Abstract
Drug-induced anaphylaxis (DIA) is a highly paradoxical disorder involving a fatal response to medicines prescribed for therapeutic purposes. This study aimed to improve the awareness on DIA and to prevent errors through an analysis of lawsuit judgments.
Sentenced judgments involving DIA from 1998 to 2017 using the database of the Korean Supreme Court Judgment System were collected. General characteristics, results, and recognized negligence of DIA litigation cases were analyzed.
Of 27 lawsuit cases included, antibiotics (n = 6, 22.2%), radiocontrast media (n = 6, 22.2%), and non-steroidal anti-inflammatory drugs (n = 5, 18.5%) were the most common drugs that had caused DIA. Cardiac arrest was reported in 23 cases (85.2%). The median time interval from drug administration to diagnosis and from diagnosis to cardiac arrest were 7 (interquartile range, IQR = 0–35) and 5 minutes (IQR = 0–33), respectively, suggesting insufficient time to cope with anaphylaxis. Consequently, either death (n = 18, 66.7%) or ischemic brain injury (n = 9, 33.3%) occurred in all cases. Violation of duty of care was recognized in 19 cases (70.4%) with median awarded amount of $106,060 (IQR = $70,296–$168,363). The recognized negligence included inadequate observation after drug administration (n = 6), delayed or missed epinephrine administration (n = 6), ignoring a history of allergy or drug hypersensitivity (n = 6), and prescription error (n = 5).
It is necessary to improve the awareness on DIA, because making a trivial error in any process of history taking, drug prescription and administration, observation, and/or emergency treatment may have fatal consequences that can lead to indemnity.
Keywords: anaphylaxis, dissent and disputes, drug hypersensitivity, jurisprudence, malpractice
1. Introduction
Drug-induced anaphylaxis (DIA) is a highly paradoxical disorder in which therapeutic medications become fatal agents. Although little is known about the epidemiology of DIA, the incidence of DIA has been estimated to be 0.04% to 3.1%,[1,2] and is responsible for one case in every 4000 emergency department visits.[3] However, other studies have reported increasing frequency and severity of DIA.[4–6] Moreover, DIA is likely to be more severe and fatal than anaphylaxis caused by other factors,[6] and accounts for up to 58% of fatal anaphylaxis.[6,7]
Despite its increasing frequency, severity, and fatality, physicians may be insufficiently aware of DIA due to its low frequency.[8] In addition, the patient and caregivers may have insufficient understanding of the sudden and fatal characteristics of DIA, which possibly leads to medical disputes. Although anaphylaxis is mainly caused by an individual's unique immunity, and is not induced intentionally by the physician, this unexpected situation may make the patient and caregivers initially suspect that the physician is at fault.
The analysis of medical lawsuit judgment is helpful for the identification and prevention of malpractice based on actual cases. However, there is no precedent in lawsuit judgment analysis studies to prevent malpractice associated with DIA. Therefore, this study performed analyses of lawsuit judgments, including presenting descriptive clinical features, culprit drugs, and physician's negligence as recognized by the court. Finally, we aimed to improve the awareness on DIA and to prevent errors that may lead to a critical outcome.
2. Materials and methods
2.1. Judgment collection
In this retrospective study, we searched for all medical civil litigation cases sentenced from January 1, 1998 to December 31, 2017 with the term “anaphylaxis” using the database of the Korean Supreme Court Judgment System.[9] We found 107 judgments, which were reviewed to extract anaphylactic events after drug administration. Cases were excluded if anaphylaxis was caused by other factors such as food or bee stings, or if the court judged the case as resulting from factors other than anaphylaxis, such as a side effect from a pharmacologic action or a severe cutaneous adverse reaction. The personal information on judgments is anonymized by the Court, and the database we used is accessible by any individual.
2.2. Clinical information on the judgments
Based on the description of the judgment texts, information on age and sex of the patient, history of allergy or drug hypersensitivity, and the culprit drug along with route of administration were collected. Data concerning the defendants such as the type of institution and medical specialty of the physician were also collected. According to the World Allergy Organization Guidelines for the Assessment and Management of Anaphylaxis,[10,11] the symptoms of anaphylaxis described in the judgment texts were also collected and classified into 5 groups: mucocutaneous, respiratory, gastrointestinal, cardiovascular, and neurologic symptoms. Furthermore, data regarding the time frame of the symptoms described, and time intervals between drug administration and the diagnosable time point for anaphylaxis were collected and analyzed. Diagnosable time point is defined as the point at which diagnosis criteria were met based on the symptom and time frame described in the judgment texts. For cases involving cardiac arrest, data regarding the time intervals between the diagnosable time point and cardiac arrest were additionally collected and analyzed. Information about the final statuses of patients after anaphylaxis attack was also collected.
2.3. Judicial decision
In terms of judgment, data regarding the progress of litigation, whether the plaintiff prevailed, and the amounts claimed and awarded for damage were also collected (the exchange rate was converted to 1 United States Dollar (USD) = 1,100 Korean Won (KRW) considering the mean exchange rate for 2018). Finally, we collected and analyzed the court rulings regarding whether physicians were negligent and violated their duty of care during the process of history taking, drug prescription and administration, observation, and emergency treatment. Among dismissed cases, we attempted to identify any measures to prevent malpractice by presenting some example cases and comparing the reasons for dismissal.
