Abstract
Purpose of review
The overarching goal of the current paper is to examine the empirical basis for neurocognitive phenotyping of HIV.
Recent findings
The pattern of cognitive symptoms associated with HIV has traditionally been referred to as a “subcortical” phenotype. Recent concern has been raised that the neurocognitive phenotype in the post-ART era has changed to reflect the addition of cortical features, suggestive of synergistic age-related neurodegeneration. Empirical evidence reviewed in this paper suggests that, when present, HIV-related cognitive impairment in the post-ART era remains “subcortical” in nature, regardless of advanced age or treatment status. Persistent neurocognitive impairment among virally suppressed individuals may reflect a combination of HIV disease factors, pre-existing risk factors, and/or emergent health comorbidities such as cerebrovascular disease in older people living with HIV.
Summary
An entrenchment of the “subcortical” neurocognitive phenotype of HIV appears to be unfolding in the post-ART era. Whether additional neurocognitive subtypes of HIV exist in the current era requires additional research utilizing harmonized test protocols and advanced computational methods capable of deep phenotyping. Recommendations from other neurological disorders are provided.
Keywords: HIV, cognition, HAND, phenotype
Introduction
HIV-associated neurocognitive disorders (HAND) are believed to affect nearly 1 in 2 persons living with HIV (PLWH) [1–3]. While HAND is not a universal manifestation of HIV, the massive scale of the HIV epidemic translates into an estimated point prevalence of 18 million PLWH who meet current research criteria for HAND [2–3]. In most cases, the symptoms are mild and do not disrupt daily living skills [3–5]. However, even mild cognitive symptoms undermine quality of life, and individuals with minor cognitive impairment are at risk of cognitive decline when co-morbid health conditions are present, despite treatment with antiretroviral therapy (ART) [6]. More profound cognitive impairment has been reported among PLWH who reside in countries where access to healthcare is suboptimal [7]. According to one estimate, HIV is the leading cause of significant brain dysfunction in young adults in resource-restricted regions of the world [8]. These trends are alarming given the absence of a cure for the disease, and a growing body of evidence that neurocognitive symptoms persist in the context of suppressive ART.
Numerous studies demonstrate that viral suppression does not fully reverse brain abnormalities in chronically infected individuals [9–10]. Additionally, initiation of ART may not be sufficient to prevent the development of new cerebral injury. Sanford et al. [11] reported neuroimaging abnormalities before ART, but no evidence of progressive injury 6 months after suppressive treatment. By contrast, preliminary results from adults with acute HIV revealed progressive brain volume loss over a two-year period despite initiation of suppressive ART during the first weeks of infection [13]. Long-term prospective studies are needed to determine whether individuals with either acute or more chronic infection are protected from de novo neurocognitive impairment. This is particularly true among PLWH on long-term suppressive ART, who are now expected to live as long as their uninfected peers. The life-saving benefits of ART introduce new health challenges including increased risk of synergistic or additive disease processes that have potential to alter the neurocognitive profile of HIV. This concern has been reported in the recent literature, noting that the neurocognitive phenotype of HIV has shifted towards a mixed subcortical-cortical pattern [14–*15]. If substantiated, the change would have high clinical significance because evidence of a cortical profile would implicate new disease mechanisms and neural substrates of neurocognitive symptoms, particularly Alzheimer’s disease (AD).
The current paper examines the empirical basis for neurocognitive phenotyping of HIV, with attention directed at the pattern of neurocognitive impairment before and after the start of the ART era. The paper concludes with recommendations to guide future studies and an example of novel research methods of deep phenotyping using unsupervised machine learning.
The subcortical cognitive phenotype of HIV
The cognitive phenotype of HIV has been described as “subcortical” since the beginning of the epidemic [16]. Independent of moderating factors (e.g., viral, host, cultural context), the cognitive symptoms in PLWH predominately include slow motor speed, bradyphrenia, reduced learning efficiency, and/or executive dysfunction, with sparing of core language and memory consolidation skills [17–20]. Impaired memory typically involves poor learning efficiency secondary to “upstream” abnormalities in attention, working memory, and/or executive function; amnestic memory dysfunction is uncommon [16–18].
