Abstract
Successful episodic memory calls upon a number of different cognitive processes that are supported by the coordination of several large-scale cortical networks. Previous work from our group has demonstrated dissociable anatomic substrates at different stages of memory in patients with dementia due to Alzheimer’s disease (AD). The aim of the current study was to extend the understanding of brain-behavior associations underlying a commonly administered neuropsychological assessment of verbal episodic memory (Rey Auditory Verbal Learning Test; RAVLT) by determining the cortical network contributions to the performance at early vs. late stages of list learning, delayed recall, and retention, in 235 very mild biomarker positive (A+/T+/N+) individuals diagnosed with amnestic mild cognitive impairment (aMCI; MMSE = 27.7). We measured cortical atrophy in four large-scale cortical networks impacted by AD: default mode (DMN), dorsal attention (DAN), frontoparietal (FPN), and language (LN) networks. We also evaluated the role of hippocampal atrophy at each stage of memory performance. Partial correlation analyses controlling for age, sex, and education and corrected for multiple comparisons revealed that early learning was most strongly associated with cortical thickness in the DAN, while late learning was most strongly associated with hippocampal volume, but also related to cortical thickness in the DAN, FPN, DMN, and LN. Delayed recall was associated most strongly with hippocampal volume, but was also related to cortical thickness in the FPN and DMN, while retention was associated only with hippocampal volume. These findings are consistent with prior models of the neural substrates of different stages of verbal list learning and retrieval, provide new insights into the cortical networks undergoing neurodegeneration even at very mild stages of prodromal AD, and inform our thinking about the networks and regions being interrogated by this kind of neuropsychological assessment of episodic memory.
Keywords: mild cognitive impairment, verbal episodic memory, neurodegeneration, cortical networks
1. INTRODUCTION
The medial temporal lobes (MTL) have historically been credited as the central regions involved in episodic memory functions (Scoville & Milner, 1957; Squire, Stark, & Clark, 2004). However, a variety of cognitive processes are necessary for successful episodic memory. In addition to the processes attributed to the MTL system, successful episodic memory also relies on top-down attentional modulation of perceptual processing, working memory, semantic processing, and controlled goal-directed retrieval. Each of these cognitive processes play a unique role at different stages of episodic memory and relies upon distinct brain networks outside the MTL. Specifically, top-down attention modulation and working memory (e.g., monitoring and mental manipulation) promote the efficient encoding of new information for later recollection (Alexander, Stuss, & Gillingham, 2009; Blumenfeld & Ranganath, 2007). These important cognitive functions are subserved by distributed frontal and parietal regions that comprise the Dorsal Attention Network (DAN), with key nodes in the superior parietal lobule (SPL)/intraparietal sulcus (IPS) and the dorsolateral prefrontal cortex (Corbetta & Shulman, 2002; Fox, et al., 2005). As information is processed perceptually and maintained and manipulated in working memory, access to conceptual representations modulates the depth and organization of information being encoded (Goldblum, et al., 1998); these processes are supported by a distributed language network (LN) anchored in the temporal pole and inclusive of regions in the middle temporal gyrus, temporo-parietal junction, and inferior parietal lobule (IPL) (Andrews-Hanna, Reidler, Sepulcre, Poulin, & Buckner, 2010; Patterson, Nestor, & Rogers, 2007; Visser, Jefferies, & Lambon Ralph, 2010). Once encoding, storage, and consolidation occur, retrieval of information relies on a set of coordinated processes which recruit regions comprising the default mode network (DMN) (Andrews-Hanna, et al., 2010; Wang, et al., 2010) which includes MTL memory systems (Kohler, et al., 1998; Walhovd, et al., 2010; Wolk, Dickerson, & ADNI, 2011), as well as the Frontoparietal Network (FPN), with key nodes in the mid-dorsolateral prefrontal cortex and the posterior inferior parietal lobule (IPL) (Vincent, Kahn, Snyder, Raichle, & Buckner, 2008).
Alzheimer’s disease (AD), the most common form of acquired episodic memory dysfunction, has served as a lens through which to study episodic memory deficits. Although the earliest site of neuronal injury is thought to be the anterior MTL in most patients, AD pathology typically spreads to other paralimbic and isocortical regions, including ventral and lateral temporal cortex, followed by parietal and frontal cortices (Arnold, Hyman, Flory, Damasio, & Van Hoesen, 1991; Braak & Braak, 1991). Previous studies in AD have shown the impact of neurodegeneration in these distributed regions on tests of executive functions (Dickerson, Wolk, & ADNI, 2011; Wolk, Dickerson, & ADNI, 2010), semantic processing (Domoto-Reilly, Sapolsky, Brickhouse, Dickerson, & ADNI, 2012; Henry, Crawford, & Phillips, 2004; Papp, et al., 2016), and visuospatial skills (Uhlhaas, et al., 2008). Dysfunction in these distributed systems that contribute to memory function likely serves to further exacerbate episodic memory impairment in AD beyond the focal injury of the MTL.
Clinical neuropsychological measurement of verbal episodic memory typically includes administration of supra-span verbal list learning tasks (e.g., Rey Auditory Verbal Learning Test or RAVLT). The RAVLT is comprised of 5 repeated learning trials of the same 15 word list, with immediate and delayed recall trials after 3 and 30 minutes, respectively, as well as recognition testing. Much of the work examining memory deficits in AD has focused on either summing words recalled across all learning trials for a measure of total encoding (e.g., Vyhnalek, et al., 2014) or delayed recall measures (e.g., Zhao, et al., 2015), or has sometimes regarded list learning performance as a single composite measure representing episodic memory (e.g., Ricci, Graef, Blundo, & Miller, 2012). Although valuable for a variety of purposes, this approach does not take account of the substantial variability between patients in performance across the different stages of episodic memory function described above. Specifically, identifying deficits in early learning (e.g., initial presentation of a word list, or Trials 1 and 2) versus deficits in late learning (after several repeated presentations, or Trials 4 and 5), or in delayed recall compared to retention (accounting for information that was successfully encoded), can help us localize dysfunction in underlying cortical networks. As previous work from our group has demonstrated in AD dementia patients, memory task performance can be fractionated meaningfully such that different stages of memory performance are associated with atrophy in different regions of the brain susceptible to AD-related neurodegeneration (Wolk, et al., 2011). Critically, Wolk et al. demonstrated that MTL atrophy in AD dementia was not associated with first trial learning, but rather, with memory retrieval only after multiple exposures to the word list (i.e., late learning). In that study, first trial encoding was associated with cortical thickness in the IPL, middle frontal gyrus, and the temporal pole, regions supporting working memory and semantic language processing, while cortical thickness in the MTL was associated with late learning, and delayed recall performance was associated with hippocampal volume, a region associated with memory retention. This initial demonstration of episodic memory fractionation in AD dementia sets the stage for the present study, as the regions that were identified with different episodic memory stages are known hubs of large-scale networks important for successful memory performance. We sought to extend those prior findings here to a group of very mild biomarker positive (amyloid- and tau-positive) patients with amnestic mild cognitive impairment (aMCI+), with the rationale that large-scale cortical network integrity or impairment may be more specific in biomarker positive individuals at an earlier stage of degeneration compared to dementia, allowing for a more sensitive investigation of the association between morphometric integrity and performance on different stages of memory performance.
We examined the neuroanatomical network correlates of performance at four stages of the RAVLT in aMCI+: Early Learning (Trials 1 and 2), Late Learning (Trials 4 and 5), Delayed Recall (30-minute delayed free recall), and Retention (Delayed Recall divided by Trial 5). In our previous work, we reported dissociable brain-behavior relationships in patients with AD dementia using the RAVLT, and discussed the regional atrophy findings in the context of large-scale cortical networks (Wolk et al., 2011). This prior work, along with relevant literature on the role of the DAN in top-down attention regulation and the FPN in goal-directed retrieval (Alexander, et al., 2009; Vincent, et al., 2008), enabled us to generate the following hypotheses. First, we predicted that Early Learning would be associated with cortical thickness in the DAN, but that Late Learning would be associated with cortical thickness in FPN and DMN (including MTL) and hippocampal volume. Next, we predicted that Delayed Recall, which requires the ability to store and retrieve information in a goal-directed fashion, would be associated with cortical thickness in the FPN and hippocampal volume, while Retention would be solely associated with hippocampal volume.
