Abstract
Heart failure is the leading cause of morbidity and mortality worldwide. Several lines of evidence suggest that physical activity and exercise can pre-condition the heart to improve the response to acute cardiac injury such as myocardial infarction or ischemia/reperfusion injury, preventing the progression to heart failure. It is becoming more apparent that cardioprotection is a concerted effort between multiple cell types and converging signaling pathways. However, the molecular mechanisms of cardioprotection are not completely understood. What is clear is that the mechanisms underlying this protection involve acute activation of transcriptional activators and their corresponding gene expression programs. Here, we review the known stress-dependent transcriptional programs that are activated in cardiomyocytes and cardiac fibroblasts to preserve function in the adult heart after injury. Focus is given to prominent transcriptional pathways such as mechanical stress or reactive oxygen species (ROS)- dependent activation of myocardin-related transcription factors (MRTFs) and transforming growth factor beta (TGFβ), and gene expression that positively regulates protective PI3K/Akt signaling. Together, these pathways modulate both beneficial and pathological responses to cardiac injury in a cell-specific manner.
Keywords: Cardioprotection, cardiomyocyte, cardiac fibroblast, transcription, exercise
1. Introduction
Physiological and pathological responses to stress are regulated by a variety of gene expression programs. In particular, the heart undergoes structural remodeling to compensate for external stresses that impede cardiac homeostasis. Cardiomyocytes (CMs) and cardiac fibroblasts (CFs) both respond to acute and chronic stress by secreting cytokines and increasing the synthesis of structural proteins. Although these changes provide temporary protection after injury, persistent stress can lead to CM death. The inability of adult CMs to proliferate partially underlies the development of heart failure following injury. As a compensatory response to preserve cardiac contractility, quiescent resident CFs transition to an activated myofibroblast state and secrete extracellular matrix (ECM). Unfortunately, this reparative mechanism of replacing lost myocardium with connective tissue ultimately hinders cardiac function and accelerates the progression to heart failure. Thus, it is of therapeutic interest to identify mechanisms that permit a hypertrophic response without triggering maladaptive changes, and that enhance cardioprotection by promoting CM survival and/or inhibiting myofibroblast activation. This review will highlight cardioprotective pathways activated in CMs and CFs based on studies of myocardial infarction (MI), ischemia/reperfusion (I/R) injury, exercise or cardiac regeneration using animal models of genetic deletion or gain-of-function.
2. Cardioprotection: insights from exercise and disease models
2.1. Evidence of cardioprotection
When subjected to oxygen deprivation or mechanical stress, cardiac cells respond by upregulating gene programs that promote survival from injury referred to as ‘cardioprotection’. This phenomenon enables the heart to defend against various stresses that impede cardiac function. A classic example of cardioprotection is through ischemic preconditioning, whereby sub-lethal episodes of ischemia can prime the heart to resist the damaging effects of subsequent MI and I/R injury. In animal models, ischemic preconditioning is achieved by temporarily occluding the coronary vasculature followed by reperfusion or reoxygenation over several episodes. Such preconditioning significantly reduces infarct size, myocardial necrosis and endothelial dysfunction in multiple animal models of permanent coronary occlusion [1–5]. The protective effects of ischemic preconditioning are orchestrated by a variety of signaling pathways. For instance, anti-apoptotic proteins such as Bcl-XL (Bcl2l1), Mcl-1 (Mcl1), and c-Flip (Cflar) are induced with ischemic preconditioning [6]. Overexpression of Bcl-XL is sufficient to protect cardiomyocytes against I/R injury [7]. Ischemic preconditioning also suppresses pro-apoptotic signals such as the activation of caspase 3 and subsequent cleavage of the DNA repair protein poly (ADP-ribose) polymerase-1 (PARP-1) [6]. While experimental study of ischemic preconditioning in humans is not feasible given obvious ethical concerns, the salutary effects of ‘preconditioning’ in humans has been observed in patients with unstable angina prior to myocardial infarction [8] or with coronary angioplasty-related preconditioning [9]. These observations suggest that the heart has an innate capacity to protect itself in response to environmental stress.
Anecdotal evidence from heart failure (HF) patients also suggests a link between exercise and improved or sustained cardiac performance [10]. Studies in rodents further gave credence to this link with the observation that rats exhibit reduced infarct size and increased vascularity with chronic exercise training [11]. The beneficial effect of exercise has prompted its use as a therapeutic for HF patients. In a multi-center, randomized study of HF patients (HF-ACTION), regular physical activity was found to be associated with reduced cardiovascular mortality and HF hospitalizations [12]. Likewise, exercise also improves myocardial perfusion in patients with stable coronary artery disease [13]. Thus, exercise serves as an example for a non-injury stimulus that affords cardioprotection.
2.2. PI3K/Akt survival signaling
To understand cardioprotective mechanisms, it is important to discuss physiological processes such as exercise, and pro-survival pathways such as the phosphoinositide-3 kinase (PI3K)/Akt pathway. Exercise induces a physiological response in the heart that promotes a protective, pro-proliferative state that extends to cells with minimal proliferative capacity such as adult CMs [14, 15]. Indeed, moderate exercise provides notable improvements in cardiac function and reduced fibrosis in mouse models of cardiomyopathy as well as human HF patients [16–18]. Traditionally, PI3K/Akt promotes cell survival by inhibiting caspase- or p53-dependent apoptosis via GSK3β and Bcl2-family members, and activating mammalian target of rapamycin (mTOR) signaling to promote cellular growth [19]. In the heart, PI3K/Akt is stimulated by exercise and participates in the canonical reperfusion injury salvage kinase (RISK) pathway of cardioprotective kinases [20]. In concert with insulin growth factor (IGF), PI3K activates Akt and triggers physiological cardiac hypertrophy and survival instead of a pathological transition to dilation and heart failure [21–24]. Transient activation of Akt via acute viral gene transfer similarly attenuates CM apoptosis and infarct size in rats subjected to I/R injury [25]. However, excessive Akt signaling can negatively impact the heart; chronic activation of Akt promotes pathological hypertrophy at baseline and exacerbates maladaptive remodeling after I/R injury [26, 27]. Thus, temporal but not persistent activation of Akt is necessary to harness its protective capacity.
2.3. Exercise, C/EBPβ and CITED4
The beneficial effects of exercise in cardioprotection are likely attributed to multiple factors, one of which may be modulation of the immune response. Indeed, chronic exercise training improves cardiac function and attenuates pro-inflammatory cytokines in rats with isoproterenol-induced HF [28]. Chronic Akt activation has been linked to tumor necrosis factor (TNF)-mediated activation of the transcription factor NFκB, which subsequently regulates the inflammatory response [29, 30]. Stimulation of the NFκB pathway is also facilitated by the transcription factor CCAAT Enhancer Binding Protein Beta (C/EBPβ) [31]. C/EBPβ induces hypertrophic gene programs and is downregulated in response to exercise [31, 32]. Mice that are haploinsufficient for C/EBPβ are resistant to pathological remodeling with pressure overload injury [32]. Inhibition of C/EBP also modulates hypoxia-induced epicardial activation, and blunts neutrophil recruitment after MI or I/R injury [33]. Of note, the transcriptional activator CITED4 is expressed reciprocally to C/EBPβ in CMs after exercise [32, 34]. Exercise stimulates microRNA (miR)-222 expression in CMs, and overexpression of miR-222 is sufficient to block pathological remodeling by upregulating the Cited4 gene [35]. CM-specific expression of CITED4 improves cardiac function, and reduces CM death and ventricular fibrosis after I/R [36]. Treating mice with anti-neoplastic 5-fluorouracil blocks exercise-induced proliferation and CITED4 stimulation, reducing the ability of exercise to protect the myocardium from I/R injury [37]. Thus, inhibiting C/EBPβ or activating CITED4 may be potential approaches to mitigate pathological remodeling and promote cell survival.
2.4. Mitochondrial Dynamics and ROS
Mitochondria are fundamental gatekeepers of cellular metabolism. In order to maintain homeostasis, CMs must produce an uninterrupted supply of energy [38]. However, oxidative phosphorylation and ATP production contribute to the production of ROS, which can promote CM dysfunction if produced in excess. Mitochondria isolated from the hearts of rodents subjected to exercise training are more resistant to calcium-induced mitochondrial permeability transition pore (mPTP) opening [39] and ROS-induced cytochrome c release [39, 40]. Exercise not only activates PI3K/Akt signaling, which in turn enhances mitochondrial cardioprotection via hexokinase-mediated resistance to mPTP opening [41], but also elicits profound changes to the mitochondrial proteome [42].
Independent of PI3K/Akt, exercise also alters mitochondrial dynamics [43]. For instance, increasing energy demand can induce protective mitochondrial fission in the heart [44]. Consistent with this concept, cardiac-specific depletion of the mitochondrial fission protein DRP1 exacerbates fibrotic remodeling after I/R, and impairs cardiac function after pressure overload [45, 46]. The fragmentation of mitochondria by fission may facilitate cardioprotection by promoting mitophagy, and/or reducing CM apoptosis in response to mitochondrial dysfunction [45–49]. For example, RhoA signaling reduces translocation of pro-apoptotic proteins such as BAX to mitochondria [50] (Fig. 1) Modulating the mitochondrial transcriptome may also facilitate cardioprotection. Mitochondrial transcription factor A (TFAM) is a critical regulator of the transcription and replication of mitochondrial DNA (mtDNA) [51]. Elevated oxidative stress and reduced mitochondrial biogenesis is associated with reduced mtDNA content in end-stage heart failure patients [52] and in mice after MI [53]. Cardiac-restricted overexpression of Tfam attenuates CM hypertrophy and apoptosis, and improves mitochondrial respiration post-MI injury [54, 55]. Thus, maintaining mitochondrial function through regulation of either gene expression or physical dynamics can protect the heart when it is burdened by stress.
Figure 1.
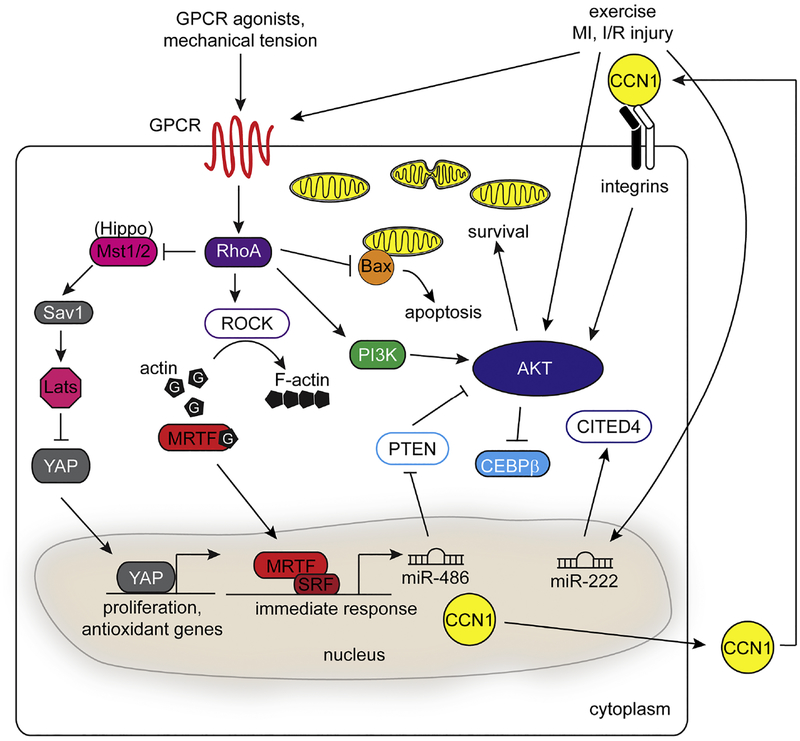
Signaling pathways that modulate cardioprotection in CMs. Mechanical and oxidative stress produced in disease or exercise can induce a complex anti-apoptotic and hypertrophic response in CMs that converges on the RhoA and PI3K/Akt signaling pathways. Stimulation of GPCRs through ligands triggers RhoA-dependent actin polymerization and nuclear localization of MRTFs to activate transcription of MRTF/SRF target genes. Expression of targets such as miR-486 and CCNs, as well as exercise-induced mechanical tension can activate the Akt response to contribute to cardioprotection. RhoA can also modulate the Hippo/YAP signaling pathway to regulate proliferation and stress responsive genes during both development and disease.
3. Cardiomyocyte Specific Transcriptional Pathways
Cardiac injury such as I/R causes CM mitochondrial dysfunction and extracellular inflammatory signaling that facilitate cell death. As a result, millions of CMs are lost that cannot be restored due to the refractoriness of CMs to re-enter the cell cycle. Limiting the loss of CMs to these stimuli is critical to maintain cardiac function. Non-ischemic stress can instead trigger a hypertrophic response in CMs. However, if the initial cardiac insult persists, this hypertrophy can transition from compensatory to maladaptive, and thus must be limited. Here, we will review gene expression pathways that occur specifically within the CM to limit both acute cell death and pathological remodeling.
