Abstract
Acinetobacter baumannii is a nosocomial pathogen that causes ventilator-associated as well as bloodstream infections in critically ill patients and the spread of multidrug-resistant (MDR) Acinetobacter strains is cause for concern. Much of the success of A. baumannii can be directly attributed to its plastic genome, which rapidly mutates when faced with adversity and stress. However, fundamental virulence mechanisms, beyond canonical drug resistance, were recently uncovered that enable A. baumannii, and to a limited extent other medically relevant Acinetobacter species, to successfully thrive in the healthcare environment. In this Review, we explore the molecular features that promote environmental persistence, including desiccation resistance, biofilm formation and motility, and we discuss the most recently identified virulence factors, such as secretion systems, surface glycoconjugates and micronutrient acquisition systems, that collectively enable these pathogens to successfully infect their hosts.
Keywords: Biological sciences, Microbiology, Bacteriology, Pathogens, Bacteria, Bacterial pathogenesis, Bacterial physiology, Bacterial secretion, Antibacterial drug resistance
Table of content:
Recently, virulence mechanisms, beyond canonical drug resistance, were uncovered that enable Acinetobacter baumannii to thrive in the healthcare environment and cause infections in critically ill patients. Feldman et al. explore the molecular features that promote environmental persistence and the most recently identified virulence factors that enable successful infection of the hosts.
Acinetobacter baumannii is an opportunistic human pathogen that predominantly infects critically ill patients. Once thought to be benign, A. baumannii is now considered a global threat in the healthcare setting mainly owing to its propensity to acquire multidrug, extensively drug and even pandrug resistance phenotypes at previously unforeseen rates1,2.
Infections caused by A. baumannii account for ~2% of all health care-associated infections in the United States3 and Europe4; however, these rates are twice as high in Asia and the Middle East 4. Although infection rates are lower compared with other Gram-negative pathogens, globally, ~45% of all isolates are considered to be multidrug resistant (MDR), with rates as high as 70% in Latin America and the Middle East1. These daunting MDR rates are nearly four times higher than those observed for other Gram-negative pathogens, such as MDR Pseudomonas aeruginosa and Klebsiella pneumoniae, for which global surveillance statistics are also available1. In light of this, the Centers for Disease Control and Prevention (CDC) categorized MDR Acinetobacter as a serious threat, thus prompting continual public health monitoring and prevention activities5. Furthermore, the World Health Organization (WHO) has included carbapenem-resistant A. baumannii in the critical group in the list of bacteria that pose the greatest threat to human health, prioritizing research and development efforts for new antimicrobial treatments6.
A. baumannii causes a range of nosocomial infections across multiple anatomical sites7. Most commonly, A. baumannii infections manifest as ventilator-associated pneumonia or central line-associated blood stream infections8. Less frequently, A. baumannii causes infections in the skin and soft tissues and at surgical sites as well as catheter-associated urinary tract infections8,9. Common to each of these scenarios is a breach in an anatomical barrier that enables the entry of A. baumannii directly to the site of infection. Community-acquired infections caused by A. baumannii have also been reported10. To date though, community-acquired infections have only presented in patients with underlying co-morbidities such as alcoholism, diabetes mellitus or other illnesses such as cancer and obstructive pulmonary disorders10.
Currently, there are more than 50 designated Acinetobacter species (List of Prokaryotic names with Standing in Nomenclature), of which the overwhelming majority are considered non-pathogenic; however, as mentioned above, a select few species are opportunistic human pathogens. The most clinically relevant members of the Acinetobacter genus phylogenetically cluster into the Acinetobacter calcoaceticus-baumannii (Acb) complex11. The Acb complex consists of five pathogenic species, A. baumannii12, Acinetobacter nosocomialis13, Acinetobacter pittii13, Acinetobacter seifertii14 and Acinetobacter dijkshoorniae15, as well as one non-pathogenic species, Acinetobacter calcoaceticus. The most clinically relevant and well-characterized Acinetobacter species is A. baumannii. This can partially be attributed to the inability to phenotypically distinguish A. baumannii from other members of the Acb complex, and until recently this has hindered appropriate species identification. However, the use of matrix-associated laser desorption ionization-time of flight (MALDI-ToF) mass spectrometry to identify species-specific outer membrane components of each member of the Acb complex has greatly enhanced species identification7,16. Nevertheless, given that all five pathogenic members of the Acb complex are frequently identified as A. baumannii, the designation of A. baumannii, unless otherwise stated, will be used in the broad sense to encompass all pathogenic members of the Acb complex.
The genetic relatedness between members of the Acb complex and their phenotypical similarities might indicate that they share common virulence factors, rendering studies in A. baumannii potentially applicable to other pathogenic Acinetobacter species. Indeed, some clinically relevant and recently described virulence attributes of pathogenic Acinetobacter species were first described in A. nosocomialis and subsequently characterized in A. baumannii (discussed below). This is particularly relevant as the number of scientific studies focusing on the pathobiology of Acinetobacter species overwhelmingly use A. baumannii as the model organism. However, we are now starting to observe discrete clinical and phenotypic characteristics between members Acb complex, particularly, A. baumannii, A. pittii and A. nosocomialis17.
Over the past decade, we have begun to unravel the exceptional and complex mechanisms that led to the emergence of A. baumannii as a formidable human pathogen, particularly beyond the canonical drug resistance mechanisms that have been extensively studied. Although many common features emerge, there is a clear absence of any discernable toxin or molecular determinant that can account for the virulence potential of a particular A. baumannii strain. Instead, our current understanding of A. baumannii virulence suggests a ‘persist and resist’ strategy. Specifically, A. baumannii has a remarkable capacity to survive in unfavorable conditions. In this Review, we discuss our current understanding of the virulence mechanisms in A. baumannii. Particularly, we explore the molecular features that promote environmental persistence, including desiccation resistance, biofilm formation and motility, and we discuss the most recently identified virulence factors, such as secretion systems, surface glycoconjugates and micronutrient acquisition systems, that facilitate A. baumannii pathogenesis. For a comprehensive review regarding the clinical and pathophysiological traits of A. baumannii, the reader is referred to a recently written article by experts in the field18.
Environmental persistence
It is largely believed that two attributes, drug resistance and environmental persistence, have enabled A. baumannii to thrive in the nosocomial environment19. Below, we discuss those features that enable A. baumannii to persist in environments that are inhospitable to many bacterial pathogens, thus, setting the stage for human colonization and subsequent infection.
Disinfection, desiccation and oxidative stress resistance mechanisms.
Commonly, healthcare environments include prolonged periods of desiccation and routine disinfection regimes. Similar to antibiotic resistance, A. baumannii has adapted to those stresses18.
Desiccation resistance, which is the ability to maintain viability under dry conditions, varies amongst clinical isolates of A. baumannii, with some isolates remaining viable for almost 100 days20,21. Desiccation resistance in A. baumannii is multi-factorial and not yet fully defined. However, it is clear that desiccation resistance depends on the ability of A. baumannii to maintain viability under conditions of water limitations. Indeed, in Acinetobacter baylyi, a non-pathogenic relative of A. baumannii, capsular polysaccharides (which are composed of repeating carbohydrate units and function as a glycan shield encompassing the entire bacterium and protecting from external threats) promotes survival during periods of desiccation22. Although direct evidence is lacking, given the similar biosynthetic pathways for capsular polysaccharide in A. baylyi and A. baumannii23, the ability of capsule [G] to retain water in A. baumannii and the presence of a capsular polysaccharide covering A. baumannii cells grown in a biofilm under dry conditions24, it is likely that the capsule contributes to resistance to desiccation in A. baumannii. Furthermore, a recent study has linked desiccation resistance to the composition of the outer membrane25. Specifically, a mutant strain that produces under-acylated lipooligosaccharide [G] (LOS) was unable to survive periods of desiccation. The authors suggested that the increased membrane fluidity resulting from the altered lipid composition of the outer membrane in this mutant would likely permit leakage of water and hydrophilic nutrients out of the cell25. Remarkably, a recent study found that the total bacterial counts and total culturable counts of A. baumannii did not change during prolonged dry periods, which indicates that transitioning to a dormant state, defined by a significant portion of the population entering a viable but non-culturable state, is not a major strategy for A. baumannii to persevere in desiccated environments26. However, the mechanisms behind desiccation persistence were not investigated and remain to be fully characterized.
Aside from the obvious loss of water during periods of desiccation, desiccation-rehydration causes various DNA lesions, including alkylation, oxidation, cross-linking, base removal and strand breaks27. To help prevent some of the DNA damage induced by desiccation-rehydration, A. baumannii relies on the protective role of the RecA protein28, which is an enzyme that is required for homologous recombination and recombination repair. A study showed an ~50 fold increase in the mutation frequency during a round of desiccation and rehydration of A. baumannii, as measured by the spontaneous appearance of rifampicin-resistant colonies29. This finding leads to the provocative hypothesis that resistance to desiccation may contribute to the MDR phenotype of A. baumannii.
Moreover, oxidative stress is also induced under periods of desiccation. As a result, A. baumannii significantly upregulates proteins that are associated with detoxifying reactive oxygen species30. Some Acinetobacter spp. are believed to have the highest tolerance to hydrogen peroxide outside of spore forming Gram-positive bacteria. A strain of Acinetobacter gyllenbergii is able to withstand 100mM hydrogen peroxide with no loss in viability and even maintain viability in 320mM hydrogen peroxide31. As A. gyllenbergii has been isolated from human specimens32, it is likely that more clinically relevant Acinetobacter spp., such as A. baumannii, will develop extremotolerances towards oxidative stressors given the genomic plasticity in members of the Acb complex. In fact, in response to oxidative stress, the emergence of A. baumannii strains that contain the insertion sequence element, ISAba1, upstream of the catalase [G] gene, katG, has been reported, which drives the expression of katG and enhances resistance to increased levels of hydrogen peroxide33. However, more experimental evidence is warranted.
Disinfectants such as chlorhexidine are extensively used in hospitals and other health care settings. Chlorhexidine, which is an antiseptic that is effective against Gram-negative and Gram-positive bacteria, disrupts cell membranes. A. baumannii has been shown to actively pump chlorhexidine out of the cell using the Acinetobacter chlorhexidine (AceI) efflux protein34, thus possibly promoting survival of the bacteria under stress conditions. Another stressor, ethanol, has been shown to promote the growth and virulence of A. baumannii35,36; albeit, at low concentrations37. Moreover, physiological concentrations of ethanol found in the blood streams of individuals with a history of alcohol use disorder sufficiently impair phagocytosis [G] and thus elimination of A. baumannii38. Expectedly, chronic alcohol consumption is one of the primary risk factors associated with community-acquired A. baumannii infections10.
