Abstract
Multiple extracellular and intracellular signals regulate the functions of oligodendrocytes as they progress through the complex process of developmental myelination and then maintain a functionally intact myelin sheath throughout adult life, preserving the integrity of the axons. Recent studies suggest that Mek/ERK1/2-MAPK and PI3K/Akt/mTOR intracellular signaling pathways play important, often overlapping roles in the regulation of myelination. However, it remains poorly understood whether they function independently, sequentially, or converge using a common mechanism to facilitate oligodendrocyte differentiation, myelin growth, and maintenance. To address these questions, we analyzed multiple genetically modified mice and asked whether the deficits due to the conditional loss-of-function of ERK1/2 or mTOR could be abrogated by simultaneous constitutive activation of PI3K/Akt or Mek, respectively. From these studies, we concluded that while PI3K/Akt, not Mek/ERK1/2, plays a key role in promoting oligodendrocyte differentiation and timely initiation of myelination through mTORC1 signaling, Mek/ERK1/2-MAPK functions largely independently of mTORC1 to preserve the integrity of the myelinated axons during adulthood. However, to promote the efficient growth of the myelin sheath, these two pathways cooperate with each other converging at the level of mTORC1, both in the context of normal developmental myelination or following forced reactivation of the myelination program during adulthood. Thus, Mek/ERK1/2-MAPK and the PI3K/Akt/mTOR signaling pathways work both independently and cooperatively to maintain a finely tuned, temporally regulated balance as oligodendrocytes progress through different phases of developmental myelination into adulthood. Therapeutic strategies aimed at targeting remyelination in demyelinating diseases are expected to benefit from these findings.
Keywords: differentiation, myelin, myelination, myelinogenesis, oligodendrocyte
1 |. INTRODUCTION
Myelination in the CNS is a complex process which includes multiple sequential steps involving proliferation and differentiation of oligodendrocyte progenitors, timely initiation of myelin wrapping, rapid expansion of the myelin sheath during peak myelination, and finally myelin maintenance throughout adulthood (Baumann & Pham-Dinh, 2001; Emery, 2010; Fancy, Chan, Baranzini, Franklin, & Rowitch, 2011; Miller, 2002). Efficient orchestration of these tightly regulated events in the life of myelinating cells must require well-coordinated integration of multiple signal transduction pathways, to elicit appropriate responses. Efficient myelination of axons is essential not only for increasing nerve conduction velocity but also for providing trophic support to axons (Nave, 2010). Damage to the myelin sheaths as in multiple sclerosis leads to axonal degeneration and severe neurological deficits. Therefore, a better understanding of the interactions between different intracellular signaling mechanisms that regulate oligodendrocyte/myelin function through development and in adulthood is critically important to identify potential therapeutic targets for repair in diseases affecting myelination.
The phosphatidyl inositol-3-phosphate kinase (PI3K)/Akt/mTOR pathway (Martini, De Santis, Braccini, Gulluni, & Hirsch, 2014) plays an important role in the regulation of oligodendrocyte differentiation and myelin growth in the CNS (Narayanan, Flores, Wang, & Macklin, 2009; Goebbels et al., 2010; Harrington et al., 2010; Guardiola-Diaz, Ishii, & Bansal, 2012; Wood et al., 2013; Dai, Bercury, & Macklin, 2014; Bercury et al., 2014; Wahl, McLane, Bercury, Macklin, & Wood, 2014; Lebrun-Julien et al., 2014; Zou et al., 2014; Figlia, Gerber, & Suter, 2018). Transgenic mice with hyperactivation of this pathway display increased myelin thickness (Flores et al., 2008; Goebbels et al., 2010; Harrington et al., 2010), which is attenuated by rapamycin, an inhibition of mTOR (Narayanan et al., 2009). Furthermore, conditional ablation of mTOR itself or Raptor, the defining subunit of the mTORC1 complexes, from oligodendrocyte lineage cells results in the impairment of oligodendrocyte differentiation and timely initiation of myelination and in the formation of myelin sheaths that were thinner than normal in the spinal cords of mutant mice (Bercury et al., 2014; Lebrun-Julien et al., 2014; Wahl et al., 2014; Zou et al., 2014).
The extracellular signal-regulated kinases-1 and −2 (ERK1/2), downstream mediators of mitogen-activated protein kinases (MAPKs) pathway, have also emerged as prominent regulators of myelin growth in the CNS (Rubinfeld & Seger, 2005; Gonsalvez et al., 2016). Conditional ablation of Erk1/2 or its upstream mediators, FGF Receptor-2 or TrkB, results in reduced myelin thickness; however, oligodendrocyte differentiation and initiation of myelination are unaffected (Furusho, Dupree, Nave, & Bansal, 2012; Ishii, Fyffe-Maricich, Furusho, Miller, & Bansal, 2012; Wong, Xiao, Kemper, Kilpatrick, & Murray, 2013). Conversely, constitutive activation of Mek, the direct upstream activator of ERK1/2, in oligodendrocyte-lineage cells results in a significant increase in myelin thickness (Fyffe-Maricich, Schott, Karl, Krasno, & Miller, 2013; Ishii, Furusho, & Bansal, 2013). Elevation of Mek/ERK1/2 activity in oligodendrocytes of FGF-Receptor-2 knock-out mice rescued the deficits in myelin thickness in these mice, suggesting that ERK1/2 is the key downstream mediator of FGF-Receptor-2 signaling that regulates myelin thickness in the CNS (Furusho, Ishii, & Bansal, 2017). Furthermore, studies in mice with tamoxifen-inducible conditional ablation of Erk1/2 in oligodendrocytes during adulthood, suggested that ERK1/2 signaling, continues to be required in oligodendrocytes throughout adulthood for the longterm preservation of myelin and axonal integrity (Ishii, Furusho, Dupree, & Bansal, 2014). In addition, when ERK1/2 are activated in mature adult oligodendrocytes during adulthood, new myelin growth is reinitiated, even after active myelination is terminated, which has important implications for understanding the mechanism underlying the plasticity of myelin in adult life (Ishii, Furusho, Dupree, & Bansal, 2016; Jeffries et al., 2016). Given that both similarities and differences were observed in the phenotypes of transgenic mice with perturbation of signaling molecules in the Mek/ERK1/2-MAPK or PI3K/Akt/mTOR pathways, it was unclear whether these two major signaling pathways play independent parallel roles in vivo or cooperate with each other using a common downstream mechanism to regulate appropriate and timely myelin formation and maintenance.
To address this question, we carried out studies on a series of genetically modified mice and examined whether the deficits due to the loss of function of a signaling protein in one pathway, could be abrogated by simultaneous constitutive activation of a signaling protein in the other pathway. We found that the constitutive activation of the PI3K or Akt, in oligodendrocytes could fully or partially abrogate deficits in myelin gene expression and myelin thickness in the Erk1/2-deficient mice, respectively. In contrast, elevating Mek/ERK1/2 activity in the mTOR-deficient mice early during developmental myelination failed to rescue deficits in oligodendrocyte differentiation and myelin thickness. However, during adulthood, while the tamoxifen-inducible deletion of Erk1/2 from adult oligodendrocytes resulted in dramatic downregulation of myelin gene expression and axonal degeneration, deletion of mTOR in parallel studies did not show these effects. Thus, Mek/ERK1/2-MAPK and PI3K/Akt/mTOR pathways play both independent and common, temporally regulated roles during developmental myelination and in the adult CNS.
2 |. MATERIALS AND METHODS
2.1 |. Mouse lines
We generated transgenic mouse line referred to as CnpCre; Erk1/2-dKO;AktDD where there was constitutive activation of Akt (AktDD) and simultaneous deletion of Erk1/2 (Erk1/2-dKO) specifically in Cnp-Cre expressing oligodendrocyte-lineage cells (2’,3’-cyclic nucleotide 3’-phosphohydrolase; Lappe-Siefke et al., 2003). This was done by appropriate mating of our Erk1/2 double knock out mouse line, CnpCre/+;Erk1−/−;Erk2flox/flox, described previously (referred to as CnpCre;Erk1/2-dKO) (Ishii et al., 2012) with mice expressing transgene carrying constitutively active Akt driven by the PLP promoter (Flores et al., 2008, Dr Wendy Macklin, University of Colorado School of Medicine, Aurora, CO). We generated another transgenic mouse line, referred to as PlpCreERT;Erk1/2-dKO;PikDD, where there was tamoxifen (Tm) inducible constitutive activation of PI3K (PikDD) and simultaneous deletion of Erk1/2 (Erk1/2-dKO), specifically in PlpCreERT expressing mature oligodendrocyte (proteolipid protein; Jackson Laboratory; Doerflinger, Macklin, & Popko, 2003; Leone et al., 2003). This was done by appropriate mating of our Tm-inducible PlpCreERT/+;Erk1−/−,Erk2flox/flox mice mouse line described previously, referred to as PlpCreERT; Erk1/2-dKO (Ishii et al., 2014) with the Rosa26StopFlP110*;EGFP/+ line (generation of these mice is described in Srinivasan et al., 2009; obtained from Jackson Laboratory). In these mice, Cre-mediated excision of floxed STOP cassette leads to the expression of constitutively active P110*-transgene which is a mutant form of P110α (Pik3ca; the catalytic subunit of PI3K) that was made constitutively active by the addition of the p85 iSH2 domain (region between the two Src homology 2 domains of p85 that is required for the enzymatic activity of p110) to the p110 N-terminus.
We conditionally ablated mTOR from oligodendrocyte lineage cells by mating mTORflox/flox (Jackson Laboratory; Risson et al., 2009) with the Cnpcre/+ mice (Lappe-Siefke et al., 2003) to generate Cnpcre/+; mTORflox/flox mice, referred to as CnpCre;mTOR-KO. Furthermore, to simultaneously elevate ERK1/2 activity in mTOR-deficient oligodendrocyte lineage cells, we generated transgenic mice by mating the Cnpcre/+;mTORflox/flox mice with our heterozygous Cnpcre/+; Rosa26StopFlMek1DD,EGFP/+ mice described previously and referred to as CnpCre MekDD/+ or CnpCre;Mek/+ (Ishii et al., 2013; Ishii et al., 2016; Srinivasan et al., 2009) to produce progeny where there is Cremediated excision of floxed STOP cassette leading to the expression of constitutively active Mek1 and simultaneous loss of mTOR gene (referred to as CnpCre;mTOR-KO;MekDD).
For some experiments, we also generated PlpCreERT/+;mTORflox/flox and PlpCreERT/+;mTORflox/flox;;Rosa26StopFlMek1DD,EGFP/+ lines (referred to as PlpCreERT;mTOR-KO and PlpCreERT;mTOR-KO;MekDD respectively) by mating the mTORflox/flox and mTORflox/flox;;Rosa26StopFlMek1DD,EGFP/+ mice with the tamoxifen inducible PlpCreERT/+ mice (Jackson Laboratory). In these mice, mTOR was conditionally ablated in PLP-expressing mature oligodendrocytes upon intraperitoneal injection of Tm to young adult mice with or without simultaneous constitutive activation of Mek1. Mice heterozygous for mTOR were also produced in the same litters. They are referred to as PlpCreERT;mTOR-het and PlpCreERT;mTOR-het;MekDD.
For all the lines of transgenic mice generated, littermates lacking Cre are referred to as “controls,” facilitating comparison among the genotypes. Additional controls for the Tm-inducible lines included sunflower injected mutants and Tm-injected controls. As these mice showed no differences compared to the “controls” lacking Cre, no further distinction was made between them for the analysis. The genetic background of all the mouse lines used in this study was C57BL/6. Genotyping of different lines of mice was performed by PCR analysis using the appropriate primers. Both male and female mice were used for the study.
2.2 |. Immunohistochemistry
As described previously (Furusho et al., 2012; Ishii et al., 2012; Kaga et al., 2006), mice of both sexes perfused with PBS or 4% paraformaldehyde/PBS were subjected to postfixation overnight in 4% paraformaldehyde/PBS, and then another overnight in 20% sucrose/PBS. Cryostat transverse sections (15 μm) of cervical spinal cord were cut. For myelin basic protein (MBP, 1:3000; Dr. E. Barbarese, UCONN) and Neurofilament M (Nf-m; 1:200, Millipore) immunolabeling, sections were delipidated with 100% ethanol for 10 min, washed with PBS (3 times, 10 min), blocked (1 hr) in PBS, 10% normal goat serum (NGS, Invitrogen, CA), 5% BSA, 0.1% fish gelatin. Prior to immunolabeling for pan-Akt (1:100; Cell Signaling Technology, MA), pan-mTOR (1:100; Cell Signaling, MA), p-S6RP (S235/S236, 1:100; Cell Signaling Technology), p-p70S6K (T389, 1:100; Cell Signaling Technology), pan-Erk1/2 (1:100; Promaga, MA), and CC1 (1:40; Millipore) spinal cord sections were subjected to antigen retrieval by 5 min of incubation at 95°C in citrate buffer, pH 6.0 followed by washes with PBS (3 times, 10 min). They were then blocked (1 hr) in PBS, 10% NGS, 0.3% TritonX100. For immunolabeling of p-mTOR (Ser2448, 1:100; Cell Signaling) alone or as double label with CC1, sections (without antigen retrieval) were incubated in TBSS, 5% methanol/1% H2O2 for 10 min, washed with TBSS (3 times, 10 min), 10% Triton X-100 for 30 min, and blocked (1 hr) then in TBSS, 10% NGS and then TBSS, 0.3% BSA, 0.02% TritonX-100. Phosphate-buffered or Tris-buffered saline containing 100 μM sodium fluoride, 100 μM o-vanadate were used for dilutions of antibodies and washes. Specimen were incubated with primary antibody in blocking buffer at 4°C for 24–72 hr. After washing the primary antibody, the specimens were incubated in appropriate secondary antibodies conjugated to biotin (1:200; Vector Lab., CA), Cy3 (1:500, Jackson Immuno Research Lab., PA), or Alexa 488 (1:500; Molecular Probes, CA) and nuclei were counterstained with Hoechst blue dye 33,342 (1 mg/mL; Sigma, MO). The Avidin/Biotinylated Enzyme Complex (ABC) system (Vector Lab., CA) was used to detect biotinylated secondary antibodies, and the color was developed by incubation in DAB (3,3′-diaminobenzidine, Sigma, MO). Negative controls were treated identically except for the exclusion of primary antibodies. Quantification of the immunofluorescence signal intensity of p-mTOR2448 in CC1 double labeled cells was done using Photoshop CS6 (Adobe) from three representative regions of the latero-ventral white matter image from each animal. Parallel measurements were made from three controls and three mutants and average values expressed in arbitrary units.