3.1. Stress-sensitive RhoA and MRTF signaling
Small GTP (guanosine 5’-triphosphate)-binding proteins or “GTPases” serve as molecular switches to regulate cellular processes. The Rho subfamily is best known for its role in regulating cell motility, myosin-based contractility and actin polymerization [56]. Upon GTP activation, RhoA stimulates myosin phosphorylation and regulates actin dynamics via the Rho-associated protein kinases, ROCK1 and ROCK2 [57, 58] (Fig. 1). Several studies demonstrate that aberrant activation of RhoA in mice can lead to pathological hypertrophy and fibrosis, typically by processes that require ROCK1 [59–61]. In contrast, inducible expression of a constitutively active RhoA transgene in CMs affords protection from I/R injury independent of the ROCK isoforms [62]. RhoA activity is also regulated by ROS [63], which may be one mechanism utilized by CMs to sense oxidative stress. In other contexts, CM-specific knockout of RhoA reveals a role for RhoA signaling in the hypertrophic response to cardiac pressure overload, but is not necessary for the progression to heart failure [64]. The seemingly disparate results between acute and chronic RhoA activity indicate that temporal regulation dictates cardioprotection versus maladaptive dysfunction.
The heart responds to hemodynamic stress by secreting peptide hormones and increasing overall size to accommodate elevated wall stress. Among the genes regulated, atrial natriuretic factor (ANF, also Nppa) is expressed and secreted in response to atrial stretch [65]. RhoA signaling is exquisitely sensitive to biophysical tension when neonatal ventricular myocytes (NVMs) are subjected to uniaxial stretching [66]. Early studies in NVMs identified the Rho effector Protein Kinase N1 (PKN) as a key regulator of ANF through Rho- and serum response factor (SRF)-dependent transcription [67]. The link between stress-activated RhoA and pro-survival pathways was further delineated by the observation that Rho kinases stimulate PI3K/Akt signaling through focal adhesion kinase (FAK) in NVMs [68]. Moreover, mice with inducible deletion of RhoA in CMs exhibit blunted FAK and Akt activation when challenged by transaortic constriction (TAC), along with a decline in cardiac function after injury [64]. Together, these findings demonstrate a RhoA-FAK-PI3K-Akt signaling circuit utilized by CMs in response to stress.
The ability of RhoA to transduce such protective signals from the plasma membrane to the nucleus requires regulators of transcription. Most notably, myocardin-related transcription factor (MRTF)-A and MRTF-B (collectively ‘MRTFs’) are Rho-stimulated activators of SRF- dependent transcription [69]. In a basal state, MRTFs are sequestered in the cytoplasm through direct interactions with globular (G)-actin, which sterically mask nuclear localization signals [70]. Upon stimulation, Rho-dependent actin polymerization promotes nuclear shuttling of MRTFs and target gene expression [71, 72] (Fig. 1). For instance, the RhoA-MRTF-SRF signaling axis drives the expression of the protective matricellular protein CCN1 (Cyr61) [73]. Secreted CCN1 signals back to CMs in an autocrine and/or paracrine manner to increase mitochondrial cardioprotection via PI3K/Akt signaling [74] (Fig. 1). CCN1 also promotes premature senescence of CMs to aid in recovery after MI [75]. Pro-survival signaling by MRTFs may also be mediated through indirect mechanisms. For instance, MRTF-A promotes PI3K/Akt signaling by repressing inhibitory PTEN and FoxO1a via upregulation of miR-486 [76] (Fig. 1).
The molecular mechanisms underlying MRTF-mediated cardioprotection are not completely understood. Genetic deletion studies in mice demonstrate a dispensable role for both RhoA and MRTFs in normal cardiac physiology [64, 77]. However, stress elicits a profound phenotype in the absence of both MRTF isoforms. CM-specific inactivation of MRTF-B in mice with a homozygous MRTF-A null background (MRTFcKO) results in rapid deterioration of cardiac function within one week after TAC [77]. Transcriptional profiling of MRTFcKO hearts revealed dysregulation of stress-dependent cytoskeletal pathways [77]. Of note, local pools of MRTFs are distributed at the intercalated discs of CMs [77]. Further investigation is necessary to elucidate if and how MRTFs translocate to CM nuclei from cytoskeletal signaling complexes. However, given the stress-dependent activation of RhoA signaling in CMs [68], tethering potent transcription factors to regions of mechanical tension may be one mechanism whereby CMs can “sense” stress.
3.2. Hippo and YAP
The adult mammalian heart has a limited regenerative capacity after birth [78, 79]. Identifying the mechanisms controlling CM proliferation could have profound impacts on heart regeneration and rejuvenation following MI. The Hippo/Yes-activated Protein (YAP) pathway has received a considerable amount of attention in this very endeavor; indeed, Hippo/YAP signaling modulates the expansion and migration of cardiac progenitor cells to sites of injury [80]. The Hippo kinase cascade restrains cell proliferation and growth by phosphorylating YAP and preventing its association with the TEAD family of transcription factors [81]. Cytoskeletal proteins, such as the alpha-catenins, may also inhibit YAP translocation to the nucleus [82]. Loss-of-function studies in the developing heart first demonstrated the pro-proliferative role of YAP in CMs. Yap1-deficient hearts display hypoplasia stemming from defects in CM proliferation, IGF1 signaling, and regulation of cell cycle genes [83–85]. Conversely, Yap1 gain-of-function [84] or inactivation of the Hippo kinases (Mst1/2, Sav1 or Lats2) [86] all stimulate CM expansion. These genetic studies support a critical role for Hippo signaling in regulating cardiac growth.
Although YAP is a potent activator of cell cycle genes and cardiac development, its role in the adult heart is less defined. Fetal heart growth occurs through cell proliferation, whereas postnatal heart growth primarily involves hypertrophy. Mice with homozygous, CM-specific loss of Yap1 exhibit signs of cardiac dysfunction and hypertrophy at baseline, which is exacerbated in the presence of ischemic injury [87]. Paradoxically, YAP-mediated induction of miR-206 may promote compensatory hypertrophy in response to I/R [88]. In contrast to these observations, other groups have convincingly shown that YAP is not required nor is sufficient to induce CM hypertrophy in vivo using cell mosaic analysis or transgenic overexpression [83, 89]. The cardiac hypertrophy associated with Yap1 deficiency may represent a secondary response to heart failure. Regardless of its role in cardiac hypertrophy, multiple studies support a cardioprotective role for YAP [89–92], or Hippo suppression that would increase YAP activity [86, 93, 94], following ischemic injury. The reverse has also been observed, as Hippo activation reduces cardioprotection [95]. Transgenic mice expressing constitutively active YAP in CMs show better preservation of heart function and approximately 2.5 fold more CM proliferation compared to control mice challenged by MI [89]. However, YAP activation may only be protective if negative feedback regulation is present. For instance, CM-specific deletion of WW45, a scaffold protein that mediates YAP inhibition, results in increased cardiac dysfunction after injury presumably due to excessive CM de-differentiation [96].
The salutary effects of YAP activation may not be solely attributed to CM expansion. I/R injury damages the myocardium through excessive oxidative stress. Forced expression of YAP blunts hydrogen peroxide-induced cell death of CMs via Akt activation, suggesting that YAP regulates the antioxidant response [87] (Fig. 1). Interestingly, TEAD binding motifs share significant co-occurrence at fetal cardiac enhancers with Pitx2, a transcription factor that is responsive to ischemic injury [97]. Yap is also required for Pitx2-induced myocardial regeneration [97]. In addition to TEAD binding, YAP associates with FoxO1 to regulate catalase and superoxide dismutase gene expression following I/R injury [98]. Together, these studies indicate that Hippo/YAP signaling modulates cardioprotection and repair following ischemic injury through a series of elaborate pathways involving stress response, cell cycle re-entry and antioxidant signaling.
3.3. GPCR-stimulated pathways of gene expression
While several transcription factors mediate cardioprotection, their activity is dependent on upstream regulators such as G-protein coupled receptors (GPCRs), a large, diverse group of transmembrane signal receptors. The lysophospholipid S1P binds to the S1PR subset of GPCRs [73, 99]. The therapeutic promise of S1PR activation has been demonstrated with the FDA approved S1P analog FTY720 (Fingolimod), which sustains cardiac function after I/R injury [100–102]. Exogenous S1P reduces infarct size and cellular apoptosis after I/R injury without stimulating CM hypertrophy [103], and promotes the proliferative function of cardiac progenitor cells through the activation of RhoA and MRTF-A dependent gene expression [104]. Mechanistically, the S1P/RhoA/MRTF pathway promotes cardioprotection in part through the induction of CCN1 [73]. CCN1 expression can also be induced by S1P-mediated activation of YAP [105, 106]. In other contexts, S1P attenuates hypertrophic signaling through increased expression of the transcription factor KLF4 [107]. Taken together, S1P-mediated cardioprotection is likely orchestrated by multiple pathways in diverse cellular contexts [108].
While the S1P signaling activates RhoA in the heart to facilitate cardioprotection [50, 73, 103], cardiac GPCR signaling is certainly not restricted to RhoA-dependent pathways [109]. GPCRs coupled to other G-proteins can also facilitate protection. Signaling through the beta-2 adrenergic receptor (β2-AR) has protective effects in CMs, primarily dependent upon anti-apoptotic signaling through the PI3K/Akt pathway [110–112] (Fig. 1). Gene programs that facilitate the protective PI3K/Akt and nitric oxide/PKG pathways can be modulated by nuclear β-ARs [113, 114]. Adenoviral mediated delivery of β2-AR to CMs can increase the expression of the anti-inflammatory cytokine IL-10 following aortic constriction [115]. β2-AR stimulation also increases the expression of the soluble inhibitory binding protein of IL-18, limiting pathological hypertrophy of the myocardium [116]. In other contexts, isoproterenol stimulation of β-AR promotes transactivation of epidermal growth factor receptor (EGFR), which signals to inhibit transcription of pro-inflammatory cytokines and pro-apoptotic factors in CMs [117–119]. Opioid receptor agonists such as morphine also facilitate cardioprotection through the delta-opioid receptor, increasing transcription of sarcomeric stress response proteins, repressing inflammation, and expressing miR-133b-5p to inhibit CM apoptosis [120–123]. Interestingly, β2-AR signaling may be responsible for delta-opioid receptor agonism and subsequent cardioprotection in CMs [124]. Common transcriptional activators could be targeted by β2-AR and delta-opioid GPCR signaling to promote downstream gene expression.
Certain members of the α-AR family are also important in transcriptional protection of CMs.Specifically, the α1a- and α1b-ARs decrease CM apoptosis, and CM-specific deletion of both α1-ARs increases apoptosis, reduces cardiac function, and reduces survival in response to TAC [125, 126]. Notably, α1-AR activation facilitates cardioprotection through ERK-dependent gene regulation and NFkB inhibition, limiting expression of pro-inflammatory stimuli such as TNFα [127]. Thus one effect of GPCR signaling at the transcriptional level, particularly stimulation of the β2-AR and α1-AR, appears to be limiting excessive pro-inflammatory expression from CMs.
4. Cardiac Fibroblast Specific Transcriptional Pathways
Cardiac fibroblasts (CFs) are a heterogeneous population of cells that have important roles in ECM secretion, paracrine signaling, electrical coupling, and the immune response. When subjected to injury such as MI or chronic pressure overload, CFs transition from a quiescent fibroblast into an activated myofibroblast, expressing contractile genes and secreting ECM as a protective mechanism to maintain myocardial wall integrity. However, excessive myofibroblast activation causes maladaptive remodeling that disrupts intracellular contacts, hinders contractility, and leads to cardiac fibrosis [128–130]. In contrast, physiological stimuli such as exercise and pregnancy do not result in fibroblast activation, indicating that CFs are important “first responders” in sensing and reacting to extracellular stress. Here, we will discuss the context-dependent mechanisms of fibroblast activation and how modulation of these pathways could promote cardioprotection or mitigate maladaptive remodeling.
4.1. Roles for fibroblasts in cardiac repair
While fibrosis in other organs can be resolved by inducing apoptosis in activated fibroblasts, subpopulations of activated CFs are less susceptible to apoptosis [131, 132]. Mechanisms to resolve cardiac fibrosis have thus proven to be challenging. Indeed, a recent study defined the matrifibrocyte, a specialized myofibroblast that has lost the ability to proliferate but persists after MI resolution to act as a scaffold [133]. Despite the repercussions of excessive CF activation, controlled activation is also required to preserve cardiac function. Conditional deletion of the ECM component periostin (Postn), or p38α (Mapk14) demonstrates that ECM production from activated fibroblasts is required to prevent cardiac rupture after MI [134–136]. Similarly, CF-specific deletion of Klf5 prevents pressure overload-induced fibrosis but is accompanied by increased mortality [137]. This may be attributed to the effects of IGF-1 on CFs; IGF-1 signaling is regulated by Klf5, and IGF-1 is enriched in CFs and can increase contraction of fetal and adult rat CFs in collagen gel assays [138]. The effects of IGF-1 on CFs are complex, as treatment of mouse CFs with IGF-1 and H2O2 (IGF/ROS) mimics exercise conditions in vitro by stimulating Akt phosphorylation without stimulating transforming growth factor beta (TGFβ) signaling [139]. Furthermore, IGF/ROS treatment in human CFs significantly downregulates transcription of activated CF markers such as POSTN, ACTA2, and COL3A1 compared to control or TGFβ-treated CFs, together suggesting that IGF signaling mitigates pathological remodeling in CFs [140].
