Abstract
Purpose
To investigate the impact of hyperglycemia and glycemic variability during intensive acute myeloid leukemia therapy (AML) on outcomes by age.
Methods
Retrospective study of 262 consecutive patients with newly diagnosed AML hospitalized for intensive induction. Hyperglycemia was assessed by mean blood glucose (BG) (mg/dL) during hospitalization and glycemic variability was determined by the standard deviation (SD) of mean BG. Outcomes were complete remission ± incomplete count recovery (CR + CRi), and overall survival (OS). We used logistic regression to evaluate CR + CRi, and Cox proportional hazard models for OS, stratified by age (< 60 vs ≥ 60 years).
Results
Older patients (N = 138, median age 70) had higher baseline comorbidity (CCI > 1 60.1% vs 25.8%) and prevalence of diabetes (20.3% vs 7.3%) compared to younger (N = 124, median age 47). The mean ± SD number of BG values obtained per patient during hospitalization was 61 ± 71. The mean (± SD) glucose (mg/dL) during hospitalization was 121.7 (25.9) in older patients (≥ 60 years) versus 111.6 (16.4) in younger. In older patients, higher mean glucose and greater glycemic variability were associated with lower odds of remission (OR 0.80, 95% CI 0.69–0.93 and OR 0.73, 95% CI 0.61–0.88 respectively, per 10-unit increase) and higher mortality rates (HR 1.13, 95% CI 1.05–1.21 and HR 1.17, 95% CI 1.09–1.26, respectively, per 10-unit increase) in multivariate analyses.
Conclusions
Our observations that hyperglycemia and increased glycemic variability were associated with lower remission rates and increased mortality in older patients suggest glycemic control may be a potentially modifiable factor to improve AML outcomes.
Keywords: Acute myeloid leukemia, Hyperglycemia, Glycemicvariability, Diabetes, Older
Introduction
In 2016, approximately 20,000 people were newly diagnosed with acute myeloid leukemia (AML) in the USA, with 10,000 people a year dying from the disease [1]. Adults ≤ 60 years now have a 60–80% complete remission rate [2] and overall survival rate of 35–40% [3]. However, patients > 60 years (older adults) continue to have a poorer prognosis, with a complete remission rate of about 50% [2] and overall survival rates of 5–15% [3]. Patient-specific characteristics (age, comorbidities and performance status) and disease-specific factors (cytogenetics, prior cytotoxic therapy, prior myelodysplastic syndrome) all influence prognosis [3]. Identification of modifiable prognostic factors would be an opportunity to intervene and potentially improve upon outcomes for older adults.
Comorbidity burden is negatively associated with outcomes in older adults with AML [4–9] but few studies have focused on the contribution of individual comorbidities to outcomes. Diabetes is associated with a near threefold increase in 30-day mortality rates among older adults compared to non-diabetic older adults undergoing intense induction therapy for AML [4]. This raises the question of whether hyperglycemia itself influences prognosis. When analyzing mean blood glucose during hospitalizations for AML therapy, Ali et al. found the relative risk of hospital mortality increased with increases in mean blood glucose levels [10]. In other studies, hyperglycemia during hospitalization for AML induction therapy associated with complicated infections, death [11], neutropenia, and greater hospital length of stay [12]. However, none of these studies focused on older adults. In a small study (N = 41) of older adults, hyperglycemia during hospitalization for AML induction therapy had an increased risk of infection [13].
Glycemic variability refers to fluctuations of blood glucose values around the mean and has been identified as a novel marker for glycemic control [14–16]. Increased glycemic variability is independently associated with higher in hospital mortality [17]. Among non-critically ill patients for every 10 mg/dL of glucose level increase in standard deviation, hospital relative risk of death rose by 8% [18].
The purpose of the present study was to evaluate the impact of the mean blood glucose level and glycemic variability on (a) complete remission ± incomplete count recovery and (b) overall survival in older adults (≥ 60 years) compared to younger patients (< 60 years) receiving induction chemotherapy for AML. To the best of our knowledge, this is the first study evaluating the impact of hyperglycemia during AML induction therapy on those outcomes and the first to examine the impact of glycemic variability.
Materials and methods
Study cohort
Utilizing electronic medical records, we retrospectively evaluated consecutive patients meeting the following inclusion criteria: newly diagnosed AML at the Wake Forest Baptist Comprehensive Cancer Center from 2002 to 2009 [4], intensive induction treatment (cytarabine and anthracycline based regimen), and age > 18 years. Patients receiving less intense treatment regimens (such as, hypomethylating agents, palliative treatment, or no treatment) and those with acute promyelocytic leukemia (APL) were excluded from the study. Patients without available recorded blood glucose measurements during induction hospitalization were also excluded. Any patients who received prior chemotherapy for hematologic malignancy or for whom the date of induction was not recorded were also excluded. Wake Forest School of Medicine’s Institutional Review Board approved this study.
