Abstract
Background:
The most appropriate treatment for early-stage hepatocellular carcinoma (HCC) remains unclear. This study compared the association of resection versus ablation with overall survival (OS) in patients with early-stage HCC.
Methods:
Using the National Cancer Database (NCDB), patients diagnosed with stage I/II HCC between 2004–2014 were identified. Cox analysis was used to determine predictors of OS.
Results:
We identified 53,161 patients, of whom 15.9% underwent ablation and 14.5% underwent resection. Patients with fewer comorbidities, larger tumors, and private insurance were more likely to undergo resection. Resection was associated with significantly improved OS compared to ablation (HR 0.58, 95% CI 0.54–0.61, p<0.001), at all tumor sizes (p<0.05) and any degree of liver fibrosis (p<0.05).
Conclusions:
Resection of HCC tumors of all sizes and any degree of underlying fibrosis was associated with significantly improved OS compared with ablation. There was pronounced variability in the use of ablation versus resection for early-stage HCC.
Keywords: Hepatocellular carcinoma, resection, ablation, survival, NCDB
SUMMARY
This study found that patients with early-stage hepatocellular carcinoma (HCC) have improved overall survival (OS) after surgical resection, compared to ablation, at all tumor sizes and any extent of liver disease. There were also marked variations in treatment patterns for early-stage HCC.
INTRODUCTION
Hepatocellular carcinoma (HCC) is the most common primary malignancy of the liver and the fourth leading cause of cancer-related death in the world.1 Surveillance of high-risk patients, such as those with hepatitis B, hepatitis C, and cirrhosis of other etiologies, is critical to detecting HCC early while curative therapies are feasible.2 Curative therapies for early-stage HCC include local therapies (such as ablation), surgical resection, and transplantation.3 For tumors that fit the Milan criteria and in patients with underlying cirrhosis, transplantation is frequently considered, although this is limited by the scarcity of donor grafts.4, 5
Between ablation and resection, it is unclear which is the most appropriate first-line treatment for early-stage HCC. While ablation was initially only offered to patients who were not surgical candidates, or as a bridge to transplantation, it is increasingly being utilized as a curative treatment particularly for smaller tumors.3, 6, 7
Several retrospective analyses have demonstrated improved survival in patients with small tumors who undergo resection compared to ablation.8, 9 However, five randomized-controlled trials (RCT’s) comparing ablation and resection have produced conflicting results.10–14 Therefore, expert opinion remains divided as to the most appropriate therapy, thus contributing to broad practice variation among treatment centers across the United States.
The primary objective of this study was to utilize the National Cancer Database (NCDB) to compare the association of resection versus ablation with overall survival (OS) in patients with early-stage HCC. We hypothesized that resection would be associated with significant improvements in OS compared to ablation. Secondary objectives included identification of predictors of undergoing surgical resection, rather than ablation, in patients with early-stage HCC.
METHODS
Study Design
We conducted a retrospective analysis of patients with early-stage HCC diagnosed between 2004–2014 in the NCDB. The NCDB is a nationwide, facility-based, dataset that captures 70% of all newly diagnosed malignancies in the United States. It is a joint project of the Commission on Cancer (CoC) of the American College of Surgeons and the American Cancer Society. The diagnosis of HCC was confirmed based on histology codes. Only patients with clinical stage I or II HCC were included, based on American Joint Commission on Cancer (AJCC) 7th edition. To identify only patients who underwent ablations with curative intent rather than as a bridge to liver transplantation, patients who underwent transplantation were excluded. This study was exempt from Institutional Review Board review due to the deidentified nature of the database.
Variable Definitions
The NCDB includes basic demographic and clinical characteristics, including age, sex, race, and Charlson/Deyo Comorbidity Score (CDCC).15 It is notable for its thorough collection of socioeconomic data, including insurance status (patient’s primary insurance carrier at the time of initial diagnosis and/or treatment), income level (median household income for each patient’s zip code between 2008–2012), and education level (percent of adults in the patient’s zip code who did not graduate from high school between 2008–2012). The NCDB additionally captures facility-level characteristics, including facility type (category classification by the CoC accreditation program, which includes community cancer programs, comprehensive community cancer programs, academic/research programs, and integrated network cancer programs) and facility region (New England/Mid Atlantic, South Atlantic, North Central, South Central, Mountain, and Pacific).
