Abstract
To investigate therapeutic effect of cuff rectum drainage tube (CDT) in preventing the postoperative complications of total mesorectal excision (TME) and promoting the recovery of the patients.
The clinical data of 84 cases of low rectal cancer performed TME from June 2015 to June 2017 in the First Affiliated Hospital of Xiamen University were analyzed retrospectively. All the cases were performed anus-retained operation without preventive colostomy. Patients were divided into 2 groups according to the material of the anorectal drainage tube placed in the colonic cavity. Group I (CDT group) was transanal cuff rectal drainage tube placement (Patent No. ZL 201320384337.8) (n = 48), and group II (conventional group) was transanal clinical conventional drainage tube placement (n = 36). Anastomotic fistula incidence, the time of anal exsufflation, postoperative first ambulation time, intestinal function recovery time, the incidence of interrelated complications of drainage tube and postoperative hospital stay between 2 groups were analyzed retrospectively.
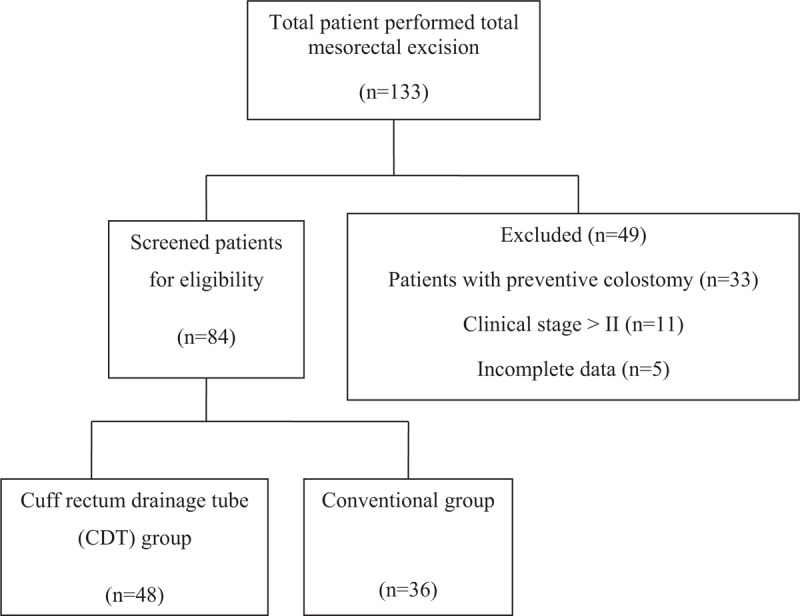
Both postoperative first ambulation and anal exhaust time in CDT group were shorter than those in the conventional group ([2.3 ± 0.4] d vs [3.0 ± 0.2] d, P < .05; [3.3 ± 0.3] d vs [3.9 ± 0.5] d, P < .05). Meanwhile, the postoperative hospital stay of CDT group was significantly decreased than that in the conventional group ([10.3 ± 1.6] d vs [11.8 ± 1.1] d, P < .05). Significant different occurrence of complications existed in anastomotic fistula (2.1% [1/48] vs 16.7% [6/36], P < .05), frequent defecation (8.3% [4/48] vs 27.8% [10/36], P < .05), defecating unfinished feeling (12.5% [6/48] vs 30.6% [11/36], P < .05), drainage tube complication (4.2% [2/48] vs 22.2% [8/36], P < .05).
The cuff rectum drainage tube may reduce incidence of anastomotic fistula after TME, shorten postoperative first ambulation and anal exsufflation time, enable faster recovery with good toleration and decrease postoperative hospital stay.
Keywords: anastomotic fistula, anus preservation surgery, drainage tube, low rectal cancer, total mesorectal excision
1. Introduction
Colorectal cancer is the third most common malignancy in the world and the fourth leading cause of cancer-related deaths, with about 1.4 million new cases and nearly 700,000 deaths each year.[1] With the further understanding of the mesenteric dilatation and total mesorectal excision (TME) as a surgery option for patients with the lower rectal cancer, local recurrence was reduced, tumor outcome and quality of life was improved, the value of such standard surgical treatment was supported.[2–5] The popularization of TME improved the rate of anus preservation in low rectal cancer, while the risks of complications associated with postoperative anastomotic fistula and lower anorectal function were increased.[6,7] Symptomatic anastomotic fistula is the most serious complication, anastomotic fistula leads to significant postoperative morbidity and mortality, and poorer long-term outcome.[8] If anastomotic fistula occurs, it can directly lead to the failure of anus-preserving operation, aggravate the pain of patients, prolong the hospitalization time, increase the medical cost, and reduce quality of life.[9] Therefore, prevention against postoperative complications, especially anastomotic fistula, is an urgent problem and very important.[10,11]
In order to minimize the risk of anastomotic fistula, several methods have been explored. Among them, synchronous diverting stoma is one of the most widely used methods, and has been recommended following low anterior resection by some previous studies.[12,13] However, it remains controversial that whether all patients with low anterior rectal resection should receive diverting stoma, because a protective stoma is associated with significant morbidity, a second surgical trauma, and increased hospital costs, and diverting stoma is unnecessary in about 80% to 95% of patients which have no anastomotic fistula.[14,15] Moreover, although the stoma is intended to protect anastomosis, the potential physiological and psychological morbidity of the stoma should also be considered.[16,17] A wide spectrum of complications can arise from stoma formation such as: wound infection, prolapse, retraction, stenosis, necrosis, parastomal hernia or fistula, skin irritation, intestinal obstruction, increased length of hospital stay, and poor patient adaptation, these are also concerned by surgeons.