2. METHODS
2.1. Participants
Data used in the preparation of this article were obtained from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) database (www.adni.loni.usc.edu). The ADNI was launched in 2003 as a public-private partnership, led by Principal Investigator Michael W. Weiner, MD. The primary goal of ADNI has been to test whether serial magnetic resonance imaging (MRI), positron emission tomography (PET), other biological markers, and clinical and neuropsychological assessment can be combined to measure the progression of mild cognitive impairment (MCI) and early Alzheimer’s disease (AD). For the current analysis, we initially selected all patients with a diagnosis of amnestic mild cognitive impairment (aMCI) at baseline who had neuropsychological data on the Rey auditory verbal learning test (RAVLT; n=622). These individuals all had a subject memory concern, received a physician CDR rating of 0.5 with a memory box score of at least 0.5, had an MMSE between 24–30, and demonstrated abnormal memory function documented by scoring below a cutoff (which varies based on the participant’s education) on the Logical Memory II from the Wechsler Memory Scale-Revised. Of these aMCI individuals, we assigned each individual to a “biomarker positive” aMCI+ group in accordance with the recently published NIA-AA research framework explicitly establishing the presence of amyloid (A+) and tau (T+) pathology (Jack, et al., 2018). aMCI+ individuals (considered prodromal AD) were so classified if they had positive levels of cerebrospinal fluid amyloid-β (≤ 192 pg/mL) and phosphorylated-tau (> 23 pg/mL), supportive of likely presence of amyloid plaques and neurofibrillary tangles (Shaw, et al., 2009). If aMCI patients were found to be negative on one or both amyloid and tau cutoffs (A-/T-), they were excluded since they did not meet both of these biomarker criteria. After restricting the sample to those who also underwent a 1.5T MRI scan of adequate quality to obtain morphometric measures, the sample size was 338 aMCI+. For all whole-cortex analysis of brain atrophy, as well as for correlational and regression analyses investigating associations between list learning performance and anatomical measures of interest, we further restricted the aMCI+ group by removing participants who performed at floor on delayed recall (zero words recalled), in order to examine brain-behavior relationships with a normally distributed range of performance in an aMCI+ sample who could perform the task, resulting in our final selected aMCI+ sample size of n=235. We also included age-matched control participants (n=158) from the ADNI cohort with a diagnosis of “cognitively normal” who were also negative on both amyloid and tau biomarker criteria (CN-); they had cerebrospinal fluid levels of amyloid-β > 192 pg/mL and phosphorylated-tau < 23 pg/mL supportive of the likely absence of amyloid plaques and neurofibrillary tangles (Shaw, et al., 2009). We used the means and standard deviations on RAVLT performance of this CN- group to calculate normative scores for neuropsychological test performance across stages of the RAVLT for the aMCI+ group. We also compared whole-cortical thickness of our aMCI+ to the CN- group (Figure 1). Due to our observations of large regions of the cortical mantle that were significantly atrophied in the aMCI+ compared to CN- group, we determined that the aMCI+ group was also positive for neurodegeneration (Figure 1), consistent with recent research framework for defining prodromal AD (Jack, et al., 2018). All other inclusion and exclusion criteria are described at: http://www.adni-info.org. Each participant gave written informed consent in accordance with institutional Human Subjects Research Committee Guidelines.
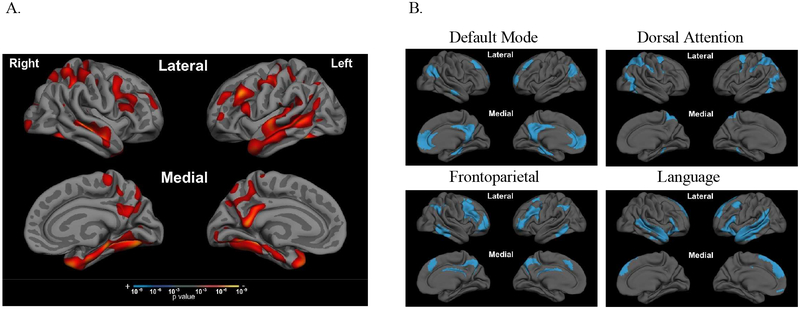
Figure 1. aMCI+ patients have cortical atrophy in multiple large-scale cortical networks outside of the MTL systems.
Compared to age-matched biomarker-negative controls (CN-), whole-brain cortical thickness analysis of aMCI+ (A) revealed atrophy in regions comprising all four of the large-scale cortical networks (B) examined in this study. Large-scale cortical networks were defined using the Yeo atlas (Yeo et al., 2015), detailed in section 2.4. Significance threshold for t-test in (A) set to p<0.001.
2.2. Episodic Memory Testing
We examined baseline memory test scores from the ADNI database. Our analysis was focused on the RAVLT (Rey, 1964), a word list learning task that is commonly administered clinically to patients with amnestic syndromes. Briefly, the RAVLT consists of five learning trials. At each trial, a list of 15 novel words is read and the subject is asked to immediately recall as many items as possible. After this repeated exposure learning process, an interference list of 15 words is read, some of which contain semantically related words. Then, participants are asked immediately to recall words from the initial repeated list (immediate recall), and again after 30 minutes (delayed recall). A 30-item recognition discrimination test follows in a forced-choice yes/no format, including 15 unstudied foils. Retention was calculated here as the number of words correctly recalled at 30 minutes divided by the number of words correctly recalled at Trial 5. Using performance on this task from the CN- group, normative z-scores were calculated for each learning trial, delayed recall, recognition, and retention. To reduce the number of comparisons made, we collapsed Trial 1 and Trial 2 into an averaged “Early Learning” measure, and Trial 4 and Trial 5 into an averaged “Late Learning” measure. We focused our study on just four variables of interest: Early Learning, Late Learning, Delayed Recall, and Retention, given our a priori hypotheses regarding specific network contributions regarding each stage of list learning performance. We present behavioral data on the larger aMCI+ group (n=338) as well as the selected aMCI+ group (n=235) in Table 1 of the Supplementary Materials.
2.3. MRI acquisition and analysis
MRI scans for ADNI were collected on a 1.5T scanner using a standardized protocol across sites. For the present analysis, the MPRAGE sequence was used with the following characteristics: sagittal plane, TR/TE, 2400/3/1000 ms, flip angle 8°, 24cm FOV, 192×192 in-plane matrix, and 1.2mm slice thickness (Jack, et al., 2008). Fully preprocessed scans were downloaded for analysis. The ADNI MRI Core at Mayo Clinic Rochester provided intensity normalized and gradient un-warped TI image volumes. All T1 images include an on-scanner non-uniformity correction. We then applied our own automated preprocessing steps in FreeSurfer version 6, which includes cortical surface extraction, segmentation of subcortical structures (Dale, Fischl, & Sereno, 1999), cortical thickness estimation (Fischl & Dale, 2000), spatial normalization onto the FreeSurfer surface template (fsaverage), and atlas-based parcellation of cortical regions (Fischl, et al., 2004) using the FreeSurfer recon-all command. T1 image volumes were examined by a cortical surface-based reconstruction and analysis of cortical thickness with the FreeSurfer version 6.0 platform, using a combination of hypothesis-driven and exploratory analysis approaches as described in multiple previous publications (Bakkour, Morris, & Dickerson, 2009; Dickerson, et al., 2009; Dickerson, et al., 2008; Dickerson, et al., 2011; Dickerson, Wolk, & ADNI, 2013). For the exploratory analyses, statistical surface maps were generated by computing general linear models with each behavioral variable of interest (z-scores of Early Learning, Late Learning, Delayed Recall, and Retention) as the independent variable, cortical thickness as the dependent variable, and age and education as covariates. The results of these analyses were inspected via maps of statistical significance at each vertex point overlaid on the average cortical surface template. For these exploratory analyses, a statistical threshold of p<0.00001 was used for the learning trials (Early Learning and Late Learning) and more liberal threshold of p<0.001 was used for the delayed recall and retention trials to be as inclusive as possible of cortical results for visualization purposes.
