Supplemental Digital Content is available in the text.
Video abstract: http://links.lww.com/WNR/A520Keywords: drug-resistant epilepsy, functional MRI, functional connectivity, generalized tonic-clonic seizure, hippocampus
Abstract
The aim of this study was to better understand the imaging features of drug-resistant epilepsy (DRE), especially in idiopathic generalized tonic-clonic seizure (GTCS), as well as to discover the associated mechanisms and functional connectivity (FC). A total of 31 idiopathic generalized epilepsy-GTCS patients and 17 healthy controls were enrolled. For each patient, resting-state functional MRI was performed. After a 12-month follow-up observation, patients were further divided into either drug-resistant (DR) or drug-sensitive (DS) groups. Compared to the DS group, DR patients had previously received more types of antiepileptic drugs and had taken more types of failed antiepileptic drugs. There were distinct FC changes toward the left thalamus, left putamen, left precuneus, and right precentral gyrus in the left hippocampus between DR and DS patients. FCs in the DR group largely decreased or remained unchanged, while DS patients exhibited compensatory enhancement. Disease duration was negatively correlated with FC between the left hippocampus and the left thalamus–putamen in patients with DRE. Further, DRE patients had an extremely high area under the curve (0.978) and a cut-off FC between the left hippocampus and thalamus–putamen of 0.282. Together, hippocampal FCs in patients with DR GTCS were impaired and time-dependently correlated with disease duration. Hippocampal FCs in DS patients showed overall compensatory enhancement, which could be used as a sensitive and specific marker to identify and predict DR GTCS.
Introduction
Epilepsies are a group of brain diseases characterized by repetitive seizure activities, which affect nearly 1% of the population in terms of lifetime prevalence 1. Approximately 17% of patients die from epilepsy onset 2. More than one-third of epilepsies are drug-resistant epilepsies (DREs) 3, which account for the largest burden of epilepsy, have a poor prognosis, and they further induce increased morbidity and mortality 4–6.
The current understanding of epilepsy is deepening with the application of noninvasive neuroimaging techniques that explore different aspects, such as volume changes or spontaneous functional organization. Functional connectivity (FC) based on functional MRI (fMRI) is one of the most useful markers for predicting epileptogenic events 7–9. For example, abnormal fundamental dynamic interactions and dysconnectivity are associated with the subcortical and cerebellar regulation of frontoparietal dysfunction in frontal-lobe epilepsy 7. There are also connectivity changes in temporal-lobe epilepsy with dysfunctional networks identified by changes in hippocampal connectivity 8.
Damaged hippocampal FC is involved in various diseases; patients with systemic lupus erythematosus, for instance, often have central nervous system involvement, and improving hippocampal connectivity in systemic lupus erythematosus patients can compensate for central memory impairment 10. In addition, Baur et al. 11 confirmed that insula–amygdala, resting-state FC could be assessed easily and in a straightforward manner, and has great potential to serve as a biomarker for anxiety. Idiopathic generalized epilepsy (IGE) is characterized by the widespread, generalized spike-and-wave/polyspike–waves and undetectable focal anatomical brain abnormalities. It has been widely accepted that thalamic–cortical network abnormalities play crucial roles in IGE development. Generalized tonic-clonic seizure (GTCS) is the most common subtype of IGE. Thus far, its pathophysiological mechanisms remain unclear, and few studies probed the mechanism of hippocampal FC abnormality in drug-resistant (DR) GTCS. This study aimed to examine resting-state-fMRI connectivity levels between the hippocampus and relative regions and to explore the relationship between hippocampal FC changes and DRE.
Materials and methods
Patients
This retrospective study was approved by the Ethics Committee of the Affiliated Drum Tower Hospital of Nanjing University Medical School (Nanjing, People’s Republic of China). Patients that had epilepsy from 2013 to 2016 were enrolled according to the following inclusion criteria: (a) those with a diagnosis of IGE-GTCS, as determined by the 2017 International League Against Epilepsy classification scheme 12; (b) those aged 14–50 years old; (c) those without structural abnormalities of the brain, as based on 3.0T resting-state-fMRI examination; (d) those with a normal intelligence level, as determined by neuropsychological assessment with Mini-Mental State Examination and Montreal Cognitive Assessment scores; (e) those who do not have growth retardation; and (f) those who do not have other diseases that might cause seizures. The exclusion criteria included: (a) those with intracranial tumors; (b) those with a history of severe brain trauma; (c) those with a history of drug or alcohol abuse; and (d) those with the presence of other mental diseases.
