Abstract
To evaluate 30-day mortality in human immunodeficiency virus (HIV) and non-HIV patients who acquired a healthcare-associated infection (HAI) while in an intensive care unit (ICU), and to describe the epidemiological and microbiological features of HAI in a population with HIV.
This was a retrospective cohort study that evaluated patients who acquired HAI during their stay in an Infectious Diseases ICU from July 2013 to December 2017 at a teaching hospital in Brazil.
Data were obtained from hospital infection control committee reports and medical records. Statistical analysis was performed using SPSS and a multivariate model was used to evaluate risk factors associated with 30-day mortality. Epidemiological, clinical, and microbiological characteristics of HAI in HIV and non-HIV patients and 30-day mortality were also evaluated.
Among 1045 patients, 77 (25 HIV, 52 non-HIV) patients acquired 106 HAI (31 HIV, 75 non-HIV patients). HIV patients were younger (45 vs 58 years, P = .002) and had more respiratory distress than non-HIV patients (60.0% vs 34.6%, P = .035). A high 30-day mortality was observed and there was no difference between groups (HIV, 52.0% vs non-HIV, 54.9%; P = .812). Ventilator-associated pneumonia (VAP) was more frequent in the HIV group compared with the non-HIV group (45.2% vs 26.7%, P = .063), with a predominance of Gram-negative organisms. Gram-positive agents were the most frequent cause of catheter associated-bloodstream infections in HIV patients. Although there was a high frequency of HAI caused by multidrug-resistant organisms (MDRO), no difference was observed between the groups (HIV, 77.8% vs non-HIV, 64.3%; P = .214). Age was the only independent factor associated with 30-day mortality (odds ratio [OR]: 1.05, 95% confidence interval [CI]: 1.01–1.1, P = .017), while diabetes mellitus (OR: 3.64, 95% CI: 0.84–15.8, P = .085) and the Sequential Organ-Failure Assessment (SOFA) score (OR: 1.16, 95% CI: 0.99–1.37, P = .071) had a tendency to be associated with death.
HIV infection was not associated with a higher 30-day mortality in critical care patients with a HAI. Age was the only independent risk factor associated with death. VAP was more frequent in HIV patients, probably because of the higher frequency of respiratory conditions at admission, with a predominance of Gram-negative organisms.
Keywords: critical care, health care-associated infections, human immunodeficiency virus infection, infection control, infectious diseases, intensive care units
1. Introduction
Infection with human immunodeficiency virus (HIV) and its complications have been important causes of admission to intensive care units (ICUs) since the beginning of HIV epidemics in the 1980s.[1,2] Thereafter, the introduction of combined antiretroviral therapy (ART) regimens caused a decrease in the incidence of opportunistic infections and mortality rates of individuals infected with HIV.[3–5]
The frequency of AIDS-related ICU admissions decreased in high-income countries in recent years,[6–9] and the frequency of HIV patient ICU admissions for causes unrelated to the HIV infection has remained stable.[6,8,10] Additionally, a high hospital mortality rate for HIV patients with no regular ART use and a low CD4+ T cell count who are admitted to the ICU with AIDS-related diseases has been reported in low- and middle-income countries.[11–13]
Healthcare-associated infections (HAIs) are complications that increase patients’ morbidity and mortality, the length of hospital stay, and costs, leading to a major impact on the health system.[14–16] The World Health Organization estimated that almost one-third of patients develop a HAI during their stay in an ICU in high-income countries, while this frequency is at least two to three-fold higher in low- and middle-income countries.[16] Multidrug-resistant organisms (MDRO), which are defined as organisms that are not susceptible to at least 1 agent from ≥3 antimicrobial classes, are common causative agents of HAI.[17] These infections have limited therapeutic options and are associated with an increase in mortality, length of hospital stay, and costs.[18–20]
There are few studies that have evaluated HAI in an HIV population. Authors of these studies suggested that HIV patients have an increased risk of acquiring an HAI because they have high hospitalization rates and longer hospital stays, and they have a high risk of acquiring infections considering their immunosuppressed status.[21–23] Using invasive devices, the presence of opportunistic disease, renal failure, and ICU stay are risk factors for acquiring HAI in an HIV-positive population.[24] Furthermore, there is no consensus on the most frequent HAI in HIV patients[21,25–27] and whether this population has a higher risk of acquiring HAIs caused by MDROs.[22,27]
The aim of this study was to evaluate 30-day mortality caused by HAI among patients with or without HIV infection in the ICU. Secondary goals were to describe epidemiological and microbiological characteristics of these infections in HIV patients.
