Abstract
This study aimed to compare the efficacy and safety of drug-eluting bead transarterial chemoembolization (DEB-TACE) vs conventional TACE (cTACE) in hepatocellular carcinoma (HCC) patients with multiple cTACE treatments history.
Eighty-one HCC patients with multiple cTACE treatments history who underwent DEB-TACE (N = 42) and cTACE treatment (N = 39) were included in this retrospective cohort study and allocated to DEB-TACE and cTACE groups accordingly. Multiple cTACE treatments history was defined as history of three or more cycles cTACE treatments. Then treatment responses were assessed according to the criteria of modified Response Evaluation Criteria in Solid Tumors (mRECIST), and progression free survival (PFS), as well as overall survival (OS), was calculated. In addition, adverse events and liver function related indexes were recorded.
Complete response (P = .167) was of no difference while objective response rate (ORR) (P = .003) was increased in DEB-TACE group compared with cTACE group. Patients in DEB-TACE group presented with more favorable PFS (P = .028) and OS (P = .037) compared with cTACE group. Further analysis revealed that DEB-TACE (vs cTACE) was an independent predictive factor for better ORR (P = .001), PFS (P = .006) and OS (P = .001). The albumin (ALB) level at first month after treatment was elevated (P = .015) while the other liver function indexes levels did not vary (all P > .05) in DEB-TACE group compared with cTACE group. The incidences of pain (P = .327), fever (P = .171) and nausea/vomiting (P = .400) during hospitalization were similar between the 2 groups.
DEB-TACE is more efficient and equally tolerant compared with cTACE in HCC patients with multiple cTACE treatments history.
Keywords: cTACE, drug-eluting bead transarterial chemoembolization, efficacy, hepatocellular carcinoma, multiple conventional transarterial chemoembolization (cTACE) treatments, safety
1. Introduction
Hepatocellular carcinoma (HCC), accounts for approximately 70% to 90% of all primary liver cancers, is the sixth most common and the third most fatal cancer worldwide.[1,2] HCC patients have been suffering from poor prognosis with 5-year survival being roughly 10% to 15% for decades despite the progress in screening, diagnosis, and treatment, which is mainly resulted from that most patients are already in the moderate or advanced stage at diagnosis, whom can only receive palliative treatments.[3,4] Among all the palliative therapies, transarterial chemoembolization (TACE) is recommended as standard therapy for inoperable HCC patients, however, as a disease requires long-term and consistent treatment, HCC always need multiple cycles of TACE treatments.[4,5] Moreover, as the most widely applied TACE treatment, conventional TACE (cTACE) presents with a problem of cTACE refractoriness in HCC patients, which is defined as a deficient response after 2 or more cycles of TACE (including cTACE) treatments.[6]
In recent years, despite that cTACE discloses an anti-tumor effect and survival benefits in HCC patients, the relatively high incidence of systemic toxicity induced by the diffusion to peripheral circulation of the chemotherapy drug loaded lipiodol, has led to the introduction of drug-eluting bead TACE (DEB-TACE).[7] DEB-TACE uses microspheres as drug carriers, which substantially reduces the concentration of chemotherapeutic in the systemic circulation of HCC patients and advances the drug concentration in tumor tissue.[8] Nonetheless, whether DEB-TACE is superior to cTACE in regard to efficacy and safety in HCC patients remains to be controversial, for instance, a meta-analysis reports that the treatment response rate and overall survival (OS) rate are increased and common adverse event rate is reduced in HCC patients receiving DEB-TACE compared with patients receiving cTACE, while another meta-analysis demonstrates that there is no difference of OS between the 2 TACE treatments.[9,10] Considering that multiple cTACE treatments history might affect the outcomes of followed repeated cTACE operation due to potential cTACE refractoriness, we hypothesized that DEB-TACE may improve the efficacy compared with cTACE in HCC patients with multiple cTACE treatments history.
Thus, the aim of our study was to compare the efficacy and safety of DEB-TACE vs cTACE in treating HCC patients with multiple cTACE treatments history.
2. Methods
2.1. Patients
From Jan 2015 to Dec 2017, 81 HCC patients who underwent the therapy of DEB-TACE or cTACE in Jining No.1 People's Hospital were included in this retrospective cohort study. The inclusion criteria were as follows:
-
1.
diagnosed as primary HCC confirmed by clinical and pathological findings according to American Association for the Study of the Liver Diseases (AASLD) guidelines;
-
2.
received DEB-TACE or cTACE treatment after relapse;
-
3.
with history of multiple cycles of cTACE treatments which was defined as 3 or more cycles cTACE (for patients underwent multiple times of TACE after 3 or more cycles cTACE, they were included only once for the first time of TACE treatment after three or more cycles cTACE);
-
4.
medical records were complete.
Following patients were excluded:
-
1.
patients with secondary liver cancer;
-
2.
patients with DEB-TACE history;
-
3.
patients who switched treatment from DEB-TACE to cTACE within 6 months;
-
4.
patients who lost follow up without any follow-up records.
Finally, 42 patients who underwent DEB-TACE therapy after 3 or more cycles of cTACE treatments were assigned to DEB-TACE group, and 39 patients who continued to receive cTACE therapy after 3 or more cycles of cTACE treatments were assigned to cTACE group, accordingly. This study was approved by the Ethics Committee of Jining No.1 People's Hospital. All the patients or their legal guardian provided the written informed consents.