2.4. Statistical analysis
Categorical variables are presented as absolute frequencies with number and relative frequencies with percentages. Continuous variables, such as time intervals and the amounts claimed and awarded for damage, were assessed using the Shapiro-Wilk test to determine its distribution (considered as non-normal distribution if P < .05).[12] Average and standard deviation (normal distribution), or median and interquartile range (non-normal distribution) were reported as appropriate. Statistical analyses were performed with SPSS 24 (IBM, Armonk, NY).
2.5. Ethics statement
Institutional Review Board (IRB) approval was not necessary in this study. This study was based on the sentenced lawsuit judgments which are publicly accessible by any individual in the database of the Korean Supreme Court Judgment System. In addition, each court provided the researcher with the judgments after deleting personally identifiable information; thus, access to sensitive personal information was impossible. This study was an analysis of medical lawsuit judgments that had already been adjudicated and did not include any infringement or threat of patients’ rights.
3. Results
3.1. General characteristics
A total of 27 DIA-related litigation cases (53 judgments) were finally analyzed. General clinical characteristics such as age, sex, and past history of allergy or drug hypersensitivity are presented in Table 1. Anaphylaxis lawsuits occurred irrespective of the size of hospitals, that is, primary clinics, district hospitals, and tertiary hospitals. In terms of medical specialty, internal medicine, emergency medicine, and pediatrics were the most common with 3 cases each. However, other medical specialties were also subject to lawsuits (Fig. 1).
Table 1.
General characteristics of cases (n = 27).
Figure 1.
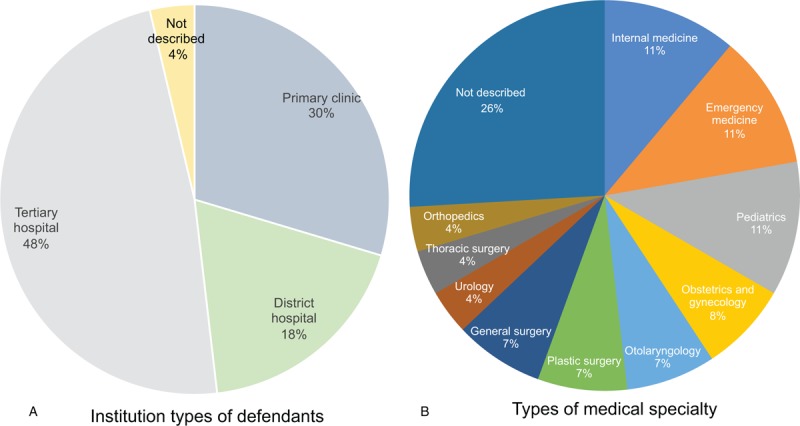
(a) The types of medical institutions and (b) medical specialties of the defendants.
3.2. Culprit drugs and administration route
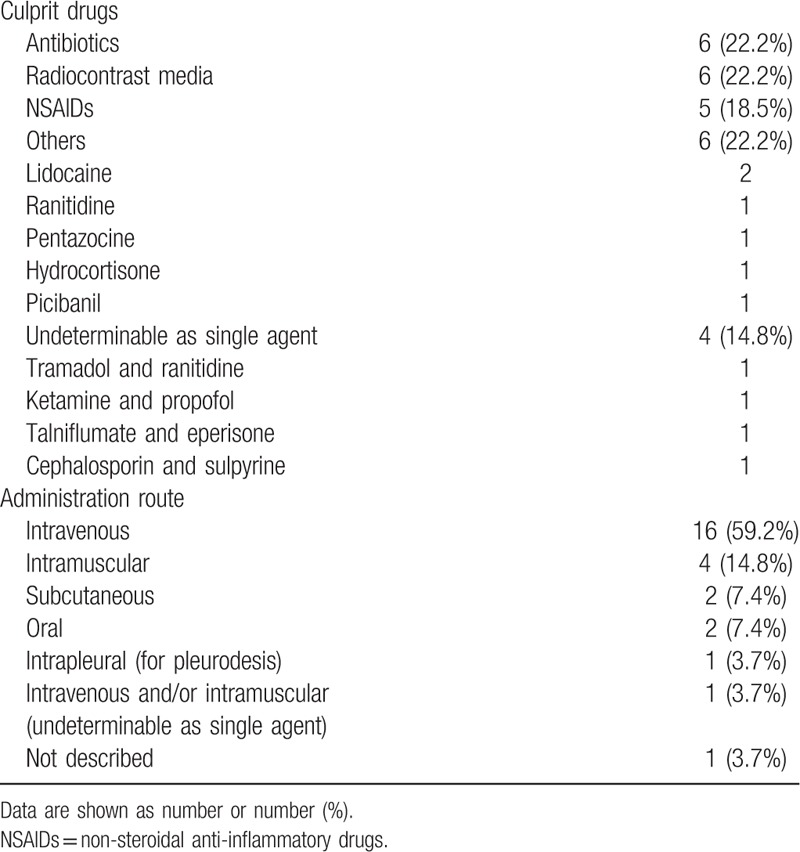
Antibiotics (n = 6, 22.2%), radiocontrast media (n = 6, 22.2%), and non-steroidal anti-inflammatory drugs (NSAIDs) (n = 5, 18.5%) were the most common culprits. Other culprit drugs were lidocaine, H2 blocker, opioids, Picibanil (Lyophilized mixture of low-virulence Streptococcus pyogenes and a high content of penicillin G potassium, used for pleurodesis), and hydrocortisone. The most common route of administration was intravenous (n = 16, 59.2%), followed by intramuscular (n = 4, 14.8%), subcutaneous (n = 2, 7.4%), and oral (n = 2, 7.4%). (Table 2)
Table 2.
