In our study of young men seeking emergency department care, the rapid syphilis test detected 76.9% of participants with active syphilis.
Abstract
Background
Syphilis transmission can be prevented by prompt diagnosis and treatment of primary and secondary infection. We evaluated the performance of a point-of-care rapid syphilis treponemal (RST) test in an emergency department (ED) setting.
Methods
Between June 2015 and April 2016, men aged 18 to 34 years seeking services in a Detroit ED, and with no history of syphilis, were screened for syphilis with the RST test, rapid plasma reagin (RPR) test, and Treponema pallidum particle agglutination assay (TP-PA). A positive reference standard was both a reactive RPR and a reactive TP-PA. We compared test results in self-reported men who have sex with men (MSM) to non-MSM.
Results
Among 965 participants, 10.9% of RST tests were reactive in MSM and only 1.5% in non-MSM (P < 0.001). Sensitivity of the RST test was 76.9% and specificity was 99.0% (positive predictive value, 50.0%) compared with the positive reference standard. Three discordant specimens found negative with the RST test but positive with the reference standard had an RPR titer of 1:1, compared with 10 specimens with concordant positive results that had a median RPR titer of 1:16. The RST sensitivity was 50.0% (positive predictive value, 68.4%) compared to the TP-PA test alone. Among men seeking care in an ED, the RST detected 76.9% of participants with a reactive RPR and TP-PA.
Conclusions
The RST test detected all of the participants with an RPR titer ≥1:2 but less than 20% of participants with a positive TP-PA and negative RPR. The RST test was useful to detect a high proportion of participants with an active syphilis in an urban ED.
In our study of young men seeking emergency department care, the rapid syphilis test detected 76.9% of participants with active syphilis.
Syphilis, a complex sexually transmitted genital ulcerative and systemic disease caused by the spirochete Treponema pallidum, is associated with significant morbidity including congenital syphilis and an increased risk of human immunodeficiency virus (HIV) infection.1,2 Nationally, reported cases of primary and secondary (P&S) syphilis have increased from 2.1 cases per 100,000 in 2001 to 8.7 cases per 100,000 population in 2016.3 Gay, bisexual, and other men who have sex with men (collectively referred to as MSM) account for the majority of syphilis cases with highest rates among young (age, 20–34 years) and black/African American (hereafter referred to as black) MSM.3 In 2014, Detroit had an ongoing syphilis outbreak primarily among young black MSM. A total number of 217 new cases of P&S syphilis were reported to the health department in 2014, a 193% increase from 74 cases reported in 2010. Of the 217 reported cases in 2014, 94% were in men, 97% in blacks, 73% in MSM. In addition, 52% of persons with P&S syphilis were coinfected with HIV.
Public health strategies to interrupt syphilis transmission depend on early diagnosis and treatment with a single intramuscular injection of benzathine penicillin in the early stages of syphilis. In addition, preventive or “epidemiologic” treatment is provided to any sexual partner exposed to infectious syphilis given the high probability of onward transmission.4 In Detroit, this strategy was complicated by the closure of Detroit's only sexually transmitted disease (STD) clinic, the Herman Kiefer Health Complex, in 2013.5 Sexually transmitted disease clinics combine testing, treatment, and partner contact tracing resources in 1 location and these services can be difficult to replicate in a non-STD specialty out-patient settings. Although young black men do not often seek routine primary care, they do access the health care system through emergency departments (EDs).6
Serologic testing for syphilis can be quite complex and involves performing multiple tests in sequence. There are 2 common algorithms used in the United States. The traditional algorithm involves screening with a nontreponemal test and, if reactive, confirming with a treponemal test.4 The second approach, termed the reverse algorithm, involves the screening with a treponemal enzyme immunoassay (EIA) and, if reactive, confirming with a nontreponemal test such as the rapid plasma reagin (RPR).7 An additional treponemal test is recommended if the EIA is reactive and the RPR is nonreactive. Both algorithms depend on laboratory assays that require some level of expertise and equipment.