2.4. Statistical analysis
Our morphometric measures of analysis included a priori hypothesized left- and right-hemisphere dorsal attention network (DAN), frontoparietal network (FPN), default mode network (DMN), and language network (LN). We defined these networks using the Yeo 17-network parcellation atlas (Yeo, et al., 2015) and combined subcomponents together based on functional specialization and visual inspection. Specifically, components 5 and 6 comprised the DAN, components 11, 12, and 13 comprised the frontoparietal network, components 14 and 17 comprised the LN, and components 15 and 16 comprised the DMN (including MTL). Using FreeSurfer version 6.0, we obtained cortical thickness measures (mm) from these 4 networks separated by hemisphere, yielding 8 network measures. We additionally calculated left- and right hemisphere hippocampal volume, divided by total intracranial volume (mm3), for each individual participant based on their FreeSurfer-defined automatically segmented (aseg) volumes.
In addition to the exploratory whole cortical GLM approach, the main question of the current study was evaluated by calculating Pearson’s partial correlations between cortical thickness in our 10 morphometric measures of interest (left and right hemisphere DAN, FPN, DMN, LN, and hippocampal volume) and RAVLT stages of Early Learning, Late Learning, Delayed Recall, and Retention, controlling for the effects of age, education, and sex. A statistical threshold of p<0.005 was considered a significant finding, after applying a Bonferroni correction for multiple comparisons in our 10 regions of interest. We present the results from this analysis in Figure 4 of this manuscript for the selected aMCI+ group that was the focus of the study, but also include the same analysis in the larger aMCI+ group in Supplementary Materials Figure 2. To further examine these partial correlations to determine which morphometric measure contributed to the most unique variance in explaining performance at each stage of list learning, we performed standard multiple hierarchical linear regression analyses. For this step, we included only left hemisphere networks and hippocampal volume because in an attempt to eliminate multicollinearity with their right hemisphere homologues; correlations between left- and right-hemisphere homologues ranged between 0.84 and 0.89. In addition, we focused only on left-hemisphere anatomical regions based on our a priori hypotheses that performance on the RAVLT list learning test would be subserved by the left-hemisphere more strongly than the right by nature of this verbal memory task as has been previously observed (e.g., de Toledo-Morrell, et al., 2000). Collinearity diagnostics (variance inflation factor <6, tolerance > 0.15) indicated that our predictor variables were all within acceptable parameters. Covariates of age, sex, and education were entered first, and then morphometric measures were entered in a standard fashion in the same block. Behavioral measures of Early Learning, Late Learning, Delayed Recall, and Retention served as dependent variables in each regression model, allowing for determination of which networks uniquely contributed to performance on each measure. Statistical analyses were performed using standard methods in IBM SPSS Version 24.0 (Armonk, NY).
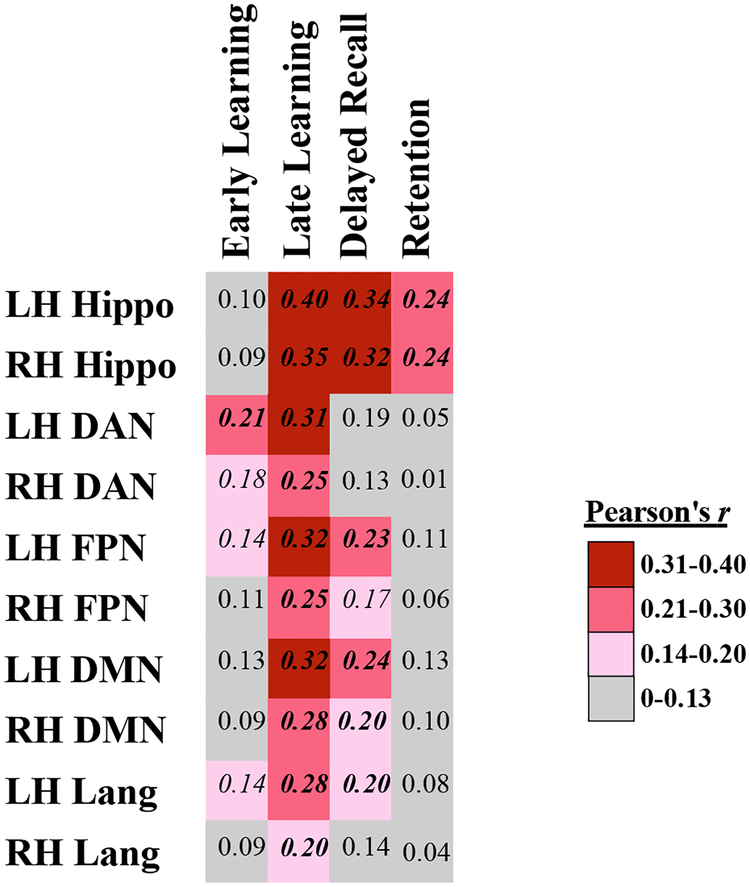
Figure 4. Partial correlation coefficients of RAVLT measures with hippocampal volume and cortical thickness across networks of interest, controlled for age, sex, and education.
LH = left hemisphere, RH= right hemisphere, Hippo = hippocampal volume / intracranial volume, DAN = Dorsal Attention Network, FPN = Frontoparietal Network, DMN = Default Mode Network, Lang = Language Network. Pearson’s r correlation coefficients written in bold italics indicates significance level of p< 0.005 (p <0.05 Bonferroni-corrected for 10 comparisons), and unbolded italics indicates uncorrected p<0.05.
3. RESULTS
3.1. Demographic characteristics
The primary study group of interest was the subgroup of aMCI+ (n=235) who did not perform at floor on the delayed recall trial of the RAVLT (n=235). For completeness, we also present some results from the larger group of 338 aMCI+ participants, including those who performed at floor, in the main manuscript and in Supplementary Material. Demographic characteristics for these groups as well as the 158 CN- participants are shown in Table 1. Consistent with the mild status of these selected aMCI+ individuals, the mean Mini Mental state Examination (MMSE) score was 27.7 out of 30. Approximately 60% of aMCI+ patients had at least one copy of the ε4 allele of the apolipoprotein E (ApoE) gene (Table 1). The CN- and aMCI+ groups did not differ on age or sex, but differed in years of education (t= 2.24, p=0.03) though all participants were very highly educated (~16 years). The groups also differed on total MMSE score and % ε4 allele carriers, as expected by diagnostic definition. Compared to age-matched CN-, whole-brain cortical thickness analysis of aMCI+ revealed an “AD-signature-like” distributed pattern of cortical atrophy (Dickerson, et al., 2009), classifying the aMCI+ group as A+/T+/N+. Comparison of Figure 1 panels A and B shows that cortical atrophy was observed in regions within all four of the large-scale cortical networks examined in this study.
Table 1. Demographic characteristics.
We present demographic characteristics of biomarker-negative cognitive normal (CN-) and biomarker-positive amnestic Mild Cognitive Impairment (aMCI+) groups, including the larger aMCI+ sample (n=338) inclusive of individuals who performed at floor levels, as well as the selected aMCI+ sample (n=235) of individuals who were able to perform the RAVLT adequately and who were the primary focus of the study.
indicates significant group differences between aMCI+ and CN- groups at p<0.05. MMSE = Mini Mental State Examination; ε4 = ε4 allele of the Apolipoprotein E (ApoE) gene. Means ± Standard Deviations are presented for continuous variables.
3.2. RAVLT performance in aMCI+ revealed greatest impairment in late learning
RAVLT performance in the larger group of aMCI+(n=338) compared to CN- (z-scores) revealed that the largest magnitude of impairment in the different stages of memory was in Late Learning (Trials 4 and 5) as well as Delayed Recall, compared to Early Learning (Trials 1 and 2) or Retention (Figure 2). Upon examining RAVLT performance in the selected group of aMCI+ (n=235; Figure 3), we found that performance on each stage of this memory test was different from each other stage (p> 0.0001), with the greatest impairment observed on the late learning trials compared to early learning, delayed recall, and retention. Because we excluded individuals who performed at floor levels on delayed recall, these impairments on delayed recall and retention are milder than we observed in the larger aMCI+ cohort.
