Abstract
Objectives:
Myasthenia gravis (MG) may be refractory to traditional therapies. Quality of life (QOL) and disease burden in patients with refractory and nonrefractory MG were compared using Myasthenia Gravis Foundation of America MG Patient Registry data.
Methods:
Adults aged 18 years or older with MG diagnosed ≥2 years before enrollment were included. Participants with refractory MG had received ≥2 previous and 1 current MG treatment and had MG Activities of Daily Living Scale total score ≥6 at enrollment; other participants had nonrefractory MG. MG QOL 15-item scale (MG-QOL15) scores were compared.
Results:
In total, 56 participants with refractory and 717 participants with nonrefractory MG enrolled. Participants with refractory MG had significantly higher mean (SD) MG-QOL15 total scores [31.4 (11.1) vs. 20.8 (15.0), P < 0.0001] and were more likely to have had exacerbations, emergency department visits, and recent hospitalizations.
Conclusions:
Participants with refractory MG experience worse QOL and greater clinical burden than those with nonrefractory disease.
Key Words: health-related quality of life, HRQOL, myasthenia gravis, quality of life, refractory, registry
INTRODUCTION
Myasthenia gravis (MG) is a rare, debilitating autoimmune disease caused by inhibition of neuromuscular transmission because of the binding of autoantibodies at the neuromuscular junction. MG often first manifests as weakness of ocular muscles (ocular MG), but approximately 75%–90% of cases progress to generalized MG (gMG) that affects other muscles including those in the face, neck, hands, and/or limbs.1–4 MG symptoms can manifest as slurred speech, inability to swallow, impaired mobility, impaired vision, and shortness of breath (with activity or at rest). Fatigue is also a common symptom and has been reported by 70%–85% of patients with gMG.5,6 The various disease manifestations can have a considerable negative impact on patients' activities of daily living (ADL), including eating and drinking, reading, driving, personal grooming, working, and meeting the needs of their families. Throughout the disease course, patients may experience exacerbations of their symptoms, which can be triggered by certain stressors (eg, some medications, infection, surgery, or emotional stress)7 and may require hospitalization. Approximately 15%–20% of patients experience respiratory weakness severe enough to cause respiratory failure, known as myasthenic crisis, which can be life-threatening and require immediate intervention.8,9
In most countries, immunosuppressant therapies (ISTs) are not approved by regulatory bodies to treat MG1,10,11; however, a range of nonspecific ISTs that interfere with autoimmune processes is used to manage the disease.12 Patients with milder forms of MG may be effectively treated with acetylcholinesterase inhibitors and/or corticosteroids. Patients who do not achieve adequate symptom control or cannot tolerate these therapies may begin treatment with an IST such as azathioprine, mycophenolate, methotrexate, or rituximab. Symptoms can be managed in up to 90% of patients with immunosuppression, with some requiring an escalation in the dosage or number of therapies to achieve an acceptable response, or relying on chronic maintenance therapy with intravenous immunoglobulin (IVIg) or plasmapheresis/plasma exchange in an attempt to control their disease.13
Approximately 10%–15% of patients with MG do not respond adequately to traditional therapies for MG, or cannot tolerate these therapies, and are considered to have refractory MG.1,11,14–16 The health-related quality of life (QOL) of these patients with MG remains diminished compared with healthy controls,17–20 particularly among the subset who do not achieve adequate symptom control and those who endure burdensome side effects of therapy.21,22 However, no studies to date have evaluated the impact of refractory MG on QOL.
A recent real-world study of the clinical burden on patients with refractory MG reported that these individuals are significantly more likely to experience myasthenic exacerbations and crises, and are significantly more likely to visit an emergency department (ED) or be admitted to hospital than those with nonrefractory MG.23 Based on these findings, it is reasonable to hypothesize that patients with refractory MG, whose disease is characterized by poorly controlled symptoms and higher levels of disease activity, experience worse QOL than patients with nonrefractory MG. The aim of the current analysis was to compare QOL and disease burden for individuals with refractory and nonrefractory MG.