Culprit drugs and the administration route.
3.3. Symptom presentation
A summary of symptom presentation is shown in Table 3. Only 29.6% of cases (n = 8) had mucocutaneous symptoms. In contrast, the frequency of respiratory (n = 20, 74.1%), cardiovascular (n = 25, 92.6%) and neurologic symptoms (n = 19, 70.3%) were higher.
Table 3.
Clinical presentation of anaphylaxis on lawsuits.
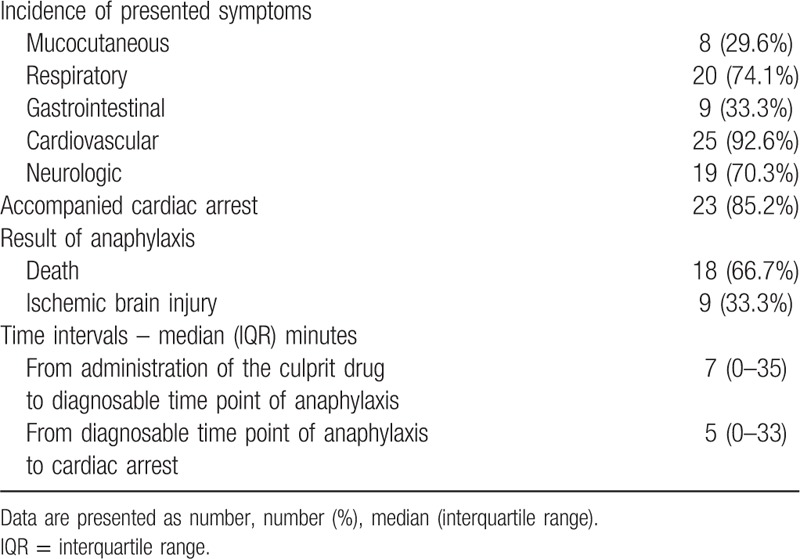
Twelve (44.4%) of 27 cases were diagnosable as anaphylaxis within 10 minutes after administering the culprit drugs; 6 were diagnosed immediately after administration. After excluding cases with missing information, the median time interval between drug administration and diagnosable time point were 7 minutes.
Progression to cardiac arrest was observed in 23 cases (85.2%). Among them, 12 cases had a diagnosable time point within 10 minutes. Eight cases were diagnosable as anaphylaxis with cardiac arrest. Cardiac arrest occurred almost immediately after administering the culprit drug in 2 cases; here, the median time interval from the diagnosable time point to cardiac arrest were 5 minutes after excluding cases with missing information. As a result of anaphylaxis, 18 patients (66.7%) died. The other 9 patients (33.3%) experienced ischemic brain injury.
Both time intervals are presented as median with interquartile range in Table 3 as it showed non-normal distribution (both P < .01). The distribution of each time interval is shown in Fig. 2.
Figure 2.
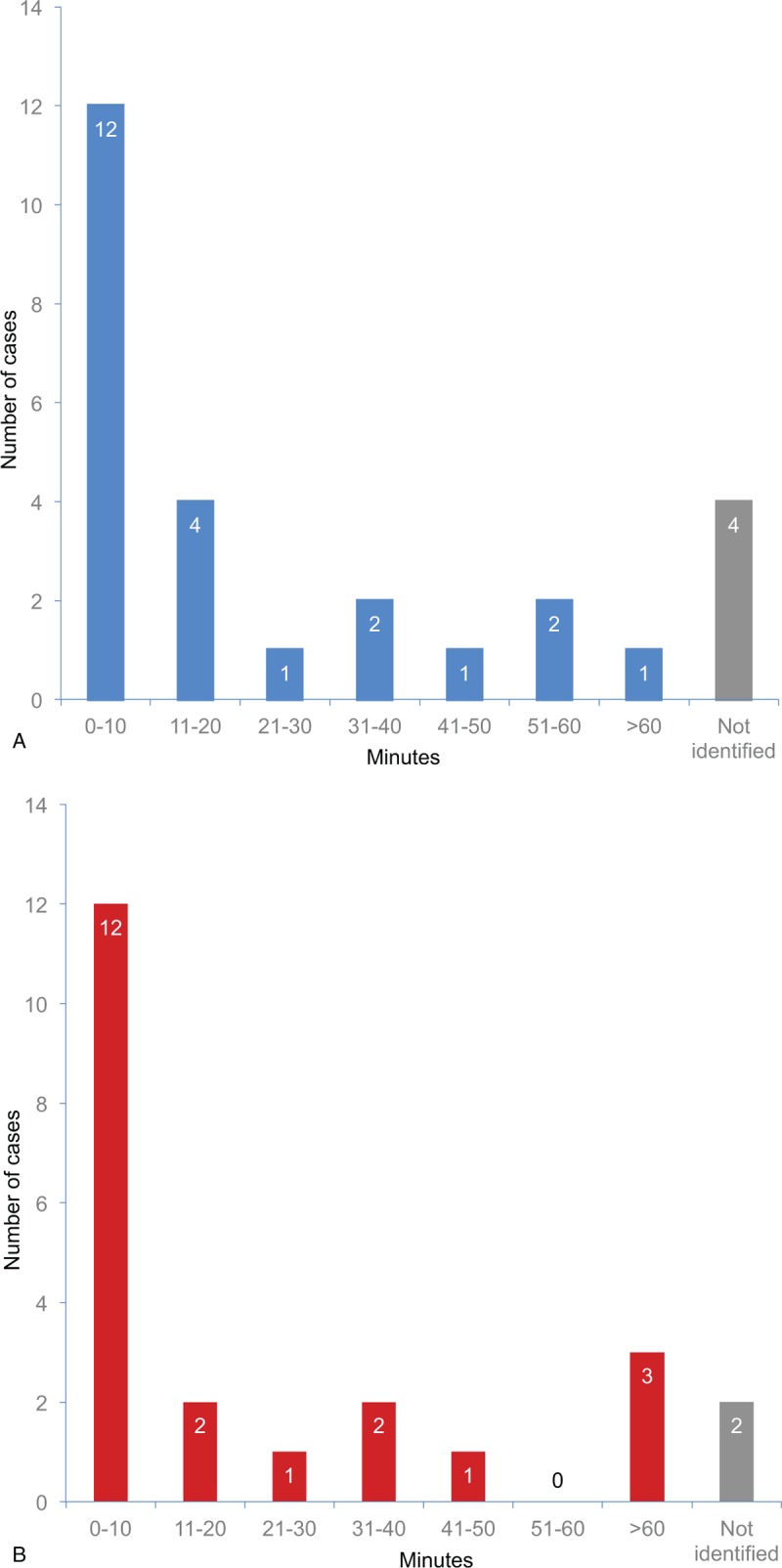
(a) Time interval from drug administration to diagnosable time point of anaphylaxis based on described symptoms (n = 27) and (b) time interval from diagnosable time point to cardiac arrest (n = 23).
3.4. Judgment status
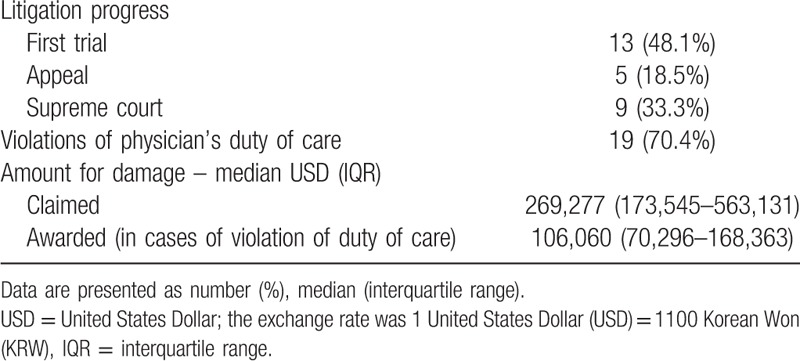