Tumor size was defined as the largest dimension of the diameter of the primary tumor in centimeters. The Ishak fibrosis score was used for subset analysis to define degree of liver fibrosis.16 The database dichotomizes patients as either F0 (scores of 0–4, indicating none to moderate fibrosis) or F1 (scores of 5–6, indicating severe fibrosis or cirrhosis).
Ablation was defined as thermal ablation of the tumor, including radiofrequency ablation (RFA) or microwave ablation (MWA). Surgical resection was defined as any type of hepatectomy. If a patient underwent multiple procedures, the registry documented the most invasive procedure and/or the cumulative effect of all primary site operations. Other treatment-related variables included days between diagnosis and procedure, length of stay (LOS) after the procedure, and unplanned readmission within 30 days after the procedure. The primary outcome was OS, defined as months from diagnosis to death.
Statistical Analysis
Variables were summarized as mean with standard error of the mean (SEM) or count with percentage. Categorical variables were compared with the Pearson’s chi-squared test. Continuous variables were compared with the 2-sample t-test. Multivariable logistic regression was used to adjust for potential confounders. Kaplan-Meier curves and Cox proportional hazard analysis were used to analyze OS. Results of the logistic regression and Cox analysis were reported as odds ratios (OR) and hazard ratios (HR), respectively, with corresponding 95% confidence intervals (CI) and p-values. Subset analysis was performed to assess the difference in differences, by comparing the hazard ratios and 95% CI from the multivariable Cox proportional hazard models in different patient subpopulations.
All statistical analyses were performed using Stata software, version SE 14.0 (StataCorp, College Station, TX, USA). All tests were 2-sided and statistical significance was accepted at the p<0.05 level.
RESULTS
We identified 59,964 patients diagnosed with stage I or II HCC between 2004–2014 of whom 6,803 (11.3%) underwent liver transplantation and were excluded from the analysis. Thus, our final cohort included 53,161 patients. Of those patients, 8,433 (15.9%) patients underwent ablation, 7,697 (14.5%) underwent surgical resection, and 37,031 (69.7%) underwent neither resection nor ablation. Fibrosis scores were available for 12,687 (23.9%) patients, of whom 2,897 (22.8%) were F0 and 9,790 (77.2%) were F1. Due to the large number of missing fibrosis scores, this variable was only used in supplemental multivariable models (Appendices A and B) and subset analysis. Mean (SEM) follow-up time was 23.8 (0.11) months for all patients in the cohort.
Characteristics Associated with Surgical Resection versus Ablation
Univariate analysis demonstrated baseline differences between patients who underwent ablation versus those who underwent surgical resection (Table 1). Patients who underwent hepatectomy were more likely to be >65 years old (p<0.001), be of Asian race (p<0.001), have fewer comorbidities (p<0.001), and have private insurance (p<0.001). More patients at community cancer programs underwent resection, while patients treated at academic and research programs tended to undergo tumor ablation (p<0.001). Patients in the South Atlantic region were more likely to undergo surgical resection, while patients in the Pacific region were more likely to undergo ablation (p<0.001).
Table 1.
