these differences in cortical thickness may be related to sex-specific cognitive functioning [24]. However, little is known about the relationship between brain morphology and sex hormones in male vs. female functional recovery following mTBI. In our study, the significant interaction between age and sex on six-month PTSD and, to a lesser extent PCS, may be attributable in part to differential aptitudes in perceiving symptoms and symptoms reporting.
Differences in symptom reporting between sexes are well-documented in the psychiatry, trauma, and TBI literatures. In general, men are less likely to seek help for depression, substance abuse, physical disabilities and stressful life events [26]. Three principal reasons are posited for this phenomenon: 1) differences in perception that there is a medical problem; 2) differences in assessing the severity of the problem; and 3) variances in likelihood of seeking help. Women are more likely to recognize and label nonspecific feelings of distress as an emotional problem, while difficulties of seeking care for men may in part be attributed to a mismatch between available services and traditional masculine roles of self-reliance and emotional control [26]. Not surprisingly, the evolving pathophysiology of mTBI in the subacute period may compound or worsen symptoms. Furthermore, societal incidence of seeking care for mental health conditions is lower than ideal given systemic and financial barriers to access, and community studies highlight that this reluctance may be stronger in men due to a multifactorial set of perceived social and cultural norms, combined with less positive attitudes on psychological openness [27,54].
Differences in diagnosis rates also exist between male and female TBI patients. In the military literature, multivariable analyses find women to be more likely diagnosed with depression, anxiety, and comorbid PTSD with depression following blast TBI [28]. After sports concussion, women have been found to report higher PCS and other neurobehavioral symptoms compared with their male counterparts [55,56]. A meta-analysis of eight studies concluded that TBI neurobehavioral problems were worse in women on 17 of 20 outcome measures [21]. In agreement with prior authors, we concede that it remains clinically challenging to differentiate symptom severity from overreporting, as a diagnosis of PCS or PTSD may create not only complexities in social stigma, but also the possibility of improved access to care and necessary insurance claims to support disability.
In our study, patients generally scored above the civilian screening cutoff of 32 on the PCLC, showing that PTSD-like symptoms may be present in all young adults after mTBI. However, women aged 30–39 years had markedly elevated adjusted PCLC scores (>45), suggesting higher likelihood that the true symptom burden indeed differs across age and sex cohorts in the young adult population. A similar effect was observed with marginal statistical significance for the RPQ13, where female patients aged 30–39y suffered high symptom severity scores, highlighting that women in this age group may be at risk for concurrent neuropsychiatric conditions leading to further disability. Similar to our findings, recent data suggests that female sex is associated with worse PCS at 3-months after mTBI, and more prominently during childbearing years [57]. While laboratory studies have described the neuroprotective effects of estrogen and progesterone in downregulating cerebral inflammation and glutamatergic excitotoxicity to improve survival in glial and animal models [58–60], the complex dynamics between primary injury, disruption to hormonal axes (e.g. pituitary-hypothalamic) and disruption of social responsibilities may constitute reasons for a lack of translation to the bedside. It is possible that in older young adults (ages 30–39y), familial responsibilities, societal norms, social anchorage and cultural perceptions are amplified in women after trauma. These factors, in conjunction with cortical and neurohormonal differences, respond, adapt and reorganize differently by sex. Effective treatment options exist for PTSD, including cognitive behavioral therapy and selective serotonin reuptake inhibitors [61], which could benefit a subset of mTBI patients if they are properly triaged to follow-up care.
While age and sex did not interact for six-month functional disability as measured by GOSE in our study, women in general scored 0.6 points lower on the GOSE compared with men. As shown in Figure 2C, the GOSE for female young adults approached 6 which by definition is moderate disability. Though the majority of TBI patients are male, women have been found to be more likely to suffer severe functional disability (OR=1.57) on GOSE after controlling for age, GCS, and polytrauma [62–64]. The reasons for this are unclear, but may be due to brain morphology, neurohormonal disruption, sociocultural norms, and self-report. Our finding of clinically significant impairment on a measure of overall functional recovery in female young adults with mTBI could in part be attributable to the spectrum of impairments experienced by this population. These findings, especially in female young adults aged over 30 years warrant future investigation in larger samples. Considerations for closer surveillance and monitoring by the treating clinician should include instructions to the patient for expectations of recovery, return precautions, and continuity of care with the patient’s primary care physician regarding their injury [65]. While not a primary focus of the current study, the importance of mental health history, assault/violent mechanism, and lower education level has been shown both in our study and others to elevate the risk of PTSD and poorer outcome [66], and collecting these data should be prioritized in what may be limited time during the clinical encounter after acute TBI. Lastly, our results further support the notion of the vigilant clinician who is mindful of patient-provider interactions, such as attention to patient identity and emotion, eliminating unconscious bias, promoting health literacy and shared decision-making [67], especially following mTBI and traumatic injuries [68], as these can influence therapeutic efforts and return to needed follow-up in the healthcare system.
