Abstract
Background
Exposure to traffic-related air pollution (TRAP) has been linked to childhood anxiety symptoms. Neuroimaging in patients with anxiety disorders indicate altered neurochemistry.
Objectives
Evaluate the impact of TRAP on brain metabolism and its relation to childhood anxiety symptoms in the Cincinnati Childhood Allergy and Air Pollution Study (CCAAPS).
Methods
Adolescents (n=145) underwent magnetic resonance spectroscopy. Brain metabolites, including myo-inositol, N-acetylaspartate, creatine, choline, glutamate, glutamate plus glutamine, and glutathione were measured in the anterior cingulate cortex. Anxiety symptoms were assessed using the Spence Children’s Anxiety Scale. TRAP exposure in early-life, averaged over childhood, and during the 12 months prior to imaging was estimated using a validated land use regression model. Associations between TRAP exposure, brain metabolism, and anxiety symptoms were estimated using linear regression and a bootstrapping approach for testing mediation by brain metabolite levels.
Results
Recent exposure to high levels of TRAP was associated with significant increases in myo-inositol (β = 0.26; 95%CI 0.01, 0.51) compared to low TRAP exposure. Recent elevated TRAP exposure (β = 4.71; 95% CI 0.95, 8.45) and increased myo-inositol levels (β = 2.98; 95% CI 0.43, 5.52) were also significantly associated with increased generalized anxiety symptoms with 12% of the total effect between TRAP and generalized anxiety symptoms being mediated by myo-inositol levels.
Conclusions
This is the first study of children to utilize neuroimaging to link TRAP exposure, metabolite dysregulation in the brain, and generalized anxiety symptoms among otherwise healthy children. TRAP may elicit atypical excitatory neurotransmission and glial inflammatory responses leading to increased metabolite levels and subsequent anxiety symptoms.
Keywords: Air pollution, neuroimaging, adolescents, mental health, metabolites
1. Introduction
Exposure to air pollution is a well-recognized global health problem associated with more than 3.3 million premature deaths annually.(1) Evidence from toxicological and epidemiologic studies suggest the central nervous system is particularly vulnerable to air pollution suggesting they might have a role also in the etiology of mental disorders. (2–5) Anxiety disorders are particularly relevant as these are among the most commonly occurring class of psychiatric disorders with lifetime prevalence of 29% (6) and a pre-adolescent prevalence ranging from 3 to 41%.(7) Childhood anxiety also carries increased risk of later-life anxiety, deviant conduct, substance abuse, and suicide, the second leading cause of death among adolescents and young adults. (8–12) Previous reports in adults and the elderly have demonstrated more recent exposure to ambient fine particulate matter (PM2.5) is associated with increased anxiety symptoms. (13, 14) While cognitive deficits and externalizing behaviors have been observed among children exposed to air pollution,(15–17) its impact on mental health outcomes, and to a lesser extent anxiety, is less clear and inconsistent. Prenatal exposure to polycyclic aromatic hydrocarbons (PAHs), a component of air pollution due to the combustion of fossil fuels, has been associated with increased anxiety in children at age 6–7 years. (18) A London-based twin study did not find evidence of an association between PM2.5 and anxiety symptoms. (19) Among 344 children participating in the Cincinnati Childhood Allergy and Air Pollution Study (CCAAPS), our group has observed significant associations between early-life, childhood, and recent exposure to traffic-related air pollution (TRAP) and symptoms of anxiety at age 12 years.(20)
Potential mechanisms by which air pollution exerts neurotoxic effects have been posited and include indirect pathways via systemic inflammation, production of reactive oxygen species, and direct exposure of pollutants to the brain resulting from translocation of particles to extrapulmonary sites, including the brain via the blood stream and directly via the olfactory nerve.(21–24) Utilizing proton magnetic resonance spectroscopy (MRS), we characterized brain metabolism within the anterior cingulate cortex (ACC), a key region implicated in the neurobiology of mental health disorders and important for executive functioning and regulating emotional processing, among otherwise healthy pre-adolescents. (25–28) Given the recently published findings by our group reporting the main effect of TRAP on anxiety symptoms (20), the primary objective of this study was to assess the mediating role of brain metabolism on the association between TRAP exposure and anxiety symptoms.
2. Methods
2.1. Study Population
Participants were enrolled in the Cincinnati Childhood Allergy and Air Pollution Study (CCAAPS), a previously described prospective birth cohort of infants born (35+ weeks of gestation) between 2001 and 2003 in the Greater Cincinnati Region.(29, 30) Briefly, children residing less than 400 meters or greater than 1500 meters from a major roadway, defined as having greater than 1,000 trucks per day, were eligible for enrollment. Enrolled children and caregivers completed demographic and clinical evaluations at ages 1, 2, 3, 4, 7, and 12 years to obtain information regarding the participant’s health and general wellbeing, housing characteristics, residential history, and secondhand smoke (SHS) exposure. Parents of children enrolled provided informed consent and informed assent was obtained from all children at the age 12 year visit. The Institutional Review Boards at the University of Cincinnati and Cincinnati Children’s Hospital Medical Center approved the study.
2.2. Nested Imaging Study
A nested magnetic resonance imaging (MRI) study was conducted at the age 12 year visit with the objective to examine differences in imaging outcomes among children exposed to high and low TRAP. Of the 762 participants enrolled in the CCAAPS cohort study at birth, 452 met the following eligibility criteria to participate in the nested imaging study: 1) children whose exposures to TRAP (estimated as described below) from birth through age one were in the highest and lowest quartiles, and 2) children whose birth record address was less than 400 m from a major road (as defined above). The nested study was oversampled for high TRAP exposure (n=262). Attempts were made to recruit all 452 eligible children; however, only 344 children were seen at the age 12 year visit and only 147 were eligible and interested in participating in the nested imaging study (n high trap = 81, n low trap = 66).
2.3. Traffic-Related Air Pollution (TRAP) Exposure Assessment
Estimates of TRAP from birth through age 12 years were derived using a previously developed and validated land-use regression (LUR) model based on 5 years of ambient air sampling for PM25 (PM with an aerodynamic diameter <2.5 microns) and elemental carbon (EC) conducted at 27 sampling sites from 2001–2006.(31–33) The fraction of elemental carbon attributable to traffic (ECAT), a marker of traffic-related particulate matter specifically related to diesel combustion, was determined for each sampling site based on UNMIX and positive matrix factorization (PMF) models and used to develop a land use regression (LUR) model. (31, 32, 34) For this study, we applied the LUR model to estimate time weighted ECAT exposures for each participant based on all reported home addresses where the child resided 1) at birth (early-life), 2) in the 12 months prior to the age 12 year visit (recent), and 3) an average childhood exposure from birth to the age 12 year clinical visit. Further, given the eligibility criteria for the nested MRI study, exposure to ECAT at each time point was modeled as a categorical variable (high vs. low) dichotomized at the 75th percentile of exposures estimated at each time period (early-life, recent, and average childhood EC AT).