Predictor variables
All blood glucose values for each patient during induction hospitalization were obtained via the electronic medical record (excluding point of care values). From these values, we calculated each patient’s mean blood glucose value and standard deviation for the entire hospitalization for AML induction therapy.
Covariates
Demographic covariates included age (stratified at the time of diagnosis as ≥ 60 vs. < 60 years) [19], sex, race/ethnicity, and insurance type. Clinical variables were baseline body mass index (BMI), comorbidities, and baseline laboratory data from the date of presentation for induction therapy in the hospital, including: hemoglobin, bilirubin, white blood cell count, creatinine, and lactate dehydrogenase (LDH). Comorbidity data were collected using ICD-9 codes and chart review via the electronic medical record on or before the date of induction chemotherapy as previously described by Tawfik et al. [4]. Comorbidity burden was then captured using the modified Charlson Comorbidity Index (CCI). Disease-specific variables included the cytogenetic risk stratification score [20]. Medication data collected included steroids, insulin, and oral anti-diabetic agents.
Outcomes
Outcomes were treatment response as defined by complete remission (CR) and overall survival (OS) from date of induction chemotherapy initiation. CR was defined as absolute neutrophil count > 1000/μl, platelets ≥ 100,000/μl, and no residual or extra medullary disease. Complete remission with incomplete count recovery (CRi) was defined as < 5% bone marrow blasts and transfusion independence with persistent cytopenia with either an absolute neutrophil count < 1000/μl or platelets < 100,000/μl, but not both [21]. For analysis, the remission outcome included CR + CRi.
Statistical analysis
Data were stratified by age (< 60, ≥ 60 years), and descriptive statistics were calculated to examine differences between the strata. All analyses were done by strata. Categorical variables were compared using an exact test and a t test or Kruskal-Wallis test was used to compare continuous measures, depending on the data distributions. For all analyses, comorbidity burden (CCI) was categorized as none vs. 1 or more based on the distribution of comorbidity scores. Some of the measures required a log transformation in order to meet the assumption of normality. Glucose mean and glucose standard deviation were categorized using cut-points determined to be the best at predicting survival, after examining multiple cut-points of the data. Specifically, we created temporary cut-offs for each group and variable using a range of values (20th to 90th percentiles). The results of log-rank tests for the range of temporary cut-offs were evaluated and the cut-off used was determined based upon the peak significance level of the test as well as the pattern of significance in cases of bimodal distribution of significance. The resulting cut-off was near the 75th percentile for each measure. Kaplan-Meier estimation was used to estimate median overall survival and produce survival plots. Cox proportional hazards models were used for bivariate analysis and fully adjusted models for overall survival. We used logistic regression to evaluate CR + CRi and 30-day mortality, stratified by age (< 60 vs. ≥ 60 years). Covariates for the multivariable models were variables with a p value of 0.1 or less in bivariate analysis. We performed two exploratory analyses in which we adjusted final models for diabetes and receipt of steroid medication during hospitalization to evaluate potential effect modification of these variables. All analyses were performed using SAS version 9.4 software (SAS Institute Inc., Cary, NC).
Results
Among 368 consecutive cases of individuals with newly diagnosed AML, 262 met our inclusion criteria. Of these, 124 were < 60 years (median age 45 years), 138 were ≥ 60 years (median age 70 years). Baseline characteristics of the 262 patients are presented in Table 1. Older patients had a higher baseline comorbidity index and a higher prevalence of diabetes. Over 80% of younger patients received the 7 + 3 + 3 regimen, whereas most older patients were treated with either 7 + 3 or 7 + 3 + 3. The mean number of blood glucose values obtained per patient during hospitalization was 61 ± 71. The mean blood glucose during hospitalization was 111.6 ± 16.4 mg/dL in younger versus 121.7 ± 25.9 mg/dL in older patients. The mean standard deviation of blood glucose values (glycemic variability) was 26.8 ± 18.6 mg/dL in younger versus 33 ± 22.8 mg/dL in older patients, with a greater value indicating increased variability. Complete remission (CR + CRi) was achieved in 82% of younger and in 61% of older patients. The median OS for younger and older adults were 23.1 months and 7.9 months, respectively. Early mortality (30 day) was 9% and 16% among younger and older adults, respectively.
Table 1.