An alternative technique with minimal invasion and at least equivalent effectiveness is mandated in solving the problem. Transanal drainage tube placement is another widely used method attempt to decrease the incidence of anastomotic fistula by keeping the anal sphincter opening and reducing the postoperative pressure of anus-intestinal cavity.[18,19] It is both time- and cost-effective, and compared with diverting stoma, it is less invasive and easier to apply. Several previous studies have identified the efficacy of transanal drainage tube placement in preventing anastomotic fistula.[16,20–23] Placing the drainage tube in the anus-intestinal cavity and keeping it unobstructed to reduce the intestinal pressure, which is an important means to reduce the incidence of the complications related to TME. The method of implementation is still up to the surgeon to decide, mainly depending on experience, patient characteristics, and the operation environment, rather than any clear evidence that one technique is superior to another. Currently, new technologies and devices are being developed and tested continuously to overcome the defects in current practices and further reduce the risks of colorectal anastomosis, which has broad prospects. However, the current clinical common anal drainage tube materials are easy to block, leakage, prolapse, even because the drainage is not smooth and lead to anastomotic fistula and other serious consequences.[24] In our study, patients with low rectal cancer who select TME surgery approach as the research object. During the operation, the cuff rectum drainage tube (Patent number: ZL201320384337.8) was transanal placement, and the efficacy was compared with that of conventional anal drainage tube commonly used in clinical practice, to explore the effect of drainage tube on preventing postoperative complications after TME and promoting patient recovery.
2. Material and methods
2.1. Ethics statement
This study was approved by the ethics committee of First Affiliated Hospital of Xiamen University. The clinical information was collected and analyzed with each participant written informed consent. All participants consented that the matched clinical information would be submitted for publication.
2.2. Patients characteristics
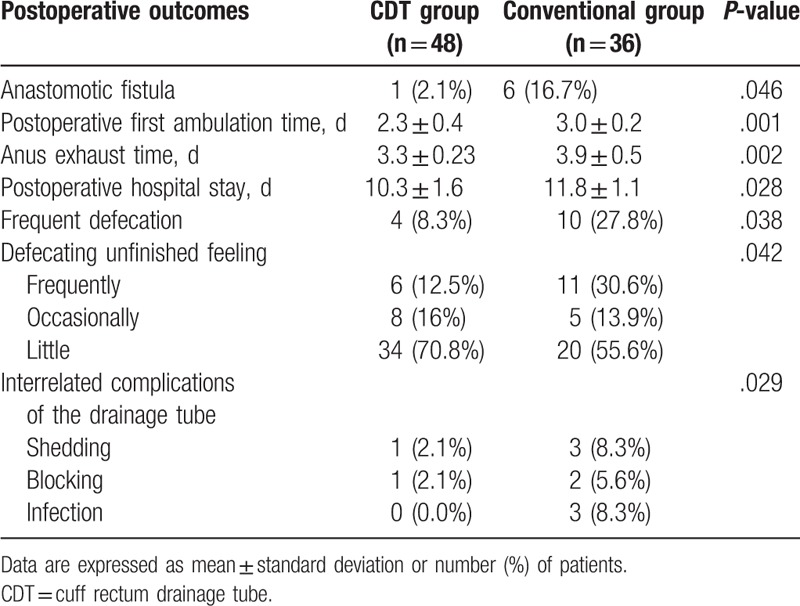
The clinical data of 84 patients who underwent TME for low rectal cancer from June 2015 to June 2017 in the First Affiliated Hospital of Xiamen University were analyzed retrospectively. Alimentary tract reconstruction was performed by end-to-end anastomosis. Among them, 51 patients were men, 33 patients were women. Age was 29.0–78.0 (59.6 ± 11.2) years old. Preoperative fibrocolonoscopy and digital rectal examination confirmed that the tumor distance from anal verge was 5.0–8.0 (6.5 ± 0.8) cm, the diameter of tumor was 2.6–7.2 (4.9 ± 1.3) cm, the body mass index (BMI) of patient was 16.5–29.0 (23.2 ± 3.2), the preoperative serum albumin was 31.2–60.1 (44.0 ± 5.0) g/L, there were 58 patients with rectal bleeding and 11 patients with anemia. We assessed rectal bleeding based on digital rectal examination, rigid rectoscopy, flexible sigmoidoscopy, and patient stool with blood. The adult men with Hb <120 g/L and adult women with Hb <110 g/L is diagnosed with anemia. According to the material of the Transanal drainage tube, patients were divided into cuff rectum drainage tube (CDT) group (n = 48) and clinical conventional drainage tube group (n = 36). The preoperative general data of patients in the 2 groups were shown in Table 1, and the difference between the groups was no statistically significant (P > .05).
