Abstract
Background:
Bladder-sparing trimodality therapy (TMT) is an alternative to radical cystectomy (RC) for muscle-invasive bladder cancer (MIBC), and biomarkers to inform therapy selection are needed.
Objective:
To evaluate the prognostic value of immune and stromal signatures in MIBC treated with TMT.
Design, setting, and participants:
We used a clinical-grade platform to perform transcriptome-wide gene expression profiling of primary tumors from 136 MIBC patients treated with TMT at a single institution. We observed 60 overall survival events at 5 yr, and median follow-up time for patients without an event was 5.0 yr (interquartile range 3.1, 5.0). Expression data from another cohort of 223 MIBC patients treated with neoadjuvant chemotherapy (NAC) and RC were also analyzed.
Outcome measurements and statistical analysis:
Molecular subtypes, and immune and stromal signatures were evaluated for associations with disease-specific survival (DSS) and overall survival (OS) in TMT patients, and in patients treated with NAC and RC.
Results and limitations:
Gene expression profiling of TMT cases identified luminal (N = 40), luminal-infiltrated (N = 26), basal (N = 54), and claudin-low (N = 16) subtypes. Signatures of T-cell activation and interferon gamma signaling were associated with improved DSS in the TMT cohort (hazard ratio 0.30 [0.14–0.65], p = 0.002 for T cells), but not in the NAC and RC cohort. Conversely, a stromal signature was associated with worse DSS in the NAC and RC cohort (p = 0.006), but not in the TMT cohort. This study is limited by its retrospective nature.
Conclusions:
Higher immune infiltration in MIBC is associated with improved DSS after TMT, whereas higher stromal infiltration is associated with shorter DSS after NAC and RC. Additional studies should be conducted to determine whether gene expression profiling can predict treatment response.
Patient summary:
We used gene expression profiling to study the association between tumor microenvironment and outcomes following bladder preservation therapy for invasive bladder cancer. We found that outcomes varied with immune and stromal signatures within the tumor. We conclude that gene expression profiling has potential to guide treatment decisions in bladder cancer.
Keywords: Bladder cancer, Bladder sparing, Bladder preservation, Chemoradiation, Radiation, Trimodality therapy, Muscle-invasive bladder cancer
1. Introduction
Although radical cystectomy (RC) with or without neoadjuvant chemotherapy (NAC) is a common treatment for muscle-invasive bladder cancer (MIBC), bladder-sparing trimodality therapy (TMT) with maximal transurethral resection of bladder tumor (TURBT) followed by concurrent chemoradiotherapy is an acceptable alternative for select patients [1–3]. A randomized trial comparing TMT and RC for MIBC failed to complete accrual [4], but multiple series demonstrate that long-term disease control and quality-of-life outcomes for TMT compare favorably with RC [5–9]. Both TMT and RC are recommended as acceptable treatment options for MIBC in multiple national and international consensus guidelines [1–3]. However, TMT remains underutilized, in part due to difficulty in identifying appropriate patients. Therefore, there is an urgent need for reliable predictive biomarkers to guide treatment selection for MIBC patients.
Transcriptional profiling of MIBC has identified molecular subtypes characterized by distinct gene expression patterns [10–13]. Although subtype characteristics and nomenclature vary across published cohorts, bladder tumors can broadly be categorized based on luminal versus basal gene expression patterns. Recent integrated analyses indicate that patterns of genomic alterations vary across subtypes [14], with important prognostic and predictive implications [10,15]. In patients undergoing RC without NAC, luminal subtype was associated with improved overall survival (OS) compared with basal subtype [15]. Interestingly, patients with basal tumors experienced a significant benefit in OS from the addition of NAC, whereas patients with luminal tumors had similar outcomes with or without NAC, suggesting that molecular subtype may serve as a predictive biomarker to guide the use of NAC [15]. However, it is not known whether molecular subtype predicts response to radiotherapy.
Radiation triggers an immune response against tumor cells through several mechanisms, including antigen release, promotion of chemokine secretion, and recruitment of immune effector cells [16]. Given the association between radiation and immune activation, we hypothesized that the presence of a tumor immune infiltrate may be associated with an improved response to TMT. In addition, fibroblasts in the tumor microenvironment have been associated with a T-cell exclusion phenotype in bladder tumors, with an impact on response to systemic therapy [17]. Thus, radiotherapy responses may also vary with the extent of tumor stromal infiltration. Recent studies demonstrated that tumor gene expression profiling can quantify immune activity, including CD8(+) T-cell infiltration [18] and interferon (IFN)-gamma signaling [19]. Here, we report results from clinical-grade whole transcriptome gene expression profiling of a large cohort of MIBC patients managed with TMT. We classified the cohort using molecular subtypes, and tested the association of immune and stromal gene signatures with clinically relevant endpoints.