Although the role of environmental persistence has generally been accepted to be a virulence strategy of A. baumannii, much work is needed to determine the full underlying molecular mechanism.
Biofilm formation and maintenance.
Microbial biofilms, which are communities that are encased in an extracellular matrix, are produced by many if not all bacteria. Biofilms are likely to have an important role in the interactions of A. baumannii with its host, and biofilm formation contributes to medical device-associated infections.
A. baumannii populations within skin and soft tissue infections form robust biofilms, both within the wound and on occlusive dressings39. A. baumannii also form biofilm communities on most abiotic surfaces; including, health care-associated equipment such as endotracheal tubes as well as polycarbonate and stainless steel40. It is well regarded that bacteria within biofilm communities, including A. baumannii, have increased tolerance to extracellular stresses40,41. As with many biofilm producing organisms, A. baumannii surface appendages, adhesins and protective surface structures, such as capsular polysaccharides, greatly contribute to the formation and maintenance of biofilms.
Although factors that contribute to biofilm formation seem to be strain-dependent, some common factors have been identified. Most A. baumannii strains encode for and produce a type I chaperone-usher pilus system designated Csu pili (Fig. 1A). Csu pili, regulated by the BfmRS two component regulatory system [G] 42, are crucial for biofilm formation and maintenance on abiotic surfaces, including polystyrene43, but, are not required for association with biotic surfaces such as human epithelial cells44. Interestingly, most A. baumannii strains seem to carry the csuA/BABCDE locus; however, a subset of clinical isolates have lost the csu cluster45, which indicates that these pili may not be required for biofilm formation and maintenance in all strains or that other pili systems may functionally replace them (see below). Moreover, a second two-component system termed GacSA46, has been shown to moderately control csu gene expression and thus indirectly biofilm formation. Interestingly, sub-inhibitory concentrations of trimethoprim-sulfamethoxazole have been shown to completely repress the expression of Csu pili in A. baumannii, which indicates that improper use of antibiotics can alter population level behaviors and may promote a planktonic lifestyle47. Other putative chaperone usher pili systems and Pap pili systems, which are homologous to the P pili of Escherichia coli, have been implicated in A. baumannii biofilm formation and maintenance; yet, a detailed molecular analysis describing their specific role is lacking48,49.
Figure 1: Protein secretion and export in Acinetobacter baumannii.
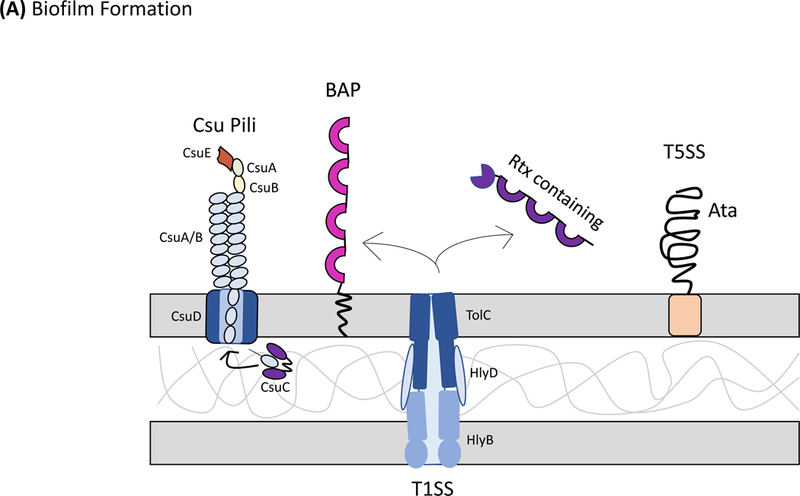
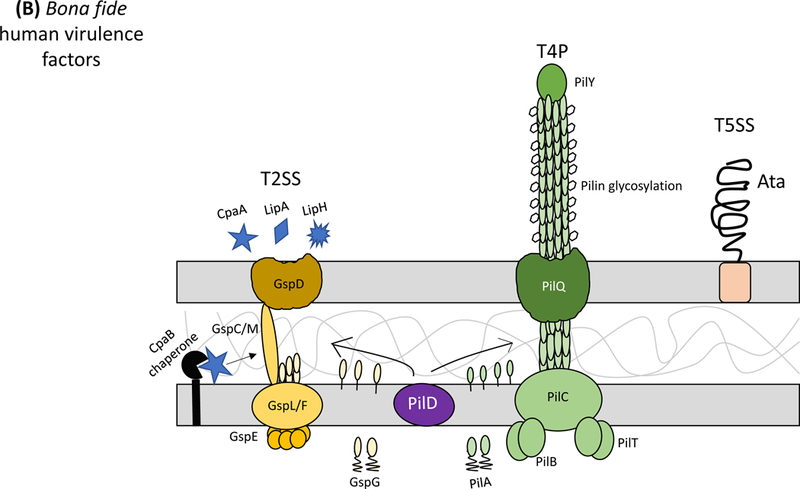
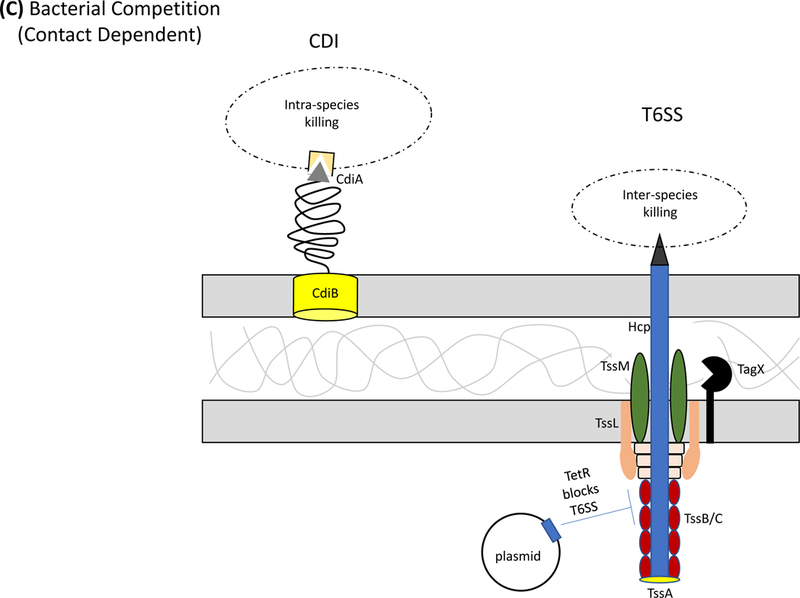
Secretion systems and extracellular appendages of Acinetobacter baumannii are involved in the formation of biofilms, in virulence and bacterial competition. (A) The Csu system extrudes a type I chaperone-usher pilus that is essential for the formation and maintenance of biofilms on abiotic surfaces, which may contribute to persistence in the hospital environments43. The CsuA/B pilin subunits are trafficked to the outer membrane CsuD usher by CsuC chaperones. The CsuD usher facilitates polymerization of CsuA/B monomers into a pilin fiber. Pilin polymerization is initiated by the CsuE tip adhesion and the minor pilins CsuA and CsuB, all of which are also trafficked to the CsuD usher by CsuC chaperones. Biofilm-associated proteins (Bap)50 and an effector protein that contains an RTX-like domain are secreted by the type I secretion system (T1SS)52 and are involved in the formation and stability of mature biofilms. The T1SS is composed of the outer membrane protein TolC, the periplasmic adaptor protein HlyD, and the inner membrane ATPase HlyB. The Acinetobacter trimeric autotransporter (Ata) type Vc secretion system consists of a membrane-associated transporter domain and a large, repetitive passenger domain that extrudes through the transporter domain60. The adhesin promotes adherence to extracellular and basal membrane components of the host. (B) The type II secretion system (T2SS) secretes multiple effectors that were shown to be required for virulence in vivo, including the lipases LipA and LipH as well as the protease CpaA103,104. Novel, dedicated chaperones are required for two of the effectors, including the CpaB chaperone, which is required for the secretion of CpaA, the most abundant type II effector. A. baumannii and A. nosocomialis also produce type IV pili, surface appendages evolutionarily related to the T2SS66. In A. baumannii and A. nosocomialis these two systems share a processing protein, PilD, required to process pre-pseudopilins and pre-pilins prior to assembly into the T2SS and type IV pilus, respectively104. Type IV pili are also glycosylated in A. baumannii and A. nosocomialis as a possible form of antigenic variation to evade host detection. Specifically, the pilin glycan is predicted to mask the major pilin subunit, PilA, from antibody recognition; furthermore, PilA displays remarkable sequence divergence across species and even between strains leading to reduced immune recognition in the case pilin subunits are not glycosylated68. (C) Contact-dependent secretion systems are used by A. baumannii for inter- and intra-species killing to eliminate bacterial competitors. Many strains of Acinetobacter have two distinct contact-dependent inhibition (CDI) systems to kill sister cells that do not have the associated immunity protein52. The type VI secretion system (T6SS)106,107 is used for inter-species competition and contains a novel L,D-endopeptidase, TagX, which is required for transit of the machinery through the peptidoglycan layer128. A large, conjugative plasmid which also contains drug-resistance genes (not indicated) regulates the expression of T6SS in some clinical isolates110. The individual components of the secretion systems are indicated in all panels.
A. baumannii also produce biofilm-associated proteins (BapAb)50, which are large surface exposed proteins orthologous to the Bap protein originally characterized in S. aureus51. BapAb, which is secreted via a type I secretion system (T1SS)52, mediates mature A. baumannii biofilm formation (Fig. 1A). Specifically, Bap has a role in cell-cell adhesion and is required for the development of higher-order structures on medically relevant materials such as polystyrene and titanium 50. Although most sequenced strains of A. baumannii carry a bap gene, many seem to have disrupted or truncated bap sequences53. It has yet to be determined if this is due to recombination events or sequence alignment errors common to the highly repetitive elements of bap coding sequences. Some A. baumannii strains also encode Bap-like proteins, BLP1 and BLP2, which coordinately contribute towards mature biofilm formation in a similar fashion as BapAb54. Finally, medically relevant Acinetobacter spp., including A. baumannii and A. nosocomialis, abundantly secrete an RTX-like domain-containing protein through the T1SS52. This family of proteins is orthologous to large repetitive RTX domain-containing proteins found in Pseudomonas putida, which mediate biofilm development55. Other notable factors in A. baumannii that might be crucial for biofilm formation include the production of poly-beta-(1–6)-N-acetylglucosamine (PNAG)56, which is produced by many Gram-negative species. Interestingly, antibodies against PNAG are able to eliminate A. baumannii in opsonophagocytosis assays, which suggests that PNAG might be a potential vaccine target57. Other factors that contribute to biofilm formation, such as capsular polysaccharides58,59, and an autotransporter system60 are discussed below.
Motility.