In contrast to pathological stress, physiological stimuli induce cardiac hypertrophy in the absence of fibrosis, highlighting CF plasticity and indicating an inherent difference in the CF response despite similar increases in cardiac demand and mechanical stress. Notably, the malleability of CFs has stimulated a series of elegant reprogramming studies that have generated functional CMs from CFs both in vitro and in vivo using cocktails of transcription factors including Gata4, Hand2, Mef2a, and Tbx5 [141, 142]. The addition of microRNAs and chemical enhancers to reprogramming cocktails further enhances CM differentiation efficiency [143–145]. Thus, resident CFs may serve as an endogenous source of cells that could rejuvenate the diseased myocardium if redirected to a CM cell fate. Although promising, this strategy may first require modulation of pro-fibrotic responses in disease [143].
4.2. TGFβ regulation of fibroblast plasticity and proliferation
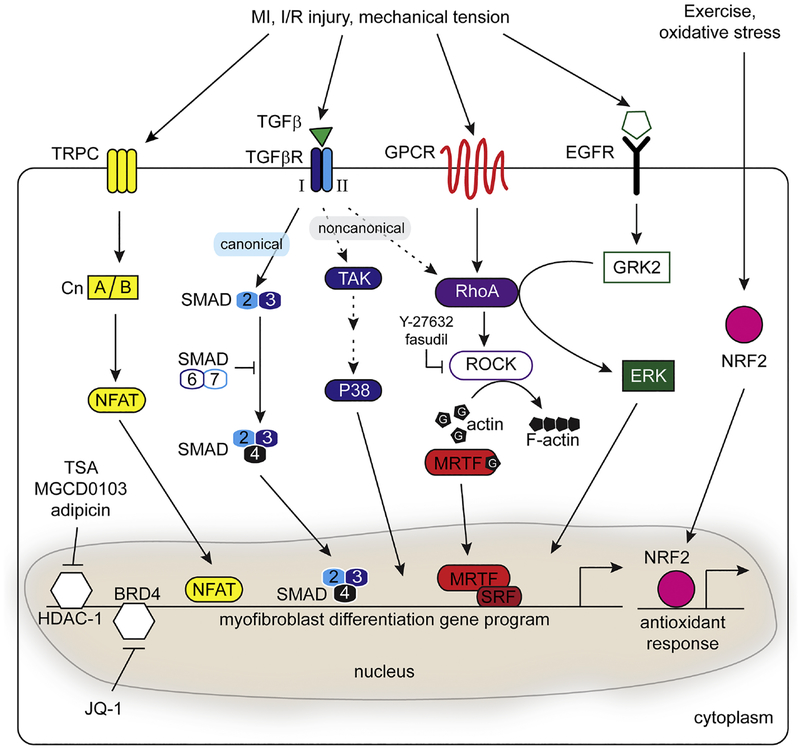
TGFβ-induced activation of canonical (SMAD) and non-canonical (TAK1/P38/MAPK) signaling pathways is critical for transcriptional regulation of myofibroblast differentiation [146–148] (Fig. 2). For instance, inhibition of SMAD3 by gene dosage or via expression of the SMAD3-inhibiting protein KLF15 decreases maladaptive diabetes-induced hypertrophy and fibrosis [149, 150]. SMAD2/3-dependent transcription can also be modulated by SMAD7, an endogenous repressor of TGFβ signaling. Reduced Smad7 levels are associated with elevated fibrosis in MI, whereas overexpression of Smad7 suppresses collagen expression [151] and attenuates angiotensin II (Ang-II) induced cardiac remodeling [152, 153]. One approach to mitigating the fibrotic response following injury is to target excessive CF proliferation. Recently, small proline rich protein 2B (Sprr2b) was shown to be induced by TGFβ/ROS treatment in CFs, and elevated in failing hearts from humans and mice [140]. In the absence of its canonical keratinocyte-binding partners, non-receptor tyrosine phosphorylation of SPRR2B promotes complex formation with USP7 and MDM2, two components of the P53 ubiquitination complex. This complex stimulates ubiquitination and degradation of P53, thereby promoting cell cycle progression and proliferation of CFs [140]. While TGFβ signaling is essential to initiate CF activation, a recent study demonstrated that ablation of TGFβR2 in activated Postn+ CFs reduced fibrosis, indicating that sustained TGFβ signaling is required to maintain CF activation [154].
Figure 2.
Signaling pathways that contribute to CF activation and stress response. Disease or mechanical stress can stimulate multiple pathways including calcineurin (Cn)/NFAT, TGFβ, GPCR, and EGFR signaling. The TGFβ pathway provides an established mechanism to stimulate SMAD-dependent CF activation and fibrosis. RhoA/MRTF-dependent signaling plays a central role in CF activation through the non-canonical TGFβ pathway, GPCR stimulation, or EGFR activation. Inhibition of TGFβ signaling by Y-27632 or Fasudil treatment, or by repressive SMAD6/7 signaling, provide nodes to control CF activation. CF activation may also be modulated through epigenetic modulation such as pharmacological inhibition of HDAC-1 or BRD4. Finally, exercise can increase mechanical load in the absence of fibrosis, potentially by maintaining an antioxidant response regulated by NRF2.
The P38 family consists of P38α/β/δ/γ that are all expressed to varying degrees in the heart and cooperate with TAK1 or TRPC6 to induce pathological remodeling [155, 156]. Inhibition of P38 signaling in the heart relieves pathological remodeling [157, 158] and knockout of tumor necrosis factor receptor associated death domain (TRADD) can blunt TAK1/P38 mediated hypertrophy and fibrosis [159]. Moreover, bleomycin-induced fibroblast activation can be blocked by the potassium ionophore, salinomyocin, in a P38-dependent manner [160]. Although these data collectively suggest that the P38/MAPK signaling axis is an attractive therapeutic target, inhibition of P38 can also induce pathological remodeling [161]. The opposing effects of P38/MAPK inhibition may stem from differences in the activity of specific P38 isoforms. For instance, in vitro models of cardiac ischemia reveal activation of P38α but deactivation of P38β, suggesting that P38α may play a central role in the progression to heart failure [162]. Furthermore, P38α is stimulated by the pro-inflammatory cytokines IL1α and TNFα, whereas P38γ promotes adaptation of skeletal muscle to exercise by upregulating PGC1α expression [163, 164]. Selective inhibition of specific P38 isoforms may be required to promote protection versus dysfunction downstream of TGFβ.
4.3. RhoA and MRTFs
While RhoA and MRTFs appear to be necessary for CM integrity and survival when mice are challenged by pressure overload, these pathways are also responsible for driving the pathological CF response to injury [128–130]. Myofibroblast differentiation requires MRTF- dependent activation of smooth muscle contractile genes (Acta2, Tagln, Cnn1) and ECM (Col1a2) in CFs [165]. In serum-depleted conditions, forced overexpression of MRTF-A is sufficient to upregulate cytoskeletal genes associated with the myofibroblast phenotype [12, 166] (Fig. 2). Conversely, MRTF-A null mice exhibit decreased scar formation induced by MI or Ang-II infusion [167]. While MRTFs appear to have dichotomous roles and maladaptive remodeling has toxic effects on the heart, wound healing is a natural defense mechanism to prevent myocardial rupture following acute cardiac injury. MRTF-dependent fibroblast activation is essential for physiological processes including cell migration and skin wound healing [71, 168, 169]. Thus, a fine balance of MRTF activity may be required in CFs, which could be accomplished by modulating upstream regulators such as RhoA in a temporal manner. Like MRTFs, RhoA/ROCK signaling also promotes pro-fibrotic pathways when CFs are stimulated with Ang-II [170]. Pharmacological inhibition of ROCK with Fasudil or Y-27632 prevents cardiac remodeling and fibrosis in vitro and in vivo [167, 169, 171, 172] (Fig. 2). More recently, screens have also identified CCG-1432 and its chemical derivatives as effective inhibitors of fibroblast activation by preventing nuclear translocation of MRTFs in fibroblasts from the lung and skin [173, 174]. An alternative strategy to regulate MRTF signaling may be through modulation of interacting proteins such as four-and-a-half LIM-only protein 2 (FHL2), as overexpression of FHL2 can also disrupt activation of a subset of MRTF/SRF target genes including Acta2 [175]. The relationship between FHL2 and MRTF/SRF is complex, as FHL2 can prevent degradation and stabilize expression of MRTF-A [176] and loss of FHL2 impairs SRF-dependent migration [177]. However, the evidence collectively suggests that targeting the RhoA/MRTF/SRF axis may attenuate maladaptive remodeling in situations of persistent stress.
RhoA can also be regulated through the G-protein coupled receptor kinase (GRK2). The function of GRK2 has been best characterized in the CMs where it promotes GPCR recycling to maintain contractility [178]. Increased GRK2 activity is commonly associated with the progression to HF, and genetic deletion of GRK2 is sufficient to prevent development of HF following MI [179, 180]. More recently, GRK2 has been shown to form a direct complex with the catalytic domain of RhoA to activate ERK in an EGFR-dependent manner [181] (Fig. 2). Consistent with this finding, ablation of GRK2 in Col1a2-positive cells attenuates CF activation, pathological remodeling, and neutrophil recruitment 48 hours after I/R injury [128, 182]. Multiple studies suggest beneficial roles for GRK2 in this inflammatory cell recruitment to initiate the reparative process and inhibition of p38/MAPK signaling in vitro [182–184]. Together, these data suggest that regulation of CF-specific GRK2 activity contributes to the reparative resolution phase following cardiac injury during which there is activation of RhoA-dependent pathways.
4.4. Exercise-induced pathways in CFs
In contrast to the cardiac response to disease, the hypertrophic response to exercise or pregnancy is not accompanied by fibrosis. Stimulation of the Akt pathway in the context of physiological cues such as microRNAs may be an important distinguishing factor between pathological and physiological hypertrophy [23]. MiR-33 is one such factor expressed by CFs that is required for CF proliferation, survival after oxidative stress, and stimulation of Akt signaling [185]. Loss of miR-33a also impairs systolic function in mouse models after TAC, consistent with the observation that miR-33 levels are decreased in human heart failure patients [185]. Another example is miR-29 which is expressed in both CFs and CMs [186, 187]. In CFs, miR-29 is upregulated by exercise and decreased after MI, and knockdown of miR-29 increases expression of collagen [186, 188]. Thus, exercise-induced miR expression may be a predictive factor for physiological rather than pathological CF responses.
The accumulation of ROS is often considered pathological and has been demonstrated to activate fibroblasts [189]. Clearance of ROS is an essential process that occurs through redundant pathways, but includes upregulation of antioxidants such as glutathiones and metallothioneins (MT) [190]. N-acetylcysteine, a glutathione precursor, has been successfully used to resolve fibrosis in a rabbit model of cardiomyopathy and reverse hypertrophy in human patients [191, 192]. Regulation of the antioxidant response program is controlled by Nuclear factor (erythroid-derived 2)-like 2 (Nfe2l2, or Nrf2) and MRTF can induce the expression of Nrf2 in vitro [193] (Fig. 2). Although acute lethality was observed when Nrf2-deficient animals were subjected to TAC, Nrf2−/− animals that survived to 14 days post TAC exhibited decreased cardiac function [194]. Consistent with these findings, transcriptional differences in CFs after exercise or disease demonstrated that an NRF-mediated ROS detoxification program is maintained in healthy CFs but is lost in CFs after pressure overload [195]. Metallothionein 1 (Mt1) was among the most highly differentially expressed genes between exercise and disease and belongs to the MT family of ROS and toxin scavengers. Animals lacking both Mt1 and Mt2 exhibited signs of exercise intolerance and cardiac dysfunction when subjected to swim training as a model of physiological remodeling [195]. These findings suggest that clearance of excessive oxidative stress in CFs is an important step in preventing pathological remodeling.
4.5. Epigenetic regulation of cardiac fibrosis
In addition to studying transcription factors and their upstream regulators, groups have recently targeted epigenetic modifiers to investigate the role of epigenetics on the fibrotic response (Fig. 2). Indeed, class I histone deacetylases (HDACs) have been implicated in Ang-II mediated fibrosis, but the class I HDAC inhibitors TSA, MGCD0103, and apicidin can block cell- cycle progression of CFs [196, 197]. While HDAC inhibitors provide an exciting therapeutic avenue for cardiac fibrosis, MGCD0103 paradoxically induces expression of pro-thrombotic plasminogen activator inhibitor 1 (PAI-1) in CFs. These findings warrant additional investigation into the ramifications of modulating epigenetics for therapeutic benefit. Epigenetic readers, such as the bromodomain-containing protein 4 (BRD4), have also been studied in the fibrotic response. Inhibition of BRD4 with miR-9 or the small molecule JQ1 potently attenuated MI or TAC-induced remodeling without affecting the ability of the heart to respond to physiological stimuli such as exercise [198, 199]. These findings suggest the possibility of blocking progression of CF-dependent pathological remodeling through epigenetic regulation, rather than targeting of transcription factors.