2. Patients and methods
2.1. Patients and clinical samples
This is a retrospective analysis of tumor samples from a previously reported cohort of 475 patients with cT2–T4aN0M0 MIBC treated with TMT at a single institution [6]. Institutional review board approval was obtained for this study. High-quality transcriptomic data were available from 136 cases and were included in the final analyses (see below). We observed 60 OS events (death by any cause) and 32 disease-specific survival (DSS) events at 5-yr follow-up; the median follow-up time for patients without an OS event was 5.0 yr (interquartile range [IQR] 3.1, 5.0). The previously reported NAC cohort comprised pretreatment TURBT specimens from a multi-institutional cohort of 223 MIBC patients treated with NAC and RC [15]. In this cohort, there were 75 OS events and 50 DSS events at 5 yr; the median follow-up time for patients without an OS event was 3.5 yr (IQR 2.1, 5.0). Detailed inclusion and exclusion criteria for both cohorts have been described previously [6,15].
2.2. Transcriptome analysis
Available formalin-fixed, paraffin-embedded TURBT specimens were reviewed by a genitourinary pathologist, and a 1 mm punch biopsy was harvested from the area of highest tumor density. Following RNA extraction, transcriptome-wide gene expression profiles were generated using Human Exon Array 1.0 ST oligonucleotide microarrays in a clinical-grade laboratory setting (GenomeDx Laboratory, San Diego, CA, USA). Genomic subtyping classifier (GSC) [15], immune content signature [18], hallmark of IFN-gamma response [19], and stromal signatures [17] were retrieved from the Decipher GRID database (GenomeDx Inc.). The GSC uses penalized multinomial logistic regression to classify tumors into four subtypes (basal, claudin low, luminal, and luminal infiltrated). To replicate The Cancer Genome Atlas (TCGA 2017) subtyping model in our cohort of 136 TMT cases, a set of 53 genes was selected from the work of Robertson et al [13], and a six-cluster solution was generated using a separate cohort of 173 TURBT samples collected through commercial use of the Decipher bladder assay (GenomeDx Inc.). Of these clusters, five of six closely matched the luminal papillary, luminal, luminal infiltrated, basal, and neuronal subtypes. The remaining cluster was labeled “unclassified” based on a lack of genomic features matching TCGA subtypes. The clustering was further adjusted using the pamr (prediction analysis for microarrays) R package to ensure tightness. A random forest classification model was then trained to predict cluster labels.
2.3. Statistical analysis
The primary endpoint was an association of immune infiltration signatures with DSS after TMT. Secondary endpoints were associations of immune infiltration signatures with OS after TMT, immune signatures with OS and DSS after NAC and RC, stromal infiltration signatures with DSS and OS after TMT or after NAC and RC, and molecular subtypes with DSS and OS after TMT or after NAC and RC. The follow-up time was censored at 5 yr prior to any analyses to facilitate comparisons between the two cohorts. Association of selected gene signatures with DSS and OS was assessed using the Kaplan-Meier method and log rank test. Cox proportional hazard models were used to perform univariable and multivariable survival analyses with individual clinicopathological variables, including age, gender, clinical stage (T2 vs T3–T4), extent of TURBT (visibly complete vs incomplete), and hydronephrosis (presence vs absence). For the signatures that we considered, cut points for dichotomization were selected based on differences observed on Kaplan-Meier plots stratified by quartiles for each signature see (Supplementary Fig. 2). Comparisons between the TMT and the NAC and RC cohorts were performed using subgroup analyses and interaction analyses (Supplementary Tables 1 and 2).
3. Results
3.1. Transcriptomic analysis identifies four gene expression subtypes in the TMT cohort
Whole transcriptome expression profiles were available from pretreatment TURBT specimens from 136 MIBC patients treated with TMT and from a previously published cohort of 223 MIBC patients treated with NAC and RC [15]. Clinical characteristics of both cohorts are summarized in Table 1 and are described in detail in prior publications [6,15]. There were several differences between the TMT and NAC cohorts, including a higher median age, a larger proportion of males, and lower T stages for TMT compared with NAC patients.
