Abstract
The aim of this study is to determine the biological function of serum interleukin-18 (IL-18) on prognosis in acute respiratory distress syndrome (ARDS).
From October 2016 to September 2017, 150 patients with ARDS in the ICU were enrolled according to the Berlin 2012 definition. The enzyme-linked immunosorbent assay (ELISA) was used to detect the expression level of IL-18 in serum isolated from the patients. Patients were divided into survival group (82 cases) and non-survival group (68 cases) and followed up for at least 2 months. The serum IL-18 expression level on the prognosis was calculated by receiver operating characteristic curve (ROC).
The expression level of serum IL-18 was significantly higher in the non-survival group than that in the survival group (P < .05). Based on the ROC curve, the sensitivity and specificity of IL-18 as a predictor of prognosis at a cutoff of 509.5 pg/mL were 88% and 82%, respectively, and the area under the curve (RUC) was 0.84 (P < .05).
The expression level of serum IL-18 could be used to evaluate the possible outcomes of patients with ARDS.
Keywords: acute respiratory distress syndrome, interleukin-18, mortality, partial pressure of oxygen in arterial blood/fraction of inspired oxygen, prognosis
1. Introduction
Acute respiratory distress syndrome (ARDS) is a medical condition with critical disorders or critically wounded patients characterized by widespread inflammation in the lungs.[1] It is well known that ARDS is closely linked with severe pulmonary[2] or systemic infection,[3] after trauma, multiple blood transfusions, severe burns,[4] severe inflammation of the pancreas.[5] Additionally, the partial cases of ARDS were associated with large volumes of fluid used during posttrauma resuscitation.[6] As motioned above, the sepsis is a crucial cause among those factors. Although many studies have been reported that ARDS was considered as a life-threatening respiratory condition, its pathogenesis still remains unknown.
The multiple upstream signaling cascades of cytokines and pro-inflammatory compounds initiate and amplify the inflammatory response in ARDS. Based on recent reports, the secreted proteins related to pro-inflammatory cytokines maintained the dynamic balance between pro-inflammatory and anti-inflammatory mediators.[7] Lack of therapeutic approach and multiple activated signaling pathways are certainly related to the pathogenic complexity of this syndrome, because this complexity is mainly depended on the type of lung injury. Currently, ARDS results in high case fatality rate.[8] Previous studies have been shown that many new biomarkers could be used to accurately determine mortality, including uric acid,[9] soluble programmed cell death receptor-1,[10] and type III procollagen.[11] Nevertheless, there is no clear and unique biomarker for predicting mortality in ARDS.
Interleukin (IL)-18, a pro-inflammatory cytokine, was first described as an interferon (IFN)-γ-inducing factor and has crucial host defense and antitumor activities. In animal studies, enhancing IL-18 expression in tissues blocks infection, tumor growth, and metastasis using gene therapy method.[12] IL-18 is secreted from macrophages, especially Kupffer cells in liver.[13] Interestingly, it was reported that IL-18 could be quantified in bronchoalveolar lavage fluid at an early stage in pulmonary inflammatory disorders associated with increased lung vascular permeability.[14] Makabe et al[15] has reported that IL-18 expression level in the serum was significantly higher in the non-survivors of ARDS patients. The possibility is that IL-18 may coordinate the development of acute lung injury. In the present study, we mainly focus on the coorelation between serum IL-18 expression level and evaluating the prognosis of patients with ARDS.
2. Methods
2.1. Patients and ethical review
One hundred fifty patients with ARDS were enrolled from October 2016 to September 2017 in the intensive care unit of our hospital, including 72 women and 78 men with ages ranging from 25 to 60 years. These patients were diagnosed according to the Berlin 2012 definition of ARDS,[16] which is based on medical history, x-ray opacities, exclusion of cardiac causes of pulmonary edema, and partial pressure of oxygen in arterial blood (PaO2)/fraction of inspired oxygen (FiO2) ratio. Patients with diabetes mellitus, chronic renal failure, cardiovascular disorders, decompensated liver disease, and malignancies were excluded from this study to prevent bias by side effect. Moreover, plain chest x-rays and echocardiography were carried out to exclude patients with cardiovascular disorders.