2. Methods
2.1. Study participants and groups
This retrospective cohort study included individuals who developed HAI during their stay in an infectious disease ICU at a teaching hospital in São Paulo, Brazil between July 2013 and December 2017. Individuals with immunosuppressed conditions, with the exception of HIV infection, such as solid organ or hematopoietic stem cell transplantation and chronic use of corticosteroids or other immunosuppressive drugs, were excluded. Two blinded investigators evaluated all patients admitted to the ICU to determine if the individuals met the entry criteria. Only patients for whom there was agreement between the investigators about HAI occurrence were included in the study. Ethics approval was provided by the Ethical and Research Commission of the Department of Infectious Diseases at the Hospital das Clinicas School of Medicine at the University of São Paulo, Brazil.
2.2. Data gathering and definitions
Data on HAIs were obtained from hospital infection control commission reports and clinical and demographic information was obtained from medical records. The Centers for Diseases Control and Prevention criteria were used to define HAI.[28–30] Device-associated HAIs were as follows: catheter-associated bloodstream infection (CA-BSI), catheter-associated urinary tract infection (CA-UTI), ventilator-associated pneumonia (VAP), ventricular catheter-associated meningitis, and peripheral access device-associated phlebitis. Based on previous standard definitions, carbapenem, or colistin-resistant Gram-negative bacteria, vancomycin-resistant Enterococcus sp. (VRE), and methicillin-resistant Staphylococcus sp. (MRSA) were considered to be the MDROs for this study.[17]
3. Microbiology
Positive blood samples that were collected in aerobic and anaerobic bottles with liquid media and incubated with BD BACTEC (BD, Franklin Lakes, NJ), and other clinical samples were spread onto blood-agar and MacConkey plates to evaluate bacterial growth. Species identification was performed in the hospital's routine microbiology laboratory using an automated Vitek 2 (bioMérieux, Lyon, France) system from July 2013 to February 2015. This system uses bacterial-growth technology as follows: after bacteria grows on the plates, a suspension was prepared using colonies and it was inoculated onto reagent cards, which were incubated at 35.5 ± 1 °C. An optical system allows interpretation of test reactions using different wavelengths that are compared with a database, leading to identification of the organism. Antimicrobial susceptibility tests (ASTs) were also used. After February 2015, matrix-assisted laser desorption ionization-time of flight (MALDI-TOF) Vitek MS (bioMérieux, Lyon, France), which included mass spectrometry techniques to identify organisms, was used. ASTs were also performed using an automated Vitek 2 system (bioMérieux), with identification cards for Gram-positive and Gram-negative agents, according to the manufacturer instructions. Additional confirmatory ASTs, such as an E-test and disc-diffusion, were performed if necessary and were based on the Clinical and Laboratory Standards Institute (CLSI) criteria.[31]
3.1. Outcomes
The primary outcome was 30-day mortality after the first HAI episode. Secondary outcomes were occurrence of HAIs caused by MDROs and device-associated HAIs. The types of infections and causative organisms were evaluated and described for each group.
3.2. Statistical analysis
A database was created using the software SPSS Statistics Version 17.0 (SPSS Inc., Chicago, IL). Chi-square test and Fisher exact test were used to analyze categorical variables and the Mann–Whitney U test was used to analyze continuous variables. To evaluate differences between survivors and non-survivors 30 days after HAI, a logistic regression analysis was performed. Multivariate analysis was performed using the direct approach including variables with P < .2 and with biological plausibility of influencing the primary outcome. Two-tailed tests were used, and P < .05 was considered to be statistically significant.
4. Results
4.1. Characteristics of the population
During the study period, 1045 patients were admitted to the ICU and 77 (25 HIV and 52 non-HIV) developed 106 episodes of HAI (Fig. 1). Figure 2 shows the number of patients admitted to the ICU, the number of HIV patients admitted to the ICU, and the number of HAIs per month during the study period. HIV patients were younger than non-HIV patients and there was a predominance of male individuals in both groups (Table 1). No statistically significant differences in the severity indexes were observed between the groups using the Simplified Acute Physiology Score (SAPS) and Sequential Organ-Failure Assessment (SOFA) score on admission to assess the frequency of vasoactive drug use, renal replacement therapy, or mechanical ventilation during their stay in the ICU (Table 1). Drug abuse was more common among HIV patients (Table 1). The length of hospitalization and frequency of antibiotic use in the last 90 days before HAI were similar between groups (Table 1). Significant differences in C-reactive protein levels and the leukocyte count 48 hours before, 48 hours after, and on the first day of HAI were not observed between HIV and non-HIV patients (Figs. 3 and 4).
Figure 1.
