Supplemental Digital Content is available in the text.
Abstract
Background:
Maternal exposure to fine particulate air pollution (PM2.5) during pregnancy has been linked to lower newborn birthweight, making it a toxic exposure because lower birthweight is a risk factor for chronic disease and mortality. However, the toxicity of major constituents of PM2.5 and how they compare to each other remain uncertain.
Methods:
We assigned address-specific exposure to PM2.5, elemental carbon (EC), organic carbon (OC), nitrate, and sulfate averaged over the entire period of pregnancy for each birth in Massachusetts from 2001 to 2012 using a high-resolution exposure model. Using multivariate regression adjusted for total PM2.5, we estimated the relative toxicity of each constituent on continuous birthweight.
Results:
EC was more toxic per interquartile range increase compared with remaining PM2.5 in single constituent models that estimated the effect of a constituent with adjustment for PM2.5. OC, nitrate, and sulfate were each less toxic than their respective remaining PM2.5 per interquartile range increase. When all constituents and total PM2.5 were included in the same model, EC was most toxic, followed by nitrate, then OC and sulfate with similar toxicities. Sensitivity analyses using term low birth weight and small for gestational age also showed that EC was most detrimental as did averaging exposures over the third trimester of pregnancy. Scaling to unit mass increases also showed EC to be most toxic.
Conclusion:
Four major constituents of PM2.5 had different relative toxicities on continuous birthweight. Our findings suggest that EC was most toxic, followed by nitrate, OC, and sulfate.
What this study adds
Most studies on the effects of exposure to particulate matter under 2.5 µm in diameter (PM2.5) constituents and birthweight relied on monitoring data, limiting spatial coverage. This study uses recently-developed high-resolution predictions of PM2.5 and its constituents to ascertain exposure to minimize exclusion because of missing exposure data. We estimate the toxicities of major PM2.5 constituents–elemental carbon, organic carbon, nitrate, and sulfate–on birthweight adjusted for total PM2.5, a step infrequently performed in prior studies but is important for reducing the potential confounding by total PM2.5.
Introduction
Particulate matter under 2.5 µm in aerodynamic diameter (PM2.5) is toxic to human health. Maternal exposure to PM2.5 has been linked to lower birthweight,1–8 which is a risk factor for cardiovascular disease, diabetes, obesity, respiratory conditions, and premature mortality for the newborn.9–14 In many of the prior studies on the relationship between PM2.5 and birthweight, PM2.5 was considered as a homogenous pollutant when in actuality, it is a mixture of particles with varying size and chemical composition.6,15 Thus, it is proposed that different chemical constituents of PM2.5 would have differing toxicities on fetal growth and consequently, newborn birthweight. Understanding the toxicity of different chemical constituents of PM2.5 on birthweight is a research need and is important for informing air pollution control policy that minimizes health detriments from air pollution.
There exists few studies that estimated the toxicity of PM2.5 chemical constituents on continuous birthweight.6 Many existing analyses estimated the effects of individual chemical constituents’ mass concentrations on birthweight without adjustment for total PM2.5.1,6,8,15–17 Although this approach leads to results that are easy to interpret, these estimates are potentially confounded by total PM2.5 and by other constituents that covary with the individual constituents.18 In this study, we estimated the effects of individual PM2.5 constituents on birthweight with concurrent adjustment for total PM2.5. With this statistical approach, we gained an understanding of the toxicity of each PM2.5 constituent compared to a general mixture of total PM2.5 and to each other.
Aside from the difference in statistical modeling, our study differs from prior research by using output from a prediction model that uses chemical transport, meteorological, and land use data to ascertain maternal exposure to PM2.5 and its constituents.19 This contrasts exposure assessment in most prior studies, which relied on measurements from monitoring networks and excluded births when the maternal residence was too far away from an air monitor, leading to losses in statistical power and potential selection bias.1,3,15,16 When predicted PM2.5 and constituents were employed in prior studies,20,21 they used kriging or land use variables and did not incorporate chemical transport model output or meteorology, which are also predictive of PM2.5 and its constituents. In the present study, we focused on estimating the associations between four major PM2.5 chemical constituents and birthweight. These were elemental carbon (EC), organic carbon (OC), nitrate, and sulfate and they contribute to a majority of PM2.5.19 We additionally conducted multi-constituent analyses to arrive at a relative toxicity ranking of PM2.5 constituents on birthweight.