MATERIALS AND METHODS
The study population was derived from the Myasthenia Gravis Foundation of America's MG Patient Registry, an active research database of treatment and patient information. The registry is open to adults at least 18 years of age who reside in the United States. An online enrollment survey is completed by all participants on entry into the registry. The survey consists of approximately 200 questions covering categories including demographics, MG history, comorbidities, past and current therapies, family history of MG, functionalities, lifestyle, employment status, and QOL. This study included data from enrollment surveys completed between July 2013 (the start of the registry) and June 2016. A shorter online survey is sent to registry participants to complete every 6 months after enrollment; no data from postenrollment surveys were included in this study. The study was approved by the institutional review board of the University of Alabama at Birmingham. Data were deidentified for research, and consent for participation was provided by participants electronically at registration, before completion of the survey.
Participants in this study were at least 18 years of age and reported having a physician-confirmed diagnosis of MG at least 2 years before completing the enrollment survey. The 2-year requirement was imposed to minimize the risk that a participant with refractory MG would be misclassified as having nonrefractory disease due to insufficient time on treatment to experience no response or inadequate response.
Information from the enrollment survey, including treatment types and durations, and MG ADL Scale (MG-ADL) total score, was used to classify participants as having either refractory or nonrefractory MG. Information on historical treatments is dependent on patients' recall when they complete the survey. The MG-ADL is a validated 8-item patient-reported outcome measure that assesses relevant MG symptoms and their functional impact on the patient.24,25 Participants report the level of functional disability [from 0 (normal) to 3 (most severe)] in the past 4 weeks (as specified by the registry survey) for each of the 8 items [ocular (2 items), bulbar (3 items), respiratory (1 item), and gross motor or limb impairment (2 items)]. Summation of item scores by severity level constitutes an MG-ADL total score ranging from 0 to 24, where a higher score indicates a higher level of functional disability. Participants were classified as having refractory MG if they met the following criteria:
-
Past treatment (before registry enrollment):
a. Past use of at least 2 of the following ISTs: prednisone, azathioprine, mycophenolate, cyclosporine, tacrolimus, methotrexate, cyclophosphamide, and/or rituximab for at least 6 months each, OR
b. Past use of at least 1 IST (listed above) for any duration AND repeated use of IVIg or plasmapheresis, defined as at least 4 rounds in the past year AND
Treatment at enrollment: use of at least one of the following: prednisone, azathioprine, mycophenolate, cyclosporine, tacrolimus, methotrexate, cyclophosphamide, rituximab, IVIg, or plasmapheresis, AND
An MG-ADL total score at enrollment of at least 6.
Participants meeting the treatment criteria for refractory MG but with an MG-ADL score less than 6 were excluded because these participants were deemed to have insufficient functional impairment to be characterized as having refractory MG. Those who did not meet the past or current (at enrollment) treatment criteria for refractory MG were considered to have nonrefractory disease, regardless of their MG-ADL score. Participants lacking complete MG-ADL data or sufficient treatment duration data to be classified as refractory or nonrefractory were excluded from the analyses.
The primary outcome measures of interest from the enrollment survey were total and item-specific MG QOL 15-item scale (MG-QOL15) scores. The MG-QOL15 is the most commonly reported MG-specific QOL measure; it was developed to reliably capture patients' perceptions of impairment and disability, and the degree to which disease manifestations are tolerated.26,27 The instrument is sensitive to the longitudinal course of the disease and the acute impact of clinical events such as crises and exacerbations.27 It comprises 15 questions about possible effects of MG, with responses to each question scored 0 (not at all), 1 (a little bit), 2 (somewhat), 3 (quite a bit), or 4 (very much), with possible total scores ranging from 0 to 60, with higher scores representing worse QOL as assessed over a recall period of the previous few weeks (as specified by the survey).28
Other variables of interest due to their potential association with QOL included MG-ADL total scores and participant demographics, MG disease characteristics, and functionalities. Disease characteristics included age at diagnosis, time since diagnosis, and indicators of MG activity, such as number of exacerbations and ED and hospital visits in the previous 6 months. Exacerbations are defined in the survey as having a duration longer than 7 days and occurring at least 30 days after the last exacerbation. Measures of functionality, fatigue, and depression included the 8-item QOL in Neurological Disorders (NeuroQoL) Lower Extremity Function Scale, the 8-item NeuroQoL Upper Extremity Function Scale, and the 8-item Short Form NeuroQoL Fatigue Scale. Depression was assessed using a single-question Likert scale (0 for no symptoms of depression to 5 for total depression), assessing symptoms in the past month in relation to depression status before developing MG. Finally, overall health status was also assessed using a single-question Likert scale (1 for excellent health to 5 for poor health).