2.2. Baseline characteristics collection
Baseline characteristics of patients were obtained from electronic medical records, which consisted of
-
1.
demographic information including age and gender,
-
2.
history of drink, hepatitis B (HB) and cirrhosis,
-
3.
disease features including tumor location, tumor distribution, largest nodule size, portal vein invasion, hepatic vein invasion, extrahepatic disease, Eastern Cooperative Oncology Group (ECOG) performance status, Child-pugh stage, and Barcelona Clinic Liver Cancer (BCLC) stage,
-
4.
history of treatments including cycles of previous cTACE, surgery, chemotherapy, and radiofrequency ablation.
2.3. Treatment procedures of DEB-TACE and cTACE
CalliSpheres microspheres (CSMs) (Jiangsu Hengrui Medicine Co., Ltd., Jiangsu Province, China) with diameters of 300 to 500 μm were used as chemoembolization reagent carriers and embolization agents in the DEB-TACE treatment; ethiodized poppyseed oil (EPO) (Jiangsu Hengrui Medicine Co., Ltd., Jiangsu Province, China) as drug carriers and Polyvinyl Alcohol (PVA) particles (Cook Medical LLC, Bloomington, IN) as embolization agents were applied in cTACE treatment. Before the DEB-TACE operation, the CSMs were loaded with pirarubicin and mixed with contrast agent following the manufacturer's instructions. All DEB-TACE and cTACE procedures were conducted in the digital subtraction angiography (DSA) room. At the initiation of DEB-TACE or cTACE operation, the hepatic angiography was carried out to detect the tumor supplying vessels under local anesthesia, then the microcatheter with diameter of 2.4F (Merit Maestro, Merit Medical System, Inc., UT) was inserted into the tumor supplying vessel that was led by a microwire. Subsequently, the mixture of preparative CSMs for the DEB-TACE treatment or the mixture of EPO, PVA, and pirarubicin for cTACE treatment was infused through the microcatheter and stopped when the flow of contrast agent stagnated. Five minutes after the embolization, the angiography was performed again to detect whether there was incomplete embolization or not. If there were remaining blushed nodules, the embolization would be continued until no more blushed tumors occurred. After completion of embolization, the microcatheter was pulled out, and the wound was bandaged.
2.4. Assessments of response and survival
Treatment response was evaluated by enhanced CT or MRI examination at first month after the operation of DEB-TACE or cTACE. According to the criteria of modified Response Evaluation Criteria in Solid Tumors (mRECIST), treatment response was classified as follows:
-
1.
complete response (CR): disappearance of any intratumoral arterial enhancement in all target lesions;
-
2.
partial response (PR): at least a 30% decrease in the sum of diameters of viable (enhancement in the arterial phase) target lesions;
-
3.
stable disease (SD): any cases that did not qualify either PR or progressive disease (PD);
-
4.
PD: an increase of at least 20% in the sum of the diameters of the viable (enhancing) target lesions.
Moreover, objective response rate (ORR) was defined as the percentage of patients who achieved CR or PR. After DEB-TACE or cTACE therapy, regular follow up was performed. Patients were followed up until Sep 2018 with a median follow-up duration of 15.0 months (range: 6.0–34.0 months). For the assessment of survival, progression free survival (PFS) was calculated from the date of the treatment to the date of disease progression or death. OS was calculated from the date of the treatment to the date of death.
2.5. Assessment of safety
Liver function indexes measured before and first month after treatment and adverse events occurred during hospitalization were used to assess safety of DEB-TACE and cTACE therapy. Liver function indexes included albumin (ALB), total protein (TP), total bilirubin (TBIL), total bile acid (TBA), alanine aminotransferase (ALT), aspartate aminotransferase (AST) and alkaline phosphatase (ALP), and the adverse events included pain, fever, nausea or vomiting.
2.6. Statistical analysis
Statistical analysis was performed with the use of SPSS 22.0 statistical software (SPSS Inc, Chicago, USA), and figures were made by GraphPad Prism 7.01 software (GraphPad Software Inc., San Diego, IL). Normal distributed continuous variable was presented as mean value ± standard deviation, skewed distributed continuous variable was described as median (25th–75th), and categorized variable was expressed as count (percentage). Comparison between 2 groups was determined by t test, Wilcoxon rank sum test or Chi-square test. Survival analysis was performed using Kaplan–Meier method and log-rank test. Univariate and multivariate logistic regression analyses with Forward Stepwise (Conditional) method were used to determine factors affecting ORR; and univariate and multivariate Cox's proportional hazards regression analyses with Forward Stepwise (Conditional LR) method were applied to determine the prognostic factors of PFS and OS. P value <.05 was considered significant.
3. Results
3.1. Baseline characteristics
No difference was discovered between DEB-TACE group and cTACE group in all the baseline characteristics (Table 1). The mean age was 53.9 ± 10.6 years in DEB-TACE group and was 56.6 ± 14.6 years in cTACE group (P = .343). The numbers of males and females were 37 (88.1%) and 5 (11.9%) in DEB-TACE group and were 33 (84.6%) and 6 (15.4%) in cTACE group (P = .648). In addition, the mean largest nodule size in DEB-TACE group and cTACE group were 7.8 ± 3.2 cm and 6.7 ± 4.0 cm (P = .167), and there were 11 (26.2%) and 6 (15.4%) patients with portal vein invasion in the 2 groups, respectively (P = .233). The numbers of patients in BCLC A, B, C, and D stage were 5 (11.9%), 22 (52.4%), 14 (33.3%) and 1 (2.4%) in the DEB-TACE group, and were 8 (20.5%), 22 (56.4%), 7 (17.9%) and 2 (5.1%) in the cTACE group (P = .345). Moreover, 25 (59.5%) patients had 3 cycles of cTACE and 17 (40.5%) patients had ≥4 cycles of cTACE in DEB-TACE group, and the numbers of patients with 3 cycles and ≥4 cycles of cTACE were 28 (71.8%) and 11 (28.2%) in cTACE group (P = .246). The other baseline characteristics of patients in the 2 groups were listed in Table 1.
