Abstract
Backgroud:
Evidence on the efficacy and safety of sertraline in patients with premature ejaculation (PE) was inconsistent. The objective of this article is to evaluate the efficacy and safety of sertraline for the treatment of PE.
Methods:
We searched Medline (OVID), Embase, the Cochrane Library, and 2 Chinese databases for randomized controlled trials (RCTs) and randomized crossover trials (RTs) that evaluated the efficacy and safety of sertraline in patients with PE. A meta-analysis was performed to calculate their pooled estimates with 95% confidence interval.
Results:
Of the 645 records obtained, we included 12 RCTs and 2 RTs (n = 977). Meta-analysis showed that sertraline prolonged intravaginal ejaculation latency time (IELT) in PE patients ((standard mean difference (SMD) = 2.14, 95% CI 1.20 to 3.08). Subgroup analyses indicated a prolonged IELT for different treatment courses: 4 weeks (SMD = 2.66, 1.06 to 4.26), 6 weeks (SMD = 0.95, 0.31 to 1.58), and 8 weeks (SMD = 1.81, 0.78 to 2.85). The sexual satisfaction rates of patients (SMD = 2.20, 1.57 to 2.84) and spouses (SMD = 2.27, 1.44 to 3.09) were also improved. We observed a significant increased risk of gastrointestinal upset (risk ratio = 2.71, 1.39 to 5.28) in the sertraline group.
Conclusion:
Sertraline can prolong IELT of PE patients, improve sexual satisfaction rates of patients and spouses, but increase risk of gastrointestinal upset.
Keywords: premature ejaculation, intravaginal ejaculation latency time, sertraline, sexual satisfaction rate, systematic review
1. Introduction
Premature ejaculation (PE) is a common sexual dysfunction which was defined by the International Society for Sexual Medicine as the following:
-
1.
Ejaculation that always or nearly always occurs prior to or within about 1 minute of vaginal penetration from the first sexual experience (lifelong premature ejaculation, LPE), or a clinically significant reduction in latency time, often to about 3 minutes or less (acquired premature ejaculation, APE);
-
2.
the inability to delay ejaculation on all or nearly all vaginal penetrations;
-
3.
negative personal consequences, such as distress, bother, frustration, and/or the avoidance of sexual intimacy.[1]
PE has been recognized for more than 100 years,[2] and the prevalence of LPE is about 4% of the general population based on previous published literatures and guidelines.[1]
Pharmacotherapy of PE includes traditional local anesthetics (such as lidocaine and prilocaine), selective serotonin reuptake inhibitors (SSRIs) and tricyclic antidepressants (TCAs) (such as dapoxetine, paroxetine and clomipramine), type 5 phosphodiesterase (PDE 5) inhibitors, tramadol and oxytocin, etc. Among the above treatments, only dapoxetine was approved in some countries, while others were off-label use.[3]
SSRIs are increasingly used for PE[4] and relevant clinical trials have been published.[5] Waldinger et al hypothesized that lifelong premature ejaculation is a neurobiological phenomenon associated with reduced transmission of central serotonin (5-HT2C and/or 5-HT1A),[6] Olivier et al have revealed that 5-HT showed a comprehensive inhibition of ejaculation, and the reduction of central 5-HT content was one of the risk factors for PE.[7] Sertraline is a highly selective SSRI that blocks the uptake of 5-HT by platelets, resulting in the increase of plasma 5-HT concentrations and the improvement of PE.[8]
However, there was no systematic review on the efficacy and safety of sertraline in patients with PE. Furthermore, due to inconsistent evidence and its off-label use in many countries, the objective of this article is to review the evidence of the efficacy and safety of sertraline for PE.
2. Methods
2.1. Search strategy
We searched Medline (OVID), Embase, the Cochrane Library, and 2 Chinese databases including Chinese Biomedicine Literature Database and Chinese Sci-tech Journals Database from inception to September 2018. The following keywords were used in search terms: “premature ejaculation”, “rapid ejaculation”, “rapid climax”, “premature climax” and “early ejaculation” for the disease, and terms “sertraline” for the medication. We used the Boolean logic “AND” to combine the two sets of terms. We limited the language of articles to English and Chinese only. We also manually searched the reference list of the included studies, journals and ClinicalTrials.gov as a supplementary source for the literature search. The systematic review with meta-analysis was registered on PROSPERO (No. CRD 42018109413). This systematic review and meta-analysis is exempt from ethical approval as the analysis involves only already published and anonymized data.
2.2. Study selection and outcome measures
Independent investigators (ZMY, SDC, and QYT) manually screened the records for potentially eligible studies by screening the title and abstract in the first stage, and full-text screening in the second. In title and abstract screening stage, studies appearing to meet the inclusion criteria, or with insufficient information to make a clear judgment, judged by either authors or both, were included in the full-text screening process. We obtained full texts of all these studies for the full-text screening. We resolved disagreements through discussion, and if necessary, a third investigator (SDZ) was consulted. We included studies if they met the following criteria:
-
1.
Randomized controlled trials (RCTs) or randomized crossover trial (RTs),
-
2.
enrolling PE patients above 18 years old; PE was defined as involuntary ejaculation before or after vaginal penetration within 1 to 2 minutes on at least 50% of occasions of attempted intercourse, or in accordance with diagnosis in the fourth edition of the American Diagnostic and Statistical Manual of Mental Disorders,[9]
-
3.
comparing sertraline with a blank control or a placebo control, both with or without another active drug or treatment. Patients combined with the following diseases were excluded: erectile dysfunction, mental illness, alcohol or drug abuse, urethritis or prostatitis.