Characteristics of patients with early-stage hepatocellular carcinoma who underwent surgical resection or ablation.
| Characteristic | Ablation (n = 8,433) |
Surgical Resection (n = 7,697) |
P-value |
|---|---|---|---|
| Age >65 years old | 3,244 (38.5%) | 3,515 (45.7%) | <0.001 |
| Male | 6,139 (72.8%) | 5,445 (70.7%) | 0.004 |
| Race | <0.001 | ||
| Charlson/Deyo Comorbidity Score | <0.001 | ||
| Fibrosis score | <0.001 | ||
| Tumor size | <0.001 | ||
| Insurance status | <0.001 | ||
| Median income quartiles (by zip code) | 0.001 | ||
| Education level* | 0.10 | ||
| Facility type | <0.001 | ||
| Facility region | <0.001 | ||
| Distance between patient zip code and hospital | 0.82 | ||
| Year of diagnosis | 0.043 | ||
| >30 days between diagnosis and procedure | 6,191 (73.4%) | 4,558 (59.2%) | <0.001 |
| LOS after procedure (days, mean) | 2.12 | 7.19 | <0.001 |
| 30-day unplanned readmissions | 189 (2.3%) | 382 (5.1%) | <0.001 |
CDCC: Charlson/Deyo Comorbidity Score; LOS: length of stay
Percent of region without high school degree
Tumors <3 cm in size were more likely to be treated with ablation (58.8% vs 25.0%), while tumors >5 cm were more likely to undergo resection (6.5% vs 40.3%) (p<0.001). Among patients who had documented fibrosis scores, patients with severe fibrosis or cirrhosis were less likely to undergo resection (83.7% vs 40.8%, p<0.001). Less time elapsed between diagnosis and resection than between diagnosis and ablation (53.9 days vs 74.2 days, p<0.001). Patients who underwent an operation had longer LOS than those who underwent ablative procedures (7.2 days vs 2.1 days, p<0.001) and were more likely to have an unplanned readmission within 30 days (5.1% vs 2.3%, p<0.001).
Independent Predictors of Undergoing Surgical Resection
On multivariable analysis, independent predictors of undergoing resection included age >65 years old (p<0.001), and black and Asian race (p<0.001) (Table 2). Hispanic race (p<0.001), CDCC scores ≥2 (p<0.001), and Medicare and Medicaid insurance (p<0.001) were associated with decreased likelihood of having an operation. Patients with tumors between 3–5 cm and >5 cm were more likely to undergo an operation than those with tumors <3 cm in diameter (OR 2.39, 95% CI 2.21–2.60, p<0.001 and OR 13.5, 95% CI 12.1–15.2, p<0.001, respectively).
Table 2.
Multivariable analysis of independent predictors of surgical resection (versus ablation) in patients with early-stage hepatocellular carcinoma.
| Variable | Odds Ratio | 95% CI | P-value |
|---|---|---|---|
| Age >65 years old | 1.19 | 1.08 – 1.30 | <0.001 |
| Male | 0.96 | 0.88 – 1.05 | 0.36 |
| Race | |||
| Charlson/Deyo Comorbidity Score | |||
| Tumor size | |||
| Insurance status | |||
| Median income quartiles (by zip code) | |||
| Facility type | |||
| Facility region | |||
| Year of diagnosis | |||
CI: confidence interval; CDCC: Charlson/Deyo Comorbidity Score
In terms of facility type, patients treated at integrated network cancer programs and academic or research programs were more likely to undergo ablation compared to community cancer programs (p<0.001 and p=0.002, respectively). Receiving care in the South Atlantic region (p=0.004) and North Central region (p=0.004) was associated with increased likelihood of undergoing resection, while being treated in the Pacific region was associated with decreased likelihood of having an operation (p<0.001), compared to receiving care in New England or the Mid Atlantic. Patients diagnosed later, between 2010–2014, were more likely to undergo surgical resection (p=0.001).
Of note, among patients in our cohort with fibrosis data, most correlations remained significant after adding fibrosis score to the multivariable model (Appendix A). Patients with severe fibrosis or cirrhosis were significantly less likely to undergo resection (OR 0.20, 95% CI 0.17–0.24, p<0.001) on adjusted analysis.