Patient characteristics
| < 60 years (N = 124) | ≥ 60 years (N = 138) | |
|---|---|---|
| Age in years: mean (SD) | 44.1 (11.5) | 70.5 (6.5) |
| Female | 52% | 47% |
| White | 81% | 94% |
| Insurance type | ||
| Medicaid | 30 (24%) | 2 (1%) |
| Medicare | 13 (11%) | 106 (77%) |
| Commercial insurance | 77 (62%) | 30 (22%) |
| No insurance | 4 (3%) | 0 (0%) |
| Clinical characteristics | ||
| Chronic obstructive pulmonary disease | 5 (4%) | 18 (13%) |
| Diabetes | 9 (7%) | 28 (20%) |
| Acute or chronic renal failure | 12 (10%) | 23 (17%) |
| Coronary artery disease/heart failure | 6 (5%) | 18 (13%) |
| Charlson comorbidity Index > 1 | 15.8% | 60.1% |
| Received steroid during hospitalization | 51.6% | 48.6% |
| Received insulin during hospitalization | 24.2% | 31.2% |
| Body mass index < 30 (kg/m2) | 79 (68%) | 92 (74%) |
| Hemoglobin median (25th, 75th) | 9.3 (8,10.4) | 9.3 (8.3,10.4) |
| White blood cell median (25th, 75th) | 18.3 (5.5, 45.1) | 10.1 (2.3, 45.6) |
| Lactate dehydrogenase median (25th, 75th) | 396 (278, 655) | 328 (212, 502) |
| Cytogenetic risk category | ||
| Favorable | 29 (24.4%) | 4 (3.1%) |
| Intermediate | 66 (55.5%) | 95 (72.5%) |
| Unfavorable | 24 (20.2%) | 32 (24.4%) |
| Induction treatment | ||
| 7 + 3 | 10 (8.1%) | 51 (37.0%) |
| 7 + 3 + 3 | 101 (81.5%) | 58 (42.0%) |
| 7 + 3 + other | 9 (7.3%) | 24 (17.4%) |
| Glycemic profiles (mg/dL) | ||
| Hospitalization mean glucose (mean (SD)) | 111.6 (16.4) | 121.7 (25.9) |
| Hospitalization glycemic variability: glucose SD (mean (SD)) | 26.8 (18.6) | 33.0 (22.8) |
SD, standard deviation; 7 + 3 + 3, cytarabine+anthracycline+etoposide
Characteristics associated with CR among younger patients were lower BMI (p < 0.01), cytogenetic risk score (p < 0.01), baseline glucose (p < 0.01), mean glucose (p < 0.01), and glycemic variability (p<0.01). After adjusting for cytogenetic risk and BMI, the odds of achieving remission remained independently associated with glycemic variability (OR 0.71 for a 10 mg/dL increase, CI 0.53–0.96, p = 0.03), but not mean blood glucose (OR 0.74 for a 10 mg/dL increase, CI 0.50–1.09, p = 0.1) (Table 2). Among older patients’ characteristics associated with remission were LDH (p = 0.02), baseline glucose (p = 0.05), mean glucose (p < 0.01), and glycemic variability (p < 0.01). In multivariate analyses adjusting for LDH, the odds of remission remained independently associated with both glycemic variability and mean blood glucose (OR 0.73, CI 0.61–0.88, p = 0.0007 and OR 0.80, CI 0.69–0.93, p = 0.004 respectively for a 10-mg/ dL increase). For example, among older adults, for each 10 mg/ dL increase in mean blood glucose during induction hospitalization, the odds of remission decreased by 27%.
Table 2.
Association between glycemic control, remission, and mortality
| Adjusted odds ratio (OR) for remission (95% CI) | ||
| < 60 years (N = 110) | ≥ 60 years (N = 136) | |
| Hospitalization mean glucose (per 10 mg/dL increase) | 0.74 (0.50–1.09) | 0.80 (0.69–0.93) |
| p = 0.1 | p = 0.004 | |
| Glycemic variability (per 10 mg/dL increase) | 0.71 (0.53–0.96) | 0.73 (0.61–0.88) |
| p = 0.03 | p = 0.0007 | |
| < 60 years, adjusted for: cytogenetic risk score, BMI | ||
| ≥ 60 years, adjusted for: LDH | ||
| Adjusted HR for Mortality (95% CI) | ||
| < 60 years (N = 119) | ≥ 60 years (N = 131) | |
| Hospitalization mean glucose (per 10 mg/dL increase) | 1.09 (0.96–1.25) | 1.13 (1.05–1.21) |
| p = 0.2 | p = 0.001 | |
| Glycemic variability (per 10 mg/dL increase) | 1.07 (0.97–1.18) | 1.17 (1.09–1.26) |
| p = 0.2 | p < 0.0001 | |
| < 60 years, adjusted for: age, comorbidities, cytogenetic risk, LDH | ||
| ≥ 60 years, adjusted for: age, comorbidities, cytogenetic risk, LDH |
BMI, body mass index; LDH, lactate dehydrogenase; OR, odds ratio; CI, confidence interval; HR, hazard ratio
Characteristics associated with OS for younger patients in univariate analyses were age (p < 0.01), comorbidity index (p = 0.03), cytogenetic risk score (p < 0.01), baseline glucose (p = 0.02), mean glucose value (p = 0.03), and glycemic variability (p = 0.04). In multivariate analyses, adjusting for age, comorbidity burden, cytogenetic risk, and LDH, mean blood glucose and glycemic variability were not significantly associated with mortality.