The acute physiology and chronic health evaluation (APACHE II) scoring was used with patients’ admission. Serum samples were collected from each patient on admission and before initiation of any treatment for basic IL-18 and standard laboratory measurements (complete blood count, serum creatinine, and blood gases). Serum IL-18 was measured by the double-antibody sandwich enzyme-linked immunosorbent assay (ELISA). According to the previous studies, we considered that the cutoff value of serum IL-18 was 300 pg/mL. The serum IL-18 values ≥300 pg/mL were considered as the higher IL-18 group. Conversely, it considered as lower IL-18 group. The mortality rate was reported accordingly. The total ICU stay, duration of mechanical ventilation, and presence or absence of complications were recored as well. All patients or their relatives signed informed consent. The study was approved by the ethics committee of Xuzhou Third People's Hospital.
2.2. Statistical analysis
Statistical analyses were performed as described in Elshafey et al.[17] Briefly, SPSS was applied for statistical testing significance. Quantitative data were analyzed by mean ± SD using the 2-tailed student unpaired t test while the chi-squared test was used to assess the significance of qualitative data. Pearson correlation coefficient tested the correlations between the groups. IL-18 and PaO2/FiO2 levels were assessed by receiver operating characteristics (ROC) analysis. Significance was achieved when P < .05 for all tests.
3. Results
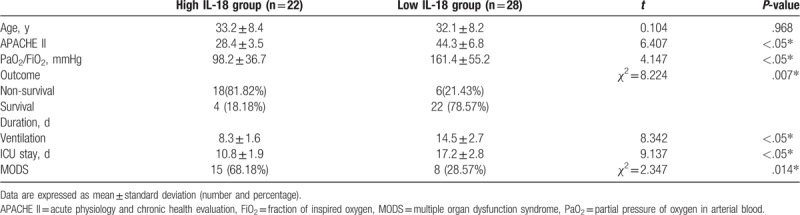
As shown in Table 1, there was no statistically significant difference with respect to age both in high and low expression level of IL-18 groups (P > .05), comparing data between these 2 groups. Strikingly, the APACHE II was significantly elevated in high IL-18 group (68.4 ± 3.5) compared with low IL-18 group (44.3 ± 6.8) (P < .05, Table 1). In addition, the PaO2/FiO2 ratio was significantly lower in high IL-18 group (94.1 ± 38.4) than that in low IL-18 group (159.3 ± 57.2) (P < .05, Table 1). Consistent to these results, the mortality rate in high IL-18 group (80.56%) was significantly higher than that in low IL-18 group (12.82%) (P < .05, Table 1). The duration of ventilation was distinctly compromised in high IL-18 group (8.3 ± 1.6) compared with low IL-18 group (14.5 ± 2.7). Further, the total ICU stay was markedly shorter in high IL-18 group (10.8 ± 1.9) compared with low IL-18 group (17.2 ± 2.8). Subsequently, the multiple organ dysfunction syndromes (MODS) were monitored and we found that MODS was frequent occurred in high IL-18 group (68.18%) than that in low IL-18 group (28.57%) (P < .05, Table 1). This result demonstrates a correlation between higher content of serum IL-18 and more serious patient outcomes.
Table 1.
Comparison between high and low serum IL-18 in ARDS patients.
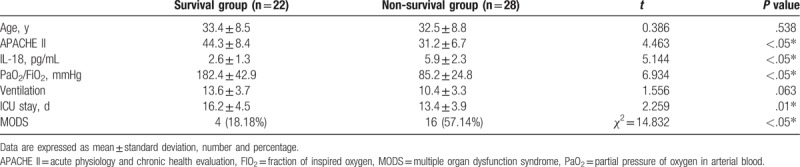
The previous results showed a highly significant correlation between mortality for patients with high level of IL-18. Comparing the data between the survival versus non-survival patients, there were also no statistically significant differences for age in either group (P > .05, Table 2). APACHE II was dramatically higher in the non-survival group (51.2 ± 6.7) than that in survival group (34.3 ± 8.4) (P < .05, Table 2). By contrast, the PaO2/FiO2 ratio was significantly lower in non-survival group (85.2 ± 24.8) than that in survival group (182.4 ± 42.9) (P < .05, Table 2). Consistent with the results from Table 1, IL-18 level in non-survival group (509.5 ± 63.7 pg/mL) was distinctly higher than that in survival group (149.8 ± 71.4 pg/mL) (P < .05, Table 2). Although the mean duration of ventilation was markedly shorter in non-survival group (9.9 ± 3.2) days compared with survival group (12.1 ± 3.4), it was not significant difference between these 2 groups (P > .05, Table 2). We also found that the total ICU stay was significantly shorter in non-survival group (13.4 ± 3.9 day) compared with (16.2 ± 4.5 day) survival group (P < .05, Table 2) and the MODS was encountered more in non-survival group (57.35%) than that in survival group (18.29%) (P < .05, Table 2). These results are consistent with the mortality associated with higher level of serum IL-18.