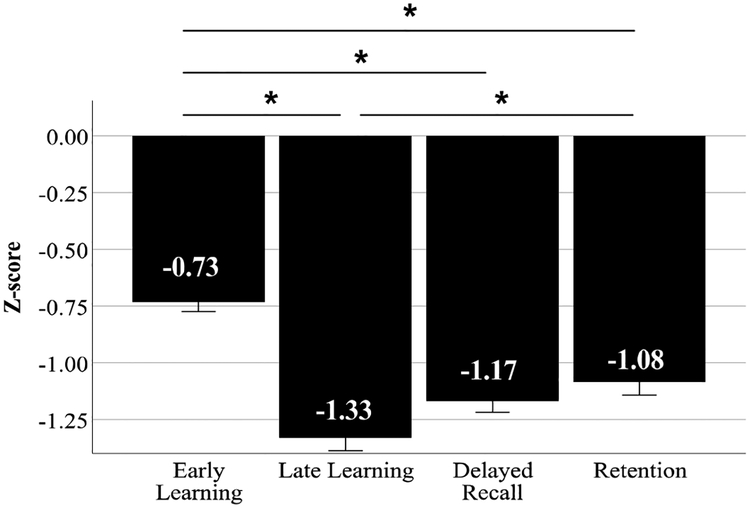
Figure 2. RAVLT performance in the entire aMCI+ group (n=338) revealed greatest impairment in Late Learning and Delayed Recall, compared to Early Learning or Retention.
Normed relative to the CN- group, aMCI+ performance on Late Learning (z-score = −1.33 ± 1.01) and Delayed Recall (z-score = −1.17 ± 0.90) were more impaired compared to Early Learning (z-score = −0.73 ± 0.77) and Retention (z-score = −1.08 ± 1.07). This aMCI+ group was inclusive of individuals who performed at floor levels on the delayed recall component of the RAVLT and who were more impaired on all other RAVLT trials as indicated in Supplementary Material Table 1. *indicates statistical significance at p <0.005. Error bars indicate ±1 standard error of the mean.
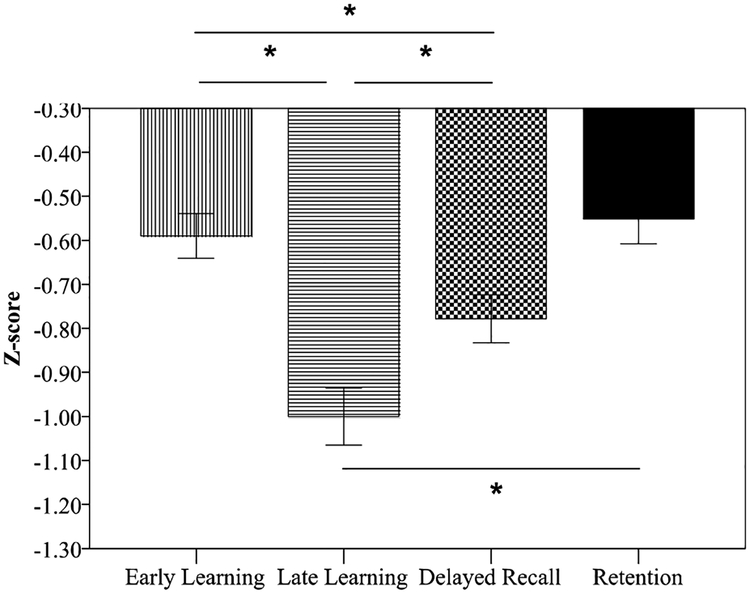
Figure 3. RAVLT performance in the selected aMCI+ group (n=235) revealed greatest impairment in Late Learning compared to Early Learning, Delayed Recall, or Retention.
Normed relative to the CN- group, aMCI+ performance on Late Learning (z-score = −1.0 ± 0.99) was reduced compared to Early Learning (z-score = −0.59 ± 0.78), Delayed Recall (z-score = −0.78 ± 0.83) and Retention (z-score = −0.55 ± 0.87). There was no difference between Early Learning and Retention. This aMCI+ group was comprised of individuals who could perform all trials of the RAVLT above floor levels.*indicates statistical significance at p <0.005. Error bars indicate ±1 standard error of the mean.
3.3. Performance on each stage of list learning is related to atrophy in different cortical networks
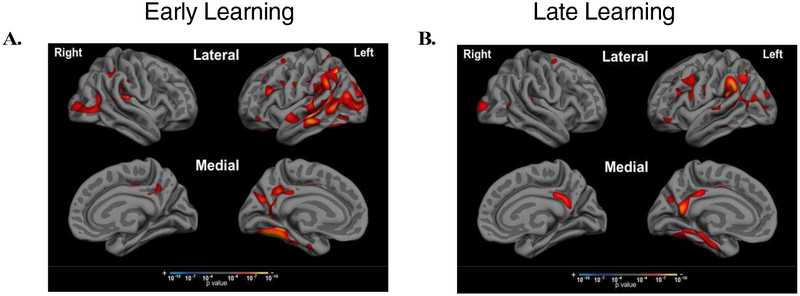
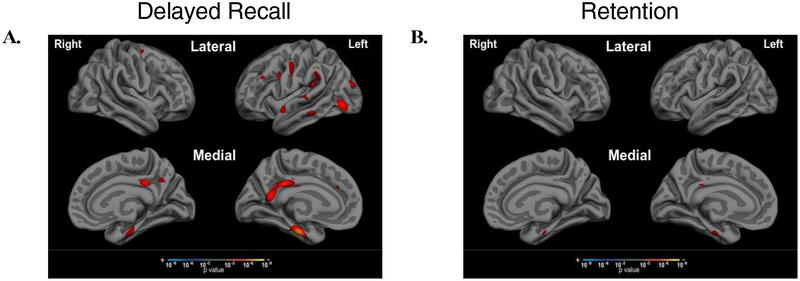
To examine the associations between performance impairments at different stages of learning and retrieval with AD-related neurodegeneration in the hypothesized brain networks, we performed Pearson’s partial correlations between each of our 10 morphometric measures and performance on RAVLT, controlling for age, education, and sex in the selected aMCI+ group. As shown in Figure 4, Early Learning performance (Trials 1 and 2) was associated most strongly with left hemisphere DAN, with weaker correlations to right hemisphere DAN (z=1.94, p=0.03). In contrast, Late Learning performance (Trials 4 and 5) was most strongly associated with left hippocampal volume, and was also associated with cortical thickness in bilateral FPN, DAN, DMN, and LN with stronger effects in left hemisphere compared to right hemisphere hippocampal volume (z=1.6, p=0.05), DAN (z=2.04, p=0.02), FPN (z=2.19, p=0.01), and LN (z=2.23, p=0.01). Figure 5 shows the exploratory whole cortical surface general linear models of Early Learning and Late Learning, controlling for age and education. Of note, MTL atrophy (part of the DMN) was related only to Late Learning and not to Early Learning, consistent with the correlation results shown in Figure 4. Delayed Recall performance was most strongly correlated with bilateral hippocampal volume and DMN atrophy, as well as to left hemisphere FPN and LN atrophy, with a weaker correlation observed with right hemisphere atrophy compared to left hemisphere atrophy in the FPN (z=1.83, p=003) and LN (z=1.64, p=0.05). Lastly, Retention (Delayed Recall divided by Trial 5 to account for initial encoding) was related only to bilateral hippocampal volume, with a strong relationship observed in left hippocampal volume compared to right hippocampal volume (z=1.99, p=0.02). Figure 6 shows the exploratory whole cortical surface general linear model maps of Delayed Recall and Retention, controlling for age and education. Of note, cortical atrophy in the left hemisphere FPN and DMN is observed in the regression map of Delayed Recall, while associations were observed only between cortical thickness in the anterior MTL and Retention, consistent with the correlation results shown in Figure 4.
Figure 5. Early Learning is associated with cortical atrophy in the DAN while Late Learning is also associated with atrophy in the DMN (including the MTL).
