Abstract
Introduction:
Cases of isolated septum pellucidum infarction have not yet been reported. To date, there are only 2 stroke reports involving septum pellucidum infarction. The etiology of septum pellucidum infarction was subcallosal artery (ScA) injury. The abnormalities were strictly confined to the septum pellucidum and the right cingulated gyrus, making this the first case to report such confined abnormalities.
Patient concerns:
In this report, we present a case of ischemic stroke confined to the septum pellucidum and cingulated gyrus in a 48-year-old male patient who presented with transient ischemic attack-like paroxysmal lower left limb weakness.
Diagnosis:
Even no obvious abnormalities were revealed by an emergency computed tomography, the infarction in the combined territories of the septum pellucidum and the cingulate gyrus was detected on magnetic resonance imaging.
Interventions:
Aspirin with clopidogrel was administered for 3 weeks as a secondary preventive drug. Clopidogrel was selected as a long-term antiplatelet drug based on a thromboelastogram.
Outcomes:
The patient showed no positive signs related to the nervous system in the hospital, and there was no recurrence during the 3-month follow-up.
Conclusions:
Infarction in the septum pellucidum and cingulate gyrus is rare and has atypical clinical manifestations. Physical examination may not yield obvious positive signs. False-negative computed tomography findings of the head may result in misdiagnosis. Thus, it is necessary to perform whole-brain magnetic resonance imaging in time. Moreover, ScA protection should be paid attention to during surgery for anterior communicating artery aneurysm.
Keywords: cingulated gyrus, ischemic stroke, septum pellucidum, subcallosal artery
1. Introduction
Septum pellucidum infarction is a rare condition caused by occlusion of one of the branches of the anterior communicating artery (ACoA), also known as the subcallosal artery (ScA). To date, cases of isolated septum pellucidum infarction have not been reported. There have been 2 stroke reports involving the septum pellucidum infarction detected on magnetic resonance imaging (MRI) (Table 1).[1–18] Here, we report a case of suspected transient ischemic attack (TIA), where MRI revealed infarctions particularly confined to the septum pellucidum and right cingulated gyrus. We have described the evolution of clinical symptoms and presented our hypothesis for the pathogenesis.
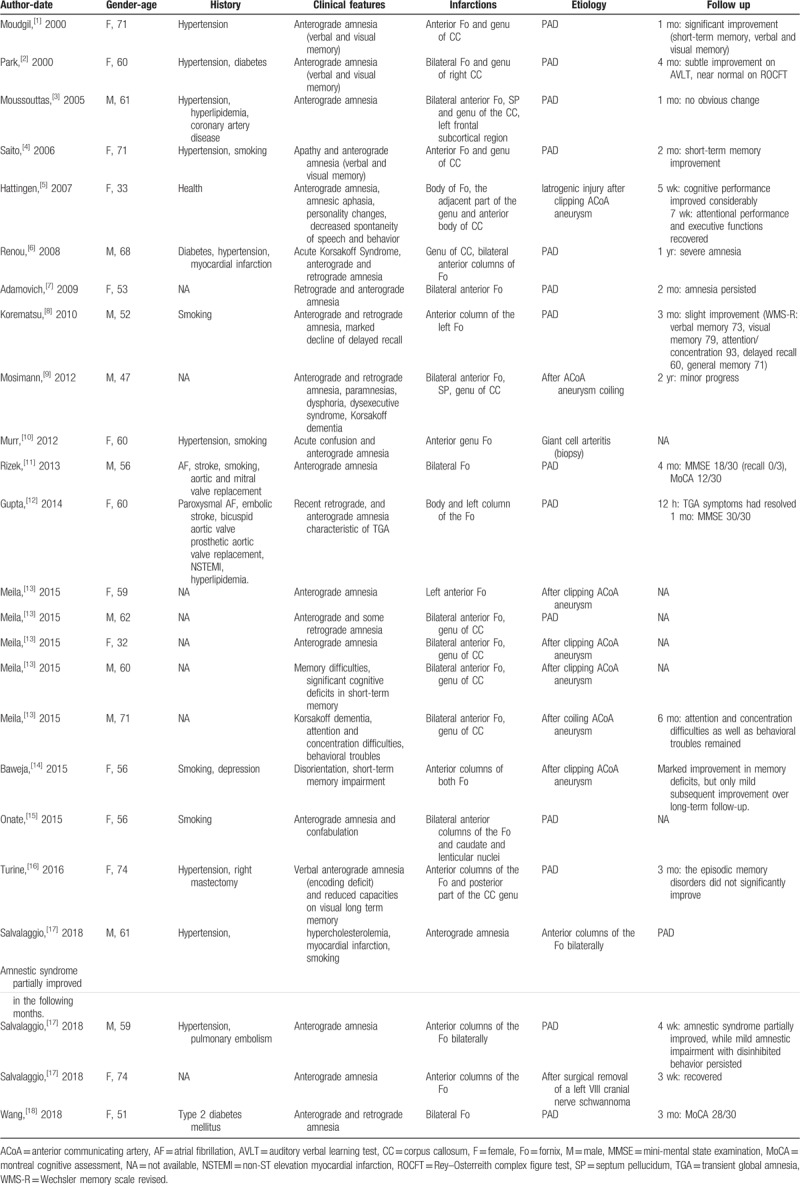
Table 1.