Age-specific differences in rewiring, plasticity, and psychosocial reporting
Rewiring consists of dendritic pruning which abolishes unused synapses, and myelination which increases the speed of impulse conduction across region-specific neurocircuitry and optimizes information communication in the central nervous system. This process is not complete in the prefrontal cortex until at least age 25 years according to well-established studies [14,69,70]. As the prefrontal cortex is responsible for cognitive analysis and abstract thought, it is unsurprising that nonverbal cognition scores were nearly 12 percentile points higher in the 30–39y group. Additionally, while cortical gray matter volumes stabilize and begin to reduce in adulthood, white matter volume has been shown to increase in a generally linear manner throughout adolescence, peaking in one’s 40s or 50s [19].
It should be noted that the older young-adults showed marginal associations with increased psychiatric symptoms burden on the BSI18 by 4.4 points (p=0.052) and decreased life satisfaction on the SWLS by 3.0 points (p=0.052). This may be due to increased demands on the cohort aged 30–39y to secure (or re-secure) financial stability and gainful employment, and to provide for families, as compared with those aged 18–29y. These post-injury socioeconomic demands, combined with the organic stress and damage from the initial brain trauma, may lead older young adults to report greater symptomatology [8].
Limitations
We recognize several limitations. The TRACK-TBI Pilot study collected a convenience sample without specific focus on the young adult population, and the sample size of young adults in this study is relatively small, which limits the generalizability of our findings. Due to the small sample size, we were limited in the number of predictors we could adjust for in our multivariable models without overfitting. The interactions and influence of age and sex on risks for trauma are complex, and while we controlled for a number of validated predictors as well as the age group*sex interaction factor, other confounders may exist. We were limited by the coding of the employment variable in NINDS TBI CDEs version 1, which did not code students, retired, or disabled subjects as separate categories [71], and thus did not analyze for student, employment and/or disability status in our cohort of young adults. Evaluation of higher education can be performed as years of education or attainable degrees. In general, the young-adult cohort is more likely to be at a dynamic point between years of education and nascent employment, and thus we did not stratify by formal degrees which may take multiple years and financial capital to complete. It is possible that with larger datasets, the effects of degrees, and other socioeconomic factors available from detailed history taking such as marriage and family can be delineated in young adults after mTBI. While we controlled for the presence of intracranial pathology, we did not evaluate for associations with individual CT findings, which has been shown to have prognostic significance for functional recovery [72]. Income and insurance data were unavailable for the TRACK-TBI Pilot, which could influence types of interventions, medications, continuity of care, and/or family support received by subjects following discharge from acute care; these data were also unavailable and would be informative for future studies on the topic.
The NINDS TBI CDEs for outcomes designated the six-month time point for data collection and earlier time points for the full outcomes battery were not available, which limits our evaluation for trajectories of recovery. While PCS is relatively specific for TBI and/or concussion, causal relationships between TBI, trauma and PTSD are more complex. Definitive analysis of effects of age and sex specific to outcomes post-TBI will necessitate trauma and/or healthy control groups, which were unavailable in TRACK-TBI Pilot. As shown with ongoing studies from the current 18-center TRACK-TBI study, rates of PTSD are indeed higher in mTBI patients vs. nonhead orthopedic trauma patients [66]. We also did not have access to steroid hormone levels at the time of injury or shortly thereafter which have been shown in rodent models to mediate sex differences in recovery from TBI [73,74]. For these reasons, findings from the current study should be regarded as exploratory, and confirmation using larger retrospective studies and prospective trials are needed.
Conclusions
Following mTBI, young-adults aged 18–29 years and aged 30–39 years may have different risks for impairment on specific outcome measures. Sex may interact with age group for PTSD, with females aged 30–39 years at highest risk for symptom severity. These results may be attributable to brain cortical maturation, biological response to injury, social modifiers during recovery, and/or differential responses to self-report outcomes. Prevention, resource allocation, rehabilitation/counseling strategies, and clinical trial design in young adults at risk for and/or suffering from mTBI may benefit from consideration of age and sex. These results warrant further study and validation in larger, more diverse samples.
Acknowledgements:
Amy J. Markowitz, JD provided editorial support. The authors would like to thank the following contributors to the development of the TRACK-TBI database and repositories by organization and in alphabetical order by last name: One Mind for Research: General Peter Chiarelli, U.S. Army (Ret.), Garen Staglin, MBA; QuesGen Systems, Inc.: Vibeke Brinck, MS, Michael Jarrett, MBA; Thomson Reuters: Sirimon O’Charoen, PhD
ClinicalTrials.gov Registration: NCT01565551
Funding Details:
This work was supported by the following grants: NINDS 1RC2NS069409-01, 3RC2NS069409-02S1, 5RC2NS069409-02, 1U01NS086090-01, 3U01NS086090-02S1, 3U01NS086090-02S2, 3U01NS086090-03S1, 5U01NS086090-02, 5U01NS086090-03; US DOD W81XWH-13-1-0441, US DOD W81XWH-14-2-0176 (to G. T. M.)