Table 2.
Comparison between survival and non-survival groups.
Finally, we found that there was a negative correlation between serum IL-18 and APACHE II (P < .05) and between serum IL-18 and PaO2/FiO2 (P < .05) (Table 3 and Fig. 1 A and B). The sensitivity and specificity of serum IL-18, as a reliable outcome indicator, at a cutoff 509.5 pg/mL were 88% and 82%, respectively, and the area under the curve (AUC) was 0.84 (P < .05). The sensitivity and specificity of PaO2/FiO2 at a 97 mmHg cutoff were 100% and 85%, respectively, and the AUC was 0.92 (P < .05) (Table 4 and Fig. 2A and B). Altogether, our findings strongly suggest that serum IL-18 is negative correlation with APACHE II and PaO2/FiO2.
Table 3.
Correlation between serum IL-18 on one side and APACHE II and PaO2/FiO2 on the other side.
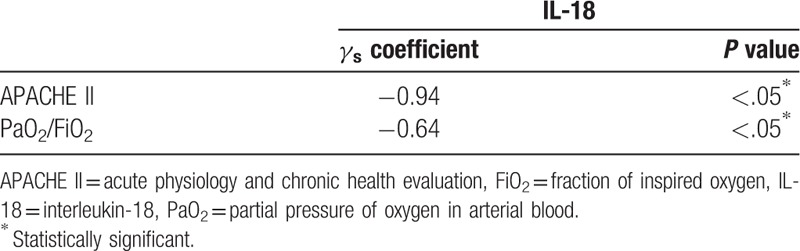
Figure 1.
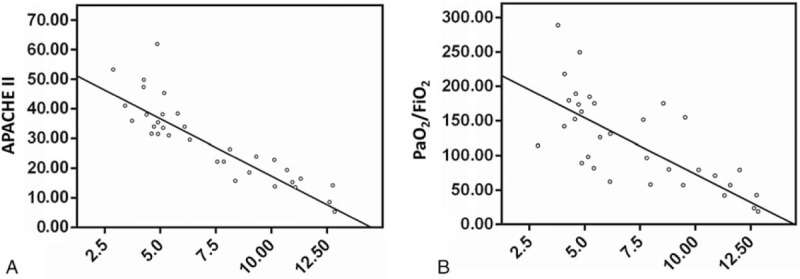
(A) Correlation between IL-18 and APACHE II; (B) Correlation between IL-18 and PaO2/FiO2. APACHE II = acute physiology and chronic health evaluation, FiO2 = fraction of inspired oxygen, IL-18 = interleukin-18, PaO2 = partial pressure of oxygen in arterial blood.
Table 4.
Sensitivity and specificity of both IL-18 and PaO2/FiO2 as outcome predictors.

Figure 2.
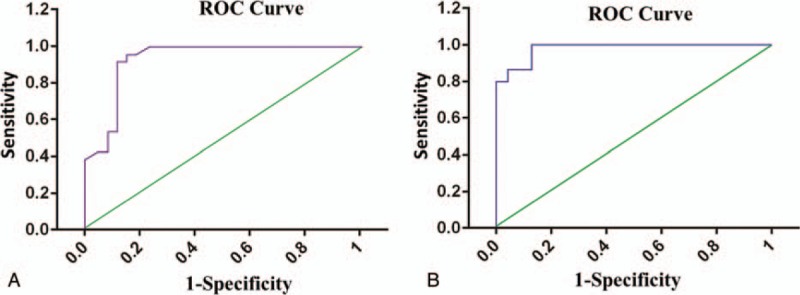
(A) ROC curve for IL-18 and (B) ROC curve for FiO2. FiO2 = fraction of inspired oxygen, IL-18 = interleukin-18, ROC = receiver operating characteristics.