2.3 |. In situ hybridization
Transverse sections of cervical spinal cord from mice of both sexes were prepared as above, and in situ hybridization (ISH) was performed as previously described (Furusho et al., 2012; Furusho, Kaga, Ishii, Hébert, & Bansal, 2011; Ishii et al., 2012), using riboprobes specific for proteolipid protein (PLP) mRNA (Dr. W.B. Macklin, Univ. of Colorado Sch. of Med., Aurora, CO) or MBP mRNA (Dr. M. Qiu, Univ. of Louisville, Kentucky). Briefly, after incubation in 1 μg/ml proteinase K at 37°C for 30 min, sections were hybridized overnight at 65°C with digoxigenin-labeled antisense cRNA probe, and washed in 50% formamide, 2× SSC, and 1% SDS at 65°C for 2–3 hr, followed by rinses in 2× SSC and 0.2× SSC at room temperature, and 0.1× SSC at 60°C. After blocking in 1% Tween20 and 1% normal goat serum (1 hr), sections were incubated (overnight) in alkaline phosphatase-conjugated antidigoxigenin antibody (1:5000; Roche Diagnostics, Penzberg, Germany). Color was developed with 4-nitroblue tetrazolium chloride and 5-bromo-4-chloro-3-indolylphosphate.
2.4 |. Quantitative real-time PCR
Total RNA from spinal cords of mice of both sexes was extracted using the TRIzol reagent (Invitrogen, Carlsbad, CA). One microgram of total RNA was reverse-transcribed to cDNA using the iScript™ Synthesis Kit (BioRad, Hercules, CA) according to the manufacturer’s instructions. Quantitative real-time PCR (qRT-PCR) was performed using an Eppendorf Mastercycler® ep realplex Thermal Cycler (Eppendorf, Hamburg, Germany) and iQ™ SYBR® Green Supermix (BioRad, Hercules, CA) according to the manufacturer’s instructions. The following primers were used: PLP forward primer, 5’-GTATA GGCAGTCTCTGCGCTGAT-3’; PLP reverse primer, 5’-AAGTGGCA GCAATCATGAAGG-3’; MBP forward primer, 5’-TACCTGGCCACAG CAAGTAC-3’; MBP reverse primer, 5’-GTCACAATGTTCTTGAAG-3’; glyceraldehyde-3-phosphate dehydrogenase (GAPDH) forward primer, 5’-TGTGTCCGTCGTGGATCTG-3’; GAPDH reverse primer, 5’-CATG TAGGCCATGAGGTCCACCAC-3’. qRT-PCR conditions were as follows: denaturation at 95°C, 30 s; primer annealing at 55.5°C or 53°C, 30 s; and elongation at 72°C, 40 s. Quantification of PCR products was performed using the 2-ΔΔCt method. Quantities of mRNA were normalized to the housekeeping gene GAPDH.
2.5 |. Electron microscopy
Transgenic and littermate control mice of both sexes were perfused with 4% paraformaldehyde, 2% glutaraldehyde in 0.1 M cacodylate buffer, pH 7.4 (Electron Microscopy Sciences, Hatfield, PA). Cervical spinal cords of mice were postfixed in 1% OsO4. Samples were dehydrated through graded ethanol, stained en bloc with uranyl acetate, and embedded in Poly/Bed812 resin (Polysciences Inc., Warrington, PA). Semithin (1 μm) sections were stained with toluidine blue. Ultrathin (0.1 μm) sections from matching areas of experimental and control tissue blocks were cut and visualized using an electron microscope (JEOL1200CX) at 80 kV. Digitized images (magnification 3,000×) were used to determine the g-ratios of randomly selected myelinated axons. Approximately 200–400 axons were measured per genotype from matched regions of the ventral cervical spinal cord. Statistical analysis was performed on average g-ratios using ANOVA using each axon as n = 1. For comparison of control and mutant mice, note that higher g-ratios indicates thinner myelin sheath.
2.6 |. Immunoblotting
Immunoblotting was performed as described previously (Fortin, Rom, Sun, Yayon, & Bansal, 2005). Briefly, equal amounts of total proteins from lysates of white matter from spinal cords of mice of both sexes were loaded onto SDS-PAGE, transferred to PVDF membranes, and immunolabeled for pan-Akt (1:1000; Cell Signaling Technology), phospho-ERK1/2 (1:10,000; Cell Signaling, Danvers, MA), and GAPDH (1:60,000; Biodesign International, Saco, ME) as a loading control. Quantification of the bands was done by Image-J software. Statistical analysis used to evaluate immunoblots was done by oneway ANOVA test.
3 |. RESULTS
3.1 |. Sustained overactivation of Akt in oligodendrocytes partly abrogated the deficit in myelin gene expression and myelin growth in mice lacking ERK1/2
We showed previously that myelin gene expression and myelin thickness were reduced in the CnpCre;Erk1/2-dKO mice (Ishii et al., 2012) suggesting a role of the Mek/ERK1/2-MAPK pathway in myelin growth. More recently we showed that in these mice, the expression of p-mTOR and p-Raptor, key components of mTORC1, and p-S6RP traditionally known as downstream effectors in the PI3K/Akt pathway were significantly downregulated, providing correlative evidence for a potential convergence of the Mek/ERK1/2-MAPK and the PI3K/Akt/mTOR pathways at the level of mTORC1 for the regulation of myelin growth (Furusho et al., 2017). However, whether there is a functional link between the two pathways was not established from this study. We therefore asked whether attenuated myelin gene expression and myelin growth in mice lacking ERK1/2 could be abrogated and restored to normal by genetically elevating Akt activity, an upstream activator of mTORC1, in Erk1/2-deficient oligodendrocytes. To address this question, we generated transgenic mice that conditionally expressed constitutively active Akt (AktDD) specifically in oligodendrocytes of mice lacking ERK1/2 (referred to as CnpCre; Erk1/2-dKO;AktDD).
We first confirmed that Akt was indeed upregulated and ERK1/2 was downregulated as expected in this transgenic mouse line. Immunoblot analysis and quantification for pan-Akt and p-ERK1/2 in white matter lysates of spinal cord white matter from control, CnpCre; Erk1/2-dKO and CnpCre;Erk1/2-dKO;AktDD mice showed that, consistent with the genotypes, pan-Akt level was significantly increased in CnpCre;Erk1/2-dKO;AktDD compared to control and CnpCre; Erk1/2-dKO, while p-ERK1/2 level was significantly decreased in the CnpCre;Erk1/2-dKO and CnpCre;Erk1/2-dKO;AktDD mice (Figure 1a (i)). Immunolabeling of spinal cord sections further confirmed that compared to control and CnpCre;Erk1/2-dKO mice, the intensity of the cellular signal for pan-Akt in the white matter was higher in CnpCre; Erk1/2-dKO;AktDD and AktDD mice (Figure 1a (ii)) and that Erk1/2 expression was completely lost from oligodendrocyte-like cells in both the white and grey matter of the CnpCre;Erk1/2-dKO mice as expected (Supporting Information Figure S1). In addition, we double labeled spinal cord tissue from CnpCre;Erk1/2-dKO;AktDD and AktDD mice for pan-Akt and CC1 expression and found that Akt is expressed in all of the CC1+ oligodendrocytes in the CnpCre;Erk1/2-dKO;AktDD, at a level comparable to that seen in the AktDD mice indicating that the recombination at the AktDD allele was efficient and that the entire population of Erk1/2-deficient oligodendrocytes was expressing AktDD (Supporting Information Figure S2). Taken together, these data confirm that the Erk1/2 expression was lost and the Akt level was truly upregulated in the entire population of oligodendrocytes in the CnpCre;Erk1/2-dKO;AktDD mice as expected.
FIGURE 1.
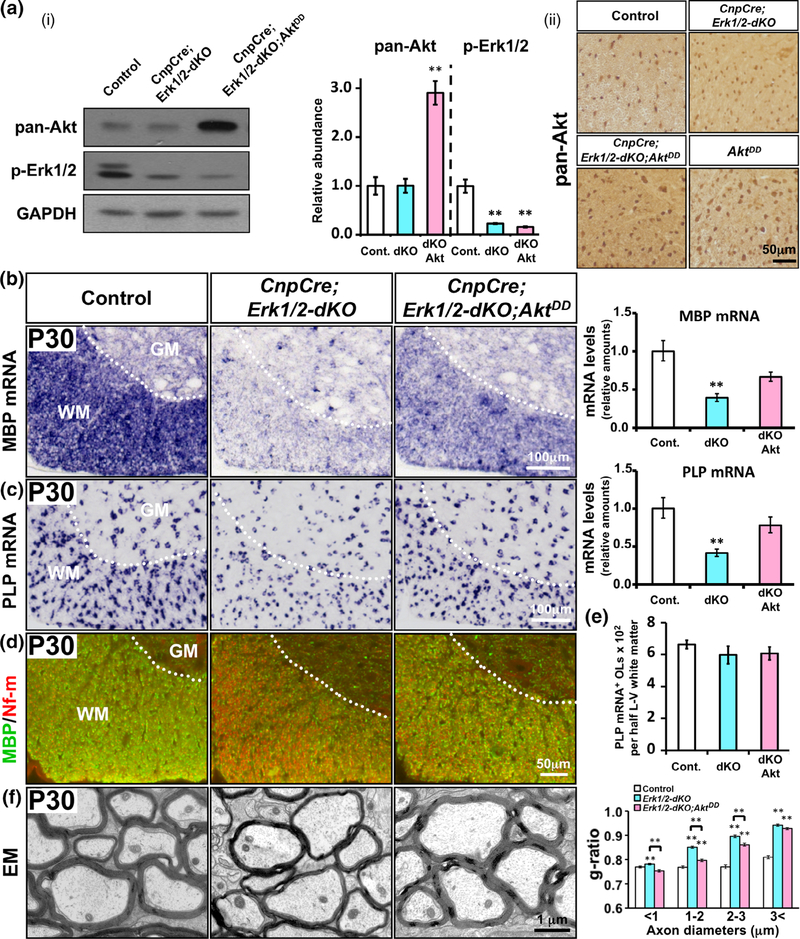
Sustained overactivation of Akt in oligodendrocytes partially abrogated the deficit in myelin gene expression and myelin growth in mice lacking ERK1/2. (a (i)) Immuno-blot analysis of equal amounts of total proteins from homogenates of spinal cord white matter and quantification of the band intensity show that pan-Akt levels are similar in control (Cont.) and CnpCre;Erk1/2-dKO (dKO), but significantly increased in the CnpCre;Erk1/2-dKO;AktDD (dKOAkt) mice. p-Erk1/2 levels are significantly reduced in both CnpCre;Erk1/2-dKO and CnpCre; Erk1/2-dKO;AktDD compared to control. GAPDH, used as a loading control, does not show a change. (a (ii)) Immunolabeling of spinal cord sections for pan-Akt shows that compared to control and CnpCre;Erk1/2-dKO, there is higher cellular signal intensity in the white matter of CnpCre; Erk1/2-dKO;AktDD and AktDD mice. (b,c) Cervical spinal cord sections, analyzed at postnatal day (P) 30 by in situ hybridization and qRT-PCR for the expression of MBP (b) or PLP (c) mRNA, show a significant reduction in their levels in the CnpCre;Erk1/2-dKO compared to controls and a partial rescue of the signal in the CnpCre;Erk1/2-dKO;AktDD mice. (d) Double immunolabeling of spinal cord sections at P30 for MBP (green) and neurofilament-m (Nf-m; red) show reduced MBP protein signal in the CnpCre;Erk1/2-dKO compared to controls and a partial rescue in the CnpCre; Erk1/2-dKO;AktDD mice. (e) Quantification of the total numbers of mature oligodendrocytes (OLs), marked by PLP mRNA expression in half latero-ventral (L-V) white matter of the cervical spinal cord, show no differences between control, dKO, and dKOAkt mice. (f) EM images of matched regions of ventral spinal cords at P30 and quantification of the myelin thickness by g-ratio analysis show reduction of myelin thickness (higher g-ratio) in the CnpCre;Erk1/2-dKO (blue bars) compared to littermate control (white bars) and a partial rescue (lower g-rato) in the CnpCre; Erk1/2-dKO;AktDD (pink bars) mice. Approximately 200–400 axons from two mice of each genotype were analyzed, significance is shown as **, p values are given in the Section 3. Error bars in (a–e) indicate SEM. **p < .01. N = 3–4. Representative images from ventral white matter region of the spinal cord are shown. WM, white matter; GM, grey matter [Color figure can be viewed at wileyonlinelibrary.com]
To investigate whether Akt overactivation would abrogate the downregulation of myelin gene expression observed in mice lacking ERK1/2, we analyzed transverse sections of cervical spinal cord for the expression of MBP and PLP mRNA by in situ hybridization at P14, P23 and P30. We found that at P14 there was only a minor reduction in the MBP mRNA signal intensity in the CnpCre;Erk1/2-dKO compared to control (Ishii et al., 2012 and data not shown). But at P23 and P30, there was a dramatic reduction in its signal intensity in CnpCre;Erk1/2-dKO mice (P30 is shown, Figure 1b). Overactivation of Akt in the Erk1/2-deficient mice led to an increase in MBP mRNA signal in the CnpCre;Erk1/2-dKO;AktDD mice, which was clearly greater than the CnpCre;Erk1/2-dKO mice. However, it remained less than control indicating that the rescue of the mRNA expression was only partial. Similarly, PLP mRNA signal intensity also showed a reduction in the CnpCre;Erk1/2-dKO at P23 and P30 compared to control mice. This attenuation was also partially abrogated upon overactivation of Akt in the CnpCre;Erk1/2-dKO;AktDD mice (P30 is shown, Figure 1c). Quantification of mRNA levels by qRT-PCR analysis confirmed the ISH results and showed that MBP and PLP mRNA levels were significantly reduced in the CnpCre;Erk1/2-dKO compared to control and that this reduction was partially abrogated in the CnpCre;Erk1/2-dKO; AktDD mice, but did not reach control levels (bar graphs, Figure 1b,c). On the other hand, the number of PLP mRNA+ oligodendrocytes in the WM of the spinal cords remained unchanged among the genotypes (Figure 1e). Therefore, the observed changes in the levels of myelin gene expression occurred per oligodendrocyte, which is consistent with the increased intensity of the PLP mRNA ISH signal (Figure 1c), where individual spinal cord oligodendrocytes can be visualized.