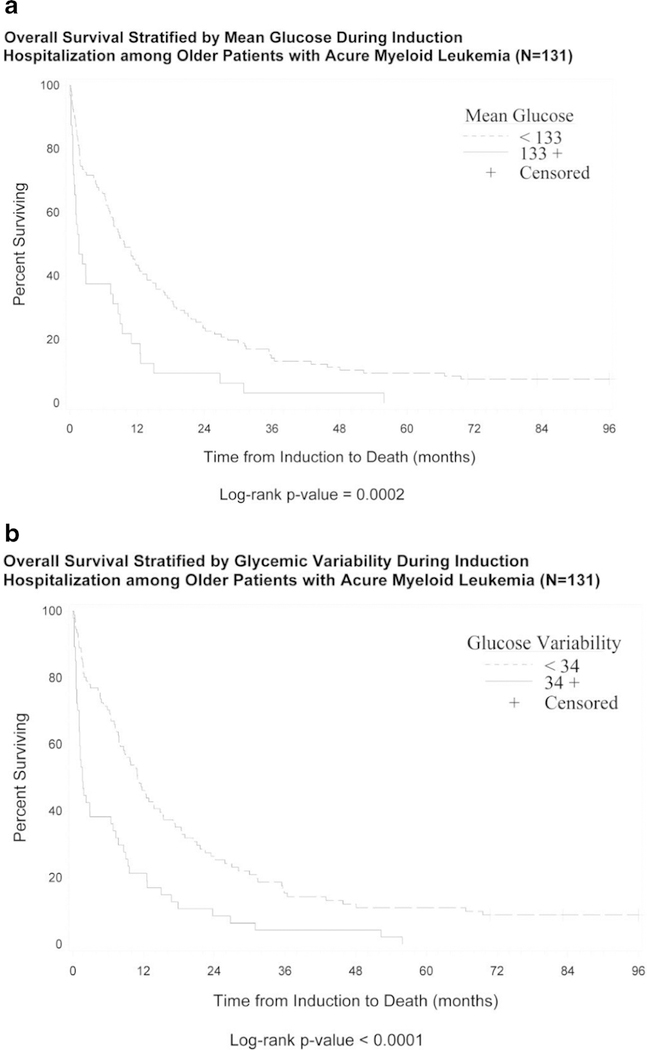
Among older patients, characteristics associated with survival in univariate analyses were age (p = 0.03), cytogenetic risk score (p = 0.03), LDH (p = 0.04), baseline glucose (p = 0.04), mean glucose value (p < 0.01), and glycemic variability (p < 0.01). The median survival for older adults with mean glucose above the cohort median (≥ 133 mg/dL) was 1.6 months (95% CI 0.8–7.7) compared to a median survival of 9.7 months (95% CI 7.2–12.9) in older adults with a lower mean glucose level (< 133 mg/dL) (see Fig. 1a). Among older adults with increased glycemic variability (greater than the cohort median 34 mg/dL), the median survival was 1.6 months (95% CI 1.1–6.5), compared to a median survival of 11 months in older adults with lower glycemic variability (glucose SD < 34 mg/dL) (see Fig. 1b).
Fig. 1.
Overall survival among older adults with acute myeloid leukemia stratified by mean hospitalization glucose (a) and glycemic variability (b)
In multivariate analyses, after adjusting for age, comorbidity burden, cytogenetic risk, and LDH, both mean blood glucose and glycemic variability were independently associated with survival in older patients (Table 2). Specifically, for every 10 mg/dL increase in mean glucose value, the hazard of overall mortality increased by 13%. Similarly, among older patients, for each 10 mg/dL increase in glycemic variability, the hazard of overall mortality increased by 17%. A diagnosis of diabetes was not associated with overall survival in either group.