Table 1 –
Clinical characteristics of the bladder-sparing TMT and NAC cohorts
| Characteristics | Levels | TMT cohort (N = 136) | NAC cohort (N = 223) | p value |
|---|---|---|---|---|
| Age | 1st qu. | 61.8 | 55.7 | <0.001 * |
| Median | 70.2 | 61.7 | ||
| 3rd qu. | 77.4 | 70.5 | ||
| Gender (%) | Female | 27 (20) | 69 (31) | 0.021 * |
| Male | 109 (80) | 154 (69) | ||
| Clinical stage (%) | Tis | 0 (0.0) | 0 (0.0) | <0.001 * |
| Ta | 0 (0.0) | 0 (0.0) | ||
| Tl | 0 (0.0) | 0 (0.0) | ||
| T2 | 91 (71) | 90 (40) | ||
| T3 | 33 (24) | 90 (40) | ||
| T4 | 6 (4.4) | 43 (19) | ||
| TURBT extent (%) | Complete | 93 (68) | 0 (0.0) | NA |
| Incomplete | 43 (32) | 0 (0.0) | ||
| NA | 0 (0.0) | 223 (100) | ||
| Hydronephrosis (%) | No | 115 (83) | 0 (0.0) | NA |
| Yes | 20 (14) | 0 (0.0) | ||
| NA | 4 (2.9) | 223 (100) |
NA = not available; NAC = neoadjuvant chemotherapy; qu. = quartile; TMT = trimodality therapy; TURBT = transurethral resection of bladder tumor.
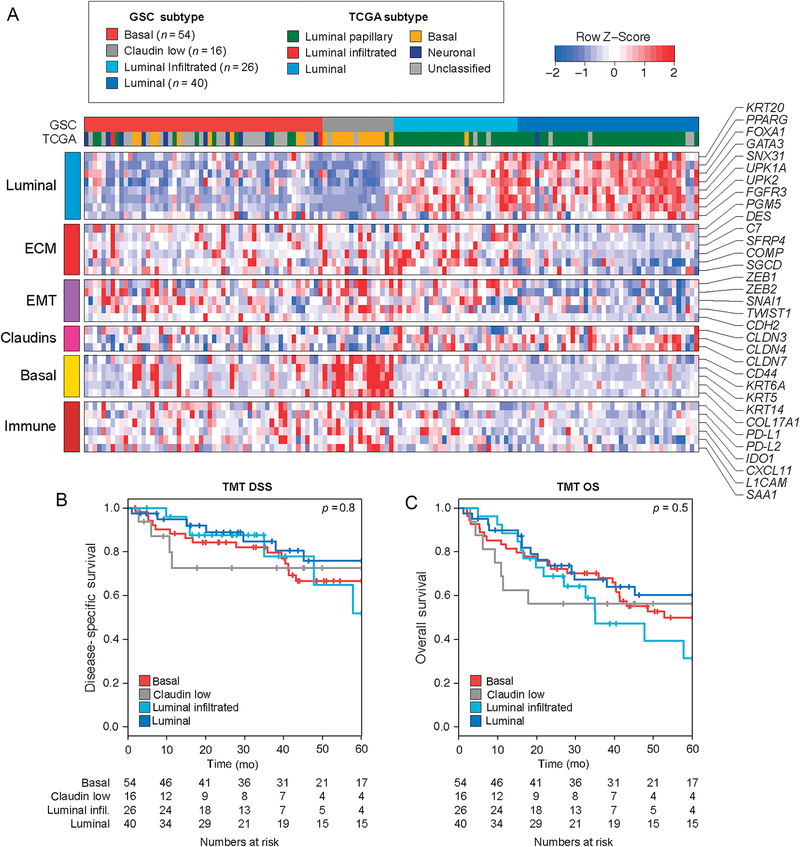
Supervised hierarchical clustering using established gene sets for molecular subtypes classified the TMT cohort into luminal (N = 40), luminal-infiltrated (N = 26), basal (N = 54), and claudinlow (N = 16) subtypes (Fig. 1A). Classification using TCGA subtypes revealed that the majority of luminal and luminal-infiltrated tumors were classified as TCGA luminal papillary subtype, whereas the majority of claudin-low and basal tumors were classified as TCGA basal or unknown subtypes. Classification of tumors revealed fewer basal tumors and more luminal tumors in the NAC cohort than in the TMT cohort (Supplementary Fig. 1A). The rate of complete response following TMT was not different across subtypes (p = 0.9; Supplementary Fig. 1B). In addition, we did not observe evidence of an effect of molecular subtype on either DSS or OS in the TMT cohort (Fig. 1B and 1C; p = 0.8 for DSS and p = 0.5 for OS). In contrast, in the NAC cohort, DSS and OS were worse among patients with claudin-low tumors (Supplementary Fig. 1C and 1D; p = 0.01 for DSS and p = 0.068 for OS) [15].