Violations of the physician's duty of care were recognized in 19 (70.4%) of the 27 cases. A summary of judgment statuses is shown in Table 4. Amount for damages of both claimed and awarded are presented as median with interquartile range, as it showed non-normal distribution (both P < .01).
Table 4.
Judgment status.
3.5. Physician's negligence recognized by the courts
Events of negligence during the process of history taking, drug prescription and administration, observation, and emergency treatment of anaphylaxis were classifiable into 8 groups. The frequencies of each class of negligence are shown in Fig. 3.
Figure 3.
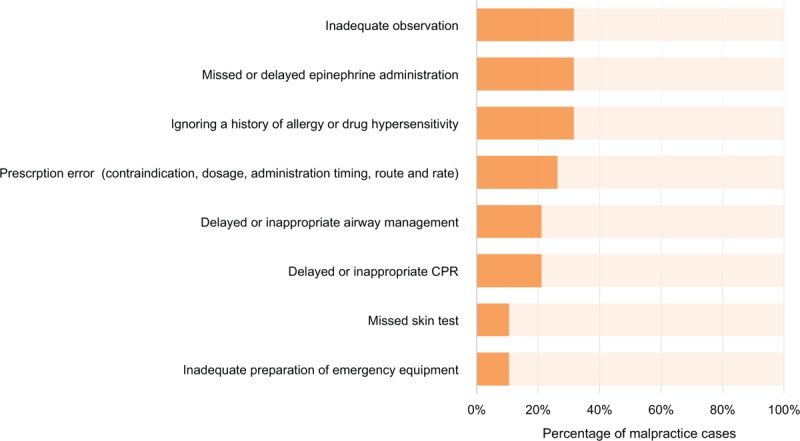
Court recognition of a physician's negligence or violation of the duty of care (n = 19).
3.6. Inadequate observation
It was pointed out in 6 cases. In 2, anaphylaxis occurred after discharge without appropriate observation after the administration of the culprit drug in outpatient clinics. In another in-patient case, there was no observation period following drug administration, and the patient was found in a state of anaphylaxis after 5 minutes. Despite treatment with epinephrine and intubation within 5 minutes of discovery, the case was recognized as negligence. In the other 2 cases, failure to contact a physician leading to delayed treatment, was the source of negligence (29 and 33 minutes, respectively). In the remaining case, although the caregiver informed the radiology technician that the patient began coughing after the administration of the radiocontrast media for computed tomography (CT) scan, no one took notice; a subsequently notified physician prescribed steroid and antihistamine without visiting the patient. The patient developed cardiac arrest within a few minutes. This event was recognized as negligence due to inadequate observation, while another case where a nurse observed the patient for 5 minutes after drug injection and left the patient after confirming that the caregiver remained by the patient's bedside was not recognized as negligence.