The description of neurocognitive strengths and weaknesses align with the neuropathological features of HIV. Specifically, studies demonstrate high viral aggregation in the basal ganglia [21], and neuroimaging abnormalities in the white matter, caudate, and putamen in the early and later stages of HIV [22–26]. Neuroimaging abnormalities in the cortex and diffuse disruption to network connectivity have also been reported in PLWH [27], particularly in advanced or untreated disease [28]. These cortical abnormalities likely reflect remote signatures of damage to interconnected subcortical regions rather than isolated injury in the cortical mantle. Nevertheless, caution is warranted when applying “subcortical” or “cortical” phenotype labels because neither are defined by exclusive neuroanatomical models.
Cognitive signatures of HIV in the pre- and post-ART era
The pattern of neurocognitive symptoms associated with HIV before and after the introduction of ART were rigorously examined in two recent publications. In the first, Cysique et al. [29] reported more significant verbal learning impairment among PLWH in the post ART era compared to the prior period. Nearly identical results were described by Heaton et al. [30], with the additional observation that motor dysfunction appeared less common in the current era when compared to the prior period. However, neither study argued for the existence of a fundamental change to the phenotype of HAND in favor of a cortical phenotype. To the contrary, both studies emphasized that poor learning, rather than loss of retained information, contributed to the expression of worse memory. Indeed, close examination of the frequencies of delayed recall performance reveals high correspondence between the two treatment periods.
Outcomes from the studies above are noteworthy on two fronts. First, neuropsychology, perhaps more so than other disciplines, is riddled with inconsistent and nonspecific nomenclature. Memory, for example, is a complex phenomenon governed by multiple neural networks that harbor different affinities for disease processes. Evidence of impairment in one component of memory does not ensure impairment across other memory systems, or the involvement of a common neural substrate. This complexity necessitates a standardized nomenclature to facilitate interpretation across studies. Second, the neurocognitive phenotype described in both studies is highly consistent with the observations from Navia and Price [16] in the earliest days of the HIV epidemic, which included descriptions of abnormal memory function among individuals with neuropathology confined to the basal ganglia and white matter, without concomitant involvement of cortical regions.
Recommendations for future research
Unsupervised machine learning classification
Cluster analyses identify intrinsic patterns that help to organize large data matrices. These methods offer a less biased approach to explore hidden features of clinical phenotypes. A relative paucity of cluster analytic studies have examined neurocognitive symptoms of HIV, and results from the existing studies are difficult to interpret due to lack of standardized methods of neurocognitive assessment. Significant differences in test selection, nomenclature for defining cognitive domains (e.g., working memory vs. short-term memory vs. executive function), assignment of individual tests to specific domains (e.g., verbal fluency as a measure of Language vs. Executive Function), definitions of “impairment” (e.g., global deficit score, NPZ) are common [31–36]. Additionally, variability in methods to calculate global dysfunction contribute to the confusion because composite scores derived from cognitive tests that assess a restricted brain network (e.g., average scores on tests of frontal function) cannot be compared to composite scores comprised of tests that examined global brain networks. These challenges emphasize the need for a common methodology to quantify and describe neurocognitive impairment.
Specialty areas in neurology (AD, cerebrovascular disease, multiple sclerosis, etc.) and psychiatry (e.g., schizophrenia) benefit from common test batteries. By comparison, cognitive protocols in HIV studies are frequently designed and implemented on a study-by-study basis without pre-meditated efforts to support harmonization. Overcoming this challenge will require a consensus panel comprised of experts with input from multiple stakeholders to select tests based on psychometrics (reliability, validity, ceiling, floor), real-world relevance, opportunity for international application, brevity, availability of multiple forms, cost, and task-sharing opportunities (i.e., administration by non-neuropsychologists). Table 1 provides an example from the vascular cognitive impairment literature [37], which provides recommendations for longer and shorter protocols.