We also examined the expression of MBP protein by double immunolabeling of spinal cord sections for MBP/Nf-m at P30 (Figure 1d). We found that the MBP signal intensity (green) was lower in CnpCre;Erk1/2-dKO compared to control. CnpCre;Erk1/2-dKO;AktDD showed a slight increase of the signal compared to CnpCre;Erk1/2-dKO but did not reach control level. Therefore, like MBP mRNA, the attenuation of MBP protein levels in mice lacking ERK1/2 could only be partially abrogated in the CnpCre;Erk1/2-dKO; AktDD mice.
We next performed Electron Microscopy (EM) analysis and asked whether sustained overactivation of Akt in mice lacking Erk1/2 could rescue the deficits in myelin thickness (Figure 1f). EM images of ventral spinal cords at P30 showed a reduction of myelin thickness in the CnpCre;Erk1/2-dKO compared to control mice. This deficit was abrogated to some extent by the elevation of Akt activity in the CnpCre;Erk1/2-dKO;AktDD mice, which showed somewhat thicker myelin sheaths than the CnpCre;Erk1/2-dKO mice. Myelin thickness in these different mice was quantified as g-ratios, and consistent with our earlier work (Ishii et al., 2012), myelin thickness was significantly decreased in the CnpCre;Erk1/2-dKO (blue bars) compared to control (white bars) [p values and average g-ratios: Control (0.77) vs CnpCre;Erk1/2-dKO (0.82): p = 5.1 × 10−22]. However, there was only a partial increase of myelin thickness in the CnpCre;Erk1/2-dKO;AktDD mice (pink bars) which remained thinner than control but thicker than CnpCre;Erk1/2-dKO [average g-ratios and p values: CnpCre;Erk1/2-dKO;AktDD (0.79) vs Control (0.77): p = 7.5 × 10−6; CnpCre;Erk1/2-dKO;AktDD (0.79) vs CnpCre;Erk1/2-dKO (0.82) p = 9.9 × 10−5]. This was more evident in axons of diameters up to 3 μm.
Taken together, we conclude that the overactivation of Akt in Erk1/2-deficient oligodendrocytes was able to partially abrogate the downregulation of major myelin gene and protein expression and thereby partially rescue the attenuation of myelin growth in mice lacking ERK1/2.
3.2 |. Sustained overactivation of Akt in oligodendrocytes deficient in ERK1/2 signaling fully rescued p-mTOR expression but partially rescued the expression of p-p70S6K and p-S6RP
As in the CnpCre;Erk1/2-dKO;AktDD mice, there was only partial rescue of myelin gene expression in oligodendrocytes, we wondered if this was due to a partial rescue of the expression of p-mTOR and its downstream signaling molecules, which are downregulated in the CnpCre;Erk1/2-dKO mice (Furusho et al., 2017). We therefore examined the expression of p-mTOR, p-S6RP, and p-p70S6K in spinal cord sections from control, CnpCre;Erk1/2-dKO, CnpCre;Erk1/2-dKO;AktDD, and AktDD mice at P14, P23, and P30 (Figure 2). At P14, the oligodendrocyte-like cells in the white matter of control spinal cords expressed p-mTOR, p-S6RP, and p-p70S6K. The same pattern was seen in CnpCre;Erk1/2-dKO, CnpCre;Erk1/2-dKO;AktDD, and AktDD mice (Figure 2a). However, by P23, the p-mTOR signal was reduced in the CnpCre;Erk1/2-dKO mice compared to control (Figure 2b, upper panel). This attenuation of the signal was completely abrogated in the CnpCre;Erk1/2-dKO;AktDD mice, which showed comparable signal intensity to the control and AktDD mice. Thus, overactivation of Akt led to an apparent full rescue of p-mTOR expression in the ERK1/2-deficient oligodendrocytes. To confirm that the p-mTOR signal seen in the white matter cells of the spinal cords was in fact in oligodendrocytes, we double-immunolabeled spinal cord sections from control, CnpCre;Erk1/2-dKO and CnpCre;Erk1/2-dKO;AktDD mice for p-mTOR and CC1 at P30 (Figure 2c). Consistent with the DAB staining (Figure 1b), the p-mTOR signal intensity was comparable in control and CnpCre;Erk1/2-dKO;AktDD mice, and it co-localized with CC1+ oligodendrocytes, while in the CnpCre;Erk1/2-dKO mice, it was almost completely lost from CC1+ cells. Quantification of the fluorescence intensity of p-mTORS2448 in CC1 positive cells by Photoshop CS6 further confirmed that the levels of p-mTORS2448 expression in oligodendrocytes of CnpCre;Erk1/2-dKO;AktDD were comparable to control mice, suggesting a full rescue of its expression (control: 153 ± 16; CnpCre;Erk1/2-dKO;AktDD: 175 ± 9, N = 3, arbitrary units).
FIGURE 2.
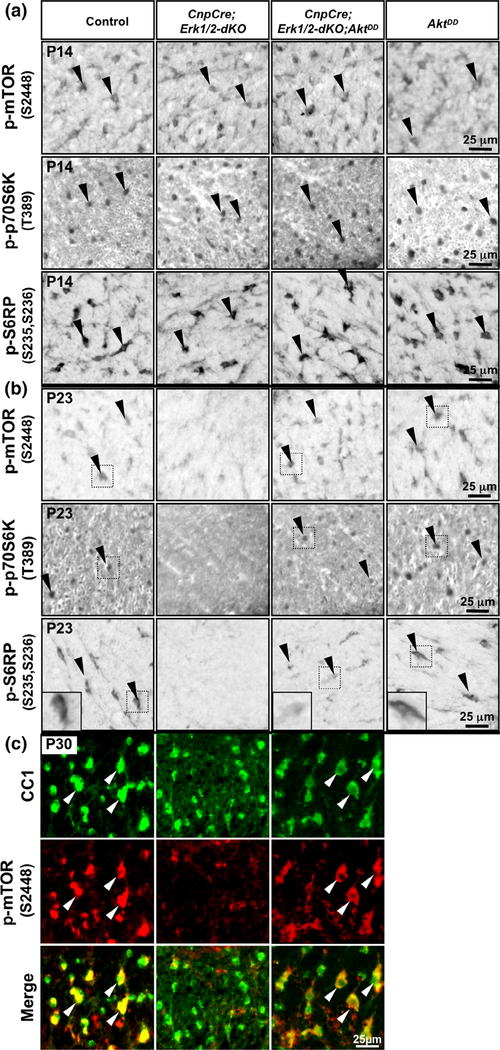
Sustained overactivation of Akt in oligodendrocytes deficient in ERK1/2 signaling fully rescued the p-mTOR expression but partially rescued the expression of p-S6RP and p-p70S6K. (a,b) Transverse sections of cervical spinal cord from P14 (a) and P23 (b) control, CnpCre; Erk1/2-dKO, CnpCre;Erk1/2-dKO;AktDD and AktDD mice immunolabeled for p-mTOR2448, p-p70S6KT389, and p-S6RPS235/236 show that at P14 (a) cellular staining of these molecules in the oligodendrocyte-like cells of the white matter is equally strong in all genotypes. At P23 (b), the staining of all these molecules is decreased dramatically in the CnpCre;Erk1/2-dKO compared to control mice. p-mTOR2448 signal is rescued completely in CnpCre;Erk1/2-dKO;AktDD and is comparable to control and AktDD. But, p-p70S6KT389 and p-S6RPS235/236 signals are partially rescued in CnpCre;Erk1/2-dKO;AktDD mice and remain less than in control and AktDD, but more than in CnpCre;Erk1/2-dKO. (c) Double immunolabeling of spinal cord sections from control, CnpCre;Erk1/2-dKO and CnpCre;Erk1/2-dKO;AktDD mice for p-mTORS2448 (red) with the mature oligodendrocyte marker CC1 (green) at P30 shows that p-mTORS2448 signal is dramatically reduced in CC1+ oligodendrocytes in the CnpCre; Erk1/2-dKO mice compared to controls and is completely rescued to control levels in the CnpCre;Erk1/2-dKO;AktDD mice. Quantification of the fluorescence intensity of p-mTORS2448 in CC1-positive cells by Photoshop CS6 shows comparable levels of expression in CnpCre;Erk1/2-dKO;AktDD and control mice (control: 153 ± 16; CnpCre;Erk1/2-dKO;AktDD: 175 ± 9, N = 3, arbitrary units). Representative images of the ventral white matter of the spinal cord from the analysis of at least three animals per genotype are shown. Black arrowheads point to cellular staining in the spinal cord white matter and white arrows show examples of CC1+/p-mTOR+ oligodendrocytes [Color figure can be viewed at wileyonlinelibrary.com]
Given our hypothesis that the Mek/ERK1/2-MAPK and the PI3K/Akt/mTOR pathways converge at the level of mTOR for the regulation of myelin gene expression and myelin growth, these results were somewhat surprising as they showed that in spite of significant rescue of p-mTOR expression in CnpCre;Erk1/2-dKO;AktDD mice, there was only a partial rescue of myelin gene expression in these mice (Figure 1). Therefore, we next asked whether p-p70S6K and p-S6RP, known downstream targets of mTORC1 signaling, which were also downregulated in the CnpCre;Erk1/2-dKO by P23, were fully or partially rescued in the CnpCre;Erk1/2-dKO;AktDD mice. Therefore, p-p70S6K and p-S6RP expression was investigated in spinal cord sections from control, CnpCre;Erk1/2-dKO, CnpCre;Erk1/2-dKO;AktDD, and AktDD mice (Figure 2b, middle and lower panels). We found a dramatic downregulation of p-p70S6K and p-S6RP intensity in oligodendrocyte-like cells in the white matter of spinal cords of the CnpCre;Erk1/2-dKO mice compared to control as expected at P23. However, although the signal intensity of p-p70S6K and particularly p-S6RP was higher in CnpCre Erk1/2-dKO;AktDD compared to CnpCre;Erk1/2-dKO mice, it did not reach control or AktDD levels as was observed for p-mTOR.
Together we conclude that in spite of the full rescue of p-mTOR expression, p-p70S6K and p-S6RP expression was only partially rescued in the CnpCre;Erk1/2-dKO;AktDD mice, similar to the partial rescue of myelin gene expression.
3.3 |. Constitutive activation of PI3K in oligodendrocytes fully abrogated the deficits in myelin gene expression and myelin growth in mice lacking ERK1/2
Analysis of CnpCre;Erk1/2-dKO;AktDD mice (above) suggested that lack of full recovery of myelin gene expression in these mice, in spite of the full rescue of p-mTOR, might have resulted from the partial rescue of p-p70S6K and p-S6RP expression, downstream of mTORC1. We therefore wondered if there were additional signals, potentially acting upstream of Akt in the PI3K pathway, that were needed for the full activation of p-p70S6K and p-S6RP and for the upregulation of myelin gene expression and myelin growth. To address this question, we developed transgenic mice where PI3K itself was conditionally overactivated by constitutive activation of the Pik gene, which encodes the catalytic subunit of PI3K. We then tested whether the myelin deficiencies in mice lacking Erk1/2 could be fully abrogated by sustained overactivation of PI3K in the Erk1/2-deficient oligodendrocytes. Using the Tm-inducible PlpCreERT mice, we injected control and mutant mice with Tm between P7–14 and analyzed MBP and PLP mRNA expression levels by ISH analysis at P30 (Figure 3, upper and middle panels). As expected, both transcripts were clearly reduced in the PlpCreERT; Erk1/2-dKO compared to control. Importantly, this deficit was completely abrogated in the PlpCreERT;Erk1/2-dKO;PikDD mice. In fact, the levels of expression rose even higher than the control. Comparable data were obtained by qRT-PCR, showing MBP and PLP mRNA was reduced in PlpCreERT;Erk1/2-dKO, but recovered in PlpCreERT;Erk1/2-dKO;PikDD mice (Figure 3a). As in the earlier studies, this RNA increase was per oligodendrocyte, as no difference in the number of PLP mRNA positive oligodendrocytes was noted among the different genotypes (Figure 3b).
FIGURE 3.