Biographies
Dr. John K. Yue, MD is a Neurosurgery Resident at University of California San Francisco (UCSF; San Francisco, CA, USA). He received his MD from UCSF School of Medicine. He has authored over 80 peer-reviewed publications in neurosurgery and neurotrauma with special focus on traumatic brain injury, spine and spinal cord injury, biomarkers, genetics, and outcomes.
Dr. Harvey S. Levin, PhD Professor in the Department of Physical Medicine & Rehabilitation, Baylor College of Medicine. He is also a Research Scientist at the Michael E. De Bakey Veterans Affairs Medical Center, Houston. Dr. Levin is a partnering PI on a VA Merit Review grant concerning functional MRI in Veterans who sustained mild TBI which is frequently complicated by post-traumatic stress disorder. Dr. Levin is also a partnering PI on an NIH grant concerning biomarkers and brain imaging in high school athletes who have been cleared to return to play following a concussion. Other relevant experience includes his DoD funded research on the chronic effects of neurotrauma. Dr. Levin is the recipient of a Distinguished Career Award by the International Neuropsychological Society.
Ms. Catherine G. Suen, BS is a Medical Student at University of Utah School of Medicine with background and special interests in critical care, neurology and neurosurgery. She has authored several publications in brain and spine injuries and presented at several national conferences.
Ms. Molly Rose Morrissey, BS is a Master’s of Science in Nursing student at UCSF School of Nursing. She has authored several publications in neurotrauma, presented at national conferences, and has a special interest in rehabilitation and improving geriatic outcomes after trauma.
Ms. Sarah J. Runyon, BA is a Neurosurgery Research Coordinator at UCSF, with interests in cognitive psychology, functional rehabilitation, and improving patient outcomes after trauma.
Dr. Ethan A. Winkler, MD, PhD is a Neurosurgery Chief Resident at UCSF. He received his MD and PhD from University of Rochester School of Medicine (Rochester, NY, USA). He is the author of over 70 peer-reviewed publications in neurovascular disorders, neurosurgical approaches, and neurotrauma. He is an expert on the role of pericytes in central nervous system disorders.
Dr. Ross C. Puffer, MD is a Neurosurgery Chief Resident at Mayo Clinic (Rochester, MN, USA). He received his MD from Mayo Clinic School of Medicine. He is the author of over 40 peer-reviewed publications in neurosurgery, with focus on operative techniques in spine and peripheral nerve injury.
Mr. Hansen Deng, BA is a Medical Student at UCSF with 20 peer-reviewed publications and several oral presentations in operative neurosurgery, neurotrauma and spine/spinal cord injury.
Ms. Caitlin K. Robinson, BS is a Trauma Research Manager at University of Colorado Denver. She has authored several publication in trauma, critical care and neurosurgery.
Mr. Jonathan W. Rick, BS is a Medical Student at UCSF and Howard Hughes Medical Scholar, with over 20 neurosurgical publications in neuro-oncology, neurotraum and functional neurosurgery.
Mr. Ryan R. L. Phelps, BA is a Medical Student at UCSF, and a current research fellow at the National Institutes of Health (NIH), with focus in neurosurgery.
Mr. Sourabh Sharma, BS is a Medical Student at Loyola University in Chicago, IL. He has authored over 20 publications in neurotrauma and radiation oncology.
Dr. Sabrina R. Taylor, PhD is the Director of Clinical Studies in the Department of Neurosurgery at UCSF. She received her PhD in Neuroscience at Dartmouth College (Hanover, NH, USA). She has presented numerous talks at national conferences and has high-level experience in neuroscience and traumatic brain injury clinical research and team management.
Ms. Mary J. Vassar, RN, MS is the Executive Director in the Department of Neurosurgery at UCSF. Her background includes research officerships in prevention, quality and costs for large academic centers and multicenter clinical trials, as well as regulatory experience in federal and local goverments. She has authored over 30 peer-reviewed publications in healthcare policy and epidemiology.
Dr. Maryse C. Cnossen, PhD is an Epidemiologist and Postdoctoral Scholar at Erasmus Medical Center (Rotterdam, The Netherlands). She received her PhD in Epidemiology from Erasmus University Rotterdam and the Netherlands Institute for Health Sciences. She is the author of over 30 peer-reviewed publications and living systematic reviews, with a special focus on global epidemiology of traumatic brain injury, and advanced statistical modeling and outcomes prediction in large multicenter trials.
Dr. Hester F. Lingsma, PhD is an Epidemiologist and Associate Professor of Medical Decision Making at Erasmus Medical Center. She received her PhD in Public Health from Erasmus University Rotterdam. She is a lead investigator in numerous large multicenter clinical trials for trauma, traumatic brain injury, stroke, and healthcare quality. She has authored over 200 peer-reviewed publications.