Table 1.
Harmonized Neurocognitive Assessment Protocols
| Cognitive Domain | Vascular Harmonization | Modified for HIV |
|---|---|---|
| Battery | Harmonization | |
| 60-Minute Battery | ||
| Premorbid Function | -- | Wide Range Achievement Test-IV |
| Focused Attention | -- | Digit Span Forward |
| 1-Back | ||
| Working Memory | Digit Span Forward | PASAT trial 1 |
| Digit Span Backward | LNS | |
| Fine Motor Speed | -- | Finger Tapping |
| Grooved Pegboard | ||
| Psychomotor Speed | -- | SDMT |
| Color Trails 1 | ||
| Executive Function^ | Animal Fluency | Letter Fluency |
| Letter Fluency | Action Fluency | |
| Color Trails 1 and 2 | Color Trails 2 | |
| Go/No-Go | ||
| Stroop Interference | ||
| Visuomotor Speed | SDMT | -- |
| Language | BNT-15 | Animal Fluency |
| BNT-15 | ||
| Visuospatial | Rey-Osterrieth CFT Copy | -- |
| Memory | Rey-Osterrieth CFT | CVLT–2 |
| HVLT-R | Alt: Rey AVLT | |
| Alt: CVLT-2 | ||
| 30-Minute Battery | ||
| Psychomotor- | Animal Fluency | Letter Fluency |
| Executive Function | Letter Fluency | Action Fluency |
| SDMT | Color Trails 1 and 2 | |
| Stroop | ||
| Go/No-Go | ||
| Memory | HVLT-R | CVLT–2 learning and delay only |
| 5-Minute Battery# | ||
| Montreal Cognitive | 5-word registration, delayed | MOCA |
| Assessment (MOCA) | recall and recognition; | Color Trails 1 and 2 |
| orientation, and letter fluency | ||
Italics represent tests that overlap with recommendations from the NIH Research Domain Criteria (RDoCS); PASAT: Paced Auditory Serial Addition Test (PASAT, trial 1 only); SDMT: Symbol Digit Modalities Test; BNT: Boston Naming Test (short form); RCFT: Rey Osterreith Complex Figure Test; HVLT-R: Hopkins Verbal Learning Test-Revised; CVLT-2: California Verbal Learning Test-2;
alternative computer-administered measures from the EXAMINER.
It is important that a harmonized test battery for HIV include domains/measures that are sensitive to diverse brain networks. While a central theme of the current paper is that available data point towards a persistent “subcortical” neurocognitive phenotype, there is high possibility that the pattern of neurocognitive symptoms will evolve in this population considering the expansive list of possible contributing factors. Recent studies identify potential causal pathways involving immune dysregulation (i.e., monocyte activation marker soluble CD163 [38–*40], increased levels of CD16 expressing monocytes [41], loss of CCR2 expressing non-classical monocytes [42], plasma and CSF levels of neopterin, MCP-1, lymphocyte markers such as IP-10 [43]), viral reservoirs, iatrogenic effects of medications, health co-morbidities (hepatitis C, cardio/cerebrovascular disease), and confounding effects of mental health symptoms. It is possible, therefore, that the neurocognitive phenotype of HIV will become more variable, and more nuanced based on the combinations of individual and interactive risk factors for each person.