Table 1.
Comparison for the characteristics of patients between CDT group and conventional group.
2.3. Inclusion and exclusion criteria
Our criteria for inclusion were as follows: patient for low rectal cancer; there were no complete bowel obstructions, tumor rupture and bleeding, severe diabetes mellitus, liver or kidney dysfunction, and other complications before operation; TNM stage II or less; postoperative pathological examination confirmed adenocarcinoma; no chemoradiotherapy was performed before operation; the anus-retained operation was performed without protective colostomy. The exclusion criteria were as follows: patients with preventive colostomy; TNM stage >II; the data are not complete.
2.4. The structure of cuff rectum drainage tube
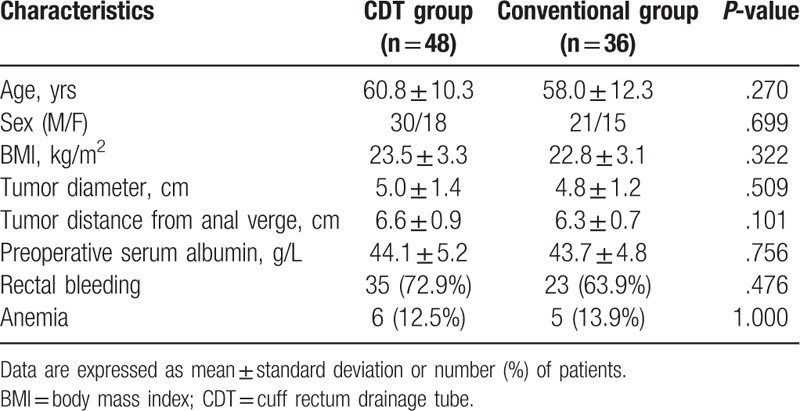
Structure as shown in Fig. 1A, the length of drainage tube is 30 cm, the diameter is 1.5 cm, there is a 1.5 cm of main hole in the front of tube, and a side-hole to facilitate drainage. A cuff is located 2 cm from the front end of the drainage tube, which a small hole attached for connecting the vent pipe. The tail end of vent pipe connected inflation inlet. When the cuff inflates, it can play a fixed and closed role. And in addition to prevent the drainage tube from escaping, it can avoid stool overflow along the pipe wall. The cuff rectum drainage tube accord with the requirement of the postoperative intestinal airtight, support, drainage, and preventing pollution.
Figure 1.
Flow diagram for screening patients of low rectal cancer who were performed total mesorectal excision and anus-retained operation without preventive colostomy.
2.5. Drainage tube placement method
After the patients of CDT group completed the digestive tract reconstruction, the tail end of cuff rectum drainage tube was connected with the drainage bag, the front end was coated with liquid paraffin and placed above the anus sphincter. Fifteen to twenty millimeter of gas was injected into the cuff by syringe, and the cuff inflated to self-fixing (Fig. 1B). After the patients of the conventional group completed the digestive tract reconstruction, the clinical conventional anal tube was applied with liquid paraffin at the end of the head, which was inserted slowly from the anus and placed above the anal sphincter. The tube was sutured and fixed on the perianal skin. The end of the tube was cut off about 8 cm from the anal verge and then connected with the drainage bag.
2.6. Data extraction
Two authors screened each patient independently and extracted data using a uniform standardized form, until an agreement was reached. Demographic and clinical variables—age, sex, body mass index (BMI), tumor diameter, tumor distance from anal verge, preoperative serum albumin, rectal bleeding, and anemia were investigated (Table 1). Discrepancies between 2 authors were resolved through discussion until reaching a general consensus. The third author was sought for opinions if a consensus could not be reached.
2.7. Postoperative observation index
Postoperative anastomotic fistula, postoperative first ambulation time, anus exhaust time, postoperative hospital stay, anus function, and drainage tube complication were recorded (Table 2). Follow-up was conducted until 3 months after surgery.
Table 2.
Comparison of primary outcome for patients after total mesorectal excision with different drainage tubes.
2.7.1. Diagnostic criteria for anastomotic fistula
-
(1)
The anal drainage tube drains out turbid pus-like or fecal-like matter.
-
(2)
There is an unexplained fever, and excluding other infection stove.
-
(3)
There are abdominal pain, increased defecation number, perianal or tailbone pain and uncomfortable feeling of anal, or rectal bladder fistula, rectal vaginal fistula, physical examination find peritoneal irritation syndrome, digital rectal examination find that anastomosis is incomplete and can feel emptiness behind fistula mouth.