4. Discussion
ARDS is the serious stage of acute lung injury. Signs and symptoms of ARDS often begin within 2 hours of an inciting event, but can occur after 1 to 3 days, including shortness of breath, fast breathing, and a low oxygen level in the blood due to abnormal ventilation. The important pathological feature of ARDS is pulmonary alveolar capillary injury.[18] The patients who suffered from respiratory distress, progressive hypoxemia, and x-ray examination showed the alveolar diffuse infiltration. Chen et al[19] reported that adrenocortical hormone administration and other anti-inflammatory treatment significantly alleviate the ARDS symptoms caused by certain pathogenic factors, indicating that pro-inflammatory and anti-inflammatory response played a crucial role in the pathogenesis of ARDS. Thus, it is a promising clinical significance to evaluate the early prognosis of ARDS patients according to the level of inflammatory factors.
IL-18 was initially reported to suppress cytokine secretion, antigen presentation, and CD4+ T cell activation.[13] IL-8 was capable to trigger Th1 cells to generate IFN-I γ under the help of L-12. It is also considered as a master regulator in different type responses. Further investigation has been shown that IL-18 predominantly prevented lipopolysaccharide (LPS) and bacterial product, mediated induction of the pro-inflammatory cytokines TNF-α and IFN-γ secretion from toll-like receptor (TLR) and triggered myeloid lineage cells.[20] Du et al[21] found that a large number of neutrophils and proinflammatory cytokines were activated in the early stage of ARDS. As a result, it destroyed the respiratory membrane, increased the permeability, and further promoted the development of ARDS. Therefore, IL-18 is considered to be a reliable promoter factor of acute lung injury[22] and detection of IL-18 in ARDS has been documented previously.[15] Dolinay et al[23] confirmed that serum IL-18 was significantly increased in patients associated with sepsis/ARDS, and it was correlated with disease severity and mortality. In the present study, serum IL-18 was detected to evaluate the prognosis of ARDS. Based on our findings, we provided a theoretical basis for the early diagnosis and treatment of ARDS and to improve the prognosis of the patients.
ARDS is a life-threatening respiratory condition characterized by hypoxemia[24] and represents a stereotypic response to many different inciting insults. The current study demonstrated that high level serum IL-18 was cloasely associated with higher mortality, more severe ARDS, and frequent occurrence of MODS. The level of serum IL-18 at 509.5 pg/mL cutoff predicted mortality in ARDS patients with 88% sensitivity and 82% specificity, respectively. It suggested that tissue injury probably caused sterile inflammation and several mediators, such as IL-2, IL-4, IL-6, IL-8, IL-18, c reaction protein, and heat shock protein 70 (HSP70) have been involved. These risky indicators were caused after lung damage, triggering inflammation, remodeling, and fibrosis.[25,26] Serum IL-18 levels significantly increase during hypoxia caused by acute lung injury[27] and elevated IL-18 levels have been associated with the presence of systemic inflammation.[28] Our results showed that APACHE II was significantly lower in ARDS patients with high level of serum IL-18 than that with low IL-18. APACHE II was also significantly lower in the non-survival group than that in survival group. There was a significant negative correlation between IL-18 and APACHE II. High APACHE II measurements are consistent with the increased severity of illness found in ARDS patients who had a higher serum IL-18 level.
The PaO2/FiO2 ratio was significantly lower in high IL-18 group than that in low IL-18 group. Additionally, PaO2/FiO2 ratio was dramatically lower in non-survival group than that in survival group. We also found that there was a significant negative correlation between IL-18 and PaO2/FiO2 ratio, suggesting that ARDS patients with severe hypoxemia had a higher serum IL-18 level. IL-18 induced a broad spectrum of COPD-like inflammatory and remodeling responses in the murine lung and mediated a mixed type 1, type 2, and type 17 cytokine responses as well.[29,30] Elevated IL-18 level was probably associated with increased inflammatory markers expression, which were also enhanced in ARDS patients. Therefore, PaO2/FiO2 ratio not only divided ARDS patients into mild, moderate, and severe, but also predicted severe outcome of patients with ARDS. In the current study, the sensitivity and specificity of PaO2/FiO2 ratio at the 97 mmHg cutoff were 100% and 82%, respectively. Our results showed that the non-survival rate of ARSD was distinctly higher in high IL-18 group (80.56%) compared with low IL-18 group (12.82%). Expectably, the serum IL-18 in non-survival group was significant higher than that in survival group (P < .05). Furthermore, the present study showed that there were more MODS in high IL-18 group than that in low IL-18 group, and the rate of MOPS was significantly higher in non-survival group than that in survival group.