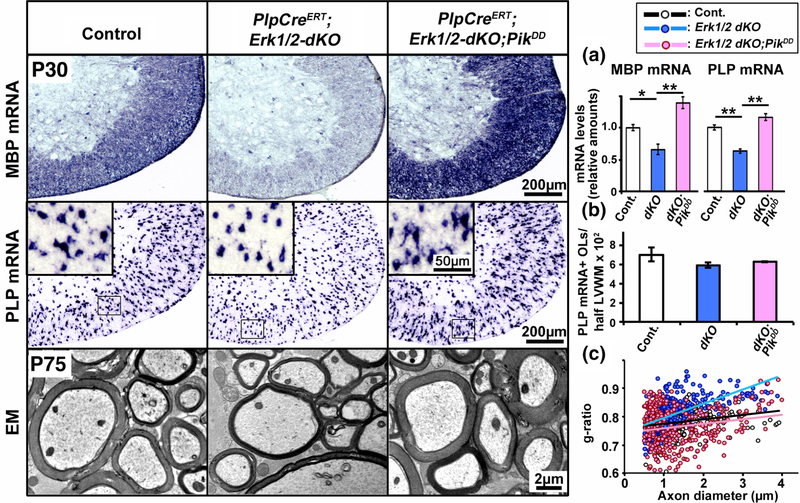
Constitutive activation of PI3K in oligodendrocytes fully abrogated the deficits in myelin gene expression and myelin growth in mice lacking ERK1/2. Transverse section of cervical spinal cord, analyzed at P30 by in situ hybridization for the expression of MBP (upper panel) or PLP (middle panel) mRNA in mice injected with tamoxifen from P7–P14 show a significant reduction in their signal intensity in the PlpCreERT;Erk1/2-dKO compared to controls and a complete rescue of the signal in the PlpCreERT;Erk1/2-dKO;PikDD mice. (a) Quantification of mRNA levels by qRT-PCR analysis also shows a statistically significant reduction of MBP and PLP mRNA levels in the spinal cords of PlpCreERT;Erk1/2-dKO (dKO) compared to controls (Cont.) and a significant increase in the PlpCreERT;Erk1/2-dKO;PikDD (dKO;PikDD) compared to PlpCreERT;Erk1/2-dKO mice. (b) Quantification of the total numbers of mature oligodendrocytes (OLs), marked by PLP mRNA expression, in half latero-ventral white matter (LVWM) of the cervical spinal cord show no differences between control, PlpCreERT;Erk1/2-dKO and PlpCreERT;Erk1/2-dKO;PikDD mice. EM images (lower panel) of matched regions of ventral spinal cords at P75 show reduction of myelin thickness in the PlpCreERT;Erk1/2-dKO compared to control and an increase in the PlpCreERT;Erk1/2-dKO;PikDD mice, which is comparable to control. (c) Quantification of myelin thickness by g-ratio analysis, presented as scatter plots relative to axon diameters shows higher g-ratios, indicative of thinner myelin sheath in the PlpCreERT;Erk1/2-dKO (blue dots) compared to littermate control (black dots) and PlpCreERT;Erk1/2-dKO;PikDD mice (red dots). Approximately 200–400 axons from two mice of each genotype were analyzed. Error bars in (a) and (b) indicate SEM. **p < .01, *p < .05. N = 3–4. Representative images from latero-ventral white matter region of spinal cord are shown [Color figure can be viewed at wileyonlinelibrary.com]
Consistent with the increases in myelin RNAs, sustained overactivation of PI3K in the PlpCreERT;Erk1/2-dKO rescued the deficits in myelin thickness in mice lacking Erk1/2 (Figure 3, lower panel). As expected, myelin thickness was reduced in the PlpCreERT;Erk1/2-dKO compared to control mice, while the elevation of PI3K activity in the PlpCreERT;Erk1/2-dKO;PikDD mice resulted in thicker myelin sheaths compared to PlpCreERT;Erk1/2-dKO. When quantified by g-ratio analysis, myelin thickness was significantly decreased (higher g-ratios) in the PlpCreERT;Erk1/2-dKO compared to control and there was a complete rescue of myelin thickness in the PlpCreERT;Erk1/2-dKO;PikDD mice (Figure 3c) (p values for average g-ratios: Control (0.78) vs PlpCreERT; Erk1/2-dKO (0.82): p = 2.0 × 10−15, Control (0.78) vs PlpCreERT; Erk1/2-dKO;PikDD (0.78): p = 0.2).
We conclude that overexpression of constitutively active PI3K in oligodendrocytes can fully rescue the loss of myelin gene expression and myelin production seen in the PlpCreERT;Erk1/2-dKO mice.
3.4 |. Constitutive activation of PI3K in oligodendrocytes deficient in ERK1/2 fully rescued p-mTOR and p-S6RP and p-70S6K expression
Since oligodendrocyte-specific constitutive activation of PI3K rescued the loss of myelin production observed in the PlpCreERT;Erk1/2-dKO mice completely, we analyzed the signaling pathways impacted in these mice, focusing as before on p-mTOR, p-S6RP, and p-p70S6K expression. Constitutive activation of PI3K fully rescued the downregulation of p-mTOR in the PlpCreERT;Erk1/2-dKO mice, that is, the signal intensity of p-mTOR in the oligodendrocyte-like cells of the spinal cord white matter of the PlpCreERT;Erk1/2-dKO;PikDD mice became comparable to control (Figure 4a, upper panel).
FIGURE 4.
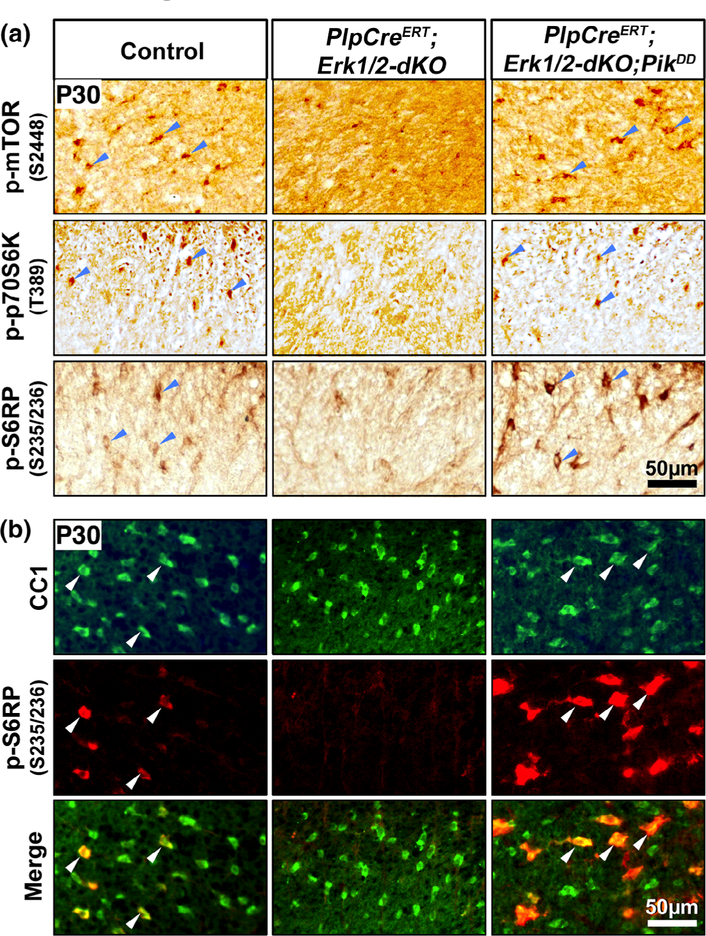
Constitutive activation of PI3K in oligodendrocytes deficient in ERK1/2 signaling fully rescued p-mTOR as well as p-S6RP and p-p70S6K expression. (a) Transverse sections of cervical spinal cord from P30 control, PlpCreERT;Erk1/2-dKO and PlpCreERT;Erk1/2-dKO;PikDD mice injected with tamoxifen from P7–P14 and immunolabeled for p-mTOR2448, p-p70S6KT389, or p-S6RPS235/236 show cellular staining of these molecules in the oligodendrocyte-like cells in the white matter of controls. These signals are dramatically reduced in the PlpCreERT;Erk1/2-dKO compared to control mice and are completely rescued in the PlpCreERT;Erk1/2-dKO;PikDD mice. (b) Double immunolabeling of spinal cord sections from these mice for p-S6RPS235/236 (red) with the mature oligodendrocyte marker CC1 (green) shows that p-S6RPS235/236 signal is dramatically reduced in CC1+ oligodendrocytes in PlpCreERT;Erk1/2-dKO mice compared to controls and increased in the CnpCre;Erk1/2-dKO;AktDD mice. Representative images of the ventral white matter from the analysis of at least three animals per genotype are shown. Blue arrowheads point to cellular staining in the spinal cord white matter and white arrows show examples of CC1+/p-S6RP+ oligodendrocytes [Color figure can be viewed at wileyonlinelibrary.com]
As p-p70S6K and p-S6RP expression had not been fully rescued by overexpression of constitutively active Akt (Figure 2b), we examined their expression in the PlpCreERT;Erk1/2-dKO;PikDD mice. Strong p-p70S6K and p-S6RP expression was seen in PlpCreERT;Erk1/2-dKO;PikDD compared to both control and PlpCreERT;ERK1/2-dKO (Figure 4a, second and third panels). Therefore, unlike CnpCre;Erk1/2-dKO;AktDD, the rescue of p-p70S6K and p-S6RP expression in the PlpCreERT;Erk1/2-dKO;PikDD mice was complete and even went above the control level. To confirm our conclusions, we double-immunolabeled spinal cord sections from control, PlpCreERT;Erk1/2-dKO, and PlpCreERT;Erk1/2-dKO;PikDD mice for p-S6RP and CC1 (Figure 4b). Consistent with the DAB staining, the p-S6RP, co-localized with CC1+ oligodendrocytes, showed a strong signal in the control and PlpCreERT;Erk1/2-dKO;PikDD mice, while the CC1+ oligodendrocytes of the PlpCreERT;Erk1/2-dKO showed negligible p-S6RP signal as expected.
We conclude that attenuation of p-S6RP and p-p70S6K expression in the PlpCreERT;Erk1/2-dKO oligodendrocytes was completely abrogated in the PlpCreERT;Erk1/2-dKO;PikDD mice unlike the partial abrogation seen in the CnpCre;Erk1/2-dKO;AktDD mice.
3.5 |. Sustained elevation of Mek/ERK1/2 activity was unable to rescue the deficits in oligodendrocyte differentiation and hypomyelination caused by the loss of mTOR early during differentiation
These studies on signaling in oligodendrocytes suggested crosstalk between the ERK1/2 signaling pathway and the PI3K/Akt signaling pathway in regulating myelination. We therefore took a converse approach and asked whether sustained elevation of ERK1/2 activity by constitutive activation of Mek1 in mice lacking mTOR could rescue the deficits in oligodendrocyte differentiation and hypo-myelination that are normally observed in the CnpCre;mTOR-KO and CnpCre;Raptor-KO mice (Bercury et al., 2014; Wahl et al., 2014; Lebrun-Julien et al., 2014). To address this question, we generated and analyzed transgenic mouse line (referred to as CnpCre;mTOR-KO;MekDD) where mTOR was deleted (mTOR-KO) and simultaneously Mek/ERK1/2 was constitutively overactivated (MekDD) specifically in Cnp-expressing oligodendrocyte lineage cells.
We first confirmed that mTOR expression was indeed lost in the CnpCre;mTOR-KO and CnpCre;mTOR-KO;MekDD mice as expected. panmTOR was strongly expressed in the white matter of control and in mice heterozygous for mTOR deletion (CnpCre;mTOR-het;MekDD) (Figure 5a). Importantly, the CnpCre;mTOR-KO and the CnpCre;mTOR-KO;MekDD mice showed a complete loss of pan-mTOR expression in the spinal cord white matter but not in the grey matter neurons, indicating that the deletion had occurred in oligodendrocytes.
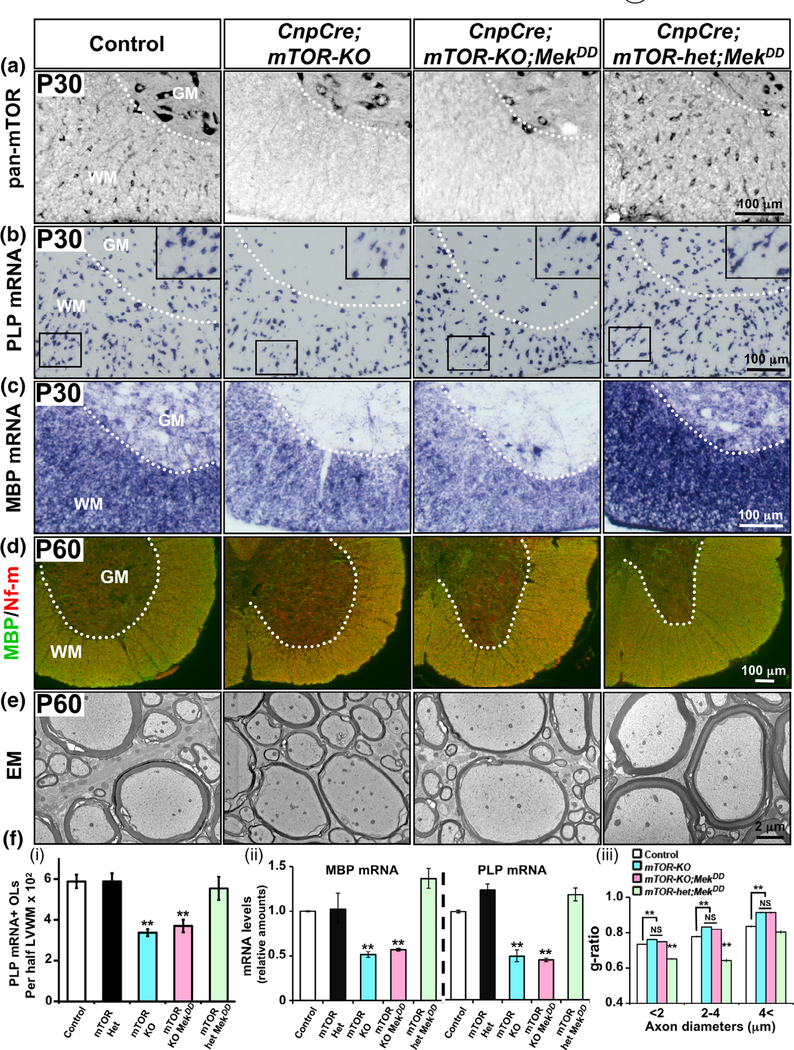
FIGURE 5.
