Abstract
Maternal cardiovascular changes during pregnancy include an expansion of plasma volume, increased cardiac output, decreased peripheral resistance, and increased uteroplacental blood flow. These adaptations facilitate the progressive increase in uteroplacental perfusion that is required for normal fetal growth and development, prevent the development of hypertension, and provide a reserve of blood in anticipation of the significant blood loss associated with parturition. Each woman’s genotype and phenotype determine her ability to adapt in response to molecular signals that emanate from the fetoplacental unit. Here, we provide an overview of the major hemodynamic and cardiac changes and then consider regional changes in the splanchnic, renal, cerebral, and uterine circulations in terms of endothelial and vascular smooth muscle cell plasticity. Although consideration of gestational disease is beyond the scope of this review, aberrant signaling and/or maternal responsiveness contribute to the etiology of several common gestational diseases such as preeclampsia, intrauterine growth restriction, and gestational diabetes.
Keywords: adaptability, cardiac output, endothelium, peripheral resistance, remodeling, vasodilation
1. INTRODUCTION
According to Merriam-Webster’s Collegiate Dictionary, biological plasticity is defined as “the capacity of organisms with the same genotype to vary in developmental pattern, in phenotype, or in behavior according to varying environmental conditions.” This definition needs to be amended, however, with regard to pregnancy, as maternal cardiovascular plasticity occurs in response to internal (i.e., the influence of the fetoplacental unit) rather than external (environmental) factors.
The primary systemic cardiovascular adaptations of mammalian pregnancy include an increase in cardiac output and an expansion of plasma volume (1–3). While these changes would normally lead to an increase in blood pressure, a concomitant decrease in peripheral resistance coupled with increased arterial compliance during gestation result in a progressive reduction in blood pressure that reaches a nadir by mid-pregnancy before increasing back to normal by term (1).
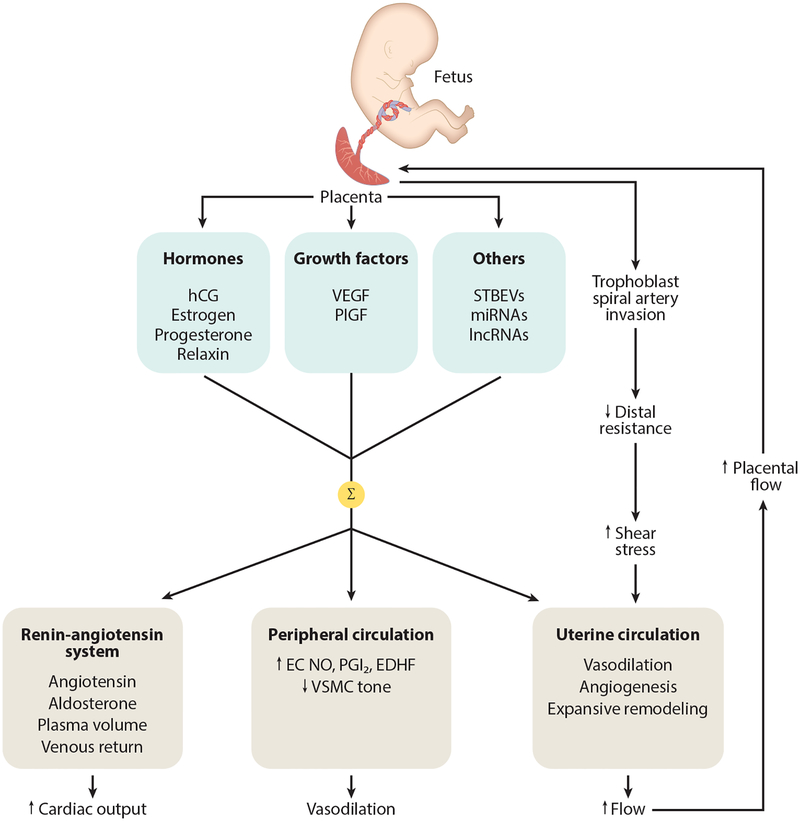
Defining plasticity in the gestational setting requires an understanding of the mechanisms that drive maternal adaptation, which begins quite early in pregnancy. For example, in humans, increases in cardiac output are already detectable by week 5 of gestation, i.e., 3 weeks postfertilization, as gestation is normally dated from the last menstrual period that occurs approximately 2 weeks before ovulation (4). At this time, the embryo is the size of a pea (5 mm), yet it is already effecting maternal cardiovascular changes by molecular signals such as human chorionic gonadotropin (hCG) secreted from the placenta. As pregnancy continues and the placenta grows, the amounts and diversity of signals increase. These include hormones, growth factors, early pregnancy proteins, miRNAs, and other—as yet, perhaps unknown—molecules. An overall schematic of the maternal gestational cardiovascular adaptive process is shown in Figure 1.
Figure 1.
Diagram showing some of the cardiovascular adaptations a woman experiences during pregnancy. The placenta secretes a variety of molecular signals (hormones, growth factors, others) into the maternal circulation. Their summative effects (Σ) result in activation of the renin-angiotensin system that acts to increase aldosterone, augmenting sodium and water reabsorption from the kidney in order to expand plasma volume; other components of the blood (cells, proteins) also increase but not as much as plasma volume, resulting in some hemodilution (not shown). Peripheral vasodilation is accomplished through increased EC release of NO, PGI2, and EDHF along with reduced VSMC myogenic tone. This leads to a reduction in peripheral resistance, which helps to accommodate the increased blood volume and maintain blood pressure at normotensive (or even slightly reduced) levels. In addition to vasodilation, the uterine circulation undergoes angiogenesis and expansive remodeling. These processes are stimulated by increased arterial wall shear stress that results from hemochorial placentation, which through trophoblast invasion and remodeling of spiral arteries, decreases distal resistance and accelerates blood in upstream vessels. The uterine circulation, uterus, and placenta grow in parallel, and uteroplacental blood flow increases progressively during pregnancy to levels that, in women, approach 1 L/min at term. Abbreviations: EC, endothelial cell; EDHF, endothelium-derived hyperpolarizing factor; hCG, human chorionic gonadotropin; lncRNA, long ncRNA; miRNA, microRNA; ncRNA, noncoding RNA; NO, nitric oxide; PGI2, prostacyclin; PlGF, placental growth factor; STBEV, syncytiotrophoblast-derived extracellular vesicle; VEGF, vascular endothelial growth factor; VSMC, vascular smooth muscle cell.
Our intent in this review is to provide an overview of the vascular adaptations that occur during normal pregnancy. After briefly considering the heart (Section 2), we discuss some of the signals and mechanisms that drive maternal vascular plasticity during gestation (Section 3) and review some of the endothelial cell (EC) and vascular smooth muscle cell (VSMC) adaptations in peripheral vessels (Section 4) that facilitate the accommodation of plasma volume expansion and increased cardiac output. Most generally, these result in peripheral vasodilation and increased compliance. The uterine (or, more accurately, uteroplacental) circulation is unique in that, in addition to changes in tone, reactivity, and matrix composition, uterine arteries and veins both undergo significant growth and remodeling in order to maintain placental perfusion and assure normal fetal development and pregnancy outcome. For this reason, this vascular bed is considered separately in Section 5.
The importance of understanding the mechanisms that drive maternal cardiovascular adaptation is underscored by the fact that some gestational diseases such as preeclampsia and intrauterine growth restriction are thought to arise from maladaptive responses that may begin early in pregnancy, such as shallow trophoblast invasion of the spiral arteries leading to placental underperfusion, attenuated or excessive fetoplacental signaling, and inappropriate maternal responsiveness. Due to space limitations, we do not consider pathological changes to any degree, nor do we discuss hematologic changes (hemodilution of the blood, altered clotting factors, etc.).
2. CARDIAC ADAPTATION DURING PREGNANCY
Although vascular plasticity is our primary focus, a few words about the cardiac adaptation are in order, as some of the vascular changes occur in response to alterations in the work of the heart. Cardiac output begins to rise within a week or two of fertilization (3–4 weeks after the last menstrual period) and continues to increase until 20–24 weeks of gestation, cresting at values 30–40% above the nonpregnant level in singleton pregnancies (e.g., increasing from 5 to 7 L/min) (5, 6). Cardiac output augmentation may be even greater in multiple pregnancies, with increases exceeding 50%. Both heart rate and stroke volume are augmented, with elevations in heart rate detectable by 5 weeks of gestation, and stroke volume increases occurring a little later, by 8 weeks. Heart rate increases 10–15 bpm, which translates into 14,000–21,000 extra beats per day! The increases in stroke volume are driven by augmented venous return secondary to plasma volume expansion, with both cardiac contractility and ejection fraction increasing accordingly (5, 6). For these reasons, pregnancy stresses the heart and, if a woman has latent heart disease or a silent congenital defect, it may be exacerbated by this and become symptomatic during her pregnancy (7, 8). Structural changes occur in the heart as well, with enlargement of the left atrial diameter (approximately 15%) and increased left ventricular mass (about 50%) (5). Cardiac function returns to nonpregnant values during the puerperium, the period of 6 weeks after delivery in humans, with reductions in heart rate occurring earlier, by 10 days postpartum (9).
3. FETOPLACENTAL SIGNALING OF THE MATERNAL ORGANISM
Signaling molecules secreted by the fetoplacental unit are primarily responsible for inducing the physiological and anatomical changes in the maternal cardiovascular system, and some of the more important ones are considered below. Their specific actions on maternal ECs and VSMCs are reviewed in Sections 4 and 5. As already mentioned, altered fetoplacental signaling is associated with gestational diseases. For example, sFlt-1 [a soluble VEGF (vascular endothelial growth factor) receptor] and endoglin (a soluble receptor for TGF-β) are both upregulated in preeclampsia, and their circulating levels are positively correlated with disease severity. By binding important signaling molecules and preventing their access to tissues, soluble receptors like sFlt-1 and endoglin prevent normal adaptation and lead to maternal endothelial dysfunction, which is a hallmark of this human disease that is characterized by new-onset hypertension and proteinuria (10, 11).
3.1. hCG and Sex Steroids
hCG is mainly produced by differentiated syncytiotrophoblasts to promote the maintenance of corpus luteum (CL) in the ovary during early pregnancy (12, 13). Following implantation, circulating levels of hCG double every 48 h, thereby preventing luteal demise (and menses, which would terminate that pregnancy). Although the CL continues to produce both estrogen and progesterone throughout pregnancy, its contribution is no longer essential after about 7–9 weeks of pregnancy, when the primary site of production becomes the placenta (14, 15). This luteoplacental shift results in progressively increasing circulating levels of sex steroids that eventually dwarf those normally present during the menstrual cycle, with estrogen concentrations increasing 50–100 fold and progesterone levels 10–20 times above cycling levels by term (16, 17). In addition, hCG plays a role in angiogenesis in the uterine endothelium (18), maintains myometrial quiescence (19), and has immunomodulatory effects at the maternal-fetal interface (20). A detailed examination of hCG and its clinical applications were recently reviewed by Nwabuobi et al. (21).
