Abstract
This study compared the efficacy of neoadjuvant chemotherapy (NACT) followed by radical surgery (RS) vs primary surgical treatment (PST) in patients diagnosed with International Federation of Gynecology and Obstetrics (FIGO) stage IB2/IIA2 cervical cancer.
Data of 303 cervical cancer patients who received primary therapy for stage IB2/IIA2 cervical cancer at 7 medical centers in Beijing, China between January 1, 2009 and December 31, 2016 and followed through December 31, 2017 were collected retrospectively. The response rates, surgical characteristics, and overall survival (OS) durations of patients who received NACT followed by RS were compared to those of patients who received PST.
An improved short-term complete response rate was observed among patients who received intra-arterial chemotherapy compared with patients who had intravenous chemotherapy (18.3% vs 4.1%, Pdifference = .020). Patients who received NACT were more likely to undergo laparoscopic surgery and to have a lower blood loss volume (555.4 ± 520.2 ml vs PST, 682.5 ± 509.8 ml; P = .036) and increased estimated operative time (249.9 ± 101.9 vs PST, 225.1 ± 76.5 min; P = .022). No differences in high-risk factors (HRFs), the effects of supplemental treatment, or 5-year OS were observed between patients who received NACT and PST.
Our findings indicate that patients who received NACT for FIGO stage IB2/IIA2 cervical cancer were more likely to undergo laparoscopic surgery. These findings have important implications regarding treatment with curative intent for stage IB2/IIA2 cervical cancer and warrant a further analysis of treatment strategies to ensure adequate treatment and patient-centered care.
Keywords: concurrent chemoradiotherapy, locally advanced cervical cancer, neoadjuvant chemotherapy, radical surgery
1. Introduction
Cervical cancer is among the most lethal diseases affecting women. In 2012,[1] 528,000 new cases of cervical cancer were diagnosed, and 266,000 women died of disease-related causes worldwide. According to the staging system proposed by the International Federation of Gynecology and Obstetrics (FIGO), cervical cancer can be classified into 3 groups[2]: early stage (FIGO stage IA–IB1), locally advanced cervical cancer (LACC, FIGO stage IB2–IIB), and advanced (FIGO stage IIIA–IVB).
Currently, the optimal management for patients with stage IB2/IIA2 cervical cancer remains controversial. Patients with tumors measuring >4 cm in the greatest dimension have a worse prognosis relative to those with smaller tumors, regardless of treatment.[3–5] Accordingly, specific treatment guidelines have not been set for IB2/IIA2 disease. Although concurrent chemoradiotherapy (CCRT) is the primary treatment in most Western countries,[6] neoadjuvant chemotherapy (NACT) followed by radical surgery (RS) have been widely introduced into clinical practice in other regions.[7–8] Since the 1980s, NACT followed by RS or CCRT has been introduced as a therapeutic strategy for LACC.[9–13] Such regimens offer the potential advantages of disease downstaging, such as tumor shrinkage and reducing the surgical difficulty and postoperative risk factors for adjuvant radiotherapy, which may increase patients’ quality of life and other outcomes.[11,14–15] However, studies have reported inconsistent findings regarding the outcomes of NACT. They found that NACT followed by RS did not improve overall survival (OS), and it can even lead to poor prognosis despite the reduction of pathological risk factors and the rate of adjuvant radiotherapy in LACC.[9,16–17]
In this study, we retrospectively analyzed data obtained from 7 medical centers in Beijing, China to evaluate and compare the effects of NACT followed by RS vs PST among patients diagnosed with LACC. We also studied the response rates associated with different NACT regimens, cycles, and pathways; high-risk postoperative profiles; and the effects of NACT and PST on OS. The results of this study may provide new evidence and insights that could support the implementation of an improved standard of NACT treatment for LACC.
