Abstract
Rationale:
3,4-Methylenedioxypyrovalerone (MDPV) is a popular synthetic cathinone reported to have a high abuse potential. Recent preclinical research indicates the psychopharmacology of MDPV is comparable to cocaine. Despite a recent influx of research on the psychopharmacology of MDPV, few studies have employed preclinical drug discrimination methods to discern the neurochemical mechanisms involved in its interoceptive stimulus effects.
Objective:
The aim of this study was to evaluate a variety of monoaminergic agents for substitution, potentiation or antagonism in rats trained to discriminate MDPV.
Methods:
Male Sprague-Dawley rats were trained to discriminate 0.5 (Experiment 1) or 1 mg/kg MDPV (Experiment 2) from saline under an FR 20 schedule of food reinforcement. In Experiment 1, MDMA, MDA and their respective optical isomers (0.75 – 3 mg/kg), cocaine (2.5 - 20 mg/kg), GBR 12909 (5-40 mg/kg), and desipramine (3.2-10 mg/kg) were assessed for substitution. GBR 12909 (40 mg/kg) and desipramine (3.2 mg/kg) were subsequently assessed for potentiation of the MDPV cue. In Experiment 2, stimulus antagonism tests were conducted with dopamine antagonists (Sch 23390, haloperidol) and serotonin antagonists (pirenperone, MDL100907, WAY 100635).
Results:
The MDMA and MDA enantiomers produced divergent results, with virtually no substitution by (−)-MDMA or (−)-MDA, partial substitution with (+)-MDA, and full substitution with (+)-MDMA, as well as full substitution by the racemates, (±)-MDMA and (±)-MDA. Consistent with previous findings, cocaine fully substituted for MDPV. Although no dose of GBR 12909 or desipramine substituted for MDPV, these reuptake inhibitors enhanced the discriminative stimulus effects of lower MDPV doses. Both D1 (Sch 23390) and D2 (haloperidol) DA antagonists attenuated 1 mg/kg MDPV discrimination, whereas none of the 5-HT antagonists assessed altered MDPV discrimination.
Conclusions:
These findings indicate MDPV’s interoceptive stimulus effects are mediated predominantly by dopaminergic actions, although serotonergic and/or noradrenergic modulation of these effects cannot be ruled out. Further investigations into the neurochemical actions involved in the discriminative stimulus effects of MDPV may serve to inform medication discovery and development for the treatment of MDPV abuse.
Recreational abuse of illicit synthetic cathinones (“bath salts”) presents a significant public health concern worldwide. The initial popularity of synthetic cathinone use arose in response to attempts to elude legal restrictions on other popular drugs of abuse, such as 3,4-methylenedioxymethamphetamine (MDMA) and cocaine (Valente et al. 2014). Although synthetic cathinones are now listed as schedule I controlled substances in the United States, illicit use remains popular among recreational users. Emergency room visits and poison control reports related to synthetic cathinones within the past decade (United Nations Office of Drugs and Crime 2015; U.S. Department of Justice National Drug Intelligence Center (NDIC) 2011) have raised public health awareness regarding these substances.
3,4-Methylenedioxypyrovalerone (MDPV) is one of several synthetic cathinone derivatives and a popular constituent of illicit “bath salts” commonly associated with emergency department reports related to “bath salt” abuse (Centers for Disease Control and Prevention (CDC) 2011; Froberg et al. 2015; NDIC 2011; Spiller et al. 2011). Previous research indicates MDPV has neuropharmacological and behavioral effects comparable to those of cocaine and the psychedelic-stimulant MDMA ("Ecstasy") (Aarde et al. 2015; Cameron et al. 2013; Fantegrossi et al. 2013; Gatch et al. 2013; Ross et al. 2012). Studies using established drug discovery techniques, such as in vitro receptor binding, have revealed that MDPV potently blocks dopamine and norepinephrine uptake, with relatively weaker effects on serotonin uptake (Eshleman et al. 2013; Simmler et al. 2013). Compared to cocaine, MDPV is up to 50-fold more potent at the dopamine transporter (DAT), approximately 10-fold more potent at the norepinephrine transporter (NET), and 10-fold less potent at the serotonin transporter (Baumann et al. 2012). This enhanced potency may be responsible for the severity of physiological and behavioral reports associated with MDPV toxicity (Froberg et al. 2015; Spiller et al. 2011).
The abuse liability of MDPV has been confirmed by several preclinical reports that this substance establishes conditioned place preference (King et al. 2015a, b) and maintains self-administration in rodents (Aarde et al. 2013; Aarde et al. 2015; Schindler et al. 2016; Watterson et al. 2014). A recent report indicates MDPV is approximately 10-fold more potent and approximately three-fold more effective at maintaining responding under a progressive ratio schedule compared to cocaine (Gannon et al. 2017). Determining the neurochemical actions contributing to MDPV’s abuse liability is critical to the discovery and development of treatment medications.
Drug discrimination is a widely accepted in vivo preclinical assay that provides both qualitative and quantitative data to evaluate the neurochemical actions underlying the interoceptive stimulus effects (i.e., subjective effects) of drugs (Baker 2017; Glennon and Young 2011). Despite the predictive utility of the drug discrimination paradigm, only two published studies have employed such methods to train animals to discriminate MDPV. These studies have noted similar interoceptive stimulus effects between MDPV and several established psychoactive stimulants, such as methamphetamine, d-amphetamine, cocaine, and MDMA (Berquist and Baker 2017; Fantegrossi et al. 2013). Some discrepancies between these studies are worth noting, particularly with regard to stimulus substitution with MDMA. Fantegrossi reported full substitution with MDMA in mice trained to discriminate 0.3 mg/kg MDPV, whereas Berquist and Baker (2017) found that MDMA failed to fully substitute in rats trained to discriminate 0.3 mg/kg MDPV. Other methodological differences besides species (e.g. reinforcer type and reinforcement schedule) were noted between these studies that could account for inconsistent findings.
Several other studies have evaluated MDPV for substitution in animals trained to discriminate other stimulants. Full substitution was observed with MDPV in rats trained to discriminate d-amphetamine (Harvey et al. 2017) or cocaine (Gannon et al. 2016; Gatch et al. 2013), although only partial substitution was observed with MDPV in rats trained to discriminate MDMA (Harvey and Baker 2016).