3.7. Delayed or missed epinephrine administration
Six cases were categorized in this group. In 4 cases of delayed administration, time intervals between onset of anaphylaxis and epinephrine administration were 19, 20, 97, and 114 minutes, respectively. In the first case involving a 19-minute delay, this interval was recognized as negligence, although other procedures, such as tracheal intubation, had been performed promptly. In contrast, a 6-minute delay in the first epinephrine administration was not recognized as negligent. In 2 missed cases, epinephrine was not administered in the defendant's institution, but was administered after transfer to a higher hospital. This was recognized as negligence even though the setting was a primary clinic.
3.8. Ignoring a history of allergy or drug hypersensitivity
Six cases were classified in this group. In 4 cases, despite the existence of related medical records, known cross-reacting drugs or the same culprit drugs were prescribed. The fact that the patients did not inform hospital staff about their drug allergy/hypersensitivity history at the time of drug administration did not affect the court's decision of physician negligence. In the other 2 cases, physicians did not ask the patient's past drug allergy/hypersensitivity history.
3.9. Prescription error (contraindication, dosage, and timing, route, and rate of administration)
Five cases fell in this category. In 2 cases, sulpyrine, which is contraindicated in persons under 12 years of age, was administered to children. The court pointed out in both cases that the physician prescribed sulpyrine without serious consideration of the associated side-effects and risks for persons less than 12 years of age. Furthermore, the court quoted that previous studies had not established the safety and efficacy of sulpyrine in children younger than 14 years and that the frequency of sulpyrine-associated anaphylaxis is relatively high, at 1 in every 5000 injections. One case of anaphylaxis resulted from incorrect dosage. The patient, who had hypersensitivity reactions during the initiation of anti-tuberculous medication, received a prescription of rifampin specifying a regular dose, which resulted in anaphylaxis. The court commented that the drug had caused a hypersensitivity reaction and should have been initiated at a lower dose, with gradual dose escalation in order to prevent anaphylaxis. Another case of anaphylaxis resulted from incorrect timing of administration. Cephalosporin (unspecified) was administered to a pregnant woman as a prophylactic antibiotic before delivery, rather than after delivery. It caused anaphylaxis of the newborn in the mother's body and led to death. In another case, the prescription order regarding the route of administration and injection rate of aspirin lysine were noted as negligence. The court determined that the order had been made without carefully considering the route of administration based on the fact that the intravenous administration of aspirin is only indicated when oral administration is impossible or has an insufficient therapeutic effect. In addition, the physician did not give specific instructions other than to slowly administer the injection, and the drug was delivered as bolus injection.
3.10. Delayed or inappropriate airway management
Four cases fell in this group. To determine whether the physician violated their duty of care due to non-performed or a delay in performing intubation, the court considered medical circumstances at that time rather than recognizing it as negligence unconditionally. For example, there was 1 case with a dismissed claim in which airway management consisted of just securing the airway and performing mouth-to-mouth breathing at a primary outpatient clinic (specialty unspecified). However, this aspect is judged more strictly in a setting capable of tracheal intubation. In a primary plastic surgery clinic, which would presumably be equipped with an operating room, a faulty intubation itself was recognized as negligent, despite the clinicians’ efforts. In addition, 10- and 17-minute delays in intubation in an emergency room setting were recognized as negligent events. Particularly, the court noted that in the case of the 10-minute delay, intubation should have been performed immediately if the airway could not adequately be secured. Although ambu-bagging had been performed before tracheal intubation, the court recognized that this procedure was not helpful, based on the poor results from a follow-up arterial blood gas analysis.
3.11. Delayed or inappropriate cardiopulmonary resuscitation (CPR)
Four cases were recognized as improper CPR performance. In 3 cases, it was pointed out that the CPR should have begun immediately. The reasons for delaying CPR included a delay because of intubation, a failure to recognize to begin CPR until the arrival of emergency service, and a delay from failing to contact the physician without any other actions performed for 33 minutes. In the remaining case, errors in CPR performance were pointed out and included performing chest compressions several times with interruptions and performing a digital rectal examination during CPR. The defendant claimed that the reason for the unprofessional-looking CPR was that the vital signs fluctuated. However, this claim was dismissed based on the submitted medical records, which revealed that the reactive changes in vital signs and electrocardiography readings had not been recorded faithfully.
3.12. Missed skin test
There were 2 cases with Picibanil and cefotaxime as culprit drugs, and both were recognized as negligence due to skipping a skin test. The court cited information about each drug from the pharmacopoeia, which states that a skin test is desirable before administration. Seven cases were dismissed on this point, because the circumstances in which the skin test was performed were either acceptable or the skin test was not an essential process. Remarkably, there was an inconsistency between judgments of using a skin test for testing allergy/sensitivity to cephalosporin. The former case of cefotaxime was concluded in 2005, while another more recent case of cefotetan was dismissed in the Supreme Court in 2017. In the latter case, the court cited the fact that the effectiveness of skin tests, except for penicillin, remains unclear, and a number of institutions do not perform skin tests for patients who do not have a history of cephalosporin hypersensitivity.