Table 1.
HCC patients’ characteristics at baseline.
3.2. Comparisons of treatment responses between the 2 groups
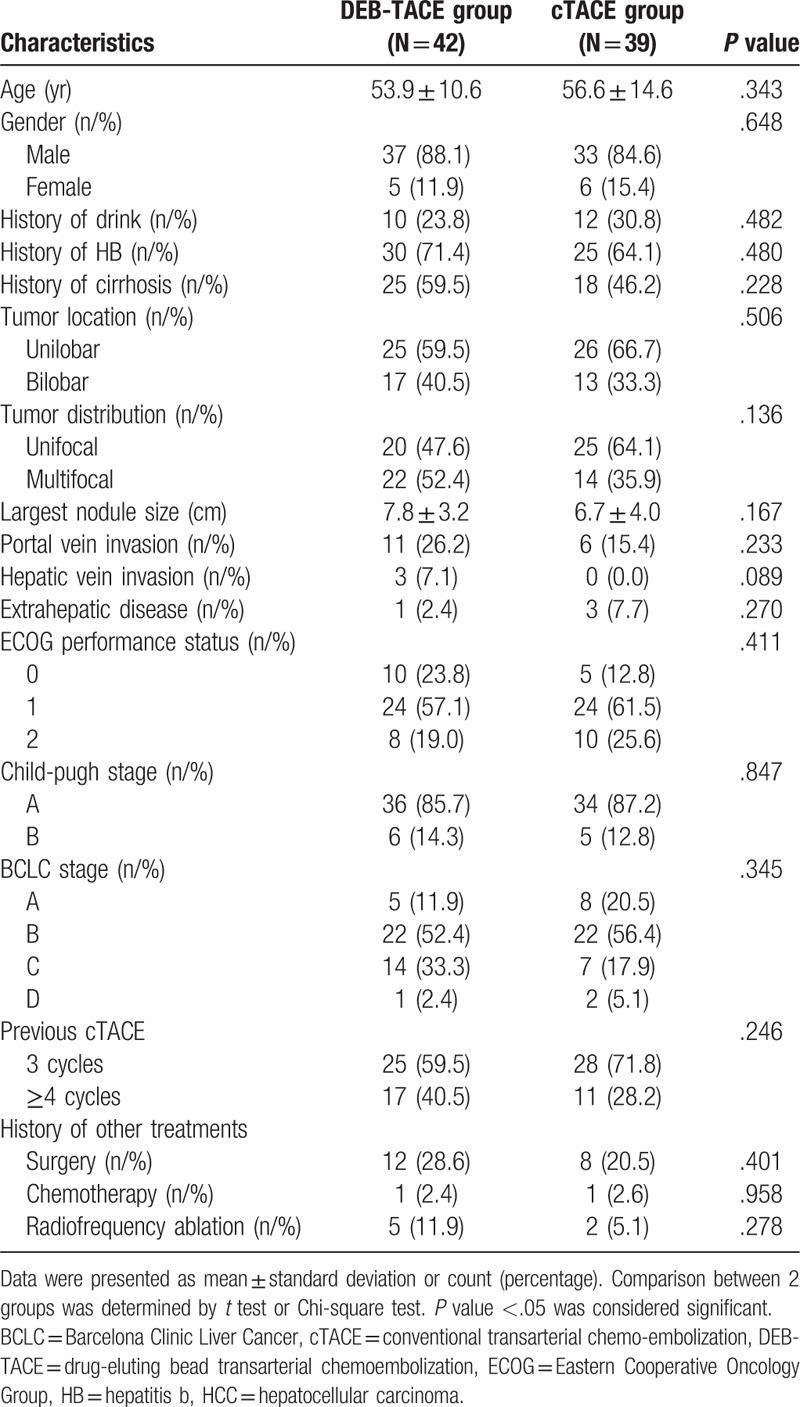
No difference of CR was found between DEB-TACE group (14.3%) and cTACE group (5.1%) (P = .167), however, the ORR was increased in DEB-TACE group (73.8%) than that in cTACE group (41.0%) (P = .003) (Fig. 1).
Figure 1.
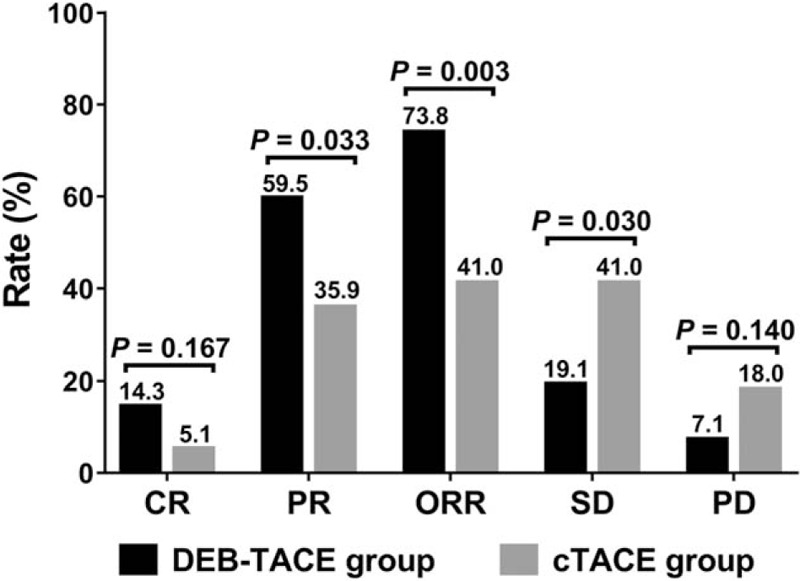
Treatment responses between the 2 groups. CR did not vary while ORR was increased in DEB-TACE group compared with cTACE group. Comparison between 2 groups was determined by Chi-square test. P < .05 was considered significant. CR = complete response, cTACE = conventional transarterial chemoembolization, DEB-TACE = drug-eluting bead transarterial chemoembolization, ORR = objective response.