Sustained elevation of ERK1/2 activity was unable to abrogate the deficits in oligodendrocyte differentiation and hypomyelination caused by the loss of mTOR early during differentiation. (a) Transverse sections of cervical spinal cord at P30 immunolabeled for pan-mTOR show a cellular signal in the white matter (WM) of control and in mice heterozygous for mTOR (CnpCre;mTOR-het;MekDD), which is completely lost in the WM but not in the grey matter (GM) of CnpCre;mTOR-KO and CnpCre;mTOR-KO;MekDD mice. (b) In situ hybridization for the expression of PLP mRNA at P30 show a significant reduction in the numbers of PLP mRNA+ oligodendrocytes and in the intensity of the signals per oligodendrocyte in the CnpCre;mTOR-KO mice compared to controls and CnpCre;mTOR-het;MekDD mice. This reduction in the CnpCre;mTOR-KO is not rescued in the CnpCre;mTOR-KO;MekDD mice. Enlarged image is shown in the insert. (c) MBP mRNA levels, also show a similar reduction in the intensity of the signals in the CnpCre;mTOR-KO and CnpCre;mTOR;MekDD mice compared to controls and CnpCre;mTOR-het;MekDD mice. (d) Double immunolabeling of spinal cord sections for MBP and neurofilament-m (Nf-m) at P60 show that compared to control and CnpCre;mTOR-het;MekDD, there is reduced signal of MBP (green) in the WM of CnpCre;mTOR-KO, which is similar to CnpCre;mTOR-KO;MekDD mice. (e) EM images of matched regions of ventral spinal cords at P60 show that axons are wrapped by thinner myelin sheaths in the CnpCre;mTOR-KO compared to control. Similarly, CnpCre;mTOR-KO;MekDD also shows thinner myelin while the CnpCre;mTOR-het;MekDD shows increased thickness compared to CnpCre;mTOR-KO;MekDD and control mice. (f (i)) Quantification of the total numbers of mature oligodendrocytes (OLs), marked by PLP mRNA expression, in half latero-ventral white matter (LVWM) of the cervical spinal cord at P30 shows no differences between control, CnpCre;mTOR-het (mTOR-het) and CnpCre;mTOR-het;MekDD (mTOR-het;MekDD) mice, while both CnpCre;mTOR-KO (mTOR-KO) and CnpCre;mTOR-KO;MekDD (mTOR-KO;MekDD) show statistically significant decrease in the numbers of oligodendrocytes compared to control. (f (ii)) Quantification of mRNA levels by qRT-PCR analysis at P30 shows that the MBP and PLP mRNA levels in the spinal cords of CnpCre;mTOR-KO and CnpCre;mTOR-KO;MekDD mice were similar and significantly reduced compared to control, CnpCre;mTOR-het or CnpCre;mTOR-het;MekDD mice. (f (iii)) Quantification by g-ratio analysis at P60 shows that myelin thickness in the CnpCre;mTOR-KO (blue bars) and CnpCre;mTOR-KO;MekDD (pink bars) mice is similar and significantly reduced (higher g-ratios) compared to littermate control (white bars), especially for axons of diameter >2 μm, while CnpCre;mTOR-het;MekDD (green bars) show increased myelin thickness (lower g-ratio) compared to control and CnpCre;mTOR-KO;MekDD mice. Approximately 400–500 axons from two mice of each genotype were analyzed, significance is shown as **, p values are given in the Section 3. Error bars in (f (i), (ii)) indicate SEM. **p < .01, N = 3–4. Representative images from ventral white matter region of the spinal cord are shown [Color figure can be viewed at wileyonlinelibrary.com]
We next examined the expression of PLP mRNA by ISH analysis of spinal cord sections. We found that at all ages examined (P14, P30, and P60), there was a significant decrease in the numbers of mature PLP mRNA+ oligodendrocytes in the CnpCre;mTOR-KO compared to control, CnpCre;mTOR-het and CnpCre;mTOR-het-MekDD mice (P30 is shown, Figure 5b,f (i)). This is consistent with the previously reported inhibition of oligodendrocyte differentiation due to the loss of mTORC1 signaling in vivo (Bercury et al., 2014; Lebrun-Julien et al., 2014; Wahl et al., 2014). Comparison of the CnpCre;mTOR-KO with the CnpCre;mTOR-KO;MekDD mice showed that the overactivation of ERK1/2 was not able to abrogate this deficit since no difference in the number of PLP mRNA+ oligodendrocytes were detected between CnpCre;mTOR-KO and CnpCre;mTOR-KO;MekDD mice. Interestingly, in addition to the reduction in the number of differentiated oligodendrocytes in the CnpCre;mTOR-KO, we observed that at P30 there was a reduction in the intensity of the PLP mRNA signal per oligodendrocyte compared to control and CnpCre;mTOR-het-MekDD (Figure 5b, inserts). This reduction of the PLP mRNA signal intensity per oligodendrocyte was also not rescued by ERK1/2 overactivation in the CnpCre;mTOR-KO;MekDD mice. Similar to PLP mRNA, attenuation of MBP mRNA expression in the CnpCre;mTOR-KO could not be abrogated by ERK1/2 overactivation in the CnpCre;mTOR-KO;MekDD mice (Figure 5c). This was consistent with the inability to rescue MBP protein (green) expression at P60 in spinal cord of the CnpCre;mTOR-KO;MekDD mice, relative to CnpCre;mTOR-KO mice, where there was a slight but clear downregulation of MBP protein signal (Figure 5d).
Quantification of mRNA by qPCR analysis (Figure 5f (ii)) confirmed the ISH results and showed that compared to control (white bars) there was a significant reduction of MBP and PLP mRNA levels in CnpCre;mTOR-KO (blue bars), which could not be abrogated in the CnpCre;mTOR-KO;MekDD (pink bars) mice. Mice heterozygous for mTOR (mTOR-het, black bars) had no significant difference in myelin gene expression compared to control (Figure 5f (ii)). Thus, the slightly elevated MBP mRNA signal intensity observed in the CnpCre;mTOR-het-MekDD compared to control and CnpCre;mTOR-het (Figure 5c,f (ii)) is due to the elevation of Mek/ERK1/2 activity as we have reported previously in the CnpCre;MekDD mice (Ishii et al., 2013, 2014).
We next examined the ultrastructure of myelin in these mice (Figure 5e). Consistent with previous reports (Wahl et al., 2014), the loss of mTOR resulted in thinner myelin. Importantly, we found that sustained over-activation of ERK1/2 in the CnpCre;mTOR-KO;MekDD mice could not abrogate this defect. Even though by P60 majority of the axons in the ventral column of the CnpCre;mTOR-KO mice were myelinated, the myelin sheaths remained thinner than normal in both the CnpCre;mTOR-KO and CnpCre;mTOR-KO;MekDD mice (Figure 5e). The g ratios for the CnpCre;mTOR-KO remained largely comparable to the CnpCre;mTOR-KO;MekDD mice [average g ratios and p values: control (0.75) vs CnpCre;mTOR-KO (0.79): p = 1.46 × 10−17; CnpCre; mTOR-KO (0.79): vs CnpCre;mTOR-KO;MekDD (0.78): p = 1 × 10−2].
Moreover, we have shown previously that sustained overactivation of Mek/ERK1/2 in oligodendrocytes led to the formation of significantly thicker myelin sheaths, accompanied by the upregulation of myelin gene expression in the CnpCre;MekDD mice (Ishii et al., 2013, 2014). Similarly, sustained overactivation of Mek/ERK1/2 in the CnpCre;mTOR-het-MekDD mice also led to the formation of significantly thicker myelin sheaths compared to controls (Figure 5e,f (iii)). This was accompanied by an upregulation of MBP mRNA expression as mentioned above (Figure 5c). Importantly, these Mek/ERK1/2-driven increases in myelin thickness and gene expression were completely lost when mTOR was simultaneously deleted in the CnpCre;mTOR-KO;MekDD. Both these analyses strongly suggest a requirement for mTOR signaling in the ERK1/2-mediated increase in myelin thickness during developmental myelination.
Taken together, we conclude that the deficits in oligodendrocyte differentiation, myelin gene expression, and myelin growth caused by the loss of mTOR early during developmental myelination could not be abrogated by simultaneous elevation of ERK1/2 activity. Conversely, the increase of myelin gene expression and myelin thickness caused by the over stimulation of ERK1/2 was lost when there was simultaneous loss of mTOR in oligodendrocytes, suggesting an impact of mTOR signaling on the stimulation of myelination induced by ERK1/2.
3.6 |. Induced ablation of mTOR in adult oligodendrocytes did not lead to myelin/axonal pathology or a significant downregulation of myelin gene expression, as found in the ERK1/2 KO mice during adulthood
Our results showed that when mTOR was deleted early during differentiation in the CnpCre-expressing oligodendrocyte-lineage cells, ERK1/2 was unable to upregulate myelin gene expression or promote myelin growth, suggesting a prominent role of mTOR as mediator of ERK1/2 signaling during the period of active myelination. However, the question remains whether mTOR continues to play an essential role in the maintenance of myelin and axonal integrity during adulthood as we have demonstrated for ERK1/2 (Ishii et al., 2014). To address this question, we analyzed mice where either mTOR or Erk1/2 ablation was conditionally induced in adult oligodendrocytes using Tm-inducible PlpCreERT and asked whether deletion of mTOR in mature oligodendrocytes had a similar detrimental effect to that caused by the deletion of Erk1/2 in the adult CNS (Figure 6).
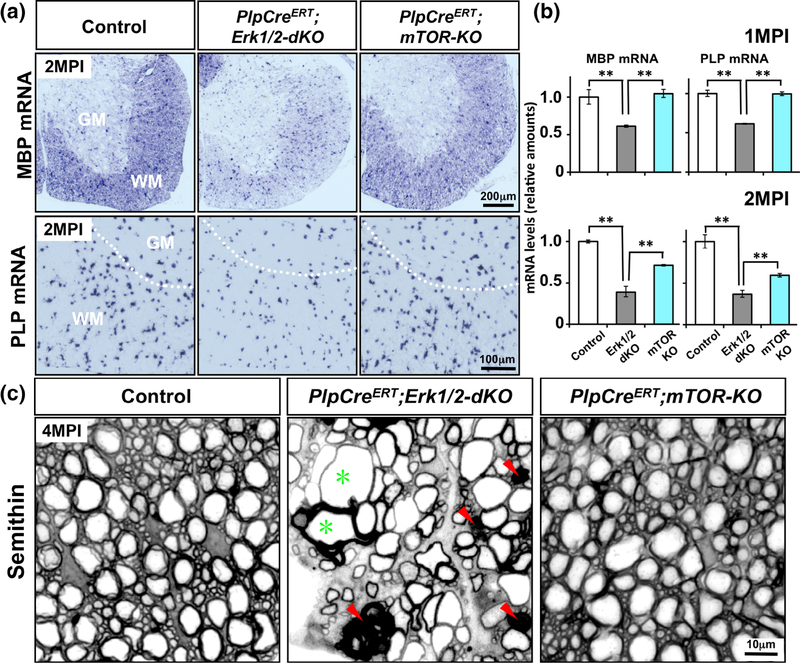
FIGURE 6.
Induced ablation of mTOR in adult oligodendrocytes did not lead to myelin and axonal pathology or a significant downregulation of myelin gene expression, compared to the sever deficit in the ERK1/2 KO mice during adulthood. (a) Control, PlpCreERT;Erk1/2-dKO and PlpCreERT;mTOR-KO mice injected with tamoxifen around 1 months of age, analyzed at 2 months postinjection (MPI) by in situ hybridization, shows that the MBP and PLP mRNA signal intensities are dramatically reduced in the spinal cords of PlpCreERT;Erk1/2-dKO mice compared to littermate controls, but PlpCreERT;mTOR-KO mice analyzed in parallel show comparable level of MBP and PLP mRNA expression as the control. (b) Quantification of MBP and PLP mRNA levels by qRT-PCR also shows a significant decrease in their levels in the PlpCreERT;Erk1/2-dKO mice compared to controls and PlpCreERT;mTOR-KO. Control values are normalized to 1. Error bars indicate SEM. **p < .01. N = 3–4 for each condition. WM, white matter; GM, grey matter. (c) Transverse semithin sections of ventral spinal cord from control, PlpCreERT;Erk1/2-dKO, and PlpCreERT;mTOR-KO mice analyzed at 4 MPI show abnormal myelin profiles with darkly stained ovals (red arrowheads) and degenerating axons, which often appeared as empty spaces surrounded by thin wraps of myelin (green asterisk) in the PlpCreERT;Erk1/2-dKO but no such abnormalities are seen in the spinal cord of PlpCreERT;mTOR-KO or control mice. Representative images from ventral white matter region of the spinal cord from 2–3 mice per genotype are shown [Color figure can be viewed at wileyonlinelibrary.com]
Recombination was induced by Tm injections around 1 month, at which point in spinal cord most oligodendrocytes are mature, myelinating cells. MBP mRNA expression was analyzed by ISH in the spinal cords of control, PlpCreERT;Erk1/2-dKO and PlpCreERT;mTOR-KO mice at 1 and 2-month post injection (MPI). It was significantly reduced in PlpCreERT;Erk1/2-dKO mice compared to control (Figure 6a, 2MPI is shown in upper panel), whereas only a slight decrease of MBP mRNA expression was noted in the PlpCreERT;mTOR-KO mice. Similarly, PLP mRNA ISH analysis showed a downregulation of the intensity of the signal per oligodendrocyte in the PlpCreERT;Erk1/2-dKO but not in the PlpCreERT;mTOR-KO (Figure 6a, lower panel). Quantification of the mRNA levels by qRT-PCR confirmed the relatively significant downregulation of MBP and PLP mRNA expression in the PlpCreERT;Erk1/2-dKO compared to the PlpCreERT;mTOR-KO and control (Figure 6b), although by 2 MPI, PLP mRNA levels were also significantly reduced in PlpCreERT;mTOR-KO mice. The number of PLP mRNA+ differentiated oligodendrocytes in PlpCreERT;Erk1/2-dKO and PlpCreERT; mTOR-KO mice were similar to control (control: 617 ± 32; PlpCreERT;Erk1/2-dKO: 556 ± 17; PlpCreERT;mTOR-KO: 570 ± 9).
In our previous studies, we have shown that progressive myelin axonal degeneration occurs in the adult PlpCreERT;Erk1/2-dKO mice and have suggested that the continued presence of ERK1/2 in mature oligodendrocytes is required for the long-term maintenance of myelin and axonal integrity during adulthood (Ishii et al., 2014). To determine if mTOR signaling plays a significant role in this function, we examined semithin sections of spinal cord in parallel from PlpCreERT;Erk1/2-dKO and PlpCreERT;mTOR-KO at 4MPI (Figure 6c). We found that consistent with our previous study (Ishii et al., 2014), the PlpCreERT;Erk1/2-dKO mice showed clear signs of myelin and axonal pathology, such as abnormal myelin profiles with darkly stained ovals (red arrowheads) and degenerating axons, which often appeared as empty spaces surrounded by thin wraps of myelin (green asterisk). In contrast, no such abnormalities appeared in the spinal cords of PlpCreERT;mTOR-KO or control mice.