Methods
Study population
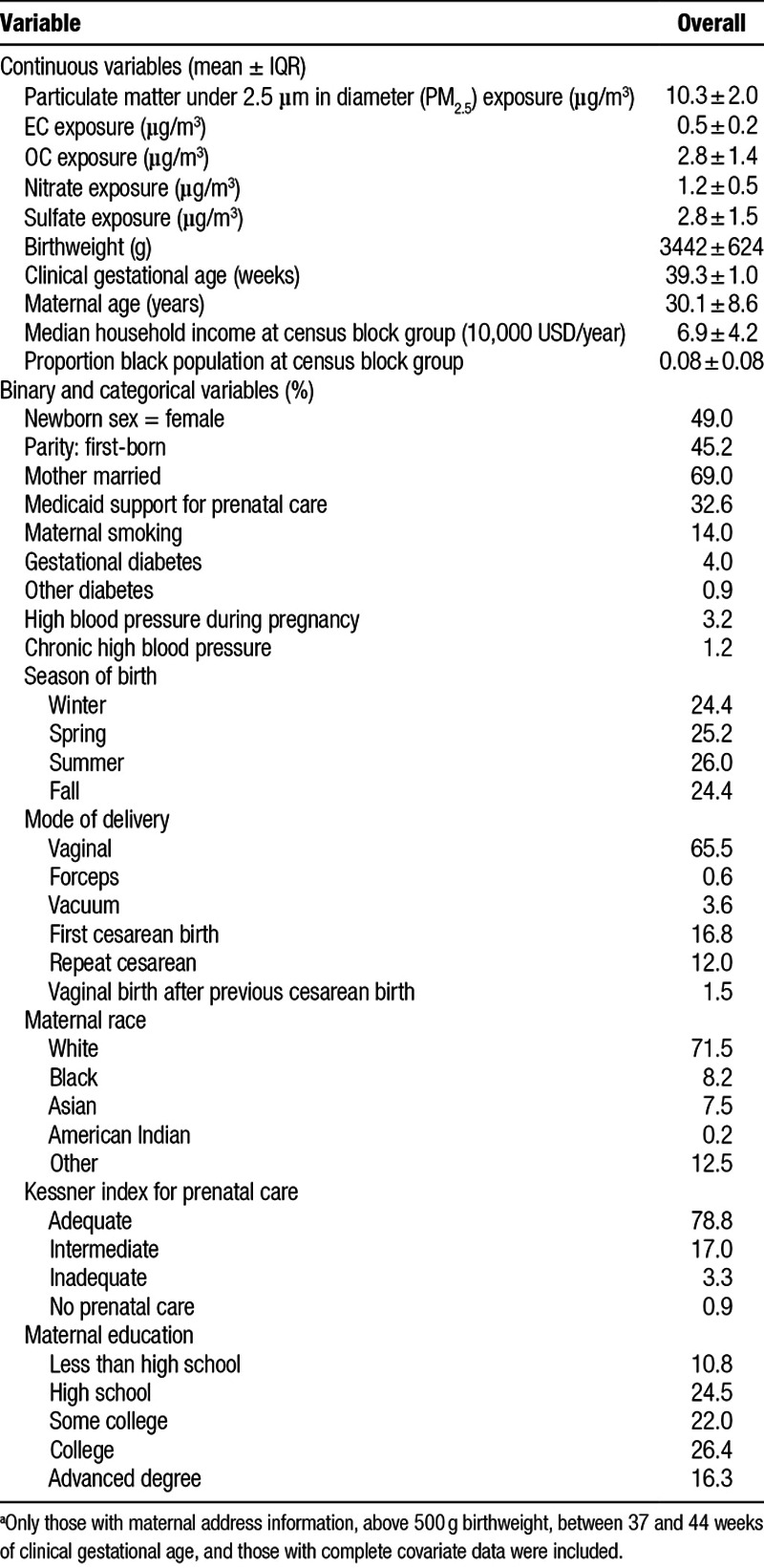
We obtained birth records from the Massachusetts Department of Public Health. The study base started with all births in Massachusetts from 1 January 2001 to 31 December 2012 (n = 907,766). In addition to birthweight, which was measured and recorded at the time of birth, the birth records contained information on maternal and individual birth characteristics. Consulting prior studies on the relationship between PM2.5 and birthweight,6,15–17,22,23 and based on their potential to confound the relationship, we selected the following covariates a priori to include in our statistical modeling: maternal age (years), maternal race (white, black, Asian, American Indian, other), maternal marital status (married, not married), maternal smoking during or before pregnancy (yes, no), maternal education (highest level of education attained: less than high school, high school, some college, college, advanced degree beyond college), parity (first-born, not first-born), maternal diabetes (yes, no), gestational diabetes (yes, no), maternal chronic high blood pressure (yes, no), maternal high blood pressure during pregnancy (yes, no), Kessner index of adequacy of prenatal care (adequate, intermediate, inadequate, no prenatal care),24 mode of delivery (vaginal, forceps, vacuum, first cesarean birth, repeat cesarean birth, vaginal birth after cesarean birth), clinical gestational age (weeks), year of birth (one of 2001–2012), season of birth (spring, summer, autumn, winter), newborn sex (male, female), and Medicaid-supported prenatal care (yes, no). In addition, we controlled for Census block group level median household income and proportion of population that was black.25 Births missing address information (n = 23,093), with lower than a 500 g birthweight (n = 713), that were not live births (n = 8,155), nor singletons (n = 39,117), nor full-term (clinical gestational age between 37 and 44 weeks; n = 62,774) were excluded from analysis. We also excluded those with missing covariate data (n = 47,991), leading to a final sample size of 725,919. Our data usage was approved by the Massachusetts Department of Public Health and the human subjects committee at the Harvard T. H. Chan School of Public Health.