Fig. 1 –
Gene expression subtypes and clinical outcomes in a TMT cohort. (A) Heat map showing genomic subtype classifier (GSC) subtype for each tumor in the TMT cohort (n = 136) and gene expression levels across several gene sets. Classification using the TCGA subtypes is also shown for each tumor. (B and C) Kaplan-Meier curves for disease-specific survival (DSS) and overall survival (OS) among GSC subtypes in the TMT cohort. Log-rank p values and the number of patients at risk are shown. ECM = extracellular matrix; infil. = infiltrated; TCGA = The Cancer Genome Atlas; TMT = trimodality therapy.
3.2. Expression signatures of immune infiltration are associated with improved outcomes following TMT
Given that radiation can activate an antitumor immune response and that tumor-infiltrating lymphocytes have been associated with a favorable prognosis in multiple tumor types including bladder cancer [20–22], we hypothesized that increased tumor immune infiltrate in MIBC is associated with an improved response to radiation therapy. Using a T-cell–inflamed gene expression signature that has been shown to correlate with CD8(+) T-cell infiltration in bladder tumors [18], we stratified patients based on quartiles of the immune score (Supplementary Fig. 2) and compared tumors having lower levels of immune infiltration (defined by an immune score in the first quartile) with tumors having higher levels of immune infiltration (Fig. 2A). In the TMT cohort, patients whose tumors had higher T-cell–inflamed scores had significantly improved DSS (p = 0.025; Fig. 2B) compared with patients whose tumors had lower levels of immune infiltration. There were no significant differences in OS (p = 0.2; Fig. 2C). This suggested that immune infiltration was associated with an improved response to TMT. In contrast, in the NAC cohort, a higher T-cell–inflamed score was associated with worse DSS, but this did not reach statistical significance (p = 0.063; Fig. 2D) and OS (p = 0.3; Fig. 2E). There were differences in T-cell–inflamed scores across subtypes in the TMT cohort, with claudin-low and luminalinfiltrated subtypes having higher scores than basal and luminal subtypes (Fig. 2F). The association between higher T-cell–inflamed scores and improved DSS in the TMT cohort remained significant in a multivariable model that included clinical factors (Table 2).
Fig. 2 –
A T-cell–inflamed gene expression signature is associated with outcomes in the TMT cohort but not in the NAC cohort. (A) Heat map showing expression of the T-cell– inflamed gene expression signature [18] across tumors from the TMT cohort. Kaplan-Meier curves for disease-specific survival (DSS) and overall survival (OS) by T-cell– inflamed expression scores in (B and C) the TMT cohort and (D and E) the NAC cohort. Log-rank p values and the number of patients at risk are shown. (F) Notched box plots showing the T-cell–inflamed scores across GSC subtypes in the TMT cohort. GSC = genomic subtyping classifier; Lum. = luminal; NAC = neoadjuvant chemotherapy; TMT = trimodality therapy.
Table 2 –
Multivariable models for DSS in the TMT cohort for T-cell–inflamed signature score and interferon-gamma signature scores
| Variable | T-cell–inflamed signature | Interferon-gamma signature | ||
|---|---|---|---|---|
| Adjusted HR | p value | Adjusted HR | p value | |
| Age | 1.01 (0.98–1.04) | 0.4 | 1.01 (0.98–1.04) | 0.4 |
| Gender (male) | 0.75 (0.31–1.81) | 0.5 | 0.85 (0.36–1.99) | 0.7 |
| Clinical stage >T2 | 2.59 (1.15–5.80) | 0.021 * | 1.82 (0.82–4.05) | 0.14 |
| Incomplete TURBT | 1.62 (0.75–3.50) | 0.2 | 1.70 (0.76–3.81) | 0.2 |
| Hydronephrosis | 1.42 (0.55–3.67) | 0.5 | 1.70 (0.66–4.39) | 0.3 |
| Signature >lst qu. | 0.30 (0.14–0.65) | 0.002 * | 0.39 (0.19–0.81) | 0.012 * |
DSS = disease-specific survival; HR = hazard ratio; qu. = quartile; TMT = trimodality therapy; TURBT = transurethral resection of bladder tumor.
Values in parentheses represent interquartile ranges.
Significant p values (p < 0.05) are denoted in bold and with asterisk.