In many different genera, bacterial motility is intimately linked with the ability of an organism to cause disease, for instance; in the case of Pseudomonas aeruginosa, the flagellum functions as a key bacterial motor that is also required for full virulence. In a related fashion, A. baumannii hyper-motility has been associated with enhanced virulence in a Caenorhabditis elegans infection model61; conversely, mutants defective in motility were shown to have an attenuated phenotype62. Furthermore, recent epidemiological studies of A. baumannii clinical isolates found blood isolates were more motile when compared against sputum isolates, indicating that motility may provide a fitness advantage in different anatomical sites63.
Paradoxically, ‘Acinetobacter’ translates to ‘non-motile rod’; however, A. baumannii and A. nosocomialis strains are capable of two independent forms of bacterial locomotion: surface-associated motility [G] and twitching motility [G]. Twitching motility64 is a well-described form of bacterial locomotion by many genera of bacteria. In A. baumannii and A. nosocomialis, twitching motility is dependent on fully functioning type IV pili65,66 for repeated rounds of extension and retraction to pull bacterial cells forward. Although a direct link between type IV pili and/or twitching motility in virulence has not been observed for A. baumannii, genes predicted to encode for proteins required for the biogenesis of type IV pili were shown to be upregulated during growth in human serum67, which indicates that type IV pili may be important during bacteremia. Moreover, almost all A. baumannii and A. nosocomialis strains carry highly homologous genes encoding for proteins required for the biogenesis of type IV pili. However, the major pilin subunit, PilA, displays remarkable sequence divergence across species and even between strains. Structural analysis of two A. baumannii PilA proteins and an A. nosocomialis PilA protein showed that these sequence divergences manifest in a high degree of structural variation, much larger than expected for a given species68. As such, these sequence and structural variations render an A. baumannii type IV pilin-specific vaccine as a likely failure.
Another form of motility, termed surface-associated motility has also been observed in A. baumannii clinical isolates. The earliest reports of Acinetobacter surface-associated motility ascribed the phenomenon to be dependent on twitching motility69. A subsequent study further strengthened these observations, as type IV pili retraction-deficient mutants in a strain of A. nosocomialis displayed impaired surface-associated motility70. A third study also observed that A. baumannii type IV pili retraction mutants had impaired surface-associated motility65. However, using the same A. nosocomialis type strain, a contradictory report found that type IV pili do not have a role in surface-associated motility66. Specifically, it was found that A. nosocomialis mutants lacking functional type IV pili were unable to exhibit twitching motility, yet, displayed completely normal surface-associated motility.
A. baumannii surface-associated motility is most similar in appearance to swarming motility of P. aeruginosa71; but, swarming is dependent on flagella and A. baumannii do not produce flagella. Currently, A. baumannii surface-associated motility has been shown to rely on the synthesis of 1,3-diaminopropane (DAP)72, quorum sensing70 and LOS production73. It is possible that DAP or a derivative of DAP functions as a signaling molecule important for regulating surface-associated motility via quorum sensing. However, further work is needed to fully establish this mode of motility. Interestingly, A. baumannii surface-associated motility is repressed at room temperature in the presence of blue light, a feature that is dependent on proteins that contain blue light-sensing domains74. Finally, a possible source for the conflicting results described above are the phase variable phenotypes of different A. baumannii and A. nosocomialis populations75. Specifically, a novel phase-variable colony opacity phenotype was discovered in A. baumannii strain AB5075, where colonies interconvert between opaque and translucent variants. Interestingly, opaque phase variants [G], have enhanced surface-associated motility and a concomitant enhanced virulence phenotype, while translucent variants are significantly less motile76. Thus it is possible that the previously characterized type IV pili mutants, which displayed impaired surface-associated motility phenotypes, were immotile due to a phase variable phenomenon and not an impairment with or absence of functioning type IV pili. Phase variation seems to be a common phenotype among most clinical isolates of A. baumannii and this phenotype is likely to control additional virulence factors. This feature must be carefully considered when designing and performing experiments assessing the virulence and fitness of pathogenic Acinetobacter species76.
Interactions with hosts and competitors
Prior to the recent antibiotic resistance epidemic, A. baumannii was a scantly studied microorganism. In the last 10 years, many virulence factors have been uncovered as well as a thorough characterization of the innate immune response during an A. baumannii infection (Box 1). Virulence factors are broadly defined as molecular features used by a bacterium that enable successful interaction with and subsequent colonization of the human host. Given that nearly half of all A. baumannii infections are caused by MDR strains1, there is a need to accelerate investigations into its pathobiology to find alternative ‘out-of-the-box’ strategies to combat A. baumannii infections77. Two main approaches have been used to discover A. baumannii virulence factors. The first, unbiased approach used high-throughput transposon screenings to identify virulence factors under different experimental conditions. These approaches have identified several candidate virulence factors, mostly predictable genes involved in cellular metabolism and cell envelope biogenesis. Although powerful, this strategy has not yet led to the identification of novel toxins or pathogenesis mechanisms. This could be due to the lack of an appropriate model that recapitulates human infections, the choice of the strains or experimental conditions, or the fact that the defect in colonization by a particular mutant may be masked by the bacterial population as a whole. The second and most applied approach revolves around identifying homologous virulence mechanisms found in other human pathogens. This approach has led to the identification of protein glycosylation and secretion systems, as well as the characterization of micronutrient acquisition mechanisms, which we discuss in the following section.
KEY POINTS
Acinetobacter baumannii is an opportunistic human pathogen that predominantly causes healthcare-associated infections.
Many members from the genus Acinetobacter, including, Acinetobacter nosocomialis, Acinetobacter pittii, Acinetobacter dijkshoorniae and Acinetobacter seifertii, are also human pathogens and increasingly identified as the cause of infections.
A. baumannii is rapidly developing resistance mechanism to antibiotics.
The ability of A. baumannii to withstand desiccation and to form biofilms promotes its success as a nosocomial pathogen.
Fundamental virulence factors, such as surface adhesins, glycoconjugates and secretion systems directly contribute to the pathogenesis of A. baumannii.
Glycoconjugates
Bacterial carbohydrates, also known as glycans, provide an interface between a pathogen and its environment. Not surprisingly glycoconjugates [G] have key structural roles and mediate the first line of defense against a variety of stresses, immune evasion and regulation, and virulence in A. baumannii (Fig 2). Common bacterial glycoconjugates include the capsular polysaccharide, glycosylated proteins, lipopolysaccharide and peptidoglycan.
Acinetobacter baumannii surface-exposed glycoconjugates.
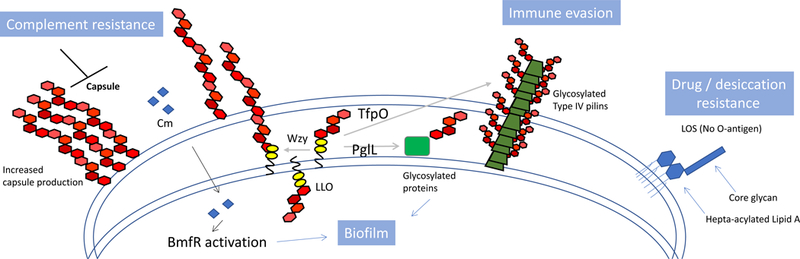
The capsular polysaccharide, glycoproteins and hepta-acylated lipooligosaccharide (LOS) all contribute to virulence of Acinetobacter baumannii. In A. baumannii and A. nosocomialis, the capsular polysaccharide and glycoproteins are formed by glycans alone or glycans attached to proteins, respectively59. Glycan synthesis is initiated at the inner membrane by dedicated glycosyltransferases that transfer sugars to a phosphorylated lipid generating a lipid-linked oligosaccharide (LLO). The LLO is then flipped to the periplasm where the glycan component can be transferred by PglL, an oligosaccharyltransferase (OTase) to proteins in the periplasm or outer membrane, or to type IV pilins by TfpO, a pilin specific OTase. The LLO can also be further processed and polymerized into a repeating polysaccharide by the Wzy polymerase prior to transport to the outer membrane for the capsule. In A. baumannii the capsular polysaccharide protects cells from complement-mediated killing58. A. baumannii glycoproteins contribute to virulence by enhancing biofilm formation and maintenance59, while glycosylated type IV pilins87 have been implied to function in immune evasion, shielding the antigenic protein from antibody recognition68. Finally, the hepta-acylated LOS, consisting of a core glycan and lipid A, lacks an O-antigen but directly contributes to drug and desiccation resistance25.
Lipopolysaccharide [G] (LPS) is a hallmark of Gram-negative bacteria and the ligand for Toll-like receptor 4 (TLR4). Similarly to Neisseria and Campylobacter species, A. baumannii does not contain an O antigen, thus, its LPS is appropriately designated lipooligosaccharide (LOS). Regardless, in Gram-negative bacteria, LPS or LOS is the primary component of the outer leaflet of the outer membrane and as such is generally considered an essential structural component required for bacterial viability. Moreover, the lipid anchor of LPS and LOS, termed lipid A, is the target of the cationic polypeptide antibiotic colistin, which is a last-line treatment option for carbapenem resistant A. baumannii78. Not surprisingly, A. baumannii isolates are adapting to this antibiotic in a multitude of ways (Fig 3). Similarly to other Gram-negative bacteria, A. baumannii can modify its lipid A composition to deter the binding of colistin. Specifically, mutations in the pmrAB two-component system of A. baumannii mediate upregulation of pmrA expression79, which is accompanied by the addition of phosphoethanolamine to lipid A and increased resistance to colistin80. Epidemiological studies show that mutations in the pmrAB system are the most commonly observed mechanism for A. baumannii strains to become colistin resistant45,81. Other lipid A modifications implicated in colistin resistance include the addition of galactosamine82 and the natural presence of a predominately hepta-acylated form of LOS25. Colistin resistance is also mediated by the complete loss of LOS in A. baumannii83. This extreme adaption, conferred by mutations in lipid A biosynthetic genes, has only been observed in A. baumannii, Neisseria meningiditis and Moraxella catarhalis. Given its role in membrane integrity and stability, loss of lipid A, and thereby LOS, in A. baumannii requires compensatory mechanisms to support viability, such as the overexpression of certain lipoproteins as a form of outer membrane stabilzation84. Another noted change in LOS-deficient strains includes an increase in the capsular polysaccharide poly-beta-1,6-N-acetylglucosamine85; however, this compensatory mechanism has not been universally observed84.
Colistin resistance mechanisms of A. baumannii.