Dr. Raquel C. Gardner, MD is an Assistant Professor at the UCSF. Dr. Gardner received her MD from Harvard Medical School and completed residency in neurology and clinical fellowship in behavioral neurology at UCSF, followed by a postdoctoral fellowship in dementia epidemiology at the San Francisco VA Medical Center and a certificate program in advanced training in clinical research methods and biostatistics at the UCSF Clinical and Translational Science Institute. She is faculty in the UCSF/SFVAMC Center for Population Brain Health and leads the geriatric TBI interest group as part of the TBI Endpoints Development (TED) initiative.
Dr. Nancy R. Temkin, PhD a Professor in the Departments of Neurological Surgery and Biostatistics and an adjunct professor in the Department of Rehabilitation. Dr. Temkin has been the PI for the data center for 5 federally funded multicenter clinical trials as well as numerous single-site trials. She has devoted her career as a biostatistician to the study of TBI, its consequences, and potential treatments. She has authored over 170 peer-reviewed publications.
Mr. Jason Barber, MS is a Statistician at University of Washington. He received his MS in Biostatistics from the University of Washington in 2003, and has been working in TBI research since 1997. For the past nine years he has served on the board of the Brain Injury Alliance of Washington, including two years as board president. He has authored over 70 peer-reviewed publications.
Dr. Sureyya S. Dikmen, PhD is a Professor in the Department of Rehabilitation Medicine, and Adjunct Professor in the Departments of Neurological Surgery and Psychiatry and Behavioral Sciences. Dr. Dikmen is one of the founding members of the UW TBI Model System, and is very active on the Research and Data Collection teams of TBIMS. Her research interests include the natural history of neuropsychological and psychosocial outcomes and the recovery and prediction of these functions after TBI. She has authored over 120 peer-reviewed publications.
Dr. Esther L. Yuh, MD, PhD is an Associate Professor of Radiology and Attending Neuroradiologist at UCSF. She received her MD and PhD from Stanford University (Stanford, CA, USA), and completed residency and fellowship in Neuroradiology at UCSF. She is an expert in diagnostic neuroradiology, traumatic brain injury, stroke and neurodegenerative disorders, and a lead investigator of numerous large multicenter trials for neurological disorders. She has authored over 40 peer-reviewed publications.
Dr. Pratik Mukherjee, MD, PhD is a Professor of Radiology and Attending Radiologist at UCSF, Director of the Neural Connectivity Lab at UCSF, and Director of the Center for Imaging of Neurodegenerative Diseases (CIND) at the San Francisco Veterans Affairs Medical Center (San Francisco, CA, USA). He received his MD from Cornell University (Ithaca, NY, USA), PhD in Neuroscience at Rockefeller University (New York City, NY, USA) and completed residency and fellowship in Neuroradiology at Washington University Medical Center in St. Louis (St. Louis, MO, USA). His primary research focuses on neurodevelopmental disorders and traumatic brain injury, technical development, neuroscience and clinical applications of advanced imaging methods. He has served as the principal investigator or co-principal investigator of numerous NIH, U.S. Department of Defense, and public foundation grants. He has authored over 140 peer-reviewed publications.
Dr. Murray B. Stein, MD, MPH is Distinguished Professor of Psychiatry and Family Medicine & Public Health, and Vice Chair for Clinical Research in Psychiatry at the University of California San Diego (UCSD). He is also a Staff Psychiatrist at the VA San Diego Healthcare System. Dr. Stein graduated from the University of Manitoba and completed his residency and post-residency fellowship at the University of Toronto and at the National Institute of Mental Health in Bethesda, Maryland, USA. He subsequently completed a Master of Public Health degree at the Johns Hopkins University Bloomberg School of Public Health in Baltimore, MD. Dr. Stein’s research interests include the epidemiology, neurobiology, and treatment of anxiety disorders especially social phobia, panic disorder, and posttraumatic stress disorder. He has written or co-written over 600 peer-reviewed scientific articles on these topics, including in journals such as JAMA, New England Journal of Medicine, The Lancet, American Journal of Psychiatry, and JAMA Psychiatry. His federally funded research has included studies of interventions for anxiety disorders in primary care, pharmacological approaches to treatment-resistant anxiety disorders, and functional neuroimaging research in anxiety and trauma-related disorders. He has authored over 500 peer-reviewed publications.
Dr. Tene A. Cage, MD is an Assistant Professor of Neurosurgery at Stanford University. She received her MD from UCSF and completed neurosurgery residency as well as neurotrauma fellowship at UCSF. She has authored over 30 publications in neurosurgery, epidemiology and health disparities.