We conclude that the induced ablation of mTOR in adult oligodendrocytes does not lead to myelin/axonal pathology or a significant downregulation of myelin gene expression, compared to the severe defects seen in the PlpCreERT;Erk1/2-dKO mice during adulthood.
3.7 |. Reactivation of myelin gene expression induced by the elevation of Mek/ERK1/2 activity in adult oligodendrocytes is abrogated in the absence of mTOR
It has been reported that overactivation of either PI3K or Mek/ERK1/2 during adulthood can reactivate oligodendrocytes to upregulate myelin gene expression which leads to new myelin growth by pre-existing adult oligodendrocytes (Goebbels et al., 2010; Ishii et al., 2016; Snaidero et al., 2014; Jeffries et al., 2016). It is not known whether PI3K and Mek/ERK1/2 act independently or converge with each other at the level of mTORC1 to achieve this function during adulthood, as they do during developmental myelination. We therefore asked whether reactivation of myelin gene expression by adult oligodendrocytes induced by the elevation of Mek/ERK1/2 activity requires the presence of mTOR activity. To this end, we analyzed transgenic mice where Mek was constitutively activated (MekDD) and simultaneously mTOR was deleted in the adult mice using tamoxifen-inducible oligodendrocyte-specific PlpCreERT mice (referred to as PlpCreERT;mTOR-KO;MekDD). These mice were compared with PlpCreERT;mTOR-het;MekDD which are in effect PlpCreERT;MekDD in phenotype as mTOR-het mice are identical to control mice.
We examined MBP and PLP mRNA expression in the spinal cord of control, PlpCreERT;mTOR-het;MekDD and PlpCreERT;mTOR-KO;MekDD mice at 1 and 2 MPI by ISH (MBP mRNA at 2 MPI is shown in Figure 7a). We found that constitutive activation of Mek in the PlpCreERT;mTOR-het;MekDD mice during adulthood led to a clear increase in MBP mRNA signal intensity compared to control as we had shown previously in the PlpCreERT;MekDD mice (Ishii et al., 2016). However, in the absence of mTOR, there was no such increase and the PlpCreERT;mTOR-KO;MekDD mice showed comparable MBP mRNA levels as the control. Quantification of MBP and PLP mRNA levels by qRT-PCR confirmed these observations (Figure 7b).
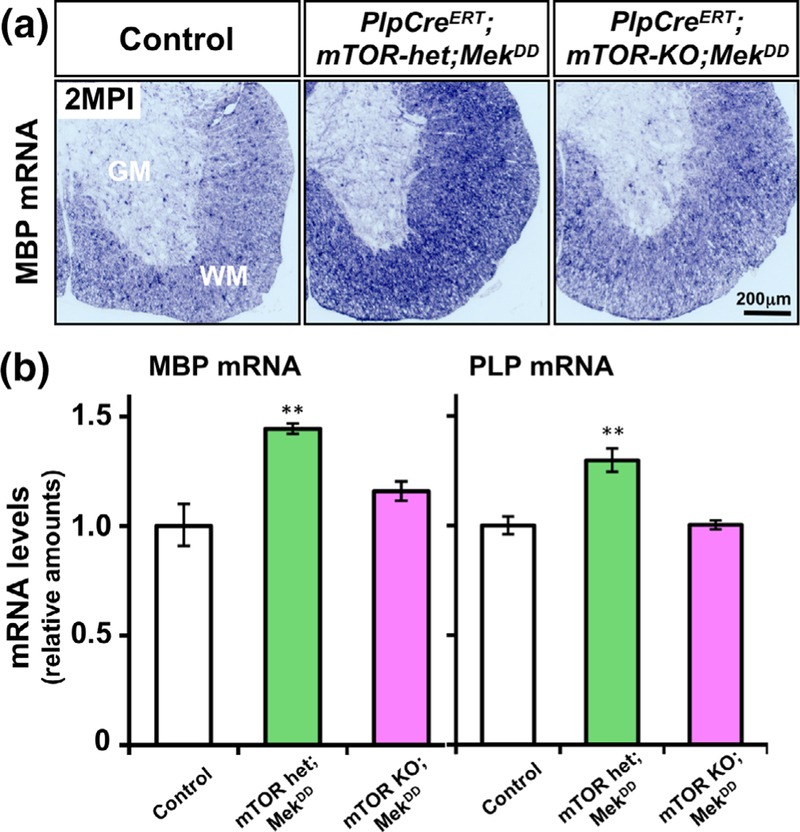
FIGURE 7.
Reactivation of myelin gene expression induced by the elevation of Mek/ERK1/2 activity in adult oligodendrocytes is abrogated in the absence of mTOR. (a) Control, PlpCreERT;mTOR-het; MekDD and PlpCreERT;mTOR-KO;MekDD mice injected with tamoxifen around 1 months of age, analyzed at 2 months postinjection (MPI) by in situ hybridization, show that the MBP mRNA signal intensity is increased in the spinal cords of PlpCreERT;mTOR-het;MekDD mice compared to littermate controls, but not in the PlpCreERT;mTOR-KO; MekDD mice. (b) Quantification of MBP and PLP mRNA levels by qRT-PCR at 1 MPI show a statistically significant increase in their levels in the PlpCreERT;mTOR-het;MekDD mice compared to control. This increase is not seen in the PlpCreERT;mTOR-KO;MekDD mice. Control values are normalized to 1. Error bars indicate SEM. **p < .01. N = 3–4 for each condition. WM, white matter; GM, grey matter [Color figure can be viewed at wileyonlinelibrary.com]
We conclude that the reactivation of myelin gene expression that normally occurs by forced overactivation of ERK1/2 during adulthood does not happen in the absence of mTOR.
4 |. DISCUSSION
The current studies establish that the Mek/ERK1/2-MAPK and the PI3K/Akt/mTOR signaling pathways work both independently and in cooperation with each other in a temporally regulated manner as oligodendrocyte lineage cells progress through different stages of developmental myelination into adulthood. Specifically, during oligodendrocyte differentiation and initiation of myelination, PI3K/Akt/mTOR pathway, not the Mek/ERK1/2-MAPK pathway, is likely to be the key regulator of these events; during active myelination, both pathways converge at the level of mTORC1, thus regulating myelin growth through a common signaling mechanism. In contrast, during adulthood, Mek/ERK1/2-MAPK is the primary pathway responsible for the preservation of the integrity of the myelinated axon, with minimal involvement of mTOR.
Transition of oligodendrocyte progenitors to terminally differentiated oligodendrocytes is an important stage during developmental myelination that is tightly controlled by multiple signals to ensure timely and efficient generation of myelinating oligodendrocytes. Numerous in vitro studies have investigated the role of different signaling pathways and suggested an involvement of both the PI3K/Akt/mTOR and the Mek/ERK1/2-MAPK pathways in the regulation of this key event (Bansal, Magge, & Winkler, 2003; Baron, Metz, Bansal, Hoekstra, & de Vries, 2000; Bhat & Zhang, 1996; Cui & Almazan, 2007; Dai et al., 2014; Frost, Zhou, Krasnesky, & Armstrong, 2009; Fortin et al., 2005; Fyffe-Maricich, Karlo, Landreth, & Miller, 2011; Guardiola-Diaz et al., 2012; Tyler et al., 2009, 2011; Van’t Veer et al., 2009; Yim, Hammer, & Quarles, 2001; Younes-Rapozo et al., 2009). in vivo genetic approaches demonstrate that conditional ablation of either mTOR or Raptor/Rictor leads to impaired oligodendrocyte progenitor differentiation and a delay in the initiation of myelination (Bercury et al., 2014; Lebrun-Julien et al., 2014; Wahl et al., 2014). Our studies confirm these earlier studies, that is, we find fewer mature oligodendrocytes in postnatal CnpCre;mTOR-KO spinal cord. Given that a role of ERK1/2 signaling in promoting oligodendrocyte differentiation and an apparent cross-talk between the PI3K/Akt/mTOR and the Mek/ERK1/2-MAPK pathways has been suggested by in vitro studies (Bansal et al., 2003; Baron et al., 2000; Bhat & Zhang, 1996; Cui & Almazan, 2007; Dai et al., 2014; Fortin et al., 2005; Frost et al., 2009; Fyffe-Maricich et al., 2011; Guardiola-Diaz et al., 2012; Rodgers et al., 2015; Van’t Veer et al., 2009; Xiao et al., 2012; Yim et al., 2001; Younes-Rapozo et al., 2009), we investigated whether conditional overactivation of ERK1/2 in oligodendrocytes could compensate for the loss of mTOR signaling in vivo. Importantly, it cannot compensate in this context, because despite expression of MekDD in oligodendrocytes, oligodendrocyte differentiation remained attenuated in the CnpCre;mTOR-KO;MekDD mice. Thus, mTOR signaling driven by PI3K/Akt, rather than ERK1/2 activation, appears to be a crucial signal that triggers the transition of oligodendrocyte progenitors to enter terminal differentiation in vivo. This dispensable role of ERK1/2 specifically in oligodendrocyte progenitor differentiation is consistent with several of our studies on CnpCre;Erk1/2-dKO mice where we find no change in the number of differentiated oligodendrocytes or in p-mTOR, p-Raptor, p-p70S6K, and p-S6RP expression during the first two postnatal weeks when rapid oligodendrocyte progenitor differentiation occurs in spinal cord (Figure 2a; Ishii et al., 2012). These findings may also explain why pharmacological inhibition of Akt/mTOR that is sufficient to inhibit oligodendrocyte progenitor differentiation in culture cannot be overcome by the increased p-ERK1/2 expression observed in these cultures (Dai et al., 2014). Taken together, these studies strongly support the model (Figure 8a (i)) that PI3K/Akt/mTOR acts independently of Mek/ERK1/2-MAPK pathway to promote oligodendrocyte progenitor differentiation.
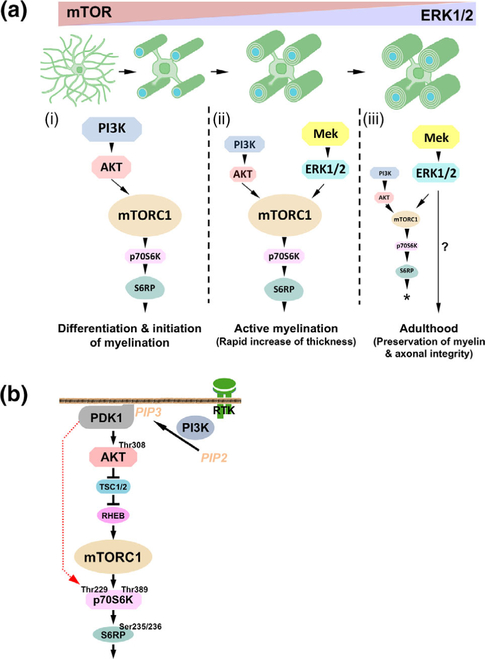
FIGURE 8.
(a) Model depicting the potential involvement of the Mek/ERK1/2-MAPK and the PI3K/Akt/mTOR signaling pathways at different stages of developmental myelination and during adulthood. From the analysis of multiple transgenic mice, a working model is proposed here: (i) During oligodendrocytes differentiation and initiation of myelination, PI3K/Akt/mTOR pathway, not the Mek/ERK1/2-MAPK pathway is the key regulator of these events through mTORC1 activation. (ii) During active myelination, both pathways cooperate to regulate the rapid increase of myelin thickness via activation of mTORC1 and its downstream signaling molecules. (iii) During adulthood, Mek/Erk1/2-MAPK is primarily responsible for the preservation of the integrity of the myelinated axon by mechanisms not yet fully understood (?), which are largely mTOR independent. Forced overactivation of ERK1/2 in adult oligodendrocytes can re-establish the mTOR-dependent, ERK1/2-mediated program of myelin growth during adulthood (*). Relative functional importance of the two pathways at different stages is depicted by the image size of the signaling proteins. (b) Simplified schematic showing the major components of the PI3K/Akt/mTOR pathway and the hypothesized involvement of PDK1. Active PI3K phosphorylates PIP2 [phosphatidylinositol (4,5)-bisphosphate], converting it to PIP3 [phosphatidylinositol (3,4,5)-trisphosphate] on the plasma membrane. PIP3 recruits Akt and PDK1 (Phosphoinositide-dependent protein kinase-1) to the plasma membrane, enabling PDK1 to phosphorylate a conserved Thr308 on Akt. The activated Akt phosphorylates the tuberous sclerosis complex 2 (TSC2) and leads to the release of TSC inhibition of RHEB (GTPase Ras homolog enriched in brain). RHEB directly activates mTORC1 which activates p70 ribosomal S6 kinase (p70S6K) by phosphorylation at Thr389, a major site important for its activation. Activated p70S6K promote the phosphorylation of S6 ribosome protein at Ser235/236. Shown in red dotted line is the hypothetical step downstream of PI3K and upstream of Akt which potentially involves PDK1. Briefly, while the mTORC1-mediated phosphorylation of p70S6K at site T389 is clearly important for its activity, a prior direct phosphorylation by PDK1 at site T229 in its catalytic domain is believed to enhance the activity of p70S6K to reach a higher threshold level of activation (Alessi et al., 1997; Martini et al., 2014; Weng et al., 1998) [Color figure can be viewed at wileyonlinelibrary.com]
Following oligodendrocyte progenitor differentiation and timely initiation of axonal wrapping, there is a period of active myelin growth when myelin thickness increases rapidly in proportion to the axon diameter. It has been speculated that the PI3K/Akt/mTOR and Mek/ERK1/2-MAPK signaling pathways also play an important role in the regulation of myelin thickness (Bercury et al., 2014; Goebbels et al., 2010; Harrington et al., 2010; Ishii et al., 2012, 2013; Lebrun-Julien et al., 2014; Narayanan et al., 2009; Wahl et al., 2014). However, a key question that remained unanswered was whether they operate independently or work together to achieve this goal. Our functional in vivo studies here clearly reveal that the Mek/ERK1/2-mediated increase in myelin thickness during this active phase of myelination is dependent on prior signaling by mTORC1, as sustained overactivation of Mek/ERK1/2 even up to P60 could not rescue the deficit in myelin thickness caused by the loss of mTOR in CnpCre-expressing oligodendrocyte progenitors and immature oligodendrocytes. This suggests that there is an essential prerequirement of mTOR “priming” early during the differentiation period, without which ERK1/2 signaling is inadequate to perform its function later during the period of active myelination to promote myelin growth and increase its thickness. Therefore, since ERK1/2 itself requires mTORC1 activation to promote myelin growth during active myelination, our data here might explain why the increased ERK1/2 activity observed in the spinal cords of CnpCre-Raptor-KO mice (Bercury et al., 2014) was unable to compensate for the absence of mTORC1 signaling and rescue myelin growth.