ScA injury-related infarctions.
2. Methods
Informed written consent was obtained from the patient for publication of this case report and accompanying images. Since the images presented in the article are entirely unidentifiable, the Ethics Committee of our institution waived the requirement for approval of this single case study with medical records.
3. Case presentation
The patient was a 48-year-old man who presented with TIA-like paroxysmal weakness in the lower left limb. In the last 2 days, when the patient became unable to stand properly and perform activities of daily life, he realized that his lower left limb was weak. He could only stand and walk after a rest of 15 minutes, and even then the walk was not proper as he was unable to lift his left leg. However, the symptoms were completely relieved after 2 hours of rest. The patient had a prior medical history of hypertension (5 years ago), which was treated with levamlodipine besylate administration. He was an occasional smoker (2–3 times per week), and consumed alcohol (250 mL every time) for more than 20 years. On admission, his blood pressure was 140/90 mm Hg. He was found to be alert at the time of admission and had no positive signs of nervous system impairment with the National Institutes of Health Stroke Scale score of 0.
No obvious abnormalities were revealed by an emergency computed tomography (CT), and electrocardiography. His serological analysis, including hematology analysis, and measurement of parameters such as blood sugar, hepatic function, renal function, glycosylated hemoglobin, electrolyte, coagulation function, blood sedimentation, myocardial enzyme, myohemoglobin, troponin, tumor markers, rheumatism series, folacin, vitamin B12, thyroid function, hepatitis antigens and antibodies, human immunodeficiency virus antibody, and treponema pallidum antibody, were found within the normal limits. However, triglycerides (2.58 mmol/L), uric acid (520 umol/L), homocysteine (16 umol/L) levels were found to be elevated. TIA was suspected, and hence, the patient was treated with aspirin (100 mg/d), clopidogrel (75 mg/d), atorvastatin (20 mg/d).
MRI on a 3.0 T scanner showed abnormal signal for the septum pellucidum and right cingulate gyrus, which was considered to be indicative of acute cerebral infarction (Fig. 1). No atherosclerotic plaque existed in carotid vessels as revealed by ultrasonography reports, and also, no obvious abnormality was found in the transcranial doppler analysis. Moreover, there was no evidence for atrial fibrillation in the 24-hour ambulatory electrocardiogram. Criminal vessels were assessed by CT angiography (CTA) and it was found that the A2 segment of the right anterior cerebral artery was severely stenosed, along with presence of basilar artery fenestration (Fig. 2). Thromboelastography was performed after 5 days of antiplatelet drug administration to guide secondary treatment. The inhibition rates of aspirin and clopidogrel were found to be 94.7% and 95.2%, respectively (Fig. 3). Aspirin with clopidogrel was administered for 3 weeks as secondary preventive drug according to the results of the CHANCE study.[30] Clopidogrel was selected as a long-term antiplatelet drug based on the results of thromboelastogram. The patient had no symptoms of neurological damage during 3-month follow-up.
Figure 1.