Analysis of Overall Survival
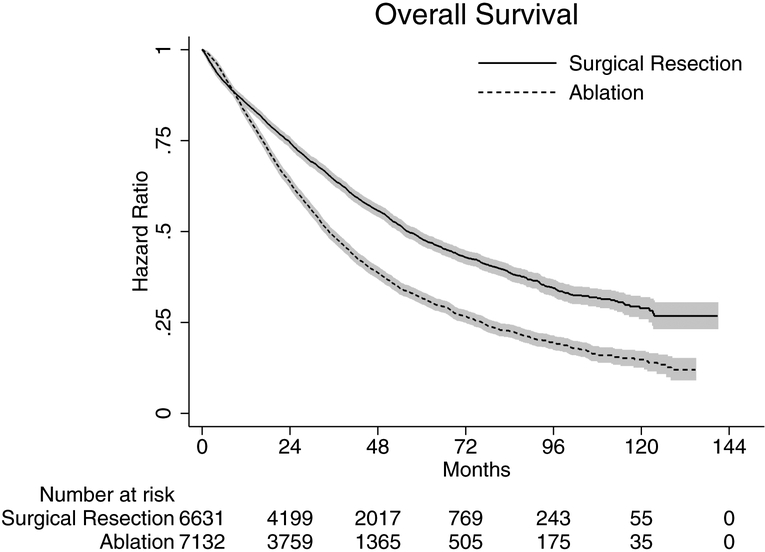
On unadjusted analysis, rates of 1-year and 5-year OS were 82.3% and 31.7% in those who underwent ablation and 85.3% and 48.0% in those who underwent surgical resection, respectively. At both time-points, resection was associated with a statistically significant improvement in OS when compared to ablation (p<0.05) (Figure 1).
Figure 1.
Kaplan-Meier curves depicting overall survival (OS) in patients with early stage hepatocellular carcinoma by type of treatment.
On multivariable Cox proportional hazard analysis, resection was significantly associated with decreased mortality compared to ablation (HR 0.58, 95% CI 0.54–0.61, p<0.001) (Table 3). Poor prognostic factors included age >65 years old (p=0.002), male sex (p=0.009), more comorbidities (p<0.001), larger tumor size (p<0.001), Medicare or Medicaid insurance (p<0.001), and lower income (p<0.001). Factors significantly associated with improved survival were Hispanic or Asian race (p<0.001), being diagnosed in the second half of the study period (p<0.001), and being treated at a comprehensive community cancer program, academic/research program, or integrated network cancer program (all p<0.05).
Table 3.
Multivariable Cox proportional hazard analysis of independent predictors of mortality in patients with early-stage hepatocellular carcinoma.
| Variable | Hazard Ratio | 95% CI | P-value |
|---|---|---|---|
| Type of intervention | |||
| Age >65 years old | 1.11 | 1.04 – 1.18 | 0.002 |
| Male | 1.08 | 1.02 – 1.14 | 0.009 |
| Race | |||
| Charlson/Deyo Comorbidity Score | |||
| Tumor size | |||
| Insurance status | |||
| Median income quartiles (by zip code) | |||
| Facility type | |||
| Facility region | |||
| Year of diagnosis | |||
CI: confidence interval; CDCC: Charlson/Deyo Comorbidity Score
Of note, among patients who had available fibrosis data, adding fibrosis score to the multivariable Cox model once again did not alter the trends in OS (Appendix B). Severe fibrosis or cirrhosis was an independent predictor of worse OS (HR 1.38, 95% CI 1.22–1.57, p<0.001). Resection remained associated with improved survival compared to ablation (HR 0.64, 95% CI 0.56–0.72, p<0.001).