We have recently shown that during active myelination, downregulation of myelin gene expression and myelin growth in conditional Erk1/2-dKO mice is accompanied by a significant reduction in the expression of p-mTOR, p-Raptor, p-p70S6K, and p-S6RP, but not of p-Akt. Since Akt is upstream of mTORC1 and p-mTOR, p-Raptor, p-p70S6K, and p-S6RP are traditionally considered downstream effectors in the PI3K/Akt/mTORC1 pathway, our earlier work suggested convergence of the two pathways at the level of mTORC1 (Figures 3 and 4 and Furusho et al., 2017). However, it was unclear whether the observed changes in the expression levels of these signaling molecules had any functional significance with respect to ERK1/2 signaling regulating myelin growth. In this study, we show that the deficits caused by loss of ERK1/2 in oligodendrocytes were completely rescued by sustained overactivation of PI3K in transgenic mice, supporting the model where the two pathways functionally converge in oligodendrocytes and use a common mechanism to promote rapid myelin growth during active myelination (Figure 8a (ii)). Surprisingly, sustained overactivation of Akt, which is downstream of PI3K in this pathway, was only partially able to rescue the deficits in myelin gene expression and myelin growth. Furthermore, although p-mTOR levels were fully rescued in these mice, the activity of its downstream effectors, p70S6K and S6RP was only partially rescued, in contrast to a complete rescue observed when PI3K was overactivated. This observation is interesting as it suggests that, in addition to Akt/mTORC1, there is another molecule upstream of Akt, most likely PDK1, that directly links PI3K to p-p70S6K in oligodendrocytes. This hypothesis, is supported by previous studies in cell lines which shows that while mTORC1-mediated phosphorylation of p70S6K at site T389 is clearly important for its activity, a prior direct phosphorylation by PDK1 at site T229 in its catalytic domain enhances the activity of p70S6K to reach a higher threshold level of activation (Alessi et al., 1997; Martini et al., 2014; Weng et al., 1998). Thus, it is possible that for oligodendrocytes to fully upregulate myelin gene expression and promote optimal myelin growth, p-p70S6K needs to reach a critical threshold level of activation that is potentially achieved by coordinated activation of p70S6K by Akt/mTORC1 and PDK1 (Figure 8b).
Given the breadth of PI3K functions (Martini et al., 2014), another explanation for the differential effects of Akt or PI3K signaling on myelin growth could be that PI3K and its principle signaling mediator PIP3 impact additional pathways besides Akt/mTOR signaling. For example, this may explain the differential impact of overexpression of constitutively active Akt vs induction of the PI3K pathway in the PNS, when constitutively active Akt, overexpressed in Schwann cells, does not increase myelination but induction of the PI3K pathway does (Flores et al., 2008; Goebbels et al., 2010). Possible mechanisms relate to PI3K/PIP3-induced activation of additional proteins besides Akt and PDK1 include Rho GTPase, Rac1, and CDC42 as well as certain Rac effector proteins, which may also contribute to p70S6K activation (Blanco-Aparicio, Renner, Leal, & Carnero, 2007; Chou & Blenis, 1996; Martini et al., 2014; Romanelli, Martin, Toker, & Blenis, 1999; Welch et al., 2002). Signaling through these proteins would be important for myelination, as they are involved in cytoskeletal remodeling, which is essential for oligodendrocyte process extension, axonal ensheathment, and formation of the myelin sheath (Bacon, Lakics, Machesky, & Rumsby, 2007; Kim et al., 2006; Thurnherr et al., 2006). Further studies are required to fully uncover the potential involvement of additional players activated by PI3K that likely work together with Akt/mTOR to coordinate various cellular processes involved in the rapid growth of the myelin sheath to increase its thickness during active myelination.
Following this period of active myelination, new myelin growth from pre-existing oligodendrocytes dramatically slows down during adulthood and the levels of major myelin mRNAs are downregulated to a basal maintenance level. We recently showed that forced overactivation of Mek/ERK1/2 in adult oligodendrocytes was able to reawaken these quiescent oligodendrocytes and induce them to upregulate myelin gene expression and reinitiate new myelin growth (Ishii et al., 2016). Reinitiation of myelin growth was also observed when the PI3K/Akt/mTOR pathway was overstimulated in adult oligodendrocytes (Goebbels et al., 2010; Snaidero et al., 2014), leaving open the question whether the Mek/ERK1/2-MAPK and PI3K/Akt/mTOR pathways converge within adult oligodendrocytes at the level of mTORC1, as they do during active phase of developmental myelination, or rather, they act as independent parallel pathways to upregulate myelin gene expression and promote the reinitiation of myelin growth during adulthood. This study addressed this question and showed that overexpression of Mek/ERK1/2 in differentiated oligodendrocytes was unable to reactivate the upregulation of myelin gene expression during adulthood if mTOR was simultaneously deleted from these oligodendrocytes. This suggests a cooperative function for the PI3K/Akt/mTOR and Mek/ERK1/2-MAPK pathways in the upregulation of myelin gene expression and promoting myelin growth, irrespective of whether it is during developmental myelination or during reinitiation of new myelin growth during adulthood. These studies are significant because they provide a molecular mechanism by which the two major signaling pathways can work together through a common mechanism to “activate” pre-existing mature quiescent oligodendrocytes, which apparently retain a certain amount of plasticity. These data establish that mature oligodendrocytes can return to a more metabolically active state to assemble new myelin when given the right signal, which is consistent with studies demonstrating myelin plasticity and its potential role in learning and memory during adulthood (Fields, 2008; Fields, 2015; Liu et al., 2012; Makinodan, Rosen, Ito, & Corfas, 2012; Mckenzie et al., 2014; Purger, Gibson, & Monje, 2015).
It is becoming increasingly clear that an important function of oligodendrocytes later in life is to preserve the long-term integrity of myelin and axons during adulthood. Our studies demonstrate that ERK1/2, largely independent of mTOR, plays a crucial role in this function during adulthood. First, in normal mice, a strong signal of p-ERK1/2 persists in the adult spinal cord, notably enriched in the paranodal loops of myelin, sites of axon–myelin interaction, while mTOR expression in oligodendrocytes is reduced to almost undetectable levels during adulthood (Ishii et al., 2014). Second, consistent with our previous study (Ishii et al., 2014), this study shows that tamoxifen-induced elimination of ERK1/2 from mature oligodendrocytes in young adults results in significant downregulation of myelin gene expression, formation of abnormal myelin structures, and onset of axonal degeneration during adulthood. By contrast, elimination of mTOR from adult oligodendrocytes in a similar manner produced no obvious myelin/axon pathology. Third, other studies show no myelin or axonal pathology even up to a year when mTORC1 or mTORC2, that is, Raptor and/or Rictor, were deleted at 2 months of age in tamoxifen-inducible PlpCreERT mice (Lebrun-Julien et al., 2014). Taken together, these findings are consistent with the hypothesis that the intracellular signals needed to preserve the integrity of the myelinated axon during adulthood are largely provided by ERK1/2, with minimal involvement of mTOR signaling (Figure 8a (iii)). It must be noted, however, that a minor reduction in myelin thickness was detected 12 months after inducible deletion of Raptor (Lebrun-Julien et al., 2014). Thus, it is possible that mTORC1 together with ERK1/2 may have a minor role in the slow growth and turnover of the myelin sheath that normally continues during adulthood. Further studies are required to fully understand the nature of ERK1/2-mediated, seemingly mTOR independent, molecular mechanisms involved in the preservation of the integrity of the myelinated axon during adulthood.
In summary, these functional in vivo studies have shown that the Mek/ERK1/2-MAPK and PI3K/Akt/mTOR pathways play both independent and interacting, temporally regulated roles during developmental myelination and in the adult CNS. Thus, establishing a finely tuned timely balance of these two signaling pathways within the oligodendrocyte lineage cells throughout the postnatal and adult life of the animal is pivotal to ensuring appropriate oligodendrocyte differentiation, myelination, and maintenance. As these pathways undoubtedly operate within complex multifaceted signaling networks, additional players are likely to be identified in future that would help to uncover the full repertoire of important intracellular interactions that may be involved in these processes.
Supplementary Material
ACKNOWLEDGMENT
This work was supported by the National Institutes of Health, Grants NS38878 and NS081948 and in part by a grant from the National Multiple Sclerosis Society, RG4878A4. The authors declare no competing financial interests. The authors thank Dr J.S. Richards (Baylor College of Medicine, Houston, TX) for the Erk1−/−;Erk2 floxed mice, and Dr K-A. Nave (Max Planck Institute of Experimental Medicine, Goettingen, Germany) for the Cnp-Cre mice.
Funding information
National Multiple Sclerosis Society, Grant/Award Number: RG4878A4; National Institutes of Health, Grant/Award Numbers: NS081948, NS38878
Footnotes
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section at the end of the article.
REFERENCES
- Alessi DR, James SR, Downes CP, Holmes AB, Gaffney PR, Reese CB, & Cohen P (1997). Characterization of a 3-phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase Bα. Current Biology, 7(4), 261–269. [DOI] [PubMed] [Google Scholar]
- Bacon C, Lakics V, Machesky L, & Rumsby M (2007). N-WASP regulates extension of filopodia and processes by oligodendrocyte progenitors, oligodendrocytes, and Schwann cells-implications for axon ensheathment at myelination. GLIA, 55(8), 844–858. 10.1002/glia.20505 [DOI] [PubMed] [Google Scholar]
- Bansal R, Magge S, & Winkler S (2003). Specific inhibitor of FGF receptor signaling: FGF-2-mediated effects on proliferation, differentiation, and MAPK activation are inhibited by PD173074 in oligodendrocyte lineage cells. Journal of Neuroscience Research, 74(4), 486–493. 10.1002/jnr.10773 [DOI] [PubMed] [Google Scholar]
- Baron W, Metz B, Bansal R, Hoekstra D, & de Vries H (2000). PDGF and FGF-2 signaling in oligodendrocyte progenitor cells: Regulation of proliferation and differentiation by multiple intracellular signaling pathways. Molecular and Cellular Neuroscience, 15(3), 314–329. 10.1006/mcne.1999.0827 [DOI] [PubMed] [Google Scholar]
- Baumann N, & Pham-Dinh D (2001). Biology of oligodendrocyte and myelin in the mammalian central nervous system. Physiological Reviews, 81(2), 871–927. 10.1152/physrev.2001.81.2.871 [DOI] [PubMed] [Google Scholar]
- Bercury KK, Dai J, Sachs HH, Ahrendsen JT, Wood TL, & Macklin WB (2014). Conditional ablation of raptor or rictor has differential impact on oligodendrocyte differentiation and CNS myelination. Journal of Neuroscience, 34(13), 4466–4480. 10.1523/JNEUROSCI.4314-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat NR, & Zhang P (1996). Activation of mitogen-activated protein kinases in oligodendrocytes. Journal of Neurochemistry, 66(5), 1986–1994. 10.1111/j.1471-4159.2012.07871.x [DOI] [PubMed] [Google Scholar]
- Blanco-Aparicio C, Renner O, Leal JF, & Carnero A (2007). PTEN, more than the AKT pathway. Carcinogenesis, 28(7), 1379–1386. 10.1093/carcin/bgm052 [DOI] [PubMed] [Google Scholar]
- Chou MM, & Blenis J (1996). The 70kD S6 kinase complexes with and is activated by the rho family G proteins Cdc42 and Rac1. Cell, 85(4), 573–583. [DOI] [PubMed] [Google Scholar]
- Cui QL, & Almazan G (2007). IGF-I-induced oligodendrocyte progenitor proliferation requires PI3K/Akt, MEK/ERK, and Src-like tyrosine kinases. Journal of Neurochemistry, 100(6), 1480–1493. 10.1111/j.1471-4159.2006.04329.x [DOI] [PubMed] [Google Scholar]
- Dai J, Bercury KK, & Macklin WB (2014). Interaction of mTOR and Erk1/2 signaling to regulate oligodendrocyte differentiation. GLIA, 62 (12), 2096–2109. 10.1002/glia.22729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doerflinger NH, Macklin WB, & Popko B (2003). Inducible sitespecific recombination in myelinating cells. Genesis, 35(1), 63–72. 10.1002/gene.10154 [DOI] [PubMed] [Google Scholar]
- Emery B (2010). Regulation of oligodendrocyte differentiation and myelination. Science, 330(6005), 779–782. [DOI] [PubMed] [Google Scholar]
- Fancy SP, Chan JR, Baranzini SE, Franklin RJ, & Rowitch DH (2011). Myelin regeneration: A recapitulation of development? Annual Review of Neuroscience, 34, 21–43. 10.1146/annurevneuro-061010-113629 [DOI] [PubMed] [Google Scholar]