In late 2014, a novel rapid point-of-care syphilis test (Syphilis Health Check; Diagnostics Direct, Cape May, NJ) was FDA-approved and CLIA-waived.8 This rapid syphilis test is a qualitative rapid membrane immunochromatographic assay that can detect T. pallidum antibodies from a finger stick specimen. The rapid syphilis test is inexpensive, simple to use with minimal training, and requires no additional laboratory instruments. The test produces a result in 10 minutes which allows for rapid preliminary results, treatment, and prompt linkage to care.9 Although this rapid point-of-care technology made it feasible to incorporate syphilis screening into a busy ED clinical setting, questions remain regarding its utility as a screening assay.10 To address these issues, we evaluated the feasibility and effectiveness of integrated rapid syphilis testing of men aged 18 to 34 years receiving care in a large urban ED.
METHODS
Study Design and Participants
The study was an evaluation study to assess the implementation of rapid syphilis testing in the ED of Henry Ford Hospital in Detroit, Michigan. All men aged 18 to 34 years who visited the ED from 8:00 am to 04:30 pm, Monday to Friday, were recruited to participate. Participants were excluded if they had (i) already screened positive for syphilis during the study period or (ii) had a life-threatening illness that made rapid syphilis testing not feasible. Participants completed a questionnaire regarding their demographic characteristics, sexual behavior, and history of syphilis, HIV, and other STDs. A review of each participant's electronic medical record was also conducted to determine if there was a history of syphilis, HIV, or other STDs. A review of previous syphilis testing results was also conducted by the health department for any participants with a positive rapid syphilis result.
Study Personnel Training
Laboratory training was conducted at the enters for Disease Control (CDC) National Reference Laboratory and Henry Ford Hospital to familiarize ED, Infectious Disease, and laboratory staff with the syphilis health check, by Trinity Biotech. Standard operating procedures, current strategies for syphilis testing algorithm (RPR and T. pallidum particle agglutination assay [TP-PA] testing) and quality assurance procedures at the Henry Ford Laboratory were also reviewed.
Laboratory Methods
A finger-stick whole blood specimen from all study participants was initially screened with a rapid syphilis test (Syphilis Health Check; Diagnostics Direct)11 by a nurse who had received training with the rapid test. This rapid syphilis test detects antibodies against T. pallidum antigens, and uses recombinant treponemal antigens. In the ED, all men in the study had blood drawn for an RPR and TP-PA that was sent to CDC. Serum specimens from all study participants were stored at −20°C and shipped as frozen specimens in batches for testing at CDC's reference laboratory. All specimens were tested at CDC with the RPR test, a nontreponemal syphilis test that detects anti-cardiolipin antibodies that are present in the majority of infected persons, and the T. pallidum particle agglutination assay, an indirect agglutination assay that detects antibodies against treponemal antigens. Reactive RPR specimens were tested quantitatively to determine the titer. If there were discordant results between the rapid syphilis treponemal (RST) and the TP-PA, specimens were tested with the Trep-Sure EIA (Phoenix Biotech, Mississauga, Ontario, Canada) which is a qualitative test that detects IgG and IgM antibodies against treponemal antigens.12
In addition to testing with RST, all patients were tested and treated for syphilis according to the standard of care in the ED. Treatment in the ED was based on the result of the RST, the patient's history and clinical presentation, and the clinical judgment of the provider.
Statistical Analysis
A sufficient sample size for this study was calculated to be 1000 participants with 12 to 29 diagnosed with syphilis. With a 90% sensitivity of the rapid syphilis test, there would be adequate power (β = 0.1 and α = 0.05) that the 95% lower bound for sensitivity would range from 73% with 12 syphilis diagnoses to 79% with 29 diagnoses. The primary analyses were within-individual comparisons of results from the rapid syphilis test and traditional laboratory tests for syphilis. Two analyses were performed to compare the rapid syphilis test to traditional laboratory tests. All study participants were screened by RST in ED, and all specimen regardless of RST results were tested by RPR and TP-PA at CDC laboratory. In the first comparison, the RST was compared to the traditional testing algorithm used to diagnose syphilis infection: screening first with RPR and confirming with TP-PA. In this comparison, specimens that were RPR nonreactive were considered negative. RPR reactive specimens (at any titer) were tested with TP-PA. If the TP-PA result was nonreactive, the specimen was classified as negative. If the TP-PA result was reactive, then the specimen was classified as positive. In the second comparison, the RST was compared to the TP-PA alone because both tests detect antibodies against treponemal antigens. The RST utilizes antigens derived from highly purified recombinant TP recombinant proteins, while TP-PA test contains sonicated T. pallidum whole organism. The sensitivity, specificity, positive predictive value, and negative predictive value were calculated for the RST compared with the traditional screening algorithm and the TP-PA. The 95% exact confidence intervals (CI) were calculated with the assumption of a Poisson distribution. McNemar tests were used to check for consistency between the RST and comparators. Participants with missing test results were excluded from the analysis. Statistical significance was indicated by a 2-sided P value less than 0.05. All analyses were performed using SAS (SAS Institute), version 9.4.