The frequencies and mean values of each study variable were compared between participants with refractory and those with nonrefractory MG using χ2 tests for categorical variables and t tests for continuous variables. In addition, a sensitivity analysis was performed to evaluate any effects on the refractory group of retaining the participants who met treatment criteria for refractory MG but had MG-ADL total scores less than 6. For participants with any missing items on the MG-QOL15, a maximum of 2 missing items were estimated using the proportional method of imputation based on participants' responses to the other items on the questionnaire. MG-ADL and MG-QOL15 total scores were compared using Pearson correlation analyses. P values of <0.05 were considered significant. Analyses were performed using SAS version 9.4.
RESULTS
A total of 799 participants had been diagnosed with MG at least 2 years before completing the enrollment survey, had provided sufficient information on the types and durations of prescribed treatments, and had complete MG-ADL data to allow for classification of refractory or nonrefractory disease. Of these 799 participants, 56 (7.0%) met the criteria for refractory MG and 717 were classified as having nonrefractory MG. A total of 26/799 participants met the treatment failure criteria for refractory MG but had MG-ADL total scores of less than 6 and were therefore excluded from the analysis; these 26 “treatment-refractory” participants were included in a sensitivity analysis with the 56 who met the full-study refractory criteria. The final study population comprised 773 individuals [56 (7.2%) with refractory and 717 (92.8%) with nonrefractory MG].
Demographic characteristics are presented in Table 1. Participants with refractory MG were significantly younger than those with nonrefractory MG at the time of initial diagnosis (mean 36.5 years and 45.8 years, respectively; P < 0.0001) and at the time of registry enrollment (mean 48.5 years and 55.4 years, respectively; P = 0.0006). A higher proportion of participants with refractory compared with nonrefractory disease was female participants (82.1% and 65.3%, respectively; P = 0.0100). No differences were seen between groups with respect to ethnicity, marital status, living status, or employment status (full-time, part-time, or unemployed; Table 1).
TABLE 1.
Participant Demographics and Disease Characteristics
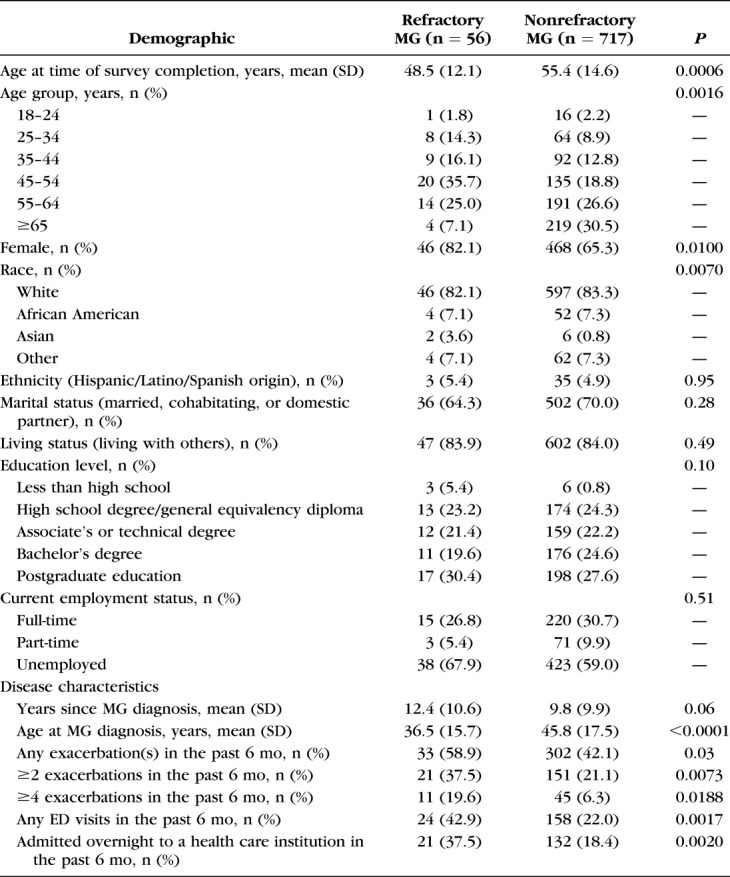
Disease activity over the 6 months before enrollment was considerably greater in the cohort with refractory MG, with 58.9% reporting at least 1 exacerbation compared with 42.1% of those with nonrefractory MG (P = 0.03), and 19.6% of participants with refractory MG reporting 4 or more exacerbations compared with 6.3% of those with nonrefractory MG (P = 0.0188; Table 1). A higher proportion of participants with refractory than with nonrefractory disease made at least 1 ED visit in the previous 6 months (42.9% vs. 22.0%, respectively; P = 0.0017), and a higher proportion of participants with refractory disease were admitted overnight to a health care institution (37.5%, vs. 18.4% of those with nonrefractory MG; P = 0.0020).