Equivocal findings regarding substitution between MDPV and MDMA may be related to MDMA’s complex cues involving serotonergic and dopaminergic actions that may be dissociable and dependent on discrimination training methods (Baker et al. 1995; Goodwin and Baker 2000; Goodwin et al. 2003). Previous studies utilizing drug discrimination procedures to assess the stereoisomers of MDMA and 3,4-methylenedioxyamphetamine (MDA) suggest that the discriminative stimulus effects of (+)-MDA are more similar to those of amphetamine, whereas the discriminative stimulus effects of (−)-MDA, and (−)-MDMA are more comparable to those of LSD (Baker et al. 1995; Broadbent et al. 1992; Callahan and Appel 1988; Glennon and Young 1984).
In an effort to evaluate the neurochemical actions underlying the discriminative stimulus effects of MDPV, the present study conducted two separate experiments. Experiment 1 consisted of stimulus substitution with MDMA, MDA, and their respective optical isomers, along with monoamine reuptake inhibitors with varying selectivity for DAT, NET, or SERT in rats trained to discriminate 0.5 mg/kg MDPV to aid in the classification of MDPV’s interoceptive stimulus effects. Additionally, Experiment 2 assessed dopamine and serotonin antagonists for attenuation of the MDPV cue in rats trained to discriminate 1.0 mg/kg MDPV.
Methods
Subjects:
Fourteen (Experiment 1: n=7; Experiment 2: n=7) male Sprague-Dawley rats (Charles River Laboratories, Wilmington, MA, USA) were housed individually in polycarbonate cages with corncob bedding (Harlan Laboratories, Haslett, MI, USA) in animal facilities maintained at a constant temperature (20 ± 2°C) and humidity (50 ± 5%) and under a 12:12 light/dark cycle (lights on from 07:00 to 19:00h). Animals were provided water ad libitum in home cages and fed restricted diets of commercial rodent chow (Purina®, Richmond, IN, USA) to maintain 85-90% of free-feeding weights (340-440g). All procedures were reviewed and approved by the Western Michigan University Institutional Animal Care and Use Committee, and were in accordance with the guidelines of the Guide for the Care and Use of Laboratory Animals (National Research Council of the National Academies 2011).
Apparatus:
Training and testing procedures were conducted in seven standard operant conditioning chambers equipped with three retractable levers, a food pellet dispenser and fan on the front panel, a 28-V house light on the back panel, and housed within sound-attenuating shells (ENV-001; Med Associates Inc. St. Albans, Vermont, USA). Experimental events were controlled using Med-PC IV software (version IV; Med Associates Inc.). Dustless Precision Pellets (45 mg; Product# F0021; BioServ, Flemington, NJ) were used as reinforcements for lever pressing.
Drugs:
Cocaine-hydrochloride, 3,4-methylenedioxypyrovalerone-hydrochloride (MDPV), (±)-3,4-methylenedioxymethamphetamine-hydrochloride (MDMA), (±)-3,4-methylenedioxyamphetamine-hydrochloride (MDA), and the optical isomers of MDMA and MDA were provided by the National Institute on Drug Abuse Drug Control Supply Program (Bethesda, MD). GBR 12909 bismethanesulfonate monohydrate was prepared in the Chemical Biology Research Branch, National Institute on Drug Abuse and the National Institute on Alcohol Abuse and Alcoholism. MDL 100,907, and WAY 100,635 were provided by the National Institutes of Mental Health Chemical Synthesis and Drug Supply Program. Pirenperone was purchased from Santa Cruz Biotechnology (Mississauga, ON). Desipramine-hydrochloride, (+)-Sch 23390-hydrochloride, and haloperidol were purchased from Sigma-Aldrich ® (St. Louis, MO). Haloperidol was dissolved in a few drops of 0.1 M HCl, diluted in sterile water, and the pH was adjusted as needed with 0.1 M NaOH. Pirenperone was dissolved in 30% cyclodextrin (2-hydroxypropyl-beta-cyclodextrin) at a concentration of 1 mg/ml and then diluted with sterile water. MDL 100,907 was dissolved with a few drops of glacial acetic acid and diluted with sterile water. All other drugs were dissolved in bacteriostatic 0.9% sodium chloride. All drugs were administered by intraperitoneal (i.p.) injection at a volume of 1 ml/kg. The range of doses and pre-session injection intervals (noted below) for MDPV and all other compounds tested were selected based on previous studies conducted in our laboratory.
Procedures:
Training procedures were identical in Experiment 1 and Experiment 2, with the exception of training dose. Initially, a training dose of 1 mg/kg was selected for both experiments. However, responding in a few animals in experiment 1 was completely disrupted by this dose, making discrimination training impossible. The training dose was subsequently lowered to 0.5 mg/kg for all animals in experiment 1. Rats used in experiment 2 continued to train with 1 mg/kg MDPV. Other than training dose, procedures were similar to previous studies conducted in our laboratory and described elsewhere (Berquist and Baker 2017). Briefly, rats were trained to discriminate either 0.5 mg/kg MDPV (Experiment 1) or 1.0 mg/kg MDPV (Experiment 2) from vehicle under a fixed ratio (FR) 20 schedule of food reinforcement. Lever assignment to stimulus condition was counterbalanced among rats in each experiment. Drug and vehicle training sessions were alternated with the stipulation that the same stimulus condition occurred no more than twice consecutively (e.g. D, D, V, D, V, V). The performance criteria for stimulus control was a minimum of eight out of ten consecutive discrimination training sessions with 80% or higher correct lever responses prior to delivery of the first reinforcer and for the total session.
After stimulus control was established, test sessions were conducted as described below for each experiment. Test sessions were similar to training sessions, with the exception that responses were not reinforced and sessions ended upon completion of the first FR 20 or after 20 minutes, whichever occurred first. Testing criteria between sessions required subjects to complete at least one drug and at least one vehicle training session consecutively with 80% or higher injection-appropriate responding.
Experiment 1.