The primary efficacy outcomes focused on intravaginal ejaculation latency time (IELT). The secondary efficacy outcomes included patient sexual satisfaction, spouse sexual satisfaction and international index of erectile function (IIEF). The safety outcome was the incidence of adverse events (AEs).
2.3. Data extraction and quality assessment
Data extraction was performed by independent investigators (ZMY, SDC, and QYT) according to a predesigned data-collection form. Extracted information included authors, publication year, participant characteristic (participation eligibility criteria, gender, and age), intervention information (the dosage and duration) and outcome of interest.
Investigators independently assessed the methodological quality of included studies. We assessed the risk of bias in the eligible RCTs and RTs with the Cochrane risk of bias assessment tool.[10] In the case of missing data, we contacted the authors of eligible studies for clarifications. All disagreements about data extraction and quality assessment were resolved through discussion among all authors.
2.4. Statistical analysis
We compared the treatment effect through meta-analysis in an intention to treat manner (following the allocation of participants in studies). Only the results of studies evaluating similar interventions in similar participants were pooled. Due to the different data units between studies, we calculated the standard mean difference (SMD) and their 95% confidence intervals (CIs) for continuous outcomes and risk ratio (RR) for categorical outcomes. We performed meta-analyses with RevMan 5.3 software using a random-effect model. Statistical heterogeneity was assessed with chi-square test and quantified with the I2 test. Subgroup analyses by duration of treatment and definitions of PE were conducted; sensitivity analysis was conducted by excluding studies that used different effect measures from other studies to test the robustness of the results. Finally, the publication bias was examined by funnel plot if the number of included studies ≥10. P < .05 was considered statistically significant.
3. Results
3.1. Study selection
The initial search identified 645 relevant records and 459 records were left after removing duplicates. Of these, 421 of 459 were excluded after title/abstract screening, and 38 reports were eligible for full-text review. After full-text review, we excluded 24 reports with following reasons: 7 studies were not RCTs or RTs; 4 studies had not reported the end-points; 7 studies were not patients with PE; 1 study was duplicated; 4 studies were not sertraline alone compared with placebo and one study was not English. Finally, we included 14 articles with 977 patients,[11–24] including 12 RCTs[12–14,16–24] and 2 RTs[11,15]. The process of literature search and study selection is presented in Figure 1.
Figure 1.
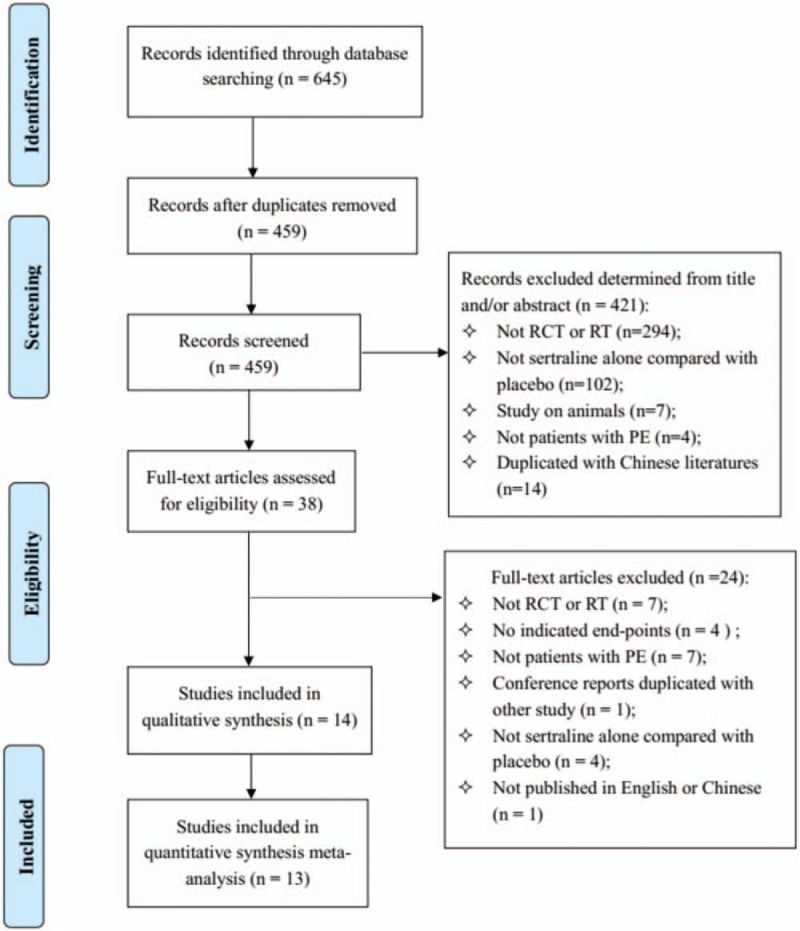
Flow diagram for study selection.