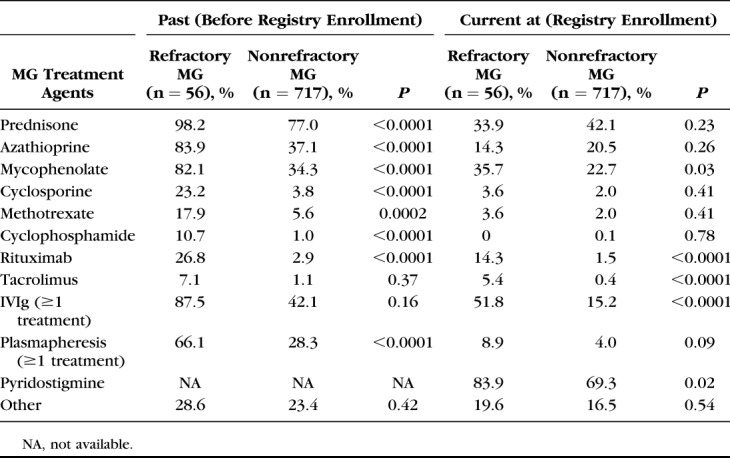
Past and current treatments at registry enrollment are summarized in Table 2. A higher proportion of participants with refractory than those with nonrefractory MG reported past plasmapheresis or current IVIg therapy.
TABLE 2.
Past and Current MG Treatment for Participants With Refractory and Nonrefractory MG
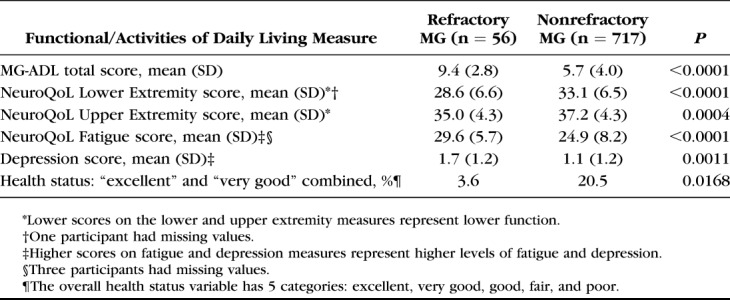
Functionality measures and ADL are described in Table 3. Mean MG-ADL total score was significantly higher for participants with refractory MG than for those with nonrefractory MG, which was consistent with the cutoff point of at least 6 to be classified as having refractory MG. Compared with participants with nonrefractory MG, those with refractory disease had significantly worse lower and upper extremity function and higher levels of fatigue and depression. A significantly lower proportion of participants with refractory MG reported their overall health status to be very good or excellent, compared with those with nonrefractory MG (3.6% vs. 20.5%; P = 0.0168).
TABLE 3.