A total of 17 normal, age-matched and sex-matched control patients were enrolled. Healthy controls did not have any personal or family history of neural/mental illness. Before our analysis, all patients had signed an informed consent form. All participants completed the Edinburgh Handedness Inventory 13, which determines hand preference during everyday tasks. In the inventory, a list of daily activities was shown in the left and right columns. Scores of 1 or 2 were written in the respective column according to the degree of dominant hand use. An individual’s score was calculated using the following formula:
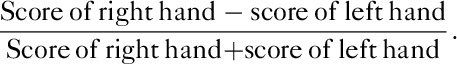 |
Scores, therefore, ranged from –1 to 1. All patients in this study scored 1, thus they were identified as right-handed. After a 12-month follow-up observation, the GTCS patients were further divided into a DR or drug-sensitive (DS) group. Imaging acquisition was performed 1–3 days prior to treatment or during treatment with standard medications, when necessary.
Drug-resistant epilepsy identification
Kwan et al. 9 proposed that the DRE phenotype should be determined using the following criteria. DRE was defined as a failure of adequate trials of two tolerated, and appropriately chosen and used antiepileptic drug (AED) schedules (whether as monotherapies or in combination) to achieve sustained seizure freedom. Specifically, if no seizure onset was observed during the 12-month follow-up for a duration that was three times the longest interval observed for a given individual, this patient was considered to be DS. If a relapse was found in the 12-month follow-up period, or if the duration was less than three times that of the longest interval for a given patient, this patient was classified into the DR group. Patients with the following situations were identified as undefined cases and were thus excluded: (a) those who withdrew due to intolerable adverse reactions; and (b) those who failed to receive enough of a therapeutic dose (<3 months or the defined daily dose) for personal reasons.
Image acquisition and preprocessing
All participants were referred for structural and resting-state-fMRI by clinicians at our hospital using the Philips 3.0T Magnetic Resonance Imaging System (Koninklijke Philips N.V., Amsterdam, The Netherlands). fMRI imaging data were acquired using the single-shot echo planar imaging sequence with the following parameters: repetition time=2000 ms; echo time=30 ms; flip angle=90°; field of view=192 mm×192 mm×140 mm; voxel size=3 mm×3 mm×4mm; matrix=64 mm×64 mm; 35 slices; and a total of 230 time points. Next, data were pre-processed within the Matlab Platform environment applying the Resting State Brain Function Data Assistance Processing tool (DPARSF V4.1). Filtered imaging data were processed to remove the sources of spurious variance, including head motion, cerebrospinal fluid signal, and white-matter signals. Data from the first 10 time points were removed, and time-level and head-motion corrections were performed using data from the remaining 220 time points. Spatial normalization was performed using the Montreal Neurological Institute template. The voxel volume was 3 mm×3 mm×3 mm. Next, spatial smoothing was performed, followed by removing linear drift. Patients with head displacement more than 1.5 mm or at an angle more than 1.5° were excluded.
Functional connectivity analysis
For seed-based FC analysis, the bilateral hippocampus was used as the seed regions of interest. The time series of the voxel in each region of interest were averaged, followed by correlation with the time series of each other voxel across the entire brain. The correlation value was z-transformed for each sample. The z-transformed FC maps were compared among the DR, DS, and normal control groups by one-way analysis of variance (ANOVA; AlphaSim corrected for multiple comparisons; P<0.01), resulting in significantly different regions employed as the mask for the next analysis. Subsequently, an assessment of FC strength between the two groups was performed using a two-samples t-test (AlphaSim corrected; P<0.05).
Statistical analysis
SPSS 18.0 (IBM Corporation, Armonk, New York, USA) was used for statistical analyses. Among groups, the clinical features were compared using χ2 (for frequencies), independent samples t-test or ANOVA (for quantitative data). Welch ANOVA test was used for the comparison of age at imaging acquisition (nonhomogeneous variances). The quantitative data for each group were expressed as the mean±SD. Different hippocampal FC levels were analyzed among groups, as mentioned previously. Spearman’s correlation analysis was applied to reveal the relationship between disease duration and FC levels. The receiver operating characteristic (ROC) curve was drawn to assess the diagnostic value of the hippocampal FC for DRE. A P value less than 0.05 was considered statistically significant.