In rats trained to discriminate 0.5 mg/kg MDPV, the following drugs (doses, pre-injection interval) were assessed for substitution: MDPV (0.05, 0.1, 0.5 mg/kg, 15 min), (±)-MDA, (±)-MDMA, (+)-MDA, (−)-MDA, (+)-MDMA, (−)-MDMA (0.75, 1.5, 3.0 mg/kg, 15 min), GBR 12909 (5, 10, 20, 40 mg/kg, 30 min), cocaine (2.5, 5, 10, 20 mg/kg, 15 min), and desipramine (0.0, 3.2, 5.6, 10 mg/kg, 30 min). Subsequently, desipramine (3.2 mg/kg, 30 min), GBR 12909 (40 mg/kg, 30 min), or vehicle (30 min) were administered as a pretreatment to each MDPV dose (0.05, 0.1, 0.5 mg/kg, 15 min) to assess potentiation of MDPV discrimination.
Experiment 2:
After determination of the dose-response curve with MDPV (0.1, 0.3, 1.0 mg/kg, 15 min), the following drugs were assessed for antagonism of 1.0 mg/kg MDPV: Sch 23990 (0.01, 0.03, 0.1, 0.3 mg/kg, 30 min), haloperidol (0.125, 0.25, 0.5 mg/kg, 60 min), pirenperone (0.16, 0.32, 0.64 mg/kg, 60 min), MDL 100,907 (0.025, 0.05, 0.1 mg/kg, 60 min), and WAY 100,635 (0.4, 0.8, 1.6 mg/kg, 60 min).
Data analysis:
Stimulus control was determined as the number of sessions required for each subject to meet specified criteria of 80% correct responses in a minimum of 8 of 10 consecutive discrimination training sessions. The number of sessions to criteria was determined from the first training session when both levers were present. For each test compound, the mean (±SEM) percentage of drug-lever responses was determined for each dose. These data were analyzed with descriptive statistics and plotted in dose response curves for visual analysis. Tests in which an animal emitted less than 10 total responses were excluded from the analysis of percentage drug-lever selection. Full substitution by a test compound was defined as ≥ 80% drug-lever selection by any particular dose. Partial substitution was defined as drug- lever selection between 40 and 80%. Full antagonism by a test compound was defined as ≤ 20% drug-lever selection by any dose. Partial antagonism was defined as between 20 and 60% drug-lever selection.
Response rates were expressed as the number responses emitted per second during test sessions. For each test compound, the mean (±SEM) response rate was determined for each dose and these data were plotted in dose response curves. Response rates were included in statistical analyses regardless of the number of responses emitted. For each test compound, response rates were statistically analyzed using a one-way repeated-measures (RM) analysis of variance (ANOVA), followed by Dunnett’s multiple comparisons to compare each test dose to the saline control. Graphical and statistical analyses were conducted using Prism GraphPad (Version 6.0).
Results
Experiment 1:
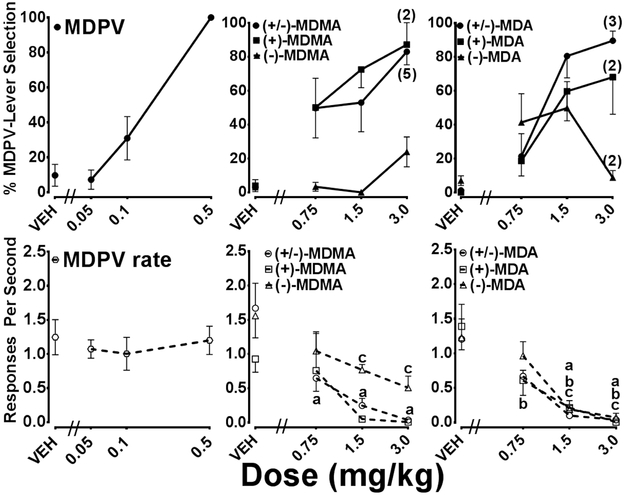
Stimulus control was established by 0.5 mg/kg MDPV within 26.7 (± 3.4, S.E.M.) training sessions (range 11-36). Dose-response curves generated from substitution tests with MDPV, MDMA, MDA and the optical isomers of MDMA or MDA are displayed in Figure 1. MDPV produced dose-dependent increases in MDPV-lever responses with full substitution at the training dose (100 ± 0 %), and with minimal effects on response rate. The MDMA and MDA enantiomers produced divergent results, with minimal MDPV-lever selection produced by (−)-MDMA or (−)-MDA, partial substitution by 1.5 mg/kg (+)-MDA (60%), and by 1.5 mg/kg (+)-MDMA (72%). Full substitution (87%) was observed with 3.0 mg/kg (+)-MDMA, but only two of the seven animals completed the response requirement following this dose. The MDMA and MDA racemates produced full substitution, with 83% MDPV-lever selection by 3.0 mg/kg (±)-MDMA and 80% by 1.5 mg/kg (±)-MDA. Full substitution (89%) was also observed following 3.0 mg/kg (±)-MDA, although only two of seven animals met the response requirement to be included in the analysis.
Figure 1.
Dose-response curves determined from substitution tests with MDPV, MDMA, MDA and the optical isomers of MDMA and MDA in rats trained to discriminate 0.5 mg/kg MDPV from saline. Percentage MDPV-lever selection is depicted by closed symbols in the top panel and response rate is shown as open symbols in the bottom panel. Individual data points represent the group mean (± SEM). N=7 except where noted in parentheses. Statistically significant Dunnett’s tests compared to saline (P < 0.05) are indicated by the letter a [(±)-MDMA or (±)-MDA], b [(+)-MDMA or (+)-MDA], or c [(−)-MDMA or (−)-MDA].
A dose-dependent decrease in response rate was observed with MDMA, MDA and their optical isomers. A one-way RM ANOVA indicated a statistically significant reduction in response rate by (−)-MDMA [F(3, 18) = 4.64, P < 0.05], (+)-MDA [F(3, 18) = 11.10, P < 0.001], (−)-MDA [F(3, 18) = 17.09, P < 0.0001] as well as with (±)-MDMA [F(3, 18) = 10.10, P < 0.001] and (±)-MDA [F(3, 18) = 13.14, P < 0.0001]. Dunnett’s multiple comparison tests indicated that the following doses and test compounds significantly reduced response rate compared to saline (P < 0.05): 1.5 mg/kg and 3.0 mg/kg (−)-MDMA; 0.75, 1.5 and 3.0 mg/kg (+)-MDA; 1.5 and 3.0 mg/kg (−)-MDA; 0.75, 1.5 and 3.0 mg/kg (±)-MDMA; 1.5 and 3.0 mg/kg (±)-MDA.