Functional and Activities of Daily Living Measures
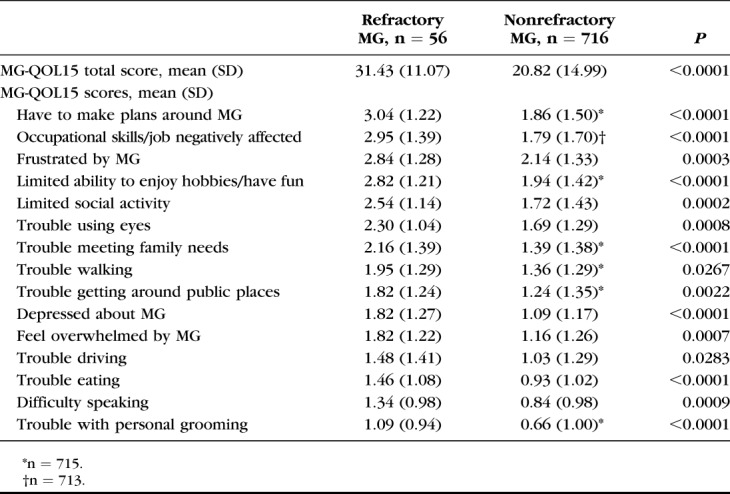
Mean MG-QOL15 total scores and item-specific scores are shown in Table 4. Mean total scores were significantly higher (indicating worse QOL) for participants with refractory than those with nonrefractory MG (31.4 and 20.8, respectively; P < 0.0001). For all of the MG-QOL15 questionnaire items, mean scores were significantly higher for participants with refractory MG (Table 4), and a significantly higher proportion of these participants reported “quite a bit” or “very much” impact of MG on QOL, compared with participants with nonrefractory MG. In a sensitivity analysis of these findings, when the 26 participants with treatment-refractory MG who did not meet the MG-ADL total score criterion were included, the mean total score for all individuals considered to have refractory MG (n = 82) remained significantly worse than for those with nonrefractory MG (26.3 and 20.8, respectively; P = 0.0014). As expected, inclusion of participants with lower MG-ADL total scores in the refractory group resulted in an attenuation of the difference between MG-QOL15 total scores for refractory versus nonrefractory disease.
TABLE 4.
MG-QOL15 Total and Individual Item Scores
Of the 717 participants with nonrefractory MG, 355 (49.5%) had MG-ADL total scores of 6 or higher. The MG-QOL15 mean total score for this subset of participants with nonrefractory MG {30.6 [95% confidence interval (CI), 29.3–31.9]} was similar to that of participants with refractory MG [31.4 (95% CI, 28.5–34.4), P = 0.63]. Disease characteristics for the 6 months before enrollment and MG treatments used before and at enrollment were also compared between these groups (nonrefractory MG with MG-ADL score of 6 or higher vs. refractory MG). There were no significant differences in percentages with disease characteristics [experiencing any exacerbations (59.8% vs. 58.9%, respectively), the number of exacerbations (59.6% vs. 58.9%), and any overnight hospitalizations (26.4% vs. 37.5%)]. For MG therapies, there were statistically significant higher treatment rates in the refractory MG group for 5 of 12 therapies evaluated at enrollment and for 8 of 11 past therapies.
For the total sample of 773 participants, there was a strong correlation between MG-ADL and MG-QOL15 total scores [r = 0.77 (95% CI, 0.74–0.80)]. When data for the subset of 355 participants with nonrefractory MG who had an MG-ADL total score of 6 or higher were evaluated, the correlation between MG-ADL and MG-QOL15 total scores was lower [r = 0.55 (95% CI, 0.48–0.62)].
DISCUSSION
The current study supports the hypothesis that individuals with refractory MG have significantly worse QOL than those with nonrefractory MG. Consistent with previous research,23 registry participants with refractory MG accounted for 7% of the overall sample, were female, significantly younger, and exhibited greater disease activity, as evidenced by higher rates of exacerbations, ED visits, and hospital admissions over the previous 6 months, than those with nonrefractory MG. Furthermore, the mean MG-QOL15 total score reported for participants with nonrefractory MG in this analysis (20.8) is consistent with that previously reported (19.3) for the same instrument among patients with gMG.29 The higher mean MG-QOL15 total score of 31.4 for participants with refractory MG reflects a clinically meaningful difference for this subpopulation compared with those with nonrefractory MG and is consistent with the baseline MG-QOL15 scores reported in the REGAIN trial of eculizumab in patients with refractory gMG.30 In addition, the sensitivity analysis that included the 26 participants with refractory MG, defined only according to treatment criteria, confirmed that the significantly worse QOL was also found in this larger refractory MG population compared with those with nonrefractory MG. The results are not unexpected because previous research has also shown that poor QOL is correlated with higher levels of disease activity, functional impairments, fatigue, and depression in patients with refractory MG.17–22 To the best of our knowledge, this is among the first reported total and item-specific MG-QOL15 scoring for individuals with refractory disease.