-
(4)
Blood routine check shows leucocyte total and percentage of neutrophils increased.
The diagnosis was verified by clinical (digital palpation, inspection of drain contents), endoscopic (rigid rectoscopy, flexible sigmoidoscopy), radiologic (rectal contrast study, computed tomography [CT] scan) investigations, or laparotomy.[24–26]
2.7.2. Anus function evaluation
The anus function is evaluated by the frequency of defecating unfinished feeling and defecation. The evaluation method of defecating unfinished feeling: according to the 3 indexes in the literature,[27] the assessment was made. Awareness of defecation: the awareness of defecation is normal for 2 points, abnormal, or not complete for 1 point, disappeared or false awareness for 0 point. Defecation control capacities: patient can control the loose stool for 2 points; patient cannot well control the loose stool for 1 point; patient cannot control the formed stool for 0 point. Sensory function: patient can distinguish the gas and feces, and percept the process of defecation for 2 points, patient can only distinguish gas and feces for 1 point, the absence of the above those for 0 point. According to the sum of the above 3 scores, the degree of defecating unfinished feeling was assessed: 0 point means there is defecating unfinished feeling frequently; 1 to 3 points mean that there is defecating unfinished feeling occasionally; >3 points mean that there is little or no defecating unfinished feeling. The evaluation method of frequent defecation: during the 3 months after surgery, the daily number of loose stool beyond 3 times.
2.7.3. Interrelated complications of the drainage tube
The condition of shedding and blocking of the drainage tube in the colonic cavity and the condition of perianal infection that due to fecal matters overflowed along the tube wall were recorded.
2.8. Statistical analysis
SPSS 16.0 statistical software (SPSS 16, Chicago, IL) was used for statistical analysis of the data. Measurement data (postoperative first ambulation time, anus exhaust time, postoperative hospital stay, medical expenses) were compared by using t test, count data (anastomotic fistula, interrelated complications of the drainage tube, the assessment of anus function) were compared by using the chi-square test or the continuous chi-square test. P < .05 was considered statistically significant for the difference.
3. Results
3.1. Patients selection and characteristics
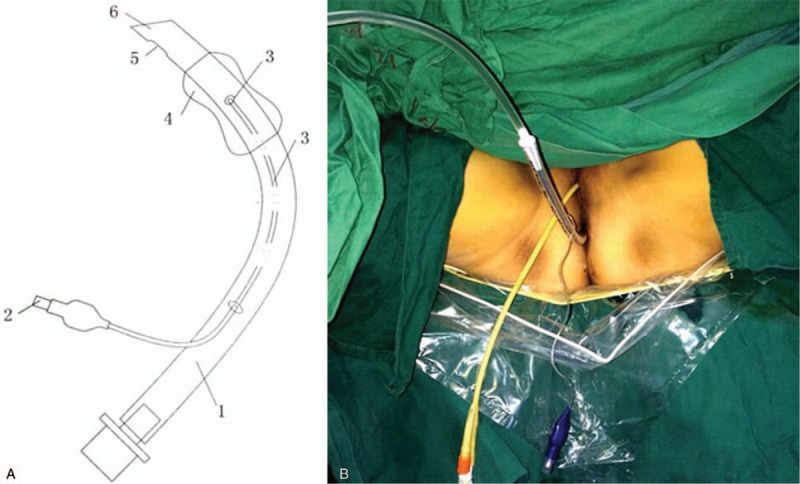
Among a total of 133 patients, data of 84 patients were included in the present analysis. The cuff rectum drainage tube group and conventional groups comprised 48 and 36 patients respectively, while 33 patients were excluded because patients performed preventive colostomy, 11 patients were excluded because of clinical stages >II, 5 patients were excluded because of incomplete data (Fig. 2). The characteristics of included patients were summarized in Table 1. Age, sex, BMI, tumor diameter, tumor distance from anal verge, preoperative serum albumin, rectal bleeding, and anemia were not considered statistically significant for the difference.
Figure 2.
The sketch of the structure of cuff rectum drainage tube and the postoperative picture. A. The sketch of the structure of cuff rectum drainage tube: drainage tube body, inflation inlet, vent pipe, cuff, side opening, main hole. B. The cuff rectum drainage tube was fixed in the anus of rectal cancer patient.