Estrogen, progesterone, and their metabolites directly facilitate several processes such as angiogenesis, trophoblast development, and invasion through an upregulation of cell proliferation, differentiation, and migration of cytotrophoblasts, as well as vasodilation via nitric oxide (NO) signaling and prostacyclin production (22). Estrogen can also have indirect effects on the vascular wall by regulating other pathways that impinge on NO signaling (such as VEGF and VEGFR-2) and, hence, support vasodilation, altered reactivity, and structural remodeling.
Human placental estrogen synthesis depends on dehydroepiandrosterone (DHEA) and its sulfated form, produced by both maternal and fetal adrenal glands (22, 23). This is a good example of how both maternal and fetal signals have the capacity to modulate placental function, vascular adaptation, and the interactive nature of gestational communication. In addition to estradiol-17β, estriol and estrone are also produced in significant amounts during pregnancy (24, 25).
3.2. Relaxin
The peptide hormone relaxin, mostly produced by the CL and placenta, is traditionally considered a pregnancy hormone because endogenous relaxin is only detectable in the circulation during pregnancy (26). At the same time, it can stimulate remodeling of various tissues such as heart, kidney, lung, liver, skin, and vasculature in nonpregnant females and males (27–29). Circulating relaxin levels are highest toward the end of the first trimester and at delivery (30). During pregnancy, it plays an important part in renal and systemic hemodynamic adaptation in view of its vasodilatory and remodeling actions on the maternal vasculature (27–29, 31), which are discussed further in later sections.
3.3. Vascular Endothelial Growth Factor and Placental Growth Factor
VEGF is expressed in villous cyto- and syncytiotrophoblasts, the invading front of the anchoring columns, and extravillous and endovascular trophoblasts in gestation (32, 33). Pleiotropic effects of VEGF consist of priming the spiral arteries for invasion, enhancing blood flow in fetal capillaries, increasing vascular permeability, stimulating angiogenesis and cell migration, and synthesizing metalloproteinases, and VEGF exerts a potent vasodilatory effect on fetoplacental vessels through action on its receptors: fms-like tyrosine kinase (Flt-1) and kinase domain receptor, also called VEGFR-1 and VEGFR-2 (10, 11, 34).
Unlike VEGF, which binds to both VEGFR-1 and VEGFR-2, placental growth factor (PlGF) only binds to VEGFR-1. Although it has sometimes been considered to be an orphan receptor, our studies in rat and human resistance arteries have shown that it is a potent vasodilator that acts primarily through endothelial NO release, with a residual component that was attributable to endothelium-derived hyperpolarizing factor (EDHF) (10).
3.4. Other Molecular Signals
Emerging evidence suggests that other molecular signals may also be critical in placental function and regulating vascular adaptation. For example, syncytiotrophoblast-derived extracellular vesicles (STBEVs), especially exosomes, which reportedly appear as early as 6 weeks of gestation (35, 36), are packed with an extensive range of proteins, microRNA (miRNA), and phospholipids that facilitate feto-maternal communication (37). Different classes of noncoding RNA molecules (ncRNAs), including miRNAs (19–25 nucleotides) and long ncRNAs (lncRNAs, >200 nucleotides), and their modulation by environment, signaling, and epigenetic pathways may represent another, less well-understood regulatory mechanism by which the placenta affects the maternal heart and blood vessels (38, 39). These molecules may also impact fetal development. For example, H19, one of the first lncRNAs discovered (40), is associated with placental and fetal growth abnormalities, and altered imprinting of H19 is related to Silver-Russell and Beckwith-Wiedemann syndromes in humans (41). Also, H19 was found to be a developmental reservoir for miR-675, whose level increased with gestation and acted as placental growth suppressor, in the mouse (42). There are three major clusters of placenta-specific miRNAs: the chromosome 14 miRNA cluster (C14MC), C19MC, and the miR-371–373 cluster (38, 43). Both miR-520g and miR-520h from C19MC have been shown experimentally to repress expression of VEGF in preeclampsia (44). Zhang and colleagues (45) recently described the downregulation of ten-eleven translocation methylcytosine dioxygenase 1 (TET1) by miR-210, leading to repression of BKca channel function in ovine uterine arteries.
At this time, the function of a vast number of ncRNAs in the human genome remains underexplored; to date, studies have mostly relied on comparing expression in normal pregnancies versus those with gestational complications. It is important to further analyze whether their altered expression and circulating levels are a cause or consequence of pathophysiological conditions and whether they may be useful as biomarkers for identifying at-risk patients or those in the early stages of gestational disease.
4. PREGNANCY-INDUCED ADAPTIVE CHANGES IN THE SPLANCHNIC, RENAL, AND CEREBRAL CIRCULATIONS
4.1. Integration of Endothelial and Vascular Smooth Muscle Cell Signaling by the Vascular Wall
As the interface between the blood and arterial vascular smooth muscle, the endothelium is well positioned to sense and integrate vascular responses to humoral factors and physical forces such as shear stress. A number of hematologic changes occur during pregnancy, e.g., hemodilution (because there is a larger increase in the fluid volume of the blood than in most of its components) and an altered humoral milieu in terms of hormones, growth factors, and sundry other placentally derived signals. Endothelial responses include altered secretion of vasoactive molecules, changes in permeability, and the formation of new types of connections between adjacent cells (e.g., gap junctions in the uterine circulation).
VSMCs are the ultimate effectors of arterial diameter via force production (contraction) that induces tone (constriction) and determines flow resistance. The ambient level of force is continually modulated by endothelial influences, which are predominantly but not exclusively vasodilatory. VSMCs can also generate contractile force directly in response to pressure or stretch (myogenic behavior) and are subject to periarterial, neural, and metabolic influences.
There is little evidence for altered autonomic regulation playing a significant role during pregnancy (46), and the primary vascular adaptations appear to be myogenic and endothelial in nature. Humoral influences can have direct effects on VSMCs, although in many cases their actions are indirect, i.e., secondary to a primary effect on the endothelium, which is directly adjacent to flowing blood and may act as a diffusion barrier that limits or prevents signal access to VSMCs in the medial layer. As discussed in Section 3, some of the major humoral influences include sex steroids, relaxin, growth factors such as VEGF and PlGF, and—particularly in diseased states—uniquely placental secretions such as soluble growth factor receptors (sFlt-1, endoglin) and miRNAs.
During pregnancy, splanchnic, renal, pulmonary, and skin blood flows are all augmented, although blood pressure is reduced or unchanged (1, 2). Therefore, the principal effect must be a reduction in arterial and arteriolar tone and, therefore, peripheral resistance. This adaptability involves changes in both endothelial and VSMC function; together, these enhance vasodilation and thereby facilitate the accommodation of increased plasma volume. Conversely, perfusion of other organs such as the brain, skeletal muscle, and liver is not measurably different, nor are the mechanisms involved in cerebral or renal autoregulation. Consideration of the splanchnic, renal, and cerebral circulations highlights the nature of organ-specific regional vascular adaptability.
4.2. Splanchnic (Mesenteric) Circulation
The splanchnic circulation receives approximately one-third of cardiac output in pregnancy and therefore contributes significantly to total peripheral vascular resistance. A number of changes in mesenteric artery (MA) structure and reactivity have been reported in a manner consistent with reduced tone and vascular resistance secondary to upregulation of endothelial vasodilator influences, particularly NO and prostacyclin (PGI2); hyperpolarization of VSMCs; and increased interendothelial gap junctional communication. Thus, studies on pressure-dependent myogenic tone have sometimes reported no change, although more often, reductions in tone were observed and related to an increased vasodilatory influence of the endothelium—particularly NO—in response to flow-induced (47) or enhanced basal release (48–50). Gap junctional communication may play a role as well, as inhibition of gap junctions increased myogenic tone in mouse MAs (50), although most of the experimental evidence in this regard comes from studies on uterine arteries (51, 52).
Endothelium-mediated vasodilation in pregnancy depends on increased production of NO, PGI2, and EDHF (53). Although there is general agreement on increased NO and prostanoid signaling in MAs from pregnant animals, conflicting results have been published on the influence of pregnancy on EDHF production. Pregnancy increased ACh-stimulated relaxation of MA, which still remained after nitroarginine (L-NNA) or indomethacin, suggesting that EDHF is the major mediator of ACh-induced dilatation in MAs from pregnant rats (54, 55). In mouse MAs, endothelial-dependent relaxation was enhanced by pregnancy and blunted after the addition of NO synthase (NOS) inhibitors although, in this species, EDHF-mediated vasodilation was not altered by pregnancy (56).
MA plasticity during pregnancy also includes alterations in VSMC membrane potential, which was found to be more hyperpolarized in cells from pregnant versus nonpregnant rats [−64 mV versus −57 mV (57)], which is consistent with reduced tone and peripheral resistance.
Several studies have shown that pregnancy decreases α-adrenergic agonist-induced tone such that sensitivity to the selective (α1) agonist phenylephrine was significantly less in MAs from pregnant versus nonpregnant rats (58, 59). Responses to norepinephrine (60) and transmural nerve stimulation (61, 62) were also diminished. The refractoriness to adrenergic vasoconstriction was abolished by NOS inhibition, suggesting that an enhanced endothelial NO production by vasoconstrictor agents was primarily responsible and related to estrogen and relaxin (55). Estrogen receptor (ER)α expression increased in both ECs and VSMCs of MA from pregnant animals and contributed to augmenting NO and PGI2 production and release (63). Relaxin augmented prostanoid production from SMCs of MA from pregnant animals; moreover, its deficiency prevented the normal blunting of vasoconstrictor responsiveness to angiotensin II (Ang II), possibly via loss of its actions on NO (64).
Although there is no evidence of MA growth during pregnancy, such as occurs in the uterine circulation, decreased distensibility was noted in one study and associated with reductions in collagen and elastin (19% and 15%, respectively) within the vascular wall (65). As the extracellular matrix derives primarily from VSMCs, plasticity-induced changes in their phenotype, in addition to changes in tone and reactivity, may extend to biomechanical properties as well.
The reasons for altered reactivity and tone are not well defined, but they may be related to increased estrogen and/or an upregulation of its receptors and to the presence of relaxin. As elucidated in a series of studies by Conrad & Shroff (66) and discussed in the next section, the actions of relaxin are particularly notable in the renal circulation, although its direct vasodilator actions extend to other vessels, including those from the mesenteric circulation (28, 29, 31, 64).
4.3. Renal Circulation
Pregnancy increases renal blood flow by reducing renal vascular resistance through diminished pressure-induced myogenic tone, refractoriness to vasoconstrictor stimuli, and an upregulation of endothelium-dependent vasodilation. Relaxin, an ovarian hormone, has been shown to play an important role in mediating maternal renal hemodynamic adaptations in pregnancy, such that correlations between reductions in renal artery resistance and relaxin concentrations have been noted (67). Relaxin acts on renal arteries by the receptors RXFP1 and LGR7, which are upregulated in renal arteries from pregnant animals (68). Relaxin-specific antibodies attenuate renal vasodilation (69) that has been shown to involve matrix metalloproteinase (MMP-2, MMP-9) activation. This, in turn, results in the cleavage of big endothelin to ET1–32, a fragment that activates the endothelial ETB receptor and stimulates the production of NO (70–72).