3.2. Study characteristics and quality assessment
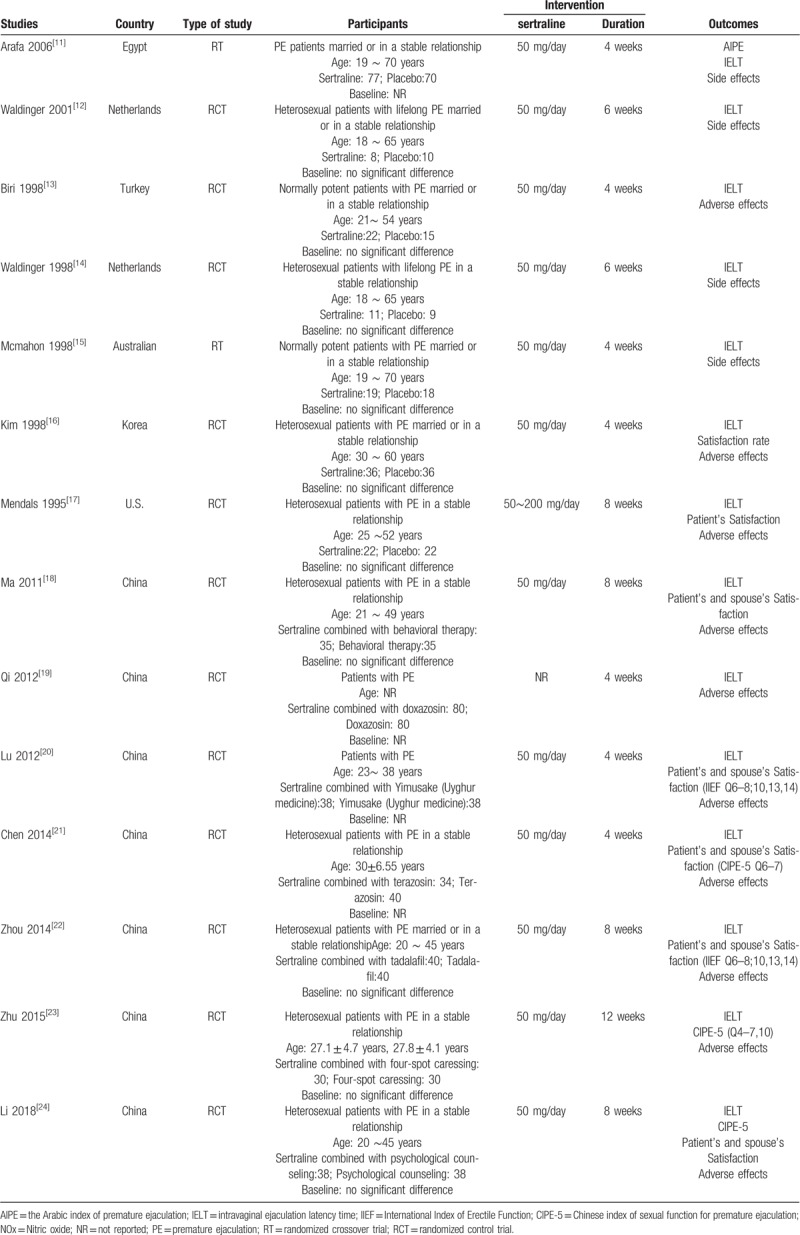
In 12 studies, the dose of sertraline was 50 mg/d,[11–16,18,20–24] and in 1 study, the dose of sertraline was 50 to 200 mg/d.[17] The duration of treatment included 4 weeks,[11,13,15,16,19–21] 6 weeks,[12,14] 8 weeks,[17,18,22,24], and 12 weeks[23] (Table 1).
Table 1.
Characteristics of included studies.
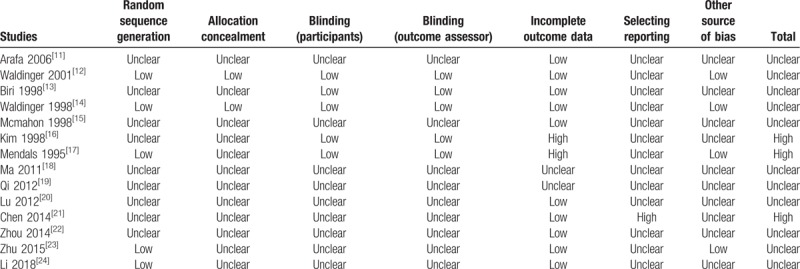
The risk of bias of 11 included literatures was unclear, and 3 were high. We classified 5 RCTs at low risk of bias in the domain of random number generation.[12,14,17,23,24] Five RCTs used the double-blind design and adopted intention-to-treat principle to analyze data.[12–14,16,17] An overall assessment in domains of risk of bias were summarized in Fig. 2. Incomplete outcome data and selective reporting were the dominant causes of high risk of bias (Table 2).
Figure 2.
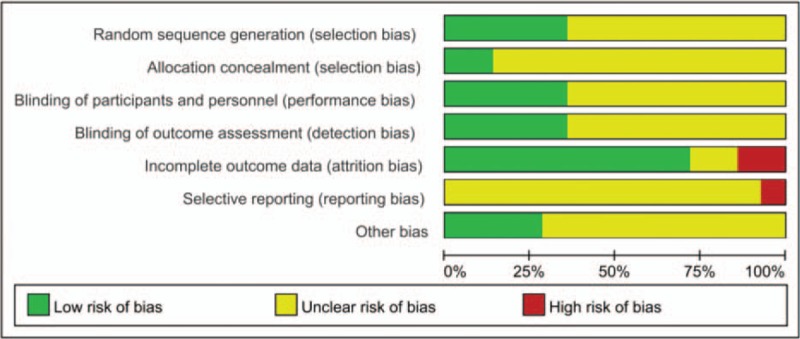
Summary of risk of bias assessment.
Table 2.
Risk of bias of included studies.