This study was approved by the Henry Ford Hospital Institutional Review Board and was approved by CDC through a research determination in accordance with federal human subject protection regulations and CDC policies and procedures.13
RESULTS
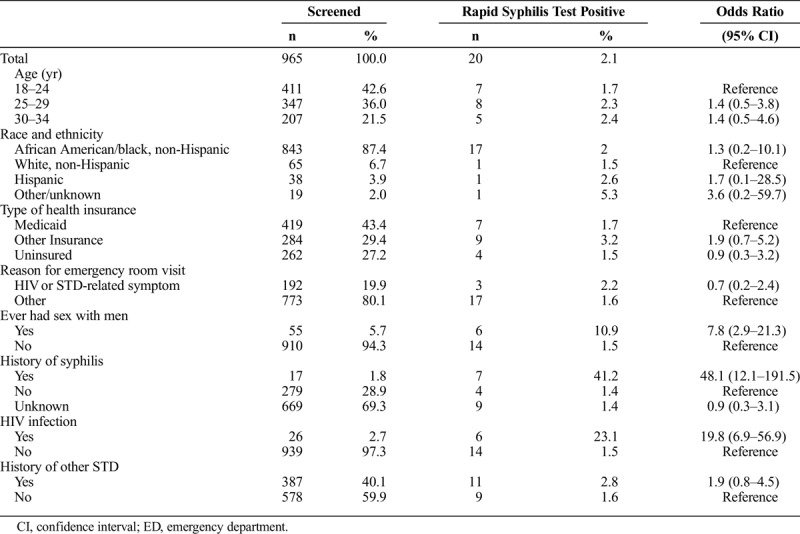
From July 2015 to April 2016, 999 men participated in our study and 965 were tested for antibodies to syphilis with the RST, RPR, and TP-PA. The median age of participants was 25 years and 87.4% were black (Table 1). Medicaid was the most prevalent health insurance (43.4%) and 27.2% of participants were uninsured. At their ED visit, symptoms related to HIV or STDs were reported by 19.9% of participants and 40.1% had a history (by either self-report or medical record) of an STD including 26 (2.7%) participants with HIV infection and 17 (1.7%) participants with a history of syphilis. The RST was reactive in 20 (2.1%) participants. A higher proportion of RSTs were reactive in MSM compared to non-MSM (10.9% vs. 1.5%; P < 0.01), in HIV-infected compared with HIV negative participants (23.1% vs. 1.5%; P < 0.01), and in participants with a self-reported history of syphilis compared to those with no history of syphilis (41.2% vs. 1.4%; P < 0.01). ED physicians made a treatment decision based only on the RST result, and their clinical evaluation. Among participants with a reactive RST, 11 were treated in the ED, 3 were verified to have already received treatment, and 6 were scheduled for a follow-up with a clinical care provider. There were no adverse events associated with IM penicillin treatment of the 11 participants in the ED, but none of the 6 untreated participants scheduled for follow-up attended their appointment.
TABLE 1.