Results
Clinical information
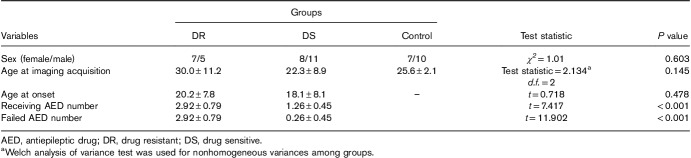
For GTCS patients, 31 individuals were excluded (four due to poor imaging quality of the resting-state data and 27 for an indefinite drug response); thus, the data of 31 patients were collected for analysis. As shown in Table 1, the 31 patients were divided into two groups according to their therapeutic outcomes, including 19 (11 males and eight females) in the DS group and 12 (five males and seven females) in the DR group. The DR group showed a slightly older age at onset (20.2±7.8 years) and imaging acquisition (30.0±11.2 years) when compared with those in the DS group (18.1±8.1 and 22.3±8.9 years, respectively), but this difference was not statistically significant. The mean epilepsy durations were slightly similar between the two epileptic groups (t=1.940, P>0.05). Compared to DS patients, DR patients had used more types of failed AEDs (t=11.902, P<0.001) or they had been taking more types of AEDs (t=7.417, P<0.001).
Table 1.
Clinical features of three groups
Different functional connectivity of the right hippocampus among groups
First, the FC of the right hippocampus for each patient was acquired using the Matlab Platform. Among all areas, the FCs between the right precentral gyrus and the right hippocampus showed significant differences among the three groups (F=13.562, P<0.001, Fig. 1a and b). The DS group, but not the DR group, had a significantly enhanced FC level compared to the control group (t=4.73, P<0.001; Fig. 1a and c). There was a highly significant difference between the two GTCS groups (t=3.85, P=0.001; Fig. 1a and d).
Fig. 1.
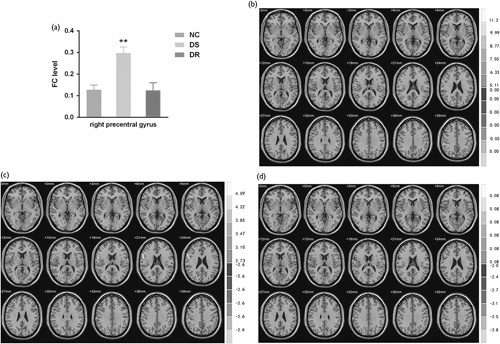
Differences in the functional connectivity (FC) levels of the right hippocampus among groups. (a) Statistical comparison of FC toward the right precentral gyrus among the control, drug-sensitive (DS), and drug-resistant (DR) groups. The DS group, but not the DR group, had a significantly enhanced FC level compared to the normal control (NC) group. There was a highly significant difference between the two GTCS groups. (b) Regions with differential FCs from the right hippocampus among the three groups; (c) FC between the right hippocampus and the right precentral gyrus was enhanced in the DS group versus controls; (d) FC between the right hippocampus and the right precentral gyrus was attenuated in the DR group versus the DS group. **P<0.01 versus the NC or DR groups.
Different functional connectivity of the left hippocampus among groups
Differently, the FCs of the left hippocampus toward the relative brain regions showed distinct trends between the DS and DR patients. The FC toward the left thalamus in the DR group was significantly attenuated compared to controls (t=−2.69, P=0.01; Fig. 2a and d) and the DS group (t=−5.48, P<0.001; Fig. 2a and e), respectively, while that in the DS group was slightly enhanced (t=2.05, P=0.048; Fig. 2a and c). Similarly, the left hippocampus/left putamen FC in the DR group was downregulated (t=−3.13, P<0.01 vs. control; Fig. 2a and d), and that in the DS group was slightly upregulated (t=2.07, P=0.046 vs. control; Fig. 2a and c). Moreover, the left hippocampus/left precuneus FC level was slightly suppressed in the DR group (t=−2.10, P=0.045 vs. control; Fig. 2a and d), but it was enhanced in the DS group (t=3.308, P<0.01 vs. control; Fig. 2a and c). Consistent with the right hippocampus, the FC towards the right precentral gyrus was dramatically upregulated in the DS group (t=2.863, P<0.01 vs. control; Fig. 2a and c), but it remained unchanged in the DR group (t=0.279, P>0.05; Fig. 2a and d). Collectively, there were distinct left hippocampal FC changes between the DR and DS patients, insofar as the FC levels in the DR group largely decreased or remained unchanged, while these parameters exhibited compensatory enhancement in DS patients.