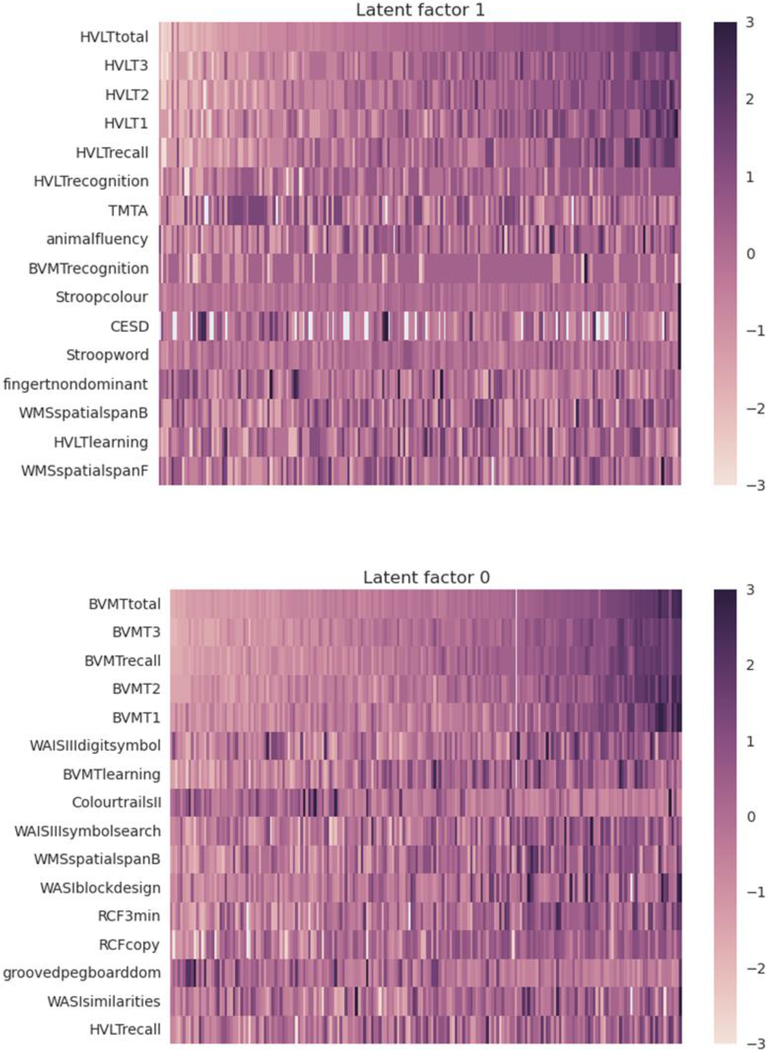
Research is also needed to leverage advances in computational methods such as machine learning algorithms. An advantage of machine learning is the opportunity to explore the underlying data structure of complex clinical phenotypes by applying algorithms that iteratively learn patterns within high dimension data. The result is a much deeper level of clinical phenotyping. An example of outcomes using the deep clustering program CorEx [44] is provided in Figure 1. CorEx (correlational explanation) identifies hierarchical representations of data (i.e., phenotypes) using a mutual information criterion. When applied to neurocognitive performance, CorEx can help identify intrinsic patterns that are not evident using traditional correlational methods. An example is provided in Figure 1, which depicts two neurocognitive phenotypes of chronic HIV. Additional methods for phenotype analyses include graphical modeling [45] and Rasche analysis [46] have been applied successfully in other neurological populations (e.g., stroke), and represent important methodological tools for deep phenotyping of HIV.
Fig. 1. Deep phenotyping of neurocognitive performance in HIV.
Heat maps depicting two deep phenotypes of neurocognitive performance in HIV. Top: verbal-dominant factor; Bottom: visual-dominant factor. Performances are depicted as raw scores.
Finally, there is a significant need to enhance methods to quantify capacity to complete activities of daily living (ADLs) in PLWH. The degree to which cognitive symptoms interfere with ADLs is a critical component of current diagnostic criteria for neurocognitive impairment, including the Frascati criteria for HAND [3]. Despite the clinical relevance, accurate assessment of real-world function is challenging in both clinical and research settings. By default, the common approach in HIV involves the use of Western-based self-report scales that were developed for use in other populations, with insights provided by informants (spouses, adult children, life partners, etc.) familiar with the individual’s prior and current functional capacity.