5. Intercellular communication
This review has highlighted multiple cell-autonomous mechanisms of cardioprotection in CMs and CFs. However, it is becoming increasingly clear that extracellular context and cell-cell communication influences how the heart responds to stress. Intricate dialogue is exchanged between CMs and CFs through direct cell contact, cytokine release, or secretion of miRNAs and other signaling molecules via exosomes [200–204]. In addition to CMs and CFs, immune and endothelial cells (ECs) also react to paracrine signals. Here we highlight examples of cellular crosstalk to describe how interactions between various cell types influence cardioprotective mechanisms.
The immune system, including cells such as neutrophils, dendritic cells, and macrophages, plays a critical role in modulating the heart’s response to ischemic injury and regeneration [205]. A recent study demonstrated that CCR2+, and not CCR2-, resident macrophages promote monocyte infiltration; suggesting that heterogeneity within sub- populations of macrophages helps to direct monocyte recruitment [206]. Depletion of CCR2+ resident macrophages also improves cardiac outcome 28 days after I/R injury [206]. Similarly, cardiac remodeling may depend on differential stimulation of ventricular CFs with cytokines such as IFNγ or IL-10 that direct recruitment of distinct subpopulations of monocytes [207]. A recent analysis of CFs isolated 0–7 days post-MI revealed dynamic shifts in the transcriptional and secretion profiles of CFs, implicating CFs as mediators of the acute inflammatory and angiogenic injury response [208].
Indeed, CFs may function as important modulatory cells to balance pro-fibrotic mechanisms. CF-derived expression of CCN1 can mediate integrin dependent CM proliferation as part of recovery from injury [73, 209]. Similarly, RhoA-dependent induction of CCN1 in CMs can provide integrin-dependent, Akt-mediated protection [73]. Overexpression of CCN1 promotes CF senescence and reduces fibrosis [210, 211]. Although conditional deletion of CCN2 results in acute lethality within 2 weeks of TAC surgery, surviving animals appeared to be protected from pathological remodeling [212]. Consistent with these findings, recent work has demonstrated that CM-secreted CCN2 had no effect on fibrosis while CF-secreted CCN2 promotes fibroblast activation and proliferation [213]. Thus, CCN2 appears to act in an autocrine manner whereas CCN1 may function in both autocrine and paracrine mechanisms. The pro-fibrotic effects of CCN2 in CFs can be mitigated by overexpression of CCN5 and miR- 133 or miR-30, which can counteract PE-induced cardiac remodeling and reduce CCN2 transcripts, respectively [214–217]. CCN proteins also have pleiotropic effects on other cardiac cell types; for instance, CCN1/2 promotes neovascularization in hibernating pig myocardium and ischemic hindlimb mouse models [218]. Thus, context and cell type are important considerations when modulating ECM-associated proteins such as the CCN family. Due to their contractile nature, CMs are subjected to a great deal of mechanical stress, and chronic pathological stress can trigger cell death. Mechanical tension is not only transmitted by direct contact between similar cell types; different cell types can form functional physical interactions. For instance, electrical current delivered to functional myocardial tissue in a transmural cryoinjury model can be detected in the scar, but depolarizations are reduced in animals lacking Cx43 in fibroblast-specific-protein-1 (FSP1) expressing cells [219]. Models of I/R injury demonstrate that necrotic CMs can elicit fibrosis and inflammation through the release of damage associated molecular patterns (DAMPs) and cytokines [220, 221]. ATP is one such DAMP secreted by CMs, which binds to the ATP-sensitive P2Y11 receptor (P2Y11R) on CFs to promote CF activation [222]. In other contexts, purinergic receptor activation can promote chemotaxis of neutrophils [223]. Although prolonged neutrophil recruitment is detrimental, neutrophils are required to initiate the reparative process that occurs 4–14 days post MI [184, 205]. Of note, P2Y11R stimulation in CFs may be beneficial; conditioned medium from human CFs treated with the P2Y11R agonist NF546 promotes pro-survival signaling in CMs subjected to hypoxia/reoxygenation (H/R) injury by activating the RISK pathway [224]. Furthermore, the secretome of P2Y11R stimulated CFs suppresses dendritic cell activation by LPS [224]. H/R injury likely promotes CF-mediated protection of CMs through multiple factors. For instance, CFs secrete tissue inhibitor of metalloproteinases-1 (TIMP-1) which promotes CM survival, in part, by stimulating PI3K/Akt signaling [225]. Altogether, these and other studies demonstrate that hypertrophic and cardioprotective responses are regulated by a complex interplay of communication between various cardiac cell types.
6. Conclusion
Numerous signaling pathways promote cardiac healing after injury through expression of protective factors in CMs and CFs; however, many of these factors also promote the progression of fibrosis and heart failure under chronic activation. For example, it remains unclear how chronic stimulation of CMs and CFs induces the RhoA/MRTF/SRF transcription pathway to sabotage cardiac function, yet interaction with other signaling pathways provide mechanisms for acute cardioprotection. Although a detailed understanding of how specific signaling pathways interact within individual cell types is critical to identifying cardioprotective factors, a number of questions remain unanswered that may resolve this paradox. How does modulating the behavior of one cell type impact the behavior of neighboring cell types? In an era of precision medicine and pharmacogenomics, are some of these consequences of greater significance to specific patient populations? Looking forward, an approach that considers intercellular communication combined with cell type heterogeneity in signaling responses will more comprehensively explain how the heart rapidly responds to cardiac injury.
Acknowledgements
CSB received support from National Institutes of Health (T32HL007444 and F32HL140851) and the American Heart Association (19POST34430051). JKL received support from a T32 training grant from the National Institutes of Health (T32 HL066988–13), and the American Heart Association (15POST25550114). MAT received support from a T32 training grant from the National Institutes of Health (T32HL07572). We would like to acknowledge the support of our advisors, Dr. Joan Heller Brown, Dr. Eric M. Small, and Dr. William T. Pu.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
The authors report no commercial or proprietary interest in any product or concept discussed in this article.
References
- [1].Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74:1124–36. [DOI] [PubMed] [Google Scholar]
- [2].Liu GS, Thornton J, Van Winkle DM, Stanley AW, Olsson RA, Downey JM. Protection against infarction afforded by preconditioning is mediated by A1 adenosine receptors in rabbit heart. Circulation. 1991;84:350–6. [DOI] [PubMed] [Google Scholar]
- [3].Gattullo D, Linden RJ, Losano G, Pagliaro P, Westerhof N. Ischaemic preconditioning changes the pattern of coronary reactive hyperaemia in the goat: role of adenosine and nitric oxide. Cardiovasc Res. 1999;42:57–64. [DOI] [PubMed] [Google Scholar]
- [4].Liu Y, Downey JM. Ischemic preconditioning protects against infarction in rat heart. The American journal of physiology. 1992;263:H1107–12. [DOI] [PubMed] [Google Scholar]
- [5].Richard V, Kaeffer N, Tron C, Thuillez C. Ischemic preconditioning protects against coronary endothelial dysfunction induced by ischemia and reperfusion. Circulation. 1994;89:1254–61. [DOI] [PubMed] [Google Scholar]
- [6].Stein AB, Bolli R, Guo Y, Wang OL, Tan W, Wu WJ, et al. The late phase of ischemic preconditioning induces a prosurvival genetic program that results in marked attenuation of apoptosis. J Mol Cell Cardiol. 2007;42:1075–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Huang J, Ito Y, Morikawa M, Uchida H, Kobune M, Sasaki K, et al. Bcl-xL gene transfer protects the heart against ischemia/reperfusion injury. Biochem Biophys Res Commun. 2003;311:64–70. [DOI] [PubMed] [Google Scholar]
- [8].Andreotti F, Pasceri V, Hackett DR, Davies GJ, Haider AW, Maseri A. Preinfarction angina as a predictor of more rapid coronary thrombolysis in patients with acute myocardial infarction. N Engl J Med. 1996;334:7–12. [DOI] [PubMed] [Google Scholar]
- [9].Jenkins DP, Pugsley WB, Alkhulaifi AM, Kemp M, Hooper J, Yellon DM. Ischaemic preconditioning reduces troponin T release in patients undergoing coronary artery bypass surgery. Heart. 1997;77:314–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Scheuer J, Greenberg MA, Zohman LR. Exercise training in patients with coronary artery disease. Modern concepts of cardiovascular disease. 1978;47:85–90. [PubMed] [Google Scholar]
- [11].McElroy CL, Gissen SA, Fishbein MC. Exercise-induced reduction in myocardial infarct size after coronary artery occlusion in the rat. Circulation. 1978;57:958–62. [DOI] [PubMed] [Google Scholar]
- [12].O’Connor CM, Whellan DJ, Lee KL, Keteyian SJ, Cooper LS, Ellis SJ, et al. Efficacy and safety of exercise training in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA. 2009;301:1439–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Niebauer J, Hambrecht R, Velich T, Hauer K, Marburger C, Kalberer B, et al. Attenuated progression of coronary artery disease after 6 years of multifactorial risk intervention: role of physical exercise. Circulation. 1997;96:2534–41. [DOI] [PubMed] [Google Scholar]
- [14].Waring CD, Vicinanza C, Papalamprou A, Smith AJ, Purushothaman S, Goldspink DF, et al. The adult heart responds to increased workload with physiologic hypertrophy, cardiac stem cell activation, and new myocyte formation. Eur Heart J. 2014;35:2722–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Vujic A, Lerchenmuller C, Wu TD, Guillermier C, Rabolli CP, Gonzalez E, et al. Exercise induces new cardiomyocyte generation in the adult mammalian heart. Nature communications. 2018;9:1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Konhilas JP, Watson PA, Maass A, Boucek DM, Horn T, Stauffer BL, et al. Exercise can prevent and reverse the severity of hypertrophic cardiomyopathy. Circ Res. 2006;98:540–8. [DOI] [PubMed] [Google Scholar]
- [17].Wei X, Liu X, Rosenzweig A. What do we know about the cardiac benefits of exercise? Trends Cardiovasc Med. 2015;25:529–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Pina IL, Apstein CS, Balady GJ, Belardinelli R, Chaitman BR, Duscha BD, et al. Exercise and heart failure: A statement from the American Heart Association Committee on exercise, rehabilitation, and prevention. Circulation. 2003;107:1210–25. [DOI] [PubMed] [Google Scholar]
- [19].Manning BD, Toker A. AKT/PKB Signaling: Navigating the Network. Cell. 2017;169:381–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Rossello X, Yellon DM. The RISK pathway and beyond. Basic research in cardiology. 2017;113:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].McMullen JR, Shioi T, Huang WY, Zhang L, Tarnavski O, Bisping E, et al. The insulin-like growth factor 1 receptor induces physiological heart growth via the phosphoinositide 3- kinase(p110alpha) pathway. J Biol Chem. 2004;279:4782–93. [DOI] [PubMed] [Google Scholar]
- [22].McMullen JR, Shioi T, Zhang L, Tarnavski O, Sherwood MC, Kang PM, et al. Phosphoinositide 3-kinase(p110alpha) plays a critical role for the induction of physiological, but not pathological, cardiac hypertrophy. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:12355–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Kemi OJ, Ceci M, Wisloff U, Grimaldi S, Gallo P, Smith GL, et al. Activation or inactivation of cardiac Akt/mTOR signaling diverges physiological from pathological hypertrophy. J Cell Physiol. 2008;214:316–21. [DOI] [PubMed] [Google Scholar]
- [24].McMullen JR, Amirahmadi F, Woodcock EA, Schinke-Braun M, Bouwman RD, Hewitt KA, et al. Protective effects of exercise and phosphoinositide 3-kinase(p110alpha) signaling in dilated and hypertrophic cardiomyopathy. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:612–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Matsui T, Tao J, del Monte F, Lee KH, Li L, Picard M, et al. Akt activation preserves cardiac function and prevents injury after transient cardiac ischemia in vivo. Circulation. 2001;104:330–5. [DOI] [PubMed] [Google Scholar]