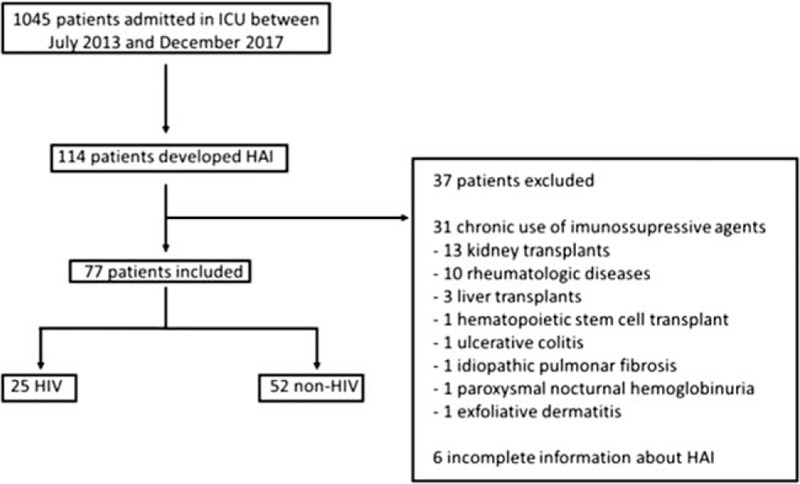
Flow chart of patients.
Figure 2.
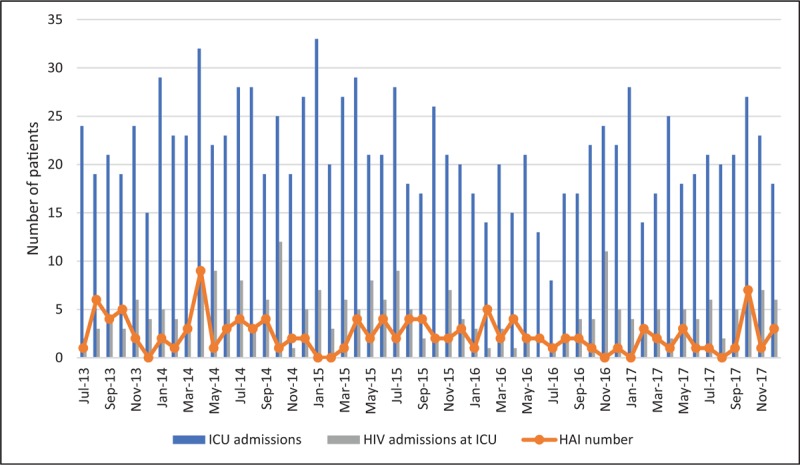
Number of ICU admissions, HIV patient admissions, and HAIs per month during the study period. HAI = healthcare associated-infection, HIV = human immunodeficiency virus, ICU = intensive care unit.
Table 1.
Clinical and demographic characteristics of individuals and types of healthcare associated-infections.
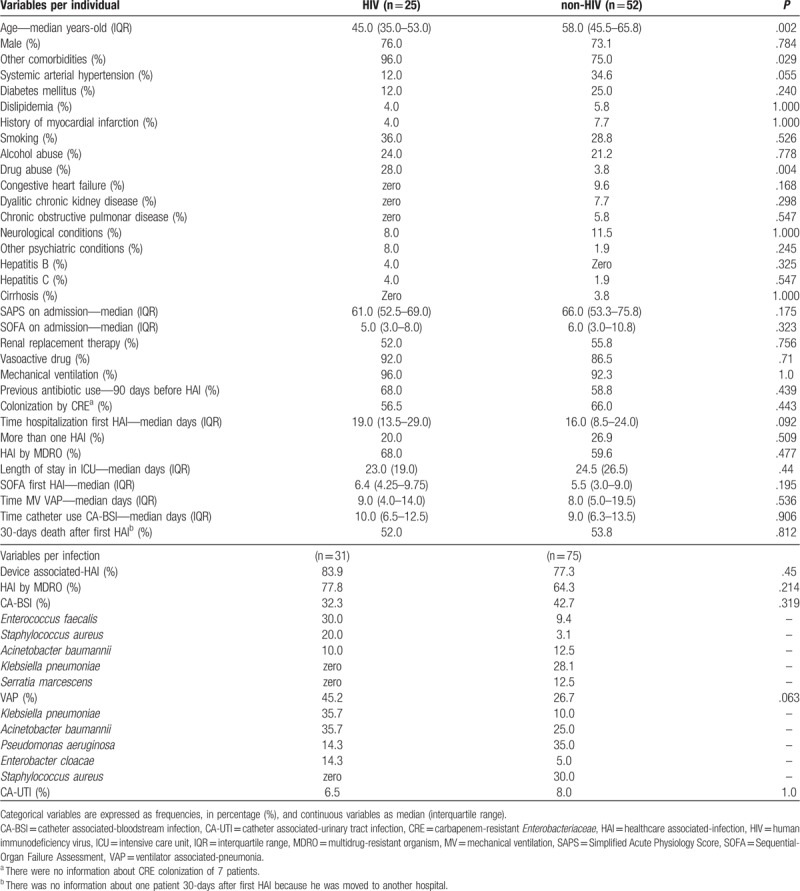
Figure 3.