3.3. Efficacy
3.3.1. IELT
Fourteen articles (977 patients) reported the IELT data. But one study, which did not distinguish data from primary premature ejaculation and secondary premature ejaculation, was excluded.[11] Finally, thirteen articles (830 patients) were included.[12–24] One study reported both the IELT change values recorded by the patient and the spouse.[17] Since there were missing data recorded by the spouse, only the IELT recorded by the patients was used. Meta-analysis showed that sertraline can prolong the IELT (changes of IELT, SMD 2.14, 95% CI 1.20 to 3.08, P < .00001; I2 = 96%) (Fig. 3 A).
Figure 3.
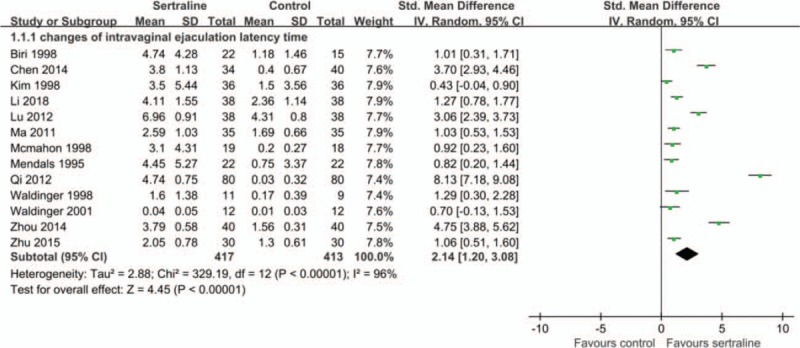
A. Effects of sertraline on changes of intravaginal ejaculation latency time (IELT). B. Effects of different courses of sertraline on changes of IELT. C. Effects of different definitions of PE on changes of IELT.
Subgroup analysis was performed according to the duration of treatment. Results indicated that 4 weeks[13,15,16,19–21,23] (516 patients, SMD 2.66, 95% CI 1.06 to 4.26, P = .001; I2 = 98%), 6 weeks[12,14] (44 patients, SMD 0.95, 95% CI 0.31 to 1.58, P = .004; I2 = 0%), 8 weeks[17–19,22–24] (330 patients, SMD 1.81, 95% CI 0.78 to 2.85, P = .0006; I2 = 94%) and 12 weeks[23] (30 patients, SMD 1.06, 95% CI 0.51 to 1.60) of sertraline treatment can all prolong IELT of PE patients (Fig. 3 B).
Of the included studies, PE was defined as uncontrollable ejaculation before/after entering the vagina within 1 min in 7 studies,[12–15,17,22–23] and within 2 minutes in 3 studies,[16,21,24] the remaining three studies had no clear time definition for PE. Subgroup analysis was performed by ejaculation time defined for PE. Meta-analysis showed that the changes of IELT in the 1-min sertraline group (302 patients, SMD 1.49, 95%CI 0.58 to 2.40, P = .001; I2 = 91%) and 2-minute group (222 patients, SMD 1.78, 95%CI 0.17 to 3.39, P = .03; I2 = 96%) was both higher than that of the control group (Fig. 3 C).
3.3.2. Patients’ sex satisfaction rate
Six studies reported patients’ sexual satisfaction rates after treatment.[16–18,20,22,24] Patients’ sexual satisfaction rates were evaluated by 6, 7, and 8 items in the IIEF (0–15 points) among 3 studies,[20,22,24] and meta-analysis showed that sertraline improved patients’ sexual satisfaction rates (SMD 2.20, 95%CI 1.57 to 2.84, P < .00001). Among the other 3 studies that cannot be pooled, 1 study[17] showed that sertraline improved patients’ sexual satisfaction rates (SMD 0.68, 95%CI 0.10 to 1.26, P = .02) by 0 to 4 items evaluation; the other 2 studies[16,18] reported percentage of patients’ sexual satisfaction and found the sexual satisfaction rates was 51.4% and 41.7% in the sertraline group after treatment, 20% and 19.4% in the control group, respectively.
3.3.3. Spouses’ sexual satisfaction rate
Seven studies reported spouses’ sexual satisfaction rates after treatment.[16–18,20–22,24] Spouses’ sexual satisfaction rates were evaluated by 6, 6, and 8 items in the IIEF (0–15 points) among 3 studies,[20,22,24] and meta-analysis showed that sertraline improved spouses’ sexual satisfaction rates (SMD 2.27, 95%CI 1.44 to 3.09, P < .00001). Among the other 4 studies that cannot be pooled, 1 study[17] showed that sertraline improved the spouses’ sexual satisfaction rates, but the difference was not statistically significant (SMD 0.45, 95%CI −0.18 to 1.09, P = .16) by 0 to 4 items evaluation; the other 3 studies[16,18,21] reported percentage of spouses’ sexual satisfaction rates and 2 studies[16,18] found the sexual satisfaction rates were 57.1% and 30.6% in the sertraline group after treatment, 14% and 11.1% in the control group, respectively; another study[21] found the sexual satisfaction rates of the sertraline group changed from 14.7% before treatment to 88.2% after treatment, and 17.5% to 25% in the control group, respectively.