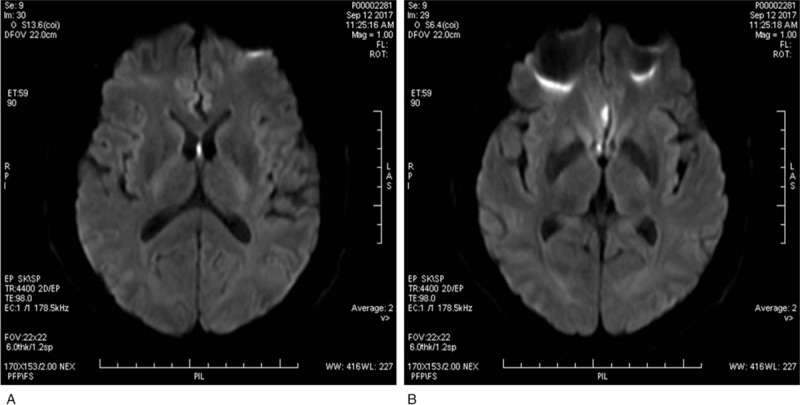
Magnetic resonance imaging findings. (A) Diffusion-weighted imaging shows high signal in the septum pellucidum. (B) Diffusion-weighted imaging shows high signal in the right cingulate gyrus.
Figure 2.
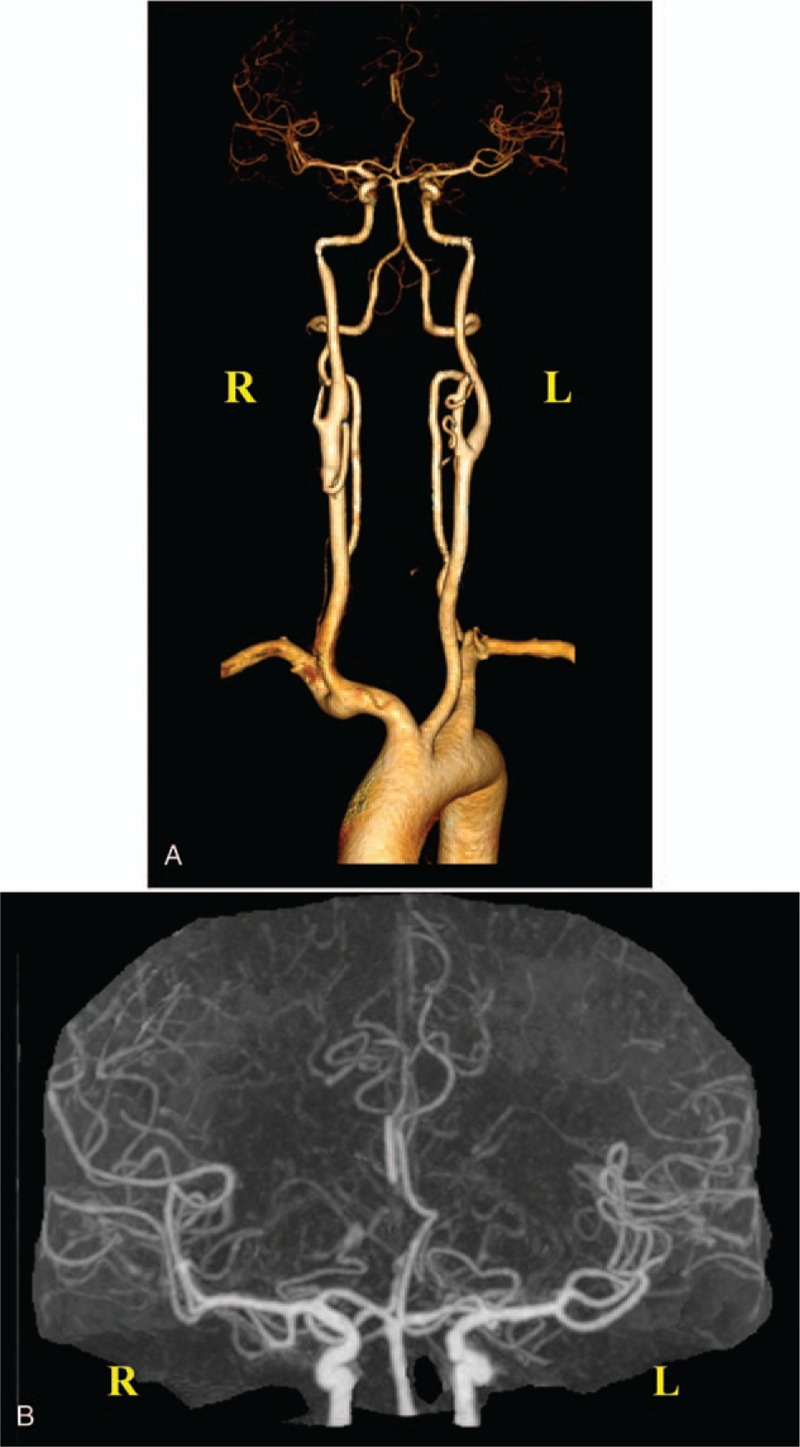
Computed tomography angiography. (A) Three-dimensional imaging shows that the A2 segment of the right anterior cerebral artery is severely stenosed, along with basilar artery fenestration. The remaining vessels are naturally shaped with no stenosis or dilatation observed in the lumen. (B) Enlarged original image confirms severe stenosis of the A2 segment of the right anterior cerebral artery.