A sensitivity analysis among only patients who survived longer than 60 days (N = 94) found that hyperglycemia (mean glucose ≥ 133 mg/dL) remained significantly associated with survival for older adults (p = 0.05) with a trend toward association for glycemic variability (p = 0.07). In exploratory analyses, diagnosis of diabetes was not associated with remission in either age group. While adjusting final models for diabetes did not significantly change effect sizes of odds ratio, after adjustment, the relationship between mean glucose and remission was significant for younger patients in addition to older adults (OR 0.67 (CI 0.48–0.93) and OR 0.78 (CI 0.65–0.93)). Odds ratios for glycemic variability remained significant when final models were adjusted for diabetes (OR 0.73 (CI 0.56–0.96) and OR 0.71 CI (0.57–0.88) for younger and older respectively. Similarly, diabetes was not associated with survival in unadjusted or adjusted analyses. When adjusting for diabetes, mean hospitalization glucose and glycemic variability remained significantly associated with mortality for older adults (HR 1.12 (CI 1.04–1.21) and HR 1.17 (CI 1.08–1.26)) and non-significant in younger adults (HR 1.11 (CI 0.98–1.27) and HR 1.09 (CI 0.98–1.22)). Additional models were evaluated adjusting for receipt of steroid medication. There were no significant changes in outcomes for remission or mortality (data not shown). Receipt of steroids was not associated with remission or mortality.
Discussion
Here, we report that higher mean blood glucose levels during induction hospitalization and increased glycemic variability were associated with lower odds of remission and shorter overall survival in older adults undergoing hospitalization for intensive AML therapy. Among patients less than 60 years old, increased glycemic variability was negatively associated with remission. These results identify glycemic variability as a risk factor that could be intervened upon during induction hospitalization.
Our results are consistent with other studies suggesting that hyperglycemia leads to poorer outcomes in AML. For example, Ali et al. (2007) found that hyperglycemia (defined as serum glucose level of > 110 mg/dL) was associated with increased hospital mortality over a 3-year period [10]. In a later study of patients with acute leukemia (including acute lymphoblastic leukemia and AML), hyperglycemia (defined as at least one fasting blood glucose > 100 mg/dL from 1 week prior to induction until 30 days after) was associated with increased risk of complicated infections and death [11]. Storey et al. found that patients hospitalized with leukemia (N = 42) experiencing hyperglycemia (defined as fasting blood glucose of > 126 mg/dL) were more likely to experience neutropenia, but risk for infection was not affected [12]. In addition, hyperglycemia in older patients (≥ 65 years) undergoing AML induction therapy was associated with increased infection risk [13]. Together, these studies have identified an association between hyperglycemia (variably defined) in hospitalized patients with leukemia and increased risk for infection [11, 13], neutropenia [12], and in-hospital mortality [10].
Our study adds to the current literature by evaluating the relationship between glycemic control, remission, and overall survival. Further, while other studies included heterogeneous acute leukemia patients (acute lymphoblastic leukemia and AML) [11, 12], we focused on patients with AML. In addition, our study analyzed patients with AML during their induction hospitalization only, prior studies included all hospitalizations for any cause over a specified time [10]. To our knowledge, only one other study has evaluated the association between glycemic control and outcomes in older AML patients undergoing intense induction hospitalization [13]. Our study’s increased sample size and specificity has reaffirmed and expanded the understanding of glycemic control’s relationship with older AML patient outcomes and suggests glycemic control may be relevant for younger adults as well.
Hyperglycemia could modulate lower CR and OS in our study through increased infection risk, as a surrogate for other vulnerabilities, or an alteration of treatment response. Our sensitivity analyses suggest that the relationship between hyperglycemia and survival is not limited to increased risk of early mortality. While hyperglycemia has been linked to increased infection risk among patients with leukemia [11, 13], its link to treatment response is unknown. However, in vitro studies have suggested that hyperglycemia may alter treatment response in colon, prostate, and breast cancers [22–24], thus making it plausible that hyperglycemia’s association with poorer outcomes in AML induction therapy may be related to tumor microenvironment and treatment response alterations. In exploratory analyses, observed relationships were not explained by use of steroids or diagnosis of diabetes at admission suggesting that hyperglycemia is not a surrogate for risk factors inherent with steroid exposure or long standing diabetes. This observation extends prior literature suggesting that hyperglycemia likely explains relationships observed between diabetes and survival rather than the other way around. This is clinically relevant as patients who do not have a diagnosis of diabetes may have hyperglycemia thereby extending the population of patients potentially at risk.
Unlike hyperglycemia, glycemic variability is an emerging paradigm through which to view glycemic control. In patients with type 2 diabetes, variability of fasting blood glucose (measured as a coefficient of variation) was an independent predictor of mortality from all causes and from cardiovascular disease, whereas the mean fasting plasma glucose or slope of the fasting plasma glucose were not [25, 26]. Within the critical care setting, glycemic variability (as measured by standard deviation) was a significant, independent, and stronger predictor of intensive care unit mortality than mean glucose concentration [27].This finding was supported by a critical care study in which increased mortality was associated with increased glycemic variability [17]. The literature suggests that increased glycemic variability is associated with worse outcomes (short-term and long-term mortality) across a variety of settings. Our study extends the association of glycemic variability and poor outcomes to patients with AML.