Not surprisingly, self-reported ADL measures are not well suited for most adult populations of PLWH, with even less relevance to pediatric cohorts and individuals residing in resource-restricted settings. Objective measures of ADL capacity that simulate real-world tasks (e.g., balancing a checking account) are viable alternatives for select adult populations [47–48], but these methods require significant investment in assessment/scoring time and the tasks do not readily translate to international settings. More work is needed to develop brief yet sensitive objective measures of ADL capacity that have strong cultural relevance in both resource-enriched and resource-restricted settings. The latter may require a paradigm shift to address superordinate skills (e.g., transportation/navigation and bartering/trade) rather than the current focus on specific subordinate tasks (e.g., driving a car and balancing a checkbook).
Conclusion
The neurocognitive phenotype of HIV remains subcortical in the post-ART era. Evidence does not support a shift towards a mixed cortical-subcortical phenotype at the present time. The neurocognitive phenotype may shift in the future, reflective of numerous candidate risk factors that persist in the context of long-term ART. Development of a common test protocol and use of standardized nomenclature will enhance data harmonization and create opportunities for deep neurocognitive phenotyping using advanced computational methods.
Footnotes
Conflict of Interest
The authors declare no conflicts of interest
Compliance with Ethics Guidelines
Human and Animal rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors
Reference List
- 1.Heaton RK, Clifford DB, Franklin DR, Woods SP, Ake C, Vaida F, Ellis RJ, Letendre SL, Marcotte TD, Atkinson JH, Rivera-Mindt M. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy CHARTER Study. Neurol, 2010: 75(23): 2087–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clifford DB, Ances BM. HIV-associated neurocognitive disorder. Lancet Infect Dis, 2013; 13(11): 976–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Antinori A, Arendt G, Becker JT, Brew BJ, Byrd DA, Cherner M, Clifford DB, Cinque P, Epstein LG, Goodkin K, Gisslen M. Updated research nosology for HIV-associated neurocognitive disorders. Neurology, 2007; 69(18): 1789–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Valcour V, Sithinamsuwan P, Letendre S, Ances B. Pathogenesis of HIV in the central nervous system. Current HIV/AIDS Reports. 2011; 8(1): 54–61.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.[dummy_reference] [Google Scholar]
- 6.Grant I, Franklin DR, Deutsch R, Woods SP, Vaida F, Ellis RJ, Letendre SL, Marcotte TD, Atkinson JH, Collier AC, Marra CM. Asymptomatic HIV-associated neurocognitive impairment increases risk for symptomatic decline. Neurol, 2014; 82(23): 2055–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saylor D, Dickens AM, Sacktor N, Haughey N, Slusher B, Pletnikov M, Mankowski JL, Brown A, Volsky DJ, McArthur JC. HIV-associated neurocognitive disorder—pathogenesis and prospects for treatment. Nature Reviews Neurol, 2016; 12(4): 234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sacktor N, Nakasujja N, Skolasky R, Robertson K, Wong M, Musisi S, Ronald A, Katabira E. Antiretroviral therapy improves cognitive impairment in HIV+ individuals in sub-Saharan Africa. Neurology, 2006: 67(2): 311–4. [DOI] [PubMed] [Google Scholar]
- 9.Cardenas VA, Meyerhoff DJ, Studholme C, Kornak J, Rothlind J, Lampiris H, Neuhaus J, Grant RM, Chao LL, Truran D, Weiner MW. Evidence for ongoing brain injury in human immunodeficiency virus–positive patients treated with antiretroviral therapy. J Neurovirol, 2009; 15(4): 324–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cohen RA, Harezlak J, Schifitto G, Hana G, Clark U, Gongvatana A, Paul R, Taylor M, Thompson P, Alger J, Brown M. Effects of nadir CD4 count and duration of human immunodeficiency virus infection on brain volumes in the highly active antiretroviral therapy era. J Neurovirol, 2010; 16(1): 25–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *11.Sanford R, Ances BM, Meyerhoff DJ, Price RW, Fuchs D, Zetterberg H, Spudich S, Collins DL. Longitudinal trajectories of brain volume and cortical thickness in treated and untreated primary human immunodeficiency virus infection. Clin Infect Dis, 2018.Initiation of ART in early infection did not reverse existing brain abnormalities but provided protection against progressive brain injury for a short period of time. Additional studies are needed to determine the durability of these findings.