Demographic Characteristics, Reason for Emergency Room Visit, and STD History Among Men Aged 18–34 Years Screened for Syphilis in an ED, 2015–2016
Compared with the traditional testing algorithm using an RPR test and TP-PA, 10 specimens tested with the RST were concordant positive and 13 specimens were discordant (Table 2; McNemar's test P = 0.05). Among the discordant results, 10 RST reactive specimens were negative by the RPR-TP-PA sequential algorithm and 3 RST negative specimens were positive by the RPR-TP-PA sequential algorithm. The RST sensitivity was 76.9%, (95% CI, 46.2–95.0%), specificity was 99.0%, and the positive predictive value was 50.0% compared with the RPR-TP-PA sequential algorithm (Table 2). The RPR titers for the 3 discordant specimens that were negative with the RST were all 1:1 compared with a median titer of 1:16 (range, 1:4 to 1:128) among the 10 concordant positive specimens. Two of the discordant RST reactive and RPR negative specimens were from patients with a chancre consistent with primary syphilis on physical examination. Among HIV-infected participants (n = 26), 4 of the 6 RST positive results were concordant positive by the RPR-TP-PA sequential algorithm and only 1 of 20 RST negative results had a discordant, positive result by the RPR-TP-PA sequential algorithm.
TABLE 2.
Performance Characteristics of the Rapid Syphilis Test in an ED, 2015–2016
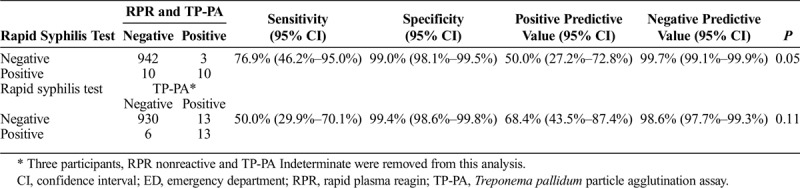
Compared with TP-PA testing only, 13 specimens tested with the RST were concordant positive and 19 specimens were discordant (Table 2; McNemar's test P = 0.11). Among the discordant results, 6 RST reactive specimens were TP-PA negative, and 13 rapid syphilis negative specimens were TP-PA positive. The RST sensitivity was 50.0%, the specificity was 99.4%, and the positive predictive value was 68.4% compared with the TP-PA testing alone (Table 2). Among these RST and TP-PA discordant results, EIA testing confirmed the TP-PA result in 16 of 18 specimens tested. Among study participants, 17 had a known history of syphilis infection. The information was obtained by self-reports (n = 13), medical chart review (n = 2), and from state surveillance data (n = 2). Of these 17, 12 participants had concordant results (7 concordant positive and 5 concordant negative) and 5 participants had discordant results (all RST negative and TP-PA positive).
DISCUSSION
In our evaluation of the performance of an RST, a substantial proportion (76.9%; 95% CI, 46.2–95.0%) of participants who were RPR and TP-PA reactive, were detected with the RST, indicating that this assay could potentially be a useful tool to screen for syphilis infection in settings where access to laboratory testing is not feasible or where an immediate result is necessary. Although the specificity of the assay was high (99.0%), the positive predictive value in this setting was 50% suggesting this test is better suited for a high prevalence (eg, MSM) than a low prevalence (eg, pregnant women) setting. Of interest, the RST did not miss a syphilis infection in any participant with a positive TP-PA and an RPR titer of 1:2 or greater dilutions, and was positive in less than 20% of participants with a positive TP-PA and negative RPR. If these findings are confirmed, it may have relevance for how the rapid syphilis test is utilized and interpreted in populations with a high prevalence of past syphilis infection. In addition, 2 persons clinically confirmed to have primary syphilis had a positive RST but a negative RPR. This finding is consistent with studies that have demonstrated that in early syphilis, treponemal antibodies are detectable earlier in the course of infection than nontreponemal antibodies.14,15 Our finding suggests that the RST might be useful to detect early primary infection, but further studies of the test's performance by stage of infection are needed.