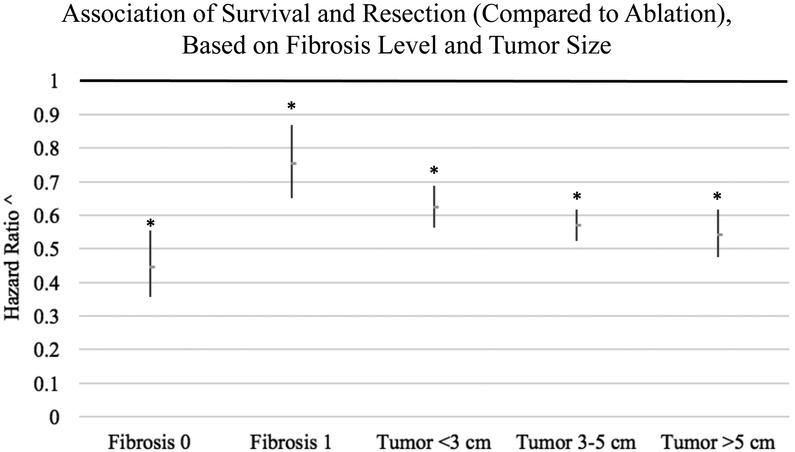
Overall Survival Subset Analysis Based on Tumor Size and Fibrosis Score
Subset analysis of the ‘difference in differences’ revealed that resection was associated with improved OS compared to ablation regardless of tumor size or fibrosis score. Subset analysis based on tumor size demonstrated that resection was associated with significantly improved OS compared to ablation at all tumor sizes investigated (<3 cm, 3–5 cm, and >5 cm) (p<0.05) (Figure 2). Even in patients with small tumors <3 cm, resection was associated with improved OS compared to ablation (HR 0.62, 95% CI 0.56–0.69, p<0.001). Additional subset analysis demonstrated that resection was associated with significantly improved OS compared to ablation irrespective of the extent of liver fibrosis (p<0.05) (Figure 2). Even patients with severe fibrosis or cirrhosis (fibrosis score F1) who underwent surgical resection had significantly improved OS compared to ablation (HR 0.75, 95% CI 0.65–0.87, p<0.001).
Figure 2.
Subset analysis of the difference in differences in the association of surgical resection (compared to ablation) with overall survival (OS) in patients with early-stage HCC, based on extent of liver fibrosis and tumor size.
Fibrosis 0, none to moderate fibrosis; Fibrosis 1, severe fibrosis or cirrhosis
* p<0.05
^ Hazard ratios obtained from multivariable Cox models of overall survival (OS) comparing surgical resection to ablative therapy (reference group). 95% confidence intervals represented by length of dash. Hazard ratios <1 indicate improved survival associated with resection, compared to ablation.
DISCUSSION
This retrospective study using a large national database demonstrates a significant survival advantage associated with surgical resection, compared to ablation, in patients with early-stage HCC. Even after adjusting for factors such as age and degree of comorbidities, this association held true at all tumor sizes investigated and regardless of the extent of liver fibrosis, which are novel findings compared with prior analyses evaluating surgical resection with ablation in the treatment of early-stage HCC.
Interestingly, of the 59,964 patients in the NCDB with early-stage HCC, only 6,803 (11.3%) patients underwent liver transplantation (excluded from the analysis), 7,697 (14.5%) underwent resection, and 8,433 (15.9%) patients were ablated. Although the dataset does not identify whether all these tumors were resectable or amenable to ablation, one would expect a greater proportion of early-stage tumors to undergo definitive, curative-intent, management. Our findings are consistent with studies based on other large databases,17–19 and demonstrate that curative-intent therapies for early-stage HCC, resection in particular, appear to be vastly under-utilized. Further standardization of treatment of early-stage HCC in the United States may increase the proportion of patients undergoing curative-intent therapies.
The reasons for the under-utilization of surgical resections are complex, and likely only partially captured by large database analyses. Our multivariable analysis of independent predictors of resection suggest that patients with fewer comorbidities and larger tumors are undergoing surgical resection, as would be expected. However, the dataset additionally revealed stark disparities associated with race and insurance status. Large differences were also noted among different facility types, in addition to significant regional variations. These findings suggest that treatment decisions may be based on healthcare access, facility-level capabilities, and provider preference or experience. Such wide variations underscore the need for stronger guidelines, regionalization of care, discussion of all cases in multidisciplinary settings, and standardization of treatment algorithms to provide appropriate, more uniform, curative-intent therapies for patients with early-stage HCC.