2. Materials and methods
2.1. Participants
We retrospectively retrieved the data of 303 cervical cancer patients who underwent primary treatment for LACC between January 1, 2009 and December 31, 2016 at 7 medical centers in Beijing, China (Peking University People's Hospital; Peking University First Hospital; Peking University Third Hospital; Beijing Obstetrics and Gynecology Hospital, Capital Medical University; Beijing Chao-Yang Hospital, Capital Medical University; Beijing Anzhen Hospital, Capital Medical University; and Beijing Shijitan Hospital, Capital Medical University). All patients had been diagnosed with histopathologically confirmed FIGO stage IB2 or IIA2 cervical cancer (including squamous cell carcinoma, adenocarcinoma, or adeno-squamous cell carcinoma) and did not present with renal, hepatic, respiratory, or cardiac failure. Computed tomography or magnetic resonance imaging was performed before (at enrollment) and after chemotherapy to assess tumor extension and exclude distant metastasis or nodal involvement. We obtained an oral agreement to participate from every participant. This retrospective study was approved by the Ethic Committee (EC) from the Beijing Obstetrics and Gynecology Hospital (2015-PHB050–01). This study has been registered in Clinicaltrials.com (No. NCT02471027) and is to be continued.
3. Neoadjuvant chemotherapy (NACT)
3.1. NACT regimens
Four NACT regimens were assessed in this study:
-
1.
Cisplatin (60–70 mg/m2 on day 1) or carboplatin [4–6 times the area under the curve (AUC) on day 1] with paclitaxel (135–175 mg/m2 on day 1) every 3 weeks for 1 to 3 cycles;
-
2.
Bleomycin (15 mg/m2 on day 1) with ifosfamide (1 g/m2 on days 1–5) and cisplatin (50 mg/m2 on day 2) every 3 weeks for 1 to 3 cycles;
-
3.
Bleomycin (20 mg/m2 on day 1) with mitomycin (10 mg/m2 on day 1) and cisplatin (80 mg/m2 on day 1) via intra-arterial infusion for 1 cycle; and
-
4.
Bleomycin (10 mg/m2 on day 1) with vincristine (1.4 mg/m2 on day 1) and carboplatin (4–6 times the AUC on day 1) via intra-arterial infusion every 2 weeks for 1 to 3 cycles.
3.2. Responses to NACT
The patients’ responses to NACT were evaluated according to the tumor size measured at the initial diagnosis and immediately before surgery, according to the Response Evaluation Criteria in Solid Tumors.[19] A complete response (CR) was defined as no visible lesion, while a partial response (PR) was defined as a ≥50% decrease in the greatest dimension. Progressive disease (PD) was defined as a ≥25% increase in the greatest dimension or the appearance of new lesions. Stable disease (SD) was defined as the status between PR and PD.
4. Observation standards
4.1. Clinical measurements
For each patient, the age, FIGO stage, gross tumor morphology, tumor size at diagnosis, pregnancy/childbirth status, menopause status, and details of the NACT regimen, course, and administration routes were obtained. The surgical approach, bleeding volume, and operative time were also recorded.
4.2. Pathological results
Pathological data, including histopathologic results, cell differentiation status, histologic type, cervical and vascular invasion statuses, lymph node involvement, parametrial infiltration, and margin status, were obtained.
4.3. Surgery
Type III radical hysterectomy plus bilateral pelvic lymphadenectomy was performed within 1 to 2 weeks of diagnosis in the PST group and within 2 to 3 weeks after the last administration of chemotherapy in the NACT group. Postoperative supplemental chemoradiotherapy was administered based on the histopathologic results in accordance with the National Comprehensive Cancer Network guideline.
5. Follow-up
All patients were followed-up via phone interviews to determine the vital status until December 31, 2017.
5.1. Statistical analyses
Statistical comparisons of inter-group differences in the NACT characteristics and responses, as well as the post-operatic adjuvant therapy rate, were performed using the chi-square test or Fisher exact test. The operation time and estimated blood loss volume between the groups of patients who underwent NACT or PST were compared using Student t test. Survival was analyzed using the Kaplan–Meier method and log-rank test, while prognostic factors affecting survival were assessed using a multivariate Cox proportional hazards analysis. All data were analyzed using the SPSS 19.0 software package (SPSS Inc., Chicago, IL). A P value <.05 was considered to indicate a statistically significant difference.