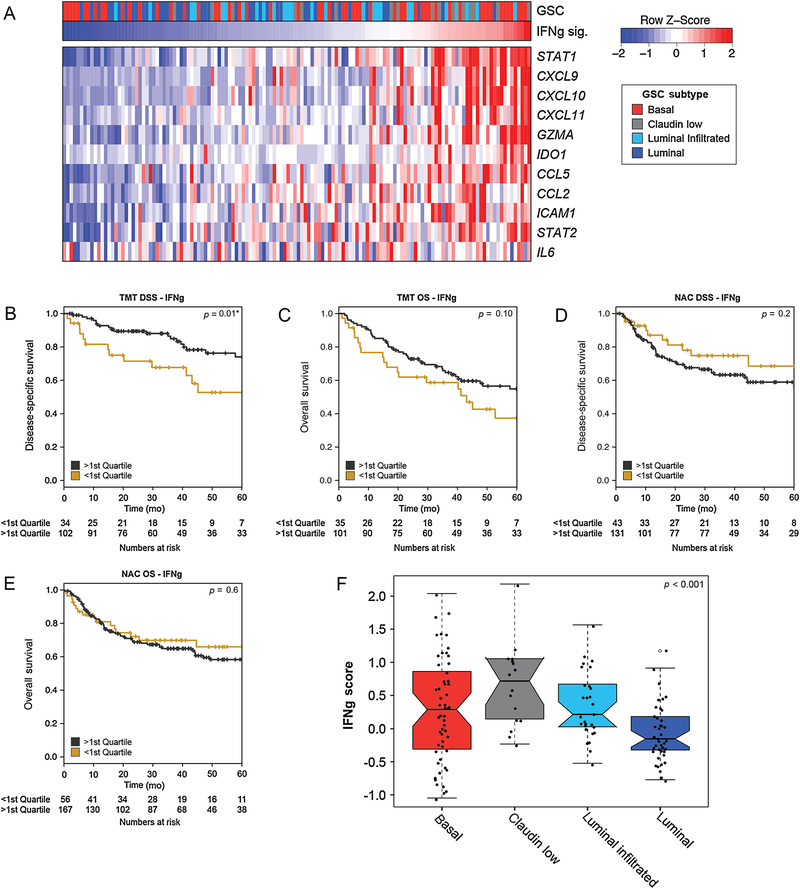
IFN signaling can drive immune activation in tumor cells, and IFN-related gene expression has been investigated as a biomarker of tumor immunogenicity and immune checkpoint inhibitor sensitivity in several clinical contexts [23,24]. Therefore, we investigated the association between IFN-gamma gene expression signature and outcomes in the TMT cohort (Fig. 3A). Similar to the T-cell–inflamed signature, patients with higher IFN-gamma signature scores had improved DSS (p = 0.01; Fig. 3B) but not improved OS (p = 0.10; Fig. 3C) in the TMT cohort, whereas in the NAC cohort, there was no association between IFN-gamma signature activity and DSS (p = 0.2; Fig. 3D) or OS (p = 0.6; Fig. 3E). We performed interaction analyses to investigate the interaction between a cohort (NAC vs TMT) and the T-cell–inflamed (Supplementary Table 1) and IFN-related (Supplementary Table 2) gene expression signatures. For both signatures, we found a significant interaction between a cohort and the activity of the gene signature with respect to DSS (p = 0.003 and p = 0.016 for the T-cell–inflamed and IFN-related gene signatures, respectively).
Fig. 3 –
An interferon-gamma (IFNg) gene expression signature is associated with outcomes in the TMT cohort but not in the NAC cohort. (A) Heat map showing expression of a subset of genes from an IFNg gene expression signature [19] across tumors from the TMT cohort. Kaplan-Meier curves for disease-specific survival (DSS) and overall survival (OS) by IFNg expression scores in (B and C) the TMT cohort and (D and E) the NAC cohort. Log-rank p values and the number of patients at risk are shown. (F) Notched box plots showing the IFNg expression scores across GSC subtypes in the TMT cohort. GSC = genomic subtyping classifier; NAC = neoadjuvant chemotherapy; TMT = trimodality therapy.
In the TCGA cohort, which consists of patients treated with RC without NAC, neither the T-cell–inflamed nor the IFN-gamma expression signatures were associated with OS (Supplementary Fig. 3A and 3B) [13]. Differences in IFN-gamma scores across molecular subtypes were noted in the TMT cohort, with claudin-low tumors having the highest scores (Fig. 3F). A higher IFN-gamma score was the only factor significantly associated with improved DSS in the TMT cohort, in a multivariable model including clinical prognostic factors (Table 2).