Exposure assessment
We obtained predictions for PM2.5 and four of its major constituents, EC, OC, nitrate, and sulfate from a model that incorporated outputs from the Goddard Earth Observing System Chemistry transport model in addition to meteorological and land use variables.19 Briefly, Goddard Earth Observing System Chemistry outputs were combined with the meteorological and land use variables then calibrated to speciation monitoring data using a backward propagation neural network, which allows for complex and nonlinear associations between model inputs. This model was used to predict daily PM2.5 and constituents mass concentrations at a 1 km × 1 km spatial resolution. Accuracy of the PM2.5 and constituents prediction model was assessed using 10-fold cross validation. The mean cross-validated R2, computed for each year by regressing monitored PM2.5 and constituents’ values against predicted values then averaged, was 0.85, 0.71, 0.69, 0.83, and 0.81 for PM2.5, EC, OC, nitrate, and sulfate, respectively.
Using the maternal residence information and reported clinical gestational age for each birth, we calculated average exposure to PM2.5 and four of its major constituents, EC, OC, nitrate, and sulfate during the pregnancy. The address information was geocoded by the Massachusetts Department of Public Health against TomTom Multinet using AccuMail address and ZIP code as the input address field and zone. For each geocode, we identified the matching 1 km2 grid from the PM2.5 and constituents data set. We then defined the entire pregnancy period as the relevant exposure window for each birth using the birthdate and clinical gestational age, which was determined by a clinician using ultrasound or physical examination during the latest prenatal visit or at birth. We averaged the daily PM2.5 and constituents predictions in the 1 km2 grid of maternal residence for the entire duration of pregnancy to ascertain exposure for each birth.
Statistical modeling
The goal of our statistical analysis was to determine the relative toxicities of the main constituents of PM2.5 on birthweight. To do this, we built multivariate linear regression models to estimate the effects of each of the four constituents on continuous birthweight. We first sought to determine the association between a single constituent and birthweight with adjustment for total PM2.5:
| (1) |
where the constituent was one of EC, OC, nitrate, and sulfate, and γ′X was the matrix of other model covariates. PM2.5 represents the remainder of PM2.5 not including the constituent. In our main analysis, we scaled the estimated effects per an interquartile range (IQR) increase per each pollutant, which is more representative of a real world scenario than mass scaling. If the coefficient for the constituent is larger in magnitude than that of PM2.5 (|β1| > |β2|), it means that an IQR increase of the constituent is more impactful on birthweight than an IQR increase in the remainder of PM2.5. In sensitivity analyses, we show results scaled to 1 µg/m3 increases in each pollutant.
We assessed the potential for each covariate to confound the results in Figure S1; http://links.lww.com/EE/A37; maternal race, parity, Census block group proportion of black population, Census block group median household income, maternal smoking, clinical gestational age, and year of birth were sources of potential confounding in the negative association between PM2.5 and birthweight. Although the models quantifying the associations of a single constituent with adjustment for PM2.5 inform us of the relative toxicity of one specific constituent relative to PM2.5, the effect estimates could be confounded by other constituents that covary with the single constituent.18 Thus, we also determined the association between a constituent and birthweight with adjustment for other constituents and PM2.5 in a multi–constituent model:
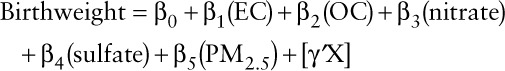 |
(2) |
We assessed the relative toxicity of each constituent on birthweight with this multi-constituent model that adjusts for PM2.5 and other covariates. For this multi-constituent model, we scaled estimates of effect to IQR increases in each respective pollutant. For comparison, we scaled estimates to 1 µg/m3 increases in each pollutant. As a sensitivity analysis, we estimated the effect of the constituents on the odds of term low birthweight (TLBW) and small for gestational age (SGA), which are binary outcomes, with multivariate logistic regression. TLBW was defined as being born under 2,500 g and SGA was defined as having a birthweight below the 10th percentile given sex and gestational age. Finally, because the most fetal weight gain occurs in the third trimester of pregnancy,6,11,26 we conducted a sensitivity analysis using third trimester averaged exposures.