6. Results
6.1. Patients’ characteristics
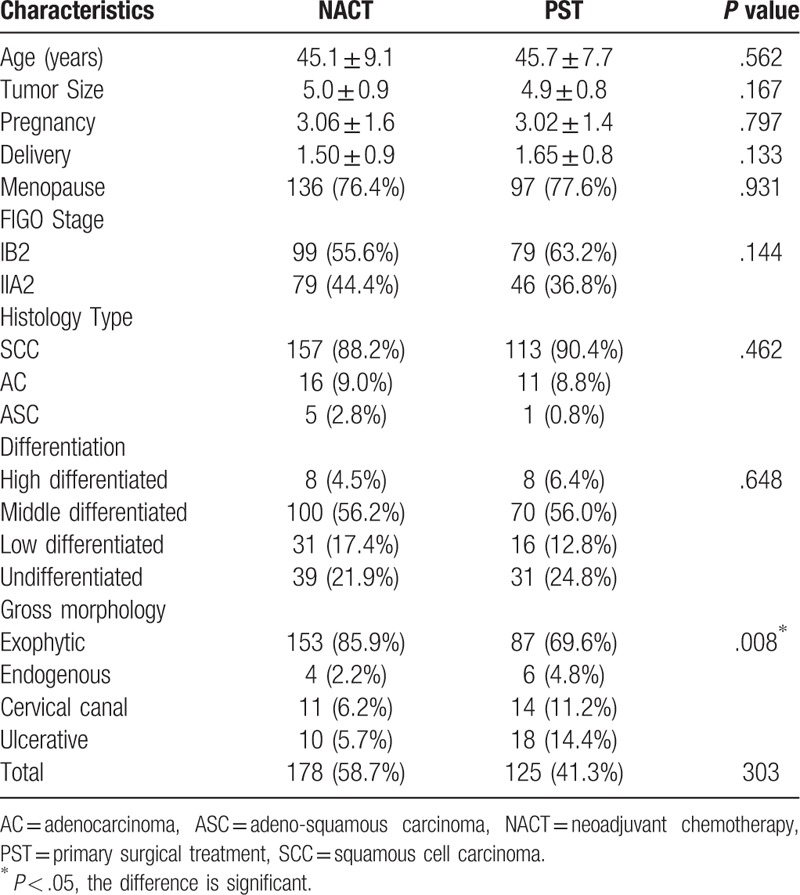
Of the 303 patients, 178 (58.7%) and 125 patients (41.3%) were classified into the NACT and PST groups, respectively. The patients in both groups were comparable with respect to the age at diagnosis, menopausal status, tumor size, FIGO stage, histology, and grade (Table 1).
Table 1.
Characteristics of patients with LACC.
6.2. Short-term responses to NACT
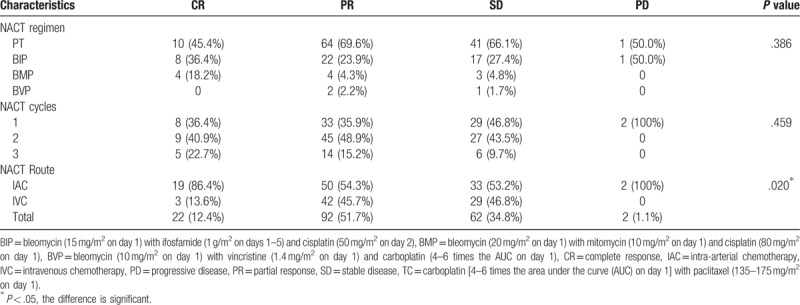
In the NACT group, 64.04% (114/178) of patients achieved an optimal clinical response; specifically, 12.35% (22/178) and 51.69% (92/178) achieved a CR and PR, respectively. Neither the NACT regimens nor the courses were found to significantly affect short-term responses. However, patients receiving intra-arterial chemotherapy (IAC) had an improved chemotherapy response rate, compared to those receiving intravenous chemotherapy (IVC) group (P = .020, Table 2).