Exploratory whole cortical surface general linear models of (A) Early Learning and (B) Late Learning show different patterns of atrophy related to each of these stages of list learning, particularly in the MTL, where atrophy is only observed in association with Late Learning, with a significance threshold set at p < 0.0001. Follow-up hierarchical linear regression revealed that Early Learning was uniquely associated with atrophy in the left hemisphere DAN, while Late Learning was uniquely associated with left hemisphere hippocampal volume.
Figure 6. Delayed Recall is associated with cortical atrophy in FPN and DMN systems, while Retention is not associated with cortical atrophy but with hippocampal volume loss.
Exploratory whole cortical surface general linear models of Delayed Recall and Retention show different patterns of related atrophy, with (A) regions of the FPN and DMN associated with Delayed Recall performance and (B) anterior MTL associated with Retention, at a significance threshold set at p < 0.001. Follow-up hierarchical linear regression revealed that Delayed Recall and Retention were associated only with hippocampal volume (not shown on these cortical surface maps).
3.4. Hierarchical regression reveals that Early Learning is uniquely associated with cortical thickness in the Dorsal Attention Network, while Late Learning, Delayed Recall, and Retention are most strongly related to hippocampal volume.
To follow up on the correlations observed with Pearson’s partial correlations (Figure 4), we performed standard hierarchical linear regression analyses on the regions established to have an association with list learning performance at each stage. Though our partial correlation matrix shows which regions of interest were significantly associated with performance on the different list learning stages, the aim of these follow-up regression analyses was to determine which morphometric measure contributed the most unique variance to performance on each list learning stage (Table 2). After controlling for demographic factors, the only region of interest contributing unique variance to performance on Early Learning was cortical thickness in the left hemisphere DAN (ß= 0.41, p=0.003). In other words, with the left hemisphere DAN thickness in the model, no other morphometric measure provided additional explanatory value in predicting Early Learning performance. In contrast, the only predictor contributing unique variance in predicting Late Learning performance was left hemisphere hippocampal volume (ß= 0.37, p=2.65×10−7). Similarly, the only morphometric measure to contribute unique variance of Delayed Recall and Retention performance was the left hemisphere hippocampal volume (ß= 0.35, p=7.0 ×10−6 and ß= 0.26, p=0.001, respectively). These findings may suggest that cortical thickness in networks predicting list learning performance at these stages may be correlated in this population and secondary to hippocampal integrity, aside from Early Learning.
Table 2. Hierarchical linear regression demonstrates anatomical associations with RAVLT performance.
Regression coefficients (standardized beta), T-values, and significance (p) values are presented below for each of the four RAVLT stages (Early Learning, Late Learning, Delayed Recall, and Retention). Demographic variables of age, sex, and education were entered first, followed by anatomical correlates of interest from left-hemisphere regions only due to our a priori hypotheses and attempt to reduce collinearity among independent variables.
indicates significance at a Bonferroni-corrected level of p< 0.005 accounting for the numerous covariates included in the model; significance noted only for measures of interest.
4. DISCUSSION
Successful episodic memory involves the coordination of a number of different cognitive processes that are supported by multiple large-scale cortical networks, including medial temporal lobe memory systems, but also including frontal, parietal, and lateral temporal cortical regions comprising the DMN, DAN, FPN, and LN. The results from the present study of individuals with prodromal AD (amyloid- and tau-positive amnestic mild cognitive impairment) revealed that neurodegeneration in multiple cortical networks outside of the classic memory systems also contribute to episodic memory impairment. We show dissociable relationships between cortical network atrophy at different stages of memory performance: early learning (after one or two exposures) was most strongly associated with atrophy in the left hemisphere DAN, late learning (after 4 and 5 exposures) was most strongly associated with atrophy in the left hippocampus but also associated with atrophy in DMN, FPN, and LN, delayed recall was most strongly associated with atrophy in the left hippocampus but also associated with atrophy in the FPN and DMN, and retention (accounting for the amount of information initially learned) was most strongly associated with atrophy in the left hemisphere hippocampus. Although performance on standard supraspan list learning tests often used in clinics by neuropsychologists to evaluate episodic memory functions (e.g., RAVLT) are typically interpreted as a monolithic construct indicative of MTL integrity, the range of cognitive processes required at each different stage of a list learning task lead us to expect that regions outside of the medial temporal lobes are also recruited to support successful performance. Specifically, successful performance of verbal list learning tasks requires a combination of attention and executive control skills, including top-down modulation of attention on task-relevant stimuli and strategic organization of new information (Alexander, et al., 2009; Blumenfeld & Ranganath, 2007; Cabeza, 2008; Long, Oztekin, & Badre, 2010), lexical semantic processing necessary to organize new verbal stimuli in a meaningful fashion to promote encoding and subsequent retrieval (e.g., semantic clustering) (Buchsbaum & D’Esposito, 2008; Craik & Lockhart, 1972; Goldblum, et al., 1998; Savill, Ellis, & Jefferies, 2017), and goal-directed retrieval skills for subsequent recall trials after a delay period (Cabeza, 2008; Cabeza, Ciaramelli, & Moscovitch, 2012).
Early Learning is most strongly related to integrity within the dorsal attention network, while Late Learning is most strongly related to the integrity of the hippocampus
The results of the present study revealed that the early stages of list learning (after one or two exposures to the word list) are most strongly associated with cortical thickness in the left hemisphere DAN, with hubs in the frontal, parietal, and occipitotemporal cortices. Early learning performance also demonstrated associations with the right hemisphere DAN, in addition to left hemisphere FPN, and left hemisphere LN. These results are consistent with prior work from our group showing the relationship between early stages of learning in AD dementia and cortical thickness of a broad set of isocortical regions implicated in supporting working memory (lateral frontal and parietal cortex) and semantic processing (lateral temporal cortex) (Wolk, et al., 2011). Encoding of novel verbal information such as a supraspan list learning test that exceeds the phonological store capacity (i.e., the RAVLT), has been shown to rely on adequate top-down attention regulation, as well as employment of learning strategies that call heavily upon verbal working memory. Such strategies may include subjective ordering of words in an idiosyncratic fashion (Alexander, Stuss, & Fansabedian, 2003; Eslinger & Grattan, 1994) or a more demanding strategy of semantic clustering, which requires one to link words based on semantically meaningful information (Eslinger & Grattan, 1994; Gershberg & Shimamura, 1995). One may even employ semantic self-cuing strategies to facilitate encoding and subsequent retrieval (Incisa della Rocchetta & Milner, 1993). One interesting property of classic list learning paradigms is the requirement of retrieving the words learned at each stage of encoding, which itself serves to assist learning in an active manner (Karpicke & Roediger, 2008). Neuroimaging studies have implicated left superior frontal and parietal cortical lesions with impairments in organizing learning strategies (Alexander, et al., 2009; Gershberg & Shimamura, 1995; Incisa della Rocchetta & Milner, 1993; Sandrini, Cappa, Rossi, Rossini, & Miniussi, 2003). In addition to organizing learning strategies, another role of the dorsolateral prefrontal cortex is in maintenance of sustained and divided attention. It has been previously proposed that divided attention is necessary in list learning tests for the individual to hold recent words in working memory while maintaining attention on upcoming words in a word list (Alexander, et al., 2009).
In contrast, the later stages of list learning (after four or five exposures) was most strongly associated with left hemisphere hippocampal volume, but was also associated with cortical thickness in all four of our hypothesized networks of interest (DAN, DMN, FPN, and LN), with stronger effects in the left hemisphere networks compared to the right hemisphere DAN, FPN, and LN. This is consistent with the brain regions previously reported to be associated with Trial 5 of list learning tests in AD dementia (Wolk et al., 2011), namely the middle frontal gyrus and inferior parietal lobule (comprising the FPN), the temporal pole (anchoring the LN), and the MTL (anchoring the DMN). As has been previously posited, information that is maintained in working memory is more deeply encoded with the aid of conceptual representations and organization of information being encoded (Goldblum, et al., 1998), processes that are supported by the LN and FPN (Andrews-Hanna, et al., 2010; Patterson, et al., 2007; Visser, et al., 2010). Critically, our whole-cortex surface exploratory results revealed associations between cortical thickness in the MTL in late learning, but not in early learning. These results are consistent with our prior findings in AD dementia which also demonstrated left hemisphere MTL atrophy in predicting Trial 5 but not Trial 1 performance on the AVLT (Wolk, et al., 2011). These converging results emphasize our conclusion that performance on initial trials of memory tests depend more strongly on attention regulation and strategic encoding efforts, while the transfer of information to long-term memory stores, supported by MTL structures including the hippocampus, contributes to performance on later trials to a greater extent.