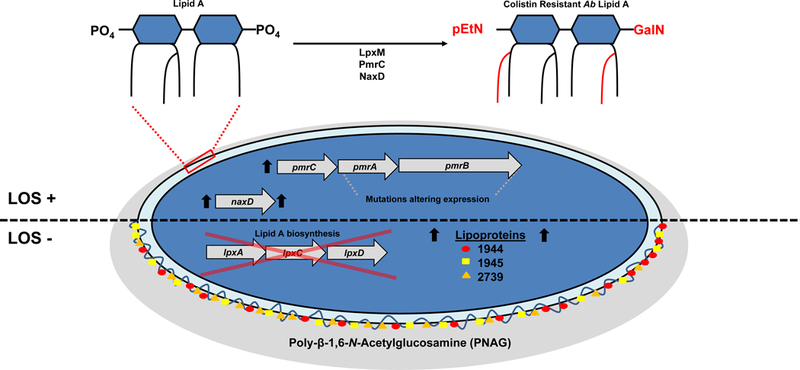
In Acinetobacter baumannii, colistin resistance manifests either through modifications of the lipid anchor of LOS, termed lipid A, or by the complete loss of LOS, both of which alter binding affinity of colistin. Most commonly, colistin-resistant clinical isolates harbor mutations in the two-component regulatory system PmrA-PmrB, which is associated with the addition of phosphoethanolamine (pEtN) by PmrC129, and galactosamine (GalN)82 to lipid A that presumably alter the binding affinity of colistin. The addition of GalN is dependent on the deacetylase activity of NaxD130, which is regulated by the PmrA-PmrB system. Moreover, the dual activity of the acyltransferase, LpxM, leads to the constitutive expression of a predominately hepta-acylated form of LOS, which provides increased resistance against colistin25. In extreme cases, A. baumannii will acquire mutations in the lipid A biosynthetic pathway thereby halting its production83. In the absence of lipid A, A. baumannii has been shown to upregulate lipoproteins, namely A1S_1944, A1S_1945 and A1S_2739, which stabilize the outer membrane84. The accumulation of the capsular polysaccharide poly-β−1,6-N-Acetylglucosamine (PNAG) has also been observed in strains lacking lipid A as a proposed mechanism of membrane stabilization85.
Most A. baumannii strains also carry a thick capsular polysaccharide58 and an O-linked protein glycosylation system59. Capsular polysaccharides protect bacteria from external threats, including host defenses. Protein glycosylation is the process of transferring a carbohydrate, usually an oligosaccharide, to a protein. In A. baumannii and A. nosocomialis, both the capsular polysaccharide and the O-glycans that decorate proteins share a biosynthetic pathway86,87. These processes start with the assembly of a lipid-linked oligosaccharide at the cytoplasmic side of the inner membrane. Once assembled, the lipid-linked oligosaccharide is flipped to the periplasmic side of the inner membrane. Upon reaching the periplasmic side, the lipid-linked oligosaccharide can be directly transferred to a protein by an oligosaccharyltransferase or further processed by the Wzy polymerase into repeating polysaccharide units required for capsule biogenesis (Fig. 2).
The capsular polysaccharide is mainly responsible for the extraordinary resistance to complement-mediated killing [G] exhibited by most A. baumannii strains58,86. In fact, the capsular polysaccharide of A. baumannii could be considered its main virulence factor as strains lacking the capsule are avirulent and readily killed by complement86. The glycans attached to proteins, which are most often located in the periplasm or the outer membrane and are exclusively membrane associated in A. baumannii, are not involved in resistance to complement, but cells lacking the glycosylation system are defective in biofilm formation and are attenuated in several infection models, which indicates a role for protein glycosylation in the successful adaption to the host environment59. Moreover, most A. baumannii and A. nosocomialis strains contain two O-glycosylation [G] systems: one system is responsible for glycosylation of multiple proteins of unknown function and the other system has been implicated in the decoration of type IV pilin with glycans87. The biological role of protein glycosylation remains elusive; however, recent structural analyses and glycan modeling indicated that the pilin glycans may have evolved to shield the protein components from antigenic recognition68.
Given the importance of capsular polysaccharides to A. baumannii virulence and the successful implementation of many capsular polysaccharide-conjugate based vaccines for the prevention of Streptococcus pneumoniae, Haemophilus influenzae type B and N. meningiditis, an A. baumannii capsule-based conjugate initially seemed promising. Unfortunately, the variability among glycan structures, including capsule and glycoproteins, and the glycan core, is outstanding, and often includes sugars that have not previously been identified in any other species23. Clearly, this will make the formulation of an effective glycan-based vaccine difficult, if not impossible. Adding to the complexity, capsule synthesis can be increased upon contact with sub-inhibitory concentrations of antibiotics, in a mechanism mediated by the two-component regulatory system BfmRS, which suggests that improper antibiotic therapy may further enhance the virulence of A. baumannii 88. Nevertheless, inhibitors of the glycan synthesis pathway would be highly valuable therapeutics to combat MDR A. baumannii infections.
Micronutrient acquisition systems.
Infecting the human host requires a coordinated response from A. baumannii that not only impairs cellular defense mechanisms, mainly in the form of protection via the capsular polysaccharide, but also enables for metabolic and nutritional flexibility. Transition metals such as iron, manganese and zinc are essential for all domains of life; as such, hosts have evolved elaborate mechanisms to sequester metals, a process that is referred to as nutritional immunity. One key to the success of A. baumannii as a nosocomial pathogen is its diverse mechanisms for scavenging these scant nutrients in vivo. This is particularly evident in the form of high-throughput transposon screens, where mutants that contain transposon insertions within iron and zinc import and/or utilization genes are severely attenuated in virulence models89–91.
The primary mechanism used by A. baumannii for scavenging iron is mediated through the action of high affinity iron chelating molecules known as siderophores [G]. The most commonly conserved iron chelating agent in A. baumannii is the catechol-hydroxymate siderophore, acinetobactin92. Depending on the pH of the extracellular environment, acinetobactin can isomerize into one of two forms, an oxazoline- or isooxazolidinone-containing form, both of which chelate iron93. This unique isomerization feature enables acinetobactin to bind iron under acidic conditions found during acute infections. Importantly, acinetobactin is absolutely required for virulence94, thus rendering its synthetic pathway an attractive antibacterial target. Another other sets of catechol-hydroxymate siderophores, fimsbactin A-F95 as well as the hydroxymate siderophores baumannoferrin A and baumannoferrin B96, are also iron scavengers used by A. baumannii; however, a detailed genetic and molecular analysis of their biosynthetic and transport machinery is lacking.
Zinc, which is a structural cofactor for many proteins, is also essential for the survival of A. baumannii. Given its essentiality to many bacterial pathogens, mammalian systems have evolved zinc sequestration mechanisms, including the production of the zinc chelating protein calprotectin97. Calprotectin production and release is robustly induced upon A. baumannii infection in the lungs of mice and persists for the entirety of the infection98. To combat calprotectin-mediated nutritional immunity, A. baumannii relies on a high affinity zinc acquisition system, termed ZnuABC99. The ZnuABC system is regulated by the zinc uptake regulator (Zur), which functions as a transcriptional repressor that binds to conserved DNA motifs upstream of many zinc-regulated genes, thus blocking their expression100. Under zinc-depleted conditions or in the presence of calprotectin, intracellular zinc levels decrease and Zur-mediated repression is relieved. A second Zn-uptake system, also regulated by Zur, relies on the coordination between the metallochaperone, ZigA, and the histidine utilization (Hut) system for the uptake and release of histidine bound zinc (His-Zn) complexes101. In this system, the His-Zn complexes are imported by the outer membrane transporter HutT. Once inside A. baumannii cells, zinc is liberated from the His-Zn complexes to a bioavailable form with the coordination of ZigA and the histidine ammonia lyase, HutH. Like siderophores, zinc utilization systems are attractive antimicrobial targets given their importance in vivo and the lack of homologous systems in eukaryotic organisms.
Recently, the importance of micronutrient acquisition and metabolism were linked in A. baumannii virulence. Specifically, A. baumannii subverts host-mediated metal limitation through the concerted action of a manganese import system and subsequent urea metabolism102. It is unclear why urea metabolism is important for A. baumannii growth under metal-limited conditions; however, it was speculated that metal limitation may cause a metabolic build-up of urea that requires manganese-mediated breakdown. Cleary, these types of studies show the importance of micronutrient acquisition systems towards the virulence potential of A. baumannii and further emphasize the potential of those systems as novel antimicrobial targets.
Protein secretion.
Similar to other Gram-negative pathogens, A. baumannii uses secreted protein products to facilitate environmental and host adaption. Within the last five years, type I52, type II103,104, type IV105, type V60 and type VI106,107 secretion systems have been uncovered in A. baumannii (Fig. 1). For a detailed review on Acinetobacter secretion systems and secreted proteins, the reader is referred to the following review108.
The first secretion system identified in A. baumannii was the Acinetobacter trimeric autotransporter (Ata)60, a type Vc autotransporter, which consists of a membrane-associated transporter domain and a large, repetitive passenger domain that extrudes through the transporter domain (Fig. 1A). Ata is important for adhesion [G] to host extracellular matrices and basal membrane components60. Ata is present in many clinical isolates and may be a potential vaccine candidate. Indeed, passive administration of anti-Ata serum protects neutropenic mice from infection109. However, Ata expression is variable amongst clinical isolates and thus may not provide adequate coverage for a comprehensive vaccine60.
A. baumannii and A. nosocomialis strains also use a type VI secretion (T6SS) system for bacterial competition106,107 (Fig. 1C). This is particularly important in the context of polymicrobial infections as the Acinetobacter T6SS has not been found to mediate eukaryotic cytotoxicity; yet, it provides an in vivo fitness advantage given its potent anti-bacterial activity. T6SS are often tightly regulated through various control mechanisms. This holds true for A. baumannii, as expression of the T6SS components and activity can vary greatly between strains. Whereas some strains are constitutively expressing their T6SS and release effectors in vitro, some strains have developed exquisite forms of regulation. For example, several A. baumannii strains contain a large conjugative plasmid that harbors many antibiotic resistance genes as well as repressors of the T6SS110. Specifically, two TetR-like regulators encoded on the plasmid repress T6SS activity, which is chromosomally encoded. Upon spontaneous loss of the plasmid, cells are relieved of T6SS repression and become potent bacterial killers. At the same time, cells that lost the plasmid now become susceptible to antibiotics, given that many resistance cassettes are located on the plasmid. This novel form of regulation may be altruistic as cells that lose the plasmid may be able to defend the entire population against competing bacteria in the polymicrobial environment. When antibiotics are introduced, cells that contain the plasmid survive and thus ensure the survival of the population.