Results
We summarize the characteristics of the 725,919 births in our analysis in Table 1. The mean maternal exposure to PM2.5 over the entire pregnancy was 10.3 µg/m3. EC, OC, nitrate, and sulfate accounted for about 70% of PM2.5. Mean exposures to OC and sulfate were highest at 2.8 µg/m3 each, followed by nitrate at 1.2 µg/m3 and EC at 0.5 µg/m3. Spearman correlations between PM2.5 and its constituents are given in Table S1; http://links.lww.com/EE/A37. Most PM2.5 and constituents were moderately-associated with each other except between PM2.5 and sulfate, which were strongly associated. More than two-thirds of mothers reported being married, less than a third received Medicaid support for prenatal care, and almost four-fifths were recorded having received adequate prenatal care according to the Kessner index. Just over 70% of the mothers were white and almost 90% had at least a high school education. Table S2; http://links.lww.com/EE/A37 shows the characteristics of births that were excluded from analysis due to missingness or exclusion criteria. On average, exposure to PM2.5 and its constituents (except EC) were slightly lower for the excluded births.
Table 1.
Characteristics of full-term live singleton births in Massachusetts from 2001 to 2012 (n = 725,919)a
Our first set of statistical models estimated the effects of EC, OC, nitrate, and sulfate on birthweight in separate regressions. Figure 1 illustrates the estimated effects on birthweight associated with an IQR increase in each of the constituents compared ith the remaining PM2.5 mixture excluding that constituent. The associations between each constituent and birthweight were negative, suggesting that exposure to each constituent was associated with lower birthweight. Focusing on EC (Figure 1: leftmost pane), the birthweight association for an IQR increase in the constituent (EC) was more negative at −10 g [95% confidence interval (CI) = −11 to −8 g] compared to an IQR increase in the remaining PM2.5 at −7 g (95% CI = −10 to −5 g). Stated differently, the associated decrease in birthweight with an IQR increase in EC exposure was slightly larger than the associated decrease in birthweight with an IQR increase in a PM2.5 mixture without EC. This suggests that an IQR increase in EC was more toxic than that of an IQR increase in PM2.5. This pattern of associations was not true in the regressions for OC, nitrate, and sulfate. For each of OC, nitrate, and sulfate, an IQR increase in each of these constituents was associated with a birthweight detriment smaller in magnitude compared to an IQR increase in the remaining PM2.5 mixture excluding the constituent. This suggests that an IQR increase in OC, nitrate, or sulfate was less toxic than an IQR increase in the remaining PM2.5 excluding each respective constituent. The differences in associations between each of these constituents and remaining PM2.5 was smallest for nitrate, followed by OC and sulfate. Point estimates and associated 95% confidence intervals are given in Table S2; http://links.lww.com/EE/A37. Sensitivity analyses found that when scaled to 1 µg/m3 increases in each pollutant, the results still suggest that EC was more toxic than the remaining PM2.5 (Figure S2 and Table S2; http://links.lww.com/EE/A37).
Figure 1.
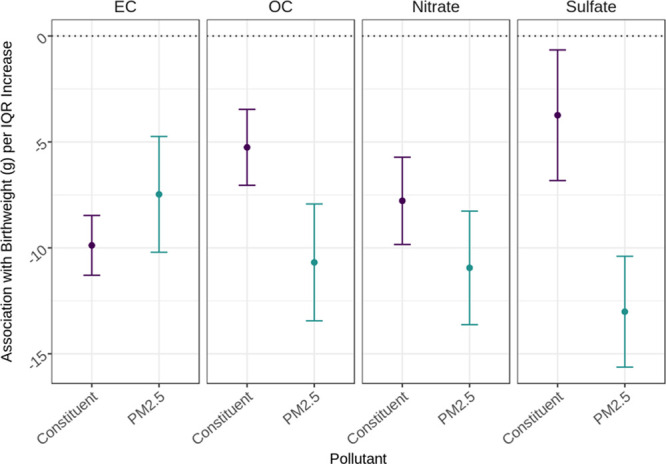
Estimated effects on birthweight per IQR increase in a PM2.5 constituent adjusted for PM2.5 in Massachusetts from 2001 to 2012 (n = 725,919). PM2.5 constituents include EC, OC, nitrate, and sulfate. Point estimate and 95% CI per individual constituent are in purple whereas those for PM2.5 are in teal. Four separate linear models for each of the four constituents were run and included adjustment for PM2.5 and the following covariates: maternal age, race, marital status, smoking, education, parity, chronic diabetes, gestational diabetes, chronic high blood pressure, high blood pressure during pregnancy, Kessner index of adequacy of prenatal care, mode of delivery, clinical gestational age, year of birth, newborn sex, and Medicaid-supported prenatal care. IQRs (µg/m3): PM2.5 = 2.0, EC = 0.2, OC = 1.4, nitrate = 0.5, and sulfate = 1.5.