3.3. Comparison of survival profile between the 2 groups
After treatment, patients in DEB-TACE group presented with more favorable PFS compared with cTACE group (P = .028) (Fig. 2A), and the mean values of PFS in DEB-TACE group and cTACE group were 26.7 months (95%CI: 23.3–30.2) and 19.5 months (95%CI: 15.7–23.2), respectively. Similarly, the OS was also better in DEB-TACE group than that in cTACE group (P = .037) (Fig. 2B), and the mean values of OS in the 2 groups were 29.8 months (95%CI: 26.9–32.6) and 23.0 months (95%CI: 19.3–26.8), respectively.
Figure 2.
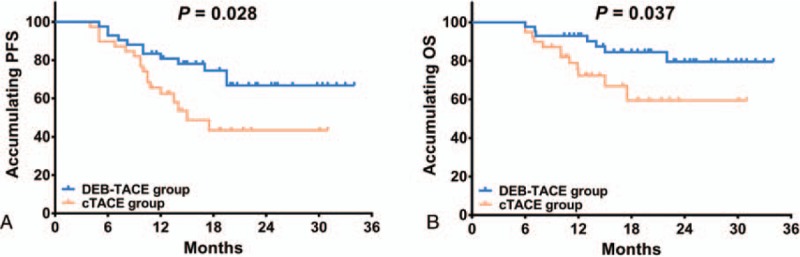
PFS and OS between the 2 groups. The PFS (A) and OS (B) were both more favorable in DEB-TACE group than those in cTACE group. Kaplan-Meier curves and log-rank test were performed to evaluate the PFS and OS between the 2 groups. P < .05 was considered significant. cTACE = conventional transarterial chemoembolization, DEB-TACE = drug-eluting bead transarterial chemoembolization, OS = overall survival, PFS = progression free survival.
3.4. Analysis of predictive factors for ORR
Univariate logistic regression model analysis revealed that DEB-TACE (vs cTACE) was correlated with higher ORR (P = .003), while portal vein invasion (vs no portal vein invasion) (P = .038) and BCLC stage C/D (vs BCLC stage A/B) (P = .017) were associated with worse ORR (Table 2). Afterward, all the factors were included in the multivariate logistic regression model analysis using Forward Stepwise (Conditional) method, which disclosed that DEB-TACE (vs cTACE) (P = .001) was an independent predictive factor for better ORR, while BCLC stage C/D (vs BCLC stage A/B) (P = .004) was an independent factor for predicting unfavorable ORR.