3.4. Safety
Ten studies reported AEs in the sertraline group and the control group during the follow-up periods. Among them, a significant increased risk of gastrointestinal upset was observed in pooled results of 8 studies[13,15–17,20,21,23,24] (RR 2.71, 95%CI 1.39 to 5.28, P = .004). While there was no statistically difference in pooled results of headache, dizziness, drowsiness, dry mouth and fatigue (Fig. 4). Another 2 studies[18,23] reported 4 cases and 1 case of headache and dizziness in the sertraline group, respectively. Two studies[12,14] reported slight decreased in sexual desire and penile rigidity in the sertraline groups.
Figure 3 (Continued).
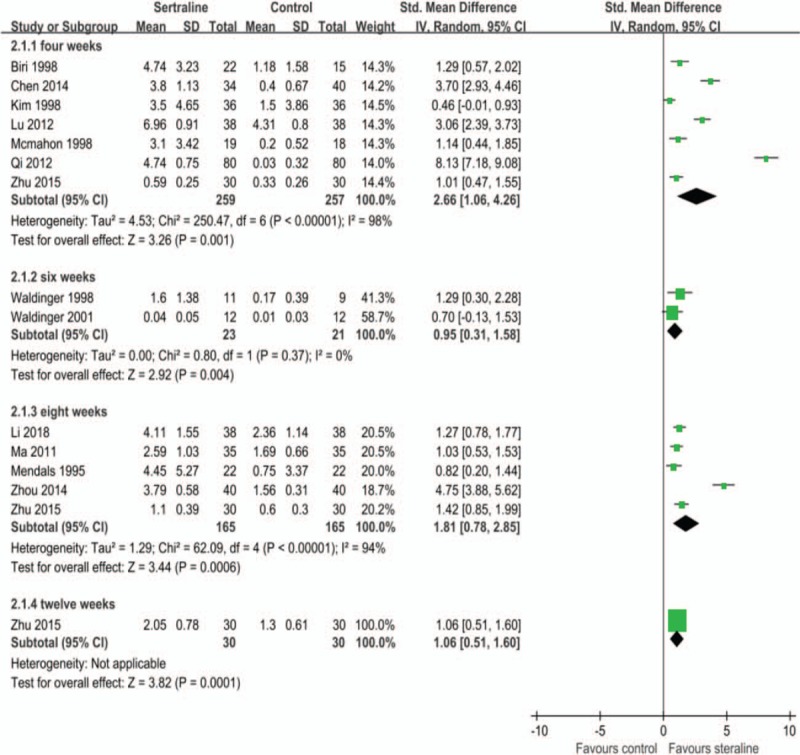
A. Effects of sertraline on changes of intravaginal ejaculation latency time (IELT). B. Effects of different courses of sertraline on changes of IELT. C. Effects of different definitions of PE on changes of IELT.
Figure 4.
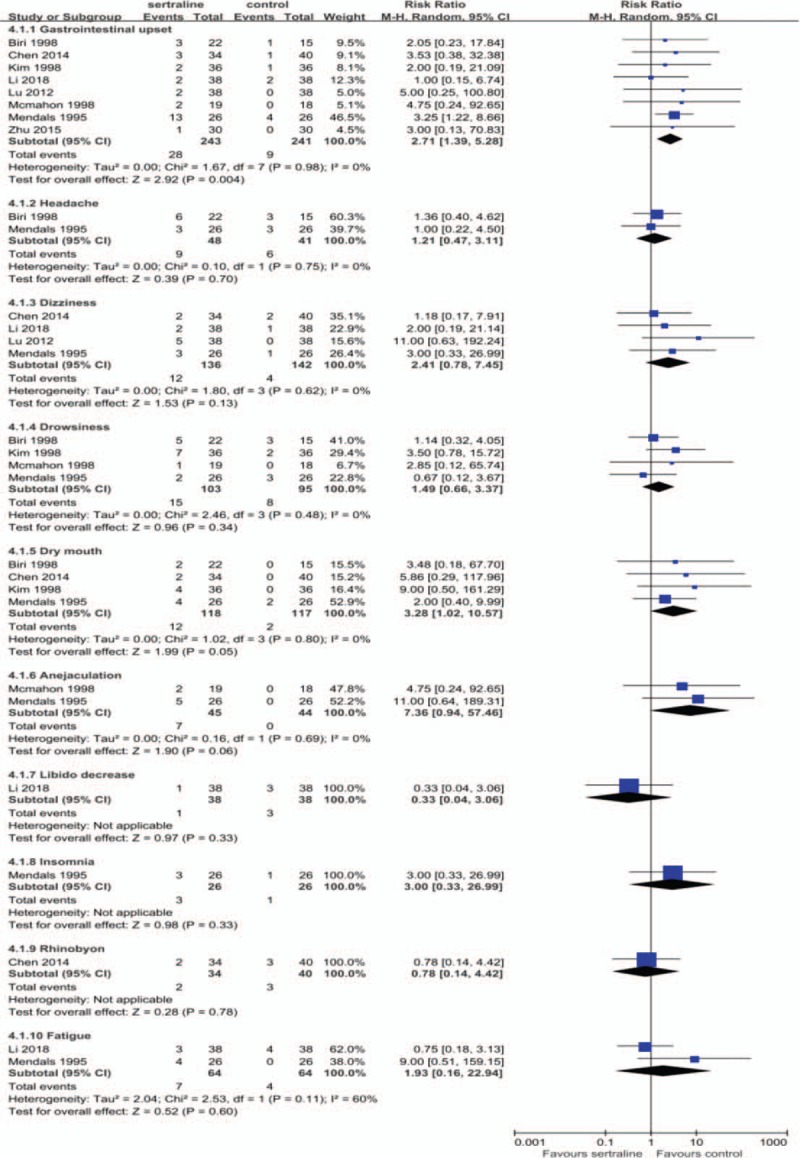
Adverse events during follow-up.