3.2. The effect of cuff rectum drainage tube for postoperative clinical indexes
After 3 months of follow-up, there was no death and tumor recurrence in the 2 groups. Table 2 shows the comparison of postoperative clinical evaluation indexes between the 2 groups of patients with low rectal cancer. Compared with the clinical conventional drainage tube group, the incidence of anastomotic fistula was reduced in cuff rectum drainage tube group, and postoperative first ambulation time, anal exhaust time, postoperative hospital stay, postoperative frequent defecation, defecating unfinished feeling, and interrelated complications of the drainage tube were significantly changed in CDT group. Both postoperative first ambulation and anal exhaust time in CDT group were shorter than those in the conventional group ([2.3 ± 0.4] d vs [3.0 ± 0.2] d, P < .05; [3.3 ± 0.3] d vs [3.9 ± 0.5] d, P < .05). Meanwhile, postoperative hospital stay of the CDT group was significantly decreased postoperative hospital stay than that in the conventional group ([10.3 ± 1.6] d vs [11.8 ± 1.1] d, P < .05). Significant different occurrence of complications existed in anastomotic fistula (2.1% [1/48] vs 16.7% [6/36], P < .05), frequent defecation (8.3% (4/48) vs 27.8% [10/36], P < .05), defecating unfinished feeling (12.5% [6/48] vs 30.6% [11/36], P < .05), drainage tube complication (4.2% [2/48] vs 22.2% [8/36], P < .05) between the 2 groups. Anastomotic fistula occurred in 7 patients after operation in the 2 groups, including 1 case in CDT group, which was cured after adequate drainage, fasting, dressing change, and symptomatic treatment. In the conventional group, 4 cases were cured after adequate drainage, fasting, dressing change, and symptomatic treatment, and 2 cases were cured after anal suture repair.
4. Discussion
The epidemiological feature of rectal cancer in China is that the proportion of low rectal cancer is high. Clinical practice has proved that the rectal wall of 1 cm above the dentate line can be retained basically normal anal defecation function after operation. This provides theoretical support for improving anal preservation rate of low rectal cancer. Symptomatic anastomotic fistula is the most serious complication, reported that the clinical fistula rate after anterior resection have varied from 5% to 16%.[8,24,28,29] Multiple studies have found as significant risk factors for anastomotic fistula: male sex, diabetes, obesity, preoperative steroid drug use, preoperative radiation, neoadjuvant therapy, American Society of Anesthesiologists (ASA) status, a lower level of anastomosis, tumor location, larger tumor size, intraoperative adverse events, postoperative pressure of the intestinal cavity.[8,19,28,30–33] Anastomotic fistula leads to significant postoperative morbidity and mortality. Patients typically show the triad of pelvic or perineal pain, temperature elevation, and serologic inflammation parameters.[19] Once anastomotic fistula occurs, it will cause infection around the anastomotic site, inflammatory tissue proliferation, resulting in anastomotic stenosis and difficult defecation and other symptoms, seriously affecting the postoperative quality of life of patients. Therefore, reducing postoperative complications, especially anastomotic fistula, is an urgent problem.[34] In the early days, patients often accompanied by abdominal distension, and from the anus out of a small amount of old blood or loose stool, suggesting that there were factors that causes anastomotic fistula during the time from recovery of bowel function to anal defecation.
High postoperative pressure of the intestinal cavity at the anastomotic site has been considered to be closely related to anastomotic fistula and other complications.[35–37] Transanal drainage tube placement is a way to reduce intestinal pressure by draining the gas and watery stool. The potential benefits include drainage, reducing intestinal pressure, promoting gastrointestinal motility and excretion of intestinal content, and avoiding the retention of content at the anastomotic, help to decrease the occurrence of postoperative anastomotic fistula.[19] Application of anus drainage tube in anterior resection of rectal cancer may be a simple, safe, and effective way to prevent or reduce the occurrence of anastomotic fistula.[22]
Transanal drainage tube placement seemed to be a safe procedure in previous studies, with very few reports of adverse events. However, perianal discomfort and skin soreness were common problems related to anal drainage tube. And common anus tube is straight and has a small diameter and is prone to clogging. Fecal easily overflow along the pipe wall and permeates the perianal area, causing perianal infection and skin ulceration, and tube is difficult to be fixed and easy to escape. Anastomotic fistula after anus-preserving surgery in patients with low rectal cancer often occurs in the postoperative early period (2–8 days), mostly around 4 days after operation, but still frequent around the 8th day, so the placement time of the anal drainage tube should be >8 days.[38] Patients with common anal tube have more complications associated with drainage tubes, so the actual placement time is often only 3 to 4 days, which does not provide effective protection.[39] Additionally, nonstandardized use of the technique, including the material used in the construction of the transanal tube, the shape of the tube, and the duration of placement, have been reported. With some using a Ficon tube (24 Fr), Malecot catheter (28 Fr), Pleats drain (10 mm), Foley catheter (24 Fr) or other material, and the tip of the tube was positioned 30 to 50 mm proximal from the anastomosis, and the tube was removed 5 to 7 days after the operation.[20,21,40,41]
In our study, the cuff rectum drainage tube (CDT) developed by our team conformed to the anatomical structure of the rectum. The use of the CDT has the following advantages: first, placement of the CDT during surgery cannot only reduce intestinal pressure, but also directly reduce the physical and chemical irritation caused by the contact of intestinal contents with the anastomotic site. Second, when the cuff inflating, it can prevent the fecal from overflowing along the pipe wall, and prevent the anal tube falling off. At the same time, the CDT has a larger diameter and is difficult to be blocked. Third, the use of CDT can effectively drain the liquid, gas, and loose stools in the intestine, reduce the pollution to the anastomosis, reduce the risk of infection and necrosis in the anastomosis, and reduce the incidence of anastomosis fistula. Fourth, since the anal sphincter may contract and spasm after surgery, CDT can provide support and stenting that the proximal and distal ends of the anastomosis are unobstructed. Fifth, the CDT is convenient for observing whether there is anastomotic bleeding during surgery. This can avoid postoperative continuous anastomotic bleeding. Finally, the CDT is very soft so that it does not cause injury to the bowel wall and anastomosis or discomfort to the patient. There are some reports about bowel perforation in association with a transanal tube.[21,42] Therefore, care should be taken to ensure that drainage tube does not perforate the colon, especially in patients with multiple diverticula. This study showed that after placement of the cuff rectal drainage tube, the patient was well tolerated.