Table 2.
Clinical responses to NACT.
6.3. Comparison of surgical profiles between the NACT and PST groups
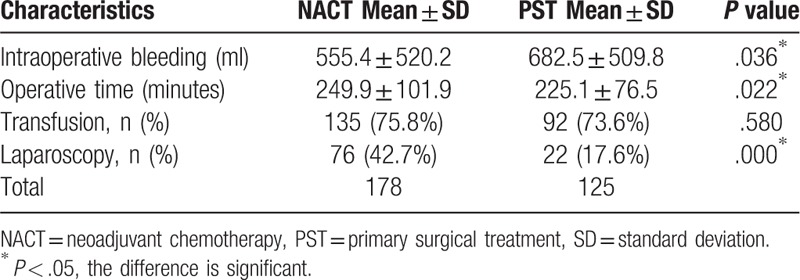
The surgery-related profiles of patients in the NACT and PST groups are listed in Table 3. Notably, the NACT and PST groups differed significantly in terms of the estimated blood loss volume (555.4 ± 520.2 vs 682.5 ± 509.8 ml, P = .036) and operative time (249.9 ± 101.9 vs 225.1 ± 76.5 min; P = .022). Specifically, although the NACT group experienced an 18.6% reduction in blood loss during surgery relative to the PST group, the former had an 11.1% longer operative time. However, the transfusion rates were similar (P = .580) between the groups. Additionally, patients in the NACT group underwent laparoscopic surgery significantly more frequently than those in the PST group (P = .000) (Table 3). Subsequently, a logistic regression analysis including NACT grouping, preoperative pathological differentiation, pathological type, gross type, age, FIGO stage, and cervical size was conducted to determine the odds ratio (OR) for laparoscopic surgery. However, the analysis identified only NACT grouping as an independent factor associated with a laparoscopic procedure [OR = 3.352, 95 confidence interval (CI): 1.07–13.18].
Table 3.
Comparison of surgical profiles between NACT and PST.
6.4. High-risk factors (HRFs) predictive of supplement treatment
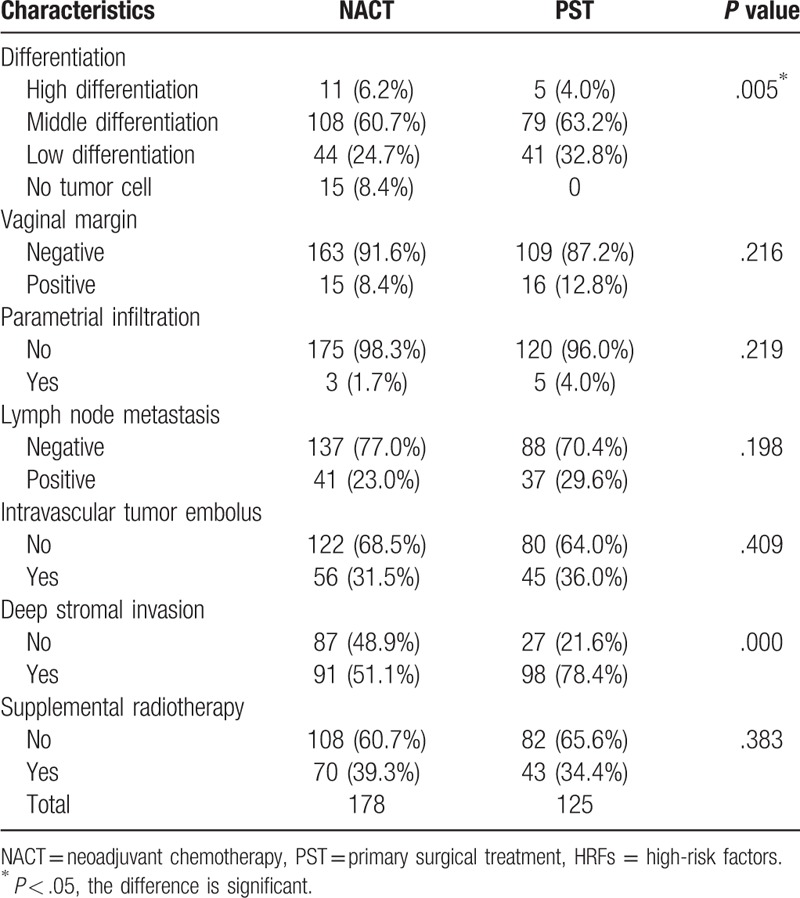
A further analysis of HRFs identified tumor differentiation as the only significant factor (P = .005); no differences in the vaginal margin, parametrial infiltration, lymph node involvement, metastasis, intravascular tumor embolus, or cervical invasion were observed between the NACT and PST groups. There was no difference in the rate of postoperative radiotherapy (Table 4).