A. baumannii and A. nosocomialis also use a type II secretion system (T2SS) for the export of multiple effector proteins103,104 (Fig 1B). Two of these effectors, the lipase LipA and the metalloprotease CpaA, require dedicated chaperones, LipB and CpaB respectively, underscoring an under recognized area of T2SS dynamics104. Previously, it was thought that the type III secretion system was the only secretion system that specifically used widespread chaperones for secreted effectors. Importantly, the A. baumannii and A. nosocomialis T2SSs functions as a bona fide virulence factor secreting effectors that mediate colonization of the lung and dissemination to other organs. Moreover, it was recently shown that CpaA may be one of the main virulence factors secreted by the T2SS as a cpaA mutant was less virulent in both an invertebrate model and a murine model of pneumonia111. Interestingly, the cpaA mutant was less able to disseminate to the spleen. This may be due to the ability of CpaA to decrease blood coagulation112, a process that may help A. baumannii disseminate throughout the bloodstream to other anatomical sites, like the spleen. However, the role of each specific effector, other than CpaA, in A. baumannii and A. nosocomialis pathogenesis remains elusive. Collectively, future studies are needed to define the secreted proteins that contribute to the success of A. baumannii as a nosocomial pathogen.
Conclusions and future perspectives
There are well over 2,000 A. baumannii genome sequences publicly available. Impressively, the paralog-collapsed pan-genome size of A. baumannii reaches almost 12,000 sets of genes113. Furthermore, these types of analyses enable the mapping of the core A. baumannii genome. From this, the global distribution across A. baumannii strains and even A. nosocomialis strains of essential virulence factors, like the capsular polysaccharide, secretion systems, and micronutrient acquisitions systems are readily identifiable. Together with the accumulation of resistance mechanisms, the success of A. baumannii as a nosocomial pathogen becomes evident. However, what if A. baumannii does acquire a toxin-like virulence factor similar to other human pathogens, such as, Vibrio cholerae or Clostridium difficile? Molecular studies have shown that A. baumannii has many of the same secretion systems as other well-known pathogens and although there is no evidence to indicate such an acquisition, the possibility does exist.
In parallel, the emergence of multidrug resistant A. baumannii emphasizes the urgency to develop alternative treatment strategies. These anti-virulence strategies may manifest in the form of phage-therapy, metabolic interference therapy, antimicrobial peptide therapy or vaccine strategies77. One such example would be targeting the phenylacetic acid (PAA) catabolic pathway with selective inhibitors as PAA functions as a potent chemoattractant for neutrophils114. Recently, an A. baumannii mutant auxotrophic for D-glutamate was shown to be a very promising live attenuated vaccine candidate given its inability to synthesize mature peptidoglycan115.
Finally, it is clear that recent clinical isolates are genetically distinct from type strains commonly used for scientific studies45. The two most used strains, A. baumannii ATCC 17978 and A. baumannii ATCC 19606, have been excellent models for over two decades; however, both strains were isolated almost 70 years ago and do not adequately reflect clinical isolates that were recovered in the past three decades. As an example, A. baumannii ATCC 17978 carries the pAB3 plasmid, a large conjugative plasmid containing a single antibiotic resistance cassette. Over the course of 60 years, this plasmid has evolved into the pAB04 plasmid which now contains 12 resistance cassettes in the same locus, which indicates that antibiotic resistance has been positively selected. Another example is CpaA, which is commonly found in recent clinical isolates but is absent in both ATCC 17978 and ATCC 19606. Therefore, the use of modern clinical isolates, such as A. baumannii AB5075116, will provide a more contemporary understanding of the molecular mechanisms important for survival and adaption in this era and may lead to the identification of more relevant virulence factors outside of those involved in the persist and resist strategy commonly recognized as the only virulence mechanism of A. baumannii.
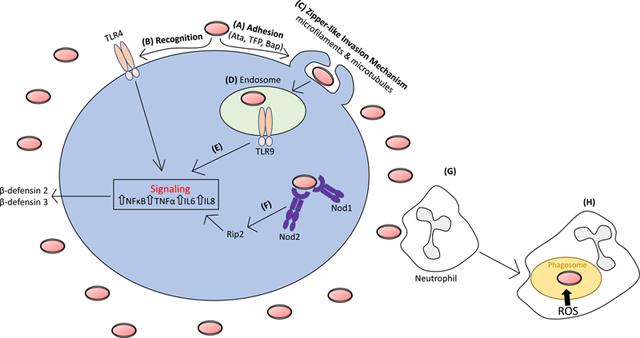
Box 1-. Innate immune response to Acinetobacter baumannii infection
As with many pathogenic microorganisms, A. baumannii interacts with mucosal epithelial cells117 as well as endothelial cells in the case of bloodstream infections118. These interactions, mediated through adhesins, like Ata118, type IV pili68, and Bap119, provide A. baumannii intimate contact with host substratum (see the figure). At the cell surface, key innate immune components known as pattern recognition receptors (PRRs) can then recognize specific pathogen associated molecular patterns (PAMPs) of A. baumannii. The extracellular PRR Toll-like receptor 4 (TLR4), which recognizes lipid A of LOS, has a prominent signaling role helping to limit A. baumannii burden in both pneumonic and systemic infection models. Although A. baumannii is considered an extracellular pathogen, it can invade epithelial cells using a zipper-like mechanism for uptake, which is dependent on both microfilaments and microtubules120. A. baumannii can persist within membrane bound vacuoles in the cytoplasm necessitating intracellular PRRs for pathogen detection. Indeed, signaling from the endolysosomal PRR, TLR9, which detects bacterial DNA, limits A. baumannii pneumonia and bacterial dissemination121. Furthermore, the cytosolic PRRs NOD1 and NOD2 as well as their downstream signaling partner RIP2 limit intracellular A. baumannii proliferation in epithelial cells, which indicates that A. baumannii can escape membrane-bound vacuoles; however, their escape mechanisms are not yet known122. Ultimately, recognition by PRRs results in downstream signaling cascades mediated by the activation of NF-κB, the mitogen-activated protein kinase pathway, and concomitant pro-inflammatory cytokine production that include TNF-α, IL-6 and IL-8122,123.
Upon detection, epithelial cells secrete antimicrobial peptides; such as; human β-defensin2 and human β-defensin 3, which functions as a first line of defense, inhibiting the growth of invading A. baumannii123,124. Simultaneously, recruitment of phagocytic cells to the site of A. baumannii infection is mediated by cytokine and chemokine signaling. Neutrophils are the first to arrive and most important phagocytic cell for controlling A. baumannii infections114. This is particularly evidenced through antibody-mediated neutrophil depletion125,126, which greatly enhances A. baumannii pathogenesis resulting in severe mortality in experimental models. Mechanistically, neutrophil clearance of A. baumannii is mediated by the activity of the NADPH oxidase system, which generates reactive oxygen species to kill phagocytosed bacteria127, with limited roles for reactive nitric species. Other phagocytic cells like macrophages and dendritic cells play minor roles in A. baumannii clearance, but may play important sensing and signaling roles.
Acknowledgements
During the preparation of this Review article, C.M.H was funded as a W.M. Keck Postdoctoral Fellow. The efforts of S.W.H, and M.F.F. were funded by a National Institutes of Health grant (1R01AI125363–01).
GLOSSARY
- Capsule
an extracellular polysaccharide layer that encompasses the entire bacterium, which acts as a glycan shield protecting the bacterium from many external threats
- Lipooligosaccharide (LOS)
a macromolecule consisting of lipid-A and a core oligosaccharide found in the outer leaflet of the outer membrane of Gram-negative bacteria. Lipid-A is also considered endotoxin and is the ligand for toll-like receptor 4
- Phagocytosis
the process used by many immune cells, including macrophages, to engulf invading bacteria
- Catalase
an enzyme that detoxifies hydrogen peroxide into water and oxygen
- Two-component regulatory system
a two part relay system employed by bacteria used for sensing and responding to environmental stimuli, consisting of a membrane bound histidine kinase and a soluble response regulator
- Surface-associated motility
a mechanism of bacterial translocation observed on semi-solid surfaces unique to Acinetobacter spp., which is not dependent on pili
- Twitching motility
a mechanism of bacterial translocation dependent on repetitive rounds of type IV pili extension and retraction broadly used by many bacteria
- Opaque phase variants
a subset of an A. baumannii population that has an opaque appearance when viewed under a dissecting microscope, which varies from the translucent form in terms of both appearance and virulence
- Glycoconjugates
macromolecules composed of a carbohydrate covalently attached to at least one other lipid or protein molecule
- Lipopolysaccharide (LPS)
a macromolecule consisting of lipid-A, a core oligosaccharide, and a polysaccharide O-antigen found in the outer leaflet of the outer membrane of Gram-negative bacteria
- Complement-mediated killing
part of the innate immune system consisting of soluble proteins in the blood that coordinately binds to an invading pathogen, triggering either lysis or the recruitment of immune cells to clear the pathogen
- O-glycosylation
the covalent attachment of a carbohydrate moiety to the hydroxyl group of a serine or threonine in a polypeptide
- Siderophores
high affinity iron binding molecules secreted by many bacterial pathogens to scavenge for iron
- Adhesion
the process of a bacteria associating with a surface, either biotic like human cells or abiotic in the form of medical equipment and devices
Author Biographies
Christian M. Harding received a Ph.D. in Biomedical Sciences from The Ohio State University. For over seven years, he has studied the molecular mechanisms of Acinetobacter pathogenesis, including Acinetobacter surface appendages, secretion systems, and glycoconjugates. He is now Co-Founder and Chief Scientific Officer of VaxNewMo LLC, a biotech startup dedicated to making conjugate vaccines using a glycoengineering approach in the lab safe Escherichia coli.
Seth W. Hennon earned a Ph.D. in Biochemistry at The Ohio State University studying membrane protein biogenesis. He is currently a postdoctoral researcher in the laboratory of Mario Feldman at the Washington University in St. Louis, where his research focuses on the biogenesis of the Type VI secretion system in Acinetobacter.
Mario F. Feldman obtained his Ph.D. at the University of Buenos Aires, Argentina. During his postdoctoral training, he focused on the study of type 3 secretion systems and on bacterial protein glycosylation. He started as independent researcher in 2006 at the University of Alberta, Canada, then moved to Washington University in St Louis in 2015. His group investigates the glycobiology and pathogenesis of Acinetobacter, and the biogenesis of outer membrane vesicles. He is the founder of VaxAlta Inc. and VaxNewMo LLC., companies focused on antibacterial vaccines.
Footnotes
There is NO Competing Interest.