2.4. Child-Reported Anxiety Assessment
The Spence Children’s Anxiety Scale (SCAS), a 45-item measure, was provided to the children with standard instructions for completion at the age 12 year visit. Trained staff offered to read the items aloud and provide assistance when needed. (35) Responses are given on a 4-point Likert scale indicative of the frequency of a given symptom- 0= never, 1= sometimes, 2= often, 3=always. The SCAS assesses total anxiety as well as six subdomains, including panic/agoraphobia, social phobia, separation anxiety, obsessive-compulsive disorder, physical injury fears, and generalized anxiety. T-scores, with a mean of 50 and a standard deviation of 10, were generated for each subscale. Higher T-scores represent a greater degree of reported anxiety symptoms.
2.5. Magnetic Resonance Spectroscopy
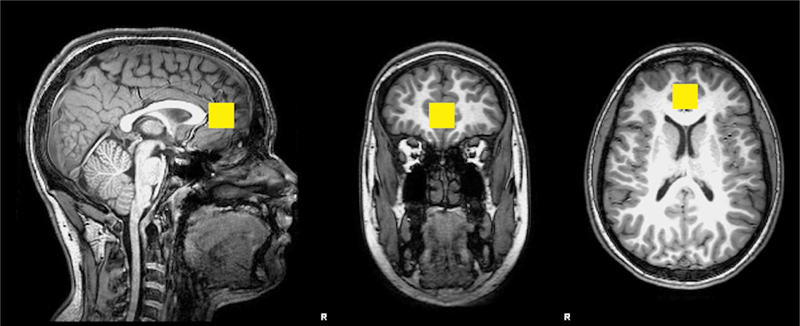
During the age 12 year visit, 147 children underwent quantitative MRS of the brain using a Philips Achieva MR scanner equipped with a 32-channel head coil operating at 3 Tesla (3T). A three-dimensional (3D), high-resolution, isotropic, T1-weighted fast Fourier echo (FFE) imaging sequence was performed using 8.2 milliseconds (ms) repetition time (TR), 3.7 ms echo time (TE), 1057 ms inversion time (TI), 8 degree flip angle, sensitivity encoding factor (SENSE) of 2, contiguous slices with a 1 mm thickness, 1 mm x 1 mm voxel sizes. A single voxel point resolved spectroscopy (PRESS) sequence was conducted using a 3000 ms TR, 30 ms TE, and 128 averages with water suppression along with an embedded unsuppressed water reference series of 16 averages. A 2 × 2 × 2 cubic centimeter single voxel was prescribed midline bilaterally about the perigenual ACC within the medial frontal lobe localized from the 3D T1-FFE anatomical imaging sequence (Figure 1). The primary neurochemicals routinely detected include N-acetyl aspartate (NAA), creatine (Cr), choline (Cho), myo-inositol (mI), glutamate (Glu), glutamate and glutamine (GLX) with the addition of glutathione (GSH).
Figure 1.
Representative location within the perigenual anterior cingulate cortex for the 8 cubic centimeter (2 cm per side) spectroscopic voxel positioned on T1 weighted imaging slices centered in the sagittal, coronal and axial plane orientations.
The raw spectra were imported for quantitative spectral processing into LCModel commercial software.(36). The raw metabolite levels were adjusted for the tissue contributions from gray matter, white matter and cerebrospinal fluid (CSF) using FSL (37, 38), adjusted to the T1 and T2 relaxation decay rate corrected water concentration, and corrected for literature reported T1 and T2 relaxation decay rates of the primary metabolites (NAA, Cr, mI).(39–41) However, secondary metabolite concentrations (Glu, GLX, and GSH) were unadjusted for T1 and T2 relaxation decay. (42) MRS data from two participants was not incorporated into this analysis due to an incomplete MRI examination and poor spectral quality.
2.6. Statistical Analyses
Descriptive statistics were used to examine child, maternal, and household characteristics at enrollment, age 12, and the subset of participants who completed the MRI study. Differences between those in the nested MRI study with adequate MRS data (n=145) and those not partaking in the nested MRI study at age 12 years (n=199) were compared using chi-square and T-tests, as appropriate.
The statistical approach consisted of four planned (a priori) phases and a series of linear regression models. First, linear regression models were used to confirm the relationship between ECAT exposure at three time points (early-life, average childhood, and recent) and each child-reported anxiety scale, modeled separately (Table S1). We focused on the following anxiety subscales significantly associated with ECAT exposure in our larger cohort analysis of 344 CCAAPS participants: generalized anxiety, total anxiety, social phobia, and panic/agoraphobia (Y=α1 + cE+e1).(20) Second, we assessed the association between brain metabolite levels (modeled individually) and child-reported anxiety symptoms focusing on anxiety scales shown to be associated with ECAT in the preceding linear regression models (Y= α3 + c’E+ bM+e3). In this phase, we adjusted for multiple comparisons using the false discovery rate of 0.05. Third, focusing on the ECAT exposures and metabolites found to be significantly associated with the previously listed anxiety subscales, we determined the effect of ECAT exposure (early-life, recent, and/or average childhood exposures) on brain metabolite levels (i.e. potential mediators) (M= α2 + aE + e2). Lastly, we formally tested the potential mediation effect on only those brain metabolites shown to be associated with both ECAT and anxiety scales. The indirect effect of the metabolites was estimated with the product of the coefficients a and b (a*b). Bootstrapping was used to estimate the 95% confidence intervals for the indirect effects through repeated sampling of the data (n=1000); 95% confidence intervals not containing zero were considered significant. (43)
Covariates included in all models were selected based on prior literature demonstrating their relationship with neurobehavioral outcomes or their potential role as a confounder in the relationship between TRAP exposure and neurobehavior. Covariates included child’s race (black/non-black), household income, maternal age at study enrollment, caregiver depression assessed by the Beck Depression Inventory, relational frustration pertaining to the parent-child relationship (assessed by the Parent Relationship Questionnaire), and serum cotinine levels. (44–51) All covariates were assessed at the age 12 year visit with the exception of maternal age and child’s race. Covariates included in the final adjusted models were either significantly (p < 0.05) associated with the outcome or their inclusion resulted in a > 10% change in the ECAT parameter estimate. Current use of anxiety and/or depression medication was considered as a potential covariate given that it might impact brain metabolite levels; however, less than 4% (3.4 % for anxiety medications, 1.36% for depression medications) of the CCAAPS nested MRI participants were currently (at the age 12 year visit) taking medication and thus we did not include current medication use in the statistical models. Statistical analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC).