3.3. A stromal infiltration signature is associated with worse outcomes following RC but not TMT
We next investigated whether gene expression signatures of other nontumor cell populations were associated with outcomes following TMT. Several studies have linked extracellular matrix and stromal genes with a poor response to systemic therapy [10,17]. We evaluated the association between expression of these genes and response to TMT. Based in part on genes identified by TCGA analysis [13], we predefined a stromal infiltration signature comprising genes typically expressed in fibroblasts or myofibroblasts (C7, SGCD, CNN1, MYH11, MFAP4, PGM5, ACTC1, DES, and PCP4; Fig. 4A). The stromal infiltration signature varied across subtypes within the TMT and NAC cohorts (p < 0.001 and p < 0.001, respectively; Fig. 4B and 4C). In each cohort, signature scores were highest in the luminal-infiltrated subgroup and lowest in the luminal subgroup. We divided each cohort into quartiles based on stromal expression signature and focused our attention on cases with high expression of this signature (defined as greater than third quartile). While there was no association between stromal infiltration signature and DSS or OS in the TMT cohort (Fig. 4D and 4E), a high stromal infiltration signature was associated with significantly worse DSS and OS in the NAC cohort (p = 0.006 and p = 0.015, respectively; Fig. 4F and 4G). Similarly, in the TCGA cohort of patients treated with RC without NAC, a high stromal score was associated with worse OS (Supplementary Fig. 2C). Taken together, these results suggest that the extent of stromal infiltration is associated with outcomes following RC with or without NAC, but not following TMT.
Fig. 4 –
A stromal gene expression signature is associated with outcomes in the NAC cohort but not in the TMT cohort. (A) Heat map showing expression of genes in the stromal gene expression signature across tumors from the TMT cohort. Notched box plots showing the stromal signature scores across GSC subtypes in the (B) TMT and (C) NAC cohorts. Kaplan-Meier curves for disease-specific survival (DSS) and overall survival (OS) by stromal signature scores in (D and E) the TMT cohort and (F and G) the NAC cohort. Log-rank p values and the number of patients at risk are shown. GSC = genomic subtyping classifier; infiltr. = infiltrated; NAC = neoadjuvant chemotherapy; TMT = trimodality therapy.
4. Discussion
Recent studies in MIBC have identified molecular subtypes with distinct biological features and clinical behaviors [13,15,25], but most studies focus on patients treated with RC. In contrast, little is known about the impact of genomic features on outcomes following TMT. As randomized data comparing RC versus TMT are not available, selection of therapy for MIBC relies on clinical factors and patient/provider preference. There is thus a critical need for biomarkers to refine therapy selection for MIBC.
To define genomic correlates of TMT response, we performed comprehensive transcriptional profiling of 136 primary tumors from patients treated with TMT. Given the emerging appreciation for the role of tumor microenvironment in driving treatment response, we sought to characterize the association between gene expression signatures of immune and stromal infiltrates and TMT outcomes. The extent and pattern of immune infiltration are known to vary among bladder tumors and are reflected in gene expression differences among subtypes. Although immune cell infiltration has been associated with response to immune checkpoint blockade in metastatic bladder cancer [17,22], the impact of tumor immune properties on response to TMT is poorly understood. We found that in patients treated with TMT, tumors with higher expression of genes associated with T-cell activation and IFN-gamma signaling had significantly improved DSS compared with tumors with lower signature scores. In contrast, among patients treated with RC, no differences in outcomes based on immune signatures were noted. These results suggest that immune-activated tumors derive a greater benefit from a TMT approach. Although the precise underlying mechanisms are unknown, the presence of immune cells prior to treatment may provide a more favorable environment in which radiation can promote immune-mediated tumor cell killing through mechanisms such as triggering additional antigen release, promoting chemokine secretion, or recruiting additional immune effector cells [16,26].
Nonimmune cell components of the tumor microenvironment also impact therapeutic response. In bladder cancer, an expression signature of transforming growth factor beta signaling from peritumoral fibroblasts was associated with an immune-excluded phenotype and a lack of response to immune checkpoint inhibition [17]. Fibroblasts and other stromal components can contribute to direct exclusion or inactivation of chemotherapy [27]. We found that high expression of a stromal gene signature was associated with worse outcomes after RC, whereas there was no significant difference in outcomes based on the stromal gene signature in the TMT cohort. Radiotherapy has diverse impacts on the tumor stroma that remain incompletely understood [28], but it is possible that radiation delivered as part of TMT could counter the treatment-resistant phenotype associated with stromal infiltration.
In an attempt to separate prognostic from predictive features of transcriptional signatures, we analyzed TMT patients as well as separate cohorts treated with NAC and RC. Despite substantial differences in clinical characteristics that make it challenging to directly compare these two cohorts, we identified interesting differences in clinical outcomes based on immune and stromal gene expression signatures. In addition, although clinical outcomes have been shown to vary across consensus molecular subtypes in MIBC patients treated with RC with or without NAC [15], we did not observe such differences within the TMT cohort. Although our study is limited by its retrospective nature and cohort size, it is possible that this observation reflects differences in biological mechanisms of radiation-based tumor killing compared with surgical resection. However, given the significantly different clinical characteristics and heterogeneity of the TMT and NAC cohorts, the differences we observed between these two groups should be considered hypothesis generating. Further studies with more events are required to determine the nature of the relationship between gene expression signatures, treatment type, and outcomes for MIBC patients. As such, prospective studies in additional cohorts are warranted.