Our findings suggest that glycemic variability may affect treatment response. Oscillations of glucose levels increase apoptosis and oxidative stress via increased superoxide production at the mitochondrial level [28–32]. In healthy subjects without diabetes, acute hyperglycemia downregulates several genes involved in free radical detoxification [28]. Therefore, although the pathophysiologic process has yet to be fully understood, our results have a plausible biologic basis that can be extended in future studies. Validation of these observations in multi-center datasets or observational studies is a next step to support design of intervention studies. In addition, investigation of these observations in preclinical models can elucidate mechanisms by which glycemic control influences outcomes.
This study has limitations such as its retrospective nature and limitation to patients seen at a single institution. We used blood glucose data points from standard laboratory testing; however, continuous blood glucose monitoring would be more informative and could allow more timely management of glucose variability and hyperglycemia. The diagnosis of diabetes is based on claims data and chart review which while comprehensive may yet underrepresent those with diagnosis of diabetes not requiring medication management. Our sample size may have been underpowered to determine the relationship between glycemic control and outcomes in younger patients since few of them had diabetes.
Our study had a large sample size of adults compared to prior studies evaluating the AML induction hospitalization time period. Standard treatment regimens (considered intense) and the hospitalization setting reduced the heterogeneity of the study population and could make it easier to replicate our findings. Finally, evaluation of glycemic variability in the context of AML is a novel contribution to the literature.
Next steps to validate these observations can include evaluating the relationship between hyperglycemia and outcomes using existing datasets from multi-site clinical trials in the National Clinical Trials Network. While existing datasets may have less comprehensive assessment of hyperglycemia, use of available lab data may provide additional confirmation of relationships observed. Prospective studies may be needed to validate the negative prognostic value of glycemic variability and carefully characterize potential confounders and outcomes which may shed further light on mechanisms. Additionally, preclinical work evaluating the impact of hyperglycemia on leukemic biology and chemo-sensitivity could inform mechanisms underlying these observations.
Conclusions
Increased glycemic variability during intense induction in adults with AML is associated with lower remission rates. Hyperglycemia and increased glycemic variability were associated with shorter overall survival in older patients. Therefore, attention and treatment of hyperglycemia and variability during induction may be a modifiable factor to improve AML outcomes.
Acknowledgments
We thank Karen Klein, MA ELS (Research Support Core Wake Forest School of Medicine Clinical Translational Science Institute), for her editorial contributions to the manuscript.
Funding information Research reported in this publication was supported by the National Cancer Institute’s Cancer Center Support Grant award number P30CA012197 issued to the Wake Forest Baptist Comprehensive Cancer Center. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute. Heidi. D. Klepin was supported by a Paul Beeson Career Development Award in Aging Research (K23AG038361; supported by NIA, AFAR, The John A. Hartford Foundation, and The Atlantic Philanthropies) and The Gabrielle’s Angel Foundation for Cancer Research.
Footnotes
Conflict of interest The authors declare that they have no conflict of interest.
Compliance with ethical standards
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Adult acute myeloid leukemia (PDR)- Health Professional Version (2016). NIH: National Cancer Institute; [PubMed] [Google Scholar]
- 2.Dohner H, Estey EH, Amadori S, Appelbaum FR, Buchner T, Burnett AK, Dombret H, Fenaux P, Grimwade D, Larson RA, Lo-Coco F, Naoe T, Niederwieser D, Ossenkoppele GJ, Sanz MA, Sierra J, Tallman MS, Lowenberg B, Bloomfield CD (2010) Diagnosis and management of acute myeloid leukemia in adults: recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood 115(3):453–474. 10.1182/blood-2009-07-235358 [DOI] [PubMed] [Google Scholar]