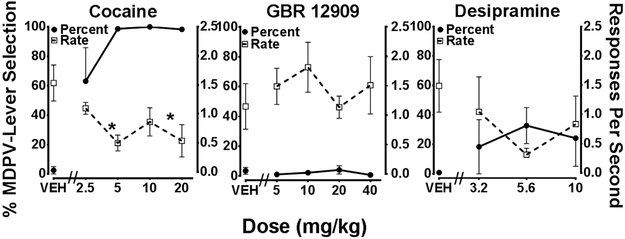
Cocaine fully substituted for MDPV at 5, 10 and 20 mg/kg and partially substituted at 2.5 mg/kg. The selective atypical DAT inhibitor, GBR 12909 and the NET/SERT inhibitor, desipramine, failed to substitute for MDPV at the doses tested. Dose-response curves generated from stimulus substitution tests with these substances are displayed in Figure 2. A one-way RM ANOVA revealed a statistically significant effect of cocaine dose on response rate [F(4, 16) = 3.87, P < 0.05]. Dunnett’s multiple comparison tests indicated 5 and 20 mg/kg cocaine significantly lowered response rate compared to saline (P < 0.05). Neither GBR 12909 nor desipramine significantly reduced response rate.
Figure 2.
Dose-response curves for cocaine (n=5),GBR 12909 (n=6), and desipramine (n=5) in animals trained to discriminate 0.5 mg/kg MDPV from saline. Percentage MDPV-lever responses (closed symbols) refers to the left Y-axis and response rate (open symbols) refers to the right Y-axis. Individual points represent the group mean (± SEM). Statistically significant Dunnett’s tests compared to saline are indicated by * (P < 0.05).
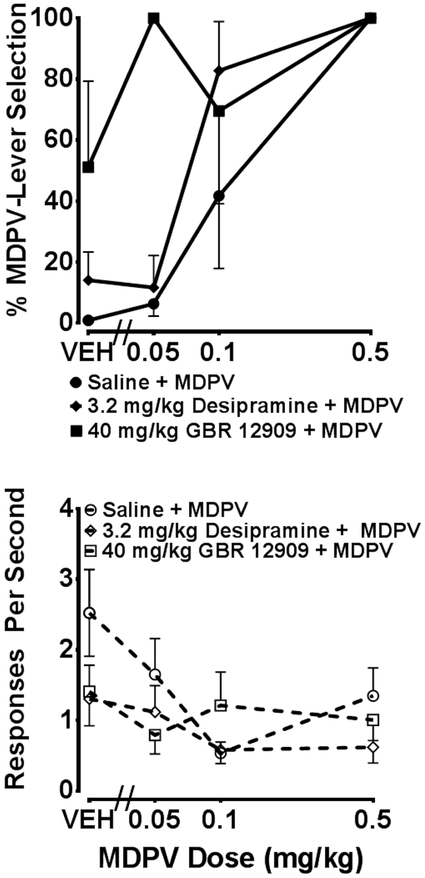
Figure 3 displays the dose-response curves generated from stimulus potentiation tests following vehicle (n=5), 3.2 mg/kg desipramine (n=5), or 40 mg/kg GBR 12909 (n=4) pretreatment with each MDPV dose. The vehicle + MDPV dose-response curve was nearly identical to the MDPV dose-response curve determined previously (see figure 1 for comparison). When vehicle was administered as a pretreatment, the percentage of MDPV-lever responses was 6.3 (±4), 41.7 (±23.8), and 100 (±0) following 0.05, 0.1, and 0.5 mg/kg MDPV, respectively. In comparison, desipramine (3.2 mg/kg) pretreatment with 0.1 mg/kg MDPV produced 82.7 (± 16) % MDPV-lever responses. GBR 12909 (40 mg/kg) pretreatment with 0.05 mg/kg MDPV produced 100% MDPV-lever responses in all four animals tested. However, only partial substitution (69.6 ± 30.4) was observed with GBR 12909 + 0.1 mg/kg MDPV; complete substitution (100%) was observed in two animals, no substitution in one animal, and the fourth animal failed to meet the response requirement to be included. ED50 values were not determined due to an insufficient number of doses assessed to yield unambiguous results. A RM ANOVA indicated no statistically significant changes in response rate by GBR 12909 + MDPV or by desipramine.
Figure 3.
MDPV dose-response curves determined with saline (n=5), 40 mg/kg GBR 12909 (n=3-4), or 3.2 desipramine (n=5) pre-treatment in animals trained to discriminate 0.5 mg/kg MDPV from saline. Percentage MDPV-lever selection is depicted by closed symbols in the upper graph and response rate is shown as open symbols in the lower graph. Individual points represent group mean (± SEM).
Experiment 2:
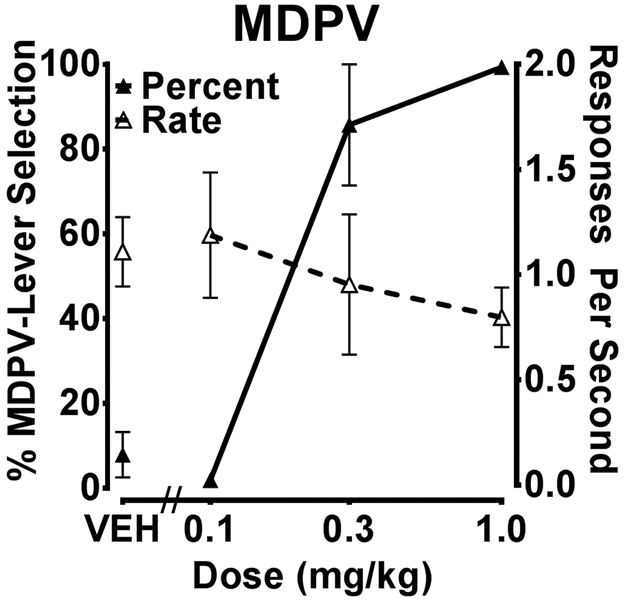
Stimulus control was established by 1.0 mg/kg MDPV within 15.3 (± 1.2, SEM) training sessions (range 12-18). As shown in figure 4, MDPV produced a dose-dependent increase in MDPV-lever responses, with full substitution produced by 0.3 and 1.0 mg/kg.