Precision medicine plays an increasingly important role in the management of MIBC [29], and additional studies of MIBC will continue to refine the prognostic and predictive roles of genomic features in the context TMT [30]. A recent study showed that an expression signature of hypoxia was associated with poorer outcomes among MIBC patients treated with radiotherapy alone, and predicted benefit from the addition of hypoxic-modifying therapy [31]. Several planned and ongoing MIBC clinical trials incorporate genomic analyses, including a randomized trial of TMT with or without the PD-L1 inhibitor atezolizumab (SWOG/NRG 1806). The association between immune infiltrate and radiation response may synergistically result in an even greater antitumor response with the addition of a PD-L1 inhibitor. These studies will further define the role of molecular subtype and immune and stromal signatures as biomarkers to guide the use of TMT.
5. Conclusions
Transcriptional profiling of tumors from patients with MIBC revealed significant associations between immune and stromal signatures, and cancer-specific outcomes. Among MIBC patients treated with TMT, decreased tumor immune infiltration was associated with shorter DSS, whereas there was no association between stromal infiltration and DSS. Conversely, among MIBC patients treated with NAC followed by RC, there was no association between immune infiltration and DSS, whereas increased tumor stromal infiltration was associated with shorter DSS. Although validation in prospective trials is necessary, our findings suggest a potential use for transcriptional profiling to guide the rational selection of patients who will benefit most from TMT.
Supplementary Material
Financial disclosures:
Jason A. Efstathiou certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Ewan A. Gibb, Yang Liu, Marguerite du Plessis, Natalie Q. Wang, and Elai Davicioni are employed by GenomeDx Biosciences.
Funding/Support and role of the sponsor: This work was supported by grants from the Ira J. Spiro Translational Research Award Program (David T. Miyamoto and Jason A. Efstathiou), the NCI (C06 CA059267, Jason A. Efstathiou and David T. Miyamoto; 1K08CA219504, Kent W. Mouw), the Burroughs Wellcome Fund (Kent W. Mouw), and the Bladder Cancer Advocacy Network (Kent W. Mouw). GenomeDx funded gene expression analyses.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].National Comprehensive Cancer Network. Bladder cancer—version 3. 2018. https://www.nccn.org/professionals/physician_gls/PDF/bladder.pdf
- [2].Chang SS, Bochner BH, Chou R, et al. Treatment of non-metastatic muscle-invasive bladder cancer: AUA/ASCO/ASTRO/SUO guideline. J Urol 2017;198:552–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Gakis G, Efstathiou J, Lerner SP, et al. ICUD-EAU International Consultation on Bladder Cancer 2012: Radical cystectomy and bladder preservation for muscle-invasive urothelial carcinoma of the bladder. Eur Urol 2013;63:45–57. [DOI] [PubMed] [Google Scholar]
- [4].Huddart RA, Hall E, Lewis R, Birtle A, SPARE Trial Management Group. Life and death of spare (selective bladder preservation against radical excision): reflections on why the spare trial closed. BJU Int 2010;106:753–5. [DOI] [PubMed] [Google Scholar]
- [5].Mak RH, Hunt D, Shipley WU, et al. Long-term outcomes in patients with muscle-invasive bladder cancer after selective bladder-preserving combined-modality therapy: a pooled analysis of Radiation Therapy Oncology Group protocols 8802, 8903, 9506, 9706, 9906, and 0233. J Clin Oncol 2014;32:3801–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Giacalone NJ, Shipley WU, Clayman RH, et al. Long-term outcomes after bladder-preserving tri-modality therapy for patients with muscle-invasive bladder cancer: an updated analysis of the Massachusetts General Hospital experience. Eur Urol 2017;71:952–60. [DOI] [PubMed] [Google Scholar]
- [7].Kulkarni GS, Hermanns T, Wei Y, et al. Propensity score analysis of radical cystectomy versus bladder-sparing trimodal therapy in the setting of a multidisciplinary bladder cancer clinic. J Clin Oncol 2017;35:2299–305. [DOI] [PubMed] [Google Scholar]
- [8].Vashistha V, Wang H, Mazzone A, et al. Radical cystectomy compared to combined modality treatment for muscle-invasive bladder cancer: a systematic review and meta-analysis. Int J Radiat Oncol Biol Phys 2017;97:1002–20. [DOI] [PubMed] [Google Scholar]
- [9].Mak KS, Smith AB, Eidelman A, et al. Quality of life in long-term survivors of muscle-invasive bladder cancer. Int J Radiat Oncol Biol Phys 2016;96:1028–36. [DOI] [PubMed] [Google Scholar]