Delayed Recall is related to integrity of the hippocampus, frontoparietal network, and default mode network, while Retention is only related to the integrity of the hippocampus
Performance on the delayed free recall trial of the RAVLT was most uniquely associated with the left hemisphere hippocampal volume, with additional correlations observed with cortical thickness in the FPN and DMN. Our exploratory whole cortical surface results also reveal associations with medial parietal and MTL regions comprising the FPN and DMN. These results are consistent with the large body of literature demonstrating the important role of the MTL cortical regions and hippocampal volume in facilitating memory recall in AD (Kohler, et al., 1998; Petersen, et al., 2000; Squire, et al., 2004; Wolk, et al., 2011). Our findings extend this previous work by showing that in addition to the classic MTL structures, the goal-directed controlled retrieval demand that is intrinsic to delayed recall trials of verbal list learning paradigms also implicate the left hemisphere FPN and medial parietal regions of the DMN. This is consistent with and builds upon previous work implicating the lateral prefrontal regions in goal-directed retrieval of newly learned information (Gershberg & Shimamura, 1995; Sandrini, et al., 2003), as well as work highlighting the functional connectivity between the precuneus and posterior cingulate cortices and hippocampal memory network in supporting successful episodic memory (Greicius, Srivastava, Reiss, & Menon, 2004; Wagner, Shannon, Kahn, & Buckner, 2005; Wang, et al., 2010) and shows that the FPN and DMN are critical systems in supporting delayed free recall. When examining retention separately, controlling for goal-directed retrieval and learning (Delayed free recall divided by Trial 5 recall), left hemisphere hippocampal volume was the only morphometric measure that predicted performance, consistent with a large body of work supporting the critical nature of the hippocampus in memory storage/retention when initial learning is accounted for (Kramer, et al., 2004).
Clinical Implications
We demonstrated neuroanatomical dissociations between large-scale cortical networks supporting different stages of list learning performance in a group of highly educated individuals with amyloid- and tau- positive aMCI who are able to perform the RAVLT, which has implications for the interpretation of classic list learning test performance in the clinic (Figure 7). We targeted this selected sample to generalize our findings to the population of aMCI individuals who commonly present for clinical evaluation who are still able to complete standard list learning tests (i.e., not at floor levels), yet who carry biomarker evidence of AD pathology and who will likely progress to dementia over time. Rather than ascribe any impairment on list learning tests to MTL pathology, we sought to refine our understanding of how other cortical networks can contribute to performance on this test that relies on multiple cognitive processes and the networks that subserve them. For example, if a patient is impaired on early stages of learning and free recall, but demonstrates a positive learning curve with repeated exposure and demonstrates strong late learning and retention, one might conclude dysfunction of the DAN and FPN but sparing of DMN and hippocampal memory systems, as is the case in some atypical hippocampal-sparing presentations of AD such as posterior cortical atrophy (Putcha, et al., 2018), dysexecutive AD (Dickerson, et al., 2011), and logopenic-variant primary progressive aphasia (Magnin, et al., 2013), as well as non-AD neurodegenerative processes such as behavioral variant frontotemporal dementia (Pasquier, Grymonprez, Lebert, & Van der Linden, 2001) and possible non-degenerative conditions such as stroke or focal cerebrovascular disease which may target specific cortical regions. Similarly, hippocampal dysfunction can be more confidently concluded if a patient demonstrates impairment in later learning stages (flat or low learning curve), as well as retention, rather than relying merely on reduced delayed recall performance, which may indicate frontoparietal dysfunction rather than MTL/hippocampal dysfunction.
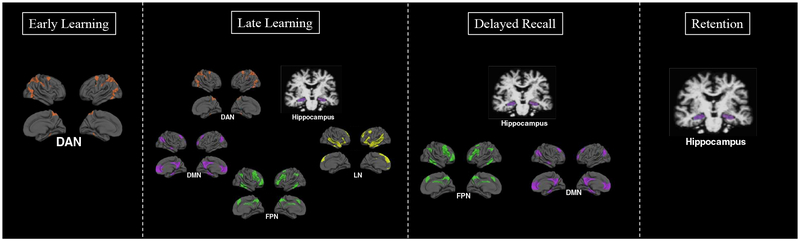
Figure 7. Neuroanatomical dissociations across stages of verbal list learning.
Here we show a summary of our correlational results, depicting the dorsal attention network (DAN) as most strongly implicated in early learning stages of list learning tasks, the hippocampus as well as dorsal attention (DAN), frontoparietal (FPN), language (LN), and default mode (DMN) networks implicated in late learning, the hippocampus, frontoparietal (FPN), and default mode network (DMN) supporting delayed recall, and only hippocampal volume supporting retention.
Strengths, Limitations, and Future Directions
Our study has several strengths including the large and well-defined cohort afforded by using the ADNI aMCI+, who fulfilled recently proposed research criteria for defining prodromal AD (A+/T+/N+) in that they were classified as positive for the presence of beta amyloid and phosphorylated tau protein (by CSF marker) and also positive for neurodegeneration as a group (Jack, et al., 2018). Further, we investigated a very commonly administered verbal list learning test to measure stages of episodic memory function (RAVLT) and used a data-driven approach to defining large-scale cortical networks of interest that are openly available and broadly utilized in neuroimaging research, allowing for replicability and generalizability across other studies of associations between network integrity and cognitive performance. However, some important limitations should also be addressed. Specifically, the prodromal AD cohort included in this study were quite mild (average MMSE = 27.7 ± 1.9) and very highly educated. As such, these individuals may not be representative of the more advanced aMCI+ individuals who are on the boundary of progression to AD dementia, nor of individuals who present with memory complaints from backgrounds of lower formal education. Additionally, our study of widely used scores from this common list learning test may illuminate the executive and lexical mechanisms that contribute to supraspan list learning abilities, but we do not offer any insights regarding the neuroanatomical bases of other aspects of memory, including source memory, temporal ordering, and sensitivity to interference. These topics would be important avenues of future investigation.
Supplementary Material
Highlights.
Early stages of learning in aMCI+ were most strongly associated with cortical thickness in the dorsal attention network, and not with hippocampal volume.
Late stages of learning in aMCI+ were most strongly associated with hippocampal volume, but also related to cortical thickness in the distributed cortical networks supporting attention, executive functions, and language.
Delayed recall was associated most strongly with hippocampal volume and cortical thickness in the frontoparietal and default mode networks, while retention was associated only with hippocampal volume.
These findings provide new insights into the cortical networks undergoing neurodegeneration in prodromal AD.
ACKNOWLEDGEMENTS
We would also like to thank and acknowledge Joseph Locascio, PhD of Massachusetts General Hospital and Harvard Medical Schools for biostatistics consultation on this project. This work was conducted with support from Harvard Catalyst | The Harvard Clinical and Translational Science Center (National Center for Advancing Translational Sciences, National Institutes of Health Award UL 1TR002541) and financial contributions from Harvard University and its affiliated academic healthcare centers. The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, Harvard University and its affiliated academic healthcare centers, or the National Institutes of Health.