Although Figure 1 illustrates the lower birthweights associated with four major PM2.5 constituents one at a time, Figure 2 shows the associations of the statistical model that simultaneous adjustment for all major constituents and PM2.5. With this multi-constituent model, an IQR increase in EC was associated with −9 g (95% CI = −11 to −8 g) difference in birthweight on average, in OC a 0 g (95% CI = −1 to 3 g) difference, in nitrate a −3 g (95% CI = −6 to −1 g) difference, in sulfate a 0 g (95% CI = −3 to 3 g) difference, and in remaining PM2.5 a −7 g (−10 to −4 g) difference. Thus, IQR increases in constituents were associated with the largest birthweight detriment if the constituent was EC, followed by remaining PM2.5, nitrate, sulfate, and OC. Estimates scaled to 1 µg/m3 increases also found that the largest decreased birthweight associated with increased EC exposure (Figure S3; http://links.lww.com/EE/A37). Point estimates and 95% CIs are found in Table S2; http://links.lww.com/EE/A37.
Figure 2.
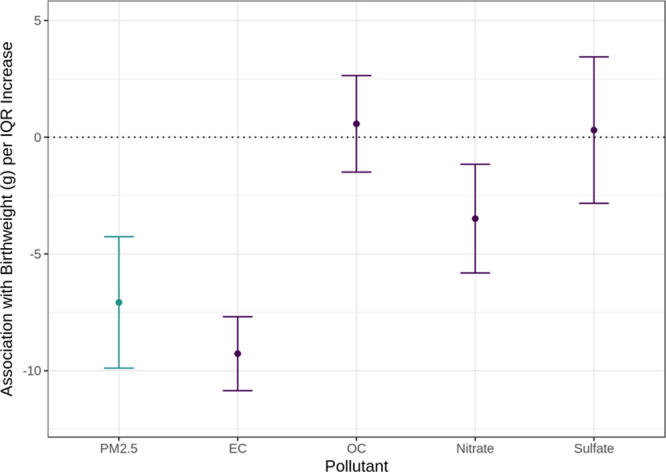
Estimated effects on birthweight per IQR increase in PM2.5, EC, OC, nitrate, and sulfate. PM2.5 constituents include EC, OC, nitrate, and sulfate in Massachusetts from 2001 to 2012 (n = 725,919). Point estimate and 95% CI per individual constituent are in purple while that per PM2.5 is in teal. A single multi-constituent linear regression with the four constituents and PM2.5 was run and included the following covariates: maternal age, race, marital status, smoking, education, parity, chronic diabetes, gestational diabetes, chronic high blood pressure, high blood pressure during pregnancy, Kessner index of adequacy of prenatal care, mode of delivery, clinical gestational age, year of birth, newborn sex, and Medicaid-supported prenatal care. IQRs (µg/m3): PM2.5 = 2.0, EC = 0.2, OC = 1.4, nitrate = 0.5, and sulfate = 1.5.
In sensitivity analyses, we explored associations with TLBW and SGA. In agreement with results of birthweight models, EC had the strongest estimated effects on odds of TLBW and SGA. The odds ratio for TLBW of an IQR increase in EC exposure was 1.039 (95% CI = 1.013–1.065) after adjustment for PM2.5 and other major constituents; for SGA, the odds ratio was 1.050 (95% CI = 1.037, 1.063) (Figures S4 and S5; http://links.lww.com/EE/A37). We additionally investigated exposures averaged to the third trimester of pregnancy. The first set of statistical models comparing the constituents individually found that increases in IQRs of EC and OC were each associated with larger birthweight detriments than remaining PM2.5 and that the difference between magnitudes was larger for EC and remaining PM2.5 (Figure S6; http://links.lww.com/EE/A37). Nitrate had comparable toxicity and sulfate had lower toxicity. With the multi-constituent model, the birthweight detriment associated with an IQR increase in EC was again the largest (Figure S7; http://links.lww.com/EE/A37). The association between OC and remaining PM2.5 was slightly more severe, and those of nitrate and sulfate were slightly less. In summary, the main and sensitivity analyses showed a consistent pattern that increased exposure to EC was associated with stronger detriments to birthweight than OC, nitrate, sulfate, and remaining PM2.5.