The debate about whether resection or ablation should be first-line therapy for early-stage HCC has intensified over the years. While resection was traditionally considered superior to ablation, therapies such as MWA are proving effective for progressively larger lesions with fewer complications, lower costs, and shorter LOS.3, 10, 20–25 However, while some retrospective analyses have demonstrated equivalence in OS between resection and ablation in small tumors,7, 21, 22, 25, 26 others have found significantly worse OS and recurrence-free survival in patients who undergo ablative therapies.8, 9, 27–29
To-date, five pivotal RCTs have provided conflicting results, likely due to small sample sizes and patient heterogeneity (in terms of degree of underlying cirrhosis and tumor size).10–14 Furthermore, these were all performed in Asian centers where most patients developed HCC in the context of chronic hepatitis B. Therefore, these findings may not be readily generalizable to Western populations, where HCC tends to arise in the setting of hepatitis C, alcoholic cirrhosis, and, most recently, fatty liver disease.
Our findings are consistent with those reported by Miura and colleagues, who also found that resection of HCC was associated with improved OS compared to ablation.9 However, their study was limited to patients with T1 tumors that were smaller than 3 cm in size and who were diagnosed before 2011. As such, only 2,804 patients were included in their analysis. Our updated analysis of the NCDB with larger tumor sizes included in subset ‘difference in differences’ analysis provides a novel component that demonstrates the potential superiority of surgical resection over ablation at all tumor sizes, and even in patients with severe fibrosis or cirrhosis. These findings provide the impetus for the need for a Western-based randomized prospective trial.
In addition to its retrospective nature, our study has some limitations. First, despite adjusting for many variables, other confounding factors may be contributing to why patients underwent ablation or resection, such as tumor resectability, operative patient candidacy, or other measures of underlying liver function. Second, it is possible that some patients underwent ablation prior to resection, but were only recorded as having undergone resection. The database does not distinguish between curative-intent treatment versus bridge to transplantation. Some patients may, therefore, have been awaiting transplantation at the time of data collection. Finally, the NCDB only records OS, not disease-free or disease-specific survival.
CONCLUSIONS
This study demonstrates that surgical resection is associated with significant improvements in OS compared to ablation in patients with early-stage HCC, at all tumor sizes. In the limited subset of patients for whom liver fibrosis data is available, the association of resection with improved survival also holds true for any degree of liver fibrosis. In addition, there is marked variation in the use of ablation and resection among different populations and across the United States, and both therapies appear to be drastically under-utilized. Given our findings are based on retrospective analysis, a Western population-based trial may be warranted to investigate the primary objective of this study and provide a more standardized approach in the treatment of patients with early-stage HCC who are not being considered for transplantation.
Supplementary Material
HIGHLIGHTS.
Stage I/II HCC: resection associated with improved survival compared to ablation.
This is true for all tumor sizes and all degrees of liver fibrosis.
There is pronounced variability in the use of ablation versus resection.
ACKNOWLEDGMENTS
GCL was supported by the NIH T32 Research Training in Alimentary Tract Surgery grant DK007754–13. The NIH had no involvement in study design; collection, analysis, or interpretation of data; writing of the report; or decision to submit the article for publication.
Appendix A. Multivariable analysis of independent predictors of surgical resection (versus ablation) in patients with early-stage hepatocellular carcinoma with available fibrosis scores.
| Variable | Odds Ratio | 95% CI | P-value |
|---|---|---|---|
| Age >65 years old | 1.14 | 0.95 – 1.39 | 0.17 |
| Male | 0.96 | 0.81 – 1.14 | 0.66 |
| Race | |||
| Charlson/Deyo Comorbidity Score | |||
| Fibrosis score | |||
| Tumor size | |||
| Insurance status | |||
| Median income quartiles (by zip code) | |||
| Facility type | |||
| Facility region | |||
| Year of diagnosis | |||
CI: confidence interval; CDCC: Charlson/Deyo Comorbidity Score
Appendix B. Multivariable Cox proportional hazard analysis of independent predictors of mortality in patients with early-stage hepatocellular carcinoma with available fibrosis scores.