Table 2.
Factors affecting ORR by logistic regression model analysis.
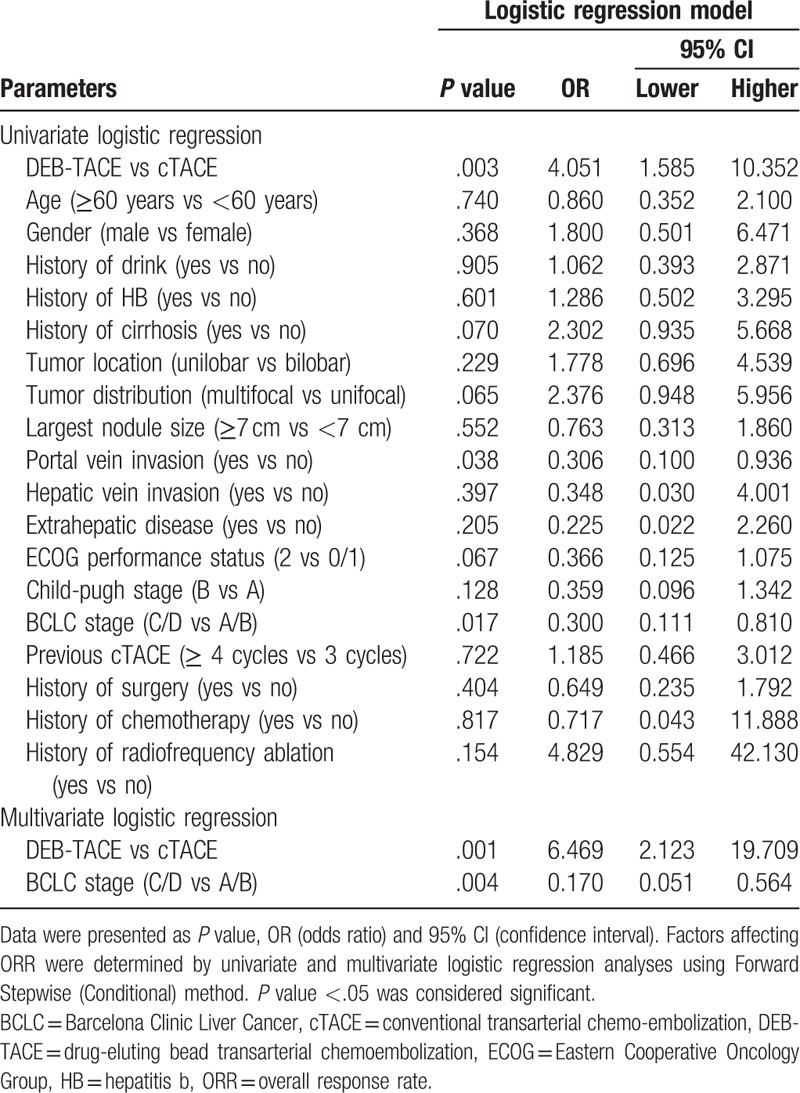
3.5. Analysis of predictive factors for survival
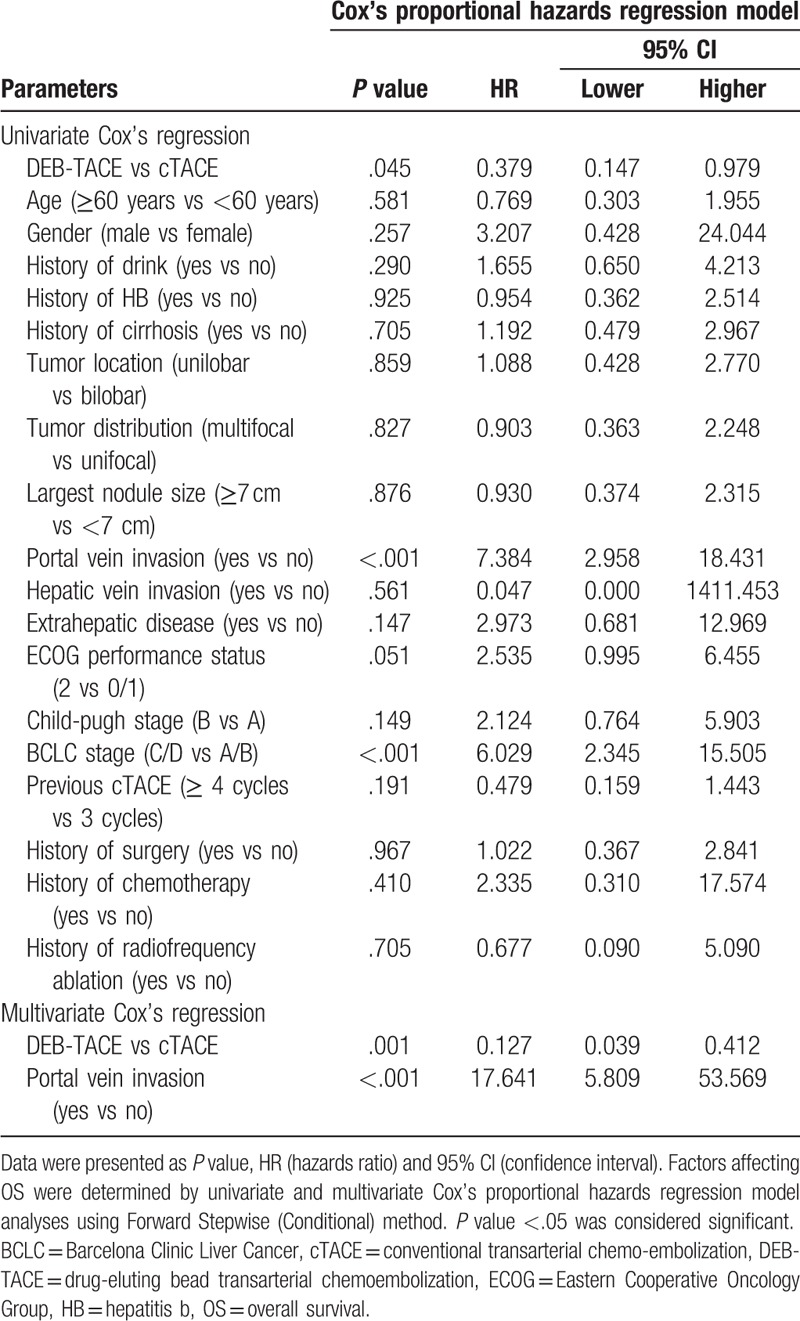
The univariate and multivariate Cox proportional hazards regression model analyses were performed to evaluate the factors affecting PFS, and the univariate Cox proportional hazards regression model analysis revealed that DEB-TACE (vs cTACE) (P = .034) associated with longer PFS, however, portal vein invasion (vs no portal vein invasion) (P = .036) and BCLC stage C/D (vs BCLC stage A/B) (P = .025) correlated with shorter PFS (Table 3). Then the multivariate Cox proportional hazards regression model analysis using Forward Stepwise (Conditional) method displayed that DEB-TACE (vs cTACE) (P = .006) was an independent predictive factor for better PFS, while, largest nodule size ≥7 cm (vs largest nodule size <7 cm) (P = .041) and BCLC stage C/D (vs BCLC stage A/B) (P = .028) were independent pejorative factors for PFS. As for OS, the univariate Cox proportional hazards regression model elucidated that DEB-TACE (vs cTACE) (P = .045) was associated with more favorable OS, while portal vein invasion (vs no portal vein invasion) (P < .001) and BCLC stage C/D (vs BCLC stage A/B) (P < .001) were correlated with unfavorable OS (Table 4). Furthermore, the multivariate Cox proportional hazards regression model analysis revealed that DEB-TACE (vs cTACE) (P = .001) was independently correlated with more prolonged OS, however, portal vein invasion (vs no portal vein invasion) (P < .001) was independently associated with shorter OS.
Table 3.
Cox's proportional hazards regression model analysis of factors affecting PFS.