Figure 4.
MDPV dose-response curve determined from substitution tests with MDPV in rats trained to discriminate 1.0 mg/kg MDPV from saline (n=7). Percentage MDPV-lever responses (closed symbols) refers to the left Y-axis and response rate (open symbols) refers to the right Y-axis.
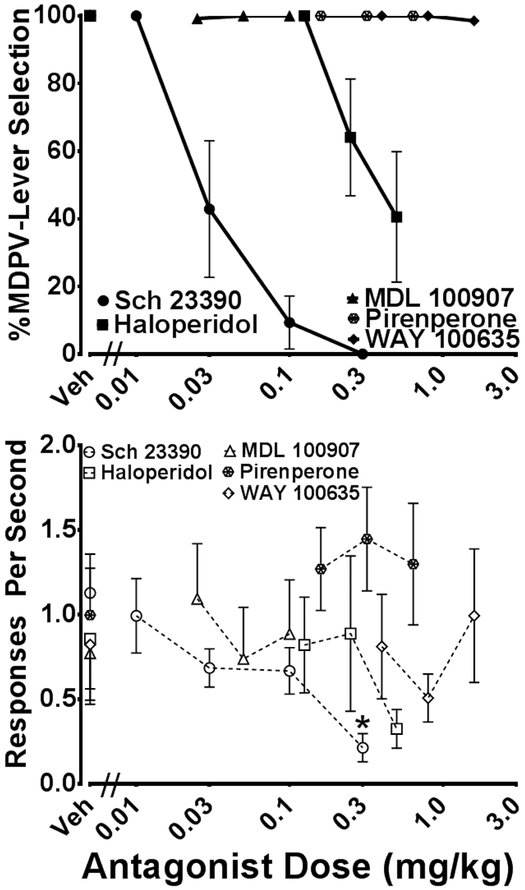
Dose-response curves generated with each antagonist combined with 1.0 mg/kg MDPV are displayed in Figure 5. The selective D1 dopamine antagonist, Sch 23390 and the prototypical D2 antagonist, haloperidol dose-dependently attenuated MDPV-lever selection. Full blockade of 1.0 mg/kg MDPV discrimination was observed with Sch 23390 0.1 mg/kg. A higher Sch 23390 dose (0.3 mg/kg) disrupted responding in nearly half of the animals. Haloperidol produced only partial antagonism of 1.0 mg/kg MDPV at the highest dose tested (0.5 mg/kg).
Figure 5.
Stimulus antagonism tests with Sch 23390, haloperidol, pirenperone, MDL 100,907, and WAY 100,635 administered in combination with 1.0 mg/kg MDPV. Percentage MDPV-appropriate lever responses (closed symbols) is depicted above and response rate (open symbols) is plotted below. Individual points represent group mean (± SEM). Statistically significant Dunnett’s tests compared to saline are indicated by * (P < 0.05).
A dose-dependent decrease in response rate was observed following tests with Sch 23390 and haloperidol. A one-way RM ANOVA found a statistically significant reduction in response rate following treatment with Sch 23390 and 1.0 mg/kg MDPV [F(4, 24) = 4.17, P < 0.05]. Dunnett’s multiple comparison tests indicated that only the highest dose of Sch 23390 (0.3 mg/kg) reduced response rate to a level that was significantly different from response rate following saline pretreatment with 1.0 mg/kg MDPV (P < 0.01). One-way RM ANOVA tests did not find any statistically significant effects on response rates following the administration of haloperidol.
The 5-HT2 antagonist, pirenperone, the 5-HT2A antagonist, MDL 100,907, and the 5-HT1A antagonist WAY 100,635 did not alter 1.0 mg/kg MDPV discrimination in any animal. Response rate was not significantly different from control rates following pirenperone, MDL 100,907, or WAY 100,635.
Discussion
The current study examined the contribution of monoaminergic mechanisms to the discriminative stimulus effects of the synthetic cathinone, MDPV through the assessment of stimulus substitution with 5-HT/DA releasers (MDMA, MDA and their optical isomers), a typical DAT inhibitor (cocaine), a highly selective atypical DAT inhibitor (GBR 12909), and a SERT/NET inhibitor (desipramine) in rats trained to discriminate 0.5 mg/kg MDPV (Experiment 1), and through the assessment of DA and 5-HT antagonists in rats trained to discriminate 1.0 mg/kg MDPV (Experiment 2).
Full substitution for MDPV was observed with cocaine, MDMA and MDA racemates, as well as (+)-MDMA. Only partial substitution was obtained with (+)-MDA at a dose that markedly reduced response rate, whereas (−)-MDMA and (−)-MDA produced virtually no substitution for MDPV. In consideration of previous evidence that MDMA and MDA have more dopaminergic activity and (−)-MDMA and (−)-MDA exert more serotonergic activity (Baker et al. 1995; Broadbent et al. 1992; Callahan and Appel 1988; Glennon and Young 1984), the current findings suggest MDPV discrimination is mediated to a greater extent by dopaminergic than serotonergic actions. Complete stimulus substitution by cocaine further supports this hypothesis and is consistent with previous reports regarding similarities in the interoceptive stimulus effects of MDPV and cocaine (Berquist and Baker 2017; Gannon et al. 2016; Gatch et al. 2013). However, the absence of stimulus substitution by GBR 12909 indicates DAT inhibition alone is not sufficient to produce the MDPV cue.