Figure 3.
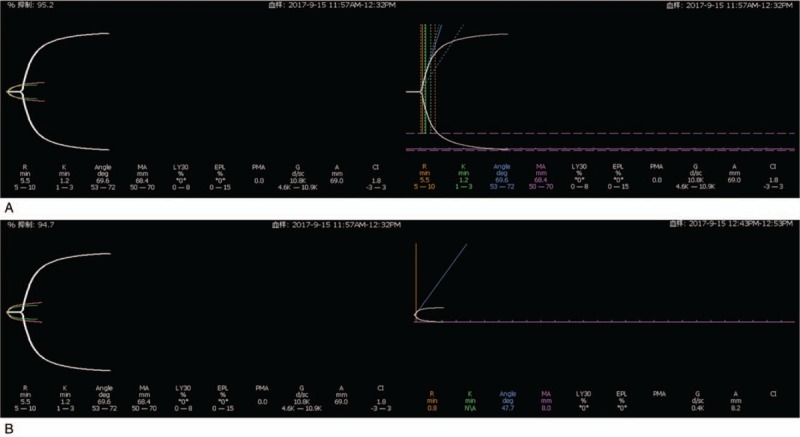
Thromboelastography analysis. (A) The platelet inhibition rate of clopidogrel is 95.2% (reference value 30%). (B) The platelet inhibition rate of aspirin is 94.7% (reference value 50%).
4. Discussion
Septum pellucidum is supplied by ScA, which is the most important branch of ACoA.[19,20] Other branches of the ACoA supply the optic nerves and chiasm, lamina terminalis, hypothalamus, and subcallosal region.[21] Microsurgical anatomy of perforating branches of the ACoA indicates that the perforating branches vary in terms of number, and direction. Corpus callosum has a lower number of perforating branches[22] than the optic chiasma. The ScA is the largest unpaired perforating branch of the ACoA, with a diameter of approximately 0.5 mm, and bilaterally perfuses the medial and ventral cerebral hemispheres (basal forebrain).[23] Therefore, infarctions in the territory of the ScA are mostly associated with bilateral effects. In this case, the cerebral infarctions were confined to the septum pellucidum and cingulated gyrus, which was consistent with the territory of ScA. We speculated hypertension to be the cause of the subcallosal arterial lesion observed in the patient owing to the absence of atherosclerosis evidence, no history of diabetes, or cerebrovascular disease, absence of plaque formation as revealed in the cervical vascular ultrasound, and absence of any significant big vascular stenosis as observed in the head and neck CTA. The etiology was consistent with that of the parent arterial disease subtype in Chinese Ischemic Stroke Subclassification,[24] and small-artery occlusion subtype in Trail of ORG 10172 in Acute Stroke classification.[25] Conventional angiography is difficult to be performed for ScA owing to the 0.5 mm diameter; however, autopsy or surgical microsurgery can be conducted. At present, the visualization of the typical course of ScA was only reported by digital subtraction angiography, and 3D rotational angiography in an AcoA aneurysm case.[13]
To date, case of isolated septum pellucidum infarction has not been reported. There were 2 reports involving infarction in the septum pellucidum region (Table 1). One of the etiologies was ScA infarction,[3] while the other was a secondary embolization which led to ACoA aneurysm.[9] ScA injury typically causes bilateral fornix infarction (Table 1). Fornix infarction is often characterized by memory impairment which can manifest as anterograde or retrograde amnesia. There are 2 hypotheses to explain this phenomenon:
-
(1)
the fornix is the key structure of the Papez circuit which can be interrupted due to fornix infarction, thereby resulting in memory impairment,[13]
-
(2)
the other hypothesis suggests that memory impairment may be related to the injury of the cholinergic fibers.[26]
Notably, the starting symptoms of the case we report here included paroxysmal weakness of left leg, and absence of amnesia. So far, there have been only 2 reports of infarction in the cingulate gyrus region.[27,28] The clinical manifestations included gelastic seizures, and development of the transient global amnesia. Although the first symptom of our patient was TIA-like paroxysmal lower left extremity weakness, a minor stroke was confirmed later by an MRI analysis. A recent study has successfully identified a novel frontal lobe pathway in the septum pellucidum tract (SPT) connected to the prefrontal cortex.[29] In addition to the right cingulate gyrus, we speculate that this pathway in the SPT may explain the TIA-like symptom.