- [10].Choi W, Porten S, Kim S, et al. Identification of distinct basal and luminal subtypes of muscle-invasive bladder cancer with different sensitivities to frontline chemotherapy. Cancer Cell 2014;25:152–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Damrauer JS, Hoadley KA, Chism DD, et al. Intrinsic subtypes of high-grade bladder cancer reflect the hallmarks of breast cancer biology. Proc Natl Acad Sci U S A 2014;111:3110–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Sjodahl G, Lauss M, Lovgren K, et al. A molecular taxonomy for urothelial carcinoma. Clin Cancer Res 2012;18:3377–86. [DOI] [PubMed] [Google Scholar]
- [13].Robertson AG, Kim J, Al-Ahmadie H, et al. Comprehensive molecular characterization of muscle-invasive bladder cancer. Cell 2017;171:540–556 e25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Choi W, Ochoa A, McConkey DJ, et al. Genetic alterations in the molecular subtypes of bladder cancer: illustration in the Cancer Genome Atlas dataset. Eur Urol 2017;72:354–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Seiler R, Ashab HAD, Erho N, et al. Impact of molecular subtypes in muscle-invasive bladder cancer on predicting response and survival after neoadjuvant chemotherapy. Eur Urol 2017;72:544–54. [DOI] [PubMed] [Google Scholar]
- [16].Buchwald ZS, Efstathiou JA. Immunotherapy and radiation—a new combined treatment approach for bladder cancer? Bladder Cancer 2015;1:15–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Mariathasan S, Turley SJ, Nickles D, et al. TGFbeta attenuates tumour response to PD-L1 blockade by contributing to exclusion of T cells. Nature 2018;554:544–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Sweis RF, Spranger S, Bao R, et al. Molecular drivers of the non-T-cell-inflamed tumor microenvironment in urothelial bladder cancer. Cancer Immunol Res 2016;4:563–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Liberzon A, Birger C, Thorvaldsdottir H, Ghandi M, Mesirov JP, Tamayo P. The Molecular Signatures Database (MSigDB) hallmark gene set collection. Cell Syst 2015;1:417–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Galon J, Costes A, Sanchez-Cabo F, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science 2006;313:1960–4. [DOI] [PubMed] [Google Scholar]
- [21].Gooden MJ, de Bock GH, Leffers N, Daemen T, Nijman HW. The prognostic influence of tumourinfiltrating lymphocytes in cancer: a systematic review with meta-analysis. Br J Cancer 2011;105:93–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Snyder A, Nathanson T, Funt SA, et al. Contribution of systemic and somatic factors to clinical response and resistance to PD-L1 blockade in urothelial cancer: an exploratory multi-omic analysis. PLoS Med 2017;14:e1002309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Brzostek-Racine S, Gordon C, Van Scoy S, Reich NC. The DNA damage response induces IFN. J Immunol 2011;187:5336–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Chen Q, Sun L, Chen ZJ. Regulation and function of the cGAS-STING pathway of cytosolic DNA sensing. Nat Immunol 2016;17:1142–9. [DOI] [PubMed] [Google Scholar]
- [25].Marzouka NA, Eriksson P, Rovira C, Liedberg F, Sjödahl G, Höglund M. A validation and extended description of the Lund taxonomy for urothelial carcinoma using the TCGA cohort. Sci Rep 2018;8:3737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Vanpouille-Box C, Formenti SC, Demaria S. Toward precision radiotherapy for use with immune checkpoint blockers. Clin Cancer Res 2018;24:259–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Wang W, Kryczek I, Dostal L, et al. Effector T cells abrogate stroma-mediated chemoresistance in ovarian cancer. Cell 2016;165:1092–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Hellevik T, Martinez-Zubiaurre I. Radiotherapy and the tumor stroma: the importance of dose and fractionation. Front Oncol 2014;4:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Felsenstein KM, Theodorescu D. Precision medicine for urothelial bladder cancer: update on tumour genomics and immunotherapy. Nat Rev Urol 2018;15:92–111. [DOI] [PubMed] [Google Scholar]
- [30].Miyamoto DT, Mouw KM, Feng FY, Shipley WU, Efstathiou JA. Molecular biomarkers in bladder preservation therapy for muscle-invasive bladder cancer. Lancet Oncol 2018;19:e683–95. [DOI] [PubMed] [Google Scholar]
- [31].Yang L, Taylor J, Eustace A, et al. A gene signature for selecting benefit from hypoxia modification of radiotherapy for high-risk bladder cancer patients. Clin Cancer Res 2017;23:4761–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.