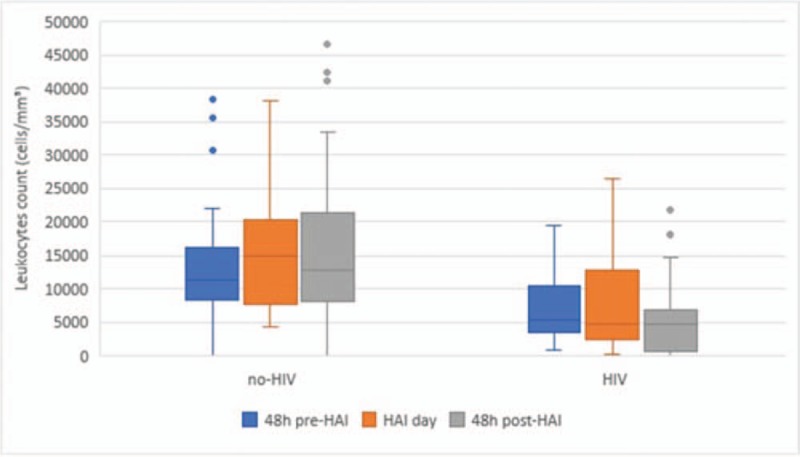
Leukocyte count 48 hours before, 48 hours after, and on day 1 of HAI. HAI = healthcare associated-infection, HIV = human immunodeficiency virus.
Figure 4.
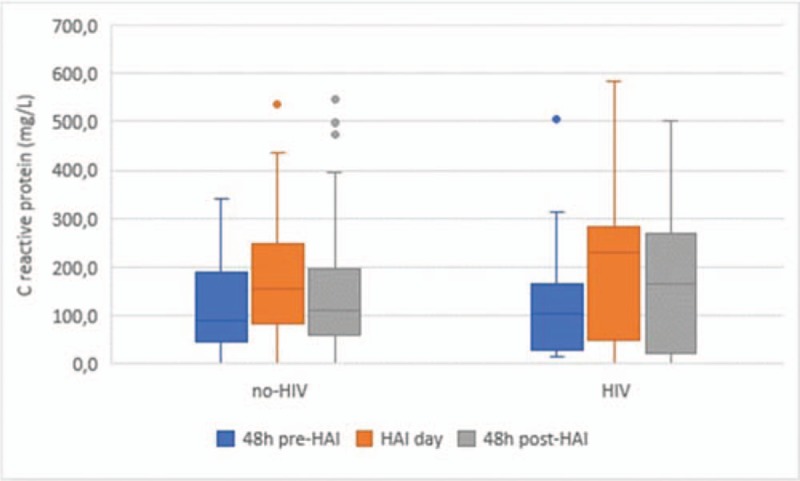
C-reactive protein 48 hours before, 48 hours after, and on day 1 of HAI. HAI = healthcare associated-infection, HIV = human immunodeficiency virus.
4.2. Characteristics of the HIV group
Among 25 patients infected by HIV, 9 (36.0%) were diagnosed previously and only 1 (4.0%) regularly used ART and had a suppressed viral load. Sixteen (64.0%) patients were admitted with an opportunistic infection and the median of the CD4+ T-cell count was 33.0 (interquartile range, 9.5–107.5) cells/mm3.
4.3. Reasons for ICU admission
Respiratory conditions were the most common cause of ICU admission (42.9%) and they were more frequent in the HIV group (60.0% vs 34.6%, P = .035). Neurological disorders were the reason for 16.9% of ICU admissions. The most common diseases present at ICU admission among HIV-positive individuals were Pneumocystis jirovecii (36.0%) and bacterial pneumonia (16.0%), while those in the non-HIV group were tetanus (17.3%), influenza (15.4%), and pneumonia (11.5%).
4.4. Thirty-day mortality after the first HAI episode
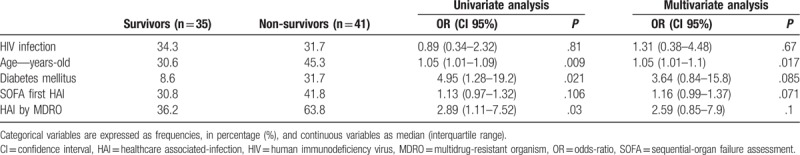
There was a high 30-day mortality rate after the first HAI episode and there was no statistically significant difference between the groups (HIV, 52.0% vs non-HIV, 53.8%, P = .812). There was no difference in the 30-day survival after HAI between HIV and non-HIV patients in the univariate analysis (P = .81), after adjusting for age, diabetes mellitus (DM), SOFA score on the day of the first HAI, and MDRO infection (P = .67; Table 2). Age was the only independent risk factor associated with 30-day death (odds ratio [OR] 1.05, 95% confidence interval [CI] 1.01–1.1, P = .017), and DM (OR 3.64, 95% CI 0.84–15.8, P = .085) and SOFA score (OR 1.16, 95% CI 0.99–1.37, P = .071) showed a trend toward association with death.
Table 2.