The sensitivity of the RST in this evaluation was similar to its sensitivity in a population tested at an STD clinic (71.4% compared with an EIA result),10 and from a meta-analysis of similar rapid assays used in resource limited settings,16 but substantially lower than the sensitivity described in the product package insert (98%).11 In the STD clinic study, as in our evaluation, most of the RPR reactive patients had a reactive RST result.10 Our observed specificity (99.0%) was higher than that in the STD clinic study (91.5%),10 and similar to the specificity of other assays used in resource limited settings (95.9–99.6%).16 Of note, the rapid syphilis test was also reactive in 2 participants with primary syphilis who had nonreactive RPR results, and this detection is a potential benefit of treponemal tests which become reactive on average 3 weeks postinfection compared with the RPR assay which becomes reactive on average 6 weeks postinfection.15
Unexpectedly, in this study, the RST was reactive in all participants with an RPR titer 1:2 or greater and a positive TP-PA but less than 20% of participants with a nonreactive RPR and positive TP-PA. We assumed the RST which detects treponemal antibodies would perform more similar to the treponemal TP-PA assay than the nontreponemal RPR test which detects nonspecific antibodies to a complex antigen that contains cardiolipin, lecithin, and cholesterol that can indicate active syphilis infection.17 In addition to being a rapid flow-through assay that can use a whole blood specimen, an important difference between the assays is that the RST detects treponemal antibodies using purified recombinant TP antigen proteins, whereas the TP-PA assay uses native treponemal antigens.11,18,19 Both assays detect treponemal IgG and IgM antibodies, and treponemal IgG antibodies usually persist for life regardless of treatment.15 Whether the total complement of treponemal antigens in the TP-PA accounts for an increased sensitivity compared with the recombinant treponemal antigens of the RST requires further evaluation.
It is notable that none of the 6 individuals with a positive RST result, and who had scheduled follow-up appointments for treatment, attended their appointments. That several patients did not follow-up with their referral suggests the RST is very useful in a setting, such as ED or other venues with large number of patients in at-risk populations.
The ability of the RST to detect early syphilis, as it did in our study in persons both with early primary infection and with both detectable treponemal and nontreponemal antibodies, offers public health advantages. Although this result should be confirmed in other settings because it has important implications for how the RST results are interpreted. Diagnosis and treatment of early syphilis infection is important to prevent the transmission of syphilis because it is most infectious in its early stages. The results from all RST should be confirmed with standard laboratory testing, especially the RPR, to be able to correctly diagnose the patient. Clinical history and examination are also critical to detect symptoms and signs of early syphilis.
Although the rapid syphilis test provides an immediate result, it still must be confirmed with nontreponemal testing. Presumptive treatment of syphilis is indicated for patients with clinical signs of primary or secondary syphilis or with a recent exposure. Although syphilis treatment with IM penicillin is generally safe,16 treatment of asymptomatic patients with a positive RST is not indicated without additional clinical or testing results. Further studies are needed to determine if there are settings where treatment would be indicated based on a positive RST, especially where treatment is immediately available but a loss to follow-up might be likely. Further studies are also needed to determine settings where an RST can be used effectively to link persons to care for confirmatory testing and treatment.
There were several limitations to our study. In this population, we had only a few positives which led to a very precise estimate of specificity, but wide CI on the estimated sensitivity. Screening for syphilis was conducted in an ED among a young population with a low prevalence of previous syphilis infection and the performance characteristics of the RST might vary in settings with either a higher prevalence of previous syphilis infection or an older population with more comorbid medical conditions. We also only included men in our study, and the performance of the rapid syphilis test may be different in pregnant women. Finally, our analysis was cross-sectional, we do not have longitudinal data on rapid syphilis test results over time in a patient previously treated and whose RPR titer declines to nonreactive.
In summary, the use of a rapid syphilis test in a busy ED was an effective screening intervention that identified people who were likely active syphilis cases enrolled in our study, and provided an opportunity for immediate treatment. Additional research, however, is needed to understand if the detection of a high proportion of participants with active syphilis is confirmed in other settings and populations, especially MSM in community settings. As the incidence of syphilis continues to rise in the United States, novel approaches that facilitate the diagnosis and treatment of syphilis may be able to improve our public health response.
Footnotes
Y.F.F. and N.M. contributed equally to this article.
Conflicts of interest: The Syphilis Health Check kits were donated by the Trinity Biotech Company.
Sources of Funding: enters for Disease Control (CDC).
Disclaimer: The conclusions, findings, and opinions expressed by authors contributing to this journal do not necessarily reflect the official position of the Centers for Disease Control and Prevention, or the authors' affiliated institutions.
Ethical approval: This study was approved by the Henry Ford Hospital Institutional Review Board (IRB 9424) and was approved by CDC through a research determination in accordance with federal human subject protection regulations and CDC policies and procedures.