3.13. Inadequate preparation of emergency equipment
Two cases were identified in this group. Both cases pointed out that despite the possibility of hypersensitivity, administration of the culprit drugs was performed in an unprotected setting without appropriate emergency equipment. As a result, the hypoxic brain damage was attributed to prolonged hypoxia while transferring the patient to the emergency room. In contrast, there was a case in which this form of negligence was dismissed because the distance between the CT room and the emergency room was only 10 m, which was recognized sufficiently close to enable prompt first aid.
4. Discussion
4.1. Culprit drugs
Previous studies have shown that antibiotics, NSAIDs, anesthetics, radiocontrast media, anti-neoplastic agents and biologic agents are the main culprit of DIA in general.[13–19] In this study, antibiotics, radiocontrast media, and NSAIDs were the most common causes of DIA-related litigations. In contrast, in an anaphylaxis-related malpractice litigation study (2011–2016) published in the United States in 2018, CT radiocontrast media was the most commonly implicated allergen, accounting for 40% of the total cases.[20] However, in principle, all drugs may induce anaphylaxis. Considering that, there was a case of hydrocortisone, even an agent for allergy treatment, the possibility of DIA should be considered in all prescriptions.
4.2. Prescription and drug administration
History taking is one of the most important measures to prevent DIA. Therefore, past history should be taken seriously. It should be recorded in a prominent area so that it can be shared with other medical staff. For a patient with a history of allergy or drug hypersensitivity, it is advisable to notify medical staff about their history repetitively. It is worthy to note that the most common malpractice causing anaphylaxis-related litigation in the United States is re-exposure to known allergens.[20]
In terms of skin test, previous studies have demonstrated a poor predictive value for radiocontrast media and antibiotics[21–24] except penicillin[25]. In addition, no standardized method has been established, and the sensitivity and specificity for such antibiotics (except for penicillin) has not been clarified. The more recent judgement regarding cefotetan suggests that the court also recognizes this practice.
If a physician does not follow the directions such as contraindication, dosage, or administration, it might be recognized as negligence. Therefore, the reason for going against the directions should be clarified and risk-benefit should be carefully considered. Particularly, for sulpyrine, which is contraindicated in children, efforts should be made to use other drugs with sufficient safety for children. Regarding the judgment related to the route and rate, the facts quoted by the court are also confirmed in the pharmacopoeia. Therefore, when prescribing a drug, it is preferable to consider information such as what is described in the pharmacopoeia prior to prescription, considering that the route or rate of administration may also be a problem.
4.3. Adequate observation and prompt recognition of anaphylaxis
Although mucocutaneous symptoms are reported in 80% to 90% of cases of anaphylaxis in general,[10,11] only about 30% of the DIA litigation cases in our study involved mucocutaneous symptoms, suggesting that in many cases reaching the diagnosis of anaphylaxis were relatively difficult.[26–29] In contrast, although cardiovascular symptoms are reported in up to 45% of cases of anaphylaxis,[7] more than 90% of cases in this study involved cardiovascular symptoms, and most cases were accompanied by cardiac arrest. Neurologic symptoms were also recognized more frequently in this study than in previous reports (up to 15%).[10,11] This discrepancy is likely due to the occurrence of end-organ dysfunction, as indicated by the pathophysiology resulting from decreased cerebral blood flow.[27] In addition, many cases progressed rapidly, suggesting that there might have been insufficient time to cope with the emergency. Therefore, when a drug, which can cause anaphylaxis is administered, careful observation is required after administration and even atypical presentations should be recognized promptly. However, there are no specified guidelines regarding how long this observation should continue after administration. Thus, specified suggestions regarding observation times should be presented.
Additionally, considering the negligence cases due to inappropriate emergency kit preparation, prompt access to epinephrine and airway management equipment should be available when administering a drug that can cause anaphylaxis. Finally, medical personnel should remember that inadequate observation and non-preparation of an emergency kit may result in failure of a prompt response to DIA.
4.4. Prompt and appropriate management of anaphylaxis
Early epinephrine treatment has been reported to reduce mortality and hospitalization rates and to improve the clinical prognosis of anaphylaxis.[11] However, several studies have shown that epinephrine is administered in as low as 24% to 30% of cases,[30,31] and close to one third of physician's are not aware of the usage of epinephrine.[32] Therefore, emphasis should be put on improving the awareness of medical staff on the ways to use this most important therapeutic entity. Even in a primary clinic, epinephrine should be available. Additionally, physicians need to make timely and early efforts to administer epinephrine in cases of DIA to prevent further significant morbidity.
Prompt airway management is also important. In a primary clinic, securing the airway and mouth-to-mouth breathing or ambu-bagging should at least be performed. Where an immediate tracheal intubation is expected to be possible (emergency room, operation room, or an inpatient ward), securing the airway should not be delayed without justified reasons. If intubation fails for reasons such as severe laryngeal edema, other methods such as cricothyroidotomy should be used to secure the airway.[33]
Cardiac arrest should be detected promptly, and chest compression (the most important step in resuscitation) should be performed immediately and correctly. It should not be delayed for any reason, even for tracheal intubation. The meticulous maintenance of medical records related to the emergency is also critical, considering that the court deemed CPR to have been inappropriate because of poor medical records regarding the course of treatment and changes in the patient's status.