In summary, our finding suggested that serum IL-18 might serve as a prognostic biomarker in ARDS. Further studies will explore the precise physiological functions of serum IL-18 in patients with ARDS. To date, the mortality of ARDS patients is very high. The accurate judgment of the patient's condition and early treatment will be helpful to greatly improve the prognosis and reduce mortality. Determining serum IL-18 is a promising approach to evaluate the prognosis of ARDS patients. It also provides a method to assess the patient's condition, facilitate early preventive medicals, and improve the prognosis of the patients with ARDS.
Author contributions
Data curation: Fei Wang.
Investigation: Min Zhu.
Methodology: Liang Xu, Bin Zhang.
Project administration: Bin Wang.
Writing – original draft: Guangsu Dong.
Footnotes
Abbreviations: APACHE II = acute physiology and chronic health evaluation, ARDS = acute respiratory distress syndrome, ELISA = enzyme-linked immunosorbent assay, FiO2 = fraction of inspired oxygen, ICU = intensive care unit, IL-18 = interleukin-18, LPS = lipopolysaccharide, MODS = multiple organ dysfunction syndrome, PaO2 = partial pressure of oxygen in arterial blood, ROC = receiver operating characteristics, TLR = toll-like receptor.
The authors declare that there is no conflict of interest.
References
- [1].Carrillo-Esper R, Vázquez-De AGF, Mejía-Pérez CI, et al. At 50 years of the description of acute respiratory distress syndrome. Gac Med Mex 2018;154:236–53. [DOI] [PubMed] [Google Scholar]
- [2].Luo J, Yu H, Hu YH, et al. Early identification of patients at risk for acute respiratory distress syndrome among severe pneumonia: a retrospective cohort study. J Thorac Dis 2017;9:3979–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Yehya N, Wong HR. Adaptation of a biomarker-based sepsis mortality risk stratification tool for pediatric acute respiratory distress syndrome. Crit Care Med 2018;46:e9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Cartotto R, Li Z, Hanna S, et al. The acute respiratory distress syndrome (ARDS) in mechanically ventilated burn patients: an analysis of risk factors, clinical features, and outcomes using the Berlin ARDS definition. Burns 2016;42:1423–32. [DOI] [PubMed] [Google Scholar]
- [5].Zhao X, Huang W, Li J, et al. Noninvasive positive-pressure ventilation in acute respiratory distress syndrome in patients with acute pancreatitis: a retrospective cohort study. Pancreas 2016;45:58–63. [DOI] [PubMed] [Google Scholar]
- [6].Marraro GA, Genovese U, Spada C, et al. Can the treatment approach of sepsis with balanced crystalloid fluids translate into therapy for acute respiratory distress syndrome if considered as “Lung-Limited Sepsis”. Crit Care Med 2017;45:1246–8. [DOI] [PubMed] [Google Scholar]
- [7].Huang SR, Ma AY, Liu Y, et al. Effects of inflammatory factors including plasma tumor necrosis factor-α in the clinical treatment of acute respiratory distress syndrome. Oncol Lett 2017;13:5016–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Butt W, Butlinski A. The outcome of patients with acute respiratory distress syndrome admitted to an ICU. Crit Care Med 2018;46:1013–4. [DOI] [PubMed] [Google Scholar]
- [9].Lee HW, Choi SM, Lee J, et al. Serum uric acid level as a prognostic marker in patients with acute respiratory distress syndrome. J Intensive Care Med 2019;34:404–10. [DOI] [PubMed] [Google Scholar]
- [10].Monaghan SF, Chung CS, Chen Y, et al. Soluble programmed cell death receptor-1 (sPD-1): a potential biomarker with anti-inflammatory properties in human and experimental acute respiratory distress syndrome (ARDS). J Transl Med 2016;14:312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Arthur A, McCall PJ, Macfie A, et al. Type III procollagen as a biomarker of susceptibility to ARDS. Intensive Care Med 2015;41:568–9. [DOI] [PubMed] [Google Scholar]
- [12].Dima E, Koltsida O, Katsaounou P, et al. Implication of Interleukin (IL)-18 in the pathogenesis of chronic obstructive pulmonary disease (COPD). Cytokine 2015;74:313–7. [DOI] [PubMed] [Google Scholar]