A closer look at the clinical characteristics of the study population (shown by MG-ADL total scores) provides greater insight into the study findings. When we compared the subset of participants with nonrefractory MG who had MG-ADL scores of at least 6 with the participants who had refractory MG, the correlation between MG-ADL and MG-QOL15 total scores was attenuated to r = 0.55, compared with the correlation between the measures for the total sample of 773 participants (r = 0.77). Furthermore, for this subset with nonrefractory MG and MG-ADL scores of at least 6 compared with those with refractory disease, the MG-QOL15 mean total scores were similar, while there was a significant difference in MG-QOL15 scores observed between all participants with nonrefractory MG and those with refractory MG. Participants in the nonrefractory MG subset and those with refractory disease also had similar levels of exacerbations and overnight admissions, and, at enrollment, generally similar rates of MG therapy use. Consequently, the driving force for the significant difference in MG-QOL15 scores is the difference in MG-ADL total scores between participants with refractory and those with nonrefractory MG, reflecting important differences in functionality between these participant groups.
Some limitations to the validity of these analyses should be noted. Data collected in the Myasthenia Gravis Foundation of America's MG Patient Registry are self-reported. It is, therefore, not possible to confirm details of clinical diagnoses, documented therapies or accuracy of recall of historical treatments, or of symptoms and exacerbations (according to the enrollment survey definitions) beyond the information that participants provide. In addition, participants enrolled in the registry might constitute a self-selected sample with MG with higher educational and socioeconomic status and better Internet access to facilitate participation than the overall MG patient population in the United States. Moreover, individuals who are severely affected by MG may find it difficult to complete the 200-question enrollment survey, meaning that worse affected patients may not have been included in the sample or may have missing data. Another limitation is that the MG-ADL and MG-QOL15 scores are derived from the instruments embedded in the enrollment survey completed online by participants. Both of these instruments have been validated for standalone completion (the MG-ADL questionnaire to be administered by a trained health care professional, while the MG-QOL15 is usually self-administered).24,27,28 Furthermore, the recall period for the MG-ADL questionnaire in the registry enrollment survey is the previous 4 weeks, compared with a recall period of the past 7 days that is used elsewhere in various clinical studies.30,31
Finally, the analyses are univariate and not adjusted for different covariate distributions between participants with refractory and nonrefractory MG. However, the observed convergence between most demographic distributions suggests comparability on these parameters. An exception is that participants with nonrefractory MG were older at diagnosis and registry enrollment than those with refractory MG. However, while older age is associated with worse QOL in patients with MG,19 in this analysis, the younger participants with refractory MG had worse QOL. This demonstrates that the negative effects of refractory status on QOL may dominate those of age. The clear differences seen in scores for functional measures, MG-ADL, and disease characteristics are reflective of the refractory definition.32 To adjust for these differences through multivariate analyses would dilute the impact of these measures on QOL, which is the basis for the underlying study hypothesis. In addition, these variables likely violate the independence assumption.
In conclusion, the findings of this study demonstrate that individuals with refractory MG not only have an increased clinical burden relative to those with nonrefractory MG, but also experience worse QOL.
ACKNOWLEDGMENTS
The authors thank the Myasthenia Gravis Foundation of America for establishing and maintaining the Myasthenia Gravis Patient Registry, including the coordinating center at the University of Alabama, Birmingham, AL, and the MGFA Patient Registry Committee. Editorial assistance for this article was provided by Catriona Scott (Oxford PharmaGenesis, Oxford, United Kingdom) and funded by Alexion Pharmaceuticals.
Footnotes
Supported by Alexion Pharmaceuticals.