5. Conclusions
Infarction in the septum pellucidum and cingulate gyrus is rare and has atypical clinical manifestations. Physical examination may not reveal obvious positive signs. False-negative CT findings of the head may lead to misdiagnosis. It is necessary to perform whole-brain MRI in a timely manner. Moreover, ScA protection should be paid attention to during surgery for ACoA aneurysm.
Acknowledgments
We would like to thank Editage (www.editage.com) for English language editing.
Author contributions
Conceptualization: Xiao-Wei Wu.
Data curation: Fu-Chun Xi.
Writing – original draft: Sui-Yi Xu.
Writing – review and editing: Chang-xin Li.
Footnotes
Abbreviations: ACoA = anterior communicating artery, AVLT = auditory verbal learning test, CTA = computed tomography angiography, MMSE = mini-mental state examination, MoCA = montreal cognitive assessment, MRI = magnetic resonance imaging, ROCFT = Rey–Osterreith complex figure test, ScA = subcallosal artery, SPT = septum pellucidum tract, TIA = transient ischemic attack, WMS-R = Wechsler memory scale revised.
This study was supported by grants from the program of Shanxi Provincial Health and Family Planning Commission, China (2017033); Doctoral Fund of the First Hospital of Shanxi Medical University, China (YB161706; BS03201631); Shanxi Applied Basic Research Program, China (201801D221426).
The authors have no conflicts of interest to disclose.
References
- [1].Moudgil SS, Azzouz M, Al-Azzaz A, et al. Amnesia due to fornix infarction. Stroke 2000;31:1418–9. [DOI] [PubMed] [Google Scholar]
- [2].Park SA, Hahn JH, Kim JI, et al. Memory deficits after bilateral anterior fornix infarction. Neurology 2000;54:1379–82. [DOI] [PubMed] [Google Scholar]
- [3].Moussouttas M, Giacino J, Papamitsakis N. Amnestic syndrome of the subcallosal artery: a novel infarct syndrome. Cerebrovasc Dis 2005;19:410–4. [DOI] [PubMed] [Google Scholar]
- [4].Saito Y, Matsumura K, Shimizu T. Anterograde amnesia associated with infarction of the anterior fornix and genu of the corpus callosum. J Stroke Cerebrovasc Dis 2006;15:176–7. [DOI] [PubMed] [Google Scholar]
- [5].Hattingen E, Rathert J, Raabe A, et al. Diffusion tensor tracking of fornix infarction. J Neurol Neurosurg Psychiatry 2007;78:655–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Renou P, Ducreux D, Batouche F, et al. Pure and acute Korsakoff syndrome due to a bilateral anterior fornix infarction: a diffusion tensor tractography study. Arch Neurol 2008;65:1252–3. [DOI] [PubMed] [Google Scholar]
- [7].Adamovich BL, Gualberto G, Roberts T, et al. Teaching NeuroImages: amnesia due to fornix infarction. Neurology 2009;73:e86. [DOI] [PubMed] [Google Scholar]
- [8].Korematsu K, Hori T, Morioka M, et al. Memory impairment due to a small unilateral infarction of the fornix. Clin Neurol Neurosurg 2010;112:164–6. [DOI] [PubMed] [Google Scholar]
- [9].Mosimann PJ, Saint-Maurice JP, Lenck S, et al. Fornix infarction and Korsakoff dementia after coiling of a large anterior communicating artery aneurysm. Neurol Clin Pract 2012;2:260–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Murr N, Thaisetthawatkul P, Helvey J, et al. Selective infarction of the anterior genu fornices associated with giant cell arteritis. J Stroke Cerebrovasc Dis 2012;21:327–9. [DOI] [PubMed] [Google Scholar]
- [11].Rizek P, Pasternak S, Leung A, et al. Acute-onset anterograde amnesia caused by isolated bilateral fornix infarction. Can J Neurol Sci 2013;40:738–9. [DOI] [PubMed] [Google Scholar]