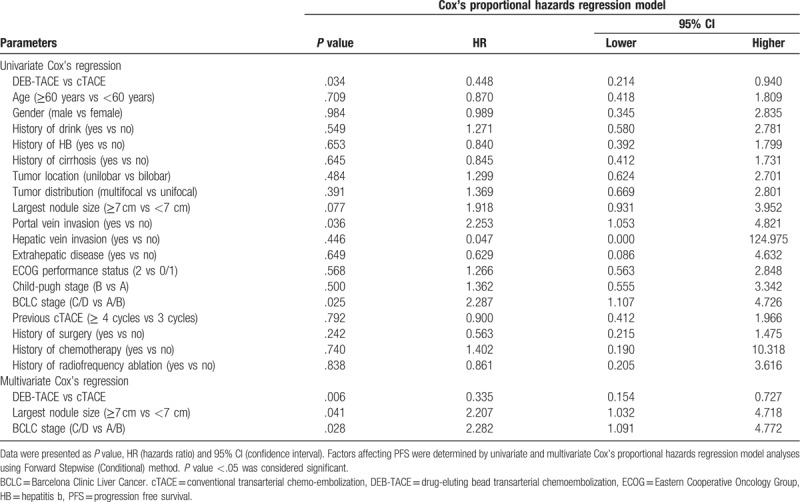
Table 4.
Cox's proportional hazards regression model analysis of factors affecting OS.
3.6. Comparison of liver function between the 2 groups
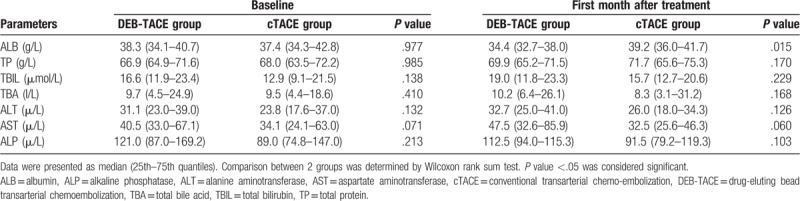
At baseline, the ALB (P = .977), TP (P = .985), TBIL (P = .138), TBA (P = .410), ALT (P = .132), AST (P = .071) and ALP (P = .213) levels were of no difference between DEB-TACE group and cTACE group (Table 5). However, at the first month after treatment, the ALB level (P = .015) was decreased in DEB-TACE group compared with cTACE group, while the levels of TP (P = .170), TBIL (P = .229), TBA (P = .168), ALT (P = .126), AST (P = .060) and ALP (P = .103) levels were similar between the 2 groups.
Table 5.
Liver function indexes before and after treatment.
3.7. Comparison of adverse events incidences between the 2 groups
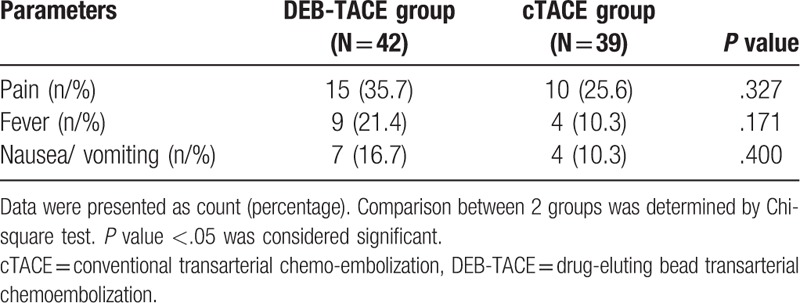
During hospitalization, no difference was found in adverse events incidences between the 2 groups (Table 6), and the numbers of patients with pain were 15 (35.7%) and 10 (25.6%) in DEB-TACE group and cTACE group, respectively (P = .3276). In addition, 9 (21.4%) patients had fever in DEB-TACE group and 4 (10.3%) patients had fever in cTACE group (P = .171). And the numbers of patients presented with nausea/vomiting were 7 (16.7%) and 4 (10.3%) in DEB-TACE group and cTACE group, respectively (P = .400).
Table 6.
Adverse events occurred during hospitalization.
4. Discussion
In this study, we discovered that in HCC patients with multiple cTACE treatments history:
-
1.
the ORR was increased in DEB-TACE group compared with cTACE group, and DEB-TACE independently correlated with better ORR;
-
2.
PFS and OS were all more favorable in DEB-TACE group than those in cTACE group, and DEB-TACE was independent predictive factors for both better PFS and OS;
-
3.
the ALB level was decreased in DEB-TACE group compared with cTACE group, while no difference of AEs during hospitalization was found between the 2 groups.
To our best knowledge, there are very few studies that aim at comparing the efficacy of DEB-TACE and cTACE in HCC patients with multiple cTACE treatments history, most of the studies are conducted in total HCC patients other than cases with history of multiple cTACE treatments. For instance, a retrospective cohort study illustrates that the disease control rate (DCR) is higher in HCC patients treated by DEB-TACE compared with patients treated by cTACE.[11] And another retrospective cohort study reports that the ORR and DCR in HCC patients are both increased in DEB-TACE group than those in cTACE group.[12] Similar results are also observed in clinical trials, a randomized controlled trial elucidates that the ORR and DCR are increased in HCC patients treated by DEB-TACE than those in patients receiving cTACE.[13] However, there is a retrospective cohort study elucidating that the DCR at 1 month after treatment is of no difference in HCC patients treated by DEB-TACE compared with patients receiving cTACE.[14] In this study, we found that in patients with history of multiple cTACE treatments, patients achieved better ORR after DEB-TACE treatment compared with patients treated by cTACE, which may be resulted from that:
-
1.
the drug-eluting beads that were used in DEB-TACE procedure reduced the drug diffusion to the peripheral system and increased the local drug concentration, which enhanced the anti-tumor effect of the chemotherapeutic;[15]
-
2.
the HCC patients with multiple cTACE treatments history may develop cTACE refractoriness, which largely contributed to the reduced treatment response in HCC patients with multiple cTACE treatments history who were treated by cTACE compared with patients treated by DEB-TACE.