Table 4.
Postoperative HRFs associated with pathology.
6.5. Survival analysis
Patients in the NACT and PST groups were followed for a median of 44 (6–104) and 64 (7–134) months, respectively. The 5-year cumulative survival rates were 83.3% in the NACT group and 87.2% in the PST group (PLogRank = .418).
7. Discussion
In this clinical study of the effects of NACT on patients diagnosed with stage IB2/IIA2 cervical cancer, we observed the greatest response rate among patients with deep stromal invasion. Compared with patients who underwent PST, those who underwent NACT were more likely to undergo a laparoscopic procedure and had a significant reduction in blood loss during surgery. During a 5-year follow-up, however, we did not notice any marked differences in OS between the groups.
According to previous reports of clinical cases or studies, the effects of NACT on stage IB2/IIA2 cervical cancer have long been controversial and ambiguous. The results of some studies have supported the use of NACT for stage IB cervical cancer, as indicated by improvements in 5-year disease-free survival and OS,[18–19] whereas other studies found no evidence of an effect of NACT[5] or improvements in OS.[5,9] Some studies of NACT even reported a worse prognosis among patients with stage IIA disease, despite reductions in intermediate-risk factors and the need for adjuvant radiotherapy,[16] as well as a reduction in the number of patients receiving postoperative radiotherapy for stage IB2, IIA2, or IIB squamous cell carcinoma. Based on the results of 5 large randomized controlled trials, in 1999, the National Cancer Institute of the USA launched an alert recommending CCRT for the treatment of LACC; since then, this regimen has become the standard of management for stage IB2–IIB cervical cancer.[6] Still, similarly efficacious treatment modalities, including platinum-based NACT followed by radical hysterectomy[5,10,16] and chemoradiotherapy followed by supplemental chemotherapy[7,13] or RS,[16] were developed in other regions of the world. Given these discrepancies, NACT is not currently a first-line treatment option for stage IB2/IIA2 cervical cancer, and the standard of treatment for women with LACC remains far from certain.
In this retrospective multicenter study, the proportion of patients harboring exogenetic tumors was clearly higher in the NACT group (85.9%) than in the PST group (69.6%). This may be attributable to the direct observability of the size of an exogenetic tumor, which may have led to the suggestion of preoperative NACT for tumor shrinkage, which would broaden the indication for the surgical treatment of LACC. By contrast, physicians are likely more willing to choose PST for cervical canal and ulcerative-type tumors, as these may not be directly observable.
According to the literature,[20–22] uterine IAC is superior to IVC in terms of improving the local effect of para-uterine infiltration. Although our study found that the effects of NACT were not associated with the chemotherapy regimen or cycle number, the chemotherapy efficacy (CR + PR) rate was significantly higher in the IAC group than in the IVC group [66.3% (69/104) vs 60.8% (45/74), P = .002], consistent with the literature.[21–22] This may be attributable to the ability of IAC to directly target the tumor, leading to a higher local drug concentration compared to that achieved with IVC.