The increased NO in renal arteries from pregnant rats reduced VSMC Ca2+ entry from the extracellular space (but did not affect intracellular Ca2+ release) and contributed to the decreased renal vascular resistance associated with normal pregnancy, a state that is characterized by increased renal blood flow and glomerular filtration (73). The gestational upregulation of NO contributes to the blunted vasoconstrictor responsiveness to Ang II, whose circulating levels are significantly increased during pregnancy. This is an important adaptation, as Ang II may stimulate sodium reabsorption and plasma volume expansion without inducing vasoconstriction and hypertension (74). Notably, this blunted vasoconstrictor responsiveness is attenuated in preeclampsia (75).
4.4. Cerebral Circulation
In contrast to other organs, including the uterus, gut, and kidney, which undergo substantial increases in flow during pregnancy, cerebral blood flow and autoregulation remain unchanged. This indicates an ability of the cerebral vasculature to be protected from the potentially damaging effects of vasoconstrictors such as Ang II (76) or to the permeability-inducing actions of VEGF (77).
In the isolated rat posterior cerebral artery (PCA, which supplies the occipital cortex) pregnancy did not alter myogenic reactivity to pressure changes, although myogenic tone was reduced at high transmural pressures (>150 mm Hg) (78). This suggests that pregnancy may predispose the cerebral circulation to forced dilatation and hypertensive encephalopathy, as may occur during eclampsia. PCAs from late pregnant animals are less sensitive to magnesium sulfate (MgSO4) than those of nonpregnant counterparts, suggesting that the beneficial effects of MgSO4 in preventing the occurrence of seizures in eclamptic patients are not due to cerebral vasodilation and improved cerebral blood flow, but are attributable to a direct effect on neurons (79). Other changes, such as significant upregulation of aquaporin 4, a water channel in the endothelium, have also been noted in vessels from pregnant animals (80).
While pregnancy does not appear to influence passive diameters or distensibility of the PCA, which is a pial artery (81), smaller penetrating parenchymal arterioles do undergo hypotrophic outward remodeling during pregnancy. This process is mediated by relaxin and occurs via the induction of peroxisome proliferator-activated receptor γ (PPARγ) (82). Interestingly, in PCAs, PPARγ is downregulated in pregnancy, possibly explaining why there is no pial artery remodeling (83).
Little information is available about venous changes in general and cerebral veins in particular. One report described outward hypotrophic remodeling and diminished myogenic tone in the vein of Galen, while endothelium-dependent and -independent vasodilations were unchanged (84).
5. THE UTEROPLACENTAL CIRCULATION
5.1. Overview
The mass of the uterus increases approximately 20-fold during pregnancy (50–1,100 g, excluding fetal tissues and fluids) in preparation for parturition, initially through hyperplasia, and then hypertrophy of myometrial smooth muscle. The phenotypic conversion to a hypertrophic phenotype in myometrial smooth muscle appears to be regulated by mechanical stretch (85).
Early studies in sheep and rabbits (86) showed that absolute myometrial flow increases progressively during pregnancy, although relative flow (per gram of tissue) stays fairly constant. In view of the increased myometrial mass, however, the architecture of the vascular tree must increase dimensionally. The combined increase in uterine and placental blood flows results in a 30- to 50-fold increase relative to the nonpregnant state in mammalian species, with increases on the order of 10- to 20-fold in the human female (700–800 ml/min by term, with values in excess of 1 L/min measured in twin pregnancy). To accomplish these hemodynamic changes, the entire maternal uterine circulation must grow considerably, and many studies have shown that uterine arteries and veins both undergo outward expansive remodeling, with uterine artery diameters increasing 50–100%, depending on species (87), with an approximate doubling measured in humans (88). Uterine vessels also increase in length; thus, the circumferential and longitudinal changes would be expected to oppose each other in terms of the effect of flow resistance. Given that the relationship between length and resistance is linear, while that of diameter to resistance is inverse fourth power, adjustments in caliber clearly have the predominant effect.
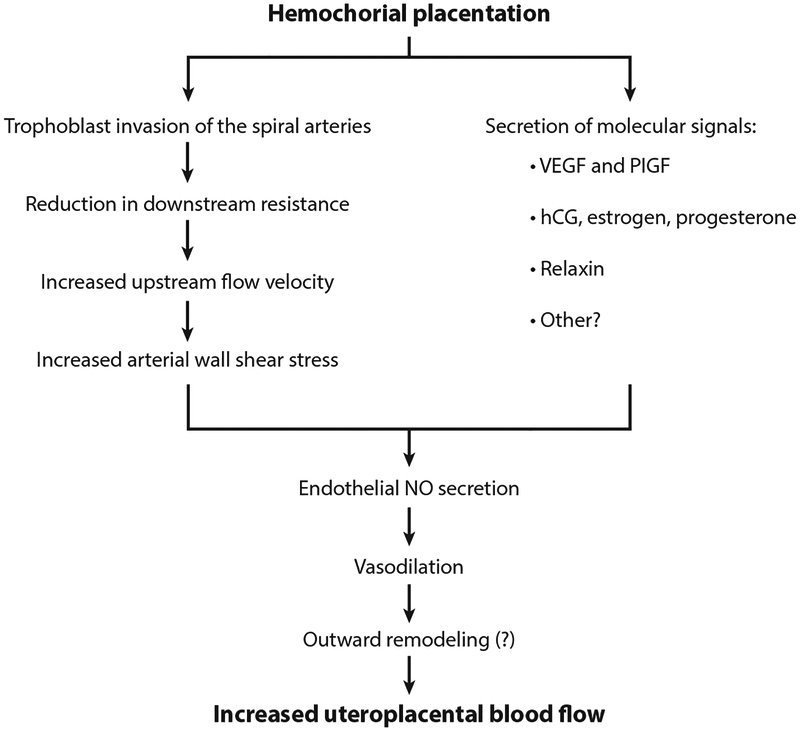
Several mechanisms have been implicated in this process of outward (expansive) hypertrophic remodeling. First, in hemochorial placentates such as humans and rats, enlargement of spiral arteries secondary to endovascular trophoblast invasion reduces downstream resistance, and placental development leads to a high-throughput, low-velocity flow through the intervillous space. This process of reducing downstream resistance accelerates blood in upstream vessels, thereby increasing wall shear stress (Figure 2).
Figure 2.
A schematic showing how hemochorial placentation leads to increased uteroplacental blood flow. Widening of the spiral arteries secondary to trophoblast invasion reduces preplacental flow resistance and thereby accelerates blood velocity in upstream arteries. This increases wall shear stress and stimulates the release of endothelial NO. Concomitant release of molecular signals such as VEGF, PlGF, hCG, and other factors such as estrogen, progesterone, and relaxin into the intervillous space lead to increased circulating levels. Many of these compounds act on the endothelium to stimulate NO (and other vasodilator) release. The combination of signaling and increased shear stress augments endothelial NO and leads to uterine artery vasodilation and outward remodeling. The question mark connotes the fact that we do not yet have experimental evidence linking vasodilation to outward remodeling, although a number of studies have shown that vasoconstriction does the opposite, i.e., it leads to inward remodeling such as occurs in hypertension. Abbreviations: hCG, human chorionic gonadotropin; NO, nitric oxide; PlGF, placental growth factor; VEGF, vascular endothelial growth factor.
Our studies in rats (89–92) and earlier published work with endothelial NOS (eNOS) knockout mice (93) support shear stress–induced endothelial NO release as a key mechanism by which vasodilation and vessel growth is achieved. Sex steroids have also been implicated in maternal uterine vascular growth early in pregnancy, as some growth was evident in pseudopregnant mice that lack a fetus and placenta but experience endocrine changes that mimic those normally seen during the first half of pregnancy (94).
The principal mechanisms that induce maternal uterine vascular remodeling appear to be local rather than systemic (at least in rats), because animals that have undergone oviductal ligation, which results in a unilateral pregnancy, show significant vascular growth and remodeling (as well as pregnancy-associated changes in myogenic tone) only in the implanted horn (95–97).
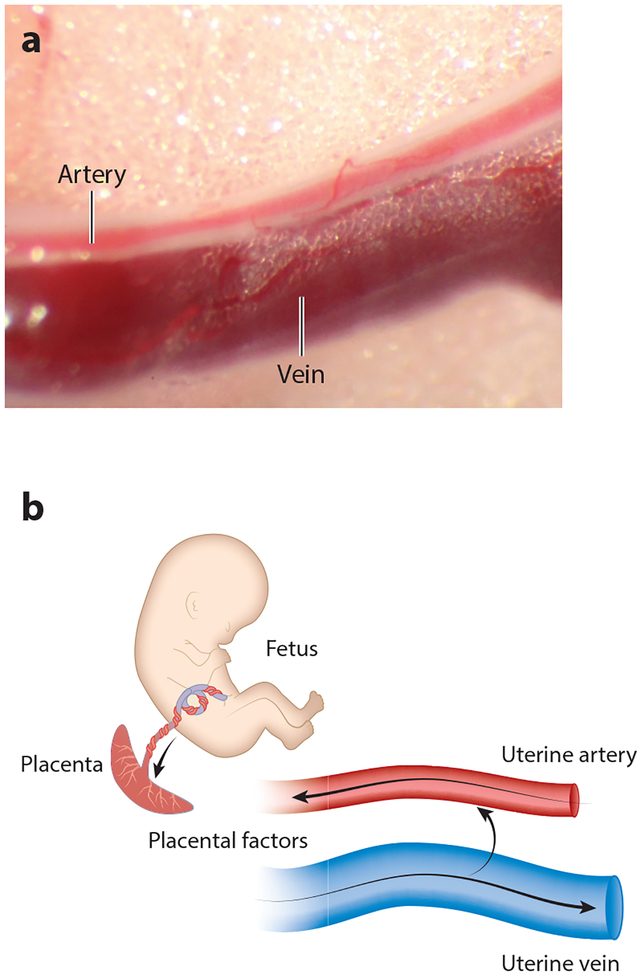
In addition to placentation-induced shear stress, a second, less-established (but provocative) mechanism involves venoarterial signaling, i.e., the transfer of placentally derived signals from postplacental veins to preplacental arteries to induce changes in tone and/or structure. There is some physiological precedent for this mechanism in the female reproductive system, as venoarterial signaling is a well-documented mechanism for luteolysis in several mammalian species (98). Also, as discussed in Section 3, the placenta secretes many vasoactive and growth-promoting signals such as sex steroids, VEGF, and PlGF, whose concentrations are highest in the venous blood exiting the uterus. And finally, the fact that uterine arteries and veins tend to run parallel to and in close apposition to each other presents an anatomical architecture that favors venoarterial communication (Figure 3). Remodeling mechanisms that may be activated by venous-borne signals are recruitment and incorporation of cells from the periarterial space into the vascular wall and stimulation of matrix remodeling by the alteration of VSMC phenotype.