The seemingly disparate results with cocaine and GBR 12909 in the current study may be due to cocaine’s nonselective reuptake inhibition of DA, NE, and 5-HT compared to the selective, atypical DAT inhibition by GBR 12909 (Andersen 1989; Matecka et al. 1996). Of interest, a previous study indicated 16 mg/kg GBR 12909 produced complete substitution in rats trained to discriminate 10 mg/kg cocaine (Cunningham and Callahan 1991). However, using intravenous drug discrimination methods, others have reported that rats can be trained to discriminate 1 mg/kg cocaine from 1 mg/kg GBR 12909 (Tella and Goldberg 2001). Nevertheless, since response rate rates were not significantly reduced by GBR 12909 in the present study, higher doses should be tested for MDPV substitution in future studies. Unfortunately, such tests were not conducted in the current study due to limited supplies.
Full substitution with (±)-MDMA for MDPV in the current study is consistent with previous findings reported by Fantegrossi et al. (2013) in a study of mice trained to discriminate 0.3 mg/kg MDPV, but contradict more recent findings reported by Berquist and Baker (2017) who trained rats to discriminate 0.3 mg/kg MDPV and found only low partial substitution (27%) with 3 mg/kg MDMA. Although the species and training procedures of the current study were similar to those reported by Berquist and Baker (2017), the training dose was slightly higher in the current study. A systematic evaluation of MDPV training dose may be required to determine if the training dose or other factors (e.g. order of test compounds assessed) may account for the discrepant results.
As previously noted, the absence of MDPV stimulus substitution by GBR 12909 or by the tricyclic antidepressant, desipramine indicates that neither DAT nor NET/SERT inhibition alone is sufficient to produce the MDPV cue. Of particular interest, both GBR 12909 and desipramine pretreatment appeared to potentiate MDPV discrimination, indicating that both DAT and NET/SERT activity may modulate the actions of MDPV. Curiously, 40 mg/kg GBR 12909 pretreatment with vehicle tests produced an average of 51% MDPV-lever selection, substituting completely in two of the four animals tested. No dose of GBR 12909 produced even partial substitution when this substance was previously tested alone for substitution. However, the current results with GBR 12909 pretreatment should be considered with caution, as these tests were completed four months after the completion of initial GBR 12909 dose-response tests and only four of the seven original animals were included in these tests. It is possible that with more extensive training or with age, the animals developed greater sensitivity to the effects of GBR 12909. However, greater sensitivity to lower doses of MDPV were not evident, as the MDPV dose-response curve generated with vehicle pretreatments was nearly identical to the initial dose-response curve determined with MDPV at the beginning of the study. At the very least, these preliminary findings may serve to prompt further investigation. Results from the potentiation tests reported here could be interpreted to suggest concurrent use of MDPV with monoamine reuptake inhibitors may have additive effects and perhaps pose an enhanced risk for abuse. Further investigation with a wider range of doses and with other selective monoamine reuptake inhibitors are required to fully assess whether these substances have additive or synergistic effects with MDPV.
Dose-dependent attenuation and full antagonism of MDPV discrimination by Sch 23390 indicate MDPV’s stimulus effects are mediated primarily by dopamine’s actions at D1 receptors, although significant partial antagonism by haloperidol indicates D2 DA receptors also contribute to these effects. Higher doses of haloperidol or other more selective D2 antagonists could be assessed to determine if MDPV discrimination is fully blocked by D2 receptor antagonism. In contrast, the absence of stimulus antagonism by pirenperone, MDL 100,907, and WAY 100,635 suggests a lack of involvement of 5-HT receptor mediation of the MDPV cue. These findings are consistent with previous reports that MDPV primarily acts as an uptake blocker at the dopamine transporter, with relatively weaker effects on serotonin release (Baumann et al. 2012; Eshleman et al. 2013; Simmler et al. 2013). It should be noted that the two highest doses of Sch 23390 (0.1 and 0.3 mg/kg) both attenuated 1.0 mg/kg MDPV discrimination and that only the highest dose (0.3 mg/kg) significantly reduced response rate. Additional tests with other selective dopamine receptor antagonists, preferably those that do not produce significant response rate disruption, are warranted to determine if the response disruptive effects of Sch 23390 contributed to the attenuation of MDPV’s discriminative stimulus effects.
Although it was not a primary aim of the current study to evaluate the influence of training dose on stimulus substitution or antagonism of the MDPV cue, a noted limitation of this study is that different MDPV training doses were utilized experiment 1 and experiment 2. Training dose has been noted to influence both qualitative and quantitative aspects of stimulus substitution, with lower training doses often producing greater sensitivity compared to higher training doses (for review, see Stolerman, 2011). Future studies will be necessary to discern if the contribution of monoaminergic actions to MDPV discrimination varies with training dose.
In summary, considering the results of the two experiments described herein, complete stimulus substitution by the typical DAT inhibitor, cocaine and antagonism by DA receptor antagonists, but not by 5-HT receptor antagonists, suggest MDPV’s interoceptive stimulus effects are mediated predominantly by dopaminergic actions, with D1 receptors contributing to these effects to a greater extent than D2 receptors. However, we cannot rule out the possibility of serotonergic and/or noradrenergic modulation of these effects, given the substitution by MDMA and MDA and potentiation by desipramine. Evaluation of additional DAT and NET inhibitors for potentiation of the MDPV cue and the assessment of additional antagonists, preferably with less disruptive effects on responding, may further assist in determining the precise neurochemical actions responsible for MDPV discrimination. Furthermore, additional studies may be warranted to determine the influence of MDPV training dose on the relative importance of D1 versus D2 receptor-mediated actions to its discriminative stimulus effects. Finally, these preclinical findings can serve to inform medication discovery and development for the treatment of MDPV abuse.
Acknowledgements:
This research was supported by a grant from the National Institutes of Health (R15 DA038295). The National Institute on Drug Abuse Drug Control Supply Program and the National Institutes of Mental Health Chemical Synthesis and Drug Supply Program provided several of the drugs used in this study. A portion of this work (GBR 12909) was supported by the intramural research programs of the National Institute on Drug Abuse and the National Institute on Alcohol Abuse and Alcoholism.
Footnotes
Conflict of Interest: The authors declare no conflict of interest.