Dr. Alex B. Valadka, MD is Professor and Chair of Neurosurgery at Virginia Commonwealth University (Richmond, VA, USA). He received his MD from University of Chicago Prizker School of Medicine (Chicago, IL, USA) and completed residency in Neurosurgery at the Medical College of Virginia. He has served as Chair of the American Association of Neurological Surgeons/Congress of Neurological Surgeons (AANS/CNS) Section on Neurotrauma and Critical Care, Chair of the Neurosurgical Specialty Group of the American College of Surgeons on Trauma, Chair of the AANS Public Relations and Membership Committees, and Chair of the Washington Committee for Neurosurgery. He is the President-Elect of the AANS and a Director of the American Board of Neurological Surgeons. He helped establish the Seton Brain and Spine Institute and served as Chair and Chief Executive Officer. His research interests include cerebral blood flow and metabolism after traumatic brain injury, biochemical markers of brain injury, and organized efforts with medical and governmental organizations to improve the delivery of emergency neurosurgical care. He has authored over 100 peer-reviewed publications.
Dr. David O. Okonkwo, MD, PhD is Professor and Executive Vice Chair of Neurosurgery and Director of the Neurotrauma Clinical Trials Center at University of Pittsburgh Medical Center. He received his MD and PhD from the Medical College of Virginia. He completed residency in Neurosurgery at University of Virginia and fellowship at Auckland Public Hospital (Auckland, New Zealand). His clinical interest are traumatic brain and spine injury, spinal deformity and scoliosis. His research endeavors include developing advanced neuroimaging modalities and novel therapeutic interventions for brain and spinal cord injury. He is lead and co-lead investigator of numerous federal and foundation grants, multicenter clinical trials, including the nationally-funded clinical core to study pathophysiology of traumatic brain injury. He has authored over 200 peer-reviewed publications.
Dr. Geoffrey T. Manley, MD, PhD is Professor and Vice Chair of Neurosurgery at UCSF, Chief of Neurosurgery and Co-Director of the Brain and Spinal Injury Center at Zuckerberg San Francisco General Hospital. He received his MD and PhD from Cornell University and completed postdoctoral fellowships in molecular neuro-oncology at Memorial Sloan Kettering Cancer Center (New York City, NY, USA) and in molecular physics at the UCSF Cardiovascular Research Institute (San Francisco, CA, USA). He completed residency in Neurosurgery at UCSF. He is an internationally recognized expert in neurotrauma. He has helped to define new molecular mechanisms of brain injury and develop advanced neuromonitoring and clinical informatic tools for critical care. He is currently leading national and international efforts to create a modern knowledge warehouse that integrates clinical, imaging, proteomic, genomic, and outcome biomarkers of TBI to drive the development of a new TBI classification system. He is the lead and co-lead investigator of numerous federal and foundation grants and multicenter clinical trials. He has authored over 200 peer-reviewed publications.
Footnotes
Disclosure Statement: No potential conflict of interest was reported by the authors.
REFERENCES
- [1].Cassidy JD, Carroll LJ, Peloso PM, et al. Incidence, risk factors and prevention of mild traumatic brain injury: results of the WHO Collaborating Centre Task Force on Mild Traumatic Brain Injury. J. Rehabil. Med 2004;28–60. [DOI] [PubMed] [Google Scholar]
- [2].Røe C, Sveen U, Alvsåker K, et al. Post-concussion symptoms after mild traumatic brain injury: influence of demographic factors and injury severity in a 1-year cohort study. Disabil. Rehabil 2009;31:1235–1243. [DOI] [PubMed] [Google Scholar]
- [3].Stulemeijer M, van der Werf S, Borm GF, et al. Early prediction of favourable recovery 6 months after mild traumatic brain injury. J. Neurol. Neurosurg. Psychiatry 2008;79:936–942. [DOI] [PubMed] [Google Scholar]
- [4].McMahon P, Hricik A, Yue JK, et al. Symptomatology and functional outcome in mild traumatic brain injury: results from the prospective TRACK-TBI study. J. Neurotrauma 2014;31:26–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].van der Naalt J Prediction of outcome in mild to moderate head injury: a review. J. Clin. Exp. Neuropsychol 2001;23:837–851. [DOI] [PubMed] [Google Scholar]
- [6].Ponsford J, Willmott C, Rothwell A, et al. Factors influencing outcome following mild traumatic brain injury in adults. J. Int. Neuropsychol. Soc 2000;6:568–579. [DOI] [PubMed] [Google Scholar]