Fig. 2.
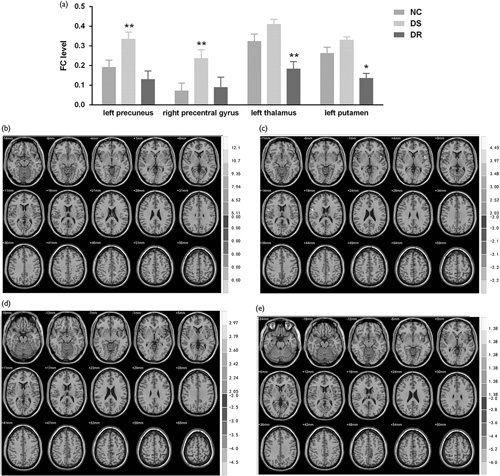
Differences in the functional connectivity (FC) levels from the left hippocampus among groups. (a) Statistical comparison of FC toward the left putamen, left thalamus, left precuneus, and right precentral gyrus among the three groups. FCs in the drug-resistant (DR) group largely decreased or remained unchanged, while they exhibited compensatory enhancement in drug-sensitive (DS) patients. (b) Regions with differential FCs from the right hippocampus among three groups; (c) FCs between the left hippocampus and the right precentral gyrus, left putamen, and left precuneus were enhanced in the DS group versus normal control (NC); (d) FCs between the left hippocampus and the left thalamus/left putamen demonstrated significant attenuation in the DR versus NC groups. The FC level between the left hippocampus and the left precuneus decreased slightly in the DR group versus the NC group. (e) FCs between the left hippocampus and left thalamus/left putamen/left precuneus were attenuated in the DR group compared to the DS group. *P<0.05 versus the NC; **P<0.01 versus the NC.
The prognostic value of functional connectivity between the left hippocampus and thalamus–putamen
Based on the differential parameters described above, the FC between the left hippocampus and the thalamus–putamen (FC-LHTP; thalamus–putamen as an entire region compared to the seeds) was further selected as one of the diagnostic and prognostic markers of GTCS, which was significantly different between groups (F=18.548, P<0.001, Fig. 3a). As expected, this index was decreased in DR patients (t=−3.320, P<0.01 vs. control), but it was increased in DS patients (t=2.335, P<0.05 vs. control). Afterward, we analyzed the correlation between disease duration and FC-LHTP in the DR group. Consistent with the above conclusion, there was a significantly negative linear correlation between disease duration and FC-LHTP (r=–0.538; P<0.01; Fig. 3b). This finding confirmed that impaired FC could be an indicator of epilepsy severity. Besides, a ROC curve was drawn to assess the diagnostic value of hippocampal FC for DRE, with an extremely high area under the curve (0.987; P<0.05; Fig. 3c). This ROC curve had a cut-off FC of 0.282 with 94.7% sensitivity and 91.7% specificity. Together, a lower FC level of FC-LHTP implied a higher risk of DRE, especially for those with an FC level less than 0.282.
Fig. 3.
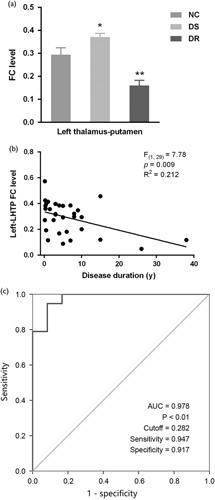
The prognostic value of functional connectivity (FC) between the left hippocampus and the left thalamus–putamen. (a) The FC between the left hippocampus and thalamus–putamen (FC-LHTP) was significantly different among groups; (b) the negative linear correlation between disease duration and FC-LHTP; (c) the receiver operating characteristic curve to assess the diagnostic value of hippocampal FC for DRE, with a high area under the curve (0.978; P<0.01) and a cutoff FC of 0.282. *P<0.05 versus normal control (NC); **P<0.01 versus NC.