- 12.Thompson PM, Dutton RA, Hayashi KM, Lu A, Lee SE, Lee JY, Lopez OL, Aizenstein HJ, Toga AW, Becker JT. 3D mapping of ventricular and corpus callosum abnormalities in HIV/AIDS. Neuroimage, 2006; 31(1): 12–23. [DOI] [PubMed] [Google Scholar]
- 13.Kallianpur KJ, Colby D, Jahanshad N, Fletcher JK, Ananworanich J, Clifford K, Benjapornpong K, Adams C, Spudich S, Valcour V for the RV254/S010 protocol teams. Brain volumetric changes after two years of ART initiated during acute HIV Infection. 23rd Conference on Retroviruses and Opportunistic Infections, February 22–25, 2016, Boston (Poster) [Google Scholar]
- 14.Sacktor N, Robertson K. Evolving clinical phenotypes in HIV-associated neurocognitive disorders. Current HIV/ AIDS Reports, 2014; 9(6): 517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *15.Sacktor N Changing clinical phenotypes of HIV-associated neurocognitive disorders. J Neurovirol, 2017; 27: 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Navia BA, Jordan BD, Price RW. The AIDS dementia complex: I. Clinical features. Ann Neurol, 1986: 19(6): 517–24. [DOI] [PubMed] [Google Scholar]
- 17.Clifford DB, Ances BM. HIV-associated neurocognitive disorder. Lancet Infect Dis, 2013: 13(11): 976–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Paul RH, Ernst T, Brickman AM, Yiannoutsos CT, Tate DF, Cohen RA, Navia BA. Relative sensitivity of magnetic resonance spectroscopy and quantitative magnetic resonance imaging to cognitive function among nondemented individuals infected with HIV. JINS, 2008: 14(5): 725–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heaton RK, Franklin DR, Ellis RJ, McCutchan JA, Letendre SL, LeBlanc S, Corkran SH, Duarte NA, Clifford DB, Woods SP and Collier AC HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. J Neurvirol, 2011: 17(1), 3–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Woods SP, Iudicello JE, Moran LM, Carey CL, Dawson MS, Grant I. HIV-associated prospective memory impairment increases risk of dependence in everyday functioning. Neuropsychol, 2008; 22(1): 110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Archibald SL, Masliah E, Fennema-Notestine C, Marcotte TD, Ellis RJ, McCutchan JA, Heaton RK, Grant I, Mallory M, Miller A, Jernigan TL. Correlation of in vivo neuroimaging abnormalities with postmortem human immunodeficiency virus encephalitis and dendritic loss. Arch Neurol, 2004: 61(3): 369–76. [DOI] [PubMed] [Google Scholar]
- 22.Becker JT, Sanders J, Madsen SK, Ragin A, Kingsley L, Maruca V, Cohen B, Goodkin K, Martin E, Miller EN, Sacktor N. Subcortical brain atrophy persists even in HAART-regulated HIV disease. Brain Imaging Behav, 2011; 5(2): 77–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cohen RA, Harezlak J, Schifitto G, Hana G, Clark U, Gongvatana A, Paul R, Taylor M, Thompson P, Alger J, Brown M. Effects of nadir CD4 count and duration of human immunodeficiency virus infection on brain volumes in the highly active antiretroviral therapy era. J Neurovirol, 2010: 16(1): 25–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Paul R, Cohen R, Navia B, Tashima K. Relationships between cognition and structural neuroimaging findings in adults with human immunodeficiency virus type-1. Neurosci Biobehav Rev, 2002; 26(3): 353–9. [DOI] [PubMed] [Google Scholar]