Figure 3.
We hypothesize that venoarterial signaling (transfer of placentally derived signals into the periarterial space via passage through the venous wall) contributes to arterial remodeling and tone. This concept is supported by a physiological precedent in terms of corpus luteum demise, which involves transfer of myometrium-derived constrictor prostaglandins to the ovarian artery, leading to corpus luteum ischemia, and by the architecture of the uterine circulation, in which close apposition of arteries and veins is often present (a). Venoarterial signaling could also function as a “short loop” mechanism for regulating placental perfusion (b). Briefly, vasoactive and/or growth-promoting signals emanating from the fetoplacental unit enter the uterine veins and are then transferred into the periarterial region, where they may act to recruit cell migration into the arterial wall and lead to changes in uterine artery tone and reactivity.
By removing a venoarterial unit and cannulating both the arterial and venous segment while they are still connected in the chamber of an arteriograph, we found that vasoactive compounds within the vein could indeed change the tone of the adjacent artery (99) and that the permeability of the venous wall was significantly increased in veins from pregnant versus nonpregnant animals. This effect might be related to increased wall tension secondary to increased venous diameter and, possibly, increased venous pressure, as the hemochorial placenta—absent a microcirculation—can be viewed as a specialized arteriovenous shunt (100).
Moreover, venous permeability was augmented in the presence of VEGF, showing that this process was subject to modulation by placental signals (101). These findings were carried out in vitro, however, and definitive proof of venoarterial signaling playing a role in the living animal is lacking.
In a very recent (2018) study of pregnant Sprague-Dawley rats, in which segments of vein were surgically removed to observe the effect on arterial remodeling, we found that arterial remodeling was reduced by approximately 50% over a 10-day period in arterial segments devoid of an accompanying vein. This provides the first evidence that the process of venoarterial signaling is an important physiological mechanism in vivo (102). Thus, increased flow and endothelial shear stress, which stimulate expansive remodeling in other vessels as well (103, 104), and venoarterial signaling appear to work synergistically to augment the process of outward remodeling.
The primary role of the endothelium in mediating changes in arterial structure in response to altered flow was first shown by Langille & O’Donnell in 1986 (105), and subsequent studies have identified NO as the active principle. Because placental signals such as estrogen, VEGF, and PlGF also stimulate endothelial NO secretion (87, 106), as does relaxin (28), the idea that shear stress and placental signals have an additive, convergent effect in stimulating NO signaling and inducing arterial vasodilation and growth is well premised.
Uterine veins grow considerably and, although shear stress is also known to stimulate venous enlargement (107), the actual mechanisms involved in venous remodeling are not known, as most studies have focused on the prerather than postplacental vessels. Compared to uterine veins from nonpregnant animals, those from late pregnant rats were larger and had elevated mitotic indices for both ECs and VSMCs. They were also more distensible and showed a reduced elastin content and adrenergic nerve density (108).
In addition to changes in overall structure, which are most reflective of extracellular matrix, there are additional changes in endothelial structure and function, in pressure-induced myogenic tone, and in vasoconstrictor and vasodilator reactivity. The following sections highlight some of the findings in this regard.
5.2. Endothelial Plasticity
By stimulating vasodilation and arterial growth, endothelial plasticity in the uterine vasculature facilitates the maintenance of normal uteroplacental perfusion and fetal development. Endothelial hyperplasia as well as VSMC hypertrophy and hyperplasia have been reported in uterine arteries during normal gestation in the rat (109).
Most of the major known endothelial vasodilatory pathways (NO, PGI2, EDHF and H2S) are augmented in pregnancy (110–113) through a combination of ionic and enzymatic mechanisms. For example, one important adaptation of uterine artery endothelial cells (UAECs) during pregnancy is an enhancement of Ca2+ signaling (111), which occurs in different species and at multiple levels and is requisite for the release of several vasodilators. As shown in a series of studies by Bird, Magness, and colleagues (114–116), capacitative Ca2+ entry was enhanced in UAECs from pregnant and nonpregnant sheep (114), as was an upregulation of connexin 43 (Cx43), a gap junctional protein. UAECs from pregnant animals produced more sustained Ca2+ bursts in response to ATP (115) and, as a result, likely increased the release of NO, EDHF, and PGI2 (116). The augmentation of Ca2+ bursts required coupling of transient receptor potential canonical type 3 (TRPC3) channels and the inositol 1,4,5-triphosphate receptor type 2 (IP3R2), both of which were upregulated in UAECs from pregnant animals in a manner dependent on Cx43 gap junctions (117). Cx43 is also located in caveolae and colocalizes with eNOS (118). The establishment of an enhanced vasodilatory endothelial phenotype by pregnancy through gap junctional mechanisms also extends to heightened ovine UAEC responses to cAMP by stimulating gap junctional trafficking and open gating. This effect was opposed by cGMP, however, illustrating the differential regulatory control of Cx43 gap junction function by cyclic nucleotides (119) that are, in turn, subject to regulation by placentally derived signals such as estrogen and VEGF. This is one example of how fetoplacental signals may induce endothelial plasticity in favor of vasodilation and thereby simultaneously impact both regional blood flows and total peripheral resistance. In a parallel mechanism, once endothelial cytosolic Ca2+ becomes elevated, it may activate endothelial potassium (K+) channels (such as IK and SK channels), which lead to hyperpolarization. In this endothelium-dependent hyperpolarization mechanism, which is upregulated in pregnancy, hyperpolarization is transmitted from the ECs to VSMCs, most likely through myoendothelial gap junctions. In VSMCs, hyperpolarization reduces cytosolic Ca2+ and induces vasorelaxation (112, 120).
The influence of augmented endothelial vasodilator during pregnancy extends to the production of PGI2 through an upregulation of its synthase. This effect, noted in sheep, was particular to the uterine circulation, as it was not present in omental vessels (110), underscoring both the importance of regional differences and the unique properties of the uteroplacental circulation that likely relate to the demands of the fetus.
Cyclooxygenase (COX) enzymes showed differential localization and expression during pregnancy. In ewes, the dramatic increase in PGI2 production during the last trimester was related to an upregulation of both message and protein for the COX-1 enzyme, which is localized in uterine artery ECs versus VSMCs. Conversely, COX-2 expression was not detectable by Western blotting in uterine artery of ECs or VSMCs (110, 121, 122).
H2S is a potent gaseous vasodilatory and angiogenic factor whose regulation and importance in pregnancy is not yet well understood. The work of Chen and colleagues (113, 123) showed that pregnancy selectively upregulates the cystathionine β-synthase (CBS) rather than the cystathionine-gamma-lyase enzyme. CBS is present in both endothelium and VSMCs, and its upregulation would favor vasodilation and lower resistance.
In summary, the increased vasodilator influence of the endothelium during pregnancy acts to reduce arterial tone and blunt vasoconstrictor reactivity. NOS inhibition reinstated mesometrial artery sensitivity to serotonin in vessels from late pregnant rats (124) and the well-known blunting of Ang II vasoconstriction during pregnancy was related to increased endothelial NO, rather than to prostanoid biosynthesis (125). This adaptation is reduced in preeclampsia, a state in which endothelial vasodilator influence, particularly that of NO, is reduced (10), reinforcing the concept of both signaling and response elements going awry in gestational diseases. Although the evidence is less categorical, newer studies also point to preeclamptic women having less estrogen than those experiencing a healthy pregnancy, and this too may favor reduced endothelial NO signaling.
Estradiol-17β and its metabolites act on UAECs through classic nuclear receptors ERα and ERβ (126) and a membrane receptor G protein–coupled estrogen receptor (GPER). Activation of GPER induces uterine artery vasodilation in a NO-dependent manner that was greater in vessels from pregnant versus nonpregnant rats (127). In ovine UAECs, estrogenic activation of ERα has been shown to induce prostacyclin synthesis through the phospholipase A2/COX-1 pathway, whose expression was higher in UAECs from pregnant versus nonpregnant animals (128).
Estrogens can act indirectly on UAECs by stimulating VEGF and PlGF, whose production by uterine arteries and the placenta is augmented during pregnancy (129). VEGF enhances uterine venous permeability, as does the increased wall tension secondary to venous growth (101). This may augment the transfer of signaling molecules from postplacental veins to adjacent arteries, as discussed above.
Studies from humans and animals have shown that VEGF acts on UAECs (123, 129) through VEGFR1, a tyrosine kinase receptor whose expression increases during pregnancy, as well as through VEGFR-2 (130). Pregnancy augments UAEC dilation to VEGF (and PlGF) by increasing the production of endothelial NO, EDHF, and H2S (112, 113, 123, 129, 131, 132).
5.3. Vascular Smooth Muscle Plasticity
Uterine artery growth during pregnancy is reflected in a 2.5-fold increase in VSMC mass per unit length of vessel (133) as a result of cellular hypertrophy and hyperplasia. VSMC proliferation rates increase significantly during pregnancy but are quite low in nonpregnant animals (109). Although uterine artery VSMCs from pregnant rats are 21% longer than those of nonpregnant ones (134), this adaptation seems relatively modest, as uterine artery diameters increase 60–200%, and arterial length increases 200–500%. Clearly, there must be significant hyperplasia as well, as shown in an earlier paper from our group (109). As discussed above, cell–cell communication is also altered, as gap junctional communication between VSMCs is enhanced in association with a 12- and 6-fold upregulation of connexin 37 and 43 expression, respectively, in uterine arteries from pregnant versus nonpregnant animals (135).
During pregnancy, growth of uterine artery VSMCs is likely driven by fetoplacentally derived humoral signals that increase in their own right (e.g., estrogen, progesterone) as pregnancy progresses. In addition, maternal responsiveness may also be further heightened by upregulation of receptors and postreceptor signaling. By studying cultured uterine artery VSMCs from pregnant guinea pigs, Moore and colleagues (136) showed that estradiol-17β and platelet-derived growth factor (PDGF) act synergistically to stimulate cellular growth and that this effect could be reproduced by protein kinase C (PKC) activation. The vascular effects of relaxin are mediated by RXFP1 receptors that are predominantly (but not exclusively) localized to the tunica media; they too are upregulated in the uterine circulation during early pregnancy (137).
Phenotypic alterations in uterine artery VSMCs may result from a combination of physical and humoral signals associated with pregnancy-induced changes in the active contractile properties of uterine artery VSMCs, e.g., heightened α-adrenergic sensitivity, intrinsic pressure-dependent tone, and myogenic reactivity (92).
There is some discrepancy with regard to myogenic tone in regard to species, in that tone is increased in myometrial arteries from pregnant women [relative to nonpregnant (138)] and in both premyometrial and preplacental radial arteries from pregnant versus nonpregnant rats (139). This effect was local in nature because it was related to implantation site (96) and was associated with an increase in VSMC cytosolic calcium secondary to diminished activity of VSMC voltagegated delayed-rectifier potassium (K+v) channels. Reduced K+v channel activity would lead to membrane depolarization and favor augmented VSMC Ca2+ influx through voltage-gated L-type Ca2+ channels (140).