Uni and multivariate analysis of clinical variables comparing survivors and non-survivors 30-day after first episode of healthcare associated-infection.
4.5. Description of HAI
Among 106 HAIs, 31 occurred in HIV patients and 75 occurred in non-HIV patients, and most were associated with devices (Table 1). CA-BSI was the most frequent HAI among all individuals (39.6%) and no difference was observed in the frequency of this infection among the groups (Table 1). The most frequent HAI in the HIV group was VAP despite the similar periods of mechanical ventilation (Table 1). A low frequency of CA-UTI was observed in both groups (Table 1). Although we have observed a high frequency of HAI caused by MDRO, no difference in frequency was found between the groups (Table 1).
There was a predominance of Gram-positive bacteria as causative agents of CA-BSI in HIV patients (60.0%), and Enterococcus faecalis was the most frequent agent with 3 isolates (30.0%), 1 (33.3%) of which was VRE, followed by 2 Staphylococcus aureus (20.0%), both of which were MRSA. Gram-negative organisms were the predominant cause of CA-BSI in the non-HIV group (78.1%), with 9 Klebsiella pneumoniae (28.1%) and 4 (12.5%) of both Serratia marcescens and Acinetobacter baumannii, which were the most common agents. Seven K pneumoniae (77.8%) and 2 A baumannii (50.0%) were resistant to carbapenems in this group. One (3.1%) S aureus, which was methicillin resistant, was observed as a causative agent of CA-BSI in non-HIV patients, and among the 3 E faecalis (9.4%), 2 (66.7%) were vancomycin resistant. In the non-HIV group, 6 (18.8%) episodes of CA-BSI were caused by Candida species (50.0% albicans and 50.0% non-albicans), whereas catheter associated-candidemia was not observed in the HIV group.
There was a predominance of Gram-negative organisms as causative agents of VAP in both groups (HIV, 92.9% vs non-HIV, 70.0%). In HIV patients, A baumannii and K pneumoniae comprised 5 isolates (35.7%) each and they were the most frequent causative agents of VAP; all of them were resistant to carbapenem and 2 (40.0%) K pneumoniae isolates were colistin-resistant. No Gram-positive organism was observed as a causative agent of VAP in the HIV group. Seven Pseudomonas aeruginosa (35.0%) and 5 A baumannii (25.0%) were the predominant Gram-negative causative agents of VAP in non-HIV patients, and 5 (71.4%) P aeruginosa and 4 A baumannii were resistant to carbapenems. Moreover, 6 VAP episodes (30.0%) were caused by S aureus in this group, and 4 (66.7%) of them were MRSA.
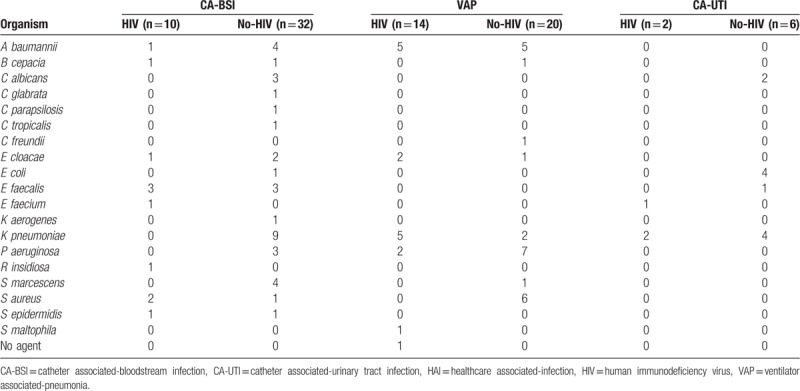
There were 2 CA-UTI in the HIV group, and one of them was caused by a carbapenem and colistin-resistant K pneumoniae, and the other was caused by 2 agents, which were an isolate of K pneumoniae that was susceptible to multiple antimicrobial classes and a vancomycin-resistant Enterococcus faecium. In the non-HIV group, there were 6 episodes of CA-UTI. The predominant agents were 4 Escherichia coli (66.7%), all of which were susceptible to multiple antimicrobial classes, and 4 K pneumoniae (66.7%), one of which was resistant to carbapenems and colistin. There were 2 (33.3%) Candida albicans and 1 (16.7%) E faecalis that were susceptible to multiple antimicrobial classes. Table 3 shows the number of each organism that was isolated for each type of device-associated infection.
Table 3.
Microrganisms identified in device-associated HAI.