| Variable | Hazard Ratio | 95% CI | P-value |
|---|---|---|---|
| Type of intervention | |||
| Age >65 years old | 1.07 | 0.94 – 1.21 | 0.34 |
| Male | 1.05 | 0.94 – 1.18 | 0.38 |
| Race | |||
| Charlson/Deyo Comorbidity Score | |||
| Fibrosis score | |||
| Tumor size | |||
| Insurance status | |||
| Median income quartiles (by zip code) | |||
| Facility type | |||
| Facility region | |||
| Year of diagnosis | |||
CI: confidence interval; CDCC: Charlson/Deyo Comorbidity Score
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Presented (oral presentation) at the Society of Surgical Oncology International Symposium on Regional Cancer Therapies, Jacksonville, FL, February 17–19, 2018.
CONFLICT OF INTEREST STATEMENT
Declarations of interest: None.
REFERENCES
- 1.Global Burden of Disease Liver Cancer C. The Burden of Primary Liver Cancer and Underlying Etiologies From 1990 to 2015 at the Global, Regional, and National Level: Results From the Global Burden of Disease Study 2015. JAMA Oncol, 2017;3:1683–1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tseng PL, Wang JH, Tung HD, et al. Optimal treatment increased survival of hepatocellular carcinoma patients detected with community-based screening. J Gastroenterol Hepatol, 2010;25:1426–1434. [DOI] [PubMed] [Google Scholar]
- 3.de Lope CR, Tremosini S, Forner A, et al. Management of HCC. J Hepatol, 2012;56 Suppl 1:S75–87. [DOI] [PubMed] [Google Scholar]
- 4.Liu JB, Baker TB, Suss NR, et al. Orthotopic liver transplantation provides a survival advantage compared with resection in patients with hepatocellular carcinoma and preserved liver function. Surgery, 2017;162:1032–1039. [DOI] [PubMed] [Google Scholar]
- 5.Colella G, Bottelli R, De Carlis L, et al. Hepatocellular carcinoma: comparison between liver transplantation, resective surgery, ethanol injection, and chemoembolization. Transpl Int, 1998;11 Suppl 1:S193–196. [DOI] [PubMed] [Google Scholar]
- 6.Nault JC, Sutter O, Nahon P, et al. Percutaneous treatment of hepatocellular carcinoma: State of the art and innovations. J Hepatol, 2017. [DOI] [PubMed] [Google Scholar]
- 7.Livraghi T, Meloni F, Di Stasi M, et al. Sustained complete response and complications rates after radiofrequency ablation of very early hepatocellular carcinoma in cirrhosis: Is resection still the treatment of choice? Hepatology, 2008;47:82–89. [DOI] [PubMed] [Google Scholar]
- 8.Liu PH, Hsu CY, Hsia CY, et al. Surgical Resection Versus Radiofrequency Ablation for Single Hepatocellular Carcinoma </= 2 cm in a Propensity Score Model. Ann Surg, 2016;263:538–545. [DOI] [PubMed] [Google Scholar]
- 9.Miura JT, Johnston FM, Tsai S, et al. Surgical resection versus ablation for hepatocellular carcinoma </= 3 cm: a population-based analysis. HPB (Oxford), 2015;17:896–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feng K, Yan J, Li X, et al. A randomized controlled trial of radiofrequency ablation and surgical resection in the treatment of small hepatocellular carcinoma. J Hepatol, 2012;57:794–802. [DOI] [PubMed] [Google Scholar]
- 11.Huang J, Yan L, Cheng Z, et al. A randomized trial comparing radiofrequency ablation and surgical resection for HCC conforming to the Milan criteria. Ann Surg, 2010;252:903–912. [DOI] [PubMed] [Google Scholar]