3.5. Sensitivity analysis and publication bias
In 1 study,[12] data of standard mean difference were converted between arithmetic means and geometric means. Therefore, the sensitivity analysis was performed by excluding this study. No significant changes in the results of IELT (SMD 2.26, 95% CI 1.26 to 3.26, P < .00001; I2 = 97%) were indicated. For Mendels et al,[17] both the IELT change values recorded by the patient and the spouse were reported, only the IELT recorded by the patients was used. No significant changes in the results of IELT (SMD 2.25, 95% CI 1.23 to 3.27, P < .00001; I2 = 97%) were indicated when excluding this study.
The funnel plot was used to assess publication bias, there has been little evidence of publication bias for the included studies that assessed changes of IELT (Fig. 5).
Figure 3 (Continued).
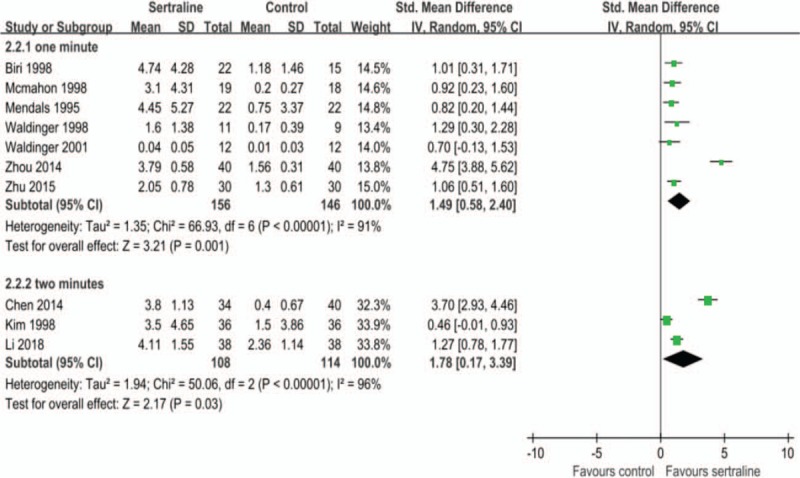
A. Effects of sertraline on changes of intravaginal ejaculation latency time (IELT). B. Effects of different courses of sertraline on changes of IELT. C. Effects of different definitions of PE on changes of IELT.
Figure 5.
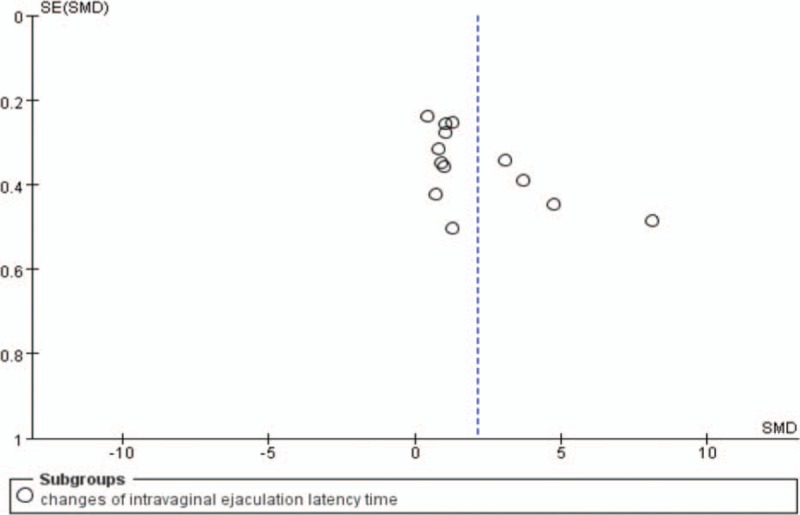
Funnel plot of publication bias.
4. Discussion
In this study, the efficacy and safety of sertraline for the treatment of PE was evaluated by a systematic review of RCTs and RTs. The results showed statistically significant difference in changes of IELT between sertraline groups and control groups, and sertraline prolonged the IELT of PE patients. In subgroup analyses, all different durations of sertraline treatment and all definitions of PE showed prolonged IELT. Studies reported sexual satisfaction rates of patients and spouses both suggested improvements by sertraline treatments. The most common AE in sertraline groups was gastrointestinal upset, which was significantly different from control groups. Headache, dizziness, drowsiness, and dry mouth were common, but no significant differences were found compared with control groups.
Guidelines by the European Association of Urology suggested that the etiology and pathophysiology of PE is largely unknown, with few data to support suggested biological and psychological hypotheses.[25] Recent researches suggested that some neurobiological and genetic variation factors might cause LPE, and psychological/environmental factors might maintain or enhance this condition. A systematic review including 18,035 patients suggested that depression increased risk of PE (OR 1.63, 95% CI 1.42 to 1.87).[26] LPE can be explained by low 5-HT concentration, low 5-HT 2C receptor sensitivity and/or 5-HT1A receptor hypersensitivity.[1] A meta-analysis of SSRIs for PE showed that paroxetine appeared to exert the strongest ejaculation delay (1492% IELT increase, 95% CI 918 to 2425%), followed by sertraline (790%, 95% CI 532 to 1173%), clomipramine (512%, 95% CI 234 to 1122%) and fluoxetine (295%, 95% CI 172 to 506%).[27]
Compared with previous studies, our study focused on sertraline. In addition to the efficacy and safety of sertraline in the treatment of PE, we also conducted subgroup analyses to further explore the treatment courses of sertraline and results by different definition of PE. Meanwhile, data of standard mean difference were converted between arithmetic means and geometric means.[12] Due to the common AEs of gastrointestinal upset, headache, dizziness, and drowsiness, sertraline may bring impacts on the daily work of PE patients whom are generally under 70 years old.