3. Results
3.1. Characteristics of Participants
Comparisons between demographic, exposure, and other characteristics between CCAAPS participants at enrollment (n = 762), those completing the age 12 year visit (n = 344), and those with MRS (n = 145) are provided in Table 1. Children included in this analysis were, on average, 12.2 years old when completing the visit, the majority were white (73.8%, n = 107), and 41.4% (n=60) were female. On average, mothers were 29.7 years old at the time of birth and 33% (n=48) of families had a total household income of greater than $90,000 (Table 1).
Table 1.
Comparison of characteristics in the CCAAPS cohort at enrollment, year 12 visit, and the nested MRI study
| Enrollment (n = 762) | Year 12 Study Visit (n=344)a | Nested CCAAPS MRI study (n=145) | ||
|---|---|---|---|---|
| Child Characteristics | n (%) or Mean (SD) | n (%) or Mean (SD) | n (%) or Mean (SD) | p-valuec |
| Sex | ||||
| Male | 415 (54.5%) | 191 (55.5%) | 85 (58.6%) | 0.33 |
| Female | 347 (45.5%) | 153 (44.5%) | 60 (41.4%) | |
| Race/Ethnicityb | ||||
| White | 587 (77.0%) | 261 (75.9%) | 107 (73.8%) | 0.44 |
| Black/More than One Race | 175 (23.0%) | 83 (24.1%) | 38 (26.2%) | |
| Birth Weight (lbs) | 7.5 (1.2) | 7.6 (1.2) | 7.5 (1.2) | 0.55 |
| Duration of Breastfeeding (months) | 5.7 (65) | 6.3 (6.9) | 5.7 (7.0) | 0.13 |
| Serum Cotinine (age 12 yr.) in ng/ml | -- | 0.32 (1.33) | 0.45 (1.86) | 0.41 |
| Child-Reported SCAS T-scores | ||||
| Generalized Anxiety T-Score | -- | 48.8 (8.4) | 49.6 (9.1) | 0.57 |
| Total Anxiety T-Score | -- | 48.4 (9.7) | 48.9 (9.8) | 0.79 |
| Obsessive Compulsive T-Score | -- | 50.3 (9.5) | 50.4 (9.9) | 0.82 |
| Panic/Agoraphobia T-Score | -- | 48.8 (9.1) | 48.8 (8.9) | 0.98 |
| Social Phobia T-Score | -- | 49.3 (9.1) | 50.2 (9.0) | 0.65 |
| Caregiver Characteristics | ||||
| Caregiver Depression using BDI-II | -- | 6.4 (4.0) | 6.9 (6.5) | 0.31 |
| Age at Enrollment in Years | 30.0 (5.7) | 30.7 (5.9) | 30.3 (5.6) | 0.22 |
| Education at Age 1 | ||||
| ≤ High School | 185 (24.9%) | 72 (21.6%) | 36 (25.7%) | 0.24 |
| Some College or Trade School | 196 (26.4%) | 94 (28.1%) | 35 (25.0%) | |
| ≥ College Degree | 361 (48.7%) | 168 (50.3%) | 63 (49.3%) | |
| Relational Frustration T-Score | 48.2 (9.5) | 48.8 (9.7) | 0.53 | |
| Household Characteristics | ||||
| Household Income (Baseline Study Visit) | 0.28 | |||
| < $20,000 | 129 (17.5%) | 58 (17.4%) | 30 (21.4%) | |
| $20,000 to < $40,000 | 129 (17.5%) | 54 (16.2%) | 22 (15.7%) | |
| $40,000 to < $70,000 | 210 (28.5%) | 96 (28.8%) | 38 (27.1%) | |
| $70,000 to < $90,000 | 196 (26.6%) | 89 (26.7%) | 38 (27.1%) | |
| > $90,000 | 73 (9.9%) | 36 (10.8%) | 12 (8.6%) | 0.46 |
| ECAT at Age 12 y Visit in μg / m3 | 0.37 (0.12) | 0.38 (0.13) | 0.41 | |
| Early-Life ECAT in μg / m3 | 0.39 (0.13) | 0.39 (0.14) | 0.43 (0.18) | < 0.01 |
| Childhood Average ECAT in μg / m3 | 0.38 (0.10) | 0.39 (0.13) | 0.36 | |
| Brain Metabolite Levels in millimolar (mM) | ||||
| mI | -- | -- | 5.63 (0.63) | |
| NAA | -- | -- | 8.38 (0.92) | |
| Cr | -- | -- | 7.72 (0.57) | |
| Cho | -- | -- | 1.61 (0.18) | |
| Glu | -- | -- | 8.78 (0.73) | |
| GLX | -- | -- | 11.18 (1.41) | |
| GSH | -- | -- | 2.44 (0.35) | |
Due to missing data, the number of children with serum cotinine at age 12 yr. visit (n=300) and nested sample (n=130), Generalized Anxiety T-scores (n=339), and caregivers with Depression BDI scores at age 12 yr. visit and the nested study (n=334 and 142, respectively) are decreased
Reported at study enrollment
Differences in means and proportions were tested using T-tests and Chi-square statistics, respectively, to test for differences in 145 MRI participants at age 12 compared to 199 non-participants. Pearson correlations between early-life, childhood average, and recent ECAT levels were 0.54 (early-life and recent), 0.82 (childhood average and recent), and 0.83 (early-life and childhood average).
Abbreviations: N-acetyl aspartate (NAA), creatine (Cr), choline (Cho), myo-inositol (ml), glutamate (Glu), glutamate and glutamine (GLX) with the addition of glutathione (GSH); standard deviation (SD); elemental carbon attributable to traffic (ECAT); Spence Children’s Anxiety Scale (SCAS).
The average (SD) ECAT concentrations during early-life [0.43 (0.18) μg/m3], at the time of the age 12 year visit [0.38 (0.13) μg/m3], and throughout childhood [0.39 (0.13) μg/m3] can be found in Table 1. Participants in the nested MRI study had higher early-life exposure to ECAT likely due to the eligibility criteria to participate in the MRI sub-study. The majority of participants (n=102, 70%) did not change exposure categories over the 12 years of follow-up with half (n=22) of the remaining participants changing from high (early-life) to low (average childhood or recent) ECAT exposure over the 12 year follow-up. Child-reported anxiety symptoms demonstrate typical distribution patterns with mean T-scores ranging from 48.8 of 50.4 (Table 1). The analytic sample size for adjusted models was 127 due to missing covariate data.
3.2. Exposure to TRAP and Anxiety Symptoms
Children exposed to high ECAT levels at the time of the age 12 year visit (recent exposure) reported significantly more generalized anxiety symptoms (β = 4.71; 95% CI 0.95, 8.45) compared to children exposed to low ECAT. Early-life, childhood average, and recent ECAT exposures were not significantly associated with the remaining anxiety subscales in our nested population (Table S1); however, the direction of effect for other ECAT exposure periods and anxiety subscales was similar to that observed in our larger (n=344) cohort analysis. (20) Further, we conducted a sensitivity analysis on just those participants that did not change exposure categories over the 12 year follow-up (n=93). This resulted in a similar magnitude and direction of effect but only a marginally significant result (p=0.15). Given that the objective of this manuscript is to investigate mediation, the remainder of our analyses focuses solely on recent ECAT exposure and generalized anxiety symptoms using our full analytic sample (n=127).