- [26].Matsui T, Li L, Wu JC, Cook SA, Nagoshi T, Picard MH, et al. Phenotypic spectrum caused by transgenic overexpression of activated Akt in the heart. J Biol Chem. 2002;277:22896–901. [DOI] [PubMed] [Google Scholar]
- [27].Nagoshi T, Matsui T, Aoyama T, Leri A, Anversa P, Li L, et al. PI3K rescues the detrimental effects of chronic Akt activation in the heart during ischemia/reperfusion injury. J Clin Invest. 2005;115:2128–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Serra AJ, Santos MH, Bocalini DS, Antonio EL, Levy RF, Santos AA, et al. Exercise training inhibits inflammatory cytokines and more than prevents myocardial dysfunction in rats with sustained beta-adrenergic hyperactivity. J Physiol. 2010;588:2431–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Ozes ON, Mayo LD, Gustin JA, Pfeffer SR, Pfeffer LM, Donner DB. NF-kappaB activation by tumour necrosis factor requires the Akt serine-threonine kinase. Nature. 1999;401:82–5. [DOI] [PubMed] [Google Scholar]
- [30].Liu T, Zhang L, Joo D, Sun SC. NF-kappaB signaling in inflammation. Signal Transduct Target Ther. 2017;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Zou J, Li H, Chen X, Zeng S, Ye J, Zhou C, et al. C/EBPbeta knockdown protects cardiomyocytes from hypertrophy via inhibition of p65-NFkappaB. Mol Cell Endocrinol. 2014;390:18–25. [DOI] [PubMed] [Google Scholar]
- [32].Bostrom P, Mann N, Wu J, Quintero PA, Plovie ER, Panakova D, et al. C/EBPbeta controls exercise-induced cardiac growth and protects against pathological cardiac remodeling. Cell. 2010;143:1072–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Huang GN, Thatcher JE, McAnally J, Kong Y, Qi X, Tan W, et al. C/EBP transcription factors mediate epicardial activation during heart development and injury. Science. 2012;338:1599–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Ryall KA, Bezzerides VJ, Rosenzweig A, Saucerman JJ. Phenotypic screen quantifying differential regulation of cardiac myocyte hypertrophy identifies CITED4 regulation of myocyte elongation. J Mol Cell Cardiol. 2014;72:74–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Liu X, Xiao J, Zhu H, Wei X, Platt C, Damilano F, et al. miR-222 is necessary for exercise- induced cardiac growth and protects against pathological cardiac remodeling. Cell Metab. 2015;21:584–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Bezzerides VJ, Platt C, Lerchenmuller C, Paruchuri K, Oh NL, Xiao C, et al. CITED4 induces physiologic hypertrophy and promotes functional recovery after ischemic injury. JCI Insight. 2016;1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Bei Y, Fu S, Chen X, Chen M, Zhou Q, Yu P, et al. Cardiac cell proliferation is not necessary for exercise-induced cardiac growth but required for its protection against ischaemia/reperfusion injury. J Cell Mol Med. 2017;21:1648–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Brown DA, Perry JB, Allen ME, Sabbah HN, Stauffer BL, Shaikh SR, et al. Expert consensus document: Mitochondrial function as a therapeutic target in heart failure. Nat Rev Cardiol. 2017;14:238–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Marcil M, Bourduas K, Ascah A, Burelle Y. Exercise training induces respiratory substrate- specific decrease in Ca2+-induced permeability transition pore opening in heart mitochondria. Am J Physiol Heart Circ Physiol. 2006;290:H1549–57. [DOI] [PubMed] [Google Scholar]
- [40].Kavazis AN, McClung JM, Hood DA, Powers SK. Exercise induces a cardiac mitochondrial phenotype that resists apoptotic stimuli. Am J Physiol Heart Circ Physiol. 2008;294:H928–35. [DOI] [PubMed] [Google Scholar]
- [41].Miyamoto S, Murphy AN, Brown JH. Akt mediates mitochondrial protection in cardiomyocytes through phosphorylation of mitochondrial hexokinase-II. Cell Death Differ. 2008;15:521–9. [DOI] [PubMed] [Google Scholar]
- [42].Kavazis AN, Alvarez S, Talbert E, Lee Y, Powers SK. Exercise training induces a cardioprotective phenotype and alterations in cardiac subsarcolemmal and intermyofibrillar mitochondrial proteins. Am J Physiol Heart Circ Physiol. 2009;297:H144–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Noh J, Wende AR, Olsen CD, Kim B, Bevins J, Zhu Y, et al. Phosphoinositide dependent protein kinase 1 is required for exercise-induced cardiac hypertrophy but not the associated mitochondrial adaptations. J Mol Cell Cardiol. 2015;89:297–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Coronado M, Fajardo G, Nguyen K, Zhao M, Kooiker K, Jung G, et al. Physiological Mitochondrial Fragmentation Is a Normal Cardiac Adaptation to Increased Energy Demand. Circ Res. 2018;122:282–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Shirakabe A, Zhai P, Ikeda Y, Saito T, Maejima Y, Hsu CP, et al. Drp1-Dependent Mitochondrial Autophagy Plays a Protective Role Against Pressure Overload-Induced Mitochondrial Dysfunction and Heart Failure. Circulation. 2016;133:1249–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Ikeda Y, Shirakabe A, Maejima Y, Zhai P, Sciarretta S, Toli J, et al. Endogenous Drp1 mediates mitochondrial autophagy and protects the heart against energy stress. Circ Res. 2015;116:264–78. [DOI] [PubMed] [Google Scholar]
- [47].Lee Y, Lee HY, Hanna RA, Gustafsson AB. Mitochondrial autophagy by Bnip3 involves Drp1-mediated mitochondrial fission and recruitment of Parkin in cardiac myocytes. Am J Physiol Heart Circ Physiol. 2011;301:H1924–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Brand CS, Tan VP, Brown JH, Miyamoto S. RhoA regulates Drp1 mediated mitochondrial fission through ROCK to protect cardiomyocytes. Cell Signal. 2018;50:48–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Twig G, Elorza A, Molina AJ, Mohamed H, Wikstrom JD, Walzer G, et al. Fission and selective fusion govern mitochondrial segregation and elimination by autophagy. EMBO J. 2008;27:433–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Xiang SY, Ouyang K, Yung BS, Miyamoto S, Smrcka AV, Chen J, et al. PLCepsilon, PKD1, and SSH1L transduce RhoA signaling to protect mitochondria from oxidative stress in the heart. Sci Signal. 2013;6:ra108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Campbell CT, Kolesar JE, Kaufman BA. Mitochondrial transcription factor A regulates mitochondrial transcription initiation, DNA packaging, and genome copy number. Biochim Biophys Acta. 2012;1819:921–9. [DOI] [PubMed] [Google Scholar]
- [52].Karamanlidis G, Nascimben L, Couper GS, Shekar PS, del Monte F, Tian R. Defective DNA replication impairs mitochondrial biogenesis in human failing hearts. Circ Res. 2010;106:1541–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Ide T, Tsutsui H, Hayashidani S, Kang D, Suematsu N, Nakamura K, et al. Mitochondrial DNA damage and dysfunction associated with oxidative stress in failing hearts after myocardial infarction. Circ Res. 2001;88:529–35. [DOI] [PubMed] [Google Scholar]
- [54].Ikeuchi M, Matsusaka H, Kang D, Matsushima S, Ide T, Kubota T, et al. Overexpression of mitochondrial transcription factor a ameliorates mitochondrial deficiencies and cardiac failure after myocardial infarction. Circulation. 2005;112:683–90. [DOI] [PubMed] [Google Scholar]
- [55].Fujino T, Ide T, Yoshida M, Onitsuka K, Tanaka A, Hata Y, et al. Recombinant mitochondrial transcription factor A protein inhibits nuclear factor of activated T cells signaling and attenuates pathological hypertrophy of cardiac myocytes. Mitochondrion. 2012;12:449–58. [DOI] [PubMed] [Google Scholar]
- [56].Hodge RG, Ridley AJ. Regulating Rho GTPases and their regulators. Nat Rev Mol Cell Biol. 2016;17:496–510. [DOI] [PubMed] [Google Scholar]
- [57].Geneste O, Copeland JW, Treisman R. LIM kinase and Diaphanous cooperate to regulate serum response factor and actin dynamics. The Journal of cell biology. 2002;157:831–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Kimura K, Ito M, Amano M, Chihara K, Fukata Y, Nakafuku M, et al. Regulation of myosin phosphatase by Rho and Rho-associated kinase (Rho-kinase). Science. 1996;273:245–8. [DOI] [PubMed] [Google Scholar]
- [59].Higashi M, Shimokawa H, Hattori T, Hiroki J, Mukai Y, Morikawa K, et al. Long-term inhibition of Rho-kinase suppresses angiotensin II-induced cardiovascular hypertrophy in rats in vivo: effect on endothelial NAD(P)H oxidase system. Circ Res. 2003;93:767–75. [DOI] [PubMed] [Google Scholar]
- [60].Sah VP, Minamisawa S, Tam SP, Wu TH, Dorn GW 2nd, Ross J Jr., et al. Cardiac- specific overexpression of RhoA results in sinus and atrioventricular nodal dysfunction and contractile failure. J Clin Invest. 1999;103:1627–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Shi J, Zhang YW, Summers LJ, Dorn GW, 2nd, Wei L. Disruption of ROCK1 gene attenuates cardiac dilation and improves contractile function in pathological cardiac hypertrophy. J Mol Cell Cardiol. 2008;44:551–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Xiang SY, Vanhoutte D, Del Re DP, Purcell NH, Ling H, Banerjee I, et al. RhoA protects the mouse heart against ischemia/reperfusion injury. J Clin Invest. 2011;121:3269–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Aghajanian A, Wittchen ES, Campbell SL, Burridge K. Direct activation of RhoA by reactive oxygen species requires a redox-sensitive motif. PLoS One. 2009;4:e8045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Lauriol J, Keith K, Jaffre F, Couvillon A, Saci A, Goonasekera SA, et al. RhoA signaling in cardiomyocytes protects against stress-induced heart failure but facilitates cardiac fibrosis. Sci Signal. 2014;7:ra100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Edwards BS, Zimmerman RS, Schwab TR, Heublein DM, Burnett JC, Jr. Atrial stretch, not pressure, is the principal determinant controlling the acute release of atrial natriuretic factor. Circ Res. 1988;62:191–5. [DOI] [PubMed] [Google Scholar]
- [66].Kawamura S, Miyamoto S, Brown JH. Initiation and transduction of stretch-induced RhoA and Rac1 activation through caveolae: cytoskeletal regulation of ERK translocation. J Biol Chem. 2003;278:31111–7. [DOI] [PubMed] [Google Scholar]
- [67].Morissette MR, Sah VP, Glembotski CC, Brown JH. The Rho effector, PKN, regulates ANF gene transcription in cardiomyocytes through a serum response element. Am J Physiol Heart Circ Physiol. 2000;278:H1769–74. [DOI] [PubMed] [Google Scholar]
- [68].Del Re DP, Miyamoto S, Brown JH. Focal adhesion kinase as a RhoA-activable signaling scaffold mediating Akt activation and cardiomyocyte protection. J Biol Chem. 2008;283:35622–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Miralles F, Posern G, Zaromytidou AI, Treisman R. Actin dynamics control SRF activity by regulation of its coactivator MAL. Cell. 2003;113:329–42. [DOI] [PubMed] [Google Scholar]
- [70].Mouilleron S, Langer CA, Guettler S, McDonald NQ, Treisman R. Structure of a pentavalent G-actin*MRTF-A complex reveals how G-actin controls nucleocytoplasmic shuttling of a transcriptional coactivator. Sci Signal. 2011;4:ra40. [DOI] [PubMed] [Google Scholar]
- [71].Esnault C, Stewart A, Gualdrini F, East P, Horswell S, Matthews N, et al. Rho-actin signaling to the MRTF coactivators dominates the immediate transcriptional response to serum in fibroblasts. Genes Dev. 2014;28:943–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Olson EN, Nordheim A. Linking actin dynamics and gene transcription to drive cellular motile functions. Nat Rev Mol Cell Biol. 2010;11:353–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Zhao X, Ding EY, Yu OM, Xiang SY, Tan-Sah VP, Yung BS, et al. Induction of the matricellular protein CCN1 through RhoA and MRTF-A contributes to ischemic cardioprotection. J Mol Cell Cardiol. 2014;75:152–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Yoshida Y, Togi K, Matsumae H, Nakashima Y, Kojima Y, Yamamoto H, et al. CCN1 protects cardiac myocytes from oxidative stress via beta1 integrin-Akt pathway. Biochemical and biophysical research communications. 2007;355:611–8. [DOI] [PubMed] [Google Scholar]