FUNDING
Data collection and sharing for this project was funded by the Alzheimer’s Disease Neuroimaging Initiative (ADNI) (National Institutes of Health Grant U01 AG024904) and DOD ADNI (Department of Defense award number W81XWH-12-2-0012). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: AbbVie, Alzheimer’s Association; Alzheimer’s Drug Discovery Foundation; Araclon Biotech; BioClinica, Inc.; Biogen; Bristol-Myers Squibb Company; CereSpir, Inc.; Cogstate; Eisai Inc.; Elan Pharmaceuticals, Inc.; Eli Lilly and Company; EuroImmun; F. Hoffmann-La Roche Ltd and its affiliated company Genentech, Inc.; Fujirebio; GE Healthcare; IXICO Ltd.; Janssen Alzheimer Immunotherapy Research & Development, LLC.; Johnson & Johnson Pharmaceutical Research & Development LLC.; Lumosity; Lundbeck; Merck & Co., Inc.; Meso Scale Diagnostics, LLC.; NeuroRx Research; Neurotrack Technologies; Novartis Pharmaceuticals Corporation; Pfizer Inc.; Piramal Imaging; Servier; Takeda Pharmaceutical Company; and Transition Therapeutics. The Canadian Institutes of Health Research is providing funds to support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the National Institutes of Health (www.fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer’s Therapeutic Research Institute at the University of Southern California. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of Southern California. This research was also supported by National Institute of Health grant R01 DC014296.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests: None
REFERENCES
- 1.Alexander MP, Stuss D, & Gillingham S (2009). Impaired list learning is not a general property of frontal lesions. J Cogn Neurosci, 21, 1422–1434. [DOI] [PubMed] [Google Scholar]
- 2.Alexander MP, Stuss DT, & Fansabedian N (2003). California Verbal Learning Test: performance by patients with focal frontal and non-frontal lesions. Brain, 126, 1493–1503. [DOI] [PubMed] [Google Scholar]
- 3.Andrews-Hanna JR, Reidler JS, Sepulcre J, Poulin R, & Buckner RL (2010). Functional-anatomic fractionation of the brain’s default network. Neuron, 65, 550–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arnold SE, Hyman BT, Flory J, Damasio AR, & Van Hoesen GW (1991). The topographical and neuroanatomical distribution of neurofibrillary tangles and neuritic plaques in the cerebral cortex of patients with Alzheimer’s disease. Cereb Cortex, 1, 103–116. [DOI] [PubMed] [Google Scholar]
- 5.Bakkour A, Morris JC, & Dickerson BC (2009). The cortical signature of prodromal AD: regional thinning predicts mild AD dementia. Neurology, 72, 1048–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blumenfeld RS, & Ranganath C (2007). Prefrontal cortex and long-term memory encoding: an integrative review of findings from neuropsychology and neuroimaging. Neuroscientist, 13, 280–291. [DOI] [PubMed] [Google Scholar]
- 7.Braak H, & Braak E (1991). Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol, 82, 239–259. [DOI] [PubMed] [Google Scholar]
- 8.Buchsbaum BR, & D’Esposito M (2008). The search for the phonological store: from loop to convolution. J Cogn Neurosci, 20, 762–778. [DOI] [PubMed] [Google Scholar]
- 9.Cabeza R (2008). Role of parietal regions in episodic memory retrieval: the dual attentional processes hypothesis. Neuropsychologia, 46, 1813–1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cabeza R, Ciaramelli E, & Moscovitch M (2012). Cognitive contributions of the ventral parietal cortex: an integrative theoretical account. Trends Cogn Sci, 16, 338–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Corbetta M, & Shulman GL (2002). Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci, 3, 201–215. [DOI] [PubMed] [Google Scholar]
- 12.Craik FIM, & Lockhart SN (1972). Levels of processing: a framework for memory research. J Verbal Learn. Verbal Behav, 11, 671–684. [Google Scholar]
- 13.Dale AM, Fischl B, & Sereno MI (1999). Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage, 9, 179–194. [DOI] [PubMed] [Google Scholar]
- 14.de Toledo-Morrell L, Dickerson B, Sullivan MP, Spanovic C, Wilson R, & Bennett DA (2000). Hemispheric differences in hippocampal volume predict verbal and spatial memory performance in patients with Alzheimer’s disease. Hippocampus, 10, 136–142. [DOI] [PubMed] [Google Scholar]
- 15.Dickerson BC, Bakkour A, Salat DH, Feczko E, Pacheco J, Greve DN, Grodstein F, Wright CI, Blacker D, Rosas HD, Sperling RA, Atri A, Growdon JH, Hyman BT, Morris JC, Fischl B, & Buckner RL (2009). The cortical signature of Alzheimer’s disease: regionally specific cortical thinning relates to symptom severity in very mild to mild AD dementia and is detectable in asymptomatic amyloid-positive individuals. Cereb Cortex, 19, 497–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dickerson BC, Fenstermacher E, Salat DH, Wolk DA, Maguire RP, Desikan R, Pacheco J, Quinn BT, Van der Kouwe A, Greve DN, Blacker D, Albert MS, Killiany RJ, & Fischl B (2008). Detection of cortical thickness correlates of cognitive performance: Reliability across MRI scan sessions, scanners, and field strengths. Neuroimage, 39, 10–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dickerson BC, Wolk DA, & ADNI. (2011). Dysexecutive versus amnesic phenotypes of very mild Alzheimer’s disease are associated with distinct clinical, genetic and cortical thinning characteristics. J Neurol Neurosurg Psychiatry, 82, 45–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dickerson BC, Wolk DA, & ADNI. (2013). Biomarker-based prediction of progression in MCI: Comparison of AD signature and hippocampal volume with spinal fluid amyloid-beta and tau. Front Aging Neurosci, 5, 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Domoto-Reilly K, Sapolsky D, Brickhouse M, Dickerson BC, & ADNI. (2012). Naming impairment in Alzheimer’s disease is associated with left anterior temporal lobe atrophy. Neuroimage, 63, 348–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eslinger PJ, & Grattan LM (1994). Altered serial position learning after frontal lobe lesion. Neuropsychologia, 32, 729–739. [DOI] [PubMed] [Google Scholar]
- 21.Fischl B, & Dale AM (2000). Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci U S A, 97, 11050–11055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fischl B, van der Kouwe A, Destrieux C, Halgren E, Segonne F, Salat DH, Busa E, Seidman LJ, Goldstein J, Kennedy D, Caviness V, Makris N, Rosen B, & Dale AM (2004). Automatically parcellating the human cerebral cortex. Cereb Cortex, 14, 11–22. [DOI] [PubMed] [Google Scholar]
- 23.Fox MD, Snyder AZ, Vincent JL, Corbetta M, van Essen DC, & Raichle ME (2005). The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A, 102, 9673–9678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gershberg FB, & Shimamura AP (1995). Impaired use of organizational strategies in free recall following frontal lobe damage. Neuropsychologia, 33, 1305–1333. [DOI] [PubMed] [Google Scholar]
- 25.Goldblum MC, Gomez CM, Dalla Barba G, Boller F, Deweer B, Hahn V, & Dubois B (1998). The influence of semantic and perceptual encoding on recognition memory in Alzheimer’s disease. Neuropsychologia, 36, 717–729. [DOI] [PubMed] [Google Scholar]
- 26.Greicius MD, Srivastava G, Reiss AL, & Menon V (2004). Default-mode network activity distinguishes Alzheimer’s disease from healthy aging: evidence from functional MRI. Proc Natl Acad Sci U S A, 101, 4637–4642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Henry JD, Crawford JR, & Phillips LH (2004). Verbal fluency performance in dementia of the Alzheimer’s type: a meta-analysis. Neuropsychologia, 42, 1212–1222. [DOI] [PubMed] [Google Scholar]
- 28.Incisa della Rocchetta A, & Milner B (1993). Strategic search and retrieval inhibition: the role of the frontal lobes. Neuropsychologia, 31, 503–524. [DOI] [PubMed] [Google Scholar]