A. N. Boscoe was previously employed by Alexion Pharmaceuticals. H. Xin is employed by the University of Alabama at Birmingham. G. J. L'Italien was previously employed by Alexion Pharmaceuticals. L. A. Harris is employed by Alexion Pharmaceuticals. G. R. Cutter is employed by the University of Alabama at Birmingham and is Professor of Biostatistics at the School of Public Health at the University of Alabama. He is the President of Pythagoras, Inc, a private consulting company located in Birmingham, AL, and has also served as a member of consulting or advisory boards (Argenix, Atara Biotherapeutics, Axon, Biogen, Brainstorm Cell Therapeutics, Charleston Laboratories Inc, Click Therapeutics, Genentech, Genzyme, GW Pharma, Klein-Buendel Incorporated, MedDay, Medimmune, Novartis, Roche, Scifluor, Somahlution, Teva pharmaceuticals, TG Therapeutics, and UT Houston), and data and safety monitoring boards [AMO Pharmaceuticals, BioLineRx, Hisun Pharmaceuticals, Horizon Pharmaceuticals, Merck, Merck/Pfizer, Neurim, NHLBI (Protocol Review Committee), NICHD (Obstetric-Fetal Pharmacology Research Unit oversight committee), Novartis, Orphazyme, Opko Biologics, Reata Pharmaceuticals, Receptos/Celgene, Sanofi-Aventis, and Teva pharmaceuticals].
REFERENCES
- 1.Conti-Fine BM, Milani M, Kaminski HJ. Myasthenia gravis: past, present, and future. J Clin Invest. 2006;116:2843–2854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grob D, Brunner N, Namba T, et al. Lifetime course of myasthenia gravis. Muscle Nerve. 2008;37:141–149. [DOI] [PubMed] [Google Scholar]
- 3.Melzer N, Ruck T, Fuhr P, et al. Clinical features, pathogenesis, and treatment of myasthenia gravis: a supplement to the Guidelines of the German Neurological Society. J Neurol. 2016;263:1473–1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Robertson NP, Deans J, Compston DA. Myasthenia gravis: a population based epidemiological study in Cambridgeshire, England. J Neurol Neurosurg Psychiatry. 1998;65:492–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hoffmann S, Ramm J, Grittner U, et al. Fatigue in myasthenia gravis: risk factors and impact on quality of life. Brain Behav. 2016;6:e00538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Paul RH, Cohen RA, Goldstein JM, et al. Fatigue and its impact on patients with myasthenia gravis. Muscle Nerve. 2000;23:1402–1406. [DOI] [PubMed] [Google Scholar]
- 7.Meriggioli MN, Sanders DB. Autoimmune myasthenia gravis: emerging clinical and biological heterogeneity. Lancet Neurol. 2009;8:475–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thomas CE, Mayer SA, Gungor Y, et al. Myasthenic crisis: clinical features, mortality, complications, and risk factors for prolonged intubation. Neurology. 1997;48:1253–1260. [DOI] [PubMed] [Google Scholar]
- 9.Jani-Acsadi A, Lisak RP. Myasthenic crisis: guidelines for prevention and treatment. J Neurol Sci. 2007;261:127–133. [DOI] [PubMed] [Google Scholar]
- 10.Murai H, Utsugisawa K, Nagane Y, et al. Rationale for the clinical guidelines for myasthenia gravis in Japan. Ann N Y Acad Sci. 2018;1413:35–40. [DOI] [PubMed] [Google Scholar]
- 11.Sanders DB, Wolfe GI, Benatar M, et al. International consensus guidance for management of myasthenia gravis: executive summary. Neurology. 2016;87:419–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Drachman DB. Myasthenia gravis. Semin Neurol. 2016;36:419–424. [DOI] [PubMed] [Google Scholar]
- 13.Li Y, Arora Y, Levin K. Myasthenia gravis: newer therapies offer sustained improvement. Cleve Clin J Med. 2013;80:711–721. [DOI] [PubMed] [Google Scholar]
- 14.Buzzard KA, Meyer NJ, Hardy TA, et al. Induction intravenous cyclophosphamide followed by maintenance oral immunosuppression in refractory myasthenia gravis. Muscle Nerve. 2015;52:204–210. [DOI] [PubMed] [Google Scholar]