Discussion
In this study, we assessed the relative toxicity of maternal exposure to PM2.5 constituents during pregnancy on newborn birthweight. In a large cohort of full-term singleton live births in Massachusetts from 2001 to 2012, we found that PM2.5 exposure was negatively associated with birthweight and that the relative toxicities of four major PM2.5 constituents, EC, OC, nitrate, and sulfate, on birthweight compared to PM2.5 and to each other were different. While the negative association between PM2.5 and continuous birthweight is well-established,1–8 the relative impacts of PM2.5 chemical constituents compared to a PM2.5 mixture are less understood.5,6 Our results suggest that EC was slightly more detrimental to birthweight, thus more toxic, than the remainder of PM2.5 per IQR increase; OC, nitrate, and sulfate were each less toxic than their respective remaining PM2.5. The multi-constituent results also suggest that EC was most toxic, followed by nitrate, then close to equally by OC and sulfate. Sensitivity analyses using TLBW and SGA also show that EC was most toxic. When exposure averaged over the third trimester was used instead, EC was again more toxic than remaining PM2.5. OC was also more toxic than remaining PM2.5 when third-trimester exposures were used. With the multi-constituent model, EC was again the most toxic in the multi-constituent model but was followed by OC, then close to equally by nitrate and sulfate. Taken together, EC appeared to be the most toxic of the four major constituents analyzed. Nitrate followed in toxicity when exposures were averaged to the entire pregnancy; OC when third-trimester exposures were used. Sulfate was least detrimental to birthweight. Prior studies, although using different methodologies, also found a negative association between EC and birthweight.1,6,15 In one study, the negative association between EC and birthweight was strongest compared to those of OC, nitrate, and sulfate.6 Sulfate was also estimated to have the weakest detrimental effect in that study. Of note, EC derives primarily from traffic emissions,15 indicating that control of traffic-derived particles could be an effective strategy for reducing detrimental effects of PM2.5 on fetal growth. While the United States Environmental Protection Agency is responsible for general emission standards for vehicles,27 there is considerable room for local action to reduce traffic through strategies such as zoning, limiting personal vehicles, and increased mass transit.
Differences in toxicities of the constituents were expected given the proposed mechanisms of how maternal exposure to air pollution affects birthweight. These proposed main pathways are pulmonary and systemic inflammation, oxidative stress, and placental function impairment.6,27 Because different constituents of PM2.5 have different chemical properties; they can also interact differently with maternal lung tissue and have varying inflammatory potentials. Another mechanism through which PM2.5 is thought to impact birthweight is oxidative stress, which leads to DNA damage. Because the different chemical constituents of PM2.5 have different oxidative potentials, they could also have different propensities to cause DNA damage and affect the growing fetus to differing degrees. Although the inflammatory and oxidative stress mechanisms through which maternal exposure to PM2.5 affect the growing fetus are unconfirmed, it is highly plausible that different chemical constituents of PM2.5 would have different consequences on birthweight given current understanding of biological systems. The evidence from the current study and prior epidemiological studies support this claim. Our finding of higher toxicity from EC compared to OC could be due to differences in chemical properties and physical properties, which affect how these particles interact with biological membranes. On average, EC is typically smaller than OC, which makes it easier to reach the deepest parts of the lungs and cross into the bloodstream through the lung parenchyma.28,29 OC undergo secondary reactions more readily than EC to form secondary organic compounds, increasing its average size.
This study had several limitations. Starting with the PM2.5 constituents analyzed, we did not consider other constituents such as metals since EC, OC, nitrate, and sulfate make up the majority of total PM2.5. Metals have previously been associated with decreased birthweight and are known to persist in fetal tissues and have long-term health consequences.6,15 Thus, the relative toxicity by each constituent on birthweight could change with the inclusion of remaining PM2.5 constituents, but we do not expect the ranking of relative toxicities of the four major PM2.5 constituents to change (Figure 2). Another limitation was missingness or inaccurate reporting of maternal residential information. Bias resulting from this was likely low and nondifferential because as <10% were excluded because of missing residential information and PM2.5 constituents were not expected to be strongly associated with missingness. There remained potential that the reported maternal residence did not accurately reflect the maternal location during pregnancy. Unfortunately, we did not have information on maternal location other than reported residence. Not accounting for behaviors such as spending time at work would likely have biased our estimates away from the null if the work environments were located in more polluted areas compared to residential areas. Finally, unlike some prior studies, we did not estimate relative toxicity between trimester-specific PM2.5 constituent exposures and birthweight.1,6,15,17,30 Estimating trimester-specific effects would help to identify important exposure windows but was beyond the scope of this study, which focused on elucidating the relative toxicity of major PM2.5 constituents. Nonetheless, identifying important exposure windows remains a research need and is a future direction.