4.5. Limitation
This study has several limitations. First, the number of cases included in the study was small. This is because not all medical disputes proceed to litigation. In the settlement process of disputes, there are solutions such as agreement or arbitration before litigation, and only a few disputes eventually proceed to litigation. Second, the clinical features described in this study may not represent the general features of DIA because of selectivity bias from litigation. Third, information that might be needed for analysis was missing because information was collected based only on anonymized sentenced judgments, in a few cases. Fourth, judges, not medical professionals wrote the contents of the judgment. Therefore, it is difficult to conclude that the facts described in the judgments are completely objective medical facts. Fifth, the analysis period is 20 years, which may be difficult to fully reflect the rapidly changing current medical environments. For example, sulpyrine (one of the causative agents in this study) has been withdrawn from the Korean market due to the known risks such as anaphylaxis and agranulocytosis since 2004. However, the basic treatment of anaphylaxis such as epinephrine and proper airway management has not changed. In addition, the grounds for judging malpractice, even 20 years ago, are still worthy of reference.
Despite several limitations, this study is expected to play an important role in improving the awareness on DIA that any physicians may encounter incidentally.
5. Conclusion
Considering that there were many atypical presentations without mucocutaneous symptom in lawsuit cases of DIA, physicians should be familiar with the diagnostic criteria of anaphylaxis and should not miss even atypical presentation. Also, since the progression showed rapid and fatal, medical institutions and the staff should be able to respond to DIA promptly. Even only 1 error during the process of history taking, drug prescription and administration, observation, and emergency treatment may lead to fatal consequences and indemnity. Therefore, it is important not to repeat the malpractice confirmed in this study, especially when prescribing drugs well-known to induce DIA. In this way, this study will help improve awareness regarding DIA and prevent related malpractice.
Author contributions
Conceptualization: SeungGyeong Jang, SoYoon Kim, Won Lee, SuHwan Shin.
Data curation: SeungGyeong Jang, Won Lee, SuHwan Shin.
Formal analysis: Cheol Won Hyeon, SeungGyeong Jang, Won Lee, SuHwan Shin.
Investigation: SuHwan Shin.
Methodology: Soo Ick Cho, SoYoon Kim, Won Lee, SuHwan Shin.
Supervision: Ji Young Lee, Soo Ick Cho, SoYoon Kim, Won Lee.
Validation: Ji Young Lee.
Visualization: SeungGyeong Jang.
Writing – original draft: Cheol Won Hyeon, Won Lee, SuHwan Shin.
Writing – review & editing: Ji Young Lee, Soo Ick Cho, Won Lee, SuHwan Shin.
Cheol Won Hyeon: https://orcid.org/0000-0003-3992-6364
Ji Young Lee: https://orcid.org/0000-0002-2260-9939
SeungGyeong Jang: https://orcid.org/0000-0001-9121-3439
Soo Ick Cho: https://orcid.org/0000-0003-3414-9869
SoYoon Kim: https://orcid.org/0000-0001-7015-357X
Won Lee: https://orcid.org/0000-0003-6948-6948
SuHwan Shin: https://orcid.org/0000-0002-2812-1985
Footnotes
Abbreviations: CPR = cardiopulmonary resuscitation, CT = computed tomography, DIA = drug-induced anaphylaxis, IQR = interquartile range, IRB = institutional review board, KRW = Korean Won, NSAIDs = non-steroidal anti-inflammatory drugs, USD = United States Dollars.
Won Lee and SuHwan Shin contributed equally to this work.
The authors have no funding and conflicts of interest to disclose.
References
- [1].Klein JS, Yocum MW. Underreporting of anaphylaxis in a community emergency room. J Allergy Clin Immunol 1995;95:637–8. [DOI] [PubMed] [Google Scholar]
- [2].Kaufman DW, Laporte JR, Latorre FJ, et al. Risk of anaphylaxis in a hospital population in relation to the use of various drugs: an international study. Pharmacoepidemiol Drug Saf 2003;12:195–202. [DOI] [PubMed] [Google Scholar]
- [3].Moro MM, Alonso MA, Hernandez JE, et al. Incidence of anaphylaxis and subtypes of anaphylaxis in a general hospital emergency department. J Investig Allergol Clin Immunol 2011;21:142–9. [PubMed] [Google Scholar]
- [4].Lieberman P, Camargo CA, Bohlke K, et al. Epidemiology of anaphylaxis: findings of the American college of allergy, asthma and immunology epidemiology of anaphylaxis working group. Ann Allergy Asthma Immunol 2006;97:596–602. [DOI] [PubMed] [Google Scholar]
- [5].Gupta R, Sheikh A, Strachan DP, et al. Time trends in allergic disorders in the UK. Thorax 2007;62:91–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Jerschow E, Lin RY, Scaperotti MM, et al. Fatal anaphylaxis in the United States, 1999-2010: temporal patterns and demographic associations. J Allergy Clin Immunol 2014;134:1318–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Liew WK, Williamson E, Tang ML. Anaphylaxis fatalities and admissions in Australia. J Allergy Clin Immunol 2009;123:434–42. [DOI] [PubMed] [Google Scholar]