Compared with the conventional group, the incidence of anastomotic fistula, frequent defecation and defecating unfinished feeling was reduced, and the first anal exhaust time postoperative and first ambulation were earlier in CDT group. These are due to the thicker rectal drainage tube and conforms to anatomical structure of the rectum, the condition of continuous anal expansion was improved, the local blood circulation was improved, the anal sphincter was quickly recovered, the edema of the crissum was reduced or even avoided, and the recovery of postoperative anal function was promote. A small number of patients may experience uncomfortable feeling of anal swelling pain, this feeling disappears after the body adapts for 1 to 2 days. The cuff can be fixed by filling 15 to 20 mL of air, and the volume is small, so it hardly increases the tension of the colonic cavity and anastomosis. In addition, after the surgery, the use of common drainage tube requires perianal suture fixation, which causes pain and patients reluctance to ambulation early, affect postoperative recovery, even some patients cannot tolerate pain so that drainage tube was spontaneously removed. Thrombosis might be caused with postoperative ambulation more difficult, so early ambulation is recommended for these patients, as is the case without a transanal tube. In this study, instead of using suture fixation, the CDT was fixed by cuff inflation. Postoperative perianal pain was mild and patients were willing to ambulation early, help to promote the recovery of intestinal function and shortened postoperative hospital stay. Patients have oral diet earlier, postoperative use of parenteral nutrition was reduced and hospitalization costs were reduced.
5. Conclusions
In summary, the cuff rectal drainage tube (CDT) conforms to the anatomical structure of the rectum, with good drainage effect and good tolerance, which can effectively reduce the occurrence of anastomotic fistula, promote postoperative recovery of the patient, shorten the hospital stay, and has a certain clinical application prospect. However, because the number of cases is relatively small, a larger-scale single-center or multi-center prospective randomized study or a meta-analysis including similar studies is necessary for further research of this issue.
Author contributions
Conceptualization: Zhengjie Huang, Weipeng Ye.
Data curation: Weipeng Ye, Zhipeng Zhu, Gang Liu, Borong Chen, Junjie Zeng, Jin Gao, Hejie Cai.
Formal analysis: Weipeng Ye, Zhipeng Zhu, Junjie Zeng, Shengjie Wang, Hejie Cai.
Funding acquisition: Guoxing Xu, Zhengjie Huang.
Investigation: Borong Chen, Jin Gao, Shengjie Wang.
Methodology: Weipeng Ye, Gang Liu, Zhengjie Huang.
Project administration: Guoxing Xu, Zhengjie Huang.
Resources: Guoxing Xu.
Software: Zhipeng Zhu, Borong Chen, Junjie Zeng.
Supervision: Zhengjie Huang, Guoxing Xu.
Validation: Zhengjie Huang, Weipeng Ye.
Visualization: Zhengjie Huang.
Writing – original draft: Weipeng Ye, Zhipeng Zhu, Gang Liu.
Writing – review & editing: Zhengjie Huang, Weipeng Ye.
Footnotes
Abbreviations: BMI = body mass index, CDT = cuff rectum drainage tube, TME = total mesorectal excision.
WPY, ZPZ, GL have contributed equally and should be regarded as co-first authors.
This study was supported by grants from the Medical Innovations Topic in Fujian Province (no.2016-CXB-8, 2012-CXB-29), the Science and Technology Project of Natural Science Foundation of Fujian Province (no. 2016J01639) and Project of Xiamen Scientific and Technological Plan (No. 3502Z20134011, 3502Z20174023,3502Z20184020) and the Youth Research Development Fund Project of The First Affiliated Hospital of Xiamen University(no. XYY2017010).
The authors have no conflicts of interest to disclose.