5. Discussion
There was no difference in 30-day mortality between HIV-infected and non-infected patients who acquired HAI during an ICU stay. Age was the only independent risk factor that was associated with outcome in our study. These findings are similar to a prospective single-center study that was conducted in a high-income country, which aimed to evaluate mortality and risk of acquiring a HAI caused by a MDRO, and they did not find a difference between HIV and non-HIV groups for both outcomes.[27] In our study, most HIV patients were recently diagnosed, had an uncontrolled disease, and were admitted for an opportunistic infection, which are considered to be factors for a poor prognosis[4,5]; however, we observed a high mortality rate in both groups, with no significant difference. These findings reinforce the evidence of high mortality associated with HAI, which is independent of other comorbidities and has a large social impact.[15,16]
There is no consensus in the literature about the impact of HIV infection on mortality of ICU patients. Some studies have observed a higher mortality rate in HIV patients admitted to the ICU.[32,33] However, a prospective study showed that HIV infection has little impact on mortality,[34] which is consistent with the results of our study. These studies evaluated patients with sepsis and none addressed HAI.[32–34]
The most common HAI observed in HIV patients in our study was VAP and its frequency in this group was higher than in non-HIV individuals, despite similar periods of device use. This could be because HIV patients were admitted more frequently with a respiratory disease or for local pulmonary changes in immune response resulting from a reduction in alveolar macrophage function caused by oxidative stress that is observed in HIV infection.[5,35] Moreover, there is no consensus in the literature about which HAI is the most frequent in HIV patients. Several studies, most of which were not restricted to the ICU setting, reported CA-BSI,[21,25,27] but there are studies that described VAP[22] and CA-UTI[26] as the most frequent HAI in HIV patients.
A high frequency of HAI caused by MDROs was found in our study, but HIV infection did not seem to be a risk factor for this outcome. This finding was similar to that described by Cobos-Trigueros et al,[27] who evaluated HAI caused by MDROs as well as mortality. There are studies that showed a higher frequency of colonization by multidrug resistant bacteria in HIV patients, suggesting possible sexual transmission of these bacteria.[36] Our study did not find a difference between HIV and non-HIV patients in the colonization rate by carbapenem-resistant Enterobacteriaceae in HAI caused by MDROs.
We observed that Gram-positive organisms were the predominant agents of CA-BSI, whereas Gram-negative organisms were the predominant cause of VAP in HIV patients. Studies showed a high frequency of Gram-positive organisms as agents of HAI in HIV patients[21,22] and most of them described these organisms as the most frequent cause of CA-BSI in this patient population. Gram-negative organisms were previously described as the predominant agents in VAP, but Gram-positive organisms were also frequently found in HIV patients.[23] Conversely, our study did not observe any Gram-positive organism as a cause of VAP in HIV patients. However, Candida species were considered to be important agents of CA-BSI in HIV patients,[26,37,38] and we observed catheter-associated candidemia only in non-HIV individuals.
This study has several limitations such as the small sample size and its design; it was based on retrospective data, with possible measurement bias and loss of information. As a retrospective study, it was not possible to evaluate death that was attributable to HAI. We did not observe an HAI outbreak during the study period, and it was not possible to evaluate if there was cross-infection among groups because molecular analysis was not performed. There was a predominance of male patients, so there is a limitation to extrapolate the results to the general population, but a multivariable analysis was performed to reduce possible confounders. There are few studies in the literature about HAI in HIV patients. To the best of our knowledge, this is the first study that evaluated 30-day mortality as a primary outcome in HIV patients after HAI. The epidemiologic and microbiologic profile of HAI in these patients is also described, contributing additional data to this controversial theme.
6. Conclusions
VAP that was mainly caused by Gram-negative bacteria was the most frequent HAI in HIV patients in our ICU. However, Gram-positive bacteria were the predominant causative agents of CA-BSI in the HIV group. There was a high frequency of HAI caused by MDROs and 30-day mortality after HAI, but there was no statistically significant difference among HIV and non-HIV patients. HIV infection is not a predictive factor that is associated with 30-day mortality of patients with HAI, and age was the only independent factor associated with outcome.
Author contributions
Conceptualization: Victor Augusto Camarinha de Castro-Lima, Igor Carmo Borges, Ho Yeh Li, Silvia Figueiredo Costa, Maria Luisa Nascimento Moura.
Data curation: Victor Augusto Camarinha de Castro-Lima, Igor Carmo Borges, Daniel Joelsons, Vivian Vieira Tenorio Sales, Ho Yeh Li, Thais Guimaraes, Silvia Figueiredo Costa, Maria Luisa Nascimento Moura.
Formal analysis: Victor Augusto Camarinha de Castro-Lima, Igor Carmo Borges, Silvia Figueiredo Costa, Maria Luisa Nascimento Moura.
Investigation: Victor Augusto Camarinha de Castro-Lima, Igor Carmo Borges, Silvia Figueiredo Costa, Maria Luisa Nascimento Moura.
Methodology: Victor Augusto Camarinha de Castro-Lima, Igor Carmo Borges, Silvia Figueiredo Costa, Maria Luisa Nascimento Moura.
Project administration: Victor Augusto Camarinha de Castro-Lima, Maria Luisa Nascimento Moura.
Software: Victor Augusto Camarinha de Castro-Lima.