- [75].Cui S, Xue L, Yang F, Dai S, Han Z, Liu K, et al. Postinfarction Hearts Are Protected by Premature Senescent Cardiomyocytes Via GATA 4-Dependent CCN 1 Secretion. J Am Heart Assoc. 2018;7:e009111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Small EM, O’Rourke JR, Moresi V, Sutherland LB, McAnally J, Gerard RD, et al. Regulation of PI3-kinase/Akt signaling by muscle-enriched microRNA-486. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:4218–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Trembley MA, Quijada P, Agullo-Pascual E, Tylock KM, Colpan M, Dirkx RA Jr., et al. Mechanosensitive Gene Regulation by Myocardin-Related Transcription Factors Is Required for Cardiomyocyte Integrity in Load-Induced Ventricular Hypertrophy. Circulation. 2018;138:1864–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Bergmann O, Bhardwaj RD, Bernard S, Zdunek S, Barnabe-Heider F, Walsh S, et al. Evidence for cardiomyocyte renewal in humans. Science. 2009;324:98–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Porrello ER, Mahmoud AI, Simpson E, Hill JA, Richardson JA, Olson EN, et al. Transient regenerative potential of the neonatal mouse heart. Science. 2011;331:1078–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Khalafalla FG, Greene S, Khan H, Ilves K, Monsanto MM, Alvarez R Jr., et al. P2Y2 Nucleotide Receptor Prompts Human Cardiac Progenitor Cell Activation by Modulating Hippo Signaling. Circ Res. 2017;121:1224–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Yu FX, Guan KL. The Hippo pathway: regulators and regulations. Genes Dev. 2013;27:355–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Li J, Gao E, Vite A, Yi R, Gomez L, Goossens S, et al. Alpha-catenins control cardiomyocyte proliferation by regulating Yap activity. Circ Res. 2015;116:70–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].von Gise A, Lin Z, Schlegelmilch K, Honor LB, Pan GM, Buck JN, et al. YAP1, the nuclear target of Hippo signaling, stimulates heart growth through cardiomyocyte proliferation but not hypertrophy. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:2394–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Xin M, Kim Y, Sutherland LB, Qi X, McAnally J, Schwartz RJ, et al. Regulation of insulin-like growth factor signaling by Yap governs cardiomyocyte proliferation and embryonic heart size. Sci Signal. 2011;4:ra70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Wang Y, Hu G, Liu F, Wang X, Wu M, Schwarz JJ, et al. Deletion of yes-associated protein (YAP) specifically in cardiac and vascular smooth muscle cells reveals a crucial role for YAP in mouse cardiovascular development. Circ Res. 2014;114:957–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Heallen T, Zhang M, Wang J, Bonilla-Claudio M, Klysik E, Johnson RL, et al. Hippo pathway inhibits Wnt signaling to restrain cardiomyocyte proliferation and heart size. Science. 2011;332:458–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Del Re DP, Yang Y, Nakano N, Cho J, Zhai P, Yamamoto T, et al. Yes-associated protein isoform 1 (Yap1) promotes cardiomyocyte survival and growth to protect against myocardial ischemic injury. J Biol Chem. 2013;288:3977–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Yang Y, Del Re DP, Nakano N, Sciarretta S, Zhai P, Park J, et al. miR-206 Mediates YAP- Induced Cardiac Hypertrophy and Survival. Circ Res. 2015;117:891–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Xin M, Kim Y, Sutherland LB, Murakami M, Qi X, McAnally J, et al. Hippo pathway effector Yap promotes cardiac regeneration. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:13839–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Lin Z, von Gise A, Zhou P, Gu F, Ma Q, Jiang J, et al. Cardiac-specific YAP activation improves cardiac function and survival in an experimental murine MI model. Circ Res. 2014;115:354–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Lin Z, Zhou P, von Gise A, Gu F, Ma Q, Chen J, et al. Pi3kcb links Hippo-YAP and PI3K- AKT signaling pathways to promote cardiomyocyte proliferation and survival. Circ Res. 2015;116:35–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Xiang FL, Guo M, Yutzey KE. Overexpression of Tbx20 in Adult Cardiomyocytes Promotes Proliferation and Improves Cardiac Function After Myocardial Infarction. Circulation. 2016;133:1081–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Leach JP, Heallen T, Zhang M, Rahmani M, Morikawa Y, Hill MC, et al. Hippo pathway deficiency reverses systolic heart failure after infarction. Nature. 2017;550:260–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Heallen T, Morikawa Y, Leach J, Tao G, Willerson JT, Johnson RL, et al. Hippo signaling impedes adult heart regeneration. Development. 2013;140:4683–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Matsuda T, Zhai P, Sciarretta S, Zhang Y, Jeong JI, Ikeda S, et al. NF2 Activates Hippo Signaling and Promotes Ischemia/Reperfusion Injury in the Heart. Circ Res. 2016;119:596–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Ikeda S, Mizushima W, Sciarretta S, Abdellatif M, Zhai P, Mukai R, et al. Hippo Deficiency Leads to Cardiac Dysfunction Accompanied by Cardiomyocyte De-Differentiation During Pressure Overload. Circ Res. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Tao G, Kahr PC, Morikawa Y, Zhang M, Rahmani M, Heallen TR, et al. Pitx2 promotes heart repair by activating the antioxidant response after cardiac injury. Nature. 2016;534:119–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Shao D, Zhai P, Del Re DP, Sciarretta S, Yabuta N, Nojima H, et al. A functional interaction between Hippo-YAP signalling and FoxO1 mediates the oxidative stress response. Nature communications. 2014;5:3315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Means CK, Xiao CY, Li Z, Zhang T, Omens JH, Ishii I, et al. Sphingosine 1-phosphate S1P2 and S1P3 receptor-mediated Akt activation protects against in vivo myocardial ischemia- reperfusion injury. Am J Physiol Heart Circ Physiol. 2007;292:H2944–51 [DOI] [PubMed] [Google Scholar]
- [100].Egom EE, Ke Y, Musa H, Mohamed TM, Wang T, Cartwright E, et al. FTY720 prevents ischemia/reperfusion injury-associated arrhythmias in an ex vivo rat heart model via activation of Pak1/Akt signaling. J Mol Cell Cardiol. 2010;48:406–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Santos-Gallego CG, Vahl TP, Goliasch G, Picatoste B, Arias T, Ishikawa K, et al. Sphingosine-1-Phosphate Receptor Agonist Fingolimod Increases Myocardial Salvage and Decreases Adverse Postinfarction Left Ventricular Remodeling in a Porcine Model of Ischemia/Reperfusion. Circulation. 2016;133:954–66 [DOI] [PubMed] [Google Scholar]
- [102].Hofmann U, Hu K, Walter F, Burkard N, Ertl G, Bauersachs J, et al. Pharmacological pre- and post-conditioning with the sphingosine-1-phosphate receptor modulator FTY720 after myocardial ischaemia-reperfusion. British journal of pharmacology. 2010;160:1243–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Yung BS, Brand CS, Xiang SY, Gray CB, Means CK, Rosen H, et al. Selective coupling of the S1P3 receptor subtype to S1P-mediated RhoA activation and cardioprotection. J Mol Cell Cardiol. 2017;103:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Castaldi A, Chesini GP, Taylor AE, Sussman MA, Brown JH, Purcell NH. Sphingosine 1- phosphate elicits RhoA-dependent proliferation and MRTF-A mediated gene induction in CPCs. Cell Signal. 2016;28:871–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Miller E, Yang J, DeRan M, Wu C, Su AI, Bonamy GM, et al. Identification of serum- derived sphingosine-1-phosphate as a small molecule regulator of YAP. Chem Biol. 2012;19:955–62. [DOI] [PubMed] [Google Scholar]
- [106].Yu FX, Zhao B, Panupinthu N, Jewell JL, Lian I, Wang LH, et al. Regulation of the Hippo- YAP pathway by G-protein-coupled receptor signaling. Cell. 2012;150:780–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Yan H, Yi S, Zhuang H, Wu L, Wang DW, Jiang J. Sphingosine-1-phosphate ameliorates the cardiac hypertrophic response through inhibiting the activity of histone deacetylase-2. Int J Mol Med. 2018;41:1704–14. [DOI] [PubMed] [Google Scholar]
- [108].Walsh CT, Stupack D, Brown JH. G protein-coupled receptors go extracellular: RhoA integrates the integrins. Mol Interv. 2008;8:165–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Offermanns S G-proteins as transducers in transmembrane signalling. Prog Biophys Mol Biol. 2003;83:101–30. [DOI] [PubMed] [Google Scholar]
- [110].Communal C, Singh K, Sawyer DB, Colucci WS. Opposing effects of beta(1)- and beta(2)- adrenergic receptors on cardiac myocyte apoptosis : role of a pertussis toxin-sensitive G protein. Circulation. 1999;100:2210–2. [DOI] [PubMed] [Google Scholar]
- [111].Chesley A, Lundberg MS, Asai T, Xiao RP, Ohtani S, Lakatta EG, et al. The beta(2)- adrenergic receptor delivers an antiapoptotic signal to cardiac myocytes through G(i)-dependent coupling to phosphatidylinositol 3’-kinase. Circ Res. 2000;87:1172–9. [DOI] [PubMed] [Google Scholar]
- [112].Fajardo G, Zhao M, Berry G, Wong LJ, Mochly-Rosen D, Bernstein D. beta2-adrenergic receptors mediate cardioprotection through crosstalk with mitochondrial cell death pathways. J Mol Cell Cardiol. 2011;51:781–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Vaniotis G, Del Duca D, Trieu P, Rohlicek CV, Hebert TE, Allen BG. Nuclear beta- adrenergic receptors modulate gene expression in adult rat heart. Cell Signal. 2011;23:89–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Vaniotis G, Glazkova I, Merlen C, Smith C, Villeneuve LR, Chatenet D, et al. Regulation of cardiac nitric oxide signaling by nuclear beta-adrenergic and endothelin receptors. J Mol Cell Cardiol. 2013;62:58–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Lin Y, Zheng C, Liu Y, Wang L, Gong H. Effect of adenovirus mediated beta2-AR overexpression on IL-10 level secreted by cardiomyocytes of heart failure rats. Exp Ther Med. 2016;12:1349–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Murray DR, Mummidi S, Valente AJ, Yoshida T, Somanna NK, Delafontaine P, et al. beta2 adrenergic activation induces the expression of IL-18 binding protein, a potent inhibitor of isoproterenol induced cardiomyocyte hypertrophy in vitro and myocardial hypertrophy in vivo. J Mol Cell Cardiol. 2012;52:206–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Talarico JA, Carter RL, Grisanti LA, Yu JE, Repas AA, Tilley DG. beta-adrenergic receptor-dependent alterations in murine cardiac transcript expression are differentially regulated by gefitinib in vivo. PLoS One. 2014;9:e99195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Grisanti LA, Talarico JA, Carter RL, Yu JE, Repas AA, Radcliffe SW, et al. beta- Adrenergic receptor-mediated transactivation of epidermal growth factor receptor decreases cardiomyocyte apoptosis through differential subcellular activation of ERK1/2 and Akt. J Mol Cell Cardiol. 2014;72:39–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Grisanti LA, Repas AA, Talarico JA, Gold JI, Carter RL, Koch WJ, et al. Temporal and gefitinib-sensitive regulation of cardiac cytokine expression via chronic beta-adrenergic receptor stimulation. Am J Physiol Heart Circ Physiol. 2015;308:H316–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Schultz JJ, Hsu AK, Gross GJ. Ischemic preconditioning and morphine-induced cardioprotection involve the delta (delta)-opioid receptor in the intact rat heart. J Mol Cell Cardiol. 1997;29:2187–95. [DOI] [PubMed] [Google Scholar]
- [121].Frassdorf J, Weber NC, Obal D, Toma O, Mullenheim J, Kojda G, et al. Morphine induces late cardioprotection in rat hearts in vivo: the involvement of opioid receptors and nuclear transcription factor kappaB. Anesth Analg. 2005;101:934–41, table of contents. [DOI] [PubMed] [Google Scholar]
- [122].Patel HH, Fryer RM, Gross ER, Bundey RA, Hsu AK, Isbell M, et al. 12-lipoxygenase in opioid-induced delayed cardioprotection: gene array, mass spectrometric, and pharmacological analyses. Circ Res. 2003;92:676–82. [DOI] [PubMed] [Google Scholar]
- [123].Ashton KJ, Tupicoff A, Williams-Pritchard G, Kiessling CJ, See Hoe LE, Headrick JP, et al. Unique transcriptional profile of sustained ligand-activated preconditioning in pre- and post- ischemic myocardium. PLoS One. 2013;8:e72278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Huang MH, Wang HQ, Roeske WR, Birnbaum Y, Wu Y, Yang NP, et al. Mediating delta-opioid-initiated heart protection via the beta2-adrenergic receptor: role of the intrinsic cardiac adrenergic cell. Am J Physiol Heart Circ Physiol. 2007;293:H376–84. [DOI] [PubMed] [Google Scholar]
- [125].McCloskey DT, Turnbull L, Swigart P, O’Connell TD, Simpson PC, Baker AJ. Abnormal myocardial contraction in alpha(1A)- and alpha(1B)-adrenoceptor double-knockout mice. J Mol Cell Cardiol. 2003;35:1207–16. [DOI] [PubMed] [Google Scholar]