Our study has some limitations. First, most of the studies included in this systematic review had a moderate risk of bias, some of the studies were heterogeneous between the subgroups and there was a certain publication bias. Second, part of the studies were not simple comparisons between sertraline and placebo but based on the combination of another active drug or intervention (behavioral therapy[18], doxazosin[19], Yimusake[20], terazosin[21], tadalafil[22], 4-spot caressing[23] and psychological counseling[24]). Third, the IELT measurements in the studies were mostly timed by stopwatch, which had the disadvantage of being intrusive and potentially disruptive of sexual pleasure. Therefore, guidelines recommend that self-estimation by the patient and partner of ejaculatory latency be accepted as the method for determining IELT in clinical practice.[1] In addition, the sample size of the included studies was small, which can affect rigor of the results. Future larger RCTs were needed to explore the drug therapy regimen, in order to obtain the maximum therapeutic effect with the least costs.
A systematic review of 103 studies also demonstrated that serotonin-noradrenaline reuptake inhibitors, tricyclic antidepressants, topical anesthetics, phosphodiesterase-5 inhibitors and opioid analgesics also increased IELT compared with placebo (P < .05).[28] Another review also presented evidence of behavioral techniques, alpha-blockers, experimental treatments such as dorsal nerve modulation, acupuncture and Yoga.[29] Further studies for the assessment of long-term (over 12 weeks) effectiveness and safety of different interventions alone as well as combination therapies are encouraged. Additionally, different treatment effects for PE with primary or acquired causes may be explored. Furthermore, time needed to the increment in the latency time following the vaginal penetration should be considered as outcomes for further studies due to its importance for patients’ and physicians’ decision-making for the off-label use of sertraline.
In conclusion, sertraline can prolong IELT of PE patients, improve sexual satisfaction rates of patients and spouses, but increase risk of gastrointestinal upset.
Acknowledgments
We extend special thanks to Ying-Ying Yan from Peking University Third Hospital and Yuan Zhang from McMaster University for their contribution to this research.
Author contributions
ZMY conceived this review. ZMY, SDC and QYT identified reports of trials and extracted data. HLT provided statistical advice. ZMY, SDC and QYT did all statistical analyses, checked for statistical inconsistency and interpreted data. SDC contributed to data extraction and interpretation. ZMY and SDC drafted the report and all other authors (QYT, HLT and SDZ) critically reviewed the article. All authors read and approved the final manuscript.
Conceptualization: Zhan-Miao Yi.
Data curation: Zhan-Miao Yi.
Formal analysis: Zhan-Miao Yi, Shi-Di Chen and Qi-Yu Tang.
Funding acquisition: Suo-Di Zhai.
Methodology: Zhan-Miao Yi and Qi-Yu Tang.
Project administration: Zhan-Miao Yi and Suo-Di Zhai.
Resources: Zhan-Miao Yi and Suo-Di Zhai.
Software: Zhan-Miao Yi, Shi-Di Chen and Qi-Yu Tang.
Validation: Hui-Lin Tang.
Visualization: Zhan-Miao Yi and Shi-Di Chen.
Writing – original draft: Zhan-Miao Yi and Shi-Di Chen.
Writing – review & editing: Hui-Lin Tang and Suo-Di Zhai.
Footnotes
Abbreviations: AEs = adverse events, APE = acquired premature ejaculation, CIs = confidence intervals, IELT = intravaginal ejaculation latency time, IIEF = international index of erectile function, LPE = lifelong premature ejaculation, PE = premature ejaculation, PDE 5 = type 5 phosphodiesterase, RCTs = randomized controlled trials, RR = risk ratio, RTs = randomized crossover trials, SMD = standard mean difference, SSRIs = selective serotonin reuptake inhibitors, TCAs = tricyclic antidepressants.
The authors report no conflicts of interest in this work.