- 3.Dohner H, Weisdorf DJ, Bloomfield CD (2015) Acute myeloid leukemia. N Engl J Med 373(12):1136–1152. 10.1056/NEJMra1406184 [DOI] [PubMed] [Google Scholar]
- 4.Tawfik B, Pardee TS, Isom S, Sliesoraitis S, Winter A, Lawrence J, Powell BL, Klepin HD (2016) Comorbidity, age, and mortality among adults treated intensively for acute myeloid leukemia (AML). J Geriatr Oncol 7(1):24–31. 10.1016/j.jgo.2015.10.182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Etienne A, Esterni B, Charbonnier A, Mozziconacci MJ, Arnoulet C, Coso D, Puig B, Gastaut JA, Maraninchi D, Vey N (2007) Comorbidity is an independent predictor of complete remission in elderly patients receiving induction chemotherapy for acute myeloid leukemia. Cancer 109(7):1376–1383 [DOI] [PubMed] [Google Scholar]
- 6.Deschler B, Ihorst G, Platzbecker U, Germing U, Marz E, de Figuerido M, Fritzsche K, Haas P, Salih HR, Giagounidis A, Selleslag D, Labar B, de Witte T, Wijermans P, Lubbert M (2013) Parameters detected by geriatric and quality of life assessment in 195 older patients with myelodysplastic syndromes and acute myeloid leukemia are highly predictive for outcome. Haematologica 98(2):208–216. 10.3324/haematol.2012.067892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Giles FJ, Borthakur G, Ravandi F, Faderl S, Verstovsek S, Thomas D, Wierda W, Ferrajoli A, Kornblau S, Pierce S, Albitar M, Cortes J, Kantarjian H (2007) The haematopoietic cell transplantation comorbidity index score is predictive of early death and survival in patients over 60 years of age receiving induction therapy for acute myeloid leukaemia. Br J Haematol 136(4):624–627 [DOI] [PubMed] [Google Scholar]
- 8.Oran B, Weisdorf DJ (2012) Survival for older patients with acute myeloid leukemia: a population-based study. Haematologica 97(12):1916–1924. 10.3324/haematol.2012.066100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Savic A, Kvrgic V, Rajic N, Urosevic I, Kovacevic D, Percic I, Popovic S (2012) The hematopoietic cell transplantation comorbidity index is a predictor of early death and survival in adult acute myeloid leukemia patients. Leukemia Res 36(4):479–482. 10.1016/j.leukres.2011.11.021 [DOI] [PubMed] [Google Scholar]
- 10.Ali NA, O’Brien JM Jr, Blum W, Byrd JC, Klisovic RB, Marcucci G, Phillips G, Marsh CB, Lemeshow S, Grever MR (2007) Hyperglycemia in patients with acute myeloid leukemia is associated with increased hospital mortality. Cancer 110(1):96–102. 10.1002/cncr.22777 [DOI] [PubMed] [Google Scholar]
- 11.Matias Cdo N, Lima V, Teixeira HM, Souto FR, Magalhaes V (2013) Hyperglycemia increases the complicated infection and mortality rates during induction therapy in adult acute leukemia patients. Revista Brasileira de Hematologia e Hemoterapia 35(1): 39–43. 10.5581/1516-8484.20130013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Storey S, Von AD (2015) Prevalence and impact of hyperglycemia on hospitalized leukemia patients. Eur J Oncol Nurs 19(1):13–17. 10.1016/j.ejon.2014.08.005 [DOI] [PubMed] [Google Scholar]
- 13.Storey S (2016) Impact of hyperglycemia and age on outcomes in patients with acute myeloid leukemia. Oncology Nurs Forum 43(5): 595–601. 10.1188/16.onf.595-601 [DOI] [PubMed] [Google Scholar]
- 14.Hirsch IB (2015) Glycemic variability and diabetes complications: does it matter? Of course it does! Diabet Care 38(8):1610–1614. 10.2337/dc14-2898 [DOI] [PubMed] [Google Scholar]
- 15.Ceriello A, Ihnat MA (2010) ‘Glycaemic variability’: a new therapeutic challenge in diabetes and the critical care setting. Diabet Med 27(8):862–867. 10.1111/j.1464-5491.2010.02967.x [DOI] [PubMed] [Google Scholar]
- 16.Ceriello A, Kilpatrick ES (2013) Glycemic variability: both sides of the story. Diabet Care 36(Suppl 2):S272–S275. 10.2337/dcS13-2030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krinsley JS (2008) Glycemic variability: a strong independent predictor of mortality in critically ill patients. Crit Care Med 36(11): 3008–3013. 10.1097/CCM.0b013e31818b38d2 [DOI] [PubMed] [Google Scholar]