3.3. Relationship between Brain Metabolites and Generalized Anxiety
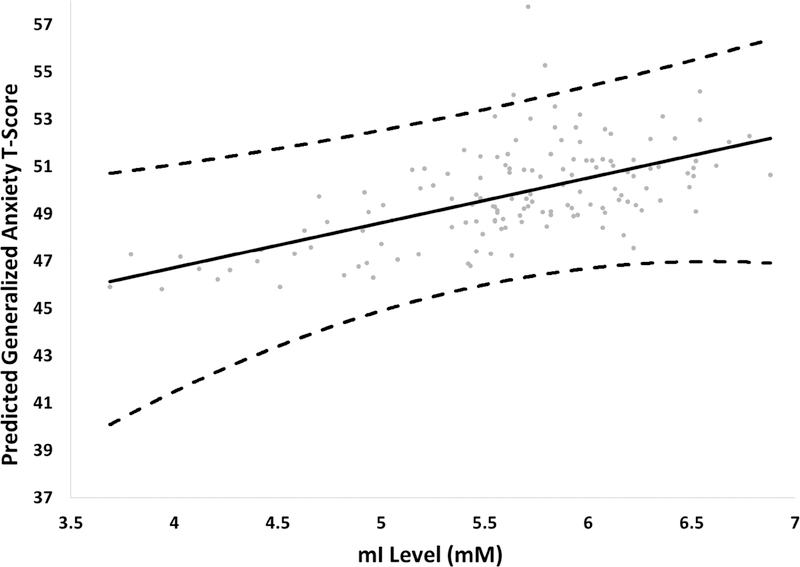
Using FDR correction (Benjamini-Hochberg) for 7 tests, we observed statistically significant (FDR = 05) associations between myo-inositol and generalized anxiety symptoms. Specifically, children reporting a greater degree of generalized anxiety symptoms exhibited elevated ml levels (β = 2.98; 95% CI 0.43, 5.52) (Figure 2). The remaining metabolites were not significantly associated with generalized anxiety symptoms (Table 2) nor were myo-inositol levels associated with other anxiety outcomes (Table S2). Thus, the remainder of our analyses focuses solely on recent ECAT exposure, myo-inositol levels, and generalized anxiety symptoms.
Figure 2. Relationship between myo-inositol (mI) levels and child-reported generalized anxiety T-scores.
Analysis is adjusted for child’s race, total family income (yr. 12), maternal age at enrollment, maternal depression (yr. 12), serum cotinine (yr. 12), and PRQ relational frustration (yr. 12). Dashed lines represent the 95% confidence intervals.
Table 2.
Effect of Brain Metabolite Levels on Child-Reported Generalized Anxiety Symptomsa
| Metabolite (Predictor) | ß | 95% CI | P-value |
|---|---|---|---|
| mI | 2.98 | 0.43, 5.52 | 0.006 |
| NAA | 0.56 | −1.21, 2.32 | 0.54 |
| Cr | 0.67 | −2.13, 3.47 | 0.64 |
| Cho | 1.91 | −7.86, 11.67 | 0.71 |
| Glu | 1.54 | −0.65, 3.74 | 0.17 |
| Glx | −0.31 | −1.42, 0.79 | 0.58 |
| GSH | 0.47 | −4.02, 4.97 | 0.83 |
Models are adjusted for child’s race, total family income (yr. 12), maternal age at enrollment, maternal depression (yr. 12), serum cotinine (yr. 12), and PRQ relational frustration (yr. 12); β represents the estimate for each metabolite (n=127)
3.4. Exposure to TRAP and Brain Metabolites and the Mediating Role of Myo-Inositol
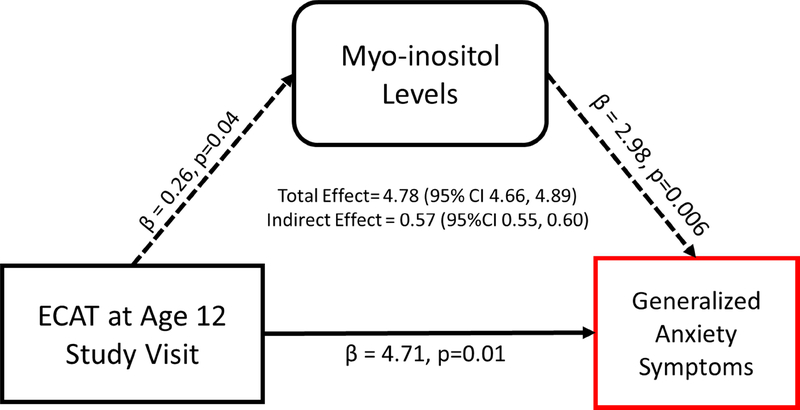
In the ACC, elevated recent ECAT exposure was significantly associated with increased mI (β = 0.26; 95% CI 0.01, 0.51) compared to children with low ECAT exposure (Table S3). In combination with a series of linear regression models outline above and bootstrapping, we identified significant indirect effects operating through mI levels (β = 0.57; 95% CI 0.55, 0.60). Specifically, 12% of the total effect (β = 4.78; 95% CI 4.66, 4.89) between recent ECAT exposure and generalized anxiety symptoms can be explained by mI levels (Figure 3).
Figure 3. Pathway linking recent ECAT exposure to generalized anxiety symptoms.
All models are adjusted for child’s race, total family income (yr. 12), maternal age at enrollment, maternal depression (yr. 12), serum cotinine (yr. 12), and PRQ relational frustration (yr. 12). Coefficients for each path are based on individual linear regression models. Bootstrapping was used to provide confidence intervals for testing indirect effects.
4. Discussion
Adolescents with elevated TRAP exposures in the previous 12 months demonstrate altered brain metabolism with increased mI concentrations measured within the ACC. They also demonstrate greater generalized anxiety symptoms accompanied by elevated mI. The mediation analysis suggests that 12% of the total effect between TRAP and generalized anxiety symptoms can be explained by mI levels. These observed metabolic alterations associated with TRAP may behaviorally manifest in adolescence with increased anxiety symptoms arising as a result of altered neurochemistry.