Visualization: Victor Augusto Camarinha de Castro-Lima.
Writing – original draft: Victor Augusto Camarinha de Castro-Lima, Silvia Figueiredo Costa.
Writing – review & editing: Daniel Joelsons, Vivian Vieira Tenorio Sales, Ho Yeh Li, Thais Guimaraes, Maria Luisa Nascimento Moura.
Supervision: Silvia Figueiredo Costa, Maria Luisa Nascimento Moura.
Footnotes
Abbreviations: ART = antiretroviral therapy, AST = antimicrobial susceptibility test, CA-BSI = catheter associated-bloodstream infection, CA-UTI = catheter associated-urinary tract infection, CLSI = Clinical and Laboratory Standards Institute, DM = diabetes mellitus, HAI = healthcare associated-infection, HIV = human immunodeficiency virus, ICU = intensive care unit, MALDI-TOF = matrix-assisted laser desorption ionization-time of flight, MDRO = multidrug-resistant organism, MRSA = methicillin-resistant Staphylococcus sp., SAPS = Simplified Acute Physiology Score, SOFA = Sequential Organ-Failure Assessment, VAP = ventilator associated-pneumonia, VRE = vancomycin-resistant Enterococcus sp.
There was no funding source for this study.
No authors have conflict of interest.
References
- [1].Masur H, Michelis MA, Greene JB, et al. An outbreak of community-acquired Pneumocystis carinii pneumonia: initial manifestation of cellular immune dysfunction. N Engl J Med 1981;305:1431–8. [DOI] [PubMed] [Google Scholar]
- [2].Wachter RM, Luce JM, Turner J, et al. Intensive care of patients with the acquired immunodeficiency syndrome. Outcome and changing patterns of utilization. Am Rev Respir Dis 1986;134:891–6. [DOI] [PubMed] [Google Scholar]
- [3].Palella FJ, Jr, Delaney KM, Moorman AC, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med 1998;338:853–60. [DOI] [PubMed] [Google Scholar]
- [4].Gupta A, Nadkarni G, Yang W-T, et al. Early mortality in adults initiating antiretroviral therapy (ART) in low- and middle-income countries (LMIC): a systematic review and meta-analysis. PLoS One 2011;6:e28691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Maartens G, Celum C, Lewin SR. HIV infection: epidemiology, pathogenesis, treatment, and prevention. Lancet 2014;384:258–71. [DOI] [PubMed] [Google Scholar]
- [6].Morris A, Creasman J, Turner J, et al. Intensive care of human immunodeficiency virus-infected patients during the era of highly active antiretroviral therapy. Am J Respir Crit Care Med 2002;166:262–7. [DOI] [PubMed] [Google Scholar]
- [7].Bonnet F, Morlat P, Chêne G, et al. Causes of death among HIV-infected patients in the era of highly active antiretroviral therapy, Bordeaux, France, 1998-1999. HIV Med 2002;3:195–9. [DOI] [PubMed] [Google Scholar]
- [8].Casalino E, Wolff M, Ravaud P, et al. Impact of HAART advent on admission patterns and survival in HIV-infected patients admitted to an intensive care unit. AIDS 2004;18:1429–33. [DOI] [PubMed] [Google Scholar]
- [9].Narasimhan M, Posner AJ, DePalo VA, et al. Intensive care in patients with HIV infection in the era of highly active antiretroviral therapy. Chest 2004;125:1800–4. [DOI] [PubMed] [Google Scholar]
- [10].Huang L, Quartin A, Jones D, et al. Intensive care of patients with HIV infection. N Engl J Med 2006;355:173–81. [DOI] [PubMed] [Google Scholar]
- [11].Croda J, Croda MG, Neves A, et al. Benefit of antiretroviral therapy on survival of human immunodeficiency virus-infected patients admitted to an intensive care unit. Crit Care Med 2009;37:1605–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Japiassú AM, Amâncio RT, Mesquita EC, et al. Sepsis is a major determinant of outcome in critically ill HIV/AIDS patients. Crit Care 2010;14:R152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Amancio FF, Lambertucci JR, Cota GF, et al. Predictors of the short- and long-term survival of HIV-infected patients admitted to a Brazilian intensive care unit. Int J STD AIDS 2012;23:692–7. [DOI] [PubMed] [Google Scholar]
- [14].Hospitals US. Estimating health care-associated infections and deaths in. Public Health Rep 2007;122:160–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Magill SS, Edwards JR, Bamberg W, et al. Multistate point-prevalence survey of health care-associated infections. N Engl J Med 2014;370:1198–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].World Health Organization. Health care-associated infections Fact sheet. World Heal Organ 2015;4. [Google Scholar]
- [17].Magiorakos AP, Srinivasan A, Carey RB, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect 2012;18:268–81. [DOI] [PubMed] [Google Scholar]