- [12].Gupta M, Kantor MA, Tung CE, et al. Transient global amnesia associated with a unilateral infarction of the fornix: case report and review of the literature. Front Neurol 2014;5:291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Meila D, Saliou G, Krings T. Subcallosal artery stroke: infarction of the fornix and the genu of the corpus callosum. The importance of the anterior communicating artery complex. Case series and review of the literature. Neuroradiology 2015;57:41–7. [DOI] [PubMed] [Google Scholar]
- [14].Baweja R, Mensinkai A, Reddy K, et al. Fornix infarction after clipping of anterior communicating artery aneurysm. Can J Neurol Sci 2015;42:205–7. [DOI] [PubMed] [Google Scholar]
- [15].Onate Miranda M, Alba Suarez EM, Frutos R, et al. Amnestic syndrome of the subcallosal artery with additional penetrating vessel involvement. J Neurol Sci 2015;359:438–9. [DOI] [PubMed] [Google Scholar]
- [16].Turine G, Gille M, Druart C, et al. Bilateral anterior fornix infarction: the “amnestic syndrome of the subcallosal artery”. Acta Neurol Belg 2016;116:371–3. [DOI] [PubMed] [Google Scholar]
- [17].Salvalaggio A, Cagnin A, Nardetto L, et al. Acute amnestic syndrome in isolated bilateral fornix stroke. Eur J Neurol 2018;25:787–9. [DOI] [PubMed] [Google Scholar]
- [18].Wang J, Ke J, Zhou C, et al. Amnesia due to the injury of papez circuit following isolated fornix column infarction. J Stroke Cerebrovasc Dis 2018;27:1431–3. [DOI] [PubMed] [Google Scholar]
- [19].Serizawa T, Saeki N, Yamaura A. Microsurgical anatomy and clinical significance of the anterior communicating artery and its perforating branches. Neurosurgery 1997;40:1211–6. [DOI] [PubMed] [Google Scholar]
- [20].Ture U, Yasargil MG, Krisht AF. The arteries of the corpus callosum: a microsurgical anatomic study. Neurosurgery 1996;39:1075–84. [DOI] [PubMed] [Google Scholar]
- [21].Jackowski AP, Meneses MS, Ramina R, et al. Perforating and leptomeningeal branches of the anterior communicating artery: an anatomical review. Crit Rev Neurosurg 1999;9:287–94. [DOI] [PubMed] [Google Scholar]
- [22].Ego H, N’Da H, Viart L, et al. Microsurgical anatomy of perforating branches of anterior communicating artery. Morphologie 2015;99:6–13. [DOI] [PubMed] [Google Scholar]
- [23].Mugikura S, Kikuchi H, Fujii T, et al. MR imaging of subcallosal artery infarct causing amnesia after surgery for anterior communicating artery aneurysm. AJNR Am J Neuroradiol 2014;35:2293–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Gao S, Wang YJ, Xu AD, et al. Chinese ischemic stroke subclassification. Front Neurol 2011;2:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Adams HP, Jr, Bendixen BH, Kappelle LJ, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke 1993;24:35–41. [DOI] [PubMed] [Google Scholar]
- [26].Mugikura S, Takahashi S. Infarction in the pars libera of the column of fornix including pre (cholinergic)- and post (circuit of Papez fiber tracts)-commissural fibers causes “basal forebrain” amnesia. Neuroradiology 2015;57:757–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Gallardo-Tur A, Romero-Godoy J, de la Cruz Cosme C, et al. Transient global amnesia associated with an acute infarction at the cingulate gyrus. Case Rep Neurol Med 2014;2014:418180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Egea-Lucas I, Martinez-Mondejar E, Piqueres-Vidal CF, et al. Gelastic seizures as the presenting symptom of infarction of the cingulate gyrus. Rev Neurol 2015;61:211–4. [PubMed] [Google Scholar]
- [29].Cho ZH, Chi JG, Choi SH, et al. A newly identified frontal path from fornix in septum pellucidum with 7.0T MRI track density imaging (TDI) – the septum pellucidum tract (SPT). Front neuroanat 2015;9:151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Wang Y, Wang Y, Zhao X, et al. Clopidogrel with aspirin in acute minor stroke or transient ischemic attack. New Engl J Med 2013;369:11–9. [DOI] [PubMed] [Google Scholar]