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Further Reading:
List of Prokaryotic names with Standing in Nomenclature: http://www.bacterio.net/acinetobacter.html
References
- 1.Giammanco A, Cala C, Fasciana T & Dowzicky MJ Global Assessment of the Activity of Tigecycline against Multidrug-Resistant Gram-Negative Pathogens between 2004 and 2014 as Part of the Tigecycline Evaluation and Surveillance Trial. mSphere 2, doi: 10.1128/mSphere.00310-16 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rolain JM et al. Real-time sequencing to decipher the molecular mechanism of resistance of a clinical pan-drug-resistant Acinetobacter baumannii isolate from Marseille, France. Antimicrob Agents Chemother 57, 592–596, doi: 10.1128/AAC.01314-12 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Magill SS et al. Multistate point-prevalence survey of health care-associated infections. N Engl J Med 370, 1198–1208, doi: 10.1056/NEJMoa1306801 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lob SH, Hoban DJ, Sahm DF & Badal RE Regional differences and trends in antimicrobial susceptibility of Acinetobacter baumannii. Int J Antimicrob Agents 47, 317–323, doi: 10.1016/j.ijantimicag.2016.01.015 (2016). [DOI] [PubMed] [Google Scholar]
- 5.(CDC), C. f. D. C. a. P. Antibiotic Resistance Threats in the United States, 2013, <https://www.cdc.gov/drugresistance/pdf/ar-threats-2013-508.pdf> (2013).
- 6.Organization WH (2017). [Google Scholar]
- 7.Mari-Almirall M et al. MALDI-TOF/MS identification of species from the Acinetobacter baumannii (Ab) group revisited: inclusion of the novel A. seifertii and A. dijkshoorniae species. Clin Microbiol Infect, doi: 10.1016/j.cmi.2016.11.020 (2016). [DOI] [PubMed] [Google Scholar]
- 8.Sievert DM et al. Antimicrobial-resistant pathogens associated with healthcare-associated infections: summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2009–2010. Infect Control Hosp Epidemiol 34, 1–14, doi: 10.1086/668770 (2013). [DOI] [PubMed] [Google Scholar]
- 9.Weiner LM et al. Antimicrobial-Resistant Pathogens Associated With Healthcare-Associated Infections: Summary of Data Reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2011–2014. Infect Control Hosp Epidemiol 37, 1288–1301, doi: 10.1017/ice.2016.174 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dexter C, Murray GL, Paulsen IT & Peleg AY Community-acquired Acinetobacter baumannii: clinical characteristics, epidemiology and pathogenesis. Expert Rev Anti Infect Ther 13, 567–573, doi: 10.1586/14787210.2015.1025055 (2015). [DOI] [PubMed] [Google Scholar]
- 11.Touchon M et al. The genomic diversification of the whole Acinetobacter genus: origins, mechanisms, and consequences. Genome Biol Evol 6, 2866–2882, doi: 10.1093/gbe/evu225 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bouvet PJM & Grimont PAD Taxonomy of the Genus Acinetobacter with the Recognition of Acinetobacter-Baumannii Sp-Nov, Acinetobacter-Haemolyticus Sp-Nov, Acinetobacter-Johnsonii Sp-Nov, and Acinetobacter-Junii Sp-Nov and Emended Descriptions of Acinetobacter-Calcoaceticus and Acinetobacter-Lwoffii. Int J Syst Bacteriol 36, 228–240 (1986). [Google Scholar]
- 13.Nemec A et al. Genotypic and phenotypic characterization of the Acinetobacter calcoaceticus-Acinetobacter baumannii complex with the proposal of Acinetobacter pittii sp. nov. (formerly Acinetobacter genomic species 3) and Acinetobacter nosocomialis sp. nov. (formerly Acinetobacter genomic species 13TU). Res Microbiol 162, 393–404, doi: 10.1016/j.resmic.2011.02.006 (2011). [DOI] [PubMed] [Google Scholar]
- 14.Nemec A et al. Acinetobacter seifertii sp. nov., a member of the Acinetobacter calcoaceticus-Acinetobacter baumannii complex isolated from human clinical specimens. Int J Syst Evol Microbiol 65, 934–942, doi: 10.1099/ijs.0.000043 (2015). [DOI] [PubMed] [Google Scholar]
- 15.Cosgaya C et al. Acinetobacter dijkshoorniae sp. nov., a member of the Acinetobacter calcoaceticus-Acinetobacter baumannii complex mainly recovered from clinical samples in different countries. Int J Syst Evol Microbiol 66, 4105–4111, doi: 10.1099/ijsem.0.001318 (2016). [DOI] [PubMed] [Google Scholar]
- 16.Espinal P, Seifert H, Dijkshoorn L, Vila J & Roca I Rapid and accurate identification of genomic species from the Acinetobacter baumannii (Ab) group by MALDI-TOF MS. Clin Microbiol Infect 18, 1097–1103, doi: 10.1111/j.1469-0691.2011.03696.x (2012). [DOI] [PubMed] [Google Scholar]
- 17.Chusri S et al. Clinical outcomes of hospital-acquired infection with Acinetobacter nosocomialis and Acinetobacter pittii. Antimicrob Agents Chemother 58, 4172–4179, doi: 10.1128/AAC.02992-14 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wong D et al. Clinical and Pathophysiological Overview of Acinetobacter Infections: a Century of Challenges. Clin Microbiol Rev 30, 409–447, doi: 10.1128/CMR.00058-16 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roca I, Espinal P, Vila-Farres X & Vila J The Acinetobacter baumannii Oxymoron: Commensal Hospital Dweller Turned Pan-Drug-Resistant Menace. Front Microbiol 3, 148, doi: 10.3389/fmicb.2012.00148 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Giannouli M et al. Virulence-related traits of epidemic Acinetobacter baumannii strains belonging to the international clonal lineages I-III and to the emerging genotypes ST25 and ST78. BMC Infect Dis 13, 282, doi: 10.1186/1471-2334-13-282 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Antunes LC, Imperi F, Carattoli A & Visca P Deciphering the multifactorial nature of Acinetobacter baumannii pathogenicity. PLoS One 6, e22674, doi: 10.1371/journal.pone.0022674 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ophir T & Gutnick DL A role for exopolysaccharides in the protection of microorganisms from desiccation. Appl Environ Microbiol 60, 740–745 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scott NE et al. Diversity within the O-linked protein glycosylation systems of acinetobacter species. Mol Cell Proteomics 13, 2354–2370, doi: 10.1074/mcp.M114.038315 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Espinal P, Marti S & Vila J Effect of biofilm formation on the survival of Acinetobacter baumannii on dry surfaces. J Hosp Infect 80, 56–60, doi: 10.1016/j.jhin.2011.08.013 (2012). [DOI] [PubMed] [Google Scholar]
- 25.Boll JM et al. Reinforcing Lipid A Acylation on the Cell Surface of Acinetobacter baumannii Promotes Cationic Antimicrobial Peptide Resistance and Desiccation Survival. MBio 6, e00478–00415, doi: 10.1128/mBio.00478-15 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bravo Z et al. The long-term survival of Acinetobacter baumannii ATCC 19606(T) under nutrient-deprived conditions does not require the entry into the viable but non-culturable state. Arch Microbiol 198, 399–407, doi: 10.1007/s00203-016-1200-1 (2016). [DOI] [PubMed] [Google Scholar]
- 27.Potts M Desiccation tolerance of prokaryotes. Microbiol Rev 58, 755–805 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aranda J et al. Acinetobacter baumannii RecA protein in repair of DNA damage, antimicrobial resistance, general stress response, and virulence. J Bacteriol 193, 3740–3747, doi: 10.1128/JB.00389-11 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Norton MD, Spilkia AJ & Godoy VG Antibiotic resistance acquired through a DNA damage-inducible response in Acinetobacter baumannii. J Bacteriol 195, 1335–1345, doi: 10.1128/JB.02176-12 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gayoso CM et al. Molecular mechanisms involved in the response to desiccation stress and persistence in Acinetobacter baumannii. J Proteome Res 13, 460–476, doi: 10.1021/pr400603f (2014). [DOI] [PubMed] [Google Scholar]
- 31.Derecho I, McCoy KB, Vaishampayan P, Venkateswaran K & Mogul R Characterization of hydrogen peroxide-resistant Acinetobacter species isolated during the Mars Phoenix spacecraft assembly. Astrobiology 14, 837–847, doi: 10.1089/ast.2014.1193 (2014). [DOI] [PubMed] [Google Scholar]
- 32.Nemec A et al. Acinetobacter beijerinckii sp. nov. and Acinetobacter gyllenbergii sp. nov., haemolytic organisms isolated from humans. Int J Syst Evol Microbiol 59, 118–124, doi: 10.1099/ijs.0.001230-0 (2009). [DOI] [PubMed] [Google Scholar]
- 33.Wright MS, Mountain S, Beeri K & Adams MD Assessment of insertion sequence mobilization as an adaptive response to oxidative stress in Acinetobacter baumannii using IS-Seq. J Bacteriol, doi: 10.1128/JB.00833-16 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hassan KA et al. Transcriptomic and biochemical analyses identify a family of chlorhexidine efflux proteins. Proc Natl Acad Sci U S A 110, 20254–20259, doi: 10.1073/pnas.1317052110 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nwugo CC et al. Effect of ethanol on differential protein production and expression of potential virulence functions in the opportunistic pathogen Acinetobacter baumannii. PLoS One 7, e51936, doi: 10.1371/journal.pone.0051936 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Camarena L, Bruno V, Euskirchen G, Poggio S & Snyder M Molecular mechanisms of ethanol-induced pathogenesis revealed by RNA-sequencing. PLoS Pathog 6, e1000834, doi: 10.1371/journal.ppat.1000834 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Smith MG, Des Etages SG & Snyder M Microbial synergy via an ethanol-triggered pathway. Mol Cell Biol 24, 3874–3884 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Asplund MB, Coelho C, Cordero RJ & Martinez LR Alcohol impairs J774.16 macrophage-like cell antimicrobial functions in Acinetobacter baumannii infection. Virulence 4, 467–472, doi: 10.4161/viru.25641 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thompson MG et al. Validation of a novel murine wound model of Acinetobacter baumannii infection. Antimicrob Agents Chemother 58, 1332–1342, doi: 10.1128/AAC.01944-13 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Greene C, Wu J, Rickard AH & Xi C Evaluation of the ability of Acinetobacter baumannii to form biofilms on six different biomedical relevant surfaces. Lett Appl Microbiol 63, 233–239, doi: 10.1111/lam.12627 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Greene C, Vadlamudi G, Newton D, Foxman B & Xi C The influence of biofilm formation and multidrug resistance on environmental survival of clinical and environmental isolates of Acinetobacter baumannii. Am J Infect Control 44, e65–71, doi: 10.1016/j.ajic.2015.12.012 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tomaras AP, Flagler MJ, Dorsey CW, Gaddy JA & Actis LA Characterization of a two-component regulatory system from Acinetobacter baumannii that controls biofilm formation and cellular morphology. Microbiology 154, 3398–3409, doi: 10.1099/mic.0.2008/019471-0 (2008). [DOI] [PubMed] [Google Scholar]