- [126].O’Connell TD, Swigart PM, Rodrigo MC, Ishizaka S, Joho S, Turnbull L, et al. Alpha1- adrenergic receptors prevent a maladaptive cardiac response to pressure overload. J Clin Invest. 2006;116:1005–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Yu X, Jia B, Wang F, Lv X, Peng X, Wang Y, et al. alpha(1) adrenoceptor activation by norepinephrine inhibits LPS-induced cardiomyocyte TNF-alpha production via modulating ERK1/2 and NF-kappaB pathway. J Cell Mol Med. 2014;18:263–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Travers JG, Kamal FA, Robbins J, Yutzey KE, Blaxall BC. Cardiac Fibrosis: The Fi broblast Awakens. Circ Res. 2016;118:1021–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Frangogiannis NG. The extracellular matrix in myocardial injury, repair, and remodeling. J Clin Invest. 2017;127:1600–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Lighthouse JK, Small EM. Transcriptional control of cardiac fibroblast plasticity. J Mol Cell Cardiol. 2016;91:52–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Driesen RB, Nagaraju CK, Abi-Char J, Coenen T, Lijnen PJ, Fagard RH, et al. Reversible and irreversible differentiation of cardiac fibroblasts. Cardiovasc Res. 2014;101:411–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Vivar R, Humeres C, Ayala P, Olmedo I, Catalan M, Garcia L, et al. TGF-beta1 prevents simulated ischemia/reperfusion-induced cardiac fibroblast apoptosis by activation of both canonical and non-canonical signaling pathways. Biochim Biophys Acta. 2013;1832:754–62. [DOI] [PubMed] [Google Scholar]
- [133].Fu X, Khalil H, Kanisicak O, Boyer JG, Vagnozzi RJ, Maliken BD, et al. Specialized fibroblast differentiated states underlie scar formation in the infarcted mouse heart. J Clin Invest. 2018;128:2127–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Molkentin JD, Bugg D, Ghearing N, Dorn LE, Kim P, Sargent MA, et al. Fibroblast-Specific Genetic Manipulation of p38 Mitogen-Activated Protein Kinase In Vivo Reveals Its Central Regulatory Role in Fibrosis. Circulation. 2017;136:549–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Shimazaki M, Nakamura K, Kii I, Kashima T, Amizuka N, Li M, et al. Periostin is essential for cardiac healing after acute myocardial infarction. J Exp Med. 2008;205:295–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Oka T, Xu J, Kaiser RA, Melendez J, Hambleton M, Sargent MA, et al. Genetic manipulation of periostin expression reveals a role in cardiac hypertrophy and ventricular remodeling. Circ Res. 2007;101:313–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Takeda N, Manabe I, Uchino Y, Eguchi K, Matsumoto S, Nishimura S, et al. Cardiac fibroblasts are essential for the adaptive response of the murine heart to pressure overload. J Clin Invest. 2010;120:254–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Diaz-Araya G, Borg TK, Lavandero S, Loftis MJ, Carver W. IGF-1 modulation of rat cardiac fibroblast behavior and gene expression is age-dependent. Cell communication & adhesion. 2003;10:155–65. [PubMed] [Google Scholar]
- [139].Lighthouse JK, Burke RM, Velasquez LS, Dirkx RA Jr., Aiezza A 2nd, Moravec CS, et al. Exercise promotes a cardioprotective gene program in resident cardiac fibroblasts. JCI Insight. 2019;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Burke RM, Lighthouse JK, Quijada P, Dirkx RA Jr., Rosenberg A, Moravec CS, et al. Small proline-rich protein 2B drives stress-dependent p53 degradation and fibroblast proliferation in heart failure. Proceedings of the National Academy of Sciences of the United States of America. 2018;115:E3436–E45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Nam YJ, Lubczyk C, Bhakta M, Zang T, Fernandez-Perez A, McAnally J, et al. Induction of diverse cardiac cell types by reprogramming fibroblasts with cardiac transcription factors. Development. 2014;141:4267–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Qian L, Huang Y, Spencer CI, Foley A, Vedantham V, Liu L, et al. In vivo reprogramming of murine cardiac fibroblasts into induced cardiomyocytes. Nature. 2012;485:593–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Zhao Y, Londono P, Cao Y, Sharpe EJ, Proenza C, O’Rourke R, et al. High-efficiency reprogramming of fibroblasts into cardiomyocytes requires suppression of pro-fibrotic signalling. Nature communications. 2015;6:8243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Muraoka N, Yamakawa H, Miyamoto K, Sadahiro T, Umei T, Isomi M, et al. MiR-133 promotes cardiac reprogramming by directly repressing Snai1 and silencing fibroblast signatures. EMBO J. 2014;33:1565–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Mohamed TM, Stone NR, Berry EC, Radzinsky E, Huang Y, Pratt K, et al. Chemical Enhancement of In Vitro and In Vivo Direct Cardiac Reprogramming. Circulation. 2017;135:978–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Dobaczewski M, Bujak M, Li N, Gonzalez-Quesada C, Mendoza LH, Wang XF, et al. Smad3 signaling critically regulates fibroblast phenotype and function in healing myocardial infarction. Circ Res. 2010;107:418–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Bujak M, Ren G, Kweon HJ, Dobaczewski M, Reddy A, Taffet G, et al. Essential role of Smad3 in infarct healing and in the pathogenesis of cardiac remodeling. Circulation. 2007;116:2127–38. [DOI] [PubMed] [Google Scholar]
- [148].Rosenkranz S TGF-beta1 and angiotensin networking in cardiac remodeling. Cardiovasc Res. 2004;63:423–32. [DOI] [PubMed] [Google Scholar]
- [149].Biernacka A, Cavalera M, Wang J, Russo I, Shinde A, Kong P, et al. Smad3 Signaling Promotes Fibrosis While Preserving Cardiac and Aortic Geometry in Obese Diabetic Mice. Circ Heart Fail. 2015;8:788–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Wang B, Haldar SM, Lu Y, Ibrahim OA, Fisch S, Gray S, et al. The Kruppel-like factor KLF15 inhibits connective tissue growth factor (CTGF) expression in cardiac fibroblasts. J Mol Cell Cardiol. 2008;45:193–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Yu H, Huang J, Wang S, Zhao G, Jiao X, Zhu L. Overexpression of Smad7 suppressed ROS/MMP9-dependent collagen synthesis through regulation of heme oxygenase-1. Mol Biol Rep. 2013;40:5307–14. [DOI] [PubMed] [Google Scholar]
- [152].Wei LH, Huang XR, Zhang Y, Li YQ, Chen HY, Yan BP, et al. Smad7 inhibits angiotensin II-induced hypertensive cardiac remodelling. Cardiovasc Res. 2013;99:665–73. [DOI] [PubMed] [Google Scholar]
- [153].Wang B, Hao J, Jones SC, Yee MS, Roth JC, Dixon IM. Decreased Smad 7 expression contributes to cardiac fibrosis in the infarcted rat heart. Am J Physiol Heart Circ Physiol. 2002;282:H1685–96. [DOI] [PubMed] [Google Scholar]
- [154].Meng Q, Bhandary B, Bhuiyan MS, James J, Osinska H, Valiente-Alandi I, et al. Myofibroblast-Specific TGFbeta Receptor II Signaling in the Fibrotic Response to Cardiac Myosin Binding Protein C-Induced Cardiomyopathy. Circ Res. 2018;123:1285–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Dingar D, Merlen C, Grandy S, Gillis MA, Villeneuve LR, Mamarbachi AM, et al. Effect of pressure overload-induced hypertrophy on the expression and localization of p38 MAP kinase isoforms in the mouse heart. Cell Signal. 2010;22:1634–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Davis J, Burr AR, Davis GF, Birnbaumer L, Molkentin JD. A TRPC6-dependent pathway for myofibroblast transdifferentiation and wound healing in vivo. Dev Cell. 2012;23:705–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Kompa AR, See F, Lewis DA, Adrahtas A, Cantwell DM, Wang BH, et al. Long-term but not short-term p38 mitogen-activated protein kinase inhibition improves cardiac function and reduces cardiac remodeling post-myocardial infarction. The Journal of pharmacology and experimental therapeutics. 2008;325:741–50. [DOI] [PubMed] [Google Scholar]
- [158].See F, Thomas W, Way K, Tzanidis A, Kompa A, Lewis D, et al. p38 mitogen-activated protein kinase inhibition improves cardiac function and attenuates left ventricular remodeling following myocardial infarction in the rat. J Am Coll Cardiol. 2004;44:1679–89. [DOI] [PubMed] [Google Scholar]
- [159].Wu L, Cao Z, Ji L, Mei L, Jin Q, Zeng J, et al. Loss of TRADD attenuates pressure overload-induced cardiac hypertrophy through regulating TAK1/P38 MAPK signalling in mice. Biochemical and biophysical research communications. 2017;483:810–5. [DOI] [PubMed] [Google Scholar]
- [160].Woeller CF, O’Loughlin CW, Roztocil E, Feldon SE, Phipps RP. Salinomycin and other polyether ionophores are a new class of antiscarring agent. J Biol Chem. 2015;290:3563–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Braz JC, Bueno OF, Liang Q, Wilkins BJ, Dai YS, Parsons S, et al. Targeted inhibition of p38 MAPK promotes hypertrophic cardiomyopathy through upregulation of calcineurin-NFAT signaling. J Clin Invest. 2003;111:1475–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Saurin AT, Martin JL, Heads RJ, Foley C, Mockridge JW, Wright MJ, et al. The role of differential activation of p38-mitogen-activated protein kinase in preconditioned ventricular myocytes. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2000;14:2237–46. [DOI] [PubMed] [Google Scholar]
- [163].Sinfield JK, Das A, O’Regan DJ, Ball SG, Porter KE, Turner NA. p38 MAPK alpha mediates cytokine-induced IL-6 and MMP-3 expression in human cardiac fibroblasts. Biochemical and biophysical research communications. 2013;430:419–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Pogozelski AR, Geng T, Li P, Yin X, Lira VA, Zhang M, et al. p38gamma mitogen- activated protein kinase is a key regulator in skeletal muscle metabolic adaptation in mice. PLoS One. 2009;4:e7934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Small EM. The actin-MRTF-SRF gene regulatory axis and myofibroblast differentiation. Journal of cardiovascular translational research. 2012;5:794–804. [DOI] [PubMed] [Google Scholar]
- [166].Leitner L, Shaposhnikov D, Mengel A, Descot A, Julien S, Hoffmann R, et al. MAL/MRTF-A controls migration of non-invasive cells by upregulation of cytoskeleton-associated proteins. J Cell Sci. 2011;124:4318–31. [DOI] [PubMed] [Google Scholar]
- [167].Small EM, Thatcher JE, Sutherland LB, Kinoshita H, Gerard RD, Richardson JA, et al. Myocardin-related transcription factor-a controls myofibroblast activation and fibrosis in response to myocardial infarction. Circ Res. 2010;107:294–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Gau D, Roy P. SRF’ing and SAP’ing - the role of MRTF proteins in cell migration. J Cell Sci. 2018;131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Velasquez LS, Sutherland LB, Liu Z, Grinnell F, Kamm KE, Schneider JW, et al. Activation of MRTF-A-dependent gene expression with a small molecule promotes myofibroblast differentiation and wound healing. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:16850–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Cavin S, Maric D, Diviani D. A-kinase anchoring protein-Lbc promotes pro-fibrotic signaling in cardiac fibroblasts. Biochim Biophys Acta. 2014;1843:335–45. [DOI] [PubMed] [Google Scholar]
- [171].Zhou Y, Huang X, Hecker L, Kurundkar D, Kurundkar A, Liu H, et al. Inhibition of mechanosensitive signaling in myofibroblasts ameliorates experimental pulmonary fibrosis. J Clin Invest. 2013;123:1096–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].Kagiyama S, Matsumura K, Goto K, Otsubo T, Iida M. Role of Rho kinase and oxidative stress in cardiac fibrosis induced by aldosterone and salt in angiotensin type 1a receptor knockout mice. Regul Pept. 2010;160:133–9. [DOI] [PubMed] [Google Scholar]
- [173].Haak AJ, Tsou PS, Amin MA, Ruth JH, Campbell P, Fox DA, et al. Targeting the myofibroblast genetic switch: inhibitors of myocardin-related transcription factor/serum response factor-regulated gene transcription prevent fibrosis in a murine model of skin injury. The Journal of pharmacology and experimental therapeutics. 2014;349:480–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174].Sisson TH, Ajayi IO, Subbotina N, Dodi AE, Rodansky ES, Chibucos LN, et al. Inhibition of myocardin-related transcription factor/serum response factor signaling decreases lung fibrosis and promotes mesenchymal cell apoptosis. The American journal of pathology. 2015;185:969–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [175].Philippar U, Schratt G, Dieterich C, Muller JM, Galgoczy P, Engel FB, et al. The SRF target gene Fhl2 antagonizes RhoA/MAL-dependent activation of SRF. Molecular cell. 2004;16:867–80. [DOI] [PubMed] [Google Scholar]