- 29.Jack CR Jr., Bennett DA, Blennow K, Carrillo MC, Dunn B, Haeberlein SB, Holtzman DM, Jagust W, Jessen F, Karlawish J, Liu E, Molinuevo JL, Montine T, Phelps C, Rankin KP, Rowe CC, Scheltens P, Siemers E, Snyder HM, Sperling R, & Contributors. (2018). NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimers Dement, 14, 535–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jack CR Jr., Bernstein MA, Fox NC, Thompson P, Alexander G, Harvey D, Borowski B, Britson PJ, L. W. J, Ward C, Dale AM, Felmlee JP, Gunter JL, Hill DL, Killiany R, Schuff N, Fox-Bosetti S, Lin C, Studholme C, DeCarli CS, Krueger G, Ward HA, Metzger GJ, Scott KT, Mallozzi R, Blezek D, Levy J, Debbins JP, Fleisher AS, Albert M, Green R, Bartzokis G, Glover G, Mugler J, & Weiner MW (2008). The Alzheimer’s Disease Neuroimaging Initiative (ADNI): MRI methods. J Magn Reson Imaging, 27, 685–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Karpicke JD, & Roediger HL (2008). The critical importance of retrieval for learning. Science, 319, 966–968. [DOI] [PubMed] [Google Scholar]
- 32.Kohler S, Black SE, Sinden M, Szekely C, Kidron D, Parker JL, Foster JK, Moscovitch M, Winocour G, Szalai JP, & Bronskill MJ (1998). Memory impairments associated with hippocampal versus parahippocampal-gyrus atrophy: an MR volumetry study in Alzheimer’s disease. Neuropsychologia, 36, 901–914. [DOI] [PubMed] [Google Scholar]
- 33.Kramer JH, Schuff N, Reed BR, Mungas D, Du AT, Rosen HJ, Jagust WJ, Miller BL, Weiner MW, & Chui HC (2004). Hippocampal volume and retention in Alzheimer’s disease. J Int Neuropsychol Soc, 10, 639–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Long NM, Oztekin I, & Badre D (2010). Separable prefrontal cortex contributions to free recall. J Neurosci, 30, 10967–10976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Magnin E, Chopard G, Ferreira S, Sylvestre G, Dariel E, Ryff I, Mertz C, Lamidieu C, Hidalgo J, Tio G, Haffen S, Galmiche J, Moulin T, Vandel P, & Rumbach L (2013). Initial neuropsychological profile of a series of 20 patients with logopenic variant of primary progressive aphasia. J Alzheimers Dis, 36, 799–808. [DOI] [PubMed] [Google Scholar]
- 36.Papp KV, Mormino EC, Amariglio RE, Munro C, Dagley A, Schultz AP, Johnson KA, Sperling RA, & Rentz DM (2016). Biomarker validation of a decline in semantic processing in preclinical Alzheimer’s disease. Neuropsychology, 30, 624–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pasquier F, Grymonprez L, Lebert F, & Van der Linden M (2001). Memory impairment differs in frontotemporal dementia and Alzheimer’s disease. Neurocase, 7, 161–171. [DOI] [PubMed] [Google Scholar]
- 38.Patterson K, Nestor PJ, & Rogers TT (2007). Where do you know what you know? The representation of semantic knowledge in the human brain. Nat Rev Neurosci, 8, 976–987. [DOI] [PubMed] [Google Scholar]
- 39.Petersen RC, Jack CR Jr., Xu YC, Waring SC, O’Brien PC, Smith GE, Ivnik RJ, Tangalos EG, Boeve BF, & Kokmen E (2000). Memory and MRI-based hippocampal volumes in aging and AD. Neurology, 54, 581–587. [DOI] [PubMed] [Google Scholar]
- 40.Putcha D, McGinnis SM, Brickhouse M, Wong B, Sherman JC, & Dickerson BC (2018). Executive dysfunction contributes to verbal encoding and retrieval deficits in posterior cortical atrophy. Cortex, 106, 36–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rey A (1964). L’examen Clinique en Psychologie Paris. In. Paris: Presses Universitaires de France. [Google Scholar]
- 42.Ricci M, Graef S, Blundo C, & Miller LA (2012). Using the Rey Auditory Verbal Learning Test (RAVLT) to differentiate alzheimer’s dementia and behavioural variant fronto-temporal dementia. Clin Neuropsychol, 26, 926–941. [DOI] [PubMed] [Google Scholar]
- 43.Sandrini M, Cappa SF, Rossi S, Rossini PM, & Miniussi C (2003). The role of prefrontal cortex in verbal episodic memory: rTMS evidence. J Cogn Neurosci, 15, 855–861. [DOI] [PubMed] [Google Scholar]
- 44.Savill N, Ellis AW, & Jefferies E (2017). Newly-acquired words are more phonologically robust in verbal short-term memory when they have associated semantic representations. Neuropsychologia, 98, 85–97. [DOI] [PubMed] [Google Scholar]
- 45.Scoville WB, & Milner B (1957). Loss of recent memory after bilateral hippocampal lesions. J Neurol Neurosurg Psychiatry, 20, 11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shaw LM, Vanderstichele H, Knapik-Czajka M, Clark CM, Aisen PS, Petersen RC, Blennow K, Soares H, Simon A, Lewczuk P, Dean R, Siemers E, Potter W, Lee VM, Trojanowski JQ, & Alzheimer’s Disease Neuroimaging I. (2009). Cerebrospinal fluid biomarker signature in Alzheimer’s disease neuroimaging initiative subjects. Ann Neurol, 65, 403–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Squire LR, Stark CE, & Clark RE (2004). The medial temporal lobe. Annu Rev Neurosci, 27, 279–306. [DOI] [PubMed] [Google Scholar]
- 48.Uhlhaas PJ, Pantel J, Lanfermann H, Prvulovic D, Haenschel C, Maurer K, & Linden DE (2008). Visual perceptual organization deficits in Alzheimer’s dementia. Dement Geriatr Cogn Disord, 25, 465–475. [DOI] [PubMed] [Google Scholar]
- 49.Vincent JL, Kahn I, Snyder AZ, Raichle ME, & Buckner RL (2008). Evidence for a frontoparietal control system revealed by intrinsic functional connectivity. J Neurophysiol, 100, 3328–3342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Visser M, Jefferies E, & Lambon Ralph MA (2010). Semantic processing in the anterior temporal lobes: a meta-analysis of the functional neuroimaging literature. J Cogn Neurosci, 22, 1083–1094. [DOI] [PubMed] [Google Scholar]
- 51.Vyhnalek M, Nikolai T, Andel R, Nedelska Z, Rubinova E, Markova H, Laczo J, Bezdicek O, Sheardova K, & Hort J (2014). Neuropsychological correlates of hippocampal atrophy in memory testing in nondemented older adults. J Alzheimers Dis, 42 Suppl 3, S81–90. [DOI] [PubMed] [Google Scholar]
- 52.Wagner AD, Shannon BJ, Kahn I, & Buckner RL (2005). Parietal lobe contributions to episodic memory retrieval. Trends Cogn Sci, 9, 445–453. [DOI] [PubMed] [Google Scholar]
- 53.Walhovd KB, Fjell AM, Dale AM, McEvoy LK, Brewer J, Karow DS, Salmon DP, Fennema-Notestine C, & Alzheimer’s Disease Neuroimaging I. (2010). Multi-modal imaging predicts memory performance in normal aging and cognitive decline. Neurobiol Aging, 31, 1107–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang L, Laviolette P, O’Keefe K, Putcha D, Bakkour A, Van Dijk KR, Pihlajamaki M, Dickerson BC, & Sperling RA (2010). Intrinsic connectivity between the hippocampus and posteromedial cortex predicts memory performance in cognitively intact older individuals. Neuroimage, 51, 910–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wolk DA, Dickerson BC, & ADNI. (2010). Apolipoprotein E (APOE) genotype has dissociable effects on memory and attentional-executive network function in Alzheimer’s disease. Proc Natl Acad Sci U S A, 107, 10256–10261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wolk DA, Dickerson BC, & ADNI. (2011). Fractionating verbal episodic memory in Alzheimer’s disease. Neuroimage, 54, 1530–1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yeo BT, Krienen FM, Eickhoff SB, Yaakub SN, Fox PT, Buckner RL, Asplund CL, & Chee MW (2015). Functional Specialization and Flexibility in Human Association Cortex. Cereb Cortex, 25, 3654–3672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhao Q, Guo Q, Liang X, Chen M, Zhou Y, Ding D, & Hong Z (2015). Auditory Verbal Learning Test is Superior to Rey-Osterrieth Complex Figure Memory for Predicting Mild Cognitive Impairment to Alzheimer’s Disease. Curr Alzheimer Res, 12, 520–526. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.