- Fields RD (2008). White matter in learning, cognition and psychiatric disorders. Trends in Neurosciences, 31(7), 361–370. 10.1016/j.tins.2008.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields RD (2015). A new mechanism of nervous system plasticity: Activity dependent myelination. Nature Reviews Neuroscience, 16(12), 756–767. 10.1038/nrn4023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figlia G, Gerber D, & Suter U (2018). Myelination and mTOR. GLIA, 66(4), 693–707. 10.1002/glia.23273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores AI, Narayanan SP, Morse EN, Shick HE, Yin X, Kidd G, … Macklin WB (2008). Constitutively active Akt induces enhanced myelination in the CNS. Journal of Neuroscience, 28(28), 7174–7183. 10.1523/JNEUROSCI.0150-082008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortin D, Rom E, Sun H, Yayon A, & Bansal R (2005). Distinct fibroblast growth factor (FGF)/FGF receptor signaling pairs initiate diverse cellular responses in the oligodendrocyte lineage. Journal of Neuroscience, 25(32), 7470–7479. 10.1523/JNEUROSCI.2120-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost EE, Zhou Z, Krasnesky K, & Armstrong RC (2009). Initiation of oligodendrocyte progenitor cell migration by a PDGF-A activated extracellular regulated kinase (ERK) signaling pathway. Neurochemical Research, 34(1), 169–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furusho M, Dupree JL, Nave KA, & Bansal R (2012). Fibroblast growth factor receptor signaling in oligodendrocytes regulates myelin sheath thickness. Journal of Neuroscience, 32(19), 6631–6641. 10.1523/JNEUROSCI.6005-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furusho M, Ishii A, & Bansal R (2017). Signaling by FGF receptor 2, not FGF receptor 1, regulates myelin thickness through activation of ERK1/2-MAPK, which promotes mTORC1 activity in an Akt-independent manner. Journal of Neuroscience, 37(11), 2931–2946. 10.1523/JNEUROSCI.3316-16.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furusho M, Kaga Y, Ishii A, Hébert JM, & Bansal R (2011). Fibroblast growth factor signaling is required for the generation of oligodendrocyte progenitors from the embryonic forebrain. Journal of Neuroscience, 31(13), 5055–5066. 10.1523/JNEUROSCI.4800-102011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fyffe-Maricich SL, Karlo JC, Landreth GE, & Miller RH (2011). The ERK2 mitogen activated protein kinase regulates the timing of oligodendrocyte differentiation. Journal of Neuroscience, 31(3), 843–850. 10.1523/JNEUROSCI.3239-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fyffe-Maricich SL, Schott A, Karl M, Krasno J, & Miller RH (2013). Signaling through ERK1/2 controls myelin thickness during myelin repair in the adult central nervous system. Journal of Neuroscience, 33(47), 18402–18408. 10.1523/JNEUROSCI.2381-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goebbels S, Oltrogge JH, Kemper R, Heilmann I, Bormuth I, Wolfer S, … Nave KA (2010). Elevated phosphatidylinositol 3,4,5-trisphosphate in glia triggers cell-autonomous membrane wrapping and myelination. Journal of Neuroscience, 30(26), 8953–8964. 10.1523/JNEUROSCI.0219-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonsalvez D, Ferner AH, Peckham H, Murray SS, & Xiao J (2016). The roles of extracellular related-kinases 1 and 2 signaling in CNS myelination. Neuropharmacology, 110(Pt B), 586–593. 10.1016/j.neuropharm.2015.04.024 [DOI] [PubMed] [Google Scholar]
- Guardiola-Diaz HM, Ishii A, & Bansal R (2012). Erk1/2 MAPK and mTOR signaling sequentially regulates progression through distinct stages of oligodendrocyte differentiation. GLIA, 60(3), 476–486. 10.1002/glia.22281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington EP, Zhao C, Fancy SP, Kaing S, Franklin RJ, & Rowitch DH (2010). Oligodendrocyte PTEN is required for myelin and axonal integrity, not remyelination. Annals of Neurology, 68(5), 703–716. 10.1002/ana.22090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii A, Furusho M, & Bansal R (2013). Sustained activation of ERK1/2 MAPK in oligodendrocytes and Schwann cells enhances myelin growth and stimulates oligodendrocyte progenitor expansion. Journal of Neuroscience, 33(1), 175–186. 10.1523/JNEUROSCI.4403-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii A, Furusho M, Dupree JL, & Bansal R (2014). Role of ERK1/2 MAPK signaling in the maintenance of myelin and axonal integrity in the adult CNS. Journal of Neuroscience, 34(48), 16031–16045. 10.1523/JNEUROSCI.3360-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii A, Furusho M, Dupree JL, & Bansal R (2016). Strength of ERK1/2 MAPK activation determines its effect on myelin and axonal integrity in the adult CNS. Journal of Neuroscience, 36(24), 6471–6487. 10.1523/JNEUROSCI.0299-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii A, Fyffe-Maricich SL, Furusho M, Miller RH, & Bansal R (2012). ERK1/ERK2 MAPK signaling is required to increase myelin thickness independent of oligodendrocyte differentiation and initiation of myelination. Journal of Neuroscience, 32(26), 8855–8864. 10.1523/JNEUROSCI.0137-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffries MA, Urbanek K, Torres L, Wendell SG, Rubio ME, & Fyffe-Maricich SL (2016). ERK1/2 activation in preexisting oligodendrocytes of adult mice drives new myelin synthesis and enhanced CNS function. Journal of Neuroscience, 36(35), 9186–9200. 10.1523/JNEUROSCI.1444-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaga Y, Shoemaker WJ, Furusho M, Bryant M, Rosenbluth J, Pfeiffer SE, … Bansal R (2006). Mice with conditional inactivation of fibroblast growth factor receptor-2 signaling in oligodendrocytes have normal myelin but display dramatic hyperactivity when combined with Cnp1 inactivation. Journal of Neuroscience, 26(47), 12339–12350. 10.1523/JNEUROSCI.3573-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HJ, DiBernardo AB, Sloane JA, Rasband MN, Solomon D, Kosaras B, … Vartanian TK (2006). WAVE1 is required for oligodendrocyte morphogenesis and normal CNS myelination. Journal of Neuroscience, 26(21), 5849–5859. 10.1523/JNEUROSCI.4921-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lappe-Siefke C, Goebbels S, Gravel M, Nicksch E, Lee J, Braun PE, … Nave KA (2003). Disruption of Cnp1 uncouples oligodendroglial functions in axonal support and myelination. Nature Genetics, 33(3), 366–374. 10.1038/ng1095 [DOI] [PubMed] [Google Scholar]
- Lebrun-Julien F, Bachmann L, Norrmén C, Trötzmüller M, Köfeler H, Rüegg MA, … Suter U (2014). Balanced mTORC1 activity in oligodendrocytes is required for accurate CNS myelination. Journal of Neuroscience, 34(25), 8432–8448. 10.1523/JNEUROSCI.1105-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leone DP, Genoud S, Atanasoski S, Grausenburger R, Berger P, Metzger D, … Suter U (2003). Tamoxifen-inducible glia-specific Cre mice for somatic mutagenesis in oligodendrocytes and Schwann cells. Molecular and Cellular Neuroscience, 22(4), 430–440. [DOI] [PubMed] [Google Scholar]
- Liu J, Dietz K, DeLoyht JM, Pedre X, Kelkar D, Kaur J, … Casaccia P (2012). Impaired adult myelination in the prefrontal cortex of socially isolated mice. Nature Neuroscience, 15(12), 1621–1623. 10.1038/nn.3263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makinodan M, Rosen KM, Ito S, & Corfas G (2012). A critical period for social experience-dependent oligodendrocyte maturation and myelination. Science, 337(6100), 1357–1360. 10.1126/science.1220845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martini M, De Santis MC, Braccini L, Gulluni F, & Hirsch E (2014). PI3K/AKT signaling pathway and cancer: An updated review. Annals of Medicine, 46(6), 372–383. 10.3109/07853890.2014.912836 [DOI] [PubMed] [Google Scholar]
- McKenzie IA, Ohayon D, Li H, de Faria JP, Emery B, Tohyama K, & Richardson WD (2014). Motor skill learning requires active central myelination. Science, 346(6207), 318–322. 10.1126/science.1254960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller RH (2002). Regulation of oligodendrocyte development in the vertebrate CNS. Progress in Neurobiology, 67(6), 451–467. [DOI] [PubMed] [Google Scholar]
- Narayanan SP, Flores AI, Wang F, & Macklin WB (2009). Akt signals through the mammalian target of rapamycin pathway to regulate CNS myelination. Journal of Neuroscience, 29(21), 6860–6870. 10.1523/JNEUROSCI.0232-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nave KA (2010). Myelination and support of axonal integrity by glia. Nature, 468(7321), 244–252. 10.1038/nature09614 [DOI] [PubMed] [Google Scholar]
- Purger D, Gibson EM, & Monje M (2015). Myelin plasticity in the central nervous system. Neuropharmacology, 110(Pt B), 563–573. 10.1016/j.neuropharm.2015.08.001 [DOI] [PubMed] [Google Scholar]
- Risson V, Mazelin L, Roceri M, Sanchez H, Moncollin V, Corneloup C, … Gangloff YG (2009). Muscle inactivation of mTOR causes metabolic and dystrophin defects leading to severe myopathy. Journal of Cell Biology, 187(6), 859–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers JM, Robinson AP, Rosler ES, Lariosa-Willingham K, Persons RE, Dugas JC, & Miller SD (2015). IL-17A activates ERK1/2 and enhances differentiation of oligodendrocyte progenitor cells. GLIA, 63(5), 768–779. 10.1002/glia.22783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanelli A, Martin KA, Toker A, & Blenis J (1999). p70 S6 kinase is regulated by protein kinase Cζ and participates in a phosphoinositide 3-kinase-regulated signalling complex. Molecular and Cellular Biology, 19(4), 2921–2928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinfeld H, & Seger R (2005). The ERK cascade: A prototype of MAPK signaling. Molecular Biotechnology, 31(2), 151–174. 10.1385/MB:31:2:151 [DOI] [PubMed] [Google Scholar]
- Snaidero N, Möbius W, Czopka T, Hekking LH, Mathisen C, Verkleij D, … Simons M (2014). Myelin membrane wrapping of CNS axons by PI(3,4,5)P3-dependent polarized growth at the inner tongue. Cell, 156(1–2), 277–290. 10.1016/j.cell.2013.11.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan L, Sasaki Y, Calado DP, Zhang B, Paik JH, DePinho RA, … Rajewsky K (2009). PI3 kinase signals BCR dependent mature B cell survival. Cell, 139(3), 573–586. 10.1016/j.cell.2009.08.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurnherr T, Benninger Y, Wu X, Chrostek A, Krause SM, Nave KA, … Relvas JB (2006). Cdc42 and Rac1 signaling are both required for and act synergistically in the correct formation of myelin sheaths in the CNS. Journal of Neuroscience, 26(40), 10110–10109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler WA, Gangoli N, Gokina P, Kim HA, Covey M, Levison SW, & Wood TL (2009). Activation of the mammalian target of rapamycin (mTOR) is essential for oligodendrocyte differentiation. Journal of Neuroscience, 29(19), 6367–6378. 10.1523/JNEUROSCI.0234-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler WA, Jain MR, Cifelli SE, Li Q, Ku L, Feng Y, … Wood TL (2011). Proteomic identification of novel targets regulated by the mammaliantarget of rapamycin pathway during oligodendrocyte differentiation. GLIA, 59(11), 1754–1769. 10.1002/glia.21221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van’t Veer A, Du Y, Fischer TZ, Boetig DR, Wood MR, & Dreyfus CF (2009). Brain-derived neurotrophic factor effects on oligodendrocyte progenitors of the basal forebrain are mediated through trkB and the MAP kinase pathway. Journal of Neuroscience Research, 87(1), 69–78. 10.1002/jnr.21841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl SE, McLane LE, Bercury KK, Macklin WB, & Wood TL (2014). Mammalian target of rapamycin promotes oligodendrocyte differentiation, initiation and extent of CNS myelination. Journal of Neuroscience, 34(13), 4453–4465. 10.1523/JNEUROSCI.4311-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch HC, Coadwell WJ, Ellson CD, Ferguson GJ, Andrews SR, Erdjument-Bromage H, … Stephens LR (2002). P-Rex1, a PtdIns (3,4,5)P3-and Gb-regulated guanine-nucleotide exchange factor for Rac. Cell, 108(6), 809–821. [DOI] [PubMed] [Google Scholar]
- Weng QP, Kozlowski M, Belham C, Zhang A, Comb MJ, & Avruch J (1998). Regulation of the p70 S6 kinase by phosphorylation in vivo. Analysis using site-specific antiphosphopeptide antibodies. Journal of Biological Chemistry, 273(26), 16621–16629. [DOI] [PubMed] [Google Scholar]
- Wong AW, Xiao J, Kemper D, Kilpatrick TJ, & Murray SS (2013). Oligodendroglial expression of TrkB independently regulates myelination and progenitor cell proliferation. Journal of Neuroscience, 33(11), 4947–4957. 10.1523/JNEUROSCI.3990-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood TL, Bercury KK, Cifelli SE, Mursch LE, Min J, Dai J, & Macklin WB (2013). mTOR: A link from the extracellular milieu to transcriptional regulation of oligodendrocyte development. ASN Neuro, 5(1), 63–79. 10.1042/AN20120092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao J, Ferner AH, Wong AW, Denham M, Kilpatrick TJ, & Murray SS (2012). Extracellular signal-regulated kinase 1/2 signaling promotes oligodendrocyte myelination in vitro. Journal of Neurochemistry, 122(6), 1167–1180. 10.1111/j.1471-4159.2012.07871.x [DOI] [PubMed] [Google Scholar]
- Yim SH, Hammer JA, & Quarles RH (2001). Differences in signal transduction pathways by which platelet-derived and fibroblast growth factors activate extracellular signal regulated kinase in differentiating oligodendrocytes. Journal of Neurochemistry, 76(6), 1925–1934. [DOI] [PubMed] [Google Scholar]
- Younes-Rapozo V, Felgueiras LO, Viana NL, Fierro IM, Barja-Fidalgo C, Manhães AC, & Barradas PC (2009). A role for the MAPK/ERK pathway in oligodendroglial differentiation in vitro: Stage specific effects on cell branching. International Journal of Developmental Neuroscience, 27(8), 757–768. 10.1016/j.ijdevneu.2009.08.014 [DOI] [PubMed] [Google Scholar]
- Zou Y, Jiang W, Wang J, Li Z, Zhang J, Bu J, … Xiao B (2014). Oligodendrocyte precursor cell-intrinsic effect of Rheb1 controls differentiation and mediates mTORC1-dependent myelination in brain. Journal of Neuroscience, 34(47), 15764–15778. 10.1523/JNEUROSCI.2267-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.