References
- Aarde SM, Huang PK, Creehan KM, Dickerson TJ, Taffe MA (2013) The novel recreational drug 3, 4-methylenedioxypyrovalerone (MDPV) is a potent psychomotor stimulant: self-administration and locomotor activity in rats. Neuropharmacology 71:130–140. 10.1016/j.neuropharm.2013.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aarde SM, Huang PK, Dickerson TJ, Taffe MA (2015) Binge-like acquisition of 3, 4-methylenedioxypyrovalerone (MDPV) self-administration and wheel activity in rats. Psychopharmacology 232(11):1867–1877. 10.1007/s00213-014-3819-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen PH (1989) The dopamine uptake inhibitor GBR 12909: Selectivity and molecular mechanism of action. European Journal of Pharmacology 166:493–504. 10.1016/0014-2999(89)90363-4 [DOI] [PubMed] [Google Scholar]
- Baker LE (2017) Hallucinogens in Drug Discrimination In: Halberstadt AL, Vollenweider FX, Nichols DE (eds) Behavioral Neurobiology of Psychedelic Drugs. Current Topics in Behavioral Neurosciences, vol 36 Springer, Berlin, Heidelberg, pp 201–219 [DOI] [PubMed] [Google Scholar]
- Baker LE, Broadbent J, Michael EK, Matthews PK, Metosh CA, Saunders RB, West WB, Appel JB (1995) Assessment of the discriminative stimulus effects of the optical isomers of ecstasy (3, 4-methylenedioxymethamphetamine; MDMA). Behavioural Pharmacology 10.1097/00008877-199504000-00007 [DOI] [PubMed] [Google Scholar]
- Baumann MH, Partilla JS, Lehner KR, Thorndike EB, Hoffman AF, Holy M, Rothman RB, Goldberg SR, Lupica CR, Sitte HH, Brandt SD, Tella SR, Cozzi NV, Schindler CW (2012) Powerful cocaine-like actions of 3, 4-methylenedioxypyrovalerone (MDPV), a principal constituent of psychoactive ‘bath salts’ products. Neuropsychopharmacology 38:552 10.1038/npp.2012.204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berquist MD, Baker LE (2017) Characterization of the discriminative stimulus effects of 3, 4-methylenedioxypyrovalerone in male Sprague-Dawley rats. Behavioural Pharmacology 28:394–400. 10.1097/FBP.0000000000000310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broadbent J, Appel JB, Michael EK, Ricker JH (1992) Discriminative stimulus effects of the optical isomers of 3, 4-methylenedioxyamphetamine (MDA). Behavioural Pharmacology 3:443–454. 10.1097/00008877-199210000-00003 [DOI] [PubMed] [Google Scholar]
- Callahan PM, Appel JB (1988) Differences in the stimulus properties of 3, 4-methylenedioxyamphetamine and 3, 4-methylenedioxymethamphetamine in animals trained to discriminate hallucinogens from saline. Journal of Pharmacology and Experimental Therapeutics 246:866–870. http://jpet.aspetjoumals.org/content/246/3/866 [PubMed] [Google Scholar]
- Cameron KN, Kolanos R, Solis E, Glennon RA, De Felice LJ (2013) Bath salts components mephedrone and methylenedioxypyrovalerone (MDPV) act synergistically at the human dopamine transporter. British Journal of Pharmacology 168:1750–1757. https://doi.org/10.1111/bph.12061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC) (2011) Emergency department visits after use of a drug sold as “bath salts”—Michigan, November 13, 2010—March 31, 2011. Morbidity and mortality weekly report 60, MMWR, pp. 624–627. https://www.cdc.gov/mmwr/preview/mmwrhtml/mm6019a6.htm. Accessed 26 February 2018 [PubMed] [Google Scholar]
- Cunningham KA, Callahan PM (1991) Monoamine reuptake inhibitors enhance the discriminative state induced by cocaine in the rat. Psychopharmacology 104:177–180. 10.1007/BF02244175 [DOI] [PubMed] [Google Scholar]
- Eshleman AJ, Wolfrum KM, Hatfield MG, Johnson RA, Murphy KV, Janowsky A (2013) Substituted methcathinones differ in transporter and receptor interactions. Biochemical pharmacology, 85:1803–1815. 10.1016/j.bcp.2013.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantegrossi WE, Gannon BM, Zimmerman SM, Rice KC (2013) In vivo effects of abused ‘bath salt’ constituent 3, 4-methylenedioxypyrovalerone (MDPV) in mice: Drug discrimination, thermoregulation, and locomotor activity. Neuropsychopharmacology 38:563 10.1038/npp.2012.233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froberg, Froberg BA, Levine M, Beuhler MC, Judge BS, Moore PW, Engebretsen KM, Mckeown NJ, Rosenbaum CD, Young AC, Rusyniak DE, ACMT Toxicology Investigators Consortium (2015) Acute methylenedioxypyrovalerone toxicity. Journal of Medical Toxicology 11:185–194. 10.1007/s13181-014-0446-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gannon BM, Galindo KI, Rice KC, Collins GT (2017) Individual differences in the relative reinforcing effects of 3, 4-methylenedioxypyrovalerone under fixed and progressive ratio schedules of reinforcement in rats. Journal of Pharmacology and Experimental Therapeutics 361:181–189. 10.1124/jpet.116.239376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gannon BM, Williamson A, Suzuki M, Rice KC, Fantegrossi WE (2016) Stereoselective effects of abused “bath salt” constituent 3, 4-methylenedioxypyrovalerone in mice: Drug discrimination, locomotor activity, and thermoregulation. Journal of Pharmacology and Experimental Therapeutics 356:615–623. 10.1124/jpet.115.229500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatch MB, Taylor CM, Forster MJ (2013) Locomotor stimulant and discriminative stimulus effects of ‘bath salt’ cathinones. Behavioural Pharmacology 24:437–447. 10.1097/fbp.0b013e328364166d [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glennon RA, Young R (1984) MDA: An agent that produces stimulus effects similar to those of 3, 4-DMA, LSD and cocaine. European Journal of Pharmacology, 99:249–250. 10.1016/0014-2999(84)90250-4 [DOI] [PubMed] [Google Scholar]