Neuroinflammation has been posited as a potential mechanism for TRAP-induced neurotoxicity.(52) Systemic inflammatory responses and/or ultrafine particles that reach the brain and result in neurodevelopmental delays are suspected to trigger neuroinflammation with brain damage exerted through many possible mechanisms including oxidative stress, mitochondrial dysfunction and glutamatergic excitotoxicity. (53–55) Specifically, the metals and hydrocarbons that contribute to TRAP’s composition have been shown to exert neuroinflammatory effects with diffuse vascular changes, increased pro-inflammatory cytokine levels [interleukin-lbeta (IL-1β), IL-6, tumor necrosis factor alpha (TNFa)], activation of astrocytes and microglia, and neurite atrophy within the brain.(53, 56–63)
Our study reports three main findings in support of the neuroinflammatory hypothesis. First, we observed increased myo-inositol levels to be associated with elevated recent TRAP exposure. This is an important finding as myo-inositol has links to neuroinflammation and is an important metabolite for many brain processes. Inositol is a natural derivative of glucose found in cellular systems and the most abundant, stable and biologically active stereoisomer is mI.(64) A sustained supply of ml is required for the synthesis of membrane phospholipids. In humans, ml is synthesized in the kidney and brain; however, in neurons the primary source is from recycling of the phosphatidyl inositol-cycle.(65) Increases in mI levels have been described in diseases with marked astrocytic gliosis, microglial activation and brain inflammation.(66) Interestingly, mI accumulates preferentially in astrocytes, which, together with endothelial tight junctions, provide selective permeability of the blood brain barrier (BBB) ultimately increasing the presence of inflammatory markers leading to toxic elevations of intracellular calcium.(67) Further, the transient nature of mI reflects active processes, as opposed to the metabolites (such as NAA, Cr, and Cho) that reflect structural nature of neural systems, supporting our finding with recent rather than early-life and average childhood TRAP exposures.
Second, mI was associated with increased generalized anxiety symptoms (Figure 2). Although the literature is limited, human studies among adults with and without psychiatric disease provide additional support of our findings that altered neurochemistry plays an important role in sub-clinical anxiety symptoms. Modi et al. evaluated 24 healthy, educated young (23.2 + 2.2 years) adults with proton MRS of the ACC and hippocampus.(68) Within the scanner, state anxiety was assessed; outside the scanning environment, trait anxiety levels were assessed using the State-Trait Anxiety Inventory (STAI). Within the ACC, significantly higher mI/Cr and GLX/Cr levels were found among the high anxiety group (STAI total trait score above 70) compared to the low anxiety group, without any group differences for Cr concentrations. Further, many in vivo studies of psychiatric disease states and cognitively normal adults report associations between brain mI levels and markers of inflammation such as plasma, CSF and serum C-reactive protein (CRP) levels.(69, 70) These findings suggest that mI may be related to the severity of anxiety symptoms and raise the possibility that dysregulation of mI within the ACC may be linked to neuroinflammation and ultimately the pathophysiology of childhood anxiety disorders even at a sub-clinical level.
Third, we observed that the effect of TRAP on generalized anxiety was partially mediated through mI levels. Independently, TRAP exposure has been associated with adverse effects on cognitive, behavior, and psychomotor development in children and changes in brain structure and function.(71) Guxens et al. observed that prenatal fine particle exposure was related to alterations in the cerebral cortex of children, and these changes partially mediated the association between fine particle exposure and impaired cognitive function.(72) It is important to keep in mind that the majority of children in our study report normal levels of anxiety symptoms for this age group (i.e. otherwise healthy children). However, it is possible that the small increases in generalized anxiety symptoms paired with ongoing physical, biological, and/or psychological factors might eventually lead to more clinically relevant anxiety symptomology. Given that generalized anxiety disorders are one of the most frequent of all psychiatric disorders seen in primary care, (73) the findings of our study are noteworthy and warrant further investigation as to whether the detected neurochemistry changes resulting from TRAP exposure are causative or the result of distinct anxiety endophenotypes. Nonetheless, to our knowledge, this is the first study to demonstrate through mediation a potential pathway linking TRAP exposure, mI dysregulation in the brain, and generalized anxiety symptoms.
The strengths of our study include a lifetime collection of address history for the estimation of TRAP exposures, a comprehensive assessment of covariates and potential confounders (i.e., SES factors, biomarkers of tobacco smoke exposure, maternal psychological functioning, and details on the parent-child relationship), a thorough assessment of anxiety, and the implementation of MRS. However, we also note limitations. We sampled only one location with proton MRS, the perigenual ACC. This approach allowed for more rigorous quantification, however it reduces comparisons of our results with others along with the functional outcomes localized to other brain regions. For instance, a study of children ages 8 to 12 years in Barcelona did not reveal any association between measures of air pollution, as reflected by elemental carbon and NO2, and brain metabolite levels in the frontal white matter (74). This may reflect the different nature of air pollutants evaluated as well as differences in MRS technique and brain region sampled. In the current study, we selected the ACC due to the significance in cognition and behavioral regulation and previous reports of deposition of TRAP within cortical regions.(53, 75) Further, while we recognize their importance, we were unable to control for other potential residual confounders such as noise from traffic or psychosocial stress resulting from neighborhood conditions. Lastly, the main effect of recent TRAP on generalized anxiety was the only significant finding we were able to replicate among the participants in the nested MRI study and thus the only relationship we could formally test for mediation by brain metabolite levels. The reasons for this are likely multifactorial including reduced power to detect significant results due to a smaller sample size, differences observed in early-life TRAP exposures between the parent and nested study (Table 1), and the nature of myo-inositol and its role in responding to recent exposures and regulating active processes likely associated with current anxiety symptoms.
5. Conclusions
High levels of TRAP exposure are associated with increased mI in the ACC, which in turn is associated with a greater degree of generalized anxiety symptoms. However, we are unable to determine if the finding is a direct result of TRAP constituents depositing within the brain, specifically the ACC. Thus, further studies are needed to determine the mechanism of action for TRAP neurotoxicity (direct/indirect) and whether a change in myo-inositol levels can behaviorally manifest as symptoms of anxiety disorders.
Supplementary Material
Highlights.
Magnetic resonance spectroscopy (MRS) was used to investigate the effects of traffic-related air pollution on brain metabolism and generalized anxiety.
Recent exposure to traffic-related air pollution was associated with increases in myo-inositol; increases in myo-inositol were also associated with increased generalized anxiety symptoms.
These findings suggest traffic pollution may elicit a change in neurochemistry, consistent with neuroinflammation, resulting in increases in anxiety symptoms.
Acknowledgements
We thank the CCAAPS study participants for their time and contribution to this research. The authors acknowledge the contribution of James Leach, MD, in reviewing the images for incidental clinical findings.
Funding Source: Funding for this project was provided by the National Institutes of Environmental Health Sciences (NIEHS) (P30 ES006096, R00 ES024116, R01 ES019890, R01 ES11170, and R01 ES027224) and the National Center for Advancing Translational Sciences (NCATS, UL1 TR001425). The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. The Institutional Review Boards at the University of Cincinnati and Cincinnati Children’s Hospital Medical Center approved the study.