Discussion
In this study, we investigated the differences in resting-state networks between DR and DS patients with IGE-GTCS and pointed out that some cases of DRE were associated with FC changes and neurophysiology. First, abnormal hippocampal FC levels were observed in GTCS patients compared to controls. Second, DS patients had significantly enhanced FCs towards different regions, while DR patients showed unchanged or attenuated FCs. Additionally, a strong, negative correlation was found between FC-LHTP and GTCS duration, and a 0.282 cutoff value of FC implied a high risk of DRE.
Hippocampal connectivities are related to various diseases in different contexts. For instance, structural atrophies in the hippocampus and aberrant patterns of FCs between the hippocampus and the rest of the brain were important characteristics of Alzheimer’s disease 14. Besides, the resting-state FC of the anterior insula and basolateral amygdala can serve as a biomarker for anxiety 11.
The hippocampus is also closely related to learning and memory functions, as were the regions linked to the hippocampus in our results. For example, episodic source memory is related to a functional network that includes the posterior precuneus 15. It is known that both the precuneus and the left inferior prefrontal cortex are important for the regeneration of rich episodic contextual associations 16. Lesions in the right precentral gyrus may cause speech difficulties, while lesions in the medial area of the left putamen may cause learning and memory deficits 17. Based on our findings, GTCS onset may lead to cognitive impairments, such as attention, memory, and language dysfunctions. In line with the findings of our study, these effects can be deduced by many published articles 18–20.
Although this is the first study to explore the relationship between hippocampal FC levels and DRE development in GTCS, our novel findings can be partially supported by published works. In the aspect of hippocampal FC variations in epilepsy, patients with nonepileptic seizures were reported to display lower cognitive performance and higher dissociation scores, as well as stronger connectivity values between areas involved in emotion (insula), executive control (inferior frontal gyrus and parietal cortex), and movement (precentral sulcus) 21. Based on a gamma-butyrolactone rat model of absence seizures, there was an overall increase in FC across most regions of the hippocampus as related to the thalamocortical circuitry 22.
Indeed, hippocampal pathology shares some relationships with DRE. It has been repeatedly noticed that DRE may reflect hippocampal sclerosis 23–27. Loss of neural stem cells or newborn neural cells in the sclerotic hippocampus were found 24, which may underlie the decreased FC levels in DRE. Also, the herpes virus can play a pathological role in DRE patients with hippocampal sclerosis 25. Recently, DRE was also proposed to be induced in different regions of the hippocampal formation, including regions that are severely affected by the neuronal loss 25. Together, hippocampal pathology may serve to directly explain our findings, and it is reasonable to take advantage of hippocampal functional changes to evaluate a potential resistance to AEDs.
Thus far, this is the first study to observe that the FC level between the hippocampus and thalamus–putamen is one of the most sensitive and specific markers of DRE. Scholars have observed that juvenile myoclonic epilepsy patients exhibited decreased gray-matter volume in the left putamen, the right hippocampus, and the right thalamus 28. The thalamus is an essential node involved in epilepsy networks; however, in genetic generalized epilepsy, thalamic structural abnormalities were revealed to be an intrinsic feature – but not a consequence – of AEDs or disease duration 29. Conversely, progressive myoclonic epilepsy type 1, an autosomal recessively inherited neurodegenerative disorder, was reportedly characterized by significant textural differences in the thalamus and right putamen. However, it currently remains unclear whether impairment of these regions confers FC abnormality. Anyhow, the negative correlation between FC-LHTP and the duration of GTCS in DR individuals provides new insights into the pathophysiological mechanisms underlying GTCS. This finding implied a gradually decreased FC along with GTCS deterioration and resistance development.
Still, there are some limitations in this study. First, some patients may have theoretically experienced some cognitive impairments due to the presence of abnormal hippocampal FCs, especially in the DR group. We did not perform cognitive assessments across different dimensions (i.e. only the Mini-Mental State Examination and Montreal Cognitive Assessment scales were used to exclude cognitive impairments), and this will be carried out in future work. Second, the sample sizes of the three groups were small. Third, there are still controversies related to whether epileptogenesis is characterized by neuronal loss, or progressive hippocampal and extrahippocampal atrophy, and whether there is a strong relationship between the severity and duration of epilepsy and hippocampal atrophy. We are still unsure about the actual role of hippocampal FC variation in GTCS or DRE. Lastly, we did not investigate the relationship between hippocampal FC and resistance to any specific drug, which may deepen the understanding of the mechanisms underlying the roles of the connectivity between the hippocampus and other specific regions in the brain.