- 43.Tomaras AP, Dorsey CW, Edelmann RE & Actis LA Attachment to and biofilm formation on abiotic surfaces by Acinetobacter baumannii: involvement of a novel chaperone-usher pili assembly system. Microbiology 149, 3473–3484, doi: 10.1099/mic.0.26541-0 (2003). [DOI] [PubMed] [Google Scholar]
- 44.de Breij A et al. CsuA/BABCDE-dependent pili are not involved in the adherence of Acinetobacter baumannii ATCC19606(T) to human airway epithelial cells and their inflammatory response. Res Microbiol 160, 213–218, doi: 10.1016/j.resmic.2009.01.002 (2009). [DOI] [PubMed] [Google Scholar]
- 45.Wright MS, Iovleva A, Jacobs MR, Bonomo RA & Adams MD Genome dynamics of multidrug-resistant Acinetobacter baumannii during infection and treatment. Genome Med 8, 26, doi: 10.1186/s13073-016-0279-y (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cerqueira GM et al. A global virulence regulator in Acinetobacter baumannii and its control of the phenylacetic acid catabolic pathway. J Infect Dis 210, 46–55, doi: 10.1093/infdis/jiu024 (2014). [DOI] [PubMed] [Google Scholar]
- 47.Moon KH, Weber BS & Feldman MF Subinhibitory concentrations of trimethoprim and sulfamethoxazole prevent biofilm formation by Acinetobacter baumannii through inhibition of Csu pili expression. Antimicrob Agents Chemother, doi: 10.1128/AAC.00778-17 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Marti S et al. Growth of Acinetobacter baumannii in pellicle enhanced the expression of potential virulence factors. PLoS One 6, e26030, doi: 10.1371/journal.pone.0026030 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Eijkelkamp BA, Stroeher UH, Hassan KA, Paulsen IT & Brown MH Comparative analysis of surface-exposed virulence factors of Acinetobacter baumannii. BMC Genomics 15, 1020, doi: 10.1186/1471-2164-15-1020 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Loehfelm TW, Luke NR & Campagnari AA Identification and characterization of an Acinetobacter baumannii biofilm-associated protein. J Bacteriol 190, 1036–1044, doi: 10.1128/JB.01416-07 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cucarella C et al. Bap, a Staphylococcus aureus surface protein involved in biofilm formation. J Bacteriol 183, 2888–2896, doi: 10.1128/JB.183.9.2888-2896.2001 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Harding CM et al. Pathogenic Acinetobacter Species have a Functional Type I Secretion System and Contact-Dependent Inhibition Systems. J Biol Chem, doi: 10.1074/jbc.M117.781575 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Goh HM et al. Molecular analysis of the Acinetobacter baumannii biofilm-associated protein. Appl Environ Microbiol 79, 6535–6543, doi: 10.1128/AEM.01402-13 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.De Gregorio E et al. Biofilm-associated proteins: news from Acinetobacter. BMC Genomics 16, 933, doi: 10.1186/s12864-015-2136-6 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Satchell KJ Structure and function of MARTX toxins and other large repetitive RTX proteins. Annu Rev Microbiol 65, 71–90, doi: 10.1146/annurev-micro-090110-102943 (2011). [DOI] [PubMed] [Google Scholar]
- 56.Choi AH, Slamti L, Avci FY, Pier GB & Maira-Litran T The pgaABCD locus of Acinetobacter baumannii encodes the production of poly-beta-1–6-N-acetylglucosamine, which is critical for biofilm formation. J Bacteriol 191, 5953–5963, doi: 10.1128/JB.00647-09 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bentancor LV, O’Malley JM, Bozkurt-Guzel C, Pier GB & Maira-Litran T Poly-N-acetyl-beta-(1–6)-glucosamine is a target for protective immunity against Acinetobacter baumannii infections. Infect Immun 80, 651–656, doi: 10.1128/IAI.05653-11 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Russo TA et al. The K1 capsular polysaccharide of Acinetobacter baumannii strain 307–0294 is a major virulence factor. Infect Immun 78, 3993–4000, doi: 10.1128/IAI.00366-10 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Iwashkiw JA et al. Identification of a general O-linked protein glycosylation system in Acinetobacter baumannii and its role in virulence and biofilm formation. PLoS Pathog 8, e1002758, doi: 10.1371/journal.ppat.1002758 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bentancor LV, Camacho-Peiro A, Bozkurt-Guzel C, Pier GB & Maira-Litran T Identification of Ata, a multifunctional trimeric autotransporter of Acinetobacter baumannii. J Bacteriol 194, 3950–3960, doi: 10.1128/JB.06769-11 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Eijkelkamp BA et al. H-NS plays a role in expression of Acinetobacter baumannii virulence features. Infect Immun 81, 2574–2583, doi: 10.1128/IAI.00065-13 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Perez-Varela M et al. Mutants in the beta-subunit of the RNA polymerase impairing the surface-associated motility and virulence of Acinetobacter baumannii. Infect Immun, doi: 10.1128/IAI.00327-17 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vijayakumar S et al. Biofilm Formation and Motility Depend on the Nature of the Acinetobacter baumannii Clinical Isolates. Front Public Health 4, 105, doi: 10.3389/fpubh.2016.00105 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Eijkelkamp BA et al. Adherence and motility characteristics of clinical Acinetobacter baumannii isolates. FEMS Microbiol Lett 323, 44–51, doi: 10.1111/j.1574-6968.2011.02362.x (2011). [DOI] [PubMed] [Google Scholar]
- 65.Wilharm G, Piesker J, Laue M & Skiebe E DNA uptake by the nosocomial pathogen Acinetobacter baumannii occurs during movement along wet surfaces. J Bacteriol 195, 4146–4153, doi: 10.1128/JB.00754-13 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Harding CM et al. Acinetobacter baumannii strain M2 produces type IV pili which play a role in natural transformation and twitching motility but not surface-associated motility. MBio 4, doi: 10.1128/mBio.00360-13 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jacobs AC et al. Characterization of the Acinetobacter baumannii growth phase-dependent and serum responsive transcriptomes. FEMS Immunol Med Microbiol 64, 403–412, doi: 10.1111/j.1574-695X.2011.00926.x (2012). [DOI] [PubMed] [Google Scholar]
- 68.Piepenbrink KH et al. Structural Diversity in the Type IV Pili of Multidrug-resistant Acinetobacter. J Biol Chem 291, 22924–22935, doi: 10.1074/jbc.M116.751099 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Henrichsen J Not gliding but twitching motility of Acinetobacter calcoaceticus. J Clin Pathol 37, 102–103 (1984). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Clemmer KM, Bonomo RA & Rather PN Genetic analysis of surface motility in Acinetobacter baumannii. Microbiology 157, 2534–2544, doi: 10.1099/mic.0.049791-0 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kearns DB A field guide to bacterial swarming motility. Nat Rev Microbiol 8, 634–644, doi: 10.1038/nrmicro2405 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Skiebe E et al. Surface-associated motility, a common trait of clinical isolates of Acinetobacter baumannii, depends on 1,3-diaminopropane. Int J Med Microbiol 302, 117–128, doi: 10.1016/j.ijmm.2012.03.003 (2012). [DOI] [PubMed] [Google Scholar]
- 73.McQueary CN et al. Extracellular stress and lipopolysaccharide modulate Acinetobacter baumannii surface-associated motility. J Microbiol 50, 434–443, doi: 10.1007/s12275-012-1555-1 (2012). [DOI] [PubMed] [Google Scholar]
- 74.Mussi MA et al. The opportunistic human pathogen Acinetobacter baumannii senses and responds to light. J Bacteriol 192, 6336–6345, doi: 10.1128/JB.00917-10 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tipton KA, Dimitrova D & Rather PN Phase-Variable Control of Multiple Phenotypes in Acinetobacter baumannii Strain AB5075. J Bacteriol 197, 2593–2599, doi: 10.1128/JB.00188-15 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tipton KA & Rather PN An ompR/envZ Two-Component System Ortholog Regulates Phase Variation, Osmotic Tolerance, Motility, and Virulence in Acinetobacter baumannii strain AB5075. J Bacteriol, doi: 10.1128/JB.00705-16 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Garcia-Quintanilla M, Pulido MR, Lopez-Rojas R, Pachon J & McConnell MJ Emerging therapies for multidrug resistant Acinetobacter baumannii. Trends Microbiol 21, 157–163, doi: 10.1016/j.tim.2012.12.002 (2013). [DOI] [PubMed] [Google Scholar]
- 78.Peleg AY & Hooper DC Hospital-acquired infections due to gram-negative bacteria. N Engl J Med 362, 1804–1813, doi: 10.1056/NEJMra0904124 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Adams MD et al. Resistance to colistin in Acinetobacter baumannii associated with mutations in the PmrAB two-component system. Antimicrob Agents Chemother 53, 3628–3634, doi: 10.1128/AAC.00284-09 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Beceiro A et al. Phosphoethanolamine modification of lipid A in colistin-resistant variants of Acinetobacter baumannii mediated by the pmrAB two-component regulatory system. Antimicrob Agents Chemother 55, 3370–3379, doi: 10.1128/AAC.00079-11 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Beceiro A et al. Biological cost of different mechanisms of colistin resistance and their impact on virulence in Acinetobacter baumannii. Antimicrob Agents Chemother 58, 518–526, doi: 10.1128/AAC.01597-13 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pelletier MR et al. Unique structural modifications are present in the lipopolysaccharide from colistin-resistant strains of Acinetobacter baumannii. Antimicrob Agents Chemother 57, 4831–4840, doi: 10.1128/AAC.00865-13 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Moffatt JH et al. Colistin resistance in Acinetobacter baumannii is mediated by complete loss of lipopolysaccharide production. Antimicrob Agents Chemother 54, 4971–4977, doi: 10.1128/AAC.00834-10 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Boll JM et al. A penicillin-binding protein inhibits selection of colistin-resistant, lipooligosaccharide-deficient Acinetobacter baumannii. Proc Natl Acad Sci U S A 113, E6228–E6237, doi: 10.1073/pnas.1611594113 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Henry R et al. Colistin-resistant, lipopolysaccharide-deficient Acinetobacter baumannii responds to lipopolysaccharide loss through increased expression of genes involved in the synthesis and transport of lipoproteins, phospholipids, and poly-beta-1,6-N-acetylglucosamine. Antimicrob Agents Chemother 56, 59–69, doi: 10.1128/AAC.05191-11 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lees-Miller RG et al. A common pathway for O-linked protein-glycosylation and synthesis of capsule in Acinetobacter baumannii. Mol Microbiol 89, 816–830, doi: 10.1111/mmi.12300 (2013). [DOI] [PubMed] [Google Scholar]
- 87.Harding CM et al. Acinetobacter strains carry two functional oligosaccharyltransferases, one devoted exclusively to type IV pilin, and the other one dedicated to O-glycosylation of multiple proteins. Mol Microbiol 96, 1023–1041, doi: 10.1111/mmi.12986 (2015). [DOI] [PubMed] [Google Scholar]