Our study also had several strengths. First, our exposure assignment used high-resolution data from a validated prediction model.19 Importantly, these predictions of PM2.5 and its constituents were created for use in epidemiological studies, and they have recently been used for assessing the relationship between PM2.5 constituents and other health outcomes, such as DNA methylation age.31–33 Combined with geocoded addresses from the birth records, we achieved a large sample size with a relatively small number of births excluded because of missing exposure data. Prior studies that relied on monitoring data had to exclude births due to low temporal or spatial resolution, and some simply had exposure data spatially averaged to larger geographic areas compared to the 1 km2 exposure data in this study.1,3,8,15,30 Taken together, exposure assignment in this study should have had lower misclassification error compared to prior studies. Second, our statistical modeling strategy was appropriate for assessing the relative toxicity of PM2.5 constituents. Several prior studies assessed the association between PM2.5 constituents and birthweight without adjustment for total PM2.5,1,6,8,15–17 which meant that the estimates were confounded by total PM2.5 and by other constituents with high correlation to the single constituent in the model.18 The single constituent models adjusted for PM2.5 to limit confounding from total PM2.5 and the multi-constituent model additionally limited confounding from other constituents. These features of our statistical approach furthered our understanding of relative toxicities of major PM2.5 constituents on birthweight, and added to the body of knowledge to consider for pollution control policy.
Conclusion
Maternal exposure to PM2.5 during pregnancy was negatively associated with birthweight and the relative toxicities of four major constituents, EC, OC, nitrate, and sulfate, were different. Our results suggest that EC was most toxic, followed by nitrate, OC, and lastly by sulfate. Our results were not likely to be confounded by PM2.5, which was adjusted for in our statistical analysis. As birthweight is an important predictor for health over the life course for newborns, our findings can inform pollution control policy to minimize long-term health detriments from air pollution.
Conflicts of interest statement
The authors declares that they have no conflicts of interest with regard to the content of this report.
Supplementary Material
Footnotes
Published online 9 April 2019
Supplemental digital content is available through direct URL citations in the HTML and PDF versions of this article (www.epidem.com).
Sponsorships or competing interests that may be relevant to content are disclosed at the end of the article.
This publication was made possible by USEPA grants RD-834798, RD-835872, RD-83615601, and NIH/NIMHD grants P50MD010428 and R00CA201542. Its contents are solely the responsibility of the grantee and do not necessarily represent the official views of the USEPA or the NIH. Further, USEPA and NIH do not endorse the purchase of any commercial products or services mentioned in the publication.
Data source and computer code: The fine particulate air pollution exposure data were based on publically-available remote sensing, land use, and meteorological variables. Details on these data have previously been published and are referenced in the main text. The births outcome data can be requested from the Massachusetts Department of Public Health. The statistical analysis was based on publically-available statistical software referenced in the main text.
References
- 1.Basu R, Harris M, Sie L, Malig B, Broadwin R, Green R. Effects of fine particulate matter and its constituents on low birth weight among full-term infants in California. Environ Res 201412842–51 [DOI] [PubMed] [Google Scholar]
- 2.Dadvand P, Parker J, Bell ML, et al. Maternal exposure to particulate air pollution and term birth weight: a multi-country evaluation of effect and heterogeneity. Environ Health Perspect 2013121267–373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ebisu K, Bell ML. Airborne PM2.5 chemical components and low birth weight in the northeastern and mid-Atlantic regions of the United States. Environ Health Perspect 20121201746–1752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kloog I, Melly SJ, Ridgway WL, Coull BA, Schwartz J. Using new satellite based exposure methods to study the association between pregnancy PM2.5 exposure, premature birth and birth weight in Massachusetts. Environ Health 20121140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stieb DM, Chen L, Eshoul M, Judek S. Ambient air pollution, birth weight and preterm birth: a systematic review and meta-analysis. Environ Res 2012117100–111 [DOI] [PubMed] [Google Scholar]
- 6.Sun X, Luo X, Zhao C, et al. The associations between birth weight and exposure to fine particulate matter (PM2.5) and its chemical constituents during pregnancy: a meta-analysis. Environ Pollut 201621138–47 [DOI] [PubMed] [Google Scholar]
- 7.Erickson AC, Ostry A, Chan LH, Arbour L. The reduction of birth weight by fine particulate matter and its modification by maternal and neighbourhood-level factors: a multilevel analysis in British Columbia, Canada. Environ Health 20161551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gray SC, Edwards SE, Schultz BD, Miranda ML. Assessing the impact of race, social factors and air pollution on birth outcomes: a population-based study. Environ Health 2014134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Belbasis L, Savvidou MD, Kanu C, Evangelou E, Tzoulaki I. Birth weight in relation to health and disease in later life: an umbrella review of systematic reviews and meta-analyses. BMC Med 201614147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boulet SL, Schieve LA, Boyle CA. Birth weight and health and developmental outcomes in US children, 1997–2005. Matern Child Health J 201115836–844 [DOI] [PubMed] [Google Scholar]