- [8].Pumphrey R. Anaphylaxis: can we tell who is at risk of a fatal reaction? Curr Opin Allergy Clin Immunol 2004;4:285–90. [DOI] [PubMed] [Google Scholar]
- [9].Shin S, Jang SG, Min K, et al. The legal doctrine on the liability of physicians in medical malpractice lawsuits involving complex regional pain syndrome. J Korean Med Sci 2018;33:e46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Simons FE, Ardusso LRF, Bilò MB. World allergy organization guidelines for the assessment and management of anaphylaxis. World Allergy Organ J 2011;4:13–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Simons FE, Ebisawa M, Scanchez-Borges M, et al. 2015 update of the evidence base: World Allergy Organization anaphylaxis guidelines. World Allergy Organ J 2015;8:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Jorge MP, Javier ES, Gloria J, et al. Characterization of medical malpractice claims against obstetricians affiliated to FEPASDE in Colombia 1999-2014. Rev Colomb Anestesiol 2018;46:112–8. [Google Scholar]
- [13].Ribeiro-Vaz I, Marques J, Demoly P, et al. Drug-induced anaphylaxis: a decade review of reporting to the Portuguese Pharmacovigilance Authority. Eur J Clin Pharmacol 2013;69:673–81. [DOI] [PubMed] [Google Scholar]
- [14].Ensina LF, de Lacerda AE, de Andrade DM, et al. Drug-induced anaphylaxis in children: nonsteroidal anti-inflammatory drugs and drug provocation test. J Allergy Clin Immunol Pract 2014;2:825. [DOI] [PubMed] [Google Scholar]
- [15].Jares EJ, Baena-Cagnani CE, Sánchez-Borges M, et al. Drug-induced anaphylaxis in Latin American countries. J Allergy Clin Immunol Pract 2015;3:780–8. [DOI] [PubMed] [Google Scholar]
- [16].Lee YS, Sun WZ. Epidemiology of anaphylaxis: a retrospective cohort study in Taiwan. Asian J Anesthesiol 2017;55:9–12. [DOI] [PubMed] [Google Scholar]
- [17].Park HK, Kang MG, Yang MS, et al. Epidemiology of drug-induced anaphylaxis in a tertiary hospital in Korea. Allergol Int 2017;66:557–62. [DOI] [PubMed] [Google Scholar]
- [18].Faria E, Rodrigues-Cernadas J, Gaspar A, et al. Drug-induced anaphylaxis survey in Portuguese Allergy Departments. J Investig Allergol Clin Immunol 2014;24:40–8. [PubMed] [Google Scholar]
- [19].Renaudin JM, Beaudouin E, Ponvert C, et al. Severe drug-induced anaphylaxis: analysis of 333 cases recorded by the Allergy Vigilance Network from 2002 to 2010. Allergy 2013;68:929–37. [DOI] [PubMed] [Google Scholar]
- [20].Lindor RA, McMahon EM, Wood JP, et al. Anaphylaxis-related Malpractice Lawsuits. West J Emerg Med 2018;19:693–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Riezzo I, Bello S, Neri M, et al. Ceftriaxone intradermal test-related fatal anaphylactic shock: a medico-legal nightmare. Allergy 2010;65:130–1. [DOI] [PubMed] [Google Scholar]
- [22].Bernstein DI, Wanner M, Borish L, et al. Twelve-year survey of fatal reactions to allergen injections and skin testing: 1990–2001. J Allergy Clin Immunol 2004;113:1129–36. [DOI] [PubMed] [Google Scholar]
- [23].Schrijvers R, Breynaert C, Ahmedali Y, et al. Skin testing for suspected iodinated contrast media hypersensitivity. J Allergy Clin Immunol Pract 2018;6:1246–54. [DOI] [PubMed] [Google Scholar]
- [24].Yoon SY, Park SY, Kim S, et al. Validation of the cephalosporin intradermal skin test for predicting immediate hypersensitivity: a prospective study with drug challenge. Allergy 2013;68:938–44. [DOI] [PubMed] [Google Scholar]
- [25].Empedrad R, Darter AL, Earl HS, et al. Nonirritating intradermal skin test concentrations for commonly prescribed antibiotics. J Allergy Clin Immunology 2003;112:629–30. [DOI] [PubMed] [Google Scholar]
- [26].Kim MY, Park CS, Jeong JW. Management and educational status of adult anaphylaxis patients at emergency department. Korean J Intern Med 2018;33:1008–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Alvarez-Perea A, Tanno LK, Baeza ML. How to manage anaphylaxis in primary care. Clin Transl Allergy 2017;7:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Brown SGA, Mullins RJ, Gold MS. Anaphylaxis: diagnosis and management. Med J Aust 2006;185:283–9. [DOI] [PubMed] [Google Scholar]
- [29].Soufras GD, Kounis GN, Kounis NG. Brain injury due to anaphylactic shock: broadening manifestations of Kounis syndrome. Int Endod J 2014;47:309–13. [DOI] [PubMed] [Google Scholar]
- [30].Ye YM, Kim MK, Kang HR, et al. Predictors of the severity and serious outcomes of anaphylaxis in Korean adults: a multicenter retrospective case study. Allergy Asthma Immunol Res 2015;7:22–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Xu YS, Kastner M, Harada L, et al. Anaphylaxis-related deaths in Ontario: a retrospective review of cases from 1986 to 2011. Allergy Asthma Clin Immunol 2014;10:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Plumb B, Bright P, Gompels MM, et al. Correct recognition and management of anaphylaxis: not much change over a decade. Postgrad Med J 2015;91:3–7. [DOI] [PubMed] [Google Scholar]
- [33].Yunginger JW, Sweeney KG, Sturner WQ, et al. Fatal food-induced anaphylaxis. JAMA 1988;260:1450–2. [PubMed] [Google Scholar]