We further observed a substantial decrease in operative blood loss and an increase in the operation time among patients who underwent NACT, consistent with some previous studies[23] but not others.[24] Additionally, we noticed that the NACT and PST groups differed in terms of the surgical route, with the former favoring laparoscopic procedures. As laparoscopy is superior to laparotomy in terms of reduced bleeding and postoperative pain, early activity and post-operative feeding, and a more rapid recovery, NACT would largely benefit patients with early-stage cervical cancer by increasing their eligibility for laparoscopy. Consistent with this speculation, our results demonstrated a higher frequency of laparoscopic operation among the NACT group, compared to the PST group. Although the survival outcomes did not differ, the increased frequency of laparoscopy surgery yielded short-term recovery benefits for patients in the NACT group.
In previous reports, Wang et al[21] and Li et al[25] reported that for early-stage bulky cervical cancer, NACT led to decreases in the frequencies of lymphovascular space invasion, deep stromal invasion, and lymph node metastasis and a reduced need for supplemental radiotherapy. Ultimately, these benefits led to improvements in the 5-year progression-free survival (PFS) and OS rates, compared to those of patients who underwent primary surgery alone. In our study, although the NACT group was superior to the PST group in terms of deep interstitial infiltration and the tumor differentiation grade, the groups did not differ significantly with respect to other HRFs. Additionally, as deep interstitial infiltration was identified as a high-risk factor for postoperative radiotherapy, the rate of supplemental radiotherapy should be less in the NACT group. By contrast, we observed no inter-group differences in the rates of supplemental radiotherapy and chemotherapy. We might thus consider that the standards used to determine the need for postoperative supplemental radiotherapy may not have been uniform. Additionally, a local tumor with a largest diameter >4 cm is itself a risk factor for recurrence. In such cases, therefore, the gynecologic oncologist may still opt for supplemental therapy even in the absence of postoperative HRFs.
8. Conclusion
In summary, this multi-center study of patients with stage IB/IIA cervical cancer indicates that NACT was associated with a relative reduction in blood loss, increased operation time, and improved likelihood of laparoscopic surgery. We also observed better chemotherapeutic efficacy when NACT was administered intraarterially, compared with intravenous administration. Our findings warrant further follow-up involving more detailed assessments to confirm whether NACT can improve patients’ prognoses and quality of life. The findings could potentially improve the standard of care for patients diagnosed with LACC.
Acknowledgments
Name of Department and Institution where work was done:
-
1.
Department of Gynecological Oncology, Beijing Obstetrics and Gynecology Hospital, Capital Medical University.
-
2.
Department of Gynecology and Obstetrics, Peking University First Hospital.
-
3.
Department of Gynecology and Obstetrics, Peking University People's Hospital.
-
4.
Department of Gynecology and Obstetrics, Peking University Third Hospital.
Author contributions
Conceptualization: Yue He, Yu-Mei Wu.
Data curation: Hui Zhao, Li-Rong Zhu, Jian-Liu Wang, Hong-Yan Guo, Ting Xu, Yi-Qin Wang, Ying Yao.
Formal analysis: Hui Zhao.
Funding acquisition: Yu-Mei Wu.
Project administration: Hui Zhao, Yu-Mei Wu.
Resources: Hui Zhao, Li-Rong Zhu, Jian-Liu Wang, Hong-Yan Guo, Ting Xu, Yi-Qin Wang, Ying Yao, Yu-Mei Wu.
Supervision: Yu-Mei Wu.
Writing – original draft: Hui Zhao.
Writing – review & editing: Yue He.