Contrary to the aforementioned findings in humans and rats, in which small uterine artery tone is increased during pregnancy, myogenic tone was reduced in the main uterine artery of mice, and this effect was related to an increased endothelial NO influence (50). Interestingly, these same authors found that myogenic tone was increased during pregnancy under conditions of moderately severe dietary restriction, which diminished the NO influence (141). As shown by Zhang and colleagues (142), myogenic tone was reduced in uterine arteries from pregnant sheep through a mechanism related to downregulation of PKCα and an upregulation of ERK1/2 signaling; this effect could be mimicked in vessels from nonpregnant sheep by 48-h treatment with estradiol-17β and progesterone, again underscoring the importance of fetoplacental signaling, in this case, of uterine artery VSMC phenotypic modulation. Although differences between rats and mice are difficult to explain, those between rodents and sheep may be related to the types of placentation (hemochorial versus epitheliochorial, respectively), which differ in the nature of their vascular adaptation and hemodynamic pattern.
Uterine arteries from pregnant rats become 4.5-fold more sensitive to the α1-adrenoceptor- mediated vasoconstriction by phenylephrine compared to those from nonpregnant rats. Sandow and colleagues (143) related this effect to underlying changes in SMC TRPC3, T-type, and L-type voltage-gated calcium channels, which act synergistically to modulate Ca2+ signaling. In an earlier study, we found that pregnancy-induced increases to α-adrenergic stimulation were related to altered G protein cycling rates, such that G proteins in smooth muscle in pressurized uterine arcuate arteries from nonpregnant rats were more susceptible to deactivation. Alternatively, myosin light chain phosphatase activity may be reduced in vessels from pregnant animals, rendering an increase in contractile filament calcium sensitivity (144). Thus, increased contractile protein content, elevated myosin light chain phosphorylation, and altered membrane channel expression and activity work in concert to change VSMC phenotype to be more contractile in response to adrenergic stimulation (133). Although the uterine circulation is highly sensitive to adrenergic influences, the physiological utility of increased arterial adrenergic reactivity in late pregnancy is not known, but it may act to limit bleeding during parturition.
A primary determinant of SMC tone and contractility is the resting membrane potential, which in turn is influenced by K+ channel activity. Small-conductance Ca2+-activated K+ (SKCa) type 2 and type 3 channels are expressed in uterine artery VSMCs and were significantly increased during pregnancy by steroid hormone (145). Similarly, large-conductance Ca2+-activated K+ (BKCa) channels in uterine artery VSMCs were upregulated during pregnancy, with 7- to 10-fold increases in the BKCa γ1-subunit transcript (146). Notably, activation of both SKCa and BKCa channels relaxed norepinephrine-preconstricted uterine arteries in pregnant sheep, but not in nonpregnant sheep, in an endothelium-independent manner (147). The pivotal role of K+ channels in uterine artery VSMCs is shown by the fact that hypoxia-mediated reactive oxygen species inhibit pregnancy-induced upregulation of SKCa channels and may contribute to the increased incidence of preeclampsia and fetal intrauterine growth restriction associated with gestational hypoxia and reduced uteroplacental blood flow (145).
In terms of channel expression, it is clear that multiple adaptations occur in channel subtypes associated with constriction, but also dilation, such as the transient receptor potential vanilloid type 3 (TRPV3) and type 4 TRPV4 ion channels. These are increased in pregnancy and may be localized on ECs and VSMCs in varying proportions (118, 148).
As already considered in the discussion of adrenergic sensitivity, nonchannel cyclic nucleotide mechanisms have also been shown to be altered by pregnancy, as have proteins that regulate actin- myosin interactions. Myosin phosphatase (MP) is the primary effector of smooth muscle relaxation and a key target of signaling pathways that regulate vascular tone. In collaboration with Fisher (149), we found that the targeting/regulatory subunit of MP (MYPT1) mRNA and protein was increased 1.7- to 2.0-fold in uterine arteries from late-pregnant rats. Furthermore, in animals made hypertensive by NOS inhibition, MYPT1 was downregulated, suggesting that MYPT1 switching is an adaptive response for regulating vascular resistance and therefore uterine blood flow.
Finally, as mentioned in Section 5.2, in human uterine artery VSMCs, pregnancy upregulated the CBS enzyme and, therefore, it augmented H2S production. By acting in a paracrine and/or autocrine manner, this gasomitter may contribute to uterine artery vasodilation. Its expression has been related to estrogen such that it is upregulated in estrogen-dominant physiological states such as pregnancy (113) and can be induced in ovariectomized nonpregnant ewes by estrogen replacement therapy (150).
SUMMARY POINTS.
Signals secreted from the fetoplacental unit induce maternal cardiovascular changes via direct and indirect actions.
The heart enlarges and increases its cardiac output and contractility, e.g., by 14,000–21,000 beats per day.
Major systemic alterations in pregnancy include increased plasma volume with some hemodilution, decreased or unchanged blood pressure, and reduced total peripheral resistance.
The magnitude of adaptation is greatest in the uterine circulation and involves changes in arterial and venous size and function.
Endothelial vasodilatory influences are generally upregulated during pregnancy, and arterial tone is reduced in most cases.
Endothelial plasticity involves changes in size, structure, intercellular connections, channel and enzyme expression, and output of vasoactive molecules.
Vascular smooth muscle plasticity involves changes in size, structure, contractile filament content, channel and enzyme expression, and intrinsic reactivity to pressure/stretch.
FUTURE ISSUES.
Epigenetic changes that modulate the process of maternal cardiovascular adaptation need to be identified.
We need to better understand the interaction between signaling systems in producing end effects, e.g., cAMP and cGMP, calcium handling, and calcium sensitivity, etc. in a way that allows us to integrate physiological information and put it into a useful, rather than a reductionist, context.
It is critical to develop biomarkers that predict development of gestational diseases.
Identifying women who are at risk for gestational disease before they become pregnant will reduce disease risk.
The field aims to improve its understanding of how gestational adaptation and maladaptation impact the future health of both mother (long-term cardiovascular effects) and child (developmental origins of adult disease).
ACKNOWLEDGMENTS
The authors gratefully acknowledge support from the US National Institutes of Health (grant 1RO1 HL134371).
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- 1.Torgersen KL, Curran CA. 2006. A systematic approach to the physiologic adaptations of pregnancy. Crit. Care Nurs. Q 29:2–19 [DOI] [PubMed] [Google Scholar]
- 2.Chang J, Streitman D. 2012. Physiologic adaptations to pregnancy. Neurol. Clin 30:781–89 [DOI] [PubMed] [Google Scholar]
- 3.Longo LD. 1983. Maternal blood volume and cardiac output during pregnancy: a hypothesis of endocrinologic control. Am. J. Physiol 245:R720–29 [DOI] [PubMed] [Google Scholar]
- 4.Robson SC, Hunter S, Boys RJ, Dunlop W. 1989. Serial study of factors influencing changes in cardiac output during human pregnancy. Am. J. Physiol 256:H1060–65 [DOI] [PubMed] [Google Scholar]
- 5.Hunter S, Robson SC. 1992. Adaptation of the maternal heart in pregnancy. Br. Heart J 68:540–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Melchiorre K, Sharma R, Thilaganathan B. 2012. Cardiac structure and function in normal pregnancy. Curr. Opin. Obstet. Gynecol 24:413–21 [DOI] [PubMed] [Google Scholar]
- 7.Place JC, Plano LR. 2015. A case report of decreased fetal movement during fetomaternal hemorrhage. J. Obstet. Gynecol. Neonatal Nurs 44:737–42 [DOI] [PubMed] [Google Scholar]
- 8.Gandhi M, Martin SR. 2015. Cardiac disease in pregnancy. Obstet. Gynecol. Clin. N. Am 42:315–33 [DOI] [PubMed] [Google Scholar]
- 9.Robson SC, Dunlop W. 1992. Letters to the editors: When do cardiovascular parameters return to their preconception values? Am. J. Obstet. Gynecol 167:1479. [DOI] [PubMed] [Google Scholar]
- 10.Osol G, Ko NL, Mandalà M. 2017. Altered endothelial nitric oxide signaling as a paradigm for maternal vascular maladaptation in preeclampsia. Curr. Hypertens. Rep 19:82. [DOI] [PubMed] [Google Scholar]
- 11.Morton JS, Care AS, Davidge ST. 2017. Mechanisms of uterine artery dysfunction in pregnancy complications. J. Cardiovasc. Pharmacol 69:343–59 [DOI] [PubMed] [Google Scholar]
- 12.Fournier T, Guibourdenche J, Evain-Brion D. 2015. Review: hCGs: different sources of production, different glycoforms and functions. Placenta 36(Suppl. 1):S60–65 [DOI] [PubMed] [Google Scholar]
- 13.Hay DL, Lopata A. 1988. Chorionic gonadotropin secretion by human embryos in vitro. J. Clin. Endocrinol. Metab 67:1322–24 [DOI] [PubMed] [Google Scholar]
- 14.Csapo AI, Pulkkinen MO, Wiest WG. 1973. Effects of luteectomy and progesterone replacement therapy in early pregnant patients. Am. J. Obstet. Gynecol 115:759–65 [DOI] [PubMed] [Google Scholar]
- 15.Devroey P, Camus M, Palermo G, Smitz J, Van Waesberghe L, et al. 1990. Placental production of estradiol and progesterone after oocyte donation in patients with primary ovarian failure. Am. J. Obstet. Gynecol 162:66–70 [DOI] [PubMed] [Google Scholar]
- 16.Levitz M, Young BK. 1977. Estrogens in pregnancy. Vitam. Horm 35:109–47 [DOI] [PubMed] [Google Scholar]
- 17.O’Leary P, Boyne P, Flett P, Beilby J, James I. 1991. Longitudinal assessment of changes in reproductive hormones during normal pregnancy. Clin. Chem 37:667–72 [PubMed] [Google Scholar]