- [7].Biswas RK, Kabir E, King R. Effect of sex and age on traumatic brain injury: a geographical comparative study. Arch. Public Health 2017;75:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Rapoport MJ, Feinstein A. Age and functioning after mild traumatic brain injury: the acute picture. Brain Inj. 2001;15:857–864. [DOI] [PubMed] [Google Scholar]
- [9].Thornhill S, Teasdale GM, Murray GD, et al. Disability in young people and adults one year after head injury: prospective cohort study. BMJ. 2000;320:1631–1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].McCauley SR, Wilde EA, Barnes A, et al. Patterns of early emotional and neuropsychological sequelae after mild traumatic brain injury. J. Neurotrauma 2014;31:914–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Lexell J, Wihlney A-K, Jacobsson LJ. Vocational outcome 6–15 years after a traumatic brain injury. Brain Inj. 2016;30:969–974. [DOI] [PubMed] [Google Scholar]
- [12].Hawley CA. Behaviour and school performance after brain injury. Brain Inj. 2004;18:645–659. [DOI] [PubMed] [Google Scholar]
- [13].Wäljas M, Iverson GL, Lange RT, et al. Return to Work Following Mild Traumatic Brain Injury. J. Head Trauma Rehabil 2014;29:443–450. [DOI] [PubMed] [Google Scholar]
- [14].Arain M, Haque M, Johal L, et al. Maturation of the adolescent brain. Neuropsychiatr. Dis. Treat 2013;9:449–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Burnett S, Blakemore S-J. The development of adolescent social cognition. Ann. N. Y. Acad. Sci 2009;1167:51–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Dikmen S, Machamer J, Temkin N. Mild Traumatic Brain Injury: Longitudinal Study of Cognition, Functional Status, and Post-Traumatic Symptoms. J. Neurotrauma 2017;34:1524–1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Dhandapani S, Manju D, Sharma B, et al. Prognostic significance of age in traumatic brain injury. J. Neurosci. Rural Pract 2012;3:131–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Witte AV, Savli M, Holik A, et al. Regional sex differences in grey matter volume are associated with sex hormones in the young adult human brain. Neuroimage. 2010;49:1205–1212. [DOI] [PubMed] [Google Scholar]
- [19].Tamnes CK, Ostby Y, Fjell AM, et al. Brain maturation in adolescence and young adulthood: regional age-related changes in cortical thickness and white matter volume and microstructure. Cereb. Cortex 2010;20:534–548. [DOI] [PubMed] [Google Scholar]
- [20].Kraus JF, Peek-Asa C, McArthur D. The independent effect of gender on outcomes following traumatic brain injury: a preliminary investigation. Neurosurg. Focus 2000;8:e5. [DOI] [PubMed] [Google Scholar]
- [21].Farace E, Alves WM. Do women fare worse? A metaanalysis of gender differences in outcome after traumatic brain injury. Neurosurg. Focus 2000;8:e6. [DOI] [PubMed] [Google Scholar]
- [22].Im K, Lee J-M, Lee J, et al. Gender difference analysis of cortical thickness in healthy young adults with surface-based methods. Neuroimage. 2006;31:31–38. [DOI] [PubMed] [Google Scholar]
- [23].Luders E, Narr KL, Thompson PM, et al. Gender effects on cortical thickness and the influence of scaling. Hum. Brain Mapp 2006;27:314–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Sowell ER, Peterson BS, Kan E, et al. Sex differences in cortical thickness mapped in 176 healthy individuals between 7 and 87 years of age. Cereb. Cortex 2007;17:1550–1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Paus T, Nawaz-Khan I, Leonard G, et al. Sexual dimorphism in the adolescent brain: Role of testosterone and androgen receptor in global and local volumes of grey and white matter. Horm. Behav 2010;57:63–75. [DOI] [PubMed] [Google Scholar]
- [26].Addis ME, Mahalik JR. Men, masculinity, and the contexts of help seeking. Am. Psychol 2003;58:5–14. [DOI] [PubMed] [Google Scholar]
- [27].Drapeau A, Boyer R, Lesage A. The influence of social anchorage on the gender difference in the use of mental health services. J. Behav. Health Serv. Res 2009;36:372–384. [DOI] [PubMed] [Google Scholar]
- [28].Iverson KM, Hendricks AM, Kimerling R, et al. Psychiatric diagnoses and neurobehavioral symptom severity among OEF/OIF VA patients with deployment-related traumatic brain injury: a gender comparison. Womens. Health Issues 2011;21:S210–S217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Iverson KM, Pogoda TK, Gradus JL, et al. Deployment-related traumatic brain injury among Operation Enduring Freedom/Operation Iraqi Freedom veterans: associations with mental and physical health by gender. J. Womens. Health 2013;22:267–275. [DOI] [PubMed] [Google Scholar]
- [30].Brickell TA, Lippa SM, French LM, et al. Female Service Members and Symptom Reporting after Combat and Non-Combat-Related Mild Traumatic Brain Injury. J. Neurotrauma 2017;34:300–312. [DOI] [PubMed] [Google Scholar]