- 12.Chen MS, Li JQ, Zheng Y, et al. A prospective randomized trial comparing percutaneous local ablative therapy and partial hepatectomy for small hepatocellular carcinoma. Ann Surg, 2006;243:321–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee HW, Lee JM, Yoon JH, et al. A prospective randomized study comparing radiofrequency ablation and hepatic resection for hepatocellular carcinoma. Ann Surg Treat Res, 2018;94:74–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fang Y, Chen W, Liang X, et al. Comparison of long-term effectiveness and complications of radiofrequency ablation with hepatectomy for small hepatocellular carcinoma. J Gastroenterol Hepatol, 2014;29:193–200. [DOI] [PubMed] [Google Scholar]
- 15.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol, 1992;45:613–619. [DOI] [PubMed] [Google Scholar]
- 16.Ishak K, Baptista A, Bianchi L, et al. Histological grading and staging of chronic hepatitis. J Hepatol, 1995;22:696–699. [DOI] [PubMed] [Google Scholar]
- 17.Sobotka L, Hinton A, Conteh L. Women receive more inpatient resections and ablations for hepatocellular carcinoma than men. World J Hepatol, 2017;9:1346–1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang J, Ha J, Lopez A, et al. Medicaid and Uninsured Hepatocellular Carcinoma Patients Have More Advanced Tumor Stage and Are Less Likely to Receive Treatment. J Clin Gastroenterol, 2018;52:437–443. [DOI] [PubMed] [Google Scholar]
- 19.Peters NA, Javed AA, He J, et al. Association of socioeconomics, surgical therapy, and survival of early stage hepatocellular carcinoma. J Surg Res, 2017;210:253–260. [DOI] [PubMed] [Google Scholar]
- 20.Bruix J, Sherman M, American Association for the Study of Liver D. Management of hepatocellular carcinoma: an update. Hepatology, 2011;53:1020–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cho YK, Rhim H, Noh S. Radiofrequency ablation versus surgical resection as primary treatment of hepatocellular carcinoma meeting the Milan criteria: a systematic review. J Gastroenterol Hepatol, 2011;26:1354–1360. [DOI] [PubMed] [Google Scholar]
- 22.Peng ZW, Lin XJ, Zhang YJ, et al. Radiofrequency ablation versus hepatic resection for the treatment of hepatocellular carcinomas 2 cm or smaller: a retrospective comparative study. Radiology, 2012;262:1022–1033. [DOI] [PubMed] [Google Scholar]
- 23.Lin SM. Local ablation for hepatocellular carcinoma in taiwan. Liver Cancer, 2013;2:73–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cucchetti A, Piscaglia F, Cescon M, et al. Cost-effectiveness of hepatic resection versus percutaneous radiofrequency ablation for early hepatocellular carcinoma. J Hepatol, 2013;59:300–307. [DOI] [PubMed] [Google Scholar]
- 25.Pompili M, Saviano A, de Matthaeis N, et al. Long-term effectiveness of resection and radiofrequency ablation for single hepatocellular carcinoma </=3 cm. Results of a multicenter Italian survey. J Hepatol, 2013;59:89–97. [DOI] [PubMed] [Google Scholar]
- 26.Hong SN, Lee SY, Choi MS, et al. Comparing the outcomes of radiofrequency ablation and surgery in patients with a single small hepatocellular carcinoma and well-preserved hepatic function. J Clin Gastroenterol, 2005;39:247–252. [DOI] [PubMed] [Google Scholar]
- 27.Ueno S, Sakoda M, Kubo F, et al. Surgical resection versus radiofrequency ablation for small hepatocellular carcinomas within the Milan criteria. J Hepatobiliary Pancreat Surg, 2009;16:359–366. [DOI] [PubMed] [Google Scholar]
- 28.Feng Q, Chi Y, Liu Y, et al. Efficacy and safety of percutaneous radiofrequency ablation versus surgical resection for small hepatocellular carcinoma: a meta-analysis of 23 studies. J Cancer Res Clin Oncol, 2015;141:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hasegawa K, Kokudo N, Makuuchi M, et al. Comparison of resection and ablation for hepatocellular carcinoma: a cohort study based on a Japanese nationwide survey. J Hepatol, 2013;58:724–729. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.