Abbreviations
- TRAP
traffic-related air pollution
- CCAAPS
Cincinnati Childhood Allergy and Air Pollution Study
- MRS
magnetic resonance spectroscopy
- PM
particulate matter
- UFPs
ultrafine particles
- MRI
magnetic resonance imaging
- ACC
anterior cingulate cortex
- SHS
secondhand smoke
- LUR
land-use regression
- ECAT
Elemental carbon attributable to traffic
- SCAS
Spence Children’s Anxiety Scale
- 3T
3 Tesla
- 3D
three-dimensional
- FFE
weighted fast Fourier echo
- ms
milliseconds
- TR
repetition time
- TE
echo time
- TI
inversion time
- SENSE
sensitivity encoding factor
- PRESS
point resolved spectroscopy
- NAA
N-acetyl aspartate
- Cr
creatine
- Cho
choline
- ml
myo-inositol
- Glu
glutamate
- GLX
glutamate and glutamine
- GSH
glutathione
- CSF
cerebrospinal fluid
- PRQ
Parenting Relationship Questionnaire
- PI
phosphatidyl inositol
- nPM
nanoscale particulate matter
- CRP
C-reactive protein
- BBB
blood brain barrier
- IL-1β
interleukin-lbeta
- TNFa
tumor necrosis factor alpha
- GAD
generalized anxiety disorders
- IFN
interferon
- STAI
State-Trait Anxiety Inventory
Footnotes
Potential Conflicts of Interest: The authors have no conflicts of interest relevant to this article to disclose.
Financial Disclosure: The authors have no financial relationships relevant to this article to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lelieveld J, Evans JS, Fnais M, et al. The contribution of outdoor air pollution sources to premature mortality on a global scale. Nature. 2015;525(7569):367–71 [DOI] [PubMed] [Google Scholar]
- 2.Babadjouni RM, Hodis DM, Radwanski R, et al. Clinical effects of air pollution on the central nervous system; a review. J Clin Neurosci. 2017 10.1016/j.jocn.2017.04.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Block ML, Calderon-Garciduenas L. Air pollution: mechanisms of neuroinflammation and CNS disease. Trends Neurosci. 2009;32(9):506–16. 10.1016/j.tins.2009.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Costa LG, Cole TB, Coburn J, et al. Neurotoxicants are in the air: convergence of human, animal, and in vitro studies on the effects of air pollution on the brain. Biomed Res Int. 2014;2014:736385. 10.1155/2014/736385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Costa LG, Cole TB, Coburn J, et al. Neurotoxicity of traffic-related air pollution. Neurotoxicology. 2015 10.1016/j.neuro.2015.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kessler RC, Berglund P, Demler O, et al. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):593–602. 10.1001/archpsyc.62.6.593 [DOI] [PubMed] [Google Scholar]
- 7.Cartwright-Hatton S, McNicol K, Doubleday E. Anxiety in a neglected population: prevalence of anxiety disorders in pre-adolescent children. Clin Psychol Rev. 2006;26(7):817–33. 10.1016/j.cpr.2005.12.002 [DOI] [PubMed] [Google Scholar]
- 8.Heron M Deaths: Leading Causes for 2014. Hyattsville, MD: National Cetner for Health Statistics; 2016. Contract No.: 5. [PubMed] [Google Scholar]
- 9.Liu J, Chen X, Lewis G. Childhood internalizing behaviour: analysis and implications. Journal of Psychiatric Mental Health Nursing. 2011;18(10):884–94. 10.1m/j.1365-2850.20n.01743.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Caraveo-Anduaga JJ, Colmenares-Bermudez E, Martinez-Velez NA. [Mental symptoms perceptions of healthcare needs, and health care seeking behaviors, among children and adolescents in Mexico City]. Salud Publica Mex. 2002;44(6):492–8 [PubMed] [Google Scholar]
- 11.Costello EJ, Pine DS, Hammen C, et al. Development and natural history of mood disorders. Biol Psychiatry. 2002;52(6):529–42 [DOI] [PubMed] [Google Scholar]
- 12.Kendall PC, Compton SN, Walkup JT, et al. Clinical characteristics of anxiety disordered youth. J Anxiety Disord. 2010;24(3):360–5. 10.1016/j.janxdis.2010.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pun VC, Manjourides J, Suh H. Association of Ambient Air Pollution with Depressive and Anxiety Symptoms in Older Adults: Results from the NSHAP Study. Environ Health Perspect. 2017;125(3):342–8. 10.1289/EHP494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Power MC, Kioumourtzoglou MA, Hart JE, et al. The relation between past exposure to fine particulate air pollution and prevalent anxiety: observational cohort study. BMJ. 2015;350:h1111. 10.1136/bmj.h1111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Allen JL, Klocke C, Morris-Schaffer K, et al. Cognitive Effects of Air Pollution Exposures and Potential Mechanistic Underpinnings. Curr Environ Health Rep. 2017;4(2):180–91. 10.1007/s40572-017-0134-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guxens M, Garcia-Esteban R, Giorgis-Allemand L, et al. Air pollution during pregnancy and childhood cognitive and psychomotor development: six European birth cohorts. Epidemiology. 2014;25(5):636–47. 10.1097/EDE.0000000000000133 [DOI] [PubMed] [Google Scholar]
- 17.Porta D, Narduzzi S, Badaloni C, et al. Air Pollution and Cognitive Development at Age 7 in a Prospective Italian Birth Cohort. Epidemiology. 2016;27(2):228–36. 10.1097/EDE.0000000000000405 [DOI] [PubMed] [Google Scholar]
- 18.Perera FP, Tang D, Wang S, et al. Prenatal polycyclic aromatic hydrocarbon (PAH) exposure and child behavior at age 6–7 years. Environmental health perspectives. 2012;120(6):921–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roberts S, Arseneault L, Barratt B, et al. Exploration of NO2 and PM2.5 air pollution and mental health problems using high-resolution data in London-based children from a UK longitudinal cohort study. Psychiatry Res. 2019;272:8–17. 10.1016/j.psychres.2018.12.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yolton K, Khoury JC, Burkle J, et al. lifetime exposure to traffic-related air pollution and symptoms of depression and anxiety at age 12 years. Environ Res. 2019;173:199–206. 10.1016/j.envres.2019.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Elder A, Gelein R, Silva V, et al. Translocation of inhaled ultrafine manganese oxide particles to the central nervous system. Environ Health Perspect. 2006;114(8):1172–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Elder A, Oberdorster G. Translocation and effects of ultrafine particles outside of the lung. Clinics in occupational and environmental medicine. 2005;5(4):785–96 [DOI] [PubMed] [Google Scholar]