Prolonging survival is the primary objective in the management of HCC patients who cannot receive curative therapies. However, in HCC patients with multiple cTACE treatments history, the existence of cTACE refractoriness has led to question about the survival benefit of continuing cTACE treatment in HCC patients.[16–18,15–17] Studies that compare the survival profile in total HCC patients other than cases with history of multiple cTACE treatments treated by DEB-TACE and cTACE reveal rather controversial results. For example, a retrospective cohort study elucidates that the OS rate is higher in HCC patients who are treated with DEB-TACE compared with patients treated by cTACE.[12] However, a retrospective case-control study reveals that no difference of the median survival of HCC patients is found between DEB-TACE group and cTACE group.[19] No study has been done to compare the survival benefit between DEB-TACE and cTACE in HCC patients with multiple cTACE treatments history. In our study, we found that the PFS and OS were both more prolonged in DEB-TACE group compared with cTACE group, which could be explained by that the multiple cTACE treatments history might induce cTACE refractoriness in HCC patients and lead to a worse ORR, which subsequently resulted in a worse survival in patients treated by cTACE compared with patients treated by DEB-TACE in our study.
In addition, we also compared the safety profile, which included the liver function indexes levels and incidences of adverse events. A previous retrospective cohort study elucidates that the ALT, AST, and TBIL levels are decreased in normal HCC patients treated by DEB-TACE compared with patients treated by cTACE.[11] And in another cohort study with large sample size, the liver function assessed by Child-pugh stage display no difference in HCC patients treated by DEB-TACE and patients treated with cTACE.[20] In this study, the results of liver function indexes levels showed that in HCC patients with multiple cTACE treatments history, at the first month after treatment, only the level of ALB was reduced in patients treated by DEB-TACE compared with patients treated by cTACE, the other liver function indexes levels were of no difference. The decreased ALB level in DEB-TACE group in our study might derive from that:
-
1.
although no significance was observed, the proportions of patients with HB, cirrhosis and history of surgery were numerically higher in DEB-TACE group compared with cTACE group, and due to that chronic liver disease and hepatic resection are demonstrated to be factors that could reduce the ALB level, therefore the patients in DEB-TACE group might have less preserved liver function to produce ALB, which finally led to the decrease of ALB level compared with patients treated by cTACE after treatment;[21,22]
-
2.
HCC patients might be more tolerant of the cTACE procedure than the DEB-TACE procedure after multiple previous cTACE treatments. As for the adverse events, chemoembolization syndrome is the most frequent adverse event in HCC patients receiving TACE treatments, including pain, fever nausea, and vomiting that are induced by embolization and tumor tissue necrosis. The studies conducted on normal HCC patients mostly reveal less adverse events in patients treated by DEB-TACE compared with patients treated with cTACE. For example, a retrospective cohort study elucidates that the complication rate in HCC patients is decreased in DEB-TACE group than that in cTACE group.[23] A randomized controlled trial reports that in HCC patients receive cTACE the post procedural pain is more frequent and severe compared with patients treated by DEB-TACE.[24] In our study, the incidences of pain, fever, and nausea/vomiting during hospitalization were of no difference between DEB-TACE group and cTACE group, which indicated that DEB-TACE and cTACE are equally tolerant in treating HCC patients with multiple cTACE treatments history.
There were still several limitations to this study:
-
1.
this was a single-center retrospective cohort study, patients were mainly from East China, which might cause selection bias;
-
2.
the sample size in our study was relatively small, which might reduce the statistical power;
-
3.
the follow-up time was rather short to observe the survival profile of patients, which should be prolonged in the future study;
-
4.
as a retrospective cohort study, there might exist confounding factors in our study, therefore a randomized controlled trial is needed in the future.
5. Conclusion
In conclusion, DEB-TACE is more efficient and equally tolerant compared with cTACE in HCC patients with multiple cTACE treatments history.
Author contributions
Data curation: Fucang Wu.
Writing – original draft: Fucang Wu, Min Duan.
Writing – review & editing: Hui Li, Guodong Zhang.
Guodong Zhang orcid: 0000-0001-6206-709X.
Footnotes
Abbreviations: AASLD = American Association for the Study of the Liver Diseases, ALB = albumin, ALP = alkaline phosphatase, ALT = alanine aminotransferase, AST = aspartate aminotransferase, BCLC = Barcelona Clinic Liver Cancer, CSMs = CalliSpheres microspheres, cTACE = conventional TACE, DCR = disease control rate, DEB-TACE = drug-eluting bead transarterial chemoembolization, DSA = digital subtraction angiography, ECOG = Eastern Cooperative Oncology Group, EPO = ethiodized poppyseed oil, HB = hepatitis B, HCC = hepatocellular carcinoma, mRECIST = modified Response Evaluation Criteria in Solid Tumors, ORR = objective response rate, OS = overall survival, PD = progressive disease, PFS = progression free survival, PR = partial response, PVA = Polyvinyl Alcohol, SD = stable disease, TACE = transarterial chemoembolization, TBA = total bile acid, TBIL = total bilirubin, TP = total protein.
The authors have no funding and conflicts of interest to disclose.