- [31].Lippa SM, Brickell TA, Bailie JM, et al. Postconcussion Symptom Reporting After Mild Traumatic Brain Injury in Female Service Members: Impact of Gender, Posttraumatic Stress Disorder, Severity of Injury, and Associated Bodily Injuries. J. Head Trauma Rehabil. [Internet] 2017; Available from: 10.1097/HTR.0000000000000353. [DOI] [PubMed] [Google Scholar]
- [32].Duhaime A-C, Gean AD, Haacke EM, et al. Common data elements in radiologic imaging of traumatic brain injury. Arch. Phys. Med. Rehabil 2010;91:1661–1666. [DOI] [PubMed] [Google Scholar]
- [33].Maas AI, Harrison-Felix CL, Menon D, et al. Common data elements for traumatic brain injury: recommendations from the interagency working group on demographics and clinical assessment. Arch. Phys. Med. Rehabil 2010;91:1641–1649. [DOI] [PubMed] [Google Scholar]
- [34].Manley GT, Diaz-Arrastia R, Brophy M, et al. Common data elements for traumatic brain injury: recommendations from the biospecimens and biomarkers working group. Arch. Phys. Med. Rehabil 2010;91:1667–1672. [DOI] [PubMed] [Google Scholar]
- [35].Wilde EA, Whiteneck GG, Bogner J, et al. Recommendations for the use of common outcome measures in traumatic brain injury research. Arch. Phys. Med. Rehabil 2010;91:1650–1660.e17. [DOI] [PubMed] [Google Scholar]
- [36].Yue JK, Vassar MJ, Lingsma HF, et al. Transforming research and clinical knowledge in traumatic brain injury pilot: multicenter implementation of the common data elements for traumatic brain injury. J. Neurotrauma 2013;30:1831–1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Marshall LF, Marshall SB, Klauber MR, et al. The diagnosis of head injury requires a classification based on computed axial tomography. J. Neurotrauma 1992;9 Suppl 1:S287–S292. [PubMed] [Google Scholar]
- [38].Majdan M, Plancikova D, Brazinova A, et al. Epidemiology of traumatic brain injuries in Europe: a cross-sectional analysis. Lancet Public Health. 2016;1:e76–e83. [DOI] [PubMed] [Google Scholar]
- [39].Agrawal A, Galwankar S, Kapil V, et al. Epidemiology and clinical characteristics of traumatic brain injuries in a rural setting in Maharashtra, India. 2007–2009. Int. J. Crit. Illn. Inj. Sci 2012;2:167–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Nott MT, Gates TM, Baguley IJ. Age-related trends in late mortality following traumatic brain injury: A multicentre inception cohort study. Australas. J. Ageing 2015;34:E1–E6. [DOI] [PubMed] [Google Scholar]
- [41].Kaul RP, Sagar S, Singhal M, et al. Burden of maxillofacial trauma at level 1 trauma center. Craniomaxillofac. Trauma Reconstr 2014;7:126–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Wilson JT, Pettigrew LE, Teasdale GM. Structured interviews for the Glasgow Outcome Scale and the extended Glasgow Outcome Scale: guidelines for their use. J. Neurotrauma 1998;15:573–585. [DOI] [PubMed] [Google Scholar]
- [43].Derogatis LR. BSI 18, Brief Symptom Inventory 18: Administration, Scoring and Procedures Manual. Bloomington, MN: NCS Pearson, Inc.; 2001. [Google Scholar]
- [44].King NS, Crawford S, Wenden FJ, et al. The Rivermead Post Concussion Symptoms Questionnaire: a measure of symptoms commonly experienced after head injury and its reliability. J. Neurol 1995;242:587–592. [DOI] [PubMed] [Google Scholar]
- [45].Using the PTSD Checklist (PCL) [Internet]. VA National Center for PTSD. 2012. [cited 2018 Jun 16]. Available from: https://sph.umd.edu/sites/default/files/files/PTSDChecklistScoring.pdf.
- [46].Diener E, Emmons RA, Larsen RJ, et al. The Satisfaction with Life Scale. J. Pers. Assess 1985;49:71–75. [DOI] [PubMed] [Google Scholar]
- [47].Reitan RM. The relation of the trail making test to organic brain damage. J. Consult. Psychol 1955;19:393–394. [DOI] [PubMed] [Google Scholar]
- [48].Wechsler D Wechsler Adult Intelligence Scale-Fourth Edition (WAIS-IV). Bloomington, MN: NCS Pearson, Inc.; 2008. [Google Scholar]
- [49].Delis DC, Kramer JH, Kaplan E, et al. California Verbal Learning Test-Second Edition (CVLT-II). Bloomington, MN: NCS Pearson, Inc.; 2000. [Google Scholar]
- [50].Levinson D A conception of adult development. Am. Psychol 1986;41:3–13. [Google Scholar]
- [51].Jacobs B, Beems T, Stulemeijer M, et al. Outcome prediction in mild traumatic brain injury: age and clinical variables are stronger predictors than CT abnormalities. J. Neurotrauma 2010;27:655–668. [DOI] [PubMed] [Google Scholar]
- [52].Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. Series B Stat. Methodol 1995;57:289–300. [Google Scholar]
- [53].Luders E, Narr KL, Thompson PM, et al. Mapping cortical gray matter in the young adult brain: effects of gender. Neuroimage. 2005;26:493–501. [DOI] [PubMed] [Google Scholar]