References
- [1].Arnold M, Sierra MS, Laversanne M, et al. Global patterns and trends in colorectal cancer incidence and mortality. Gut 2017;66:683–91. [DOI] [PubMed] [Google Scholar]
- [2].Heald RJ, Husband EM, Ryall RD. The mesorectum in rectal cancer surgery--the clue to pelvic recurrence? Br J Surg 1982;69:613–6. [DOI] [PubMed] [Google Scholar]
- [3].Wibe A, Eriksen MT, Syse A, et al. Total mesorectal excision for rectal cancer--what can be achieved by a national audit? Colorectal Dis 2003;5:471–7. [DOI] [PubMed] [Google Scholar]
- [4].Shirouzu K, Ogata Y, Araki Y. Oncologic and functional results of total mesorectal excision and autonomic nerve-preserving operation for advanced lower rectal cancer. Dis Colon Rectum 2004;47:1442–7. [DOI] [PubMed] [Google Scholar]
- [5].Kim NK, Kim YW, Min BS, et al. Operative safety and oncologic outcomes of anal sphincter-preserving surgery with mesorectal excision for rectal cancer: 931 consecutive patients treated at a single institution. Ann Surg Oncol 2009;16:900–9. [DOI] [PubMed] [Google Scholar]
- [6].Beirens K, Penninckx F, PROCARE Defunctioning stoma and anastomotic leak rate after total mesorectal excision with coloanal anastomosis in the context of PROCARE. Acta Chir Belg 2012;112:10–4. [DOI] [PubMed] [Google Scholar]
- [7].Law WL, Chu KW. Anterior resection for rectal cancer with mesorectal excision: a prospective evaluation of 622 patients. Ann Surg 2004;240:260–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].McDermott FD, Heeney A, Kelly ME, et al. Systematic review of preoperative, intraoperative and postoperative risk factors for colorectal anastomotic leaks. Br J Surg 2015;102:462–79. [DOI] [PubMed] [Google Scholar]
- [9].Kang CY, Halabi WJ, Chaudhry OO, et al. Risk factors for anastomotic leakage after anterior resection for rectal cancer. JAMA Surg 2013;148:65–71. [DOI] [PubMed] [Google Scholar]
- [10].Walker KG, Bell SW, Rickard MJ, et al. Anastomotic leakage is predictive of diminished survival after potentially curative resection for colorectal cancer. Ann Surg 2004;240:255–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Ziegler MA, Catto JA, Riggs TW, et al. Risk factors for anastomotic leak and mortality in diabetic patients undergoing colectomy: analysis from a statewide surgical quality collaborative. Arch Surg 2012;147:600–5. [DOI] [PubMed] [Google Scholar]
- [12].Huser N, Michalski CW, Erkan M, et al. Systematic review and meta-analysis of the role of defunctioning stoma in low rectal cancer surgery. Ann Surg 2008;248:52–60. [DOI] [PubMed] [Google Scholar]
- [13].Tan WS, Tang CL, Shi L, et al. Meta-analysis of defunctioning stomas in low anterior resection for rectal cancer. Br J Surg 2009;96:462–72. [DOI] [PubMed] [Google Scholar]
- [14].Bakx R, Busch OR, Bemelman WA, et al. Morbidity of temporary loop ileostomies. Dig Surg 2004;21:277–81. [DOI] [PubMed] [Google Scholar]
- [15].Kaiser AM, Israelit S, Klaristenfeld D, et al. Morbidity of ostomy takedown. J Gastrointest Surg 2008;12:437–41. [DOI] [PubMed] [Google Scholar]
- [16].Thoker M, Wani I, Parray FQ, et al. Role of diversion ileostomy in low rectal cancer: a randomized controlled trial. Int J Surg 2014;12:945–51. [DOI] [PubMed] [Google Scholar]
- [17].Gao F, Xu M, Song F, et al. Prevention of anastomotic fistula formation after low-position Dixon Operation. Pak J Med Sci 2014;30:1007–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Morks AN, Havenga K, Ploeg RJ. Can intraluminal devices prevent or reduce colorectal anastomotic leakage: a review. World J Gastroenterol 2011;17:4461–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Xiao L, Zhang WB, Jiang PC, et al. Can transanal tube placement after anterior resection for rectal carcinoma reduce anastomotic leakage rate? A single-institution prospective randomized study. World J Surg 2011;35:1367–77. [DOI] [PubMed] [Google Scholar]
- [20].Hidaka E, Ishida F, Mukai S, et al. Efficacy of transanal tube for prevention of anastomotic leakage following laparoscopic low anterior resection for rectal cancers: a retrospective cohort study in a single institution. Surg Endosc 2015;29:863–7. [DOI] [PubMed] [Google Scholar]
- [21].Nishigori H, Ito M, Nishizawa Y, et al. Effectiveness of a transanal tube for the prevention of anastomotic leakage after rectal cancer surgery. World J Surg 2014;38:1843–51. [DOI] [PubMed] [Google Scholar]