- Glennon RA, Young R (2011) Drug Discrimination: Applications to Medicinal Chemistry and Drug Studies. John Wiley and Sons, Inc., New Jersey [Google Scholar]
- Goodwin AK, Baker LE (2000) A three-choice discrimination procedure dissociates the discriminative stimulus effects of d-amphetamine and (±)-MDMA in rats. Experimental and Clinical Psychopharmacology 8:415–423. 10.1037//1064-1297.8.3.415 [DOI] [PubMed] [Google Scholar]
- Goodwin AK, Pynnonen DM, Baker LE (2003) Serotonergic–dopaminergic mediation of MDMAs discriminative stimulus effects in a three-choice discrimination. Pharmacology Biochemistry and Behavior 74:987–995. 10.1016/s0091-3057(03)00029-7 [DOI] [PubMed] [Google Scholar]
- Harvey EL, Baker LE (2015) Differential effects of 3,4-methylenedioxypyrovalerone (MDPV) and 4-methylmethcathinone (mephedrone) in rats trained to discriminate MDMA or a d-amphetamine MDMA mixture. Psychopharmacology 233:673–680. 10.1007/s00213-015-4142-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey EL, Burroughs RL, Baker LE (2017) Effects of D1 and D2 receptor antagonists on the discriminative stimulus effects of methylendioxypyrovalerone and mephedrone in male Sprague-Dawley rats trained to discriminate D-amphetamine. Behavioural Pharmacology 28:586–589. 10.1097/fbp.0000000000000328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King HE, Wakeford A, Taylor W, et al. (2015) Sex differences in 3,4-methylenedioxypyrovalerone (MDPV)-induced taste avoidance and place preferences. Pharmacology Biochemistry and Behavior 137:16–22. 10.1016/j.pbb.2015.07.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King HE, Wetzell B, Rice K, Riley A (2015) An assessment of MDPV-induced place preference in adult Sprague-Dawley rats. Drug and Alcohol Dependence 10.1016/j.drugalcdep.2015.07.310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matecka D, Rothman RB, Radesca L, de Costa BR, Dersch CM, Partilla JS, Pert A, Glowa JR, Wojnicki FH, Rice KC (1996) Development of novel, potent, and selective dopamine reuptake inhibitors through alteration of the piperazine ring of 1-[2-(Diphenylmethoxy)ethyl]- and 1-[2-[Bis(4-fluorophenyl)methoxy] ethyl]-4-(3-phenylpropyl)piperazines (GBR 12935 and GBR 12909). Journal of Medicinal Chemistry 39:4704–4716. 10.1021/jm960305h [DOI] [PubMed] [Google Scholar]
- Rosenbaum CD, Carreiro SP, Babu KM (2012) Here today, gone tomorrow…and back again? A review of herbal marijuana alternatives (k2, spice), synthetic cathinones (bath salts), kratom, salvia divinorum, methoxetamine, and piperazines. Journal of Medical Toxicology 8:15–32. 10.1007/s13181-011-0202-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross EA, Reisfield GM, Watson MC, et al. (2012) Psychoactive “Bath Salts” intoxication with methylenedioxypyrovalerone. The American Journal of Medicine 125:854–858. 10.1016/j.amjmed.2012.02.019 [DOI] [PubMed] [Google Scholar]
- Schindler CW, Thorndike EB, Goldberg SR, Lehner KR, Cozzi NV, Brandt SD, Baumann MH (2016) Reinforcing and neurochemical effects of the “bath salts” constituents 3,4-methylenedioxypyrovalerone (MDPV) and 3,4-methylenedioxy-N-methylcathinone (methylone) in male rats. Psychopharmacology 233:1981–1990. 10.1007/s00213-015-4057-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmler L, Buser T, Donzelli M, Schramm Y, Dieu LH, Huwyler J, Chaboz S, Hoener MC, Liechti ME (2013) Pharmacological characterization of designer cathinones in vitro. British Journal of Pharmacology 168:458–470. 10.1111/j.1476-5381.2012.02145.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiller HA, Ryan ML, Weston RG, Jansen J (2011) Clinical experience with and analytical confirmation of “bath salts” and “legal highs” (synthetic cathinones) in the United States. Clinical Toxicology 49:499–505. 10.3109/15563650.2011.590812 [DOI] [PubMed] [Google Scholar]
- Stolerman IP, Mariathasan EA, White JA, Olufsen KS (1999) Drug mixtures and ethanol as compound internal stimuli. Pharmacology Biochemistry and Behavior 64:221–228. 10.1016/S0091-3057(99)00087-8 [DOI] [PubMed] [Google Scholar]
- Stolerman IP, Childs E, Ford MM, Grant KA (2011) Role of training dose in drug discrimination: A review. Behavioural Pharmacology 22:415–429. doi: 10.1097/FBP.0b013e328349ab37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tella SR, Goldberg SR (2001) Subtle differences in the discriminative stimulus effects of cocaine and GBR-12909. Progress in Neuro-Psychopharmacology and Biological Psychiatry 25:639–656. 10.1016/S0278-5846(00)00180-9 [DOI] [PubMed] [Google Scholar]
- UNODC (2015) United Nations Office on Drugs and Crime: World Drug Report 2015. United Nations publication, New York: http://www.undoc.org/documents/wdr2015/World_Drug_Report_2015.pdf. Accessed 29 June 2018 [Google Scholar]
- National Drug Intelligence Center. (2011) Situation report: synthetic cathinones (bath salts): an emerging domestic threat National Drug Intelligence Center; Johnstown, PA: pp 15901–1622. http://www.justice.gov/archive/ndic/pubs44/44571/44571p.pdf [Google Scholar]
- Valente MJ, De Pinho PG, de Lourdes Bastos M, Carvalho F, Carvalho M (2014) Khat and synthetic cathinones: A review. Archives of Toxicology 88:15–45. 10.1007/s00204-013-1163-9 [DOI] [PubMed] [Google Scholar]
- Watterson LR, Kufahl PR, Nemirovsky NE, Sewalia K, Grabenauer M, Thomas BF, Marusich JA, Wegner S, Olive MF (2014) Potent rewarding and reinforcing effects of the synthetic cathinone 3, 4-methylenedioxypyrovalerone (MDPV). Addiction biology Mar; 19:165–74. https://doi.org/10.1111/j.1369-1600.2012.00474.x [DOI] [PMC free article] [PubMed] [Google Scholar]