- 88.Geisinger E & Isberg RR Antibiotic modulation of capsular exopolysaccharide and virulence in Acinetobacter baumannii. PLoS Pathog 11, e1004691, doi: 10.1371/journal.ppat.1004691 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wang N, Ozer EA, Mandel MJ & Hauser AR Genome-wide identification of Acinetobacter baumannii genes necessary for persistence in the lung. MBio 5, e01163–01114, doi: 10.1128/mBio.01163-14 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Subashchandrabose S et al. Acinetobacter baumannii Genes Required for Bacterial Survival during Bloodstream Infection. mSphere 1, doi: 10.1128/mSphere.00013-15 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gebhardt MJ et al. Joint Transcriptional Control of Virulence and Resistance to Antibiotic and Environmental Stress in Acinetobacter baumannii. MBio 6, e01660–01615, doi: 10.1128/mBio.01660-15 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Antunes LC, Imperi F, Towner KJ & Visca P Genome-assisted identification of putative iron-utilization genes in Acinetobacter baumannii and their distribution among a genotypically diverse collection of clinical isolates. Res Microbiol 162, 279–284, doi: 10.1016/j.resmic.2010.10.010 (2011). [DOI] [PubMed] [Google Scholar]
- 93.Shapiro JA & Wencewicz TA Acinetobactin Isomerization Enables Adaptive Iron Acquisition in Acinetobacter baumannii through pH-Triggered Siderophore Swapping. ACS Infect Dis 2, 157–168, doi: 10.1021/acsinfecdis.5b00145 (2016). [DOI] [PubMed] [Google Scholar]
- 94.Gaddy JA et al. Role of acinetobactin-mediated iron acquisition functions in the interaction of Acinetobacter baumannii strain ATCC 19606T with human lung epithelial cells, Galleria mellonella caterpillars, and mice. Infect Immun 80, 1015–1024, doi: 10.1128/IAI.06279-11 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Proschak A et al. Structure and biosynthesis of fimsbactins A-F, siderophores from Acinetobacter baumannii and Acinetobacter baylyi. Chembiochem 14, 633–638, doi: 10.1002/cbic.201200764 (2013). [DOI] [PubMed] [Google Scholar]
- 96.Penwell WF et al. Discovery and Characterization of New Hydroxamate Siderophores, Baumannoferrin A and B, produced by Acinetobacter baumannii. Chembiochem, doi: 10.1002/cbic.201500147 (2015). [DOI] [PubMed] [Google Scholar]
- 97.Zackular JP, Chazin WJ & Skaar EP Nutritional Immunity: S100 Proteins at the Host-Pathogen Interface. J Biol Chem 290, 18991–18998, doi: 10.1074/jbc.R115.645085 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Moore JL et al. Imaging mass spectrometry for assessing temporal proteomics: analysis of calprotectin in Acinetobacter baumannii pulmonary infection. Proteomics 14, 820–828, doi: 10.1002/pmic.201300046 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hood MI et al. Identification of an Acinetobacter baumannii zinc acquisition system that facilitates resistance to calprotectin-mediated zinc sequestration. PLoS Pathog 8, e1003068, doi: 10.1371/journal.ppat.1003068 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mortensen BL, Rathi S, Chazin WJ & Skaar EP Acinetobacter baumannii response to host-mediated zinc limitation requires the transcriptional regulator Zur. J Bacteriol 196, 2616–2626, doi: 10.1128/JB.01650-14 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Nairn BL et al. The Response of Acinetobacter baumannii to Zinc Starvation. Cell Host Microbe 19, 826–836, doi: 10.1016/j.chom.2016.05.007 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Juttukonda LJ, Chazin WJ & Skaar EP Acinetobacter baumannii Coordinates Urea Metabolism with Metal Import To Resist Host-Mediated Metal Limitation. MBio 7, doi: 10.1128/mBio.01475-16 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Johnson TL, Waack U, Smith S, Mobley H & Sandkvist M Acinetobacter baumannii Is Dependent on the Type II Secretion System and Its Substrate LipA for Lipid Utilization and In Vivo Fitness. J Bacteriol 198, 711–719, doi: 10.1128/JB.00622-15 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Harding CM, Kinsella RL, Palmer LD, Skaar EP & Feldman MF Medically Relevant Acinetobacter Species Require a Type II Secretion System and Specific Membrane-Associated Chaperones for the Export of Multiple Substrates and Full Virulence. PLoS Pathog 12, e1005391, doi: 10.1371/journal.ppat.1005391 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Liu CC, Kuo HY, Tang CY, Chang KC & Liou ML Prevalence and mapping of a plasmid encoding a type IV secretion system in Acinetobacter baumannii. Genomics 104, 215–223, doi: 10.1016/j.ygeno.2014.07.011 (2014). [DOI] [PubMed] [Google Scholar]
- 106.Weber BS et al. Genomic and functional analysis of the type VI secretion system in Acinetobacter. PLoS One 8, e55142, doi: 10.1371/journal.pone.0055142 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Carruthers MD, Nicholson PA, Tracy EN & Munson RS Jr. Acinetobacter baumannii utilizes a type VI secretion system for bacterial competition. PLoS One 8, e59388, doi: 10.1371/journal.pone.0059388 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Weber BS, Kinsella RL, Harding CM & Feldman MF The Secrets of Acinetobacter Secretion. Trends Microbiol, doi: 10.1016/j.tim.2017.01.005 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bentancor LV et al. Evaluation of the trimeric autotransporter Ata as a vaccine candidate against Acinetobacter baumannii infections. Infect Immun 80, 3381–3388, doi: 10.1128/IAI.06096-11 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Weber BS, Ly PM, Irwin JN, Pukatzki S & Feldman MF A multidrug resistance plasmid contains the molecular switch for type VI secretion in Acinetobacter baumannii. Proc Natl Acad Sci U S A 112, 9442–9447, doi: 10.1073/pnas.1502966112 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kinsella RL et al. Defining the interaction of the protease CpaA with its type II secretion-chaperone CpaB and its contribution to virulence in Acinetobacter species. J Biol Chem, doi: 10.1074/jbc.M117.808394 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Tilley D, Law R, Warren S, Samis JA & Kumar A CpaA a novel protease from Acinetobacter baumannii clinical isolates deregulates blood coagulation. FEMS Microbiol Lett 356, 53–61, doi: 10.1111/1574-6968.12496 (2014). [DOI] [PubMed] [Google Scholar]
- 113.Chan AP et al. A novel method of consensus pan-chromosome assembly and large-scale comparative analysis reveal the highly flexible pan-genome of Acinetobacter baumannii. Genome Biol 16, 143, doi: 10.1186/s13059-015-0701-6 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Bhuiyan MS et al. Acinetobacter baumannii phenylacetic acid metabolism influences infection outcome through a direct effect on neutrophil chemotaxis. Proc Natl Acad Sci U S A 113, 9599–9604, doi: 10.1073/pnas.1523116113 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Cabral MP et al. Design of live attenuated bacterial vaccines based on D-glutamate auxotrophy. Nat Commun 8, 15480, doi: 10.1038/ncomms15480 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Jacobs AC et al. AB5075, a Highly Virulent Isolate of Acinetobacter baumannii, as a Model Strain for the Evaluation of Pathogenesis and Antimicrobial Treatments. MBio 5, e01076-01014, doi: 10.1128/mBio.01076-14 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lee JC et al. Adherence of Acinetobacter baumannii strains to human bronchial epithelial cells. Res Microbiol 157, 360–366, doi: 10.1016/j.resmic.2005.09.011 (2006). [DOI] [PubMed] [Google Scholar]
- 118.Weidensdorfer M et al. Analysis of Endothelial Adherence of Bartonella henselae and Acinetobacter baumannii Using a Dynamic Human Ex Vivo Infection Model. Infect Immun 84, 711–722, doi: 10.1128/IAI.01502-15 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Brossard KA & Campagnari AA The Acinetobacter baumannii biofilm-associated protein plays a role in adherence to human epithelial cells. Infect Immun 80, 228–233, doi: 10.1128/IAI.05913-11 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Choi CH, Lee JS, Lee YC, Park TI & Lee JC Acinetobacter baumannii invades epithelial cells and outer membrane protein A mediates interactions with epithelial cells. BMC Microbiol 8, 216, doi: 10.1186/1471-2180-8-216 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Noto MJ et al. Toll-Like Receptor 9 Contributes to Defense against Acinetobacter baumannii Infection. Infect Immun 83, 4134–4141, doi: 10.1128/IAI.00410-15 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Bist P et al. The Nod1, Nod2, and Rip2 axis contributes to host immune defense against intracellular Acinetobacter baumannii infection. Infect Immun 82, 1112–1122, doi: 10.1128/IAI.01459-13 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.March C et al. Dissection of host cell signal transduction during Acinetobacter baumannii-triggered inflammatory response. PLoS One 5, e10033, doi: 10.1371/journal.pone.0010033 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Feng Z et al. Epithelial innate immune response to Acinetobacter baumannii challenge. Infect Immun 82, 4458–4465, doi: 10.1128/IAI.01897-14 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Breslow JM et al. Innate immune responses to systemic Acinetobacter baumannii infection in mice: neutrophils, but not interleukin-17, mediate host resistance. Infect Immun 79, 3317–3327, doi: 10.1128/IAI.00069-11 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.van Faassen H et al. Neutrophils play an important role in host resistance to respiratory infection with Acinetobacter baumannii in mice. Infect Immun 75, 5597–5608, doi: 10.1128/IAI.00762-07 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Qiu H, Kuolee R, Harris G & Chen W Role of NADPH phagocyte oxidase in host defense against acute respiratory Acinetobacter baumannii infection in mice. Infect Immun 77, 1015–1021, doi: 10.1128/IAI.01029-08 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Weber BS et al. Genetic Dissection of the Type VI Secretion System in Acinetobacter and Identification of a Novel Peptidoglycan Hydrolase, TagX, Required for Its Biogenesis. MBio 7, doi: 10.1128/mBio.01253-16 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Arroyo LA et al. The pmrCAB operon mediates polymyxin resistance in Acinetobacter baumannii ATCC 17978 and clinical isolates through phosphoethanolamine modification of lipid A. Antimicrob Agents Chemother 55, 3743–3751, doi: 10.1128/AAC.00256-11 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Chin CY, Gregg KA, Napier BA, Ernst RK & Weiss DS A PmrB-Regulated Deacetylase Required for Lipid A Modification and Polymyxin Resistance in Acinetobacter baumannii. Antimicrob Agents Chemother 59, 7911–7914, doi: 10.1128/AAC.00515-15 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]