- [22].Zhao WT, Hu FL, Li YY, et al. Use of a transanal drainage tube for prevention of anastomotic leakage and bleeding after anterior resection for rectal cancer. World J Surg 2013;37:227–32. [DOI] [PubMed] [Google Scholar]
- [23].Goto S, Hida K, Kawada K, et al. Multicenter analysis of transanal tube placement for prevention of anastomotic leak after low anterior resection. J Surg Oncol 2017;116:989–95. [DOI] [PubMed] [Google Scholar]
- [24].Peeters KC, Tollenaar RA, Marijnen CA, et al. Risk factors for anastomotic failure after total mesorectal excision of rectal cancer. Br J Surg 2005;92:211–6. [DOI] [PubMed] [Google Scholar]
- [25].Matthiessen P, Hallböök O, Andersson M, et al. Risk factors for anastomotic leakage after anterior resection of the rectum. Colorectal Dis 2004;6:462–9. [DOI] [PubMed] [Google Scholar]
- [26].Bruce J, Krukowski ZH, Al-Khairy G, et al. Systematic review of the definition and measurement of anastomotic leak after gastrointestinal surgery. Br J Surg 2001;88:1157–68. [DOI] [PubMed] [Google Scholar]
- [27].Zhuo HQ, Zhou YB, Lu L, et al. [Prognostic analysis of middle and lower rectal cancer with different degrees of infiltration in mesorectum]. Zhonghua Yi Xue Za Zhi 2009;89:820–2. [PubMed] [Google Scholar]
- [28].Rullier E, Laurent C, Garrelon JL, et al. Risk factors for anastomotic leakage after resection of rectal cancer. Br J Surg 1998;85:355–8. [DOI] [PubMed] [Google Scholar]
- [29].Shiomi A, Ito M, Maeda K, et al. Effects of a diverting stoma on symptomatic anastomotic leakage after low anterior resection for rectal cancer: a propensity score matching analysis of 1,014 consecutive patients. J Am Coll Surg 2015;220:186–94. [DOI] [PubMed] [Google Scholar]
- [30].Park JS, Choi G-S, Kim SH, et al. Multicenter analysis of risk factors for anastomotic leakage after laparoscopic rectal cancer excision: The Korean Laparoscopic Colorectal Surgery Study Group. Ann Surg 2013;257:665–71. [DOI] [PubMed] [Google Scholar]
- [31].Cong ZJ, Fu CG, Wang HT, et al. Influencing factors of symptomatic anastomotic leakage after anterior resection of the rectum for cancer. World J Surg 2009;33:1292–7. [DOI] [PubMed] [Google Scholar]
- [32].Kawada K, Hasegawa S, Hida K, et al. Risk factors for anastomotic leakage after laparoscopic low anterior resection with DST anastomosis. Surg Endosc 2014;28:2988–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Eberl T, Jagoditsch M, Klingler A, et al. Risk factors for anastomotic leakage after resection for rectal cancer. Am J Surg 2008;196:592–8. [DOI] [PubMed] [Google Scholar]
- [34].Eckmann C, Kujath P, Schiedeck TH, et al. Anastomotic leakage following low anterior resection: results of a standardized diagnostic and therapeutic approach. Int J Colorectal Dis 2004;19:128–33. [DOI] [PubMed] [Google Scholar]
- [35].Bertelsen CA, Andreasen AH, Jorgensen T, et al. Anastomotic leakage after anterior resection for rectal cancer: risk factors. Colorectal Dis 2010;12:37–43. [DOI] [PubMed] [Google Scholar]
- [36].Buchs NC, Gervaz P, Secic M, et al. Incidence, consequences, and risk factors for anastomotic dehiscence after colorectal surgery: a prospective monocentric study. Int J Colorectal Dis 2008;23:265–70. [DOI] [PubMed] [Google Scholar]
- [37].Shada AL, Rosenberger LH, Mentrikoski MJ, et al. Endoluminal negative-pressure therapy for preventing rectal anastomotic leaks: a pilot study in a pig model. Surg Infect (Larchmt) 2014;15:123–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Lee WS, Yun SH, Roh YN, et al. Risk factors and clinical outcome for anastomotic leakage after total mesorectal excision for rectal cancer. World J Surg 2008;32:1124–9. [DOI] [PubMed] [Google Scholar]
- [39].Amin AI, Ramalingam T, Sexton R, et al. Comparison of transanal stent with defunctioning stoma in low anterior resection for rectal cancer. Br J Surg 2003;90:581–2. [DOI] [PubMed] [Google Scholar]
- [40].Okuda J, Tanaka K, Kondo K, et al. Safe anastomosis in laparoscopic low anterior resection for rectal cancer. Asian J Endosc Surg 2011;4:68–72. [DOI] [PubMed] [Google Scholar]
- [41].Matsuda M, Tsuruta M, Hasegawa H, et al. Transanal drainage tube placement to prevent anastomotic leakage following colorectal cancer surgery with double stapling reconstruction. Surg Today 2016;46:613–20. [DOI] [PubMed] [Google Scholar]
- [42].Lee SY, Kim CH, Kim YJ, et al. Impact of anal decompression on anastomotic leakage after low anterior resection for rectal cancer: a propensity score matching analysis. Langenbecks Arch Surg 2015;400:791–6. [DOI] [PubMed] [Google Scholar]