- [18].Brooklyn Antibiotic Resistance Task Force. The cost of antibiotic resistance: effect of resistance among Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, and Pseudomonas aeruginosa on length of hospital stay. Infect Control Hosp Epidemiol 2002;23:106–8. [DOI] [PubMed] [Google Scholar]
- [19].Siegel JD, Rhinehart E, Jackson M, et al. Management of multidrug-resistant organisms in health care settings. Am J Infect Control 2007;3510 suppl 2:S165–93. [DOI] [PubMed] [Google Scholar]
- [20].Giske CG, Monnet DL, Cars O, et al. Clinical and economic impact of common multidrug-resistant gram-negative bacilli. Antimicrob Agents Chemother 2008;52:813–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Padoveze MC, Trabasso P, Branchini MLM. Nosocomial infections among HIV-positive and HIV-negative patients in a Brazilian infectious diseases unit. Am J Infect Control 2002;30:346–50. [DOI] [PubMed] [Google Scholar]
- [22].Panis C, Matsuo T, Reiche EMV. Nosocomial infections in human immunodeficiency virus type 1 (HIV-1) infected and AIDS patients: major microorganisms and immunological profile. Braz J Microbiol 2009;40:155–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Pathak V, Rendon ISH, Atrash S, et al. Comparing outcomes of HIV versus non-HIV patients requiring mechanical ventilation. Clin Med Res 2012;10:57–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Tchakoute CT, Liu J, Cohen B, et al. Risk factors and temporal trends of hospital-acquired infections (HAIs) among HIV positive patients in Urban New York City Hospitals: 2006 to 2014. Rev Recent Clin Trials 2017;12:44–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Petrosillo N, Pugliese G, Girardi E, et al. Nosocomial infections in HIV infected patients. Epidemiol Soc 1999;13:599–605. [DOI] [PubMed] [Google Scholar]
- [26].Petrosillo N, Viale P, Nicastri E, et al. Nosocomial bloodstream infections among human immunodeficiency virus-infected patients: incidence and risk factors. Clin Infect Dis 2002;34:677–85. [DOI] [PubMed] [Google Scholar]
- [27].Cobos-Trigueros N, Rinaudo M, Solé M, et al. Acquisition of resistant microorganisms and infections in HIV-infected patients admitted to the ICU. Eur J Clin Microbiol Infect Dis 2014;33:611–20. [DOI] [PubMed] [Google Scholar]
- [28].Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med 2005;171:388–416. [DOI] [PubMed] [Google Scholar]
- [29].Healthcare Infection Control Practices Advisory Committee (HICPAC), O’Grady NP, Alexander M, Burns LA, et al. Guidelines for the Prevention of Intravascular Catheter-Related Infections, 2011 Oklahoma Foundation for Medical Quality, Vol. 39. 2011. [Google Scholar]
- [30].Gould CV, Umscheid CA, Agarwal RK, et al. Guideline for prevention of catheter - associated urinary tract infections. Healthc Infect Control Pract Advis Comm 2017;1–61. [Google Scholar]
- [31].CLSI. Clinical and Laboratory Standards Institute. Vol. 32; 2017. 18 p. [Google Scholar]
- [32].Medrano J, Alvaro-Meca A, Boyer A, et al. Mortality of patients infected with HIV in the intensive care unit (2005 through 2010): significant role of chronic hepatitis C and severe sepsis. Crit Care 2014;18:475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Akgun KM, Tate JP, Pisani M, et al. Medical ICU admission diagnoses and outcomes in human immunodeficiency virus-infected and virus-uninfected veterans in the combination antiretroviral era. Crit Care Med 2013;41:1458–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Wiewel MA, Huson MA, van Vught LA, et al. Impact of HIV infection on the presentation, outcome and host response in patients admitted to the intensive care unit with sepsis; a case control study. Crit Care 2016;20:322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Fan X, Staitieh B, Neveu W, et al. HIV-1 decreases Nrf2/anti-oxidant response element (ARE) activity and phagocytic function in alveolar macrophages. In: C55 HIV-Associated Lung Diseases And Infections. American Thoracic Society; 2015. p. A4718 (American Thoracic Society International Conference Abstracts). [Google Scholar]
- [36].Reinheimer C, Keppler OT, Stephan C, et al. Elevated prevalence of multidrug-resistant gram-negative organisms in HIV positive men. BMC Infect Dis 2017;17:206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Tacconelli E, Tumbarello M, Donati KDG, et al. Morbidity associated with central venous catheter-use in a cohort of 212 hospitalized subjects with HIV infection. J Hosp Infect 2000;44:186–92. [DOI] [PubMed] [Google Scholar]
- [38].Declercq S, De Munter P, Derdelinckx I, et al. Characteristics, causes, and outcome of 54 episodes of bloodstream infections in a cohort of HIV patients. Infect Dis (Auckl) 2015;47:611–7. [DOI] [PubMed] [Google Scholar]