- 18.Zygmunt M, Herr F, Keller-Schoenwetter S, Kunzi-Rapp K, Munstedt K, et al. 2002. Characterization of human chorionic gonadotropin as a novel angiogenic factor. J. Clin. Endocrinol. Metab 87:5290–96 [DOI] [PubMed] [Google Scholar]
- 19.Ambrus G, Rao CV. 1994. Novel regulation of pregnant human myometrial smooth muscle cell gap junctions by human chorionic gonadotropin. Endocrinology 135:2772–79 [DOI] [PubMed] [Google Scholar]
- 20.Bansal AS, Bora SA, Saso S, Smith JR, Johnson MR, Thum MY. 2012. Mechanism of human chorionic gonadotrophin-mediated immunomodulation in pregnancy. Expert Rev. Clin. Immunol 8:747–53 [DOI] [PubMed] [Google Scholar]
- 21.Nwabuobi C, Arlier S, Schatz F, Guzeloglu-Kayisli O, Lockwood CJ, Kayisli UA. 2017. hCG: biological functions and clinical applications. Int. J. Mol. Sci 18:2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Berkane N, Liere P, Oudinet JP, Hertig A, Lefevre G, et al. 2017. From pregnancy to preeclampsia: a key role for estrogens. Endocr Rev. 38:123–44 [DOI] [PubMed] [Google Scholar]
- 23.Escobar JC, Patel SS, Beshay VE, Suzuki T, Carr BR. 2011. The human placenta expresses CYP17 and generates androgens de novo. J. Clin. Endocrinol. Metab 96:1385–92 [DOI] [PubMed] [Google Scholar]
- 24.Milewich L, MacDonald PC, Carr BR. 1986. Estrogen 16α-hydroxylase activity in human fetal tissues. J. Clin. Endocrinol. Metab 63:404–6 [DOI] [PubMed] [Google Scholar]
- 25.Mueller JW, Gilligan LC, Idkowiak J, Arlt W, Foster PA. 2015. The regulation of steroid action by sulfation and desulfation. Endocr Rev 36:526–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hisaw FL. 1926. Experimental relaxation of the pubic ligament of the guinea pig. Proc. Soc. Exp. Biol. Med 23:661–63 [Google Scholar]
- 27.Jelinic M, Marshall SA, Stewart D, Unemori E, Parry LJ, Leo CH. 2018. Peptide hormone relaxin: from bench to bedside. Am. J. Physiol. Regul. Integr. Comp. Physiol 314:R753–60 [DOI] [PubMed] [Google Scholar]
- 28.Leo CH, Jelinic M, Ng HH, Marshall SA, Novak J, et al. 2017. Vascular actions of relaxin: nitric oxide and beyond. Br. J. Pharmacol 174:1002–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Conrad KP. 2011. Maternal vasodilation in pregnancy: the emerging role of relaxin. Am. J. Physiol. Regul. Integr. Comp. Physiol 301:R267–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stewart DR, Cragun JR, Boyers SP, Oi R, Overstreet JW, Lasley BL. 1992. Serum relaxin concentrations in patients with out-of-phase endometrial biopsies. Fertil. Steril 57:453–55 [PubMed] [Google Scholar]
- 31.Sherwood OD. 2004. Relaxin’s physiological roles and other diverse actions. Endocr Rev 25:205–34 [DOI] [PubMed] [Google Scholar]
- 32.Demir R, Kayisli UA, Seval Y, Celik-Ozenci C, Korgun ET, et al. 2004. Sequential expression of VEGF and its receptors in human placental villi during very early pregnancy: differences between placental vasculogenesis and angiogenesis. Placenta 25:560–72 [DOI] [PubMed] [Google Scholar]
- 33.Shiraishi S, Nakagawa K, Kinukawa N, Nakano H, Sueishi K. 1996. Immunohistochemical localization of vascular endothelial growth factor in the human placenta. Placenta 17:111–21 [DOI] [PubMed] [Google Scholar]
- 34.Valdés G, Corthorn J. 2011. Challenges posed to the maternal circulation by pregnancy. Integr. Blood Press. Control 4:45–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sabapatha A, Gercel-Taylor C, Taylor DD. 2006. Specific isolation of placenta-derived exosomes from the circulation of pregnant women and their immunoregulatory consequences. Am. J. Reprod. Immunol 56:345–55 [DOI] [PubMed] [Google Scholar]
- 36.Orozco AF, Lewis DE. 2010. Flow cytometric analysis of circulating microparticles in plasma. Cytometry A 77:502–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thery C, Zitvogel L, Amigorena S. 2002. Exosomes: composition, biogenesis and function. Nat. Rev. Immunol 2:569–79 [DOI] [PubMed] [Google Scholar]
- 38.Bounds KR, Chiasson VL, Pan LJ, Gupta S, Chatterjee P. 2017. MicroRNAs: new players in the pathobiology of preeclampsia. Front. Cardiovasc. Med 4:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jairajpuri DS, Malalla ZH, Mahmood N, Almawi WY. 2017. Circulating microRNA expression as predictor of preeclampsia and its severity. Gene 627:543–48 [DOI] [PubMed] [Google Scholar]
- 40.Bartolomei MS, Zemel S, Tilghman SM. 1991. Parental imprinting of the mouse H19 gene. Nature 351:153–55 [DOI] [PubMed] [Google Scholar]
- 41.Hirasawa R, Feil R. 2010. Genomic imprinting and human disease. Essays Biochem 48:187–200 [DOI] [PubMed] [Google Scholar]
- 42.Keniry A, Oxley D, Monnier P, Kyba M, Dandolo L, et al. 2012. The H19 lincRNA is a developmental reservoir of miR-675 that suppresses growth and Igf1r. Nat. Cell Biol 14:659–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Morales-Prieto DM, Ospina-Prieto S, Schmidt A, Chaiwangyen W, Markert UR. 2014. Elsevier Trophoblast Research Award Lecture: origin, evolution and future of placenta miRNAs. Placenta 35(Suppl. 1):S39–45 [DOI] [PubMed] [Google Scholar]
- 44.Ye W, Lv Q, Wong CK, Hu S, Fu C, et al. 2008. The effect of central loops in miRNA:MRE duplexes on the efficiency of miRNA-mediated gene regulation. PLOS ONE 3:e1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hu XQ, Dasgupta C, Xiao D, Huang X, Yang S, Zhang L. 2017. MicroRNA-210 targets ten-eleven translocation methylcytosine dioxygenase 1 and suppresses pregnancy-mediated adaptation of large conductance Ca2+-activated K+ channel expression and function in ovine uterine arteries. Hypertension 70:601–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fu Q, Levine BD. 2009. Autonomic circulatory control during pregnancy in humans. Semin. Reprod. Med 27:330–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Meyer MC, Osol G, McLaughlin M. 1997. Flow decreases myogenic reactivity of mesenteric arteries from pregnant rats. J. Soc. Gynecol. Investig 4:293–97 [PubMed] [Google Scholar]
- 48.Cockell AP, Poston L. 1997. Flow-mediated vasodilatation is enhanced in normal pregnancy but reduced in preeclampsia. Hypertension 30:247–51 [DOI] [PubMed] [Google Scholar]
- 49.Learmont JG, Cockell AP, Knock GA, Poston L. 1996. Myogenic and flow-mediated responses in isolated mesenteric small arteries from pregnant and nonpregnant rats. Am. J. Obstet. Gynecol 174:1631–36 [DOI] [PubMed] [Google Scholar]
- 50.Veerareddy S, Cooke CL, Baker PN, Davidge ST. 2002. Vascular adaptations to pregnancy in mice: effects on myogenic tone. Am. J. Physiol. Heart Circ. Physiol 283:H2226–33 [DOI] [PubMed] [Google Scholar]
- 51.Kenny LC, Baker PN, Kendall DA, Randall MD, Dunn WR. 2002. The role of gap junctions in mediating endothelium-dependent responses to bradykinin in myometrial small arteries isolated from pregnant women. Br. J. Pharmacol 136:1085–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Boeldt DS, Bird IM. 2017. Vascular adaptation in pregnancy and endothelial dysfunction in preeclampsia. J. Endocrinol 232:R27–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Carbillon L, Uzan M, Uzan S. 2000. Pregnancy, vascular tone, and maternal hemodynamics: a crucial adaptation. Obstet. Gynecol. Surv 55:574–81 [DOI] [PubMed] [Google Scholar]
- 54.Kim TH, Weiner CP, Thompson LP. 1994. Effect of pregnancy on contraction and endothelium-mediated relaxation of renal and mesenteric arteries. Am. J. Physiol 267:H41–47 [DOI] [PubMed] [Google Scholar]
- 55.Dalle Lucca JJ, Adeagbo ASO, Alsip NL. 2000. Influence of oestrous cycle and pregnancy on the reactivity of the rat mesenteric vascular bed. Hum. Reprod 15:961–68 [DOI] [PubMed] [Google Scholar]
- 56.Cooke CL, Davidge ST. 2003. Pregnancy-induced alterations of vascular function in mouse mesenteric and uterine arteries. Biol. Reprod 68:1072–77 [DOI] [PubMed] [Google Scholar]
- 57.Meyer MC, Brayden JE, McLaughlin MK. 1993. Characteristics of vascular smooth muscle in the maternal resistance circulation during pregnancy in the rat. Am. J. Obstet. Gynecol 169:1510–16 [DOI] [PubMed] [Google Scholar]
- 58.Davidge ST, McLaughlin MK. 1992. Endogenous modulation of the blunted adrenergic response in resistance-sized mesenteric arteries from the pregnant rat. Am. J. Obstet. Gynecol 167:1691–98 [DOI] [PubMed] [Google Scholar]
- 59.D’Angelo G, Osol G. 1993. Regional variation in resistance artery diameter responses to alpha-adrenergic stimulation during pregnancy. Am. J. Physiol 264:H78–85 [DOI] [PubMed] [Google Scholar]
- 60.Crandall ME, Keve TM, McLaughlin MK. 1990. Characterization of norepinephrine sensitivity in the maternal splanchnic circulation during pregnancy. Am. J. Obstet. Gynecol 162:1296–301 [DOI] [PubMed] [Google Scholar]
- 61.Crandall ME, Heesch CM. 1990. Baroreflex control of sympathetic outflow in pregnant rats: effects of captopril. Am. J. Physiol 258:R1417–23 [DOI] [PubMed] [Google Scholar]
- 62.Chu ZM, Beilin LJ. 1998. Neuropeptide Y and mesenteric sympathetic vasoconstriction in pregnant and non-pregnant Wistar-Kyoto rats. Clin. Exp. Pharmacol. Physiol 25:630–32 [DOI] [PubMed] [Google Scholar]
- 63.Mata KM, Li W, Reslan OM, Siddiqui WT, Opsasnick LA, Khalil RA. 2015. Adaptive increases in expression and vasodilator activity of estrogen receptor subtypes in a blood vessel-specific pattern during pregnancy. Am. J. Physiol. Heart Circ. Physiol 309:H1679–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Marshall SA, Leo CH, Senadheera SN, Girling JE, Tare M, Parry LJ. 2016. Relaxin deficiency attenuates pregnancy-induced adaptation of the mesenteric artery to angiotensin II in mice. Am. J. Physiol. Regul. Integr. Comp. Physiol 310:R847–57 [DOI] [PubMed] [Google Scholar]
- 65.Mackey K, Meyer MC, Stirewalt WS, Starcher BC, McLaughlin MK. 1992. Composition and mechanics of mesenteric resistance arteries from pregnant rats. Am. J. Physiol 263:R2–8 [DOI] [PubMed] [Google Scholar]
- 66.Conrad KP, Shroff SG. 2011. Effects of relaxin on arterial dilation, remodeling, and mechanical properties. Curr. Hypertens. Rep 13:409–20 [DOI] [PubMed] [Google Scholar]