- 11.Demerath EW, Choh AC, Czerwinski SA, et al. Genetic and environmental influences on infant weight and weight change: the Fels Longitudinal Study. Am J Hum Biol 200719692–702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hack M, Klein NK, Taylor HG. Long-term developmental outcomes of low birth weight infants. Future Child 19955176–196 [PubMed] [Google Scholar]
- 13.Korten I, Ramsey K, Latzin P. Air pollution during pregnancy and lung development in the child. Paediatr Respir Rev 20172138–46 [DOI] [PubMed] [Google Scholar]
- 14.Reyes L, Mañalich R. Long-term consequences of low birth weight. Kidney Int Suppl 200597S107–S111 [DOI] [PubMed] [Google Scholar]
- 15.Bell ML, Belanger K, Ebisu K, et al. Prenatal exposure to fine particulate matter and birth weight. Epidemiol Camb Mass 201021884–891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Darrow LA, Klein M, Strickland MJ, Mulholland JA, Tolbert PE. Ambient air pollution and birth weight in full-term infants in Atlanta, 1994–2004. Environ Health Perspect 2011119731–737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ebisu K, Belanger K, Bell ML. The association between airborne PM2.5 chemical constituents and birth weight-implication of buffer exposure assignment. Environ Res Lett 20149Available at: https://iopscience.iop.org/article/10.1088/1748-9326/9/8/084007/meta [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mostofsky E, Schwartz J, Coull BA, et al. Modeling the association between particle constituents of air pollution and health outcomes. Am J Epidemiol 2012176317–326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Di Q, Koutrakis P, Schwartz J. A hybrid prediction model for PM2.5 mass and components using a chemical transport model and land use regression. Atmos Environ 2016131390–399 [Google Scholar]
- 20.Laurent O, Hu J, Li L, et al. Sources and contents of air pollution affecting term low birth weight in Los Angeles County, California, 2001–2008. Environ Res 2014134488–495 [DOI] [PubMed] [Google Scholar]
- 21.Wilhelm M, Ghosh JK, Su J, Cockburn M, Jerrett M, Ritz B. Traffic-related air toxics and term low birth weight in Los Angeles County, California. Environ Health Perspect 2012120132–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ng C, Malig B, Hasheminassab S, Sioutas C, Basu R, Ebisu K. Source apportionment of fine particulate matter and risk of term low birth weight in California: exploring modification by region and maternal characteristics. Sci Total Environ 2017605–606647–654 [DOI] [PubMed] [Google Scholar]
- 23.Hao H, Chang HH, Holmes HA, et al. Air pollution and preterm birth in the U.S. state of georgia (2002–2006): associations with concentrations of 11 ambient air pollutants estimated by combining community multiscale air quality model (CMAQ) simulations with stationary monitor measurements. Environ Health Perspect 2016124875–880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kessner DM, Singer J, Kalk CW, Shlesinger ER. Infant Death: An Analysis by Maternal Risk and Health Care. Chapter 2 1973Washington, DC: Institute of Medicine and National Academy of Sciences; 22–30 [Google Scholar]
- 25.U.S. Census Bureau. American Community Survey, 2010 5-Year Estimates. 2017 Available at: https://factfinder.census.gov. Accessed 29 November 2017.
- 26.Slama R, Darrow L, Parker J, et al. Meeting report: atmospheric pollution and human reproduction. Environ Health Perspect 2008116791–798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.United States Environmental Protection Agency. Regulations for Smog, Soot, and Other Air Pollution from Passenger Cars & Trucks. 2016 Available at: https://www.epa.gov/regulations-emissions-vehicles-and-engines/regulations-smog-soot-and-other-air-pollution-passenger. Accessed 29 October 2018.
- 28.Wang GH, Wei NN, Liu W, et al. [Size distributions of organic carbon (OC) and elemental carbon (EC) in Shanghai atmospheric particles]. Huan Jing Ke Xue 2010311993–2001 [PubMed] [Google Scholar]
- 29.Sahan E, ten Brink HM, Weijers EP. Carbon in Atmospheric Particulate Matter. 2008Available at: http://www.ecn.nl/docs/library/report/2008/e08060.pdf. Accessed 29 October 2018
- 30.Geer LA, Weedon J, Bell ML. Ambient air pollution and term birth weight in Texas from 1998 to 2004. J Air Waste Manag Assoc 2012621285–1295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nwanaji-Enwerem JC, Colicino E, Dai L, et al. miRNA processing gene polymorphisms, blood DNA methylation age and long-term ambient PM2.5 exposure in elderly men. Epigenomics 201791529–1542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nwanaji-Enwerem JC, Dai L, Colicino E, et al. Associations between long-term exposure to PM2.5 component species and blood DNA methylation age in the elderly: the VA normative aging study. Environ Int 201710257–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peng C, Cayir A, Sanchez-Guerra M, et al. Associations of annual ambient fine particulate matter mass and components with mitochondrial DNA abundance. Epidemiology 201728763–770 [DOI] [PMC free article] [PubMed] [Google Scholar]