- [54].Mackenzie CS, Gekoski WL, Knox VJ. Age, gender, and the underutilization of mental health services: the influence of help-seeking attitudes. Aging Ment. Health 2006;10:574–582. [DOI] [PubMed] [Google Scholar]
- [55].Colvin AC, Mullen J, Lovell MR, et al. The role of concussion history and gender in recovery from soccer-related concussion. Am. J. Sports Med 2009;37:1699–1704. [DOI] [PubMed] [Google Scholar]
- [56].Dick RW. Is there a gender difference in concussion incidence and outcomes? Br. J. Sports Med 2009;43 Suppl 1:i46–i50. [DOI] [PubMed] [Google Scholar]
- [57].Bazarian JJ, Blyth B, Mookerjee S, et al. Sex differences in outcome after mild traumatic brain injury. J. Neurotrauma 2010;27:527–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Brotfain E, Gruenbaum SE, Boyko M, et al. Neuroprotection by Estrogen and Progesterone in Traumatic Brain Injury and Spinal Cord Injury. Curr. Neuropharmacol 2016;14:641–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Johann S, Beyer C. Neuroprotection by gonadal steroid hormones in acute brain damage requires cooperation with astroglia and microglia. J. Steroid Biochem. Mol. Biol 2013;137:71–81. [DOI] [PubMed] [Google Scholar]
- [60].Arevalo M-A, Santos-Galindo M, Bellini M-J, et al. Actions of estrogens on glial cells: Implications for neuroprotection. Biochim. Biophys. Acta 2010;1800:1106–1112. [DOI] [PubMed] [Google Scholar]
- [61].Yue JK, Burke JF, Upadhyayula PS, et al. Selective Serotonin Reuptake Inhibitors for Treating Neurocognitive and Neuropsychiatric Disorders Following Traumatic Brain Injury: An Evaluation of Current Evidence. Brain Sci. 2017;7:93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Haarbauer-Krupa J, Taylor CA, Yue JK, et al. Screening for Post-Traumatic Stress Disorder in a Civilian Emergency Department Population with Traumatic Brain Injury. J. Neurotrauma 2017;34:50–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Cnossen MC, Winkler EA, Yue JK, et al. Development of a Prediction Model for Post-Concussive Symptoms following Mild Traumatic Brain Injury: A TRACK-TBI Pilot Study. J. Neurotrauma [Internet] 2017; Available from: 10.1089/neu.2016.4819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].van der Horn HJ, Spikman JM, Jacobs B, et al. Postconcussive complaints, anxiety, and depression related to vocational outcome in minor to severe traumatic brain injury. Arch. Phys. Med. Rehabil 2013;94:867–874. [DOI] [PubMed] [Google Scholar]
- [65].Seabury SA, Gaudette E, Goldman DP, et al. Assessment of follow-up care after emergency department presentation for mild traumatic brain injury and concussion: Results from the TRACK-TBI study. JAMA Netw Open 2018;1:e180210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Stein MB, Jain S, Giacino JT, et al. Risk of Posttraumatic Stress Disorder and Major Depression in Civilian Patients After Mild Traumatic Brain Injury: A TRACK-TBI Study. JAMA Psychiatry [Internet]. 2019; Available from: 10.1001/jamapsychiatry.2018.4288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Schattner A The clinical encounter revisited. Am. J. Med 2014;127:268–274. [DOI] [PubMed] [Google Scholar]
- [68].Zatzick DF, Russo J, Rajotte E, et al. Strengthening the patient-provider relationship in the aftermath of physical trauma through an understanding of the nature and severity of posttraumatic concerns. Psychiatry. 2007;70:260–273. [DOI] [PubMed] [Google Scholar]
- [69].Baumrind D A developmental perspective on adolescent risk taking in contemporary America. New Dir. Child Dev 1987;93–125. [DOI] [PubMed] [Google Scholar]
- [70].Sinclair D, Purves-Tyson TD, Allen KM, et al. Impacts of stress and sex hormones on dopamine neurotransmission in the adolescent brain. Psychopharmacology . 2014;231:1581–1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Yue JK, Rick JW, Morrissey MR, et al. Preinjury employment status as a risk factor for symptomatology and disability in mild traumatic brain injury: A TRACK-TBI analysis. NeuroRehabilitation. 2018;43:169–182. [DOI] [PubMed] [Google Scholar]
- [72].Yue JK, Winkler EA, Puffer RC, et al. Temporal lobe contusions on computed tomography are associated with impaired 6-month functional recovery after mild traumatic brain injury: a TRACK-TBI study. Neurol. Res 2018;40:972–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Roof RL, Duvdevani R, Stein DG. Gender influences outcome of brain injury: progesterone plays a protective role. Brain Res. 1993;607:333–336. [DOI] [PubMed] [Google Scholar]
- [74].Roof RL, Hall ED. Gender differences in acute CNS trauma and stroke: neuroprotective effects of estrogen and progesterone. J. Neurotrauma 2000;17:367–388. [DOI] [PubMed] [Google Scholar]