- [176].Hinson JS, Medlin MD, Taylor JM, Mack CP. Regulation of myocardin factor protein stability by the LIM-only protein FHL2. Am J Physiol Heart Circ Physiol. 2008;295:H1067–H75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [177].Wixler V, Hirner S, Muller JM, Gullotti L, Will C, Kirfel J, et al. Deficiency in the LIM-only protein Fhl2 impairs skin wound healing. The Journal of cell biology. 2007;177:163–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [178].Lodowski DT, Pitcher JA, Capel WD, Lefkowitz RJ, Tesmer JJ. Keeping G proteins at bay: a complex between G protein-coupled receptor kinase 2 and Gbetagamma. Science. 2003;300:1256–62. [DOI] [PubMed] [Google Scholar]
- [179].Choi DJ, Koch WJ, Hunter JJ, Rockman HA. Mechanism of beta-adrenergic receptor desensitization in cardiac hypertrophy is increased beta-adrenergic receptor kinase. J Biol Chem. 1997;272:17223–9. [DOI] [PubMed] [Google Scholar]
- [180].Raake PW, Vinge LE, Gao E, Boucher M, Rengo G, Chen X, et al. G protein-coupled receptor kinase 2 ablation in cardiac myocytes before or after myocardial infarction prevents heart failure. Circ Res. 2008;103:413–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [181].Robinson JD, Pitcher JA. G protein-coupled receptor kinase 2 (GRK2) is a Rho-activated scaffold protein for the ERK MAP kinase cascade. Cell Signal. 2013;25:2831–9. [DOI] [PubMed] [Google Scholar]
- [182].Woodall MC, Woodall BP, Gao E, Yuan A, Koch WJ. Cardiac Fibroblast GRK2 Deletion Enhances Contractility and Remodeling Following Ischemia/Reperfusion Injury. Circ Res. 2016;119:1116–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [183].Peregrin S, Jurado-Pueyo M, Campos PM, Sanz-Moreno V, Ruiz-Gomez A, Crespo P, et al. Phosphorylation of p38 by GRK2 at the docking groove unveils a novel mechanism for inactivating p38MAPK. Curr Biol. 2006;16:2042–7. [DOI] [PubMed] [Google Scholar]
- [184].Horckmans M, Ring L, Duchene J, Santovito D, Schloss MJ, Drechsler M, et al. Neutrophils orchestrate post-myocardial infarction healing by polarizing macrophages towards a reparative phenotype. Eur Heart J. 2017;38:187–97. [DOI] [PubMed] [Google Scholar]
- [185].Nishiga M, Horie T, Kuwabara Y, Nagao K, Baba O, Nakao T, et al. MicroRNA-33 Controls Adaptive Fibrotic Response in the Remodeling Heart by Preserving Lipid Raft Cholesterol. Circ Res. 2017;120:835–47. [DOI] [PubMed] [Google Scholar]
- [186].van Rooij E, Sutherland LB, Thatcher JE, DiMaio JM, Naseem RH, Marshall WS, et al. Dysregulation of microRNAs after myocardial infarction reveals a role of miR-29 in cardiac fibrosis. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:13027–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [187].Sassi Y, Avramopoulos P, Ramanujam D, Gruter L, Werfel S, Giosele S, et al. Cardiac myocyte miR-29 promotes pathological remodeling of the heart by activating Wnt signaling. Nature communications. 2017;8:1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [188].Soci UP, Fernandes T, Hashimoto NY, Mota GF, Amadeu MA, Rosa KT, et al. MicroRNAs 29 are involved in the improvement of ventricular compliance promoted by aerobic exercise training in rats. Physiological genomics. 2011;43:665–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [189].Ma Y, Iyer RP, Jung M, Czubryt MP, Lindsey ML. Cardiac Fibroblast Activation Post- Myocardial Infarction: Current Knowledge Gaps. Trends Pharmacol Sci. 2017;38:448–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [190].Ma Q Role of nrf2 in oxidative stress and toxicity. Annual review of pharmacology and toxicology. 2013;53:401–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [191].Lombardi R, Rodriguez G, Chen SN, Ripplinger CM, Li W, Chen J, et al. Resolution of established cardiac hypertrophy and fibrosis and prevention of systolic dysfunction in a transgenic rabbit model of human cardiomyopathy through thiol-sensitive mechanisms. Circulation. 2009;119:1398–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [192].Marian AJ, Tan Y, Li L, Chang J, Syrris P, Hessabi M, et al. Hypertrophy Regression With N-Acetylcysteine in Hypertrophic Cardiomyopathy (HALT-HCM): A Randomized, Placebo- Controlled, Double-Blind Pilot Study. Circ Res. 2018;122:1109–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [193].Xu Y, Luo Y, Wang ZY, Li X, Zheng P, Zhang TC. MRTF-A can activate Nrf2 to increase the resistance to doxorubicin. Oncotarget. 2017;8:8436–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [194].Li J, Ichikawa T, Villacorta L, Janicki JS, Brower GL, Yamamoto M, et al. Nrf2 protects against maladaptive cardiac responses to hemodynamic stress. Arterioscler Thromb Vasc Biol. 2009;29:1843–50. [DOI] [PubMed] [Google Scholar]
- [195].Lighthouse JK, Burke RM, Velasquez LS, Dirkx RA, Aiezza A, Moravec C, Alexis JD, Rosenberg A, Small EM Exercise promotes a cardioprotective gene program in resident cardiac fibroblasts. JCI Insight. 2018;Accepted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [196].Williams SM, Golden-Mason L, Ferguson BS, Schuetze KB, Cavasin MA, Demos-Davies K, et al. Class I HDACs regulate angiotensin II-dependent cardiac fibrosis via fibroblasts and circulating fibrocytes. J Mol Cell Cardiol. 2014;67:112–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [197].Schuetze KB, Stratton MS, Blakeslee WW, Wempe MF, Wagner FF, Holson EB, et al. Overlapping and Divergent Actions of Structurally Distinct Histone Deacetylase Inhibitors in Cardiac Fibroblasts. The Journal of pharmacology and experimental therapeutics. 2017;361:140–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [198].Duan Q, McMahon S, Anand P, Shah H, Thomas S, Salunga HT, et al. BET bromodomain inhibition suppresses innate inflammatory and profibrotic transcriptional networks in heart failure. Science translational medicine. 2017;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [199].Stratton MS, Lin CY, Anand P, Tatman PD, Ferguson BS, Wickers ST, et al. Signal- Dependent Recruitment of BRD4 to Cardiomyocyte Super-Enhancers Is Suppressed by a MicroRNA. Cell reports. 2016;16:1366–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [200].Adamiak M, Sahoo S. Exosomes in Myocardial Repair: Advances and Challenges in the Development of Next-Generation Therapeutics. Molecular therapy : the journal of the American Society of Gene Therapy. 2018;26:1635–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [201].Bang C, Batkai S, Dangwal S, Gupta SK, Foinquinos A, Holzmann A, et al. Cardiac fibroblast-derived microRNA passenger strand-enriched exosomes mediate cardiomyocyte hypertrophy. J Clin Invest. 2014;124:2136–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [202].Vicencio JM, Yellon DM, Sivaraman V, Das D, Boi-Doku C, Arjun S, et al. Plasma exosomes protect the myocardium from ischemia-reperfusion injury. J Am Coll Cardiol. 2015;65:1525–36. [DOI] [PubMed] [Google Scholar]
- [203].Hulsmans M, Sager HB, Roh JD, Valero-Munoz M, Houstis NE, Iwamoto Y, et al. Cardiac macrophages promote diastolic dysfunction. J Exp Med. 2018;215:423–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [204].Danielson KM, Shah R, Yeri A, Liu X, Camacho Garcia F, Silverman M, et al. Plasma Circulating Extracellular RNAs in Left Ventricular Remodeling Post-Myocardial Infarction. EBioMedicine. 2018;32:172–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [205].Prabhu SD, Frangogiannis NG. The Biological Basis for Cardiac Repair After Myocardial Infarction: From Inflammation to Fibrosis. Circ Res. 2016;119:91–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [206].Bajpai G, Bredemeyer A, Li W, Zaitsev K, Koenig AL, Lokshina I, et al. Tissue Resident CCR2- and CCR2+ Cardiac Macrophages Differentially Orchestrate Monocyte Recruitment and Fate Specification Following Myocardial Injury. Circ Res. 2019;124:263–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [207].Pappritz K, Savvatis K, Koschel A, Miteva K, Tschope C, Van Linthout S. Cardiac (myo)fibroblasts modulate the migration of monocyte subsets. Scientific reports. 2018;8:5575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [208].Mouton AJ, Ma Y, Rivera Gonzalez OJ, Daseke MJ 2nd, Flynn ER, Freeman TC, et al. Fibroblast polarization over the myocardial infarction time continuum shifts roles from inflammation to angiogenesis. Basic research in cardiology. 2019;114:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [209].Ieda M, Tsuchihashi T, Ivey KN, Ross RS, Hong TT, Shaw RM, et al. Cardiac fibroblasts regulate myocardial proliferation through beta1 integrin signaling. Dev Cell. 2009;16:233–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [210].Jun JI, Lau LF. Taking aim at the extracellular matrix: CCN proteins as emerging therapeutic targets. Nat Rev Drug Discov. 2011;10:945–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [211].Meyer K, Hodwin B, Ramanujam D, Engelhardt S, Sarikas A. Essential Role for Premature Senescence of Myofibroblasts in Myocardial Fibrosis. J Am Coll Cardiol. 2016;67:2018–28. [DOI] [PubMed] [Google Scholar]
- [212].Fontes MS, Kessler EL, van Stuijvenberg L, Brans MA, Falke LL, Kok B, et al. CTGF knockout does not affect cardiac hypertrophy and fibrosis formation upon chronic pressure overload. J Mol Cell Cardiol. 2015;88:82–90. [DOI] [PubMed] [Google Scholar]
- [213].Dorn LE, Petrosino JM, Wright P, Accornero F. CTGF/CCN2 is an autocrine regulator of cardiac fibrosis. J Mol Cell Cardiol. 2018;121:205–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [214].Care A, Catalucci D, Felicetti F, Bonci D, Addario A, Gallo P, et al. MicroRNA-133 controls cardiac hypertrophy. Nat Med. 2007;13:613–8. [DOI] [PubMed] [Google Scholar]
- [215].Duisters RF, Tijsen AJ, Schroen B, Leenders JJ, Lentink V, van der Made I, et al. miR-133 and miR-30 regulate connective tissue growth factor: implications for a role of microRNAs in myocardial matrix remodeling. Circ Res. 2009;104:170–8, 6p following 8. [DOI] [PubMed] [Google Scholar]
- [216].Jeong D, Lee MA, Li Y, Yang DK, Kho C, Oh JG, et al. Matricellular Protein CCN5 Reverses Established Cardiac Fibrosis. J Am Coll Cardiol. 2016;67:1556–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [217].Yoon PO, Lee MA, Cha H, Jeong MH, Kim J, Jang SP, et al. The opposing effects of CCN2 and CCN5 on the development of cardiac hypertrophy and fibrosis. J Mol Cell Cardiol. 2010;49:294–303. [DOI] [PubMed] [Google Scholar]
- [218].Hinkel R, Trenkwalder T, Petersen B, Husada W, Gesenhues F, Lee S, et al. MRTF-A controls vessel growth and maturation by increasing the expression of CCN1 and CCN2. Nature communications. 2014;5:3970. [DOI] [PubMed] [Google Scholar]
- [219].Mahoney VM, Mezzano V, Mirams GR, Maass K, Li Z, Cerrone M, et al. Connexin43 contributes to electrotonic conduction across scar tissue in the intact heart. Scientific reports. 2016;6:26744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [220].Zhang W, Lavine KJ, Epelman S, Evans SA, Weinheimer CJ, Barger PM, et al. Necrotic myocardial cells release damage-associated molecular patterns that provoke fibroblast activation in vitro and trigger myocardial inflammation and fibrosis in vivo. J Am Heart Assoc. 2015;4:e001993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [221].Liu L, Wang Y, Cao ZY, Wang MM, Liu XM, Gao T, et al. Up-regulated TLR4 in cardiomyocytes exacerbates heart failure after long-term myocardial infarction. J Cell Mol Med. 2015;19:2728–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [222].Chen JB, Liu WJ, Che H, Liu J, Sun HY, Li GR. Adenosine-5’-triphosphate up-regulates proliferation of human cardiac fibroblasts. British journal of pharmacology. 2012;166:1140–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [223].Chen Y, Corriden R, Inoue Y, Yip L, Hashiguchi N, Zinkernagel A, et al. ATP release guides neutrophil chemotaxis via P2Y2 and A3 receptors. Science. 2006;314:1792–5. [DOI] [PubMed] [Google Scholar]
- [224].Lefort C, Benoist L, Chadet S, Piollet M, Heraud A, Babuty D, et al. Stimulation of P2Y11 receptor modulates cardiac fibroblasts secretome toward immunomodulatory and protective roles after Hypoxia/Reoxygenation injury. J Mol Cell Cardiol. 2018;121:212–22. [DOI] [PubMed] [Google Scholar]
- [225].Abrial M, Da Silva CC, Pillot B, Augeul L, Ivanes F, Teixeira G, et al. Cardiac fibroblasts protect cardiomyocytes against lethal ischemia-reperfusion injury. J Mol Cell Cardiol. 2014;68:56–65. [DOI] [PubMed] [Google Scholar]