REFERENCES
- 1.Radolf JD, Tramont EC, Salazar JC. Syphilis (Treponema pallidum). In: Bennett JE, Dolin R, Blaser MJ, eds. Mandell, Douglas, and Bennett's Principles and Practice of Infectious Diseases, Updated Edition, 8th ed. Philadelphia, PA: Elsevier Saunders, 2015. [Google Scholar]
- 2.Lynn WA, Lightman S. Syphilis and HIV: A dangerous combination. Lancet Infect Dis 2004; 4:456–466. [DOI] [PubMed] [Google Scholar]
- 3.Centers, for Disease Control. Sexually Transmitted Disease Surveillance 2016. US Department of Health and Human Services 2017. [Google Scholar]
- 4.Workowski KA, Bolan GA. Sexually Transmitted Diseases Treatment Guidelines, 2015. MMWR Recomm Rep 2015; 64:1–137. [PMC free article] [PubMed] [Google Scholar]
- 5.Ham DC, Lentine D, Hoover KW, et al. Strengthening sexually transmitted disease Services in Detroit, Michigan: A call to action. Sex Transm Dis 2016; 43:65–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention. 2016. National Center for Health Statistics, United States 2016. https://www.cdc.gov/nchs/hus/contents2016.htm#074. Accessed April 12.
- 7.Radolf JD, Bolan G, Park I, et al. Discordant results from reverse sequence syphilis screening–five laboratories, United States, 2006-2010. MMWR Morb Mortal Wkly Rep 2011; 60:133–137. [PubMed] [Google Scholar]
- 8.Anonymous. 2014. FDA grants CLIA waiver expanding the availability of rapid screening test for syphilis. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm426843.htm. Accessed April 12.
- 9.Trinity Biotech. 2014. Syphilis Health Check package insert. https://www.diagnosticsdirect2u.com/images/PDF/SHC%20products/Syphilis%20Health%20Check%20%20test_PI%20rev%20N_121014_Final[1].pdf. Accessed April 12.
- 10.Matthias J, Dwiggins P, Totten Y, et al. Notes from the field: Evaluation of the sensitivity and specificity of a commercially available rapid syphilis test—Escambia County, Florida, 2016. MMWR Morb Mortal Wkly Rep 2016; 65:1174–1175. [DOI] [PubMed] [Google Scholar]
- 11.Trinity Biotech Diagnostics Direct CM, New Jersey. Syphilis Health Check package insert. https://www.diagnosticsdirect2u.com/images/pdf/syphilishc.pdf. Accessed April 17.
- 12.Park IU, Fakile YF, Chow JM, et al. Performance of treponemal tests for the diagnosis of syphilis. Clin Infect Dis 2018; doi:10.1093/cid/ciy558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.US Department of Health and Human Services. Protection of human subjects. 45 CFR § 46. http://archive.hhs.gov/ohrp/humansubjects/guidance/45cfr46.html. Accessed April 17, 2018.
- 14.Peeling RW, Ye H. Diagnostic tools for preventing and managing maternal and congenital syphilis: An overview. Bull World Health Organ 2004; 82:439–446. [PMC free article] [PubMed] [Google Scholar]
- 15.Ballard R, Hook EW., 3rd 3rd, SYPHILIS IN Laboratory diagnosis of sexually transmitted infections, including human immunodeficiency virus. WHO 2013 2013; CH.10:107–129. [Google Scholar]
- 16.Jafari Y, Peeling RW, Shivkumar S, et al. Are Treponema pallidum specific rapid and point-of-care tests for syphilis accurate enough for screening in resource limited settings? Evidence from a meta-analysis. PLoS One 2013; 8:e54695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peterman TA, Fakile YF. What is the use of rapid syphilis tests in the United States? Sex Transm Dis 2016; 43:201–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoover KW, Radolf JD. Serodiagnosis of syphilis in the recombinant era: Reversal of fortune. J Infect Dis 2011; 204:1295–1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Larsen SA, Steiner BM, Rudolph AH. Laboratory diagnosis and interpretation of tests for syphilis. Clin Microbiol Rev 1995; 8:1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]