- 23.Maher BA, Ahmed IA, Karloukovski V, et al. Magnetite pollution nanoparticles in the human brain. Proc Natl Acad Sci U S A. 2016;113(39):10797–801. 10.1073/pnas.1605941113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Calderon-Garciduenas L, Reynoso-Robles R, Vargas-Martinez J, et al. Prefrontal white matter pathology in air pollution exposed Mexico City young urbanites and their potential impact on neurovascular unit dysfunction and the development of Alzheimer’s disease. Environ Res. 2016;146:404–17. 10.1016/j.envres.2015.12.031 [DOI] [PubMed] [Google Scholar]
- 25.Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci. 2000;4(6):215–22 [DOI] [PubMed] [Google Scholar]
- 26.de la Vega A, Chang LJ, Banich MT, et al. Large-Scale Meta-Analysis of Human Medial Frontal Cortex Reveals Tripartite Functional Organization. J Neurosci. 2016;36(24):6553–62. 10.1523/JNEUROSCI.4402-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Holroyd CB, Umemoto A. The research domain criteria framework: The case for anterior cingulate cortex. Neurosci Biobehav Rev. 2016;71:418–43. 10.1016/j.neubiorev.2016.09.021 [DOI] [PubMed] [Google Scholar]
- 28.Yucel M, Wood SJ, Fornito A, et al. Anterior cingulate dysfunction: implications for psychiatric disorders? J Psychiatry Neurosci. 2003;28(5):350–4 [PMC free article] [PubMed] [Google Scholar]
- 29.LeMasters GK, Wilson K, Levin L, et al. High prevalence of aeroallergen sensitization among infants of atopic parents. J Pediatr. 2006;149(4):505–11. 10.1016/j.jpeds.2006.06.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ryan PH, LeMasters G, Biagini J, et al. Is it traffic type, volume, or distance? Wheezing in infants living near truck and bus traffic. Journal of Allergy and Clinical Immunology. 2005;116(2):279–84. 10.1016/j.jaci.2005.014 [DOI] [PubMed] [Google Scholar]
- 31.Ryan PH, LeMasters GK, Biswas P, et al. A comparison of proximity and land use regression traffic exposure models and wheezing in infants. Environmental Health Perspectives. 2007;115(2):278–84. 10.1289/ehp.9480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ryan PH, LeMasters GK, Levin L, et al. A land-use regression model for estimating microenvironmental diesel exposure given multiple addresses from birth through childhood. Science of the Total Environment. 2008;404(1):139–47. 10.1016/j.scitotenv.2008.05.051 [DOI] [PubMed] [Google Scholar]
- 33.Ryan PH, Lemasters GK, Biswas P, et al. A comparison of proximity and land use regression traffic exposure models and wheezing in infants. Environ Health Perspect. 2007;115(2):278–84. 10.1289/ehp.9480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hu S, McDonald R, Martuzevicius D, et al. UNMIX modeling of ambient PM 2.5 near an interstate highway in Cincinnati, OH, USA. Atmospheric environment. 2006;40:378–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Spence SH. A measure of anxiety symptoms among children. Behaviour Research and Therapy. 1998;36(5):545–66 [DOI] [PubMed] [Google Scholar]
- 36.Provencher SW. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med. 1993;30(6):672–9 [DOI] [PubMed] [Google Scholar]
- 37.Jenkinson M, Beckmann CF, Behrens TE, et al. Fsl. Neuroimage. 2012;62(2):782–90. 10.1016/j.neuroimage.2011.09.015 [DOI] [PubMed] [Google Scholar]
- 38.Zhang Y, Brady M, Smith S. Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE Trans Med Imaging. 2001;20(1):45–57. 10.1109/42.906424 [DOI] [PubMed] [Google Scholar]
- 39.Wansapura JP, Holland SK, Dunn RS, et al. NMR relaxation times in the human brain at 3.0 tesla. J Magn Reson Imaging. 1999;9(4):531–8 [DOI] [PubMed] [Google Scholar]
- 40.Traber F, Block W, Lamerichs R, et al. 1H metabolite relaxation times at 3.0 tesla: Measurements of T1 and T2 values in normal brain and determination of regional differences in transverse relaxation. J Magn Reson Imaging. 2004;19(5):537–45. 10.1002/jmri.20053 [DOI] [PubMed] [Google Scholar]
- 41.Tal A, Kirov II, Grossman RI, et al. The role of gray and white matter segmentation in quantitative proton MR spectroscopic imaging. NMR Biomed. 2012;25(12):1392–400. 10.1002/nbm.2812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gussew A, Erdtel M, Hiepe P, et al. Absolute quantitation of brain metabolites with respect to heterogeneous tissue compositions in (1)H-MR spectroscopic volumes. MAGMA. 2012;25(5):321–33. 10.1007/s10334-012-0305-z [DOI] [PubMed] [Google Scholar]
- 43.Tavakoli AS, Heiney SP. Using SAS to Examine Mediator, Direct and Indirect Effects of Isolation and Fear on Social Support Using Baron& Kenny Combined with Bootstrapping Methods. South Eastern SAS User Group2014. [Google Scholar]
- 44.Ashford J, Van Lier PA, Timmermans M, et al. Prenatal smoking and internalizing and externalizing problems in children studied from childhood to late adolescence. Journal of the American Academy of Child & Adolescent Psychiatry. 2008;47(7):779–87 [DOI] [PubMed] [Google Scholar]
- 45.Bandiera FC, Arheart KL, Caban-Martinez AJ, et al. Secondhand smoke exposure and depressive symptoms. Psychosomatic medicine. 2010;72(1):68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bouchard MF, Bellinger DC, Weuve J, et al. Blood lead levels and major depressive disorder, panic disorder, and generalized anxiety disorder in US young adults. Archives of general psychiatry. 2009;66(12):1313–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kamphaus RW, Reynolds CK. Parenting Relationship Questionnaire. SanAntonio, TX: Pearson Clinical Assessment; 2008 [Google Scholar]
- 48.Wood JJ, McLeod BD, Sigman M, et al. Parenting and childhood anxiety: theory, empirical findings, and future directions. J Child Psychol Psychiatry. 2003;44(1):134–51 [DOI] [PubMed] [Google Scholar]
- 49.Moller EL, Nikolic M, Majdandzic M, et al. Associations between maternal and paternal parenting behaviors, anxiety and its precursors in early childhood: A meta-analysis. Clin Psychol Rev. 2016;45:17–33. 10.1016/j.cpr.2016.03.002 [DOI] [PubMed] [Google Scholar]
- 50.Wen DJ, Poh JS, Ni SN, et al. Influences of prenatal and postnatal maternal depression on amygdala volume and microstructure in young children. Transl Psychiatry. 2017;7(4):e1103. 10.1038/tp.2017.74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Beck AT, Steer RA, Brown G. Manual for the Beck Depression Inventory-II. San Antonio, TX: Psychological Corporation; 1996 [Google Scholar]