Conclusion
The hippocampal FCs in patients with DR GTCS were impaired, and impairment degree correlated with disease duration. The FCs in DS patients showed overall compensatory enhancement. Therefore, the hippocampal FC can be used as a sensitive and specific marker to identify and predict DR GTCS.
Supplementary Material
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's website, www.neuroreport.com.
Acknowledgements
English-language editing of this manuscript was provided by Journal Prep Services.
This work was supported by National Natural Science Foundation of China (grant number: 81301198), the Health Institute of Nanjing (grant number: ZKX 16038).
Conflicts of interest
There are no conflicts of interest.
Reference
- 1.Fisher RS, Carlos A, Alexis A, Alicia B, Helen CJ, Elger CE, et al. ILAE official report: a practical clinical definition of epilepsy. Epilepsia 2014; 55:475–482. [DOI] [PubMed] [Google Scholar]
- 2.Ficker DM. Sudden unexplained death and injury in epilepsy. Epilepsia 2000; 41 (Suppl 2):S7–S12. [DOI] [PubMed] [Google Scholar]
- 3.Brodie MJ, Barry SJ, Bamagous GA, Norrie JD, Kwan P. Patterns of treatment response in newly diagnosed epilepsy. Neurology 2012; 78:1548–1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mann MW, Pons G. Drug resistance in partial epilepsy: epidemiology, mechanisms, pharmacogenetics and therapeutical aspects. Neurochirurgie 2008; 54:259. [DOI] [PubMed] [Google Scholar]
- 5.Sillanpää M, Schmidt D. Is incident drug-resistance of childhood-onset epilepsy reversible? A long-term follow-up study. Brain 2012; 135:2256. [DOI] [PubMed] [Google Scholar]
- 6.Voll A, Hernández-Ronquillo L, Buckley S, Téllez-Zenteno JF. Predicting drug resistance in adult patients with generalized epilepsy: a case–control study. Epilepsy Behav 2015; 53:126–130. [DOI] [PubMed] [Google Scholar]
- 7.Klugah-Brown B, Luo C, He H, Jiang S, Armah GK, Wu Y, et al. Altered dynamic functional network connectivity in frontal lobe epilepsy. Brain Topogr 2019; 32:394–404. [DOI] [PubMed] [Google Scholar]
- 8.Haneef Z, Lenartowicz A, Yeh HJ, Levin HS, Engel J, Jr, Stern JM. Functional connectivity of hippocampal networks in temporal lobe epilepsy. Epilepsia 2014; 55:137–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kwan P, Arzimanoglou AT, Berg A, Brodie MJ, Allen HW, Mathern G, et al. Definition of drug resistant epilepsy: consensus proposal by the ad hoc Task Force of the ILAE Commission on Therapeutic Strategies. Epilepsia 2010; 51:1069–1077. [DOI] [PubMed] [Google Scholar]
- 10.Shapiralichter I, Vakil E, Litinsky I, Oren N, Glikmannjohnston Y, Caspi D, et al. Learning and memory-related brain activity dynamics are altered in systemic lupus erythematosus: a functional magnetic resonance imaging study. Lupus 2013; 22:562–573. [DOI] [PubMed] [Google Scholar]
- 11.Baur V, Hänggi J, Langer N, Jäncke L. Resting-state functional and structural connectivity within an insula–amygdala route specifically index state and trait anxiety. Biol Psychiatry 2013; 73:85–92. [DOI] [PubMed] [Google Scholar]
- 12.Scheffer IE, Berkovic S, Capovilla G, Connolly MB, French J, Guilhoto L, et al. ILAE classification of the epilepsies: position paper of the ILAE commission for classification and terminology. Epilepsia 2017; 58:512–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 1971; 9:97–113. [DOI] [PubMed] [Google Scholar]
- 14.Kim J, Kim YH, Lee JH. Hippocampus–precuneus functional connectivity as an early sign of Alzheimer’s disease: a preliminary study using structural and functional magnetic resonance imaging data. Brain Res 2013; 1495:18–29. [DOI] [PubMed] [Google Scholar]