References
- [1].Althof SE, McMahon CG, Waldinger MD, et al. An update of the International Society of Sexual Medicine's guidelines for the diagnosis and treatment of premature ejaculation (PE). J Sex Med 2014;11:1392–422. [DOI] [PubMed] [Google Scholar]
- [2].Gross S. A Practical treatise on impotence and sterility and allied disorders of the male sexual organs. Edinburg: Nabu Press 2010. [Google Scholar]
- [3].McMahon CG, Althof SE, Kaufman JM, et al. Efficacy and safety of dapoxetine for the treatment of premature ejaculation: Integrated analysis of results from five phase 3 trials. J Sex Med 2011;8:524–39. [DOI] [PubMed] [Google Scholar]
- [4].Waldinger MD. Chapter 15: Rapid ejaculation. In: Levine SB, Risen CB, Althof SE. (Eds) Handbook of Clinical Sexuality for Mental Health Professionals. New York: Brunner-Routledge; 2003:257–74. [Google Scholar]
- [5].Tuncel A, Aslan Y, Basar MM, et al. Efficacy of clomipramine, sertraline and terazosin treatments in premature ejaculation. Turk J Med Sci 2008;38:59–64. [Google Scholar]
- [6].Waldinger MD. Premature ejaculation and SSRI-induced delayed ejaculation: the involvement of the serotonergic system. Behav Brain Res 1998;928:111–8. [DOI] [PubMed] [Google Scholar]
- [7].Olivier B, van Oorschot R, Waldinger MD. Serotonin, serotonergic receptors, selective serotonin reuptake inhibitors and sexual behaviour. Int Clin Psychopharmacol 1998;13:9–14. [DOI] [PubMed] [Google Scholar]
- [8].Cheng B, Shao F, Liu Y, et al. Sertraline in combination with vardenafil in the treatment of premature ejaculation. Chin J Hum Sexuality 2013;22:13–5. [Google Scholar]
- [9].American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th editionText revision. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- [10].Higgins JP, Altman DG, Gøtzsche PC, et al. The Cochrane collaboration's tool for assessing risk of bias in randomized trials. BMJ 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Arafa M, Shamloul R. Efficacy of sertraline hydrochloride in treatment of premature ejaculation: a placebo-controlled study using a validated questionnaire. Int J Impot Res 2006;18:534–8. [DOI] [PubMed] [Google Scholar]
- [12].Waldinger MD, Zwinderman AH, Olivier B, et al. A double-blind, randomized, placebo-controlled, fixed-dose study with paroxetine, sertraline, and nefazodone. J Clin Psychopharm 2001;21:293–7. [DOI] [PubMed] [Google Scholar]
- [13].Biri H, Isen K, Sinik Z, et al. Sertraline in the treatment of premature ejaculation: a double-blind placebo controlled study. Int Uro Nephrol 1998;30:611–5. [DOI] [PubMed] [Google Scholar]
- [14].Waldinger MD, Hengeveld MW, Zwinderman AH, et al. Effect of SSRI antidepressants on ejaculation: A double-blind, randomized, placebo-controlled study with fluoxetine, fluvoxamine, paroxetine, and sertraline. J Clin Psychopharm 1998;18:274–81. [DOI] [PubMed] [Google Scholar]
- [15].McMahon CG. Treatment of premature ejaculation with sertraline hydrochloride: a single-blind placebo controlled crossover study. J Urology 1998;159:1935–8. [DOI] [PubMed] [Google Scholar]
- [16].Kim CS, Seo KK. Efficacy and safety of fluoxetine, sertraline and clomipramine in patients with premature ejaculation: a double-bind, placebo controlled study. J Urol 1998;159:425–7. [DOI] [PubMed] [Google Scholar]
- [17].Mendels J, Camera A, Sikes C. Sertraline treatment for premature ejaculation. J Clin Psychopharm 1995;15:341–6. [DOI] [PubMed] [Google Scholar]
- [18].Ma SL, Li ZG. Comparison of efficacy of sertraline or tadalafil combined with behavioral therapy for premature ejaculation. Natl J Androl 2011;17:189–91. [Google Scholar]
- [19].Tao Q, Bin Z, Jun C, et al. Effect of sertralin combined with doxazosin on the treatment of premature ejaculation: a 240 cases report. J Sex Med 2012;9:141. [Google Scholar]
- [20].Lu Y, Yang D. Clinical study of Yimusake tablets combined with sertraline hydrochloride in the treatment of primary premature ejaculation. Seek Med Ask Med 2012;10:30–1. [Google Scholar]
- [21].Chen ZJ, Jiang SK, Zhang WS, et al. Clinical study of sertraline combined with terazosin in the treatment of premature ejaculation. J Mod Med Health 2014;30:3129–31. [Google Scholar]
- [22].Zhou XP, Yang YG, Xu HP, et al. Clinical observation of sertraline combined with tadalafil in the treatment of primary premature ejaculation. Jiangxi Med J 2014;49:903–4. [Google Scholar]
- [23].Zhu Y, Le J, Liu Z, et al. Sertraline hydrochloride combined with four-spot caressing for primary premature ejaculation. Natl J Androl 2015;21:1116–20. [PubMed] [Google Scholar]
- [24].Li G, Li N, Qiu JH, et al. Clinical study of sertraline combined with psychological counseling in the treatment of premature ejaculation and the effects on life satisfaction of patients. Chin J Hum Sexuality 2018;27:25–8. [Google Scholar]
- [25]. EAU Guidelines. Edn. Presented at the EAU Annual Congress Barcelona 2019. ISBN 978-94-92671-04-2. Available at: https://uroweb.org/guideline/male-sexual-dysfunction/. Accessed March 6, 2019. [Google Scholar]
- [26].Xia Y, Li J, Shan G, et al. Relationship between premature ejaculation and depression: a PRISMA-compliant systematic review and meta-analysis. Medicine (Baltimore) 2016;95:e4620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Waldinger MD, Zwinderman AH, Schweitzer DH, et al. Relevance of methodological design for the interpretation of efficacy of drug treatment of premature ejaculation: a systematic review and meta-analysis. Int J Impot Res 2004;16:369–81. [DOI] [PubMed] [Google Scholar]
- [28].Cooper K, Martyn-St James M, Kaltenthaler E, et al. Interventions to treat premature ejaculation: a systematic review short report. Health Technol Assess 2015;19:1–80. v-vi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Martin C, Nolen H, Podolnick J, et al. Current and emerging therapies in premature ejaculation: where we are coming from, where we are going. Int J Urol 2017;24:40–50. [DOI] [PubMed] [Google Scholar]