- 67.Smith MC, Murdoch AP, Danielson LA, Conrad KP, Davison JM. 2006. Relaxin has a role in establishing a renal response in pregnancy. Fertil. Steril 86:253–55 [DOI] [PubMed] [Google Scholar]
- 68.Ferreira VM, Gomes TS, Reis LA, Ferreira AT, Razvickas CV, et al. 2009. Receptor-induced dilatation in the systemic and intrarenal adaptation to pregnancy in rats. PLOS ONE 4:e4845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Novak J, Danielson LA, Kerchner LJ, Sherwood OD, Ramirez RJ, et al. 2001. Relaxin is essential for renal vasodilation during pregnancy in conscious rats. J. Clin. Investig 107:1469–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jeyabalan A, Novak J, Danielson LA, Kerchner LJ, Opett SL, Conrad KP. 2003. Essential role for vascular gelatinase activity in relaxin-induced renal vasodilation, hyperfiltration, and reduced myogenic reactivity of small arteries. Circ. Res 93:1249–57 [DOI] [PubMed] [Google Scholar]
- 71.Novak J, Ramirez RJ, Gandley RE, Sherwood OD, Conrad KP. 2002. Myogenic reactivity is reduced in small renal arteries isolated from relaxin-treated rats. Am. J. Physiol. Regul. Integr. Comp. Physiol 283:R349–55 [DOI] [PubMed] [Google Scholar]
- 72.Gandley RE, Conrad KP, McLaughlin MK. 2001. Endothelin and nitric oxide mediate reduced myogenic reactivity of small renal arteries from pregnant rats. Am. J. Physiol. Regul. Integr. Comp. Physiol 280:R1–7 [DOI] [PubMed] [Google Scholar]
- 73.Murphy JG, Fleming JB, Cockrell KL, Granger JP, Khalil RA. 2001. [Ca2+]i signaling in renal arterial smooth muscle cells of pregnant rat is enhanced during inhibition of NOS. Am. J. Physiol. Regul. Integr. Comp. Physiol 280:R87–99 [DOI] [PubMed] [Google Scholar]
- 74.Chu ZM, Beilin LJ. 1997. Demonstration of the existence of nitric oxide-independent as well as nitric oxide-dependent vasodilator mechanisms in the in situ renal circulation in near term pregnant rats. Br. J. Pharmacol 122:307–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Anumba DO, Robson SC, Boys RJ, Ford GA. 1999. Nitric oxide activity in the peripheral vasculature during normotensive and preeclamptic pregnancy. Am. J. Physiol 277:H848–54 [DOI] [PubMed] [Google Scholar]
- 76.Amburgey OA, Reeves SA, Bernstein IM, Cipolla MJ. 2010. Resistance artery adaptation to pregnancy counteracts the vasoconstricting influence of plasma from normal pregnant women. Reprod. Sci 17:29–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Schreurs MP, Houston EM, May V, Cipolla MJ. 2012. The adaptation of the blood-brain barrier to vascular endothelial growth factor and placental growth factor during pregnancy. FASEB J. 26:355–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cipolla MJ, Vitullo L, McKinnon J. 2004. Cerebral artery reactivity changes during pregnancy and the postpartum period: a role in eclampsia? Am. J. Physiol. Heart Circ. Physiol 286:H2127–32 [DOI] [PubMed] [Google Scholar]
- 79.Euser AG, Cipolla MJ. 2005. Resistance artery vasodilation to magnesium sulfate during pregnancy and the postpartum state. Am. J. Physiol. Heart Circ. Physiol 288:H1521–25 [DOI] [PubMed] [Google Scholar]
- 80.Quick AM, Cipolla MJ. 2005. Pregnancy-induced up-regulation of aquaporin-4 protein in brain and its role in eclampsia. FASEB J. 19:170–75 [DOI] [PubMed] [Google Scholar]
- 81.Chapman AC, Cipolla MJ, Chan SL. 2013. Effect of pregnancy and nitric oxide on the myogenic vasodilation of posterior cerebral arteries and the lower limit of cerebral blood flow autoregulation. Reprod. Sci 20:1046–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chan SL, Cipolla MJ. 2011. Relaxin causes selective outward remodeling of brain parenchymal arterioles via activation of peroxisome proliferator-activated receptor-γ. FASEB J. 25:3229–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chan SL, Chapman AC, Sweet JG, Gokina NI, Cipolla MJ. 2010. Effect of PPARγ inhibition during pregnancy on posterior cerebral artery function and structure. Front. Physiol 1:130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.van der Wijk AE, Schreurs MP, Cipolla MJ. 2013. Pregnancy causes diminished myogenic tone and outward hypotrophic remodeling of the cerebral vein of Galen. J. Cereb. Blood Flow Metab 33:542–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Shynlova O, Kwong R, Lye SJ. 2010. Mechanical stretch regulates hypertrophic phenotype of the myometrium during pregnancy. Reproduction 139:247–53 [DOI] [PubMed] [Google Scholar]
- 86.Duncan SL. 1969. The partition of uterine blood flow in the pregnant rabbit. J. Physiol 204:421–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Osol G, Mandalà M. 2009. Maternal uterine vascular remodeling during pregnancy. Physiology 24:58–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Palmer SK, Zamudio S, Coffin C, Parker S, Stamm E, Moore LG. 1992. Quantitative estimation of human uterine artery blood flow and pelvic blood flow redistribution in pregnancy. Obstet. Gynecol 80:1000–6 [PubMed] [Google Scholar]
- 89.Osol G, Barron C, Gokina N, Mandalà M. 2009. Inhibition of nitric oxide synthases abrogates pregnancy-induced uterine vascular expansive remodeling. J. Vasc. Res 46:478–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ni Y, Meyer M, Osol G. 1997. Gestation increases nitric oxide-mediated vasodilation in rat uterine arteries. Am. J. Obstet. Gynecol 176:856–64 [DOI] [PubMed] [Google Scholar]
- 91.Hale SA, Weger L, Mandalà M, Osol G. 2011. Reduced NO signaling during pregnancy attenuates outward uterine artery remodeling by altering MMP expression and collagen and elastin deposition. Am. J. Physiol. Heart Circ. Physiol 301:H1266–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Barron C, Mandalà M, Osol G. 2010. Effects of pregnancy, hypertension and nitric oxide inhibition on rat uterine artery myogenic reactivity. J. Vasc. Res 47:463–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.van der Heijden OWH, Essers YPG, Fazzi G, Peeters LLH, De Mey JGR, van Eys GJJM. 2005. Uterine artery remodeling and reproductive performance are impaired in endothelial nitric oxide synthase deficient mice. Biol. Reprod 72:1161–68 [DOI] [PubMed] [Google Scholar]
- 94.van der Heijden OWH, Essers YPG, Spaanderman MEA, De Mey JGR, van Eys GJJM, Peeters LLH. 2005. Uterine artery remodeling in pseudopregnancy is comparable to that in early pregnancy. Biol. Reprod 73:1289–93 [DOI] [PubMed] [Google Scholar]
- 95.Fuller R, Colton I, Gokina N, Mandalà M, Osol G. 2011. Local versus systemic influences on uterine vascular reactivity during pregnancy in the single-horn gravid rat. Reprod. Sci 18:723–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gokina NI, Kuzina OY, Fuller R, Osol G. 2009. Local uteroplacental influences are responsible for the induction of uterine artery myogenic tone during rat pregnancy. Reprod. Sci 16:1072–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Fuller R, Barron C, Mandalà M, Gokina N, Osol G. 2009. Predominance of local over systemic factors in uterine arterial remodeling during pregnancy. Reprod. Sci 16:489–500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mapletoft RJ, Ginther OJ. 1975. Adequacy of main uterine vein and the ovarian artery in the local venoarterial pathway for uterine-induced luteolysis in ewes. Am. J. Vet. Res 36:957–63 [PubMed] [Google Scholar]
- 99.Celia G, Osol G. 2002. Venoarterial communication as a mechanism for localized signaling in the rat uterine circulation. Am. J. Obstet. Gynecol 187:1653–59 [DOI] [PubMed] [Google Scholar]
- 100.Celia G, Osol G. 2005. Mechanism of VEGF-induced uterine venous hyperpermeability. J. Vasc. Res 42:47–54 [DOI] [PubMed] [Google Scholar]
- 101.Celia G, Osol G. 2005. Uterine venous permeability in the rat is altered in response to pregnancy, vascular endothelial growth factor, and venous constriction. Endothelium 12:81–88 [DOI] [PubMed] [Google Scholar]
- 102.Ko NL, John L, Gelinne A, Mandalà M, Osol G. 2018. Venoarterial communication mediates arterial wall shear stress-induced maternal uterine vascular remodeling during pregnancy. Am. J. Physiol. Heart Circ. Physiol 315:H709–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Vita JA, Holbrook M, Palmisano J, Shenouda SM, Chung WB, et al. 2008. Flow-induced arterial remodeling relates to endothelial function in the human forearm. Circulation 117:3126–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bakker EN, Matlung HL, Bonta P, de Vries CJ, van Rooijen N, Vanbavel E. 2008. Blood flow-dependent arterial remodelling is facilitated by inflammation but directed by vascular tone. Cardiovasc. Res 78:341–48 [DOI] [PubMed] [Google Scholar]
- 105.Langille BL, O’Donnell F. 1986. Reductions in arterial diameter produced by chronic decreases in blood flow are endothelium-dependent. Science 231:405–7 [DOI] [PubMed] [Google Scholar]
- 106.Osol G, Moore LG. 2014. Maternal uterine vascular remodeling during pregnancy. Microcirculation 21:38–47 [DOI] [PubMed] [Google Scholar]
- 107.Hwang M, Berceli SA, Garbey M, Kim NH, Tran-Son-Tay R. 2012. The dynamics of vein graft remodeling induced by hemodynamic forces: a mathematical model. Biomech. Model. Mechanobiol 11:411–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Page KL, Celia G, Leddy G, Taatjes DJ, Osol G. 2002. Structural remodeling of rat uterine veins in pregnancy. Am. J. Obstet. Gynecol 187:1647–52 [DOI] [PubMed] [Google Scholar]
- 109.Cipolla M, Osol G. 1994. Hypertrophic and hyperplastic effects of pregnancy on the rat uterine arterial wall. Am. J. Obstet. Gynecol 171:805–11 [DOI] [PubMed] [Google Scholar]
- 110.Magness RR, Shideman CR, Habermehl DA, Sullivan JA, Bird IM. 2000. Endothelial vasodilator production by uterine and systemic arteries. V. Effects of ovariectomy, the ovarian cycle, and pregnancy on prostacyclin synthase expression. Prostaglandins Lipid Mediat. 60:103–18 [DOI] [PubMed] [Google Scholar]
- 111.Gokina NI, Goecks T. 2006. Upregulation of endothelial cell Ca2+ signaling contributes to pregnancy-enhanced vasodilation of rat uteroplacental arteries. Am. J. Physiol. Heart Circ. Physiol 290:H2124–35 [DOI] [PubMed] [Google Scholar]