- [13].Nakanishi K. Unique action of interleukin-18 on T cells and other immune cells. Front Immunol 2018;9:763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Antoniou KM, Tzouvelekis A, Alexandrakis MG, et al. Upregulation of Th1 cytokine profile (IL-12, IL-18) in bronchoalveolar lavage fluid in patients with pulmonary sarcoidosis. J Interferon Cytokine Res 2006;26:400–5. [DOI] [PubMed] [Google Scholar]
- [15].Makabe H, Kojika M, Takahashi G, et al. Interleukin-18 levels reflect the long-term prognosis of acute lung injury and acute respiratory distress syndrome. J Anesth 2012;26:658–63. [DOI] [PubMed] [Google Scholar]
- [16].Ferguson ND, Fan E, Camporota L, et al. The Berlin definition of ARDS: an expanded rationale, justification, and supplementary material. Intensive Care Med 2012;38:1573–82. [DOI] [PubMed] [Google Scholar]
- [17].Elshafey M, Mossalam AMA, Makharita MY, et al. Prognostic role of serum uric acid in acute respiratory distress syndrome patients: a preliminary study. Egypt J Chest Dis Tubercul 2015;64:197–202. [Google Scholar]
- [18].Yadav H, Kor DJ. Platelets in the pathogenesis of acute respiratory distress syndrome. Am J Physiol Lung Cell Mol Physiol 2015;309:L915–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Chen C, Shi L, Li Y, et al. Disease-specific dynamic biomarkers selected by integrating inflammatory mediators with clinical informatics in ARDS patients with severe pneumonia. Cell Biol Toxicol 2016;32:169–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Reuter BK, Pizarro TT. Commentary: the role of the IL-18 system and other members of the IL-1R/TLR superfamily in innate mucosal immunity and the pathogenesis of inflammatory bowel disease: friend or foe. Eur J Immunol 2004;34:2347–55. [DOI] [PubMed] [Google Scholar]
- [21].Du H, Wang PZ, Li J, et al. Clinical characteristics and outcomes in critical patients with hemorrhagic fever with renal syndrome. BMC Infect Dis 2014;14:191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Chen S, Xu L, Tang J. Association of interleukin 18 gene polymorphism with susceptibility to the development of acute lung injury after cardiopulmonary bypass surgery. Tissue Antigens 2010;76:245–9. [DOI] [PubMed] [Google Scholar]
- [23].Dolinay T, Kim YS, Howrylak J, et al. Inflammasome-regulated cytokines are critical mediators of acute lung injury. Am J Respir Crit Care Med 2012;185:1225–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Claar DD, Hyzy RC. Refractory hypoxemia and acute respiratory distress syndrome adjunctive therapies: an open question. Ann Am Thorac Soc 2017;14:1768–9. [DOI] [PubMed] [Google Scholar]
- [25].Tokuriki S, Igarashi A, Okuno T, et al. Treatment with geranylgeranylacetone induces heat shock Protein 70 and attenuates neonatal hyperoxic lung injury in a model of bronchopulmonary dysplasia. Lung 2017;195:469–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Li T, Zhao B, Wang C, et al. Regulatory effects of hydrogen sulfide on IL-6, IL-8 and IL-10 levels in the plasma and pulmonary tissue of rats with acute lung injury. Exp Biol Med (Maywood) 2008;233:1081–7. [DOI] [PubMed] [Google Scholar]
- [27].Arndt PG, Fantuzzi G, Abraham E. Expression of interleukin-18 in the lung after endotoxemia or hemorrhage-induced acute lung injury. Am J Respir Cell Mol Biol 2000;22:708–13. [DOI] [PubMed] [Google Scholar]
- [28].Nakamura K, Asano Y, Taniguchi T, et al. Serum levels of interleukin-18-binding protein isoform a: clinical association with inflammation and pulmonary hypertension in systemic sclerosis. J Dermatol 2016;43:912–8. [DOI] [PubMed] [Google Scholar]
- [29].Briend E, Ferguson GJ, Mori M, et al. IL-18 associated with lung lymphoid aggregates drives IFNγ production in severe COPD. Respir Res 2017;18:159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Dias-Melicio LA, Fernandes RK, Rodrigues DR, et al. Interleukin-18 increases TLR4 and mannose receptor expression and modulates cytokine production in human monocytes. Mediators Inflamm 2015;2015:236839. [DOI] [PMC free article] [PubMed] [Google Scholar]