- 25.Wright PW, Vaida FF, Fernández RJ, Rutlin J, Price RW, Lee E, Peterson J, Fuchs D, Shimony JS, Robertson KR, Walter R. Cerebral white matter integrity during primary HIV infection. AIDS (London, England). 2015; 29(4): 433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Paul RH, Yiannoutsos CT, Miller EN, Chang L, Marra CM, Schifitto G, Ernst T, Singer E, Richards T, Jarvik GJ, Price R. Proton MRS and neuropsychological correlates in AIDS dementia complex: evidence of subcortical specificity. J Neuropsych Clin Neurosci, 2007; 19(3): 283–92. [DOI] [PubMed] [Google Scholar]
- 27.Thompson PM, Dutton RA, Hayashi KM, Toga AW, Lopez OL, Aizenstein HJ, Becker JT. Thinning of the cerebral cortex visualized in HIV/AIDS reflects CD4+ T lymphocyte decline. PNAS, 2005; 102(43): 15647–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baker LM, Cooley SA, Cabeen RP, Laidlaw DH, Joska JA, Hoare J, Stein DJ, HeapsWoodruff JM, Salminen LE, Paul RH. Topological organization of whole-brain white matter in HIV infection. Brain connectivity. 2017;7(2):115–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cysique LA, Maruff P, Brew BJ. Prevalence and pattern of neuropsychological impairment in human immunodeficiency virus-infected/acquired immunodeficiency syndrome (HIV/AIDS) patients across pre-and post-highly active antiretroviral therapy eras: a combined study of two cohorts. J Neurovirol, 2004; 10(6): 350–7. [DOI] [PubMed] [Google Scholar]
- 30.Heaton RK, Franklin DR, Ellis RJ, McCutchan JA, Letendre SL, LeBlanc S, Corkran SH, Duarte NA, Clifford DB, Woods SP, Collier AC. HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. J Neurovirol, 2011; 17(1): 3–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Solomon IH, De Girolami U, Chettimada S, Misra V, Singer EJ, Gabuzda D. Brain and liver pathology, amyloid deposition, and interferon responses among older HIV-positive patients in the late HAART era. BMC Infect Dis, 2017; 17(1): 151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van Gorp WG, Hinkin C, Satz P, Miller EN, Weisman J, Holston S, Drebing C, Marcotte TD, Dixon W. Subtypes of HIV-related neuropsychological functioning: A cluster analysis approach. Neuropsychol, 1993; 7(1): 62. [Google Scholar]
- 33.Fazeli PL, Crowe M, Ross LA, Wadley V, Ball K, Vance DE. Cognitive functioning in adults aging with HIV: a cross-sectional analysis of cognitive subtypes and influential factors. J Clin Res HIV AIDS Prev, 2014; 1(4): 155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lojek E, Bornstein RA. The stability of neurocognitive patterns in HIV infected men: Classification considerations. J Clin Exp Neuropsychol, 2005: 27(6): 665–82. [DOI] [PubMed] [Google Scholar]
- 35.Dawes S, Suarez P, Casey CY, Cherner M, Marcotte TD, Letendre S, Grant I, Heaton RK. Variable patterns of neuropsychological performance in HIV-1 infection. J Clin Exp Neuropsychol, 2008; 30(6): 613–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Molsberry SA, Cheng Y, Kingsley L, Jacobson L, Levine AJ, Martin E, Miller EN, Munro CA, Ragin A, Sacktor N, Becker JT. Neuropsychological phenotypes among men with and without HIV disease in the multicenter AIDS cohort study. AIDS. 2018. July 31;32(12):1679–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hachinski V, Iadecola C, Petersen RC, Breteler MM, Nyenhuis DL, Black SE, Powers WJ, DeCarli C, Merino JG, Kalaria RN, Vinters HV. National Institute of Neurological Disorders and Stroke–Canadian stroke network vascular cognitive impairment harmonization standards. Stroke, 2006; 37(9): 2220–41. [DOI] [PubMed] [Google Scholar]