Footnotes
Abbreviations: CCRT = concurrent chemoradiotherapy, CR = complete response, EC = ethic committee, FIGO = international federation of gynecology and obstetrics, HRFs = high-risk factors, IAC = intra-arterial chemotherapy, IVC = intravenous chemotherapy, LACC = locally advanced cervical cancer, NACT = neoadjuvant chemotherapy, OR = odds ratio, OS = overall survival, PD = progressive disease, PFS = progression-free survival, PR = partial remission, PST = primary surgical treatment, RS = radical surgery, SD = stable disease.
This study is funded by Beijing Municipal Science and Technology Commission (D151100001915001 and D131100005313009) and Beijing Municipal Administration of Hospitals Clinical Medicine Development of Special Funding Support (ZYLX201705).
The authors declare that they have no conflict of interest.
References
- [1].World Health Organization (second ed.).December 2014 WHO Report: Comprehensive Cervical Cancer Control: A Guide to Essential Practice. Available at: http://www/who.int/reproductivehealth/publications/cancers/cervical-cancer-guide/en/ [accessed December 2014]. [PubMed] [Google Scholar]
- [2].Sardi JE, Giaroli A, Sananes C, et al. Long-term follow-up of the first randomized trial using neoadjuvant chemotherapy in stage Ib squamous carcinoma of the cervix: the final results. Gynecol Oncol 1997;67:61–9. [DOI] [PubMed] [Google Scholar]
- [3].Koh WJ, Greer BE, Abu-Rustum NR, et al. Cervical cancer, version 2.2015. J Natl Compr Canc Netw 2015;13:395–404. [DOI] [PubMed] [Google Scholar]
- [4].Landoni F, Maneo A, Colombo A, et al. Randomised study of radical surgery versus radiotherapy for stage Ib-IIa cervical cancer. Lancet 1997;350:535–40. [DOI] [PubMed] [Google Scholar]
- [5].Katsumata N, Yoshikawa H, Kobayashi H, et al. Phase III randomised controlled trial of neoadjuvant chemotherapy plus radical surgery vs radical surgery alone for stages IB2, IIA2, and IIB cervical cancer: a Japan Clinical Oncology Group trial (JCOG 0102). Br J Cancer 2013;108:1957–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].National Comprehensive Cancer Network. Cervical Cancer (Version 3. 2019). Available at: https://www.nccn.org/professionals/physician_gls/pdf/cervical.pdf. [access date December 17, 2018]. [Google Scholar]
- [7].Mikami M, Aoki Y, Sakamoto M, et al. Surgical principles for managing stage IB2, IIA2, and IIB uterine cervical cancer (bulky tumors) in Japan: a survey of the Japanese Gynecologic Oncology Group. Int J Gynecol Cancer 2014;24:1333–40. [DOI] [PubMed] [Google Scholar]
- [8].Takatori E, Shoji T, Omi H, et al. Analysis of prognostic factors for patients with bulky squamous cell carcinoma of the uterine cervix who underwent neoadjuvant chemotherapy followed by radical hysterectomy. Int J Clin Oncol 2015;20:345–50. [DOI] [PubMed] [Google Scholar]
- [9].Azevedo CRAS, Thuler LCS, Mello MJG, et al. Phase II trial of neoadjuvant chemotherapy followed by chemoradiation in locally advanced cervical cancer. Gynecol Oncol 2017;146:560–5. [DOI] [PubMed] [Google Scholar]
- [10].Tanioka M, Yamaguchi S, Shimada M, et al. Cisplatin with dose-dense paclitaxel before and after radical hysterectomy for locally advanced cervical cancer: a prospective multicenter phase II trial with a dose-finding study. Med Oncol 2017;34:134. [DOI] [PubMed] [Google Scholar]
- [11].Koensgen D, Sehouli J, Belau A, et al. Clinical outcome of neoadjuvant radiochemotherapy in locally advanced cervical cancer: result of an open prospective, multicenter phase 2 Study of the North-Eastern German Society of Gynecological Oncology. Int J Gynecol Cancer 2017;27:500–6. [DOI] [PubMed] [Google Scholar]