- 15.Silvestri NJ, Wolfe GI. Treatment-refractory myasthenia gravis. J Clin Neuromuscul Dis. 2014;15:167–178. [DOI] [PubMed] [Google Scholar]
- 16.Suh J, Goldstein JM, Nowak RJ. Clinical characteristics of refractory myasthenia gravis patients. Yale J Biol Med. 2013;86:255–260. [PMC free article] [PubMed] [Google Scholar]
- 17.Raggi A, Leonardi M, Antozzi C, et al. Concordance between severity of disease, disability and health-related quality of life in myasthenia gravis. Neurol Sci. 2010;31:41–45. [DOI] [PubMed] [Google Scholar]
- 18.Leonardi M, Raggi A, Antozzi C, et al. The relationship between health, disability and quality of life in myasthenia gravis: results from an Italian study. J Neurol. 2010;257:98–102. [DOI] [PubMed] [Google Scholar]
- 19.Winter Y, Schepelmann K, Spottke AE, et al. Health-related quality of life in ALS, myasthenia gravis and facioscapulohumeral muscular dystrophy. J Neurol. 2010;257:1473–1481. [DOI] [PubMed] [Google Scholar]
- 20.Twork S, Wiesmeth S, Klewer J, et al. Quality of life and life circumstances in German myasthenia gravis patients. Health Qual Life Outcomes. 2010;8:129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boldingh MI, Dekker L, Maniaol AH, et al. An up-date on health-related quality of life in myasthenia gravis -results from population based cohorts. Health Qual Life Outcomes. 2015;13:115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Utsugisawa K, Suzuki S, Nagane Y, et al. Health-related quality-of-life and treatment targets in myasthenia gravis. Muscle Nerve. 2014;50:493–500. [DOI] [PubMed] [Google Scholar]
- 23.Engel-Nitz NM, Boscoe AN, Wolbeck R, et al. Burden of illness in patients with treatment refractory myasthenia gravis. Muscle Nerve. 2018;58:99–105. [DOI] [PubMed] [Google Scholar]
- 24.Wolfe GI, Herbelin L, Nations SP, et al. Myasthenia gravis activities of daily living profile. Neurology. 1999;52:1487–1489. [DOI] [PubMed] [Google Scholar]
- 25.Muppidi S, Wolfe GI, Conaway M, et al. MG-ADL: still a relevant outcome measure. Muscle Nerve. 2011;44:727–731. [DOI] [PubMed] [Google Scholar]
- 26.Burns TM, Grouse CK, Conaway MR, et al. Construct and concurrent validation of the MG-QOL15 in the practice setting. Muscle Nerve. 2010;41:219–226. [DOI] [PubMed] [Google Scholar]
- 27.Burns TM, Grouse CK, Wolfe GI, et al. The MG-QOL15 for following the health-related quality of life of patients with myasthenia gravis. Muscle Nerve. 2011;43:14–18. [DOI] [PubMed] [Google Scholar]
- 28.Burns TM, Conaway M, Sanders DB. The MG Composite: a valid and reliable outcome measure for myasthenia gravis. Neurology. 2010;74:1434–1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martinez-Lapiscina EH, Erro ME, Ayuso T, et al. Myasthenia gravis: sleep quality, quality of life, and disease severity. Muscle Nerve. 2012;46:174–180. [DOI] [PubMed] [Google Scholar]
- 30.Howard JF, Jr, Utsugisawa K, Benatar M, et al. Safety and efficacy of eculizumab in anti-acetylcholine receptor antibody-positive refractory generalised myasthenia gravis (REGAIN): a phase 3, randomised, double-blind, placebo-controlled, multicentre study. Lancet Neurol. 2017;16:976–986. [DOI] [PubMed] [Google Scholar]
- 31.Howard JF, Jr, Barohn RJ, Cutter GR, et al. A randomized, double-blind, placebo-controlled phase II study of eculizumab in patients with refractory generalized myasthenia gravis. Muscle Nerve. 2013;48:76–84. [DOI] [PubMed] [Google Scholar]
- 32.Howard JF, Jr, Freimer M, O'Brien F, et al. QMG and MG-ADL correlations: study of eculizumab treatment of myasthenia gravis. Muscle Nerve. 2017;56:328–330. [DOI] [PubMed] [Google Scholar]