- 18.Mendez CE, Mok KT, Ata A, Tanenberg RJ, Calles-Escandon J, Umpierrez GE (2013) Increased glycemic variability is independently associated with length of stay and mortality in noncritically ill hospitalized patients. Diabet Care 36(12):4091–4097. 10.2337/dc12-2430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Appelbaum FR, Gundacker H, Head DR, Slovak ML, Willman CL, Godwin JE, Anderson JE, Petersdorf SH (2006) Age and acute myeloid leukemia. Blood 107(9):3481–3485. 10.1182/blood-2005-09-3724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Slovak ML, Kopecky KJ, Cassileth PA, Harrington DH, Theil KS, Mohamed A, Paietta E, Willman CL, Head DR, Rowe JM, Forman SJ, Appelbaum FR (2000) Karyotypic analysis predicts outcome of preremission and postremission therapy in adult acute myeloid leukemia: a southwest oncology group/eastern cooperative oncology group study. Blood 96(13):4075–4083 [PubMed] [Google Scholar]
- 21.Cheson BD, Bennett JM, Kopecky KJ, Buchner T, Willman CL, Estey EH, Schiffer CA, Doehner H, Tallman MS, Lister TA, Lo-Coco F, Willemze R, Biondi A, Hiddemann W, Larson RA, Lowenberg B, Sanz MA, Head DR, Ohno R, Bloomfield CD (2003) Revised recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. J Clin Oncol 21(24):4642–4649. 10.1200/jco.2003.04.036 [DOI] [PubMed] [Google Scholar]
- 22.Ma YS, Yang IP, Tsai HL, Huang CW, Juo SH, Wang JY (2014) High glucose modulates antiproliferative effect and cytotoxicity of 5-fluorouracil in human colon cancer cells. DNA Cell Biol 33(2): 64–72. 10.1089/dna.2013.2161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Biernacka KM, Uzoh CC, Zeng L, Persad RA, Bahl A, Gillatt D, Perks CM, Holly JM (2013) Hyperglycaemia-induced chemoresistance of prostate cancer cells due to IGFBP2. Endocrine-Related Cancer 20(5):741–751. 10.1530/erc-13-0077 [DOI] [PubMed] [Google Scholar]
- 24.Zeng L, Biernacka KM, Holly JM, Jarrett C, Morrison AA, Morgan A, Winters ZE, Foulstone EJ, Shield JP, Perks CM (2010) Hyperglycaemia confers resistance to chemotherapy on breast cancer cells: the role of fatty acid synthase. Endocrine-Related Cancer 17(2):539–551. 10.1677/erc-09-0221 [DOI] [PubMed] [Google Scholar]
- 25.Muggeo M, Verlato G, Bonora E, Ciani F, Moghetti P, Eastman R, Crepaldi G, de Marco R (1995) Long-term instability of fasting plasma glucose predicts mortality in elderly NIDDM patients: the Verona diabetes study. Diabetologia 38(6):672–679 [DOI] [PubMed] [Google Scholar]
- 26.Muggeo M, Verlato G, Bonora E, Zoppini G, Corbellini M, de Marco R (1997) Long-term instability of fasting plasma glucose, a novel predictor of cardiovascular mortality in elderly patients with non-insulin-dependent diabetes mellitus: the Verona diabetes study. Circulation 96(6):1750–1754 [DOI] [PubMed] [Google Scholar]
- 27.Egi M, Bellomo R, Stachowski E, French CJ, Hart G (2006) Variability of blood glucose concentration and short-term mortality in critically ill patients. Anesthesiology 105(2):244–252 [DOI] [PubMed] [Google Scholar]
- 28.Meugnier E, Faraj M, Rome S, Beauregard G, Michaut A, Pelloux V, Chiasson JL, Laville M, Clement K, Vidal H, Rabasa-Lhoret R (2007) Acute hyperglycemia induces a global downregulation of gene expression in adipose tissue and skeletal muscle of healthy subjects. Diabetes 56(4):992–999. 10.2337/db06-1242 [DOI] [PubMed] [Google Scholar]
- 29.Quagliaro L, Piconi L, Assaloni R, Martinelli L, Motz E, Ceriello A (2003) Intermittent high glucose enhances apoptosis related to oxidative stress in human umbilical vein endothelial cells: the role of protein kinase C and NAD(P)H-oxidase activation. Diabetes 52(11):2795–2804 [DOI] [PubMed] [Google Scholar]
- 30.Piconi L, Quagliaro L, Assaloni R, Da Ros R, Maier A, Zuodar G, Ceriello A (2006) Constant and intermittent high glucose enhances endothelial cell apoptosis through mitochondrial superoxide overproduction. Diabet/Metab Res Rev 22(3):198–203. 10.1002/dmrr.613 [DOI] [PubMed] [Google Scholar]
- 31.Quagliaro L, Piconi L, Assaloni R, Da Ros R, Maier A, Zuodar G, Ceriello A (2005) Intermittent high glucose enhances ICAM-1, VCAM-1 and E-selectin expression in human umbilical vein endothelial cells in culture: the distinct role of protein kinase C and mitochondrial superoxide production. Atherosclerosis 183(2): 259–267. 10.1016/j.atherosclerosis.2005.03.015 [DOI] [PubMed] [Google Scholar]
- 32.Piconi L, Quagliaro L, Da Ros R, Assaloni R, Giugliano D, Esposito K, Szabo C, Ceriello A (2004) Intermittent high glucose enhances ICAM-1, VCAM-1, E-selectin and interleukin-6 expression in human umbilical endothelial cells in culture: the role of poly (ADP-ribose) polymerase. J Thromb Haemos 2(8):1453–1459. 10.1111/j.1538-7836.2004.00835.x [DOI] [PubMed] [Google Scholar]