References
- [1].IARC. Fact sheets by Population-Globocan-IARC.; Available from: http://globocan.iarc.fr/Pages/fact_sheets_population.aspx. [Google Scholar]
- [2].London WT MK. Liver cancer 2006. [Google Scholar]
- [3].De Angelis R, Sant M, Coleman MP, et al. Cancer survival in Europe 1999–2007 by country and age: results of EUROCARE–5-a population-based study. Lancet Oncol 2014;15:23–34. [DOI] [PubMed] [Google Scholar]
- [4].Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet 2018;391:1301–14. [DOI] [PubMed] [Google Scholar]
- [5].Liu Y, Huang W, He M, et al. Efficacy and safety of CalliSpheres(R) drug-eluting beads transarterial chemoembolization in Barcelona Clinic Liver Cancer stage C patients. Oncol Res 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Kudo M, Matsui O, Izumi N, et al. Transarterial chemoembolization failure/refractoriness: JSH-LCSGJ criteria 2014 update. Oncology 2014;87Suppl 1:22–31. [DOI] [PubMed] [Google Scholar]
- [7].Baur J, Ritter CO, Germer CT, et al. Transarterial chemoembolization with drug-eluting beads versus conventional transarterial chemoembolization in locally advanced hepatocellular carcinoma. Hepat Med 2016;8:69–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Song JE, Kim DY. Conventional vs drug-eluting beads transarterial chemoembolization for hepatocellular carcinoma. World J Hepatol 2017;9:808–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Zou JH, Zhang L, Ren ZG, et al. Efficacy and safety of cTACE versus DEB-TACE in patients with hepatocellular carcinoma: a meta-analysis. J Dig Dis 2016;17:510–7. [DOI] [PubMed] [Google Scholar]
- [10].Xie ZB, Wang XB, Peng YC, et al. Systematic review comparing the safety and efficacy of conventional and drug-eluting bead transarterial chemoembolization for inoperable hepatocellular carcinoma. Hepatol Res 2015;45:190–200. [DOI] [PubMed] [Google Scholar]
- [11].Wu B, Zhou J, Ling G, et al. CalliSpheres drug-eluting beads versus lipiodol transarterial chemoembolization in the treatment of hepatocellular carcinoma: a short-term efficacy and safety study. World J Surg Oncol 2018;16:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Song MJ, Chun HJ, Song DS, et al. Comparative study between doxorubicin-eluting beads and conventional transarterial chemoembolization for treatment of hepatocellular carcinoma. J Hepatol 2012;57:1244–50. [DOI] [PubMed] [Google Scholar]
- [13].Lammer J, Malagari K, Vogl T, et al. Prospective randomized study of doxorubicin-eluting-bead embolization in the treatment of hepatocellular carcinoma: results of the PRECISION V study. Cardiovasc Intervent Radiol 2010;33:41–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Lee YK, Jung KS, Kim DY, et al. Conventional versus drug-eluting beads chemoembolization for hepatocellular carcinoma: emphasis on the impact of tumor size. J Gastroenterol Hepatol 2017;32:487–96. [DOI] [PubMed] [Google Scholar]
- [15].Zhang S, Huang C, Li Z, et al. Comparison of pharmacokinetics and drug release in tissues after transarterial chemoembolization with doxorubicin using diverse lipiodol emulsions and CalliSpheres Beads in rabbit livers. Drug Deliv 2017;24:1011–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Raoul JL, Gilabert M, Piana G. How to define transarterial chemoembolization failure or refractoriness: a European perspective. Liver Cancer 2014;3:119–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Kudo M, Izumi N, Kokudo N, et al. Management of hepatocellular carcinoma in Japan: Consensus-Based Clinical Practice Guidelines proposed by the Japan Society of Hepatology (JSH) 2010 updated version. Dig Dis 2011;29:339–64. [DOI] [PubMed] [Google Scholar]
- [18].Park JW, Amarapurkar D, Chao Y, et al. Consensus recommendations and review by an International Expert Panel on Interventions in Hepatocellular Carcinoma (EPOIHCC). Liver Int 2013;33:327–37. [DOI] [PubMed] [Google Scholar]
- [19].Cheung AH, Lam CS, Tam HS, et al. Nine-year experience of doxorubicin-eluting beads chemoembolization for hepatocellular carcinoma. Hepatobiliary Pancreat Dis Int 2016;15:493–8. [DOI] [PubMed] [Google Scholar]
- [20].Kloeckner R, Weinmann A, Prinz F, et al. Conventional transarterial chemoembolization versus drug-eluting bead transarterial chemoembolization for the treatment of hepatocellular carcinoma. BMC Cancer 2015;15:465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Kumar PA, Subramanian K. The role of ischemia modified albumin as a biomarker in patients with chronic liver disease. J Clin Diagn Res 2016;10: BC09-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Andreatos N, Amini N, Gani F, et al. Albumin-bilirubin score: predicting short-term outcomes including bile leak and post-hepatectomy liver failure following hepatic resection. J Gastrointest Surg 2017;21:238–48. [DOI] [PubMed] [Google Scholar]
- [23].Ferrer Puchol MD, la Parra C, Esteban E, et al. Comparison of doxorubicin-eluting bead transarterial chemoembolization (DEB-TACE) with conventional transarterial chemoembolization (TACE) for the treatment of hepatocellular carcinoma. Radiologia 2011;53:246–53. [DOI] [PubMed] [Google Scholar]
- [24].Golfieri R, Giampalma E, Renzulli M, et al. Randomised controlled trial of doxorubicin-eluting beads vs conventional chemoembolisation for hepatocellular carcinoma. Br J Cancer 2014;111:255–64. [DOI] [PMC free article] [PubMed] [Google Scholar]