- 112.Gokina NI, Kuzina OY, Vance AM. 2010. Augmented EDHF signaling in rat uteroplacental vasculature during late pregnancy. Am. J. Physiol. Heart Circ. Physiol 299:H1642–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sheibani L, Lechuga TJ, Zhang H, Hameed A, Wing DA, et al. 2017. Augmented H2S production via cystathionine-beta-synthase upregulation plays a role in pregnancy-associated uterine vasodilation. Biol. Reprod 96:664–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Gifford SM, Yi FX, Bird IM. 2006. Pregnancy-enhanced Ca2+ responses to ATP in uterine artery endothelial cells is due to greater capacitative Ca2+ entry rather than altered receptor coupling. J. Endocrinol 190:373–84 [DOI] [PubMed] [Google Scholar]
- 115.Boeldt DS, Hankes AC, Alvarez RE, Khurshid N, Balistreri M, et al. 2014. Pregnancy programming and preeclampsia: identifying a human endothelial model to study pregnancy-adapted endothelial function and endothelial adaptive failure in preeclamptic subjects. Adv. Exp. Med. Biol 814:27–47 [DOI] [PubMed] [Google Scholar]
- 116.Boeldt DS, Grummer MA, Magness RR, Bird IM. 2014. Altered VEGF-stimulated Ca2+ signaling in part underlies pregnancy-adapted eNOS activity in UAEC. J. Endocrinol 223:1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Alvarez RE, Boeldt DS, Pattnaik BR, Friedman HL, Bird IM. 2017. Pregnancy-adapted uterine artery endothelial cell Ca2+ signaling and its relationship with membrane potential. Physiol. Rep 5:e13452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Senadheera S, Bertrand PP, Grayson TH, Leader L, Murphy TV, Sandow SL. 2013. Pregnancy-induced remodelling and enhanced endothelium-derived hyperpolarization-type vasodilator activity in rat uterine radial artery: transient receptor potential vanilloid type 4 channels, caveolae and myoendothelial gap junctions. J. Anat 223:677–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ampey BC, Ampey AC, Lopez GE, Bird IM, Magness RR. 2017. Cyclic nucleotides differentially regulate Cx43 gap junction function in uterine artery endothelial cells from pregnant ewes. Hypertension 70:401–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kenny LC, Baker PN, Kendall DA, Randall MD, Dunn WR. 2002. Differential mechanisms of endothelium-dependent vasodilator responses in human myometrial small arteries in normal pregnancy and pre-eclampsia. Clin. Sci 103:67–73 [DOI] [PubMed] [Google Scholar]
- 121.Janowiak MA, Magness RR, Habermehl DA, Bird IM. 1998. Pregnancy increases ovine uterine artery endothelial cyclooxygenase-1 expression. Endocrinology 139:765–71 [DOI] [PubMed] [Google Scholar]
- 122.Habermehl DA, Janowiak MA, Vagnoni KE, Bird IM, Magness RR. 2000. Endothelial vasodilator production by uterine and systemic arteries. IV. Cyclooxygenase isoform expression during the ovarian cycle and pregnancy in sheep. Biol. Reprod 62:781–88 [DOI] [PubMed] [Google Scholar]
- 123.Zhang HH, Chen JC, Sheibani L, Lechuga TJ, Chen DB. 2017. Pregnancy augments VEGF-stimulated in vitro angiogenesis and vasodilator (NO and H2S) production in human uterine artery endothelial cells. J. Clin. Endocrinol. Metab 102:2382–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Mandalà M, Gokina N, Osol G. 2002. Contribution of nonendothelial nitric oxide to altered rat uterine resistance artery serotonin reactivity during pregnancy. Am. J. Obstet. Gynecol 187:463–68 [DOI] [PubMed] [Google Scholar]
- 125.Nathan L, Cuevas J, Chaudhuri G. 1995. The role of nitric oxide in the altered vascular reactivity of pregnancy in the rat. Br. J. Pharmacol 114:955–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Pastore MB, Talwar S, Conley MR, Magness RR. 2016. Identification of differential ER-alpha versus ER-beta mediated activation of eNOS in ovine uterine artery endothelial cells. Biol. Reprod 94:139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Tropea T, De Francesco EM, Rigiracciolo D, Maggiolini M, Wareing M, et al. 2015. Pregnancy augments G protein estrogen receptor (GPER) induced vasodilation in rat uterine arteries via the nitric oxide–cGMP signaling pathway. PLOS ONE 10:e0141997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Jobe SO, Ramadoss J, Wargin AJ, Magness RR. 2013. Estradiol-17β and its cytochrome P450- and catechol-O-methyltransferase-derived metabolites selectively stimulate production of prostacyclin in uterine artery endothelial cells: role of estrogen receptor-α versus estrogen receptor-β. Hypertension 61:509–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ni Y, May V, Braas K, Osol G. 1997. Pregnancy augments uteroplacental vascular endothelial growth factor gene expression and vasodilator effects. Am. J. Physiol 273:H938–44 [DOI] [PubMed] [Google Scholar]
- 130.Osol G, Celia G, Gokina N, Barron C, Chien E, et al. 2008. Placental growth factor is a potent vasodilator of rat and human resistance arteries. Am. J. Physiol. Heart Circ. Physiol 294:H1381–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Storment JM, Meyer M, Osol G. 2000. Estrogen augments the vasodilatory effects of vascular endothelial growth factor in the uterine circulation of the rat. Am. J. Obstet. Gynecol 183:449–53 [DOI] [PubMed] [Google Scholar]
- 132.Burger NZ, Kuzina OY, Osol G, Gokina NI. 2009. Estrogen replacement enhances EDHF-mediated vasodilation of mesenteric and uterine resistance arteries: role of endothelial cell Ca2+. Am. J. Physiol. Endocrinol. Metab 296:E503–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Annibale DJ, Rosenfeld CR, Stull JT, Kamm KE. 1990. Protein content and myosin light chain phosphorylation in uterine arteries during pregnancy. Am. J. Physiol 259:C484–89 [DOI] [PubMed] [Google Scholar]
- 134.Osol G, Cipolla M. 1993. Pregnancy-induced changes in the three-dimensional mechanical properties of pressurized rat uteroplacental (radial) arteries. Am. J. Obstet. Gynecol 168:268–74 [DOI] [PubMed] [Google Scholar]
- 135.Morschauser TJ, Ramadoss J, Koch JM, Yi FX, Lopez GE, et al. 2014. Local effects of pregnancy on connexin proteins that mediate Ca2+-associated uterine endothelial NO synthesis. Hypertension 63:589–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Keyes LE, Moore LG, Walchak SJ, Dempsey EC. 1996. Pregnancy-stimulated growth of vascular smooth muscle cells: importance of protein kinase C-dependent synergy between estrogen and platelet-derived growth factor. J. Cell Physiol 166:22–32 [DOI] [PubMed] [Google Scholar]
- 137.Vodstrcil LA, Tare M, Novak J, Dragomir N, Ramirez RJ, et al. 2012. Relaxin mediates uterine artery compliance during pregnancy and increases uterine blood flow. FASEB J. 26:4035–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Eckman DM, Gupta R, Rosenfeld CR, Morgan TM, Charles SM, et al. 2012. Pregnancy increases myometrial artery myogenic tone via NOS- or COX-independent mechanisms. Am. J. Physiol. Regul. Integr. Comp. Physiol 303:R368–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Gokina NI, Mandalà M, Osol G. 2003. Induction of localized differences in rat uterine radial artery behavior and structure during gestation. Am. J. Obstet. Gynecol 189:1489–93 [DOI] [PubMed] [Google Scholar]
- 140.Telezhkin V, Goecks T, Bonev AD, Osol G, Gokina NI. 2008. Decreased function of voltage-gated potassium channels contributes to augmented myogenic tone of uterine arteries in late pregnancy. Am. J. Physiol. Heart Circ. Physiol 294:H272–84 [DOI] [PubMed] [Google Scholar]
- 141.Veerareddy S, Campbell ME, Williams SJ, Baker PN, Davidge ST. 2004. Myogenic reactivity is enhanced in rat radial uterine arteries in a model of maternal undernutrition. Am. J. Obstet. Gynecol 191:334–39 [DOI] [PubMed] [Google Scholar]
- 142.Xiao D, Huang X, Yang S, Zhang L. 2009. Direct chronic effect of steroid hormones in attenuating uterine arterial myogenic tone: role of protein kinase C/extracellular signal–regulated kinase 1/2. Hypertension 54:352–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Senadheera S, Bertrand PP, Grayson TH, Leader L, Tare M, et al. 2013. Enhanced contractility in pregnancy is associated with augmented TRPC3, L-type, and T-type voltage-dependent calcium channel function in rat uterine radial artery. Am. J. Physiol. Regul. Integr. Comp. Physiol 305:R917–26 [DOI] [PubMed] [Google Scholar]
- 144.D’Angelo G, Osol G. 1994. Modulation of uterine resistance artery lumen diameter by calcium and G protein activation during pregnancy. Am. J. Physiol 267:H952–61 [DOI] [PubMed] [Google Scholar]
- 145.Zhu R, Huang X, Hu XQ, Xiao D, Zhang L. 2014. Gestational hypoxia increases reactive oxygen species and inhibits steroid hormone-mediated upregulation of Ca2+-activated K+ channel function in uterine arteries. Hypertension 64:415–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Lorca RA, Wakle-Prabagaran M, Freeman WE, Pillai MK, England SK. 2018. The large-conductance voltage- and Ca2+ -activated K+ channel and its γ1-subunit modulate mouse uterine artery function during pregnancy. J. Physiol 596:1019–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Zhu R, Hu XQ, Xiao D, Yang S, Wilson SM, et al. 2013. Chronic hypoxia inhibits pregnancy-induced upregulation of SKCa channel expression and function in uterine arteries. Hypertension 62:367–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Murphy TV, Kanagarajah A, Toemoe S, Bertrand PP, Grayson TH, et al. 2016. TRPV3 expression and vasodilator function in isolated uterine radial arteries from non-pregnant and pregnant rats. Vasc. Pharmacol 83:66–77 [DOI] [PubMed] [Google Scholar]
- 149.Lu Y, Zhang H, Gokina N, Mandalà M, Sato O, et al. 2008. Uterine artery myosin phosphatase isoform switching and increased sensitivity to SNP in a rat L-NAME model of hypertension of pregnancy. Am. J. Physiol. Cell Physiol 294:C564–71 [DOI] [PubMed] [Google Scholar]
- 150.Lechuga TJ, Zhang HH, Sheibani L, Karim M, Jia J, et al. 2015. Estrogen replacement therapy in ovariectomized nonpregnant ewes stimulates uterine artery hydrogen sulfide biosynthesis by selectively up-regulating cystathionine β-synthase expression. Endocrinology 156:2288–98 [DOI] [PMC free article] [PubMed] [Google Scholar]