- 15.Lundstrom BN, Petersson KM, Andersson J, Johansson M, Fransson P, Ingvar M. Isolating the retrieval of imagined pictures during episodic memory: activation of the left precuneus and left prefrontal cortex. Neuroimage 2003; 20:1934–1943. [DOI] [PubMed] [Google Scholar]
- 16.Lundstrom BN, Ingvar M, Petersson KM. The role of precuneus and left inferior frontal cortex during source memory episodic retrieval. Neuroimage 2005; 27:824–834. [DOI] [PubMed] [Google Scholar]
- 17.Kengo M, Takahiro I, Nobuhiro O, Atsushi N, Mitsuru S, Hiromichi K. A case of agrammatism due to cerebral infarction of the middle-lower part of the right precentral gyrus. Rinsho Shinkeigaku 2009; 49:414–418. [DOI] [PubMed] [Google Scholar]
- 18.Zhu L, Li Y, Wang Y, Li R, Zhang Z, Lu G, et al. Aberrant long-range functional connectivity density in generalized tonic-clonic seizures. Medicine 2016; 95:e3893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bailet LL, Turk WR. The impact of childhood epilepsy on neurocognitive and behavioral performance: a prospective longitudinal study. Epilepsia 2010; 41:426–431. [DOI] [PubMed] [Google Scholar]
- 20.Caroline H, Sauerwein HC, Bertrand DT, Maryse L. Idiopathic epileptic syndromes and cognition. Neurosci Biobehav Rev 2006; 30:85–96. [DOI] [PubMed] [Google Scholar]
- 21.Van der Kruijs SJ, Bodde NM, Vaessen MJ, Lazeron RH, Kristl V, Paul B, et al. Functional connectivity of dissociation in patients with psychogenic non-epileptic seizures. J Neurol Neurosurg Psychiatry 2012; 83:239–247. [DOI] [PubMed] [Google Scholar]
- 22.Mousavi SR, Arcaro JA, Leung LS, Tenney JR, Mirsattari SM. Functional connectivity of the hippocampus to the thalamocortical circuitry in an animal model of absence seizures. Epilepsy Res 2017; 137:19–24. [DOI] [PubMed] [Google Scholar]
- 23.Tezer FI, Firat A, Tuzun E, Unal I, Soylemezoglu F, Bilginer B, et al. Immunopathology in drug resistant mesial temporal lobe epilepsy with different types of hippocampal sclerosis. Int J Neurosci 2017; 128:421–428. [DOI] [PubMed] [Google Scholar]
- 24.D’Alessio L, Konopka H, Escobar E, Acuña A, Oddo S, Solís P, et al. Dentate gyrus expression of nestin-immunoreactivity in patients with drug-resistant temporal lobe epilepsy and hippocampal sclerosis. Seizure 2015; 27:75–79. [DOI] [PubMed] [Google Scholar]
- 25.Li JM, Huang C, Yan B, Wang W, Zhou Q, Sander JW, et al. HHV-7 in adults with drug-resistant epilepsy: a pathological role in hippocampal sclerosis? J Clin Virol 2014; 61:387–392. [DOI] [PubMed] [Google Scholar]
- 26.Reyesgarcia SZ, Scorza CA, Araújo NS, Ortizvillatoro NN, Jardim AP, Centeno R, et al. Different patterns of epileptiform-like activity are generated in the sclerotic hippocampus from patients with drug-resistant temporal lobe epilepsy. Sci Rep 2018; 8:7116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ingmar B, Maria T, Eleonora A, Dawna D A, Fabrice B, Andrea B, et al. International consensus classification of hippocampal sclerosis in temporal lobe epilepsy: a Task Force report from the ILAE Commission on Diagnostic Methods. Epilepsia 2013; 54:1315–1329. [DOI] [PubMed] [Google Scholar]
- 28.Zhong C, Liu R, Luo C, Jiang S, Dong L, Peng R, et al. Altered structural and functional connectivity of juvenile myoclonic epilepsy: an fMRI study. Neural Plast 2018; 2018:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Perani S, Tierney TM, Centeno M, Shamshiri EA, Yaakub SN, O’Muircheartaigh J, et al. Thalamic volume reduction in drug-naive patients with new-onset genetic generalized epilepsy. Epilepsia 2018; 59:226. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's website, www.neuroreport.com.