- 38.Burdo TH, Weiffenbach A, Woods SP, Letendre S, Ellis RJ, Williams KC. Elevated sCD163 in plasma but not cerebrospinal fluid is a marker of neurocognitive impairment in HIV infection. AIDS (London, England), 2013; 27(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.D’Antoni ML, Byron MM, Chan P, Sailasuta N, Sacdalan C, Sithinamsuwan P, Tipsuk S, Pinyakorn S, Kroon E, Slike BM, Krebs SJ. Normalization of Soluble CD163 after Institution of Antiretroviral Therapy During Acute HIV Infection Tracks with Fewer Neurological Abnormalities. JID, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *40.Imp BM, Rubin LH, Tien PC, Plankey MW, Golub ET, French AL, Valcour VG. Monocyte activation is associated with worse cognitive performance in virologically suppressed HIVinfected women. JID, 2016. 27:jiw506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ndhlovu LC, Umaki T, Chew GM, Chow DC, Agsalda M, Kallianpur KJ, Paul R, Zhang G, Ho E, Hanks N, Nakamoto B. Treatment intensification with maraviroc (CCR5 antagonist) leads to declines in CD16-expressing monocytes in cART-suppressed chronic HIV-infected subjects and is associated with improvements in neurocognitive test performance: implications for HIVassociated neurocognitive disease (HAND). J Neurovirol 2014; 20(6): 571–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ndhlovu LC, D’Antoni ML, Ananworanich J, Byron MM, Chalermchai T, Sithinamsuwan P, Tipsuk S, Ho E, Slike BM, Schuetz A, Zhang G. Loss of CCR2 expressing non-classical monocytes are associated with cognitive impairment in antiretroviral therapy-naive HIV-infected Thais. J Neuroimmunol, 2015; 288: 25–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Krebs SJ, Slike BM, Sithinamsuwan P, Allen IE, Chalermchai T, Tipsuk S, Phanuphak N, Jagodzinski L, Kim JH, Ananworanich J, Marovich MA. Sex differences in soluble markers vary before and after the initiation of antiretroviral therapy in chronically HIV infected individuals. AIDS (London, England). 2016. June 19;30(10):1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ver Steeg G, Galstyan A. Maximally informative hierarchical representations of high-dimensional data. InArtificial Intelligence and Statistics 2015. February 21 pp. 1004–1012. [Google Scholar]
- 45.Massa MS, Wang N, Bickerton WL, Demeyere N, Riddoch MJ, Humphreys GW. On the importance of cognitive profiling: A graphical modelling analysis of domain-specific and domain-general deficits after stroke. Cortex. 2015. October 1;71:190–204. [DOI] [PubMed] [Google Scholar]
- 46.Li Y, Root JC, Atkinson TM, Ahles TA. Examining the association between patient-reported symptoms of attention and memory dysfunction with objective cognitive performance: a latent regression rasch model approach. Archives of Clinical Neuropsychology. 2016. April 24;31(4):365–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Patton DE, Woods SP, Franklin D, Cattie JE, Heaton RK, Collier AC, Marra C, Clifford D, Gelman B, McArthur J, Morgello S. Relationship of Medication Management Test-Revised (MMT-R) performance to neuropsychological functioning and antiretroviral adherence in adults with HIV. AIDS and Behavior. 2012. November 1;16(8):2286–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Woods SP, Iudicello JE, Morgan EE, Cameron MV, Doyle KL, Smith TV, Cushman C, HIV Neurobehavioral Research Program (HNRP) Group, Grant I, Atkinson JH, Ellis RJ. Healthrelated everyday functioning in the internet age: HIV-associated neurocognitive disorders disrupt online pharmacy and health chart navigation skills. Archives of Clinical Neuropsychology. 2016. January 6;31(2):176–85. [DOI] [PMC free article] [PubMed] [Google Scholar]