- [12].Naik A, Gurjar OP, Gupta KL, et al. Comparison of dosimetric parameters and acute toxicity of intensity-modulated and three-dimensional radiotherapy in patients with cervix carcinoma: a randomized prospective study. Cancer Radiother 2016;20:370–6. [DOI] [PubMed] [Google Scholar]
- [13].Ferrandina G, Gambacorta A, Gallotta V, et al. Chemoradiation with concomitant boosts followed by radical surgery in locally advanced cervical cancer: long-term results of the ROMA-2 prospective phase 2 study. Int J Radiat Oncol Biol Phys 2014;90:778–85. [DOI] [PubMed] [Google Scholar]
- [14].Yee GP, de Souza P, Khachigian LM. Current and potential treatments for cervical cancer. Curr Cancer Drug Targets 2013;13:205–20. [DOI] [PubMed] [Google Scholar]
- [15].Di Donato V, Schiavi MC, Ruscito I, et al. Effects of neoadjuvant chemotherapy plus radical surgery as front line treatment strategy in patients affected by FIGO stage III Cervical Cancer. Ann Surg Oncol 2016;23:841–84. [DOI] [PubMed] [Google Scholar]
- [16].Kim HS, Kim JY, Park NH, et al. Matched-case comparison for the efficacy of neoadjuvant chemotherapy before surgery in FIGO stage IB1-IIA cervical cancer. Gynecol Oncol 2010;119:217–24. [DOI] [PubMed] [Google Scholar]
- [17].Lee J, Kim TH, Kim GE, et al. Neoadjuvant chemotherapy followed by surgery has no therapeutic advantages of concurrent chemoradiotherapy in International Federation of Gynecology and Obstetrics stage IB-IIB cervical cancer. J Gynecol Oncol 2016;27:e52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Hu T, Li S, Chen Y, et al. Matched-case comparison of neoadjuvant chemotherapy in patients with FIGO stage IB1–IIB cervical cancer to establish selection criteria. Eur J Cancer 2012;48:2353–60. [DOI] [PubMed] [Google Scholar]
- [19].Yavas G, Yavas C, Sen E, et al. Adjuvant carboplatin and paclitaxel after concurrent cisplatin and radiotherapy in patients with locally advanced cervical cancer. Int J Gynecol Cancer 2019;29:42–7. [DOI] [PubMed] [Google Scholar]
- [20].Ujihira T, Ota T, Kusunoki S, et al. Outcome of neoadjuvant intra-arterial chemotherapy and radical hysterectomy for treatment of bulky stage IIB uterine cervical cancer: can postoperative irradiation be avoided? Int J Gynecol Cancer 2016;26:1258–63. [DOI] [PubMed] [Google Scholar]
- [21].Wang Y, Wang G, Wei LH, et al. Neoadjuvant chemotherapy for locally advanced cervical cancer reduces surgical risks and lymph-vascular space involvement. Chin J Cancer 2011;30:645–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Matsuo K, Shimada M, Yamaguchi S, et al. Neoadjuvant chemotherapy with taxane and platinum followed by radical hysterectomy for stage IB2-IIB cervical cancer: impact of histology type on survival. J Clin Med 2019;8:pii: E156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Wang PH, Chang YH, Yang YH, et al. Outcome of patients with bulky IB (≥ 6 cm) cervical squamous cell carcinoma with and without cisplatin-based neoadjuvant chemotherapy. Taiwan J Obstet Gynecol 2014;53:330–6. [DOI] [PubMed] [Google Scholar]
- [24].Liu SP, Yang JX, Cao DY, et al. Efficacy of neoadjuvant cisplatin and 5-flourouracil prior to surgery in FIGO stage IB2/IIA2 cervical cancer. Mol Clin Oncol 2014;2:240–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Li R, Lu ST, Si JG, et al. Prognostic value of responsiveness of neoadjuvant chemotherapy before surgery for patients with stage IB(2)/IIA(2) cervical cancer. Gynecol Oncol 2013;128:524–9. [DOI] [PubMed] [Google Scholar]