- 52.Brockmeyer S, D’Angiulli A. How air pollution alters brain development: the role of neuroinflammation. Transl Neurosci. 2016;7(1):24–30. 10.1515/tnsci-2016-0005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Guxens M, Sunyer J. A review of epidemiological studies on neuropsychological effects of air pollution. Swiss Med Wkly. 2012;141:w13322. 10.4414/smw.2011.13322 [DOI] [PubMed] [Google Scholar]
- 54.Lubczynska MJ, Sunyer J, Tiemeier H, et al. Exposure to elemental composition of outdoor PM2.5 at birth and cognitive and psychomotor function in childhood in four European birth cohorts. Environ Int. 2017;109:170–80. 10.1016/j.envint.2017.09.015 [DOI] [PubMed] [Google Scholar]
- 55.Ehsanifar M, Tameh AA, Farzadkia M, et al. Exposure to nanoscale diesel exhaust particles: Oxidative stress, neuroinflammation, anxiety and depression on adult male mice. Ecotoxicol Environ Saf. 2019;168:338–47. 10.1016/j.ecoenv.2018.10.090 [DOI] [PubMed] [Google Scholar]
- 56.Calderon-Garciduenas L, Mora-Tiscareno A, Gomez-Garza G, et al. Effects of a cyclooxygenase-2 preferential inhibitor in young healthy dogs exposed to air pollution: a pilot study. Toxicol Pathol. 2009;37(5):644–60. 10.1177/0192623309340277 [DOI] [PubMed] [Google Scholar]
- 57.Campbell A, Araujo JA, Li H, et al. Particulate matter induced enhancement of inflammatory markers in the brains of apolipoprotein E knockout mice. J Nanosci Nanotechnol. 2009;9(8):5099–104 [DOI] [PubMed] [Google Scholar]
- 58.Gerlofs-Nijland ME, van Berlo D, Cassee FR, et al. Effect of prolonged exposure to diesel engine exhaust on proinflammatory markers in different regions of the rat brain. Part Fibre Toxicol. 2010;7:12. 10.1186/1743-8977-7-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Levesque S, Taetzsch T, Lull ME, et al. Diesel exhaust activates and primes microglia: air pollution, neuroinflammation, and regulation of dopaminergic neurotoxicity. Environ Health Perspect. 2011;119(8):1149–55. 10.1289/ehp.1002986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Levesque S, Surace MJ, McDonald J, et al. Air pollution & the brain: Subchronic diesel exhaust exposure causes neuroinflammation and elevates early markers of neurodegenerative disease. J Neuroinflammation. 2011;8:105. 10.1186/1742-2094-8-105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.van Berlo D, Albrecht C, Knaapen AM, et al. Comparative evaluation of the effects of short-term inhalation exposure to diesel engine exhaust on rat lung and brain. Arch Toxicol. 2010;84(7):553–62. 10.1007/s00204-010-0551-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Woodward NC, Levine MC, Haghani A, et al. Toll-like receptor 4 in glial inflammatory responses to air pollution in vitro and in vivo. J Neuroinflammation. 2017;14(1):84. 10.1186/s12974-017-0858-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Campbell A, Oldham M, Becaria A, et al. Particulate matter in polluted air may increase biomarkers of inflammation in mouse brain. Neurotoxicology. 2005;26(1):133–40. 10.1016/j.neuro.2004.08.003 [DOI] [PubMed] [Google Scholar]
- 64.Fisher SK, Novak JE, Agranoff BW. Inositol and higher inositol phosphates in neural tissues: homeostasis, metabolism and functional significance. J Neurochem. 2002;82(4):736–54 [DOI] [PubMed] [Google Scholar]
- 65.Best JG, Staff CJ, Dennis A. Other Significant Metabolites: Myo-Inositol, GABA, Glutamine and Lactate In: Rothman D, Stagg C, editors. Magnetic Resonance Spectroscopy. Oxford: Academic Press; 2014. p. 122–38. [Google Scholar]
- 66.Bokemeyer M, Ding XQ, Goldbecker A, et al. Evidence for neuroinflammation and neuroprotection in HCV infection-associated encephalopathy. Gut. 2011;60(3):370–7. 10.1136/gut.2010.217976 [DOI] [PubMed] [Google Scholar]
- 67.Abbott NJ. Astrocyte-endothelial interactions and blood-brain barrier permeability. J Anat. 2002;200(6):629–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Modi S, Rana P, Kaur P, et al. Glutamate level in anterior cingulate predicts anxiety in healthy humans: a magnetic resonance spectroscopy study. Psychiatry Res. 2014;224(1):34–41. 10.1016/j.pscychresns.2014.03.001 [DOI] [PubMed] [Google Scholar]
- 69.Haroon E, Fleischer CC, Felger JC, et al. Conceptual convergence: increased inflammation is associated with increased basal ganglia glutamate in patients with major depression. Mol Psychiatry. 2016;21(10):1351–7. 10.1038/mp.2015.206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Eagan DE, Gonzales MM, Tarumi T, et al. Elevated serum C-reactive protein relates to increased cerebral myoinositol levels in middle-aged adults. Cardiovasc Psychiatry Neurol. 2012;2012:120540. 10.1155/2012/120540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.de Prado Bert P, Mercader EMH, Pujol J, et al. The Effects of Air Pollution on the Brain: a Review of Studies Interfacing Environmental Epidemiology and Neuroimaging. Curr Environ Health Rep. 2018 10.1007/s40572-018-0209-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Guxens M, Lubczynska MJ, Muetzel RL, et al. Air Pollution Exposure During Fetal Life, Brain Morphology, and Cognitive Function in School-Age Children. Biol Psychiatry. 2018;84(4):295–303. 10.1016/j.biopsych.2018.01.016 [DOI] [PubMed] [Google Scholar]
- 73.Kroenke K, Spitzer RL, Williams JB, et al. Anxiety disorders in primary care: prevalence, impairment, comorbidity, and detection. Ann Intern Med. 2007;146(5):317–25 [DOI] [PubMed] [Google Scholar]
- 74.Pujol J, Martinez-Vilavella G, Macia D, et al. Traffic pollution exposure is associated with altered brain connectivity in school children. Neuroimage. 2016;129:175–84. 10.1016/j.neuroimage.2016.01.036 [DOI] [PubMed] [Google Scholar]
- 75.Calderon-Garciduenas L, Solt AC, Henriquez-Roldan C, et al. Long-term air pollution exposure is associated with neuroinflammation, an altered innate immune response, disruption of the blood-brain barrier, ultrafine particulate deposition, and accumulation of amyloid beta-42 and alpha-synuclein in children and young adults. Toxicol Pathol. 2008;36(2):289–310. 10.1